Hypofractionated radiotherapy (RT) combined with dihydroartemisinin (DHA): No synergistic effect observed in a preliminary animal study
Abstract
Purpose
Colorectal cancer (CRC) is one of the leading causes of cancer-related death worldwide. Dihydroartemisinin (DHA) is an anti-malaria agent and recent evidence indicates a broad anti-cancer activity. Radiotherapy (RT) is the cornerstone of cancer treatment and often has synergistic effects when combined with chemotherapy or targeted therapy. We aim to investigate whether synergistic effect exists for RT combined with DHA in a preliminary animal study of CRC treatment.
Methods
Twenty-four BALB/c nude mice were subcutaneously injected with 5.0 × 106 cells of the murine CRC cell line CT26. When tumor xenografts were formed (∼ 100 mm3), mice were randomly allocated into four groups (n = 6 per group) and the tumor-bearing mice were intra-abdominally injected at Day 7, 9, 11 with PBS (control), 6Gy irradiation (RT), DHA (50mg/Kg), and DHA with irradiation (DHA+RT). All RT was performed on a medical linear accelerator using collimated anterior posterior 6MV photon beam conformal to tumor xenografts. The tumor volume was measured using an electronic caliper and was calculated based on the length (L) and the width of the tumor using the formula V = (L x W2)/2. Tumor weight was also measured after mice sacrificed. Histological assay was conducted, including using gammaH2AX for DNA double strand breaks (DSB) analysis.
Results
The tumor weight was 2.4±0.8g, 2.5±0.6g, 0.4±0.2g, and 0.4±0.1g for the control, DHA, RT, and DHA+RT groups, respectively. Significant difference was observed between the control and RT groups, and between the control and DHA+RT groups (p<0.05). However, there was almost no difference between the RT alone and DHA+RT groups. The longitudinal change in tumor volume showed tumor progression inhibition in the RT and DHA+RT groups, but not so obvious in the DHA group, which was consistent with histological assay.
Conclusion
Similar treatment efficacy is observed in the RT alone and concurrent DHA+RT group. No significant difference in tumor volume or weight or tumor progression inhibition is observed between the RT alone and concurrent DHA+RT groups, demonstrating that DHA might not provide synergistic effect with RT for the proposed hypofractionated radiation dose regimen.
1 INTRODUCTION
Colorectal cancer (CRC) is the third most commonly diagnosed cancer and the fourth leading cause of cancer-related death worldwide.1 In the United States, CRC is the fourth most frequently diagnosed cancer and the second leading cause of cancer death.2 The incidence rates of CRC are increasing in developing countries due to the adoption of western lifestyle and the global burden is expected to further rise because of population growth and aging. Moreover, the incidence rates in young adults (<50 years old) have been increasing in high-income countries across North America, Europe and Oceania.3 More than half patients diagnosed with CRC develop colorectal metastases and 80 ∼ 90% of those patients have unresectable metastatic liver disease.2 For surgically unresectable CRC lesions and metastasis, an alternative local treatment approach towards oligometastatic CRC is stereotactic body radiotherapy (SBRT), which is a high precision hypofractionated radiation technique using ablative doses to treat isolated tumor sites. Compared to surgery, SBRT does not need general anesthesia and its high biological equivalent dose (BED) can yield optimal local control rates, which may lead to increased progression free survival (PFS) and overall survival (OS).4
Artemisinin from the Sweet wormwood (qinhao) in Chinese herbal garden, or its derivatives artesunate and dihydroartemisinin (DHA), was identified as an effective anti-malaria agent by Youyou Tu and colleagues.5 The discovery and use of artemisinin have saved millions of lives across the globe and Youyou Tu was honored with the Nobel Prize for Medicine or Physiology 2015.6 Recently, there has been evidence of a broader anti-cancer activity in artemisinin-derived compounds.7-9 Since artemisinin and its derivatives bear peroxide-containing sesquiterpene lactone, they may form free radicals with high reactivity and possess anti-cancer pharmaceutical properties.7 Artemisinin related compounds have cytotoxic effects against cancer cells through pleiotropic effects, including inhibiting tumor cell proliferation, promoting apoptosis, and preventing angiogenesis.8 Specifically, DHA, a semi-synthetic derivative of artemisinin, has been suggested as a natural product and a weapon against cancer to induce non-canonical cell death.9 Some studies show that artemisinin derivatives could reduce cancer cell viability with high sensitivity to drug-resistant cancer cells with minor side effects to normal cells.10-12 For example, DHA or artesunate was found to induce ROS-mediated apoptosis to counteract tumor growth,13 or apoptosis through the mitochondria-dependent pathway14 or through EGFR/RAS mutation related mechanism,15 or ferroptosis16, 17 and autophagy,18 or inhibiting tumorigenesis19 and suppressing colony formation,20 promoting cell death via lysosomal function21 or via cellular senescence.22 All those properties of DHA may lead to its drug repurposing in oncology.23 In fact, DHA has been used as a sensitizing agent in cancer therapy to facilitate chemotherapy or targeted therapy in ovarian, pancreatic and lung cancer cells.24-27 There are also published studies of DHA enhancing photodynamic therapy (PDT) against breast tumor and human papillomavirus infection.28, 29
DHA may play a role in sensitizing cancer therapy of CRC. Bader et al30, 31 studied hypoxic CRC cell line HCT116 treated with DHA and ionizing radiation (IR). Because DHA demonstrated pro-oxidative characteristics, hypoxic CRC cells returned to normoxia with a delayed cell death in vitro. They found DHA improved efficacy of IR of low energy (320kV), low dose rate (3.7Gy/min) and a single fraction of 2Gy in cell culture. Kim et al32 found that DHA radiosensitization of 6Gy gamma radiation in cell culture of human glioma in vitro but did not specify the radiation energy and dose rate. Fei et al33 treated esophageal cancer cells using 6MV photon 4Gy-irradiation (IR) with DHA and found the survival rate of combined therapy was slightly lower than the monotherapy of radiation in cell culture. Zhao et al34 conducted similar experiments in non-small cell lung cell (NSCLC) A549 and found DHA induced cell cycle arrest together with IR of beta-ray (electron RT) of a single dose of 5Gy in cell culture and nude mice, where they suggested a further investigation was needed to validate DHA's efficacy in vivo. Luo et al35 applied 2Gy or 6Gy IR in vitro and 10Gy IR in vivo with DHA to treat cervical cancer using X-rays from a linear accelerator but did not specify IR dose rate or beam energy. Although they claimed artemisinin compounds potently induced radiosensitivity of cervical cancer cells, yet tumor volume almost had no difference in mice between DHA+RT and RT alone groups. Reichert et al36 reported treating glioblastoma (GBM) cells with artesunate and irradiation resulted in an increased apoptosis and pronounced G2/M cell cycle arrest, which showed artesunate might radiosensitize glioma cells in vitro. In terms of treating CRC, it has been reported that DHA is a hypoxia-active drug in cell culture.37 The oral artesunate treatment prior to surgical resection of CRC was well-tolerated and might reduce Ki67 expression in a single-center, randomized, double-blind, placebo-controlled pilot trial study.38 There were also nanoparticles designed to simultaneously deliver paclitaxel (PTX) and DHA to CRC, where increased apoptosis in CRC cell line HT-29 was observed and nanoparticles exhibited higher accumulation in the tumor site. The combination of PTX and DHA in a dual-drug co-delivery nanoparticle system minimized severe side effects.39
Radiotherapy (RT) is the cornerstone of cancer treatment and has been used clinically for more than a century. RT often has synergistic effects when combined with chemotherapy or targeted therapy. Since Meanwhile, DHA has potential to increase radiotherapy efficacy of CRC with minimal side effects in vitro. Therefore, whether synergy exists in the combined RT and DHA therapy in CRC needs to be investigated in vivo. Specifically, we aim to investigate whether DHA can sensitize SBRT in CRC treatment in well-controlled animal experiments.40
2 MATERIALS AND METHODS
2.1 Cell culture and animal preparation
All experimental procedures in this study were conducted in accordance with the Animal Care and Use Regulations to ensure ethical treatment of the animals. The murine colorectal cancer cell line CT26 was purchased from The Cell Bank of Chinese Academy of Sciences (Shanghai, China) and cultured in RPMI 1640 medium (Hyclone, USA) containing 10% fetal bovine serum (Gibco, USA), penicillin (100 U/mL) and streptomycin (100 µg/mL) in an incubator with 5% CO2 and 95% air at 37°C. All cells were used within 20 passages. The cell line's original source was validated using short-tandem-repeat (STR) profiling.
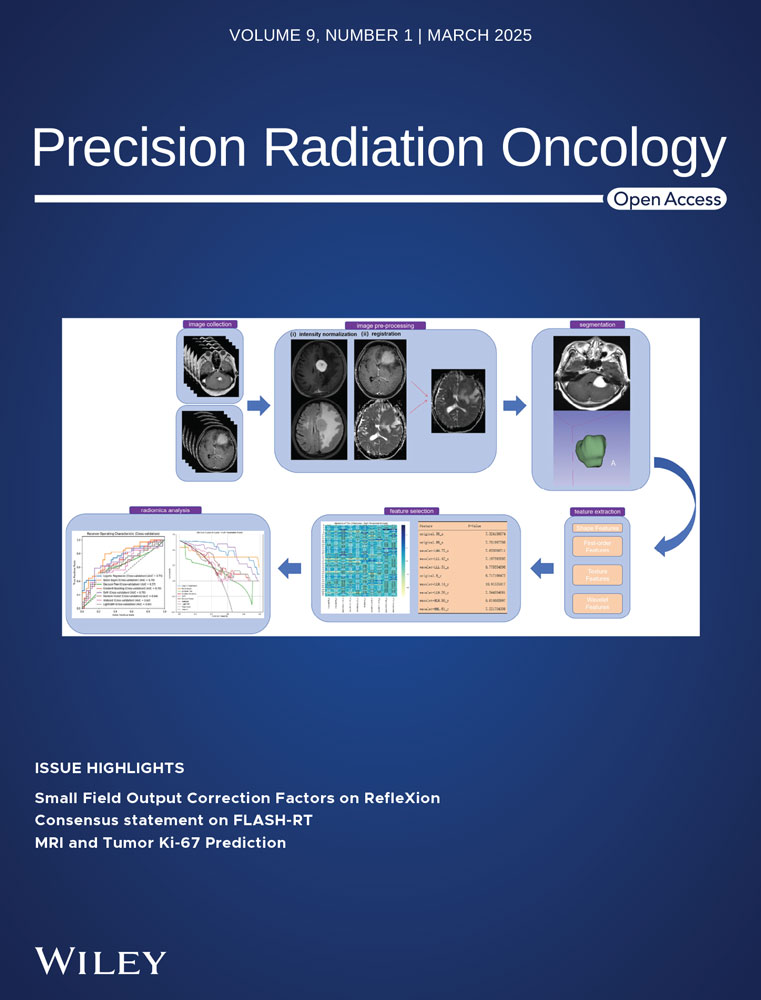
Twenty-four BALB/c female nude mice (6∼8 weeks old) were purchased from GemPharmatech Company (Guangdong, China). The well-mixed CT26 cell suspensions of 5 × 106 cells in 0.1 mL of PBS solution were injected subcutaneously to the right limb to generate CRC tumor xenografts. Following inoculation, all mice were monitored until they fully recovered and were moving. Subcutaneous xenografts grew to approximately 100mm3 (or 0.1 cc) in 1∼2 weeks and that day was defined as Day 0. Then, the tumor-bearing mice were randomly allocated into four groups with each group's sample size n = 6. The four groups were (1) control (abdominal injection of PBS); (2) DHA (abdominal injection of 50mg/Kg on Day 7, 9 and 11); (3) RT (6Gy on Day 7, 9 and 11); and (4) DHA+RT (abdominal injection of 50mg/Kg one hour prior to RT of 6Gy on Day 7, 9 and 11).
Prior to RT, mice were anesthetized with ketamine and xylazine (100 mg/kg and 10 mg/kg) by intraperitoneal injection. All mice were sacrificed on Day 15, and tumors of different groups was dissected for immunohistochemistry (IHC) assay. The animal experimental workflow was shown in Figure 1.
2.2 Drug treatment
Dihydroartemisinin (DHA) has been reported to have higher bioavailability and less side effects than artemisinin.41 The DHA drug used in our study was obtained from Sigma-Aldrich (D7439, St Lious, USA) by the Department of Pharmaceutical Sciences at Shenzhen University. The abdominal injection of 50mg/Kg was applied as a typical in vivo dose suggested by previously published animal studies in mice.16, 27 For Group 2 and Group 4, mice had three abdominal injections of 50mg/Kg DHA on Day 7, 9 and 11, respectively. In mice of Group 4, DHA injection happened one hour prior to RT. All DHA treated mice were monitored closely and no abnormal behaviors were observed.
2.3 Radiotherapy
For Group 3 and Group 4, mice had three RT fractions of 6Gy using anterior posterior (AP) collimated 6MV photon beam conformal to tumor xenografts on Day 7, 9 and 11, respectively. In mice of Group 4, DHA injection happened one hour prior to RT. All RT was performed on a clinically operated medical linear accelerator (Varian Medical Systems, Palo Alto, USA) with 6 megavoltage (MV) photon and dose rate of 600 monitor unites (MU)/min, where output and energy were monitored daily, monthly and annually with routinely quality assurance (QA) procedures according to AAPM task group report (TG) 142.42 All mice were set up in prone positioning with an anterior posterior (AP) 6MV photon beam with collimated jaws conformal to tumor xenografts with the source to surface distance (SSD) of 100 cm. Delivered dose of 6Gy per fraction was verified using calibrated EBT3 Gafchromic films beneath mice (Ashland, USA).
2.4 Tumor volume and IHC evaluation
The tumor volume was measured using an electronic caliper every three days throughout the experimental study. The tumor volume (V) was calculated based on the length (L) and the width of the tumor (W) using the formula V = (L x W2)/2. Tumor weight of all groups was also measured after sacrifice.
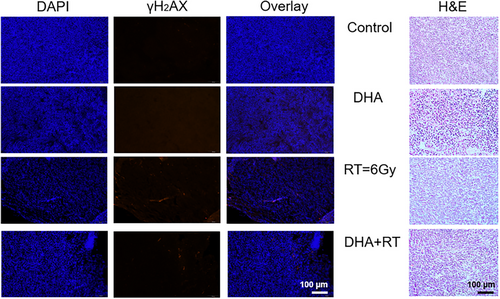
On day 15, all mice were sacrificed, and xenograft tumor was resected from mice. Photos were taken to record the tumor size. Tumor tissue was stained with hematoxylin and eosin (H&E) obtained from ThermoFisher Scientific (Waltham, MA, USA) for histological assay. H&E staining as a laboratory technique was provide microscopic examination of tissue sections, where hematoxylin (H) was shown as a deep blue-purple color for cell nuclei and eosin (E) was shown as orange-pink-red color for collagen and connective tissue. Together, H&E staining could show abnormal structure. Tumor tissue was also stained with gammaH2AX purchased from American Master Tech Scientific (Lodi, CA, USA) for DNA double strand breaks (DSB) analysis.43 For all digital imaging of HE and gammaH2AX assay, all samples were imaged with identical settings on a Leica microscope (Leica Biosystems, Wetzel Gmbh Germany) and quantification of images was performed using ImageJ software (v.2.0.0, NIH).
Statistical comparisons between different groups were calculated by ANOVA, and *p < 0.05 was considered statistically significant. All values were presented in the figures and table as mean ± standard error of mean (SEM). Statistical significance between two groups was determined by unpaired, two-tailed Student's t test.
3 RESULTS
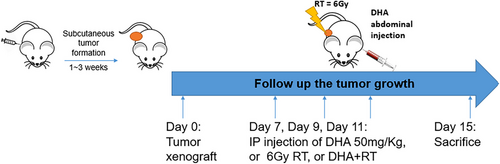
The tumor volume increased longitudinally in the control and DHA groups, while tumor growth was inhibited in the RT and DHA+RT groups (Figure 2).
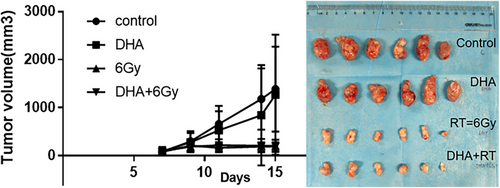
The tumor bearing mice with the murine colorectal cancer cell line CT26 had significantly lower tumor weight of the RT or DHA+RT groups than that of the control group (p<0.05) (Table 1). However, there was no significant difference between the RT alone and RT+DHA groups (Figure 3), which demonstrated that RT and DHA+RT might be effective therapies, yet the addition of DHA to RT might not have synergistic effect.
| Subcutaneous colorectal cancer (CRC) xenograft | Mean (g) | ± Standard error of mean (g) | Sample size (n) |
|---|---|---|---|
| Control (PBS) | 2.39 | 0.76 | 6 |
| DHA (abdominal injection 50mg/Kg x 3) | 2.49 | 0.61 | 6 |
| RT (6Gy x3) | 0.36 | 0.16 | 6 |
| DHA+RT (50mg/Kg+6Gy) x3 | 0.36 | 0.22 | 6 |
| p-value | Control vs. RT | 0.030* | |
| Control vs. DHA+RT | 0.019* | ||
| DHA vs. RT | 0.010* | ||
| DHA vs. DHA+RT | 0.022* | ||
| Control vs. DHA | p > 0.05 | ||
| RT vs. DHA+RT | p > 0.05 | ||
- * p < 0.05 Significant difference.
In histological analysis, a little more blurred red spots were observed in the DHA group compared to the control group in the overlay DAPI and gammaH2AX staining results, which meant more DSB of DNA damage was observed microscopically in the DHA group. However, DSB of DNA damage was much more obvious in the RT group (Figure 4 left panel), which demonstrated that RT was much more effective than DHA in treating CRC in vivo. Similarly, abnormal microscopic structures were observed in the DHA, RT and DHA+RT groups compared to the control group (Figure 4 right panel).
4 DISCUSSION AND CONCLUSION
Significant difference is observed between the control and RT groups, and also between the control and DHA+RT groups (p<0.05). However, there was almost no difference between the RT alone and DHA+RT groups. The longitudinal change in tumor volume showed tumor progression inhibition in the RT and DHA+RT groups, but not very obvious in the DHA group, which was consistent with histological assay.
The in vitro results of DHA combined with targeted therapy, radiotherapy or chemotherapy showed synergistic effects clearly in cancer cell lines (see references,27-37 which motivated us to pursue whether DHA would provide similar synergistic effect with radiotherapy in the animal study. The in vivo DHA dosage and abdominal injection were used based on previously published animal experiments.16, 27 We closely monitored the mice after DHA injection and all of them showed tolerance after ten minutes. The RT dose and energy was used based on clinical and pre-clinical practice of stereotactic body radiation (SBRT) for oligometastatic colorectal cancer.4 The animal study was designed to prepare for a potential combined DHA and RT pre-clinical trial. Unfortunately, no significant difference in tumor volume or weight or tumor progression inhibition is observed between the RT alone and concurrent DHA+RT groups, indicating that DHA might not provide synergistic effect with RT for the proposed hypofractionated radiation dose regimen. We observe treatment efficacy in the RT alone and concurrent DHA+RT group, but the combined DHA and RT regimen might not have a greater benefit than the sum of the individual effect, which might be because RT has dominant treatment efficacy in the animal experiments and the addition of DHA could not further sensitize or enhance colorectal cancer cell apoptosis. There were several limitations in this study. One of them was that biological assays were not comprehensive for treatment efficacy analysis. We focused on the double strand breaks (DSB) of DNA in this preliminary study. Yet many other assays, such as Ki-67 for apoptosis, colony formation assay for overall survival, or flow cytometry for cell cycle analysis, were not performed. The second limitation of this preliminary study was that we only focused on the hypofractionated radiotherapy dose regimen to mimic CRC oligometastatic reality in clinical setting. Future studies may be designed to explore conventional radiotherapy dose regimens, such as 2Gy per fraction, to investigate whether such synergistic effect exists in animal studies. The third limitation was that the underlying cell pathway mechanisms were not discussed in this study due to the lack of expertise. We authors were trained as physicists who emphasized the precision dose delivery and tracking of tumor volume reduction. Nonetheless, this well-controlled dose-verified concurrent DHA and radiotherapy animal study provides insights to future design in combined cancer therapies.
CONFLICT OF INTEREST STATEMENT
All authors declare no conflict of interest.
FUNDING INFORMATION
No.
ETHICS STATEMENT
All experimental procedures in this study were conducted in accordance with the Animal Care and Use Regulations to ensure ethical treatment of the animals.
Open Research
DATA AVAILABILITY STATEMENT
The experimental data generated in the current study are available within the manuscript.