New technologies and machines for stereotactic radiation therapy
Abstract
Stereotactic radiosurgery and stereotactic body radiation therapy have been increasingly utilized in radiation oncology to treat early stage tumors, metastatic targets, and retreatment of relapsed diseases due to their efficiency, treatment effect, and cost effectiveness over the past two decades. Stereotactic radiosurgery and stereotactic body radiation therapy both demand high specifications for their delivery machines, as they deliver radiation doses with fewer treatment fractions and higher doses per fraction. Manufacturers have either invented specialized technologies solely or customized their existing machines for this purpose. In this paper, we review the major technologies and treatment machines for stereotactic radiosurgery and stereotactic body radiation therapy, describe their main features, and discuss the advantages and disadvantages.
1 INTRODUCTION
Stereotactic radiosurgery (SRS) and stereotactic body radiation therapy (SBRT) are treatment methods that deliver a high dose per fraction, typically in less than five fractions. The high fractional dose does not have a clear definition, but it is usually higher than 5 Gy/faction. It depends on the biological equivalent dose calculation to treat mostly early-stage tumors. The dose is more conformal to the target and has a rapid falloff. In contrast, the toxicity needs to be carefully planned for organs at risk. Thus, stereotactic treatments have strong requirements on the treatment quality and need stricter restrictions on the treatment modalities. Those restrictions include, but are not limited to, a submillimeter delivery accuracy, image guidance integration, good management of respiratory motion programs, qualified personnel, and direct supervision by a physicist and a physician.1
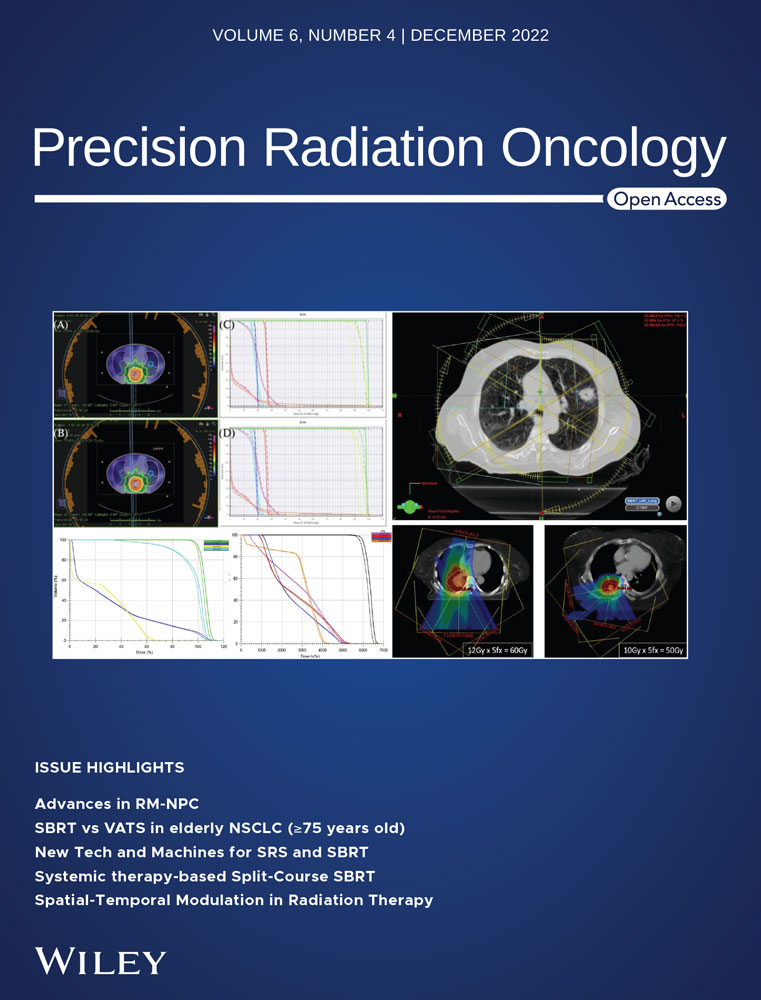
The general workflow for SBRT is listed in Figure 1. The whole SBRT involves personnel of therapists, dosimetrists, physicists, and physicians. Each personnel, as noted by the American Association of Physicists in Medicine practice guideline 9.a, has different roles and responsibilities. For example, the physicist must be qualified for SBRT/SRS treatment direct supervision with enough training before being able to be a consultant for the technical aspects of SBRT. The radiation oncologist must be trained according to the guidelines of the American College of Radiology and American Society for Radiation Oncology best practices. The medical dosimetrist must be certified by the Medical Dosimetry Certification Board or similar board program and have a specific training certificate for SBRT planning. The radiation therapist should have similar training for SBRT, and certification by regulations and license by the state.
In addition to the personnel qualification, the workflow from simulation to treatment also needs to be carefully designed. For instance, the simulation of computed tomography (CT) slice thickness selection, immobilization device choice, patient conformability, and motion management strategies. Multimodality images are usually required for target delineation and fusion. For treatment planning, the radiation treatment planning system should have the ability to calculate and predict the dose with accuracy required by SBRT. The target and organ at risk delineation should have an accuracy of a millimeter for small targets and organs. The fusion algorithm should have similar accuracy. A forward/inverse optimization algorithm should be effective in finding a solution for the plan. For the treatment modality, the acceptance should meet the requirements of geometric and dosimetric accuracy. The commission of the modality also needs a qualified medical physicist to fully understand the scope of SBRT in immobilization, imaging, motion management, treatment delivery, and integration of the whole system. Small field dosimetry must meet the SBRT requirements also. The peer review process is highly recommended by a third party. Well-documented policies and procedures need to be established.
In this article, we discuss the choice of a treatment modality to meet the SRS and SBRT requirements. We provide an analysis of their advantages and disadvantages, mainly based on literature reviews. Readers should create an SBRT committee to decide which modality to select in their department.
2 MODALITIES FOR AN SRS/SBRT PROGRAM
2.1 Tomotherapy
Helical TomoTherapy has a special design using a helical method to deliver the radiation from 51 beam angles while the couch moves in continually. The binary multileaf collimator (MLC) can be either open or closed to optimize the fluence map. The jaws will create a 0.6-cm, 1.0-cm, 2.5-cm, or 5.0-cm opening in the patient's superior and inferior directions.2 The synchronization of the gantry, MLC, and couch creates a unique way for so-called helical tomotherapy to deliver the radiation map to the target area. The software platform was specifically designed to meet the system requirements for optimization and dose calculation accuracy. The final optimized plan will consider the MLC delivery latency effect to achieve the deliverable plan. Usually, it is close to the optimized plan with minor round-off errors. The system was further developed with a dynamic jaw and dynamic couch settings to reduce the whole treatment time.
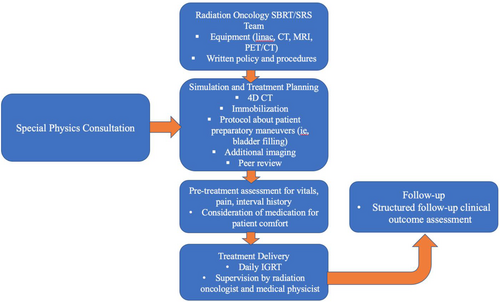
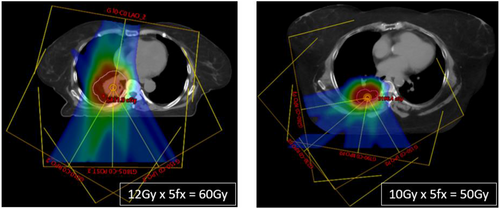
For SBRT treatment, tomotherapy utilizes narrower jaw settings, such as 0.6 cm and 1.0 cm, with fine dose calculation and delivery. Tomotherapy has the integrated on-board mega-volts (MV) CT ability, and the imaging and treatment radiation by default have the same isocenter, which eliminates the additional steps to correlate these two isocenters. The treatment delivery system has the auto-alignment function to assist the patient setup. The limitations of the system include lower MV image quality, especially for soft tissues, and longer treatment delivery time with the narrow jaws. A motion management feature is being developed by the vendor for intrafractional monitoring of patient motion. That said, the treatment planning system of tomotherapy has optimization and dose calculation algorithms to reduce potential delivery uncertainty with geometrical margins. These factors should be considered when using tomotherapy as an SBRT modality. Figure 2 shows an example of an optimized tomotherapy plan for a liver SBRT case.
The Radixact system is built on the TomoTherapy platform with several new features that are especially valuable for SBRT treatments.3 First, a new kV helical fan beam CT function is integrated in addition to the MV imaging capability. Its image quality approaches diagnostic CT qualities, and the field of view is up to 135 cm in the superior and inferior directions. These intrinsic features of a fan beam CT are better than those on a cone beam CT. The large field of view is especially valuable when multiple metastatic targets are treated in one session, and some bulky late-stage tumors are managed by spatially fractionated radiation therapy.4 Second, the compact Linac has a higher output of 1000 MU/min than the 850 MU/min on the Hi-ART system, making SBRT delivery more efficient. Last, the Radixact system has integrated CyberKnife's Synchrony motion management technology, allowing real-time tracking of a moving target during the treatment delivery. With this feature, the internal target volume margin can be reduced with less normal tissues irradiated.
2.2 Novalis Tx
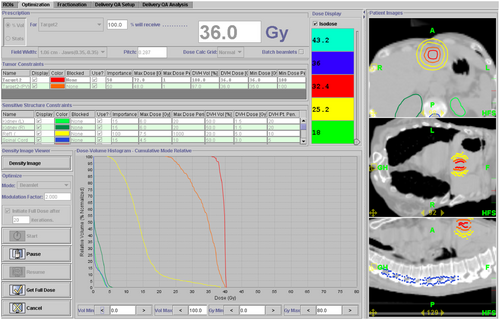
Novalis Tx is a modality integrated with Varian and Brainlab technologies, and provides the ability of 2-D and 3-D multi-imaging (kV, MV) for treatment. Treatment delivery can either use intensity-modulated radiation therapy or conformal radiation therapy to deliver static beam or arc therapy. Novalis Tx can provide intrafraction monitoring for motion management with its ExacTrac system. The 6-D degrees of freedom (couch also makes it easy for initial patient alignment with submillimeter accuracy.5 All the patient body sites can be treated by SBRT, such as the brain, lung, liver, spine, and prostate, with a carefully-commissioned machine. Either the Eclipse or the iPlan treatment planning system can be used to design customized plans. With a high definition of a MLC, the isocenter field resolution is as fine as 2.5 mm in width. For simple targets not too close to critical organs, the conformal arc technique is also a good choice for treatment owing to its simplicity and robustness. Figure 3 shows a typical lung SBRT case using Novalis Tx and conformal arc. Here, six partial arcs were designed to deliver 8–12 Gy per fraction dose to the target off-center. The choice of off-center is to avoid a potential collision with the patient's body when the target is close to the chest wall, as in this case. Similar techniques can be applied to other sites, such as the liver or prostate. The limitation of Novalis Tx is the separation of the two sets of parts belonging to two different vendors of Varian and Brainlab. The integration of the whole system is not seamless and issues could occur, such as data transfer interruption and repair/maintenance difficulty.
2.3 TrueBeam
TrueBeam has high definition MLC or regular MLC, both can be used for SBRT treatments. The TrueBeam system can provide multiple degrees of freedom for SBRT planning and treatment with RapidArc, and even the HyperArc delivery method. The 6D degrees of freedom couch can easily assist the patient setup. The whole system is an integrated product of one single vendor, which enables a smooth process from simulation to treatment. The imaging capabilities include kV, MV 2-D, 3-D, and 4-D to guide target localization before delivery and to provide monitoring during treatment.6 It also has intrafraction monitoring of motion by either surface imaging system (OSMS or Identify system) or IMR imaging ability for intrafraction kV/MV co-alignment of the target. The Calypso system is also integrated to monitor the motion for sites, such as the prostate, liver, and lung, with implanted transponders. TrueBeam uses the RPM or RGSC system to monitor the patient surrogate for respiratory motion control. All these image-guidance or tracking systems are critical for SRS/SBRT treatments, as the fractional dose is larger and PTV margins are less than those of conventional fractionated treatments. The system has the flattening filter free mode for 6-MV and 10-MV photon beams, with dose rates of 1400 MU/min and 2400 MU/min, respectively. Such a high dose rate feature is especially beneficial for SBRT delivery with high fractional prescription dose. In the current Eclipse TPS, beam data is only accepted down to 1 × 1 cm2 in the dose algorithm, making small-field dosimetry more challenging. Such limitations can be overcome by using a specific model built in Eclipse by fine-tuning the output factor, spot size, and dosimetric leaf gap of the MLC. Owing to multiple functions and complexities in the TrueBeam system, quality assurance of the whole system is much more complicated and time-consuming.
2.4 Gamma Knife
A Swedish neurosurgeon, Lars Leksell, invented Gamma Knife, which is a machine used for stereotactic radiosurgery for intracranial lesions in the 1960s. Dr Leksell, defined radiosurgery as “a single high dose fraction of radiation, stereotactically directed to an intracranial region of interest”.7 The Gamma Knife machine has undergone several improvements with technological advancements. The latest version of this machine, known as the Leksell Gamma Knife (LGK) Icon, was introduced in 2016 as an upgrade to the previous LGK Perfexion model from 2006.8 Same as the previous models, the LGK Icon consists of 192 sealed sources of Co-60 arranged on eight motorized sectors, each containing 24 sources. Each sector can move over a drilled tungsten collimation ring system, which has three collimating diameters of 4, 8, and 16 mm.9 The LGK Icon model differs from its predecessors mainly in its capabilities of non-invasive frameless procedures.10 Two image-guidance systems are introduced to the machine: a cone beam CT imaging system with an X-ray tube and image detector, and an intra-fraction motion management system based on infrared lights. These two systems enabled the LGK machine to treat lesions without the need of invasive frames for stereotactic coordinates that have been used since the beginning of this modality. The LGK machine has the largest body of clinical evidence for SRS, with more than 10,000 peer-reviewed articles in the literature because of its long history. The limitation is that it can only treat intracranial and some upper neck lesions, while not for targets at other body sites.
2.5 Proton therapy
Proton therapy is a modality that is increasingly used to provide radiation therapy services for cancer patients. The technology has advanced in recent years; however, the cost of a proton center is a socioeconomic concern. The pencil beam scanning technique can deliver radiation to the target with great accuracy and less uncertainty if the whole system is properly commissioned and maintained. Protons can be used for SBRT treatment, with unique considerations different than photon modality from the simulation to the treatment.11
2.5.1 CT simulation
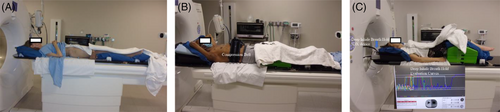
The inhomogeneity environment is not preferred for proton therapy planning; therefore, the CT simulation of a patient will need to reduce the metal artifacts if there are some high-density materials inside the patient's body, especially near the target area. To avoid variations of daily tissue deformations from the immobilization devices, the bags and devices are typically applied above and below the target area during simulation. Motion management and mitigation methods need to be considered for thoracic and abdominal targets. Motion evaluation based on diaphragm motion is performed during the CT simulation with free-breathing by clinical persons (dosimetrists, physicists, and physicians). When the motion is equal to or less than 7 mm, deep inspiration breath-hold (DIBH) is not required, and regular 4-D CT or 4-D CT with abdominal compression scans are acquired. An abdominal compression belt can also be used as an alternative motion mitigation method for upper gastrointestinal cases when the patient is not able to tolerate DIBH. Both DIBH and abdominal compression are only considered for lower thorax cases, who often show large motion in free-breathing scans. Other mitigation strategies, such as volumetric repainting and purposely using the compression belt to increase the spot size, can be used for cases with tumor motion more than 7 mm. Figure 4 shows the simulation examples of free-breathing, compression belt, and DIBH.
For patients who received 4-D CT simulation, the target motion amplitude and the water equivalent thickness of each beam can be quantified statistically and evaluated through the deformable image registration method, such as an in-house script developed on the software platform of Open source software called REGGUI (https://openreggui.org/). Generally, the 90th percentiles of the motion amplitude in the histogram are used as the clinical metrics to determine the motion management strategy for treatment planning.
2.5.2 Treatment planning
Compared with regular fractionated intensity-modulated proton therapy planning, SBRT treatment planning would involve more beam angles (typically 3–5 fields) to improve the conformality of the high dose volume and the robustness of the treatment. When the motion amplitude is larger than 7 mm, volumetric repainting is typically used to further reduce the motion interplay effects, owing to limited fractionations in SBRT treatment. Field beam selections are preferably from the posterior angles, as they are less sensitive to motion setup uncertainties, whereas other angles would be used with a planning advantage for the tumor coverage. The field beam selection also needs to consider the water equivalent thickness changes for that particular beam angle. Fields should not pass through high-density implants to maintain the treatment robustness. In that case, the robust target volume (beam-specific volume) would be created to define the volumes in which the proton spots can be placed. Single-field optimization is recommended as the default planning technique for SBRT cases, whereas multifield optimization can also be considered to maximize sparing of the nearby organs at risk, especially for patients who have had prior radiotherapy treatment. Perturbation settings for CTV (or iCTV)-based robust optimization are 3.5% or 5% for range uncertainties in lung or massive CT artifacts at the treatment level; and setup errors are ±3 mm for patients with masks or ±5 mm in other immobilization cases. To further reduce the sensitivity of the treatment to motion effect, increased spot sizes using a range shifter with a large air gap on purpose might be used if the motion effect is still a concern.
2.5.3 Treatment supervision
For all the SBRT patient treatments, a physicist of the day and a doctor of the day would be on site for the IGRT review and clinical decision in case that there are major discrepancies between the simulation and setup positions. In general, a pair of kV images are acquired first for the initial alignment, and the patient can be adjusted to achieve less than 3-mm uncertainties. After positioning based on kV images, cone beam CT is acquired to further achieve a finer alignment of the patient treatment position. The kV and cone beam CT images are reviewed and approved by both the physicist of the day and doctor of the day before the treatment starts. Another pair of kV images can be acquired between treatment beams to monitor potential intrafraction motion.
2.6 Zap-X
Zap-X (ZAP Surgical Systems, Inc., San Carlos, CA, USA) is a self-shielded Linac-based machine optimized specifically for SRS procedures. The self-shielding feature is designed by mounting all the beam-generating components into the primary spherical supporting structure, specifically a primary beam stop with more than 5.5 tenth value layers. Additionally, scatter radiation is reduced by a rotatable iron shell and a shielded, pneumatically elevated door at the foot of the treatment.12, 13 With these designs, the traditional shielding, provided by the vault walls, ceiling, and floor, is accomplished by the shielded patient spherical chamber, the treatment table enclosure, and the rotating beam stop. The self-shielding feature makes it possible to place an SRS system in a location where a traditional Linac would not fit, thus benefiting a larger patient population. SRS procedures typically require many non-coplanar beam angles. Zap-X uses a combination of dual-axis gimbals, such as a gyroscope, enabling the Linac beam to be isocentrically positioned across a solid angle of over 2π steradian. The S-band 2.7-MV Linac can produce a dose rate of 1500 MU/min at a 45 cm source axis distance. Sharp penumbra is another required feature for SRS systems. Instead of multileaf collimators, the Zap-X system uses divergent circular collimators which eliminate the interleaf leakage radiation. A range of diameters from 4 to 25 mm are designed to cut through a rotating tungsten collimator wheel. Such a design enables the automatic change of various collimator sizes with the least amount of time.14 With all these designs centered around radiosurgery procedures, the Zap-X system can generate and deliver conformal, high-gradient treatment plans (Figure 5). The major limitation of the ZAP-X system is that it can only treat intracranial and upper neck targets, which excludes its use from many other body sites.
2.7 CyberKnife
Dr John Adler, a USA neurosurgeon, invented CyberKnife as a modernized, dedicated, stereotactic radiosurgery system. Since the treatment of the first patient at the Stanford University Medical Center in 1994 by Dr Adler, CyberKnife has undergone numerous technological improvements. They help track targets during treatments using implanted fiducial markers,15 and enable tracking of tumors that move with respiration16 and fiducial-free spinal tracking.17 The linear accelerator on the CyberKnife system is mounted on a robotic manipulator (KR240-2, Series 2000; Kuka Roboter GmbH, Augsburg, Germany) with six degrees of positional freedom, enabling non-coplanar beam geometry. The linear accelerator uses a magnetron-based 6-MV X-band technology that is capable of delivering radiation at a rate of 1000 MU/min. With the integrated image guidance system, target motion is constantly tracked and automatically corrected by the robotic arm throughout treatment delivery. The real-time image guidance feature uses a pair of stereotactic X-ray imaging systems and an optic motion tracking system. Two X-ray sources are mounted to the vault ceiling, and the X-ray detectors are installed beneath the vault floor. Orthogonal X-ray images are obtained throughout treatments at a frequency defined by the operator. The real-time X-ray images are compared with previously generated digitally reconstructed radiographs to calculate the patient position and target location via the tracking software systems. This unique robotic architecture with an integrated image guidance system has presented a new paradigm in the clinical application of SRS and SBRT.
2.8 Magnetic resonance-guided radiotherapy
Magnetic resonance-guided radiotherapy (MRgRT) is a new treatment technology, especially for SRS/SBRT applications. With the on-board magnetic resonance imaging (MRI), the system can provide better image contrast of the target and surrounding healthy tissue. It monitors the organ motion in real time and has the ability to adapt the treatment if needed. Two commercial systems in clinical use are the Elekta Unity (Elekta, Stockholm, Sweden) and ViewRay MRIdian (ViewRay, Cleveland, OH, USA). Both systems can acquire online MRI during radiation treatments and adapt the plan. The magnetic field strength is different with 0.35 T for the MRIdian system and 1.5 T for the Unity machine.18, 19 MRIs provide incomparable image quality compared with the conventional X-ray-based techniques, especially for soft-tissue targets. MRgRT is increasingly used for SBRT in the abdominal sites, such as liver and pancreatic tumors. Promising clinical outcomes have been achieved with reduced margins and escalated dose with its feature of tracking targets during treatments.19 In addition, researchers have been actively investigating other benefits of MRgRT, such as the use of functional imaging during treatment delivery and unique opportunities for MR-guided radio-immunotherapy.20 With all these features, MRgRT could make a paradigm change in SBRT treatments, especially for abdominal tumors.
3 DISCUSSION AND SUMMARY
The hardware of new technologies has two common features. First, most have a ring gantry instead of the traditional C-shape or L-shape Linacs. The gantry rotation speed could be much higher with an enclosed weight-balanced ring gantry design. In addition, potential collisions with patient or treatment accessories are no longer a concern. Second, MLCs are either the binary type or double stack, double focus. The leaf speed of a binary design is much faster. A double stack MLC design can eliminate the secondary collimator jaw, leaving more clearance space. By offsetting the center of upper and lower stacks, double stack MLCs can effectively achieve half of the leaf thickness, providing a better physical modulation which is a requirement for SRS/SBRT procedures.
The choice of an SBRT modality is complex and should consider multiple aspects, such as cost, efficiency, accuracy, robustness, motion management, IGRT, and intrafraction motion. However, the modality is only one side of the whole story. The other important side is the personnel and well-controlled policy and procedures. With well-trained personnel and good quality-controlled program, the modality disadvantages can be overcome and still can be used for SBRT treatment in a certain way, such as add-on micro-MLC, cones, and proper clinical target margins. Therefore, there are no absolute criteria to define which modality is suitable for SBRT treatment. The whole SBRT program needs to be evaluated by the department committee to choose the proper approaches.
ACKNOWLEDGMENTS
We thank our colleagues for providing several figures for this manuscript: Dr Chengyu Shi at City of Hope in Los Angeles, CA, USA, and Dr Christoph Furweger at European Radiosurgery Center in Munich, Germany.
CONFLICT OF INTEREST
The authors declare that they have read the article and there are no competing interests.
ETHICAL STATEMENT
Not applicable.