Crystal structure of the TM1442 protein from Thermotoga maritima, a homolog of the Bacillus subtilis general stress response anti-anti-sigma factor RsbV
Introduction.
In Bacillus subtilis, the activities of several sigma (σ) factors and their cognate anti-σ factors regulate important cellular processes such as sporulation and stress response.1, 2 Sporulation in B. subtilis requires four σ factors: σE, σF, σG, and σK. The activities of σF and σG are controlled by the regulatory proteins SpoIIAB (anti-σ factor and serine kinase), SpoIIAA (anti-anti-σ factor), and SpoIIE (phosphatase). The anti-σ factor SpoIIAB inhibits σF and σG by direct interaction. It has also kinase activity that inactivates the anti-anti-σ factor SpoIIAA by phosphorylation at Ser58. SpoIIAB is regulated in turn by unphosphorylated SpoIIAA, which releases σF and σG from the SpoIIAB-σF and SpoIIAB-σG complexes. The solution structure of B. subtilis SpoIIAA revealed that it is a monomeric protein with a novel fold consisting of a four-stranded β-sheet and four α-helices.3, 4 Subsequently, the crystal structures of SpoIIAA from B. sphaericus in both phosphorylated and unphosphorylated forms were determined.5 A comparison of these structures shows that phosphorylation has no significant effect on the global structure of the protein.
Regulation of gene expression in response to the metabolic stress due to starvation and the environmental stress such as elevated temperatures, high salt concentrations, and high manganese ion concentrations is essential for bacterial survival. The general stress responses of B. subtilis involve an estimated 200 genes, which are regulated by σB, an alternative σ factor.6 The activity of σB itself is tightly controlled by a regulatory module consisting of RsbU or RsbP (phosphatase)-RsbV (antagonist)-RsbW (kinase).7 RsbV plays an important role in both the environmental stress and the energy stress. Likewise, σF-dependent expression of sporulation genes is controlled by the module comprising SpoIIE (phosphatase)-SpoIIAA (antagonist)-SpoIIAB (kinase). Significant homology was noted between the RsbU, RsbP, and SpoIIE phosphatases; between the RsbV and SpoIIAA antagonists; between RsbW and SpoIIAB kinases; and between the σF and σB transcription factors. It was concluded that the higher affinity of RsbW for RsbV than for σB is the driving force that is responsible for the switch of RsbW to unphosphorylated RsbV.8
The TM1442 gene of T. maritima encodes a 110-residue polypeptide chain of 12,300 Da. Its gene product shows significant levels (between 36 and 31%) of amino acid sequence identity to RsbV-like anti-anti-σ factors. The level of sequence identity to B. subtilis SpoIIAA and its homologs is lower (between 29 and 17%). In order to provide further structural data on anti-anti-σ factors necessary for a better understanding of their mechanism of action and functional differences, we have determined the three-dimensional structure of the TM1442 protein from T. maritima, a homolog of RsbV-like anti-anti-σ factors.
Materials and Methods.
Purification and crystallization of the recombinant TM1442 protein was reported previously.9 For the structure determination by the multi-wavelength anomalous dispersion (MAD) method, the selenomethionine (SeMet)-substituted protein was overexpressed in E. coli B834(DE3) cells using the M9 cell culture medium containing extra amino acids. The SeMet-substituted protein was crystallized using the reservoir solution consisting of 100 mM sodium acetate (pH 4.6), 100 mM magnesium chloride, and 18% (v/v) polyethylene glycol 400. X-ray diffraction data from a SeMet-substituted crystal were collected at three different wavelengths at 100 K on an Area Detector Systems Corporation Quantum 4R CCD detector at the BL-18B experimental station of Photon Factory (Table I, ). The raw data were processed and scaled with the program MOSFLM.10 The SeMet-substituted crystal belongs to space group P21, with unit cell parameters of a = 31.14 Å, b = 107.53 Å, c = 31.11 Å, α = 90.00°, β = 118.90°, γ = 90.00°. Collection of X-ray diffraction data from a native crystal of TM1442 to 2.0-Å resolution was reported previously.9
| Phasing | |||
|---|---|---|---|
| Data set | Remote | Peak | Edge |
| Wavelength (Å) | 0.9500 | 0.9794 | 0.9797 |
| Resolution (Å) | 20–1.9 | 20–1.9 | 20–1.9 |
| Unique reflections | 13,625 | 13,317 | 13,288 |
| Completeness (%) | 96.8 | 94.6 | 94.3 |
| Rsym (%)a | 3.5 (9.7) | 3.0 (10.3) | 4.0 (9.5) |
| Structure refinement | |
|---|---|
| Data set | Native |
| Wavelength (Å) | 1.0000 |
| Unit cell parameters (Å, °) | a = 31.54, b = 116.83, c = 31.39, β = 119.84 (P21) |
| Resolution (Å) | 20–2.0 |
| Number of reflections (working/free set)c | 11,503/1,322 |
| Completeness (%)d | 96.5 (96.5) |
| Amino acid residues (B-factor, Å2) | 110 × 2 (31.0) |
| Waters (B-factor, Å2) | 177 (43.0) |
| RMS bond lengths (Å)e | 0.014 |
| Completeness (%)d | 96.5 (96.5) |
| Rwork/Rfree (%)e | 19.2/24.8 |
- a Rsym = ΣhΣi|I(h)i − 〈I(h)〉|/ΣhΣi I(h)i, where I(h) is the intensity of reflection h, Σh is the sum over all reflections, and Σi is the sum over i measurements of reflection h. Numbers in parentheses reflect statistics for the last shell (2.00–1.90 Å).
- b Figure of merit = <|ΣP(α)eiα/ΣP(α)|>, where α is the phase and P(α) is the phase probability distribution.
- c R = Σ||Fobs| − |Fcalc||/Σ|Fobs|, where Rfree is calculated for a randomly chosen 10% of reflections, which were not used for structure refinement and Rwork is calculated for the remaining reflections. Reflections with |Fobs|/σ(F) < 2.0 were rejected.
- d Completeness for I/σ(I) > 1.0, high-resolution shell (2.11–2.00 Å) in parentheses.
- e Deviations from ideal bond lengths and angles.
The structure was determined by the MAD method and the model of the native protein was subsequently refined against data extending to 2.0 Å resolution (Table I). All of the four expected selenium atoms in two monomers of TM1442 in the crystallographic asymmetric unit were located with the program SOLVE11 and the selenium sites were used to calculate the phases with SHARP.12 Phasing statistics are summarized in Table I. Model building was done with the program O.13 The native model, refined with the program CNS,14 gave Rwork and Rfree values of 19.2 and 24.8%, respectively, for 20–2.0 Å data. The model (Protein Data Bank ID code 1VC1) has excellent stereochemistry, with no Ramachandran outliers.
Results and Discussion.
The two monomers of TM1442 in the asymmetric unit are related by a noncrystallographic two-fold symmetry and form a stable dimer [Fig. 1(A)]. A monomer of TM1442 forms a compact, single domain, the central feature of which is a mixed five-stranded β-sheet of topology −1, +1, +1, +1, and +1 [Fig. 1(A)]. One side of this central β-sheet is covered completely by three α-helices (α1–α3) and a 310-helix extending from helix α3, while the other side is covered only partially by a short winding loop at the C-terminus of the polypeptide chain [Fig. 1(A)]. A structural similarity search using the program DALI15 indicates that the overall fold of TM1442 monomer is shared by B. subtilis and B. sphaericus SpoIIAA,3-5 and the structural similarities are limited to them only. These SpoIIAA proteins show 23 and 17% sequence identity to TM1442, respectively. The most prominent structural difference is the replacement of a C-terminal loop of TM1442 by an α-helix at the C-terminus of SpoIIAA structures [Fig. 1(B)].
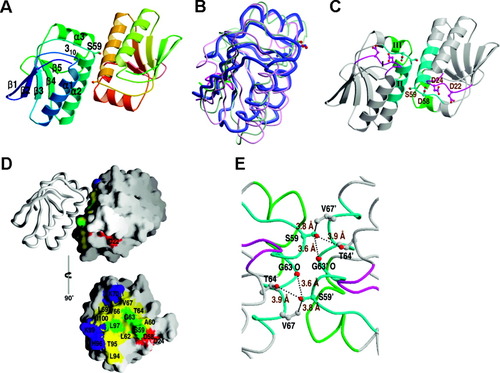
Structure of TM1442 from T. maritima. A: Stereo ribbon diagram of a dimer showing the secondary structure elements. Ser59 is drawn in a ball-and-stick model. Oxygen atoms are colored in red and the side chain carbon atoms of each Ser59 are colored in the same color as the secondary structure, to which the residue belongs. B: Superposition of three anti-anti-σ factor monomer structures. T. maritima TM1442, B. subtilis SpoIIAA, and B. sphaericus SpoIIAA are drawn in thick blue, thin green, and thin scarlet coils, respectively. The C-terminal α-helices of the B. subtilis and B. sphaericus SpoIIAA structures are highlighted in dark color. Ser59 of TM1442 and corresponding residues are drawn in a ball-and-stick model. C: Ribbon diagram of a TM1442 dimer showing the conserved regions I–III, which are colored in violet, cyan, and green, respectively. Ser59 and three conserved, negatively charged residues (Asp22, Asp24, and Asp58) are drawn in the same manner as in A. Labeling is shown in one subunit only. D: Surface representation of the dimer interface. The top view has the same orientation as in A. Strictly conserved and highly conserved residues in the dimer interface are colored in green and yellow, respectively. Of the highly conserved residues in the dimer interface, the positively- and negatively-charged residues are colored in blue and red, respectively. One monomer is omitted for clarity in the bottom view obtained by a 90°-rotation around a vertical axis. E: A detailed view around Ser59 in the same orientation as in C. The closest distances between side-chain atoms of Ser59 and other three residues are indicated. Primed residues belong to the second subunit of the dimer.
When we align the sequences of RsbV and SpoIIAA homologs, conserved residues are clustered around three distinct sequence regions I–III of TM1442 [Fig. 1(C)]. Region I (residues G21DIDAYN27, with the strictly conserved residues in bold) is located mostly in the β2–α1 loop. It is not involved in the formation of a dimer; instead it may possibly provide part of the interaction site with the cognate anti-σ factor and/or the cognate phosphatase. Highly conserved residues Asp22 and Asp24 from region I, together with another conserved residue Asp58 from region II, form a negatively charged surface patch adjacent to the phosphorylation site Ser59 [Fig. 1(D)]. Asp22 is invariably Glu in other homologs. The surface region defined by these residues may play a crucial role in binding to the cognate anti-σ factor and/or the cognate phosphatase. Region II (residues Y56MDSAGLGTLVVILK70), covering the C-terminal part of the β3–α 2 loop and the N-terminal part of helix α2, contributes to the formation of the dimeric interface. It includes the phosphorylation site Ser59 at the N-terminus of α-helix 2. The residues Ser59, Ala60, Leu62, Gly63, Thr64, Val66, Val67, Leu69, and Lys70 make contacts with the other subunit in a dimer [Fig. 1(D)]. Strictly conserved Gly61 seems to play a structural role for proper folding of the polypeptide chain. The third conserved region III (residues R90ILKLTHLDKIF101), encompassing the C-terminal part of α-helix 3 and the following 310-helix, contributes to the dimeric interface as well. The residues Leu94, Thr95, His96, Leu97, Lys99, and Ile100 make contacts with the other subunit in the dimer interface [Fig. 1(D)]. Lys99 in one monomer of the dimer makes a hydrogen bond with the main chain carbonyl oxygen atom of Lys99′ in the other monomer directly or through a water molecule. The primed residue belongs to the other subunit of the dimer.
We previously observed that the recombinant M1442 protein exists both as a monomer and as a dimer in solution.9 In this study, we have determined the crystal structure of the TM1442 protein in its dimeric form. Generation of other stable dimers different from the present dimer by the crystallographic symmetry operations is not possible. Thus we conclude that the observed dimeric structure of TM1442 in the present crystal represents the dimeric form in solution. Although further studies are needed to clarify the functional significance of the dimeric structure of TM1442, it appears difficult for the TM1442 dimer to accommodate two phosphate groups upon phosphorylation of Ser59 and Ser59′, if we assume that phosphorylation induces little structural change in each subunit as observed in B. sphaericus SpoIIAA.5 This is because the side-chain atoms of Ser59 are located within the distances of 3.6–3.9 Å from the main chain oxygen atom of Gly63′ and the side-chain atoms of Thr64′ and Val67′ [Fig. 1(E)]. The steric hindrance and the charge repulsion due to the presence of bulky phosphate groups at Ser59 and Ser59′ (side-chain oxygen–oxygen distances being 9.3 Å) could possibly lead to the dissociation of the dimer into monomers. We propose that the possible phosphorylation-dependent dimer-monomer transition may be important for the function of RsbV-like anti-sigma factor antagonists.
Acknowledgements
We thank Dr. N. Sakabe, Dr. M. Suzuki, and Dr. N. Igarashi for assistance during data collection at BL-6B of Photon Factory, Japan (approval no. 01G147). HJA and KSH are recipients of the BK21 fellowship.




