Miscible blends containing a liquid crystalline polymer via optimized hydrogen bonding: Correlation to theory
Abstract
Blending a liquid crystalline polymer (LCP) with an amorphous polymer to create a molecular composite offers a method to use the desirable properties of an LCP at a more modest cost. However, very few such blends are miscible. Our earlier findings (Viswanathan, S.; Dadmun, M. D. Macromol Rapid Commun 2001, 22, 779–782; Macromolecules 2001, 35, 5049–5060; Macromolecules 2003, 36, 3196–3205) demonstrate that it is possible to create a true molecular composite by inducing miscibility in a blend containing an LCP and an amorphous polymer by slightly modifying the structure of the polymer constituents to promote hydrogen bonding between the two polymers. This result is interpreted to indicate that separation of the hydroxyl groups along the amorphous polymer chain enhances the accessibility of the OH to intermolecularly hydrogen bond to CO groups and increases the miscibility of the blends. In this report, the phase diagrams for these blends are correlated to the theoretical phase diagrams that are determined using Coleman and Painter's association model, indicating excellent agreement between theory and experiment. This correlation also provides quantification of the functional group accessibility (via K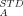 ) as a function of copolymer composition, which agrees very well with the previous phase behavior results and interpretation. © 2004 Wiley Periodicals, Inc. J Polym Sci Part B: Polym Phys 42: 1010–1022, 2004
) as a function of copolymer composition, which agrees very well with the previous phase behavior results and interpretation. © 2004 Wiley Periodicals, Inc. J Polym Sci Part B: Polym Phys 42: 1010–1022, 2004




