Proton beam radiation therapy treatment for head and neck cancer
PBT for head and neck cancer
Abstract
Proton beam therapy has gained popularity over recent years. This is likely due to improved affordability; that is, lower cost, and increasing reports on excellent patient-reported outcomes. Protons’ physical properties provide dosimetric advantages over photon therapy due to the unique ability to have little-to-no “exit” dose, potentially translating to reduced toxicities and improved patient quality of life. The increased delivery of proton beam therapy to treat numerous head and neck cancers, including oropharynx, nasopharynx, sinonasal, in the re-irradiation setting, and unilateral malignancies, has led to more studies elucidating the clinical risks and benefits. In this review, we aim to summarize the recent literature on proton beam therapy utilization in head and neck cancer. In addition, we discuss the process of treatment and planning, clinical treatment toxicities and outcomes, limitations, and future directions.
1 INTRODUCTION
Radiation therapy is an important treatment for head and neck cancer. Many patients either undergo definitive radiation with or without chemotherapy or postoperative radiation with or without chemotoherapy.1 Although the main priority of radiation therapy is to improve overall survival (OS) and locoregional control (LRC), it is critical to minimize toxicities and enhance the quality of life (QoL) of patients by sparing essential structures. In the late 1990s and early 2000s, the development of intensity-modulated radiotherapy (IMRT) improved target coverage with a lower radiation dose delivered to nearby structures, thereby minimizing toxicities. However, despite these advancements, the physical properties of photon beams still expose healthy tissues to radiation due to the large exit dose, leading to acute and chronic toxicities that impact patients’ QoL.2 An alternative radiation delivery method was required to improve the therapeutic window.
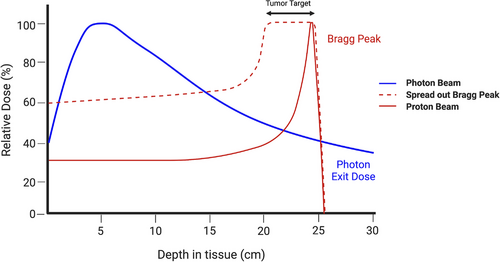
In 1946, Robert R. Wilson, a Harvard physicist, postulated the utilization of protons to precisely target small volumes within the body, and almost a decade later, the first patients were treated with proton beam therapy (PBT).3, 4 PBT is an external beam radiation therapy that has little-to-no exit dose. When protons are accelerated to the tumor, the heavy, charged particles deposit their radiation dose at a narrow range of tissue depth, termed the “Bragg peak” (Figure 1). The Bragg peak is localized at any depth in the tissue to precisely radiate the tumor and limit radiation-related toxicities in surrounding healthy tissue. The large therapeutic window of PBT coupled with its improved affordability has generated more popularity around this treatment option over the past decade.
In this review, we aim to summarize the recent literature on PBT utilization in head and neck cancer. In addition, we discuss the process of treatment and planning, clinical treatment toxicities and outcomes, limitations, and future directions.
2 REFERENCES SEARCH
We did not attempt a systematic review of PBT; instead, we comprehensively summarized the literature on PBT in head and neck cancer. A review of PubMed and Web of Science was conducted using a search syntax with the keywords (“proton therapy” OR “proton beam therapy” OR “proton radiation” OR “proton beam radiation AND “head and neck cancer”). Terms were included to specify individual anatomical sites. Articles that reported clinical results were included in this review. We identified ongoing interventional clinical trials by searching ClinicalTrials.gov using the terms “proton therapy” and “head and neck cancer,” and excluded trials that are terminated, completed, withdrawn, or have an unknown status.
3 TREATMENT PLANNING AND DELIVERY
Radiation planning for PBT differs from IMRT due to its inherent physical properties. On an atomic level, protons’ heavier mass decreases the scattering angle and the dose distribution, allowing for a more finite and defined range of radiation. In addition, the localization of the Bragg peak to the designated tumor volume and the virtually non-existent exit dose favors a more precise dose delivery. However, tumors are complex targets with varying thickness and depths, requiring a spread-out Bragg peak to cover the entire selected volume (Figure 1). This method can eliminate the skin-sparing effects of the entrance dose and lead to skin toxicities, especially in more superficial tumors.5
Relative to photons, protons are more sensitive to the varying densities it travels through. A considerable challenge for PBT planning is considering the factors that can shift the Bragg peak location, potentially leading to poor treatment and inappropriate radiation to healthy tissue. Some of the uncertainties that need to be considered to avoid this dilemma are artifacts (e.g. dental or surgical hardware), anatomical variations due to tumor response or weight change, and daily changes in patient position.5, 6 To circumnavigate the issue of artifacts and other heterogeneous structures interfering with Bragg peak localization, a short and reliable beam path needs to be selected that avoids areas such as the mouth, spinal cord, and other hollow structures. Implementation of automated adaptive replanning is highly recommended to account for the anatomical variations and patient's weight change.7-9
The two primary modes of PBT delivery are passive-scatter protons therapy or intensity-modulated proton therapy (IMPT). Similar to three-dimensional photon therapy, passive-scatter protons therapy uses scattering foils to spread the proton beam, which is less flexible than active scanning. The utilization of apertures in passive-scatter protons therapy allows for impressive lateral conformality. Conversely, IMPT – the most recent advancement of PBT – utilizes pencil-beam scanning, which uses two pairs of scanning magnets that guide the proton beam to varying directions and depth. The modulation of the proton beamline allows for more precise coverage of the 3-D, irregular targets, improving proximal and distal conformality. In IMPT planning, single-field optimization (i.e. each proton beam separately covers the target volume) or multiple-field optimization (i.e. the proton beams collectively cover the target volume) can be utilized to personalize patients’ treatments. Multiple-field optimization allows for a higher degree of conformality and intensity modulation and is more sensitive to treatment uncertainties than single-field optimization. Overall, IMPT provides for an increased relative biological effectiveness and decreased radiation to healthy surrounding tissue.
4 MANAGEMENT OF HEAD AND NECK CANCER
4.1 Oropharyngeal cancer
Alongside surgery and chemotherapy, radiation therapy is an essential component in the definitive and adjuvant treatment of oropharyngeal carcinoma (OPC). IMRT is commonly used to treat oropharyngeal carcinoma with limited toxicities, such as dysphagia and xerostomia; however, these adverse effects significantly impact patient QoL.10 The incidence of young, human papilloma virus-positive patients is on the rise,11 so further efforts are required to reduce radiation-related toxicities due to the longer lives these patients will live after treatment. The physical properties of photon therapy lead to incidental radiation of healthy oropharyngeal and nasopharyngeal tissue. PBT is an emerging modality to reduce toxicities and improve patient QoL. Given the virtually non-existent exit dose of PBT, there is potentially a dosimetric advantage in using PBT compared with IMRT because of the minimal radiation to nearby major organs, which can translate to minimal toxicities in OPC treatment.12, 13
Slater et al. reported the first prospective trial of PBT in OPC patients. The 29 locally advanced OPC patients tolerated accelerated fractionation using photon therapy and concomitant proton boost well, with just three patients (11%) experiencing grade 3 toxicities with an 84% 5-year LRC rate.14 Gunn et al. reported the toxicities of 50 OPC patients (98% human papilloma virus-positive) who were treated with IMPT, were grade 2 or higher xerostomia (25%), grade 3 mucositis (58%), and grade 3 dysphagia (24%). No grade 4 toxicities or death were mentioned.15 A retrospective review of 46 patients treated with IMPT and a median follow-up period of 19.2 months reported LRC, progression-free survival (PFS), and OS of 100%, 93.5%, and 95.7%.16 This analysis is limited by the relatively short follow-up period, but it is an early report of the clinical benefits of IMPT. We reported our experience of 27 OPC patients treated with PBT, and showed a 1-year OS, LC, regional control, and distant metastasis free survival were 100%, 100%, 100%, and 96.3%, respectively. Grade 3 toxicities observed were mucositis, dysphagia, and skin, with only one patient experiencing each.17
Blanchard et al. conducted a case-matched study comparing the same 50 OPC patients radiated with IMPT to 100 OPC patients radiated with IMRT. OPC patients treated with IMPT had a 2-year OS of 94.5% and PFS of 88.6%, with no statistically significant difference noted in OS or PFS between groups. The investigators reported a decrease in grade 3 weight loss at the 3-month follow-up visit (OR 0.44; 95% CI 0.19–1.0) and gastronomy tube placement during treatment (OR 0.53; 95% CI 0.24–1.15).18 Another case-matched study comparing 25 OPC patients treated with IMPT with 25 patients treated with IMRT reported lower mean doses of radiation delivered to nearby healthy structures.12 However, further evaluation would be required to determine the clinical significance of reduced radiation on toxicities.
A study using prospectively collected patient-reported outcome surveys from 35 patients treated with chemotherapy and IMPT, and 46 patients treated with chemotherapy and IMRT showed fewer changes in taste and appetite among the IMPT patients. Based on the MD Anderson Symptom Inventory for Head and Neck Cancer survey, the collective symptom burden from the top five MD Anderson Symptom Inventory for Head and Neck Cancer scores was worse in IMRT patients.19 Another study that prospectively collected patient-reported outcome surveys from 31 postoperative PBT patients and 33 postoperative IMRT patients reported less radiation to healthy structures among PBT patients. The decreased radiation was reflected in patients’ QoL, where patients had higher general scores and significantly less xerostomia at 6 and 12 months post-radiation.20 Cao et al. compared OPC patients who received IMPT (n = 103) to IMRT (n = 429). Although the proportion of moderate-to-severe xerostomia was similar in both groups up to 18 months post-radiation, the IMPT group had less common moderate-to-severe xerostomia at 18–24 months (6% vs. 20%; p = 0.025) and 24–36 months (6% vs. 20%; p = 0.01).21
The rise of human papilloma virus-related OPC in the US incentivizes further investigation of the potential benefits of IMPT over IMRT, as outcomes are excellent and patients are living longer. Randomized clinical trials are necessary to evaluate the efficacy and reduction of toxicities of IMPT critically. The benefits of PBT observed in retrospective studies are being tested currently in an ongoing multi-institutional, randomized clinical trial, NCT01893307, comparing the toxicities between IMRT and IMPT for oropharyngeal squamous cell carcinoma (Table 1).
| NCT number | Study title | Institution | Inclusion criteria | Treatment | Primary endpoints |
|---|---|---|---|---|---|
| NCT01893307 | Randomized Trial of Intensity-Modulated Proton Beam Therapy (IMPT) Versus Intensity-Modulated Photon Therapy (IMRT) for the Treatment of Oropharyngeal Cancer of the Head and Neck | MDACC, Mayo Clinic, UF Health, NMH, UMD, MGH, UPHS, UW | SCC of oropharynx (AJCC v7 stage III–IV) | Randomized to IMRT or IMPT | Rates of late grade 3–5 toxicity between 90 days and 2 years post-RT |
| NCT04870840 | Image-guided Proton Therapy for the Treatment of Locally Advanced Unresectable Head and Neck Cancer | MDACC | Locally advanced HPV negative HNC | PBT | Grade 4–5 toxicities up to 6 months post-RT |
| NCT03164460 | Stereotactic Body Radiation Therapy or Intensity Modulated Radiation/Proton Therapy in Treating Patients With Recurrent Head and Neck Cancer | MDACC | Recurrent HNC or second primary HNC and have previously received at least 30 Gy for HNC | Randomized to SBRT or IMRT/IMPT | 2-year rate of grade ≥3 toxicity at 2 years post-RT |
| NCT03217188 | Proton Re-Irradiation for Recurrent Head and Neck Cancer | MSKCC | Recurrent HNC or second primary HNC and have previously received at least 40 Gy for HNC | PBT | Locoregional control 12 months |
| NCT01973179 | Re-irradiation of Recurrent Head and Neck Cancer | Technische Universität Dresden | Previously irradiated HNC | PBT | Late toxicity 2 years post-RT |
| NCT04609280 | Selective Avoidance of Nodal Volumes at Minimal Risk (GCC 20110) | UMD | HPV + oropharyngeal SCC | IMRT/VMAT or PBPT | Elective out-of-field contralateral nodal failure 2 years post-RT |
| NCT02923570 | Study of Proton Versus Photon Beam Radiotherapy in the Treatment of Head and Neck Cancer | MKSCC, Mayo Clinic, Mount Sinai Hospital | Unilateral cancers in head and neck region (e.g., salivary gland or skin cancer) | Randomized to PBT or IMRT | Acute toxicities |
| NCT03981068 | DAHANCA 37. Re-irradiation With Proton Radiotherapy | Danish Head and Neck Cancer Group | Recurrent HNC or second primary HNC and have previously received RT | IMPT | Any new grade ≥3 toxicity within 3 years post-RT |
| NCT03513042 | Early Response Evaluation of Proton Therapy by PET-imaging in Squamous Cell Carcinoma Located in the Head and Neck | Leiden University Medical University | Primary unresected invasive HNSCC | IMPT | 3-year local recurrence-free survival |
| NCT04607694 | DAHANCA 35: Proton Versus Photon Therapy for Head-neck Cancer | Danish Head and Neck Cancer Group | SCC of pharynx or larynx | Randomized to PBT or IMRT | Acute toxicities within 6 months post-RT |
| NCT03539198 | Study of Proton SBRT and Immunotherapy for Recurrent/Progressive Locoregional or Metastatic Head and Neck Cancer | Mayo Clinic | Recurrent/progressive locoregional or metastatic HNC | Proton SBRT + nivolumab | Objective response rate |
| NCT04671667 | Testing What Happens When an Immunotherapy Drug (Pembrolizumab) is Added to Radiation or Given by Itself Compared to the Usual Treatment of Chemotherapy with Radiation After Surgery for Recurrent Head and Neck Squamous Cell Carcinoma | NCI | Recurrent HNC or second primary HNC and have previously received RT | Randomized to pembrolizumab with IMRT or PBT, cisplatin/carboplatin with IMRT or PBT, or pembrolizumab alone | Overall survival at 2 years and incidence of adverse effects up to 5 years post-RT |
- AJCC, American Joint Committee of Cancer; HNC, head and neck cancer; HNSCC, head and neck squamous cell carcinoma; HPV, human papillomavirus; IMPT, intensity modulated proton therapy; IMRT, intensity modulated radiation therapy; MDACC, MD Anderson Cancer Center; NMH, Northwestern Memorial Hospital; MSKCC, Memorial Sloan Kettering Cancer Center; NCI, National Cancer Institute; PBPT, pencil beam proton therapy; RT, radiation therapy; SBRT, stereotactic body radiation therapy; SCC, squamous cell carcinoma; UF Health, University of Florida Health Proton Therapy Institute; UMD, University of Maryland; MGH, Massachusetts General Hospital; UPHS, University of Pennsylvania Health System; UW, University of Washington; VMAT, volumetric modulated arc therapy
4.2 Nasopharyngeal cancer
The standard of care for locoregionally advanced nasopharyngeal carcinoma (NPC) consists of radiation and chemotherapy in the definitive setting. The challenge of radiating the nasopharynx is limiting the radiation dose to nearby integral structures, such as the major salivary glands, pharyngeal constrictors, brain stem, optic chiasm, cranial nerves, and spinal cord. IMRT is utilized for optimal tumor coverage while limiting radiation to critical tissue, leading to reductions in toxicity and better QoL than conventional radiotherapy in NPC.22, 23 Despite IMRT's superiority over conformal radiotherapy, the physical properties of photon therapy result in increased radiation delivered to non-target, healthy structures. Additionally, IMRT is suboptimal in advanced NPC disease, such as T4 tumors, Epstein–Barr virus-negative tumors, or previously irradiated, locally recurrent tumors.24-26 PBT may offer an alternative radiotherapy option to allow for dose escalation while sparing non-target structures to improve outcomes among advanced NPC patients.
Chan et al. conducted a phase II trial on 23 locally advanced NPC patients treated with combined photon and proton therapy, and reported 90% DFS, 100% local control (LC), and 100% OS at 2-year follow up. The most common grade 3 or worse toxicities were hearing loss in 29% of patients and weight loss in 38% of patients, whereas no grade 3 or worse xerostomia was observed.27 In another study by Chan et al., 17 patients with T4 nasopharyngeal carcinoma were treated with PBT. The median follow-up period was 43 months, with only one local failure, two systemic failures, and no neck recurrences. Late toxicities were radiographic temporal lobe changes (n = 5), endocrine dysfunction (n = 1), and mandibular osteoradionecrosis (n = 1).28 In a matched case–control study by Holliday et al., they compared NPC patients treated with IMPT (n = 10) and IMRT (n = 20), and reported lower frequencies of gastrostomy tube among IMPT-treated patients (20% vs. 65%, p = 0.02), which is possibly due to the lower radiation dose to the oral cavity among IMPT patients.29 A retrospective review by McDonald et al. noted that nasopharyngeal and paranasal cancers treated with PBT had lower rates of G-tube dependence and opioid requirement.30 In another retrospective study of 40 NPC patients treated with IMPT, Jiri et al. reported 2-year OS, DFS, and LC were 80%, 75%, and 84%, respectively. G-tube placement was required in four cases (10%), and toxicities grade >3 were observed in just two patients (5%).31 We conducted a retrospective review of 77 newly diagnosed NPC treated with IMPT or IMRT and reported a 2-year locoregional failure-free survival of 100% and 86.2% in the IMPT and IMRT groups, respectively (p = 0.08). Compared with IMRT, patients treated with IMPT were less likely to develop grade 2 or higher acute toxicities (OR 0.15, p = 0.01).32
Overall, PBT is an alternative radiotherapy for treating NPC with excellent treatment outcomes and reduced toxicities. More prospective data are required to confirm the improved outcomes and reduction in late toxicities. Multiple clinical trials are underway evaluating PBT in any head and neck cancer, including NPC, and will elucidate the efficacy of PBT (Table 1). Of note, the currently on-going NRG Oncology HN001 (NCT02135042), a randomized phase II/III trial investigating the utilization of Epstein–Barr virus as a blood biomarker for treating locoregionally advanced NPC, allows proton therapy.
4.3 Sinonasal cancer
Most primary sinonasal cancers have acceptable treatment outcomes with surgical resection followed by radiation with or without chemotherapy. However, in advanced cancers, surgery can potentially result in facial disfiguration and neurovascular injury due to the proximity of the paranasal sinuses and nasal cavity to critical structures.33 Patients with unresectable sinonasal tumors are treated with radiation with or without chemotherapy, but this treatment results in suboptimal outcomes, because the radiation dose is limited to preserve nearby critical structures and prevent radiation-induced hypopituitarism.34 Dosimetry studies showed that dose escalation could be achieved safely with PBT compared with photon therapy.35-37 In a clinical setting, PBT has been shown to reduce the radiation dose to nearby tissue, such as optic structures and brain stem, thereby reducing toxicities.38-41
A systematic review and meta-analysis of 41 observational studies reported the clinical outcomes of paranasal sinus and nasal cavity cancer patients treated with charged particle therapy (n = 286) to photon therapy (n = 1186). The two radiotherapies had no significant difference between the median doses administered. At 5-year follow up, the pooled OS and DFS were significantly higher for charged particle therapy than for photon therapy. A subgroup analysis comparing PBT versus IMRT showed significantly greater DFS and LC at 5 years and the longest follow-up time point, respectively. Of note, the authors acknowledged potential biases in their study, such as selection and publication bias, and the limitations to using retrospective studies. However, this study still provides strong, consistent evidence for the improved outcomes of PBT among sinonasal cancer patients.42 A multi-institutional study with 69 sinonasal cancer patients treated with PBT, including 27 treated for re-irradiation, was conducted by Yu et al. They reported a 3-year OS, freedom from distant metastasis (FFDM), freedom from disease progression (FF), and freedom from locoregional recurrence rates of 100%, 84.0%, 77.3%, and 92.9%, respectively. Among re-RT patients, 3-year OS, freedom from distant metastasis, freedom from disease progression, and freedom from locoregional recurrence rates were 76.2%, 47.4%, 32.1%, and 33.8%, respectively. In 15% of patients, late toxicities were reported, but none of the toxicities exceeded grade 2. None of the patients experienced vision loss or symptomatic brain necrosis.43
In a retrospective review with 90 sinonasal cancer patients treated with PBT, the median follow-up period was 57.5 months, and 17 patients (19%) had grade 3 toxicities and six patients (7%) had grade 4 toxicities.44 Russo et al. reported outcomes of 54 sinonasal cancer patients treated with PBT who had a median follow-up period of 82 months. LC and OS at 2-year follow up were 80% and 67%, respectively, and at 5-year follow up were 80% and 47%, respectively. There were nine grade 3 and six grade 4 toxicities observed.45 Both studies reported toxicities occurring over 5 years post-treatment, emphasizing the necessity of prospective trials to follow patients for the long term to assess toxicities attributed to PBT accurately.
We reported our institutional experience of treating 86 sinonasal cancer patients with PBT – 68 patients were radiation-naïve, and 18 patients were re-irradiated. Approximately 53% of patients received IMPT. The median follow-up period was 23.4 months, and the 2-year LC, distant control, DFS, and OS rates were 83%, 84%, 74%, and 81%, respectively, for radiation-naïve patients, and 77%, 80%, 54%, and 66%, respectively, for re-irradiated patients. Patients treated with IMPT had significantly improved LC (91%) compared with the 3-D conformal proton technique (72%, p < 0.01). Lower grade 3 toxicities were observed compared with historical controls.46
In summary, these studies support the use of PBT for its improved local control, overall survival, and reduced toxicities among sinonasal cancer patients. Ongoing clinical trials will further support the benefits of PBT delivery among sinonasal cancer patients (Table 1).
4.4 Re-irradiation for recurrent head and neck cancer
When head and neck cancer patients develop recurrence of disease after radiation treatment, they require salvage therapy to control the tumor and prevent severe declines in QoL and painful death. Salvage therapy may consist of surgery and subsequent re-irradiation or re-irradiation without surgery. High doses of radiation would be required to control the radioresistant tumors; however, delivering high doses is limited by the surrounding tissue previously exposed to radiation from prior treatment. Re-irradiation carries the risk of damaging surrounding tissue, leading to irreversible toxicity. This challenge limits the dose delivered and occasionally limits the dose given in the re-irradiation setting. Given the near absence of an exit dose, PBT has been used to overcome the obstacle of limiting radiation to spare nearby structures and allows for dose escalation, resulting in reduced toxicity to surrounding tissue and improve disease control.
Based on a review of re-irradiation of head and neck cancer, the 1–2-year LRC rates are approximately 50–60%.47 In contrast, the study of Phan et al. with 60 patients with recurrent head and neck cancer re-irradiated with PBT showed LC, OS, PFS, and distant metastasis-free survival at 1 year were 68.4%, 83.8%, 60.1%, and 74.9%, respectively. Acute grade 3 toxicities were observed in 18 patients (30%), including 13 patients (22%) who required gastronomy tube placement. However, at 1-year follow up, the grade 3 toxicities decreased to 16.7%, with just 2% feeding tubes. Three deaths may have occurred due to re-irradiation-related toxicities.48 McDonald et al. analyzed 61 head and neck patients who completed PBT re-irradiation and observed a 2-year cumulative local failure rate of 19.7% of patients. Grade ≥3 toxicities were noted acutely in 14.7% of patients and late in 24.6% of patients, including three treatment-associated deaths.49 A multi-institutional study of 92 patients re-irradiated with PBT for recurrent head and neck cancer by Romesser et al. showed the 1-year locoregional failure, distant metastasis free survival, and OS rate to be 25.1%, 84.0%, and 65.2%, respectively. The acute grade 3 toxicities were dysphagia (n = 6, 9.1%), mucositis (n = 9, 9.9%), esophagitis (n = 6, 9.1%), and dermatitis (n = 3, 3.3%). Compared with IMRT, PBT had lower grade 3 or 4 late toxicities rates, including skin complications (n = 6, 8.7%) and dysphagia (n = 4, 7.1%). Two deaths occurred from bleeding.50 These studies suggest re-irradiation with PBT has a relatively safe toxicity profile with acceptable outcomes, but it is important to note the small percentage of patient deaths, and for practitioners to be mindful of reducing toxicity. Predictive factors for severe late toxicity include shorter intervals to re-irradiation (<20 months) and larger re-irradiated planning tumor volumes (PTVs >100 cm3).51
A retrospective study of recurrent NPC patients treated with PBT showed OS and LC rates of 54.4% and 66.6%, respectively. No acute grade ≥3 toxicities were observed, and late grade ≥3 toxicities were observed in 23.5%, with hearing impairment (17.6%) being the most frequent.52 In another disease-specific retrospective review, recurrent oral cancer patients treated with PBT were reported to have 1-year OS and LC rates of 62% and 77%, respectively, and 2-year OS and LC rates of 42% and 60%, respectively. No treatment-related deaths were observed.53
Despite the challenges of re-irradiating recurrent tumors, PBT appears to have a relatively safe toxicity profile with acceptable outcomes compared with historical photon use. Of note, the frequencies of acute and late adverse events are still high. Re-irradiation treatment planning needs to be personalized for patients because of the heterogeneity in cases to limit toxicities and improve disease control. Prospective clinical trials are required to further assess the advantages of PBT compared with traditional photon radiotherapy. Ongoing prospective clinical trials with recurrent head and neck cancer patients will continue to evaluate PBT's efficacy in the re-irradiation setting (Table 1).
4.5 Unilateral head and neck irradiation
Head and neck cancers may involve only one side, and pose minimal risk for contralateral spread, such as malignancies of major salivary glands, oral cavity, oropharynx, or skin cancers. Compared with IMRT, PBT is an ideal alternative in treating unilateral head and neck cancers due to the minimal exit dose.54
Romesser et al. compared toxicities between unilateral PBT and IMRT treatment for 41 patients with major salivary gland tumors or cutaneous squamous cell carcinoma. Although both treatments provided similar tumor target coverage, PBT had minimal radiation to the oral cavity, contralateral major salivary glands, and brainstem. This translated to patients treated with PBT having fewer grade ≥2 acute toxicities, including dysgeusia, mucositis, and nausea (all p < 0.05).55
Dagan et al. assessed acute adverse effects from treatment and nutritional status among patients who received ipsilateral PBT for parotid malignancies. They observed no grade 4 toxicities and only one grade 3 dysphagia. There were low rates of mucositis, with 35% experiencing grade 2 and 43% not experiencing mucositis. Patients preserved their weight and nutritional status, wherein no patient required a feeding tube or intermittent intravenous hydration.56 Holliday et al. reported on postoperative, ipsilateral head and neck radiation using IMPT in 16 head and neck adenoid cystic carcinoma patients. At a median follow-up period of 24.9 months, they noted a LC of 93.8%, with four patients experiencing acute grade 3 toxicities and one patient developing one chronic grade 4 toxicity.57 Chuong et al. evaluated acute toxicities from unilateral PBT in the treatment of major salivary gland tumors and observed acute grade ≥2 toxicities, including nausea (1.5%), dysgeusia (4.8%), xerostomia (7.6%), mucositis (10.5%) and dysphagia (10.5%).58 Our retrospective review on major salivary gland tumors treated with PBT reported a 3-year 95.1% LRC (95% CI 89.9–100), 80.7% PFS (95% CI 70.2–92.7), and 96.1% OS (95% CI 90.9–100).59
An on-going randomized trial, NCT02923570, aims to compare PBT and IMRT for unilateral radiation, while primarily assessing acute toxicities (Table 1). RTOG 1008 (NCT01220583), a phase II/III randomized trial assessing radiation therapy with or without chemotherapy treatment for resected malignant salivary gland tumors, has permitted proton therapy.
5 LIMITATIONS
PBT has been used clinically for over two decades, but the costly expenses to build and operate proton therapy facilities have limited its utilization. Recent technological advancements have increased its affordability and use, allowing more studies to investigate its clinical advantages. Nevertheless, the current literature has minimal multi-institutional prospective, randomized studies, with most reports being single-institutional retrospective studies. Although these retrospective trials have shown favorable outcomes across head and neck sites, higher evidence studies are required to support PBT delivery fully.
Given the physical characteristics of protons, PBT has a dosimetric advantage over photons, allowing for lower dose delivery to non-target structures. This translates to fewer acute toxicities. However, protons’ scattering characteristics increase relative biological effectiveness along the distal edge of the Bragg peak.60, 61 This results in adverse events stemming from the increased dose to tissue directly surrounding the target volume. Some studies report that patients might experience worse adverse events with PBT, such as skin toxicities, temporal lobe necrosis, and neurological toxicities.30, 42, 55 Radiation oncologists and physicists should further investigate ways to limit the overlap of distal Bragg peaks with integral structures. The limited use of PBT has hindered efforts to improve proton techniques; however, the growing number of proton centers will ease the endeavor to optimize and innovate proton planning and delivery.
As mentioned previously, PBT is expensive to establish and operate, leading to higher costs of treatments. Without supporting evidence that PBT is cost-effective, insurance companies infrequently provide full coverage for treatment.62 The cost of PBT compared with IMRT is approximately two- to threefold more expensive, but this difference could be reduced when accounting for the reduction in managing fewer adverse events.63 Prospective trials are required to evaluate the cost-effectiveness of reducing toxicities. For head and neck cancer patients, the American Society for Radiation Oncology deems PBT as an appropriate treatment for those enrolled in institutional review board approved clinical trials.64 In a study analyzing the National Cancer Database of patients treated with PBT, patients who are treated in an academic setting (p < 0.001) and in the highest median household income quartile (>$63,000, p = 0.002) were more likely to receive PBT. These findings suggest that patients from more affluent backgrounds are more likely to receive PBT.65 This trend is noteworthy to recognize early to ensure the inclusion of economically disadvantaged patients in future studies. These patients are necessary to acquire an accurate representation of both the beneficial outcomes and the toxicities. More importantly, providing marginalized patients with this potentially superior radiotherapy will be one step closer to narrowing the disparities gap.
6 FUTURE DIRECTIONS
The rise in proton facilities and increase in PBT delivery has expanded the literature, providing more insight into the benefits and areas of improvement. Efforts to strengthen techniques, including on-board imaging and automating proton plan adaption, will establish a system to precisely deliver protons, thereby permitting an even wider adoption of this radiotherapy.66, 67 Furthermore, these technological improvements and increased availability of sites offering this radiotherapy may reduce treatment costs. The reduction in adverse effects will lead to decreased expenses on toxicity management, which can minimize the total costs spent throughout the entire duration of care.
Prospective, randomized studies are required to directly compare the clinical benefits and adverse effects of PBT to photon therapy. Reports from these trials will provide more robust evidence supporting the clinical benefits, leading to increased adoption of protons as a treatment option. Current trials are evaluating the cost-effectiveness, toxicities, QoL, and suitable patient populations (Table 1). As these factors will vary among head and neck cancer patients, the decision to pursue PBT needs to be personalized, based on the individual characteristics of each patient. Other factors to consider are the proton interactions with other treatment modalities, such as immunotherapy.68 Investigating the relationship between proton radiotherapy and immunotherapy will provide insight into the immunological response to cancer cells. Furthermore, the effect of proton radiation on different biological mechanisms needs to be continuously studied, as it has been shown that RNA and protein expression varies in (lymph) angiogenic, inflammatory, proliferative, and anti-tumor immune responses.69, 70 Besides the superior physical characteristics that protons have over photons, these findings elucidate the biological advantages of protons and their potential to be supplemented with anti-angiogenic or anti-immune checkpoint drugs to improve the therapeutic window.70
7 CONCLUSION
Given the physical properties of protons to maximize radiation dose to target volume with minimal dose to normal tissue, PBT is theoretically advantageous over IMRT. The clinical evidence summarized in this review suggests PBT is a favorable radiotherapy option for head and neck cancer treatment. Improvements in its technologies and increased proton therapy sites will allow for more accessible treatment. With more patients receiving protons, we can further conduct clinical trials to improve our understanding of proton radiotherapy, increasing the therapeutic ratio. As prospective studies are underway to highlight its benefits, and proton radiotherapy becomes more accessible and effective, practitioners will provide better quality of life and health outcomes to head and neck cancer patients.
ETHICAL CONSIDERATIONS
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest to disclose.
FUNDING
The research is funded by P30 Cancer Center Support Grant (P30 CA008748).