Consensus statement on the exploration of clinical translation and application of electron ultra-high dose rate FLASH radiotherapy
Abstract
Ultra-high dose rate FLASH Radiotherapy (FLASH-RT) has attracted wide attention because the well-known FLASH effect and the extremely short irradiation time. During FLASH-RT, high radiation doses and dose rate (usually thousands of times compared with conventional radiotherapy (CONV-RT)) are delivered to the tumor area. This novel irradiation technique shows a reduction of normal tissue injury (20-40%) in comparison to CONV-RT. Meanwhile, FLASH-RT maintaining comparable tumor killing effect as CONV-RT. With the progress of basic research on FLASH-RT in reducing radiation-induced injury to normal tissues, clinical trials of FLASH-RT have been carried out across the world. To date, there is no consensus in China focused on the exploration of clinical transformation and application of electron FLASH-RT. Therefore, the China Anti-Cancer Association Radiation Oncology Committee and the Chinese Medical Doctor Association Radiation Oncology Physician Committee gathered a group of experts together to develop this consensus statement. The authors discuss their current views on electron FLASH-RT, demonstrate the unresolved questions, provide insights for the further application of this technology in clinical practice.
Radiotherapy is a vitally important strategy in the management of malignant tumor. It is estimated that radiotherapy is needed in 50–70% of all patients with tumor across the world, either in curative or palliative treatment.1 The aim of radiotherapy is dedicated to deliver large doses of radiation to tumors, while reducing dose-limiting toxicity to organs at risk and the surrounding normal tissues.2 According to the dose rate administrated on tumors, the present radiotherapy utilized in clinic can be divided into the following: conventional dose rate radiotherapy (CONV-RT, 0.01 Gy/second), high dose rate brachytherapy (0.3 Gy/second), stereotactic body radiotherapy (1 Gy/second), etc.3 All the radiation modalities give the highest chance of curing or shrinking the tumor, however, radiation-associated side effects becomes an important bottleneck in achieving satisfactory tumor control.
Over the last decade, the development of technology allows linear accelerators provide multiple radiation modalities, for example ultra-high-dose-rate FLASH radiotherapy (FLASH-RT).4 FLASH-RT is a cutting-edge treatment strategy to deliver large doses into the target volume by ultra-high dose rate in an extremely short time.5 FLASH-RT offers superior tissue protection than CONV-RT in normal tissues without compromising anticancer efficacy in various in vivo modes.6 This normal tissue sparing after exposure to ultra-high dose rate irradiation is known as the FLASH effect. FLASH-RT is characterized with unique and revolutionary radiobiological advantages; and becomes an emerging science-driven advance in radiotherapy.7 Two completed clinical trials demonstrated the safety and feasible of FLASH-RT in patients with cancer.8, 9 There are several ongoing clinical trials focused on the efficacy and toxicities of FLASH-RT (NCT04986696, NCT05724875, NCT05524064, NCT06549439, ChiCTR2400080935).
Although researchers and clinicians are dedicated to promote the clinical translation of FLASH-RT, this innovative technology is still in the early stage of clinical trials. Consensus statement on the exploration of clinical transformation and application of electron ultra-high dose rate FLASH radiotherapy is rare. Therefore, the China Anti-Cancer Association Radiation Oncology Committee, the Chinese Medical Doctor Association Radiation Oncology Physician Committee collaborated to develop this consensus statement, this will contribute to standardize the clinical transformation and application of electron ultra-high dose rate FLASH radiotherapy.
1 DEFINATION
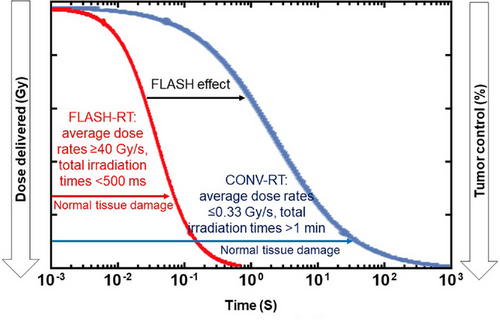
FLASH radiotherapy is an innovative irradiation technology defined as a single or several fractions ultra-high dose rate (average dose rates ≥40 Gy/s, and instantaneous dose rate, up to 106 Gy/second), with total irradiation times <500 milliseconds and preferably microseconds (Figure 1).5 These irradiation parameters are very challenging to achieve, especially with photon. Therefore, the first FLASH-RT preclinical study and clinical application were all performed with elections.10, 11 Although electron FLASH-RT is limited by their low penetration depth (approximately 3 cm), the FLASH effect is most commonly observed with electron beams.
2 IRRADIATION DEVICES
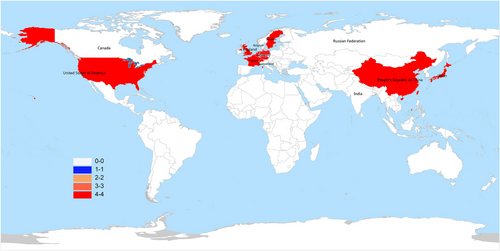
FLASH-RT can instantaneously deliver high-energy beams, and a powerful FLASH irradiation system is required. To date, the widely used electron FLASH-RT facilities including dedicated electron accelerators, well-modified medical accelerators, and originally designed devices. As illustrated in Figure 2, a total of 9 countries in the world have the device to perform FLASH-RT. The Oriatron eRT6 electron linear accelerator was developed by PMB-ALCEN (France) and utilized in the first human clinical trial at Lausanne University Hospital. The dosimetric parameters used for electron FLASH-RT including an energy of 5.6 MeV, an average dose rate of 166 Gy/s, an instantaneous dose rate of 1.8 × 105 Gy/s, and can only penetrate tissues to a depth of about 3 cm. This device is mainly designed for superficial tumors.12 The phase I clinical trial of dose escalated FLASH-RT (22-34 Gy) for cutaneous malignant melanoma was carried out by using the Mobetron intraoperative radiotherapy system.13 The Mobetron is a self-shielded electron beam linear accelerator designed by IntraOp Medical company in the United States. The average dose rate of Mobetron was 100 Gy/s, the instantaneous dose rate was 1000 Gy/s, and can penetrate tissues to a depth of about 4 cm.14 In 2022, our FLASH-RT group successfully delivered electron FLASH irradiation by modifying a Varian 23CX medical linear accelerator. The average dose rate achieved 120 Gy/s at the source skin distance of 100 cm, and the instantaneous dose rate achieved 86500 Gy/s; all the dose rate were confirmed by radiochromic films (Gafchromic HD-V2 films, Ashland, USA) and alanine-ESR dosimeter.15 Furthermore, there were several groups from Europe and USA successfully established electron FLASH-RT platforms by modifying the existing linear accelerators.16-19
FLASH-RT needs the delivery of electron beam fluences several orders higher than those used for CONV-RT. The design of novel devices contributes to achieve ultra-high dose rate, high enough for FLASH-RT. Of note, very-high-energy electron (VHEE) beams have been explored recently. VHEE can penetrate deeply into the tissues with energy above 100 MeV.20 Compared with conventional energy electron beams that commonly used in clinical practice, VHEE beams with energy of 250 MeV can be utilized to treat deep-seated tumors (in the region 5–30 cm).21 Moreover, VHEE has uniform dose distribution, high conformity and low scattering rate, which is superior to X-ray.22, 23 It was confirmed that VHEE could effectively cause DNA damage based on the plasmid model, and approximately 99 % of the damage was caused by indirect effects of radiation.24 However, whether VHEE beams can spare normal tissue remains to be elucidated.
3 DOSE RATE VERTIFICATION
Verifying dose distribution during FLASH-RT is vitally important. FLASH-RT is a novel technique for tumor treatment with an ultra-high does rate of more than 40 Gy/s, the irradiation time is extremely short compared to CONV-RT.7 The integrated process of FLASH-RT is complex and involves understanding of the principles of radiochemistry, medical physics, radiation biology, treatment planning, radiation dosimetry, simulation, radiation safety and protection to ensure accurate and safe delivery of treatment.8, 11 Because the instantaneous dose rate of FLASH-RT is significantly higher than that of CONV-RT, ionization chambers and semiconductors will achieve saturation during FLASH-RT.25, 26 Alanine dosimeter, thermoluminescence dosimeter, radiochromic films, and photoluminescence dosimeter has been utilized to measure the does rate of FLASH-RT.27, 28
The diamond detector exhibits a considerable linearity of does response to both electron and photon FLASH-RT, and could provide a relatively rapid and accurate dosimetry.29 The group from Tsinghua University designed a large dynamic range current measurement circuit based on the pre-integration method, and developed a diamond detector system prototype with real-time current output using a CIVIDECTM B1HV diamond sensor.30 The device is expected to be applied in the quality assurance of FLASH-RT. In addition, image-guided FLASH-RT can be considered to ensure real-time tracking of tumors and improve dose delivery. In recent years, Cherenkov imaging has emerged as a unique method of radiation therapy verification, it enabled the visualization of radiation dose on tumor surface because the produce of Cherenkov light.31 Notably, though diamond detector and Cherenkov imaging are now available, its clinical application are still ongoing. All the eligible equipment that can be utilized for dose rate verification has been summarized in supplementary Table S1. Overall, new dosimeters and dosimetric protocols capable of detecting electron beams at ultra-high dose are required, these will facilitate the trigger of optimal normal tissue spare effect.
4 THE MECHANISM OF FLASH EFFECT
Since the middle of the 20th century, especially in 2014, the FLASH effect has been observed in zebrafish and various mammal models.10, 32 Compared with CONV-RT, FLASH-RT significantly decreased radiation damage to normal tissues including brain, skin, lung, intestine, mesenchymal tissue, muscle, and hematopoietic stem cells; and maintained the same tumor killing effect compared with CONV-RT.33-37 The biological mechanisms of FLASH effect are mainly focused on oxygen consumption, free radical production, DNA integrity, reactive oxygen species, nuclear and mitochondria damage, and circulating immune cell exemption.38-42
Oxygen content is one of the influencing factors of cell survival fraction.43 With the increase of oxygen content, the survival fraction of normal cells decreases after CONV-RT.44 This is known as the “oxygen effect”. Indeed, the “oxygen effect” during FLASH-RT has been investigated. Based on the plasmid model, FLASH effect is affected by oxygen content.45 The oxygen depletion hypothesis emphasized the significance of instantaneous dose rate within FLASH-RT.46, 47 FLASH-RT can consume a large amount of oxygen molecules in normal tissues instantaneously, this transient hypoxia is beneficial to protect normal tissues; while the effect on hypoxic tumor tissues is slight.48 In contrast, a group from University of Pennsylvania believed the oxygen consumption capacity of FLASH-RT was inferior to that of CONV-RT at the cellular level in vitro; nevertheless, the continuous oxygen supply during CONV-RT can offset its inherent oxygen consumption capacity in vivo.49 Another group from Lausanne University Hospital confirmed the outcomes using an oxygen-sensitive phosphorescent probe (Oxyphor PtG4).50 Therefore, FLASH-RT does consume oxygen, but it cannot fully explain the FLASH effect.
The ‘DNA integrity’ hypothesis has been proposed to interpret FLASH effect.40 The Soochow University group found that FLASH-RT (average dose rate ≥ 100 Gy/s) can alleviates intestinal damage in immune checkpoint PD-L1 knockout mice and significantly improve the survival rate of irradiated mice compared with CONV-RT. FLASH X-Ray produces less cytosolic DNA and elicits less cGAS-STING activation compared with CONV-RT in the intestinal crypts. The group emphasized the significance of the duration of dose delivery. FLASH-RT can deliver the prescribed radiation dose into the target volume in milliseconds, this short-term dose delivery contributes to maintain the DNA integrity. Therefore, the ‘DNA integrity’ hypothesis might play a crucial role in interpreting the FLASH effect.
Reactive oxygen species (ROS) has been demonstrated to play an important role in alleviating radiation induced damage to normal tissues.51 In classic radiation biology, both direct effect and indirect effect account for eradicating tumor cells. Previous study indicated the indirect effect of ionizing radiation initiate FLASH effect rather than the direct effect.52 The water radiolysis analysis by Kacem et al revealed that the production of free radicals was significantly decreased during the homogenous chemistry phases in the FLASH-RT group compared with the CONV-RT group using deaerated samples; the radical-radical interaction have taken place at this stage, and the generated free radicals begin to diffuse.39 In comparison to CONV-RT, radiation-induced neuroinflammation was markedly resolved after exposure to FLASH-RT by reducing the production of ROS, the oxidative stress involved in regulating the brain injury.53 Bogaerts et al suggested that FLASH-RT was associated with decreased normal cell injury by limiting the mitochondrial ROS in comparison to CONV-RT.54 Therefore, the group from Lausanne University Hospital proposed that ROS generated by the indirect effect of ionizing radiation might involve in mediating the FLASH effect.52
The nuclear and mitochondria damage hypothesis has also been proposed to interpret the FLASH effect. Mitochondrial plays an essential role in regulating cell damage and inflammatory pathway.55 Han et al demonstrated the loss of mitochondrial function contributed to alleviated late apoptosis and necrosis in mouse embryonic fibroblast cells after exposure to FLASH-RT.56 Lv et al implied FLASH-RT reduce the immune damage to normal tissues by inhibiting the IFN-I inflammatory response, and the mitochondrial DNA triggered cGAS-STING signal pathway involved in regulating the IFN-I inflammatory response.57 Further investigation of mitochondria damage is required, especially in multiple animal models.
In addition, several studies attempt to elucidate the FLASH effect from the perspective of ‘immunization exemption’ hypothesis.58, 59 The irradiation dose of CONV-RT to circulating immune cells was associated to lymphopenia.60 The irradiation time of FLASH-RT is extremely shorter than that of CONV-RT. A single high-dose FLASH-RT is beneficial to spare the proportion of radiation-induced lymphocytes and decrease the incidence of chromosome aberration.54 The glioblastoma model showed that FLASH-RT has the potential to mitigate depletion of circulating blood and lymphocytes (about 4%) compared with CONV-RT.58 Overall, the mechanism of FLASH effect is complicated, the physico-chemical and biological response to FLASH-RT and CONV-RT was inconsistent, further exploration is needed.
5 RADIATION SENSITIVITY
FLASH-RT is different from CONV-RT by sparing normal tissues, whether tumor radiation sensitivity of CONV-RT is similar in comparison to FLASH-RT is unclear. Tumors sensitive to FLASH irradiation will facilitate further screening of eligible patients of FLASH-RT. Previous bioinformatics analysis of human acute lymphoblastic leukemia showed that genotype may involve in regulating the sensitivity of FLASH-RT.37 In the phase 3 animal trial of primary nasal squamous cell carcinoma in cats, one cat in the FLASH-RT group experienced disease progression 1-year post irradiation, while there was no tumor progression observed in the CONV-RT group.61 These results suggested that individual sensitivity to radiation may lead to different results. Nevertheless, the sample size for studies estimated the sensitivity of FLASH-RT is small, and further exploration is necessary.
6 ANIMAL TRIALS
The efficacy and safety of FLASH-RT have been investigated in various of animal trials. Favaudon et al established an orthotopic lung cancer model and performed whole thoracic FLASH-RT and CONV-RT (single does, 17 Gy), after 5–7 weeks, the pulmonary fibrosis was more severe in CONV-RT group compared with FLASH-RT group.10 In mini-pigs, electron FLASH-RT significantly reduced radiation damage to normal skins compared with CONV-RT by using a single irradiation dose (28-34 Gy). 36 weeks post irradiation, histological analysis showed that radiation-induced skin fibrosis, necrosis, and keratosis were severe in the CONV-RT site, whereas it was mild to moderate in the FLASH-RT group.62 Further exploration revealed both inflammatory infiltration and epithelial cell remodeling involved in regulating the protective effect. Moreover, the number of hair follicles preserved in the FLASH-RT lesions were better than that in the CONV-RT lesions. Immunofluorescence staining showed that the epidermal CD34+ stem cells were effectively preserved in the FLASH-RT site. Moreover, the group also implemented electron FLASH-RT (single irradiation, dose 25–41 Gy) in 6 cats (stage T2/T3N0M0) with primary nasal squamous cell carcinoma, while successfully eradicating the tumors, depilation was observed in all the cats, and only 3 cats exhibited mild to moderate transient acute mucositis.62 Therefore, the acute radiation-associated injury is mild and controllable after a single high dose of FLASH-RT.
Subsequently, a phase 3 animal clinical trial was performed, the cats with primary nasal squamous cell carcinoma were treated by FLASH-RT (7 cases, irradiation with a single dose of 30 Gy) and CONV-RT (9 cases, 4.8 Gy × 10 fractions). There was mild acute radiation-associated injury in the two groups of cats, and the tumor was well controlled within 1 year. However, 3 cats in the FLASH-RT group showed maxillary bone necrosis 9–15 months post-irradiation.61 This may be attributed to the hot spot (42 Gy) in the irradiated field, which exceeds the dose tolerance limits of cat ’s maxilla and oral mucosa.
7 CLINICAL TRANSLATION
In 2019, the first clinical trial of electron FLASH-RT was carried out in Lausanne University Hospital.11 The patient of refractory cutaneous lymphoma had previously treated with CONV-RT including 20 Gy/10 fractions and 21 Gy/6 fractions (average dose rate 0.08 Gy/s) for various ulcerative and/or painful cutaneous lesions. The patient had experienced up to grade 3 acute radiation induced skin injury. In order to decrease toxic for skin, a single dose of 15 Gy FLASH-RT (average dose rate 166 Gy/s, a total of 10 pulses, each pulse in 1 millisecond) was delivered to a progressing lesion, transient grade 1 acute cutaneous reaction to radiotherapy was observed; the irradiated tumor achieved rapid, lasting and complete remission. The tumor control was comparable between FLASH-RT and CONV-RT with a follow up period of 2 years, and the late radiation injury was similar between FLASH-RT and CONV-RT.9 This study suggests that the application of electron FLASH-RT is safe and feasible with favorable results both on normal skin and tumor.
The first human clinical trial has shown promising results. Hence, the Lausanne University Hospital registered two clinical trials. The first one is a phase I clinical trial focused on dose escalated FLASH-RT (22-34 Gy) for patients with cutaneous malignant melanoma (NCT04986696). The second one is a phase II clinical trial of FLASH-RT for basal cell and squamous cell carcinoma of the skin (NCT05724875). Another group from Zurich University Hospital started a study with electron FLASH-RT in melanoma using a combined hypo-fractionated regimen composed of 3 fractions of 9 Gy, where 2 fractions will be delivered with FLASH-RT and 1fraction delivered with CONV-RT to compensate for possible uncertainty compared with the 3 fractions delivered using CONV-RT. Biopsies are also scheduled to investigated possible difference in radiation response (NCT06549439). Our group designed a phase I clinical trial of electron FLASH-RT and aimed to estimate the safety and acute radiation-associated skin injury, eligible patients are being recruited now (ChiCTR2400080935).
8 CLINICAL TRIAL DESIGN
8.1 Ethics
FLASH radiotherapy is an emerging antitumor technology, its clinical transformation and application should be complied with ethical requirements. Problematic aspects including informed consent, use of placebo, protection of participants, and randomization need to be examined.63 The clinical trial should be performed under the framework of International Ethical Guidelines for Health-related Research Involving Humans organized by World Health Organization. The project should be reviewed and approved by the ethics committee of the local institution.
8.2 Indication
Although FLASH-RT has the advantage of alleviating radiation damage to normal tissues, clinical trials are an indispensable step in moving FLASH-RT from laboratory discoveries into daily practice. The development of detailed implementation plans prior to FLASH-RT is necessary. The indications of FLASH-RT should be strictly controlled during radiotherapy.8, 9, 11 We suggest to consider the following criterions before patients’ enrollment. (a) Disease progression may lead to life-threatening complications; (b) Resistant to standard treatment; (c) FLASH-RT is expected to bring benefits; (d) Expected treatment adverse effects are limited and controllable; (e) Patients have a strong intentions to receive FLASH-RT and sign the informed consent; (f) A FLASH-RT group that consists of at least two senior radiation oncologists, two medical physicists and one radiographer should be established before patients recruitment.
8.3 Dose and fractionation schedules
Generally, the incidence of radiation damage to healthy tissues directly correlates with the amount of radiation exposure.64 In CONV-RT, fractional irradiation is applied to maximize the destruction of tumor cells while minimizing injury to normal tissues.65 Current evidence for the influence of dose rate upon radiation-related injury is encouraging, and FLASH-RT was reported to reduce damage to normal tissues and organs with a single high dose prescription.32 However, it is unclear whether the fractionation schemes and treatment modalities utilized in CONV-RT are also applicable with FLASH-RT. Thus, the FLASH effect needs to be replicated and validated extensively in various models before large-scale applications.
Böhlen et al indicated that the FLASH effect was tissue-specific, and the normal tissue sparing effect was much more significant along with the increase of irradiation dose.66 The FLASH effect was insignificant compared with CONV-RT in the first patient treated with FLASH-RT at Lausanne University Hospital.11 In the phase 3 animal trials, severe late radiation injury was observed in cats with primary squamous cell carcinoma.61 These findings indicated the potential limitations of delivering FLASH-RT in a single high dose. Maity et al implied that the late radiation-associated side effects of FLASH-RT was correlated with large radiation field.67 The group from Lausanne University Hospital suggested the FLASH-RT associated radiation injury was about 1/3 less than that of CONV-RT, and a single high-dose irradiation may concealed the potential protective effect of normal tissues behind FLASH-RT.5 In the meantime time, basic research suggest that a single irradiation dose less than 5 Gy is not enough to trigger the FLASH effect.52 Therefore, the implementation of a single high-dose (greater than 15 Gy) or low-dose (less than 5 Gy) fractionated FLASH-RT needs to be cautioned in clinical trials.
To achieve optimal normal tissue protection without attenuating tumor control rates, fractional radiotherapy with a moderate dose modality might be appropriate for FLASH-RT. Thus, a noval approach, FLASH-stereotactic body radiation therapy has been proposed (SBRT).68 FLASH-SBRT is believed to deliver highly conformal doses to the target volume within 1 second in each fraction while sparing surrounding normal tissues. Due to the unique radiobiological mechanisms of FLASH-RT, whether biologically effective dose (BED) or equivalent dose in 2-Gy fractions (EQD2) can be used to evaluate the biological effect of fractional FLASH-RT, or FLASH-SBRT has not yet been understood, further investigation is necessary. In addition, the published studies only focus on the acute radiation-associated toxicities during and after FLASH-RT, long-term follow-up of late adverse effects after FLASH-RT is needed, and detailed mechanism of late side effects of FLASH-RT remains to be fully elucidated.
8.4 Quality control and quality assurance
To prevent errors and give high confidence that patients will receive the prescribed treatment precisely, quality control and quality assurance are necessary in each stage of FLASH-RT. For CONV-RT, the American Association of Physicists in Medicine Task Group (TG) 142 report itself is considered a comprehensive report that covers the recommendations for linac quality assurance in detail, the TG 198 report is an implementation guide to ensure appropriate conduction in routine clinical settings.69 Based on the TG 142/TG 198 reports, dosimetric parameters correlated to ultra-high dose rate should be monitored closely to minimize errors and maintain treatment accuracy. It is recommended that the FLASH-RT quality control and quality assurance report contain the following parameters: beam energy, pulse repetition rate, duty cycle, temporal pulse structure, beam intensity, cumulative dose per delivery, dose per pulse, instantaneous dose rate, average dose rate per beam, mean does rate per fraction, dose distribution per beam and per fraction.70 Nevertheless, the optimal techniques to obtain dose distributions of FLASH-RT in real-time and actual clinical situations remains unclear, further improvements are required in dose verification methods. Overall, when implementing FLASH-RT, optimal quality control and quality assurance is the cornerstone of giving correct and accurate dose to tumor, and reducing radiation damage to normal tissues.
8.5 Implementations
Redundant dosimetric checks should be performed as described by Bourhis et al before, after, and during FLASH-RT.11 Prior to FLASH-RT, the dose profiles should be measured by radiochromic films, the absolute dose at isocenter should be detected by alanine pellets. A bolus is required to increase irradiation dose on tumor. During electron FLASH-RT, the average dose should be measured using alanine pellets, the dose distributions should be calculated by radiochromic films. A comparison of dose profiles and average dose rates is necessary before and during FLASH-RT to ensure the accuracy of dose delivery. Novel methods also can be used as a supplement for vitrification of the dose distribution during FLASH-RT.
9 PERSPECTIVES
Despite the encouraging preclinical and clinical findings, FLASH-RT is currently in its early stage of clinical transformation and application. There are several significant uncertainties including physical, chemical, dosimetric, and radiation biological aspects. The measurement of oxygen depletion remains to be optimized. Free radicals produced during FLASH-RT remains to be elucidated. For low linear energy transfer FLASH-RT, a comprehensive understanding of the biological mechanisms of FLASH effect from both direct and indirect effects is necessary. Furthermore, combination of dose verification techniques during FLASH-RT may an option to assure the optimum dose delivery. Several temporal beam parameters involved in triggering the FLASH effect needs to be clarified before clinical translation of electron FLASH-RT.
10 CONCLUSIONS
In conclusion, FLASH-RT is a novel milestone in the field of radiotherapy. Preliminary results from preclinical and clinical trials showed that FLASH-RT is feasible and safe with encouraging outcomes. The dose delivery time of FLASH-RT is less than 200 milliseconds, this technique not only greatly improves treatment efficiency but also reduces radiation damage to normal tissues. More trials are needed to promote the translation of FLASH-RT in clinical practice.
ACKNOWLEDGMENT
Guiding experts: Jinming Yu (Shandong Cancer Hospital).
Writing experts: Hui Luo (The Affiliated Cancer Hospital of Zhengzhou University), Chengliang Yang (The Affiliated Cancer Hospital of Zhengzhou University), Jinbo Yue (Shandong Cancer Hospital), Hong Ge (The Affiliated Cancer Hospital of Zhengzhou University).
Editorial Board Members: (Alphabetize by Last Name)
Sen Bai, West China Hospital of Sichuan University
Yanling Bai, The Affiliated Cancer Hospital of Harbin Medical University
Jing Cai, The Hong Kong Polytechnic University
Jianrong Dai, The Cancer Hospital of Chinese Academy of Medical Sciences
Lehui Du, The People's Liberation Army General Hospital
Xiaobo Du, Mianyang Central Hospital
Mei Feng, Sichuan Third People's Hospital
Xiaolong Fu, Shanghai Chest Hospital
Yuan Hao, The Chinese Clinical Trial Registry
Weigang Hu, Fudan University Shanghai Cancer Center
Baosheng Li, Shandong Cancer Hospital
Jie Li, Shanxi Provincial Cancer Hospital
Yexiong Li, The Cancer Hospital of Chinese Academy of Medical Sciences
Hongchang Lei, Henan Cancer Hospital
Fei Liu, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology
Shixin Liu, Jilin Cancer Hospital
Zhong Lu, The Affiliated Hospital of Shangdong Second Medical University
Mei Shi, Xijing Hospital of Air Force MilitaryMedical University
Jie Qiu, Peking Union Medical College Hospital Academy of Medical Sciences & Peking UnionMedical College
Ying Sun, Sun Yat-sen University Cancer Center
Xiangbo Wan, First Affiliated Hospital of Zhengzhou University
JunWang, The Fourth Hospital of Hebei Medical University
Ping Wang, Tianjin Medical University Cancer Institute and Hospital
QifengWang, Sichuan Cancer Hospital
Ruozheng Wang, Cancer Hospital Affiliated to Xinjiang Medical University
Xianliang Wang, Sichuan Cancer Hospital
Yongzhong Wu, Chongqing University Cancer Hospital
Wei Wei, Hubei Cancer Hospital
Dehuan Xie, Guangdong Provincial Peoples Hospital
Xingyu Lu, Southern Medical University
Pei Yang, Hunan Cancer Hospital
Junlin Yi, The Cancer Hospital of Chinese Academy of Medical Sciences
Yong Yin, Shandong Cancer Hospital
Shuanghu Yuan, Anhui Provincial Hospital
Zhiyong Yuan, Tianjin Medical University Cancer Institute and Hospital
Jinbo Yue, Shandong Cancer Hospital
Jiandong Zhang, Shandong Qianfoshan Hospital
Wencheng Zhang, Tianjin Medical University Cancer Institute and Hospital
Zhen Zhang, Fudan University Shanghai Cancer Center
Lujun Zhao, Tianjin Medical University Cancer Institute and Hospital
Wei Zhou, Chongqing University Cancer Hospital
Hongyu Zhu, Sun Yat-sen University Cancer Center
CONFLICTS OF INTEREST STATEMENT
Jinming Yu is the Editor-in-Chief of the journal and co-author of this article. He was excluded from the peer-review process and all editorial decisions related to the acceptance and publication of this article. Peer review was handled independently by the other editor to minimize bias.
ETHICS STATEMENT
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part are appropriately investigated and resolved.