Cellular Senescence in Cancer: Mechanisms, Roles in Tumor Progression, and Therapeutic Implications
Jingrui Yan, Yu Zhang, and Guohua Mao contributed equally to this study.
ABSTRACT
Cellular senescence, a state of irreversible cell cycle arrest accompanied by a senescence-associated secretory phenotype (SASP), plays dual roles in cancer biology. Initially recognized as a tumor-suppressive mechanism by halting the proliferation of damaged cells, senescence paradoxically fosters tumor progression through SASP-mediated immunosuppression and chronic inflammation. Thus, the role of senescent cells in tumors still needs to be further elucidated. Our review comprehensively examines the triggers and molecular pathways of senescence. We also summarized the characteristics and functions of senescent tumor and nontumor cells, delineating the heterogeneous tumor senescence microenvironment. Here, we highlight the functional reprogramming of senescent cells, including enhanced stemness, secretome and metabolome reprogramming, which can sustain tumorigenesis and therapeutic resistance. Furthermore, we discuss emerging therapeutic strategies, notably the “one-two punch” approach to overcome therapy resistance. By integrating recent advances in senescence-targeted therapies, our review underscores the necessity of context-specific strategies to harness senescence's tumor-suppressive effects while mitigating its protumorigenic consequences. These insights provide a roadmap for developing precision therapies and refining biomarker-driven approaches to improve cancer treatment outcomes.
Graphical Abstract
1 Introduction
Cellular senescence is a double-edged sword in oncology, contributing to both tumor suppression and progression [1]. Initially characterized as an antiproliferative mechanism, cellular senescence is defined as permanent cell cycle arrest that prevents damaged or dysfunctional cells from dividing [2]. In addition to cell cycle arrest, senescent cells can also develop a distinct secretory profile known as the senescence-associated secretory phenotype (SASP) [3]. SASP comprises a wide range of pro-inflammatory cytokines, chemokines, proteases, and growth factors, which can facilitate immune surveillance and the removal of pre-malignant cells [4]. This function positions senescence as an essential barrier against early tumorigenesis, preventing the unchecked proliferation of damaged cells and thus reducing the risk of cancer.
However, a paradoxical role for senescence has recently emerged since the same SASP factors that promote immune clearance can also create a protumorigenic environment in the later stages of cancer. SASP promotes chronic inflammation, immune suppression, and tissue remodeling that collectively foster a tumor microenvironment (TME) supportive of cancer cell survival, invasion, and metastasis [5]. In particular, SASP contributes to immunosuppression within the TME by recruiting myeloid-derived suppressor cells (MDSCs) and modulating the behavior of surrounding immune cells [6]. These interactions facilitate immune evasion by tumor cells, allowing them to thrive despite the efforts of the immune system to eliminate them. Consequently, SASP-producing senescent cells often promote cancer progression, metastasis, and resistance to therapy, highlighting a critical challenge in targeting senescence as a therapeutic strategy.
In addition to SASP, senescent cells undergo extensive metabolic reprogramming, such as enhanced glycolysis, altered lipid metabolism, and increased dependency on amino acid pathways like glutaminolysis [7]. These metabolic shifts, while necessary for sustaining the energy-intensive production of SASP, mirror the metabolic flexibility of cancer cells and underscore the ability of senescent cells to actively influence their microenvironment. Within the TME, these reprogrammed metabolic pathways in senescent cells can fuel tumor progression by providing nutrients and pro-survival signals to neighboring cancer cells. Thus, understanding and targeting the metabolic adaptations of senescent cells represent a novel and promising avenue for cancer therapy.
The induction of senescence within the TME occurs via several pathways. Oncogene-induced senescence (OIS) and therapy-induced senescence (TIS) are the main mechanisms through which senescence is triggered by genetic mutations and anticancer treatments respectively [8, 9]. OIS, typically activated by hyperactive oncogenes, functions as an early protective mechanism against tumor formation by halting cell division in potentially malignant cells. However, some cells undergoing OIS can eventually escape senescence, and re-enter the cell cycle with heightened tumorigenic potential. Similarly, TIS, induced by chemotherapy or radiotherapy, can also lead to the accumulation of senescent cells within the TME. While initially beneficial in preventing further cancer cell division, TIS cells contribute to an immunosuppressive milieu and can drive therapy resistance, which poses a significant barrier to successful cancer treatment.
Thus, cellular senescence embodies a complex interplay within the TME, making it a challenging but essential therapeutic target. In this review, we focus on the mechanisms and functional alterations of senescent tumor and nontumor cells in the TME. The reprogramming of senescent cells including increased stemness, secretome reprograming and metabolome reprogramming, led to functional alterations in senescent cells. Given the pivotal role of senescent cells, therapeutic strategies targeting cellular senescence are undergoing continuous evolution, with promising potential for tumor growth control. In this review, we comprehensively depict multiple facets of senescent cells, encompassing their molecular mechanisms, functional alterations, dualistic roles in tumorigenesis and tumor suppression, and potential therapeutic targets. Through this multifaceted discussion of cellular senescence characteristics, we aim to propose future directions for both developmental and research endeavors in the field of cellular senescence.
2 Mechanisms of Cellular Senescence in Tumors
Cellular senescence is frequently associated with the upregulation of cell cycle-related proteins, a reduction in cell proliferation, and the accumulation of DNA damage. Recently, the role of senescence in cancer has emerged as a focal point of research. Therefore, an in-depth understanding of the mechanisms, functions, and triggers of cellular senescence within the tumor microenvironment is of significant importance for targeting senescence, developing novel therapeutic strategies, and improving patient outcome.
2.1 Triggers of Senescence
Cellular senescence is induced by multiple stress factors and involves an irreversible proliferative arrest that is accompanied by the upregulation of antiapoptotic pathways [10-12]. Various stimulators, including oncogenic stress, DNA damage, oxidative stress, and epigenetic modulation, can lead to permanent cell cycle arrest and the onset of senescence [13]. Within the TME, cellular senescence is mainly induced by the pro-inflammatory microenvironment, oncogene mutations and chemotherapy or radiotherapy. However, other factors, such as nutrient depletion, metabolic stress and microorganisms can also contribute to cellular senescence in tumors. Understanding the factors that mediate cellular senescence in tumors is crucial for designing potential interventions to target the onset of senescence. The key stimulators involved in inducing the onset of senescence in the TME are summarized in Figure 1.
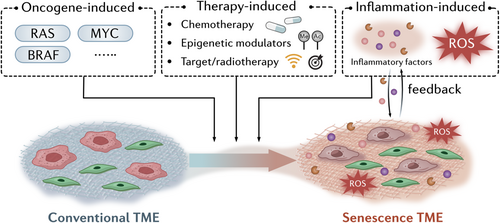
2.1.1 Oncogene-Induced Senescence (OIS)
OIS is a cellular response that acts as a protective mechanism to prevent uncontrolled cell proliferation caused by the abnormal activation of oncogenes, such as RAS, MYC, or BRAF [14-16]. Overactivation of these oncogenes initiates a stress response in cells, which leads to permanent cell cycle arrest and the induction of senescence. OIS serves as a barrier against tumorigenesis by halting the growth of potentially malignant cells [17]. OIS induced by aberrant RAS activation leads to the arrest of pre-malignant cells, which secrete SASP factors to enhance innate and adaptive immune clearance, ultimately blocking tumorigenesis [18]. However, OIS cells can also re-enter the cell cycle and escape their senescent state. These transformed cells display more aggressive tumorigenic features, such as enhanced migration, increased resistance to therapy compared to normal cells [19] and increased stemness [20], which may be due to overactivation of the Wnt signaling pathway [20].
2.1.2 Inflammation-Induced Senescence
The TME is rich in pro-inflammatory factors, including cytokines such as interleukin-6 (IL-6), IL-1β, tumor necrosis factor-α (TNF-α), and chemokines, which contribute to the chronic inflammatory state [21]. The inflammatory microenvironment can generate reactive oxygen species (ROS) and trigger DNA damage, which induces cellular senescence, while the accumulation of senescent cells and SASP can form a positive feedback loop which drives the development of cancer [22]. IL-6 and IL-8 treatment have been reported to induce cellular senescence accompanied by increased stemness [23]. In the hypoxic microenvironment, suppressing TGF-β signaling has been shown to abrogate endogenous senescent cells and decrease SASP secretion and immune infiltration [24]. Recently, Widjaja and colleagues identified a crucial role for IL-11 in senescence [25]. They demonstrated that stimulating human fibroblasts or hepatocytes with IL-11 activated extracellular signal-regulated kinase (ERK) and mammalian target of rapamycin complex 1 (mTORC1) and increased p16 and p21 levels. Together, the findings from these studies indicate that chronic inflammation in the TME triggers cellular senescence and therefore could be a potential target for tumor senotherapy.
2.1.3 Therapy-Induced Senescence
Chemotherapy- and radiotherapy-induced cellular senescence occur as a result of DNA damage, oxidative stress, and activation of stress response pathways, such as the p53-p21 and p16-RB pathways [26, 27]. These treatments cause irreversible cell cycle arrest in damaged cells, preventing further proliferation in cells.
Conventional chemotherapy regimens, such as doxorubicin, etoposide and camptothecin, have been shown to cause significant DNA damage and induce senescence through increased expression of p53 [28-30]. Docetaxel and vinca alkaloids trigger a p53–p21-facilitated senescence response [31, 32], while senescence biomarkers have been detected following treatment with epigenetic modulators, such as DNA methyltransferase inhibitors and histone deacetylase inhibitors [33, 34]. Treatment with trastuzumab or pertuzumab, anticancer agents that target human epidermal growth factor receptor 2 (HER2)/neu, were found to induce senescence in breast cancer cells [35]. Treatment with low doses of radiotherapy induced cellular senescence through activation of ataxia telangiectasia mutated (ATM) or ataxia-telangiectasia mutated and Rad3-related (ATR) and the p53–p21 pathway, while high doses of radiation induced apoptosis [27, 36-38]. Together, these studies indicate that TIS may increase the senescent cell burden in the TME, thereby creating an immunosuppressive microenvironment.
2.2 Molecular Pathways of Senescence
As various stimulis may trigger cellular senescence, several signaling pathways are involved during the onset of cellular senescence. Lin et al. demonstrated that oncogenic Ras promotes senescence with upregulating the downstream effectors p53 and p16INK4A through the MEK/MAPK activation [39]. At the same time, Zhu et al. found that MEK/MAPK activation also takes place in fibroblasts after RAF activation [40]. PI3K/AKT pathway also plays a cole role in OIS. Another study demonstrated that AKT induced senescence is p53-dependent and is characterized by mTORC1-dependent regulation of p53 translation and stabilization of p53 protein following nucleolar localization and inactivation of MDM2, promotes accumulation of p53 and p21 [41]. What's more, ROS may also triggers cellular senescence by regulating several signaling pathways. For example, ROS leads to hyperactivation of ERK signaling pathway by oxidation of cysteine residues in protein tyrosine phosphatases, inducing cellular senescence [42-44]. Recently, Borodkina et al. revealed that the accumulation of ROS leads to the activation of MAPK/P38 signaling pathway [45]. The SASP also plays an important role in cellular senescence, while the regulation of the SASP is complex from numerous studies. HMGB1, NF-κb, NOTCH1, and m TOR pathways are the main contributors which promotes the onset of the SASP [46-49]. In conclusion, the molecular pathways of senescence are complex and multifaceted. Further elucidation of these pathways will not only enhance our understanding of the biology of aging but also pave the way for novel therapeutic strategies targeting age-related diseases and cancer.
2.3 DNA Damage Response and Cell Cycle Arrest
The DNA Damage Response (DDR) and cell cycle arrest are key hallmarks of senescent cells. Various triggers of senescence, such as OIS, reactive oxygen species (ROS), and tumor therapies, can induce DNA damage and subsequently activate the DDR signaling pathway. For instance, oncogene activation may lead to DNA double-strand breaks (DSBs), thereby activating p53 and establishing a barrier to tumor progression [49]. ATM, a central kinase protein, serves as the initial and primary transducer of DNA DSBs and activates downstream checkpoint kinases, CHK1, and CHK2 [50]. These kinases, in turn, phosphorylate p53 in conjunction with ATM, leading to increased p53 levels [51]. Phosphorylated p53 upregulates the expression of p21WAF1CIP1, which inhibits the kinase activity of CDK4/6 and cyclin E/CDK2, ultimately resulting in cell cycle arrest [52]. Moreover, the p16INK4A/pRB pathway also mediates cell cycle arrest, either independently or simultaneously with the DDR pathway. These two pathways are intricately interconnected, with p53, p21, p16, and pRB serving as critical regulatory components [53-55]. p21WAF1CIP1, which regulates the kinase activity of CDK4/6 and cyclin E/CDK2, plays a dual role in cell cycle arrest. High levels of p21WAF1CIP1 inhibit CDK4/6 activation, while low levels may promote it [56]. Similarly, p16INK4A directly binds to and inhibits CDK4/6, preventing the phosphorylation of pRB by cyclin E/CDK2 [57]. This leads to the retention of pRB in its hypophosphorylated state, which in turn sequesters E2F transcription factors, thereby inhibiting the transcription of genes required for cell cycle progression [58, 59].
3 The Dual Role of Tumor Cell Senescence in Tumor Progression
Cellular senescence, characterized by an irreversible cell cycle arrest, has emerged as a crucial player in tumor biology. There has been reported that cell senescence has the ability to predict patient outcomes in glioma, stomach cancer, colon cancer, with lower progression-free survival and overall survival for groups with higher cell senescence scores [60, 61]. Patients with a high cellular senescence score for ban exhibit a high abundance of immunosuppressive cells and generally have poor clinical outcomes after immunotherapy. Besides, it has been reported that anti-senescence drugs or gene-editing techniques aimed at eliminating senescent cells in tumor-bearing mice have been shown to enhance the efficacy of chemotherapy and immunotherapy, significantly reduce tumor volume, and improve survival rates [62, 63]. These findings underscore the critical impact of senescence on tumor progression.
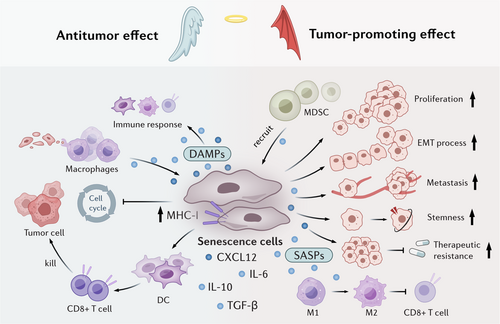
However, as the alterations of trigger intensity, disease stage or age, senescence elicits diverse effects [64]. In tumor microenvironment, senescent cells can induce immune cell-induced clearance and promote elimination of pre-neoplastic cells, which can delay tumor progression. On the other hand, the progressive accumulation of senescent cells can secret various factors or metabolites to induce immunosuppression microenvironment, exacerbate tumor progression [65]. Here, we have discussed the paradoxical effects of tumor cell senescence in tumor niche and outlined the underlying reasons (Figure 2).
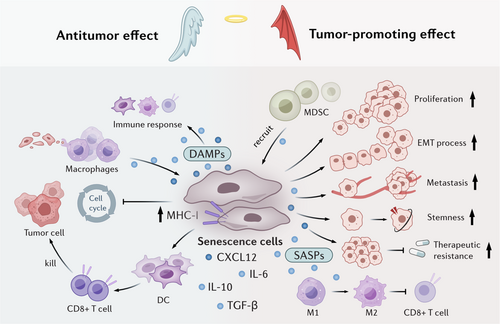
3.1 Senescence as a Tumor-Suppressive Mechanism
The antitumor activity of senescent cells is multifaceted. Senescent cells induce cell cycle arrest and suppress tumor cell proliferation, thereby inhibiting cancer progression. Moreover, senescent cells facilitate immunosurveillance by presenting distinctive surface markers that trigger immune-mediated clearance. Their presence may also sensitize tumors to therapeutic interventions like radiotherapy and chemotherapy, potentially enhancing treatment efficacy.
The aberrant activation of oncogenes, such as BRAF, RAS, or c-MYC, facilitate OIS and temporarily arrest tumor cells in a pre-malignant state [16, 66, 67], which serves as a barrier to tumor initiation. Activation of P53 has also been shown to induce liver tumor cell senescence, which further delays tumor growth and extends survival [68, 69]. NrasG12V-induced senescent liver tumor cells have been shown to recruit CD4+ T cells and induce macrophage-dependent clearance of pre-malignant cells. Senescent tumor cells have been shown to upregulate IFN-γ of T cells and robustly enhance the antigen-presenting capacity of tumor cells, thereby promoting the recruitment of innate cells, including natural killer (NK) cells and macrophages to the tumor bed, where they facilitate immune clearance [68, 70]. Furthermore, after tumor cell senescence, macrophages have also been found to transition from suppressive PD-L1+ cells to the antigen-presenting phenotype, synergistically enhancing T cell-mediated immune clearance of senescent cells [68]. Neutrophils can also be recruited and activated by IL-8 and acute-phase serum amyloid A1 (A-SAA) seZcreted by senescent cells, which further the phagocytic removal of senescent tumor cells by neutrophil extracellular traps (NETs) [71].
Senescent cells also exhibit potent antigenicity and adjuvanticity to act as an immunoadjuvant. Marin and colleagues reported that regardless of the senescence-inducing stimuli, senescent cells display high processing and presentation of antigens through MHC class I molecules, some of which were senescence-specific antigens [72, 73], rendering them highly effective at activating dendritic cells (DCs) and antigen-specific CD8 T cells. These phenotypes may be induced by activation of self-sustained IFN-I signaling in senescent cells. Furthermore, compared with cells undergoing immunogenic cell death (ICD), senescent cells also release damage-associated molecular patterns (DAMPs) and have an increased ability to activate an immune response [73]. These reports highlight the potential therapeutic effects of senescent cell-derived vaccines to delay tumor progression. It is also intriguing to hypothesize about the potential synergistic effects of using senescent cell-derived vaccines in conjunction with immune checkpoint inhibitors (ICIs).
The senescence response also sensitize the effects of chemotherapy or targeted anticancer treatments. It has been reported that using MEKi + CDK4/6i to treat pancreatic ductal adenocarcinoma (PDAC) promotes vascular remodeling through induction of a proangiogenic SASP, leading to enhanced drug delivery and T cell infiltration, which could sensitize the efficacy of anti-PD-1 therapy [74]. In addition, reducing c-MYC levels has been shown to significantly inhibit bladder cancer cell proliferation, promote cellular senescence, and enhance cisplatin chemosensitivity [75], indicating a novel prospective to change cisplatin chemosensitivity by modulating bladder cancer cell senescence.
3.2 Senescence as a Tumor-Promoting Mechanism
Senescent tumor cells promote tumor progression through various mechanisms. SASP factors and metabolites released from senescent cells promote tumor cell proliferation, survival, invasiveness and metastasis [76]. They modulate the immune microenvironment by releasing immunosuppressive factors like IL-10 and TGF-β, which foster a tumor-promoting niche [77, 78]. Notably, once senescent cells escape cell cycle arrest and re-enter the proliferative phase to form tumor cells, they display more aggressive tumorigenic features than other tumor cells [19]. Evidence for this concept comes from studies showing that genetically eliminating p16+ cells in a transgenic mouse model is enough to extend the lifespan of naturally aged mice and alleviate the rate of spontaneous tumor formation [79].
Senescence-associated stemness is a cell-autonomous feature that exerts detrimental, highly aggressive growth potential and is enriched in relapse tumors [20, 80]. For example, Lee et al. demonstrated that in a dense monolayer of breast cancer cells, senescent cells acted as aggregation centers, attracting surrounding nonsenescent cells and thus forming localized 3D cell clusters within the 2D tumor layer [81]. These findings indicated that senescent cells have the ability to promote cell aggregation and remodel tissue structure. Several studies have reported that senescent cells that resume proliferation (postsenescent cells) differ fundamentally from those that never entered senescence [82, 83]. Post-senescent cells display therapy resistance [19], metabolic reprogramming, immunogenicity, stemness, and have epigenetic memory [20].
Senescent tumor cells create a cytokine shield that safeguards neighboring non-senescent tumor cells from immune system attacks [78]. Multiple studies in murine models of HCC, prostate, and skin cancers have indicated that this process is mediated by the recruitment of MDSCs, which are chemoattracted by factors such as IL-6, IL-8, and CCL2 secreted by senescent cells [84-86]. In colorectal cancer (CRC), senescent tumor cells have been shown to enhance monocyte differentiation into M2 macrophages and inhibit CD8+ T cell infiltration through the secretion of CXCL12 and CSF1 [87]. Consistent with this observation, neutralization of CXCL12/CSF1 enhances the efficacy of anti-PD1 antibodies in mice. CCL20 secreted by senescent cells also promotes M2 polarization, while non-senescent tumor cells stimulate M1 differentiation and antigen presentation [88], which induces immunosuppression and promotes the epithelial-mesenchymal transition (EMT) [89]. In thyroid cancer, senescent cells increase the survival of cancer cells and promote the invasion and metastasis through CXCL12/CXCR4 signaling [90]. In addition, the secretion of IL-6 by senescent osteoblasts has been shown to drive the colonization of metastatic breast cancer cells in bone niche [91].
TIS not only induces tumor cell cycle arrest to suppress tumor progression, but can also act as a tumor-promoting factor by promoting the proliferation of bystander cells and facilitating metastasis [69]. Studies have demonstrated that elimination of chemotherapy- or radiotherapy-induced senescent cells reduces tumor recurrence in glioblastoma and skin cancer [92, 93]. TIS cells release an increased number of extracellular vesicles, which promote the progression of recipient cancer cells [94]. Chemotherapy has been shown to promote enhancer of zeste homolog 2 (EZH2) expression and inhibit the secretion of immunomodulatory SASP factors, which diminish NK and T cell infiltration [95]. Furthermore, unlike other senescent cells, TIS cells fail to elicit immune clearance due to the overexpression of immune checkpoint ligands, such as PD-L1, which leads to T cell inhibition [96]. To address these issues, the use of some drugs, such as antineoplastic drugs or senolytics in combination with pro-senescence chemotherapy has been examined in tumor treatment [97, 98].
Additionally, the influence of senescent cells on drug resistance have also been highlighted. Following the induction of senescent cells by chemotherapy and radiotherapy, subsequent immunotherapy revealed that the presence of senescent cells significantly reduced the survival rate compared to the control group without senescent cells [99]. This indicates that senescent cells impair the tumor immune response, while their removal can restore the effectiveness of immunotherapy. Furthermore, drug resistance to chemotherapy is more prominently associated with the effect of SASP on the stemness of tumor cells. Studies have demonstrated that TSPAN8 + myCAFs can enhance the stemness of breast cancer cells by secreting factors related to the senescent phenotype (such as IL-6 and IL-8), thereby conferring resistance to chemotherapy [100]. In another study, it was found that human stromal cells would passively enter the senescent state during chemotherapy, generate and release an exogenic factor named amphiregulin, which on the one hand promoted the malignant phenotype of residual cancer cells in the lesion and led to their drug resistance, thus causing clinical treatment resistance. On the other hand, cancer cells are induced to up-regulate the expression of PD-L1, forming an immunosuppressive microenvironment, making cancer cells escape immune surveillance [101].
Besides, small extracellular vesicles derived from senescent human stromal cells aggravate the malignant progression of residual cancer cells in the lesions and promote their acquisition of drug resistance, resulting in significantly poor prognosis in clinical patients [83].
3.3 Context-Dependent Outcomes of Senescence
Cellular senescence is a complex and multifaceted biological process whose outcomes are highly context-dependent. The phenotypic and functional manifestations of senescence can vary significantly based on the cellular environment, tissue type, and the specific signals and stressors encountered by the cell. The dual function of senescent cells can be influenced by the disease stage. In HCC, senescence-associated CCL2-CCR2 signaling suppresses tumor progression in the early stages of the disease, but promotes tumor progression in later stages [84]. In the early stages of HCC, senescent cells secrete CCL2 and recuit CCR2+ myeloid cells to clear pre-malignant hepatocytes. However, in established tumors, senescent cells recruit CCR2+ immunosuppressive myeloid cells that inhibit the activity of NK cells, and promote tumor growth and progression. However, at present, Most animal studies on tumors rely on xenograft models, which bypass the tumor initiation stage, so there are few studies focus on the role of senescent cells in the initial stage of tumorigenesis. Future research are suggested to be conducted in spontaneous models such as KrasG12Dp53R172HPdx1-Cre(KPC) spontaneous pancreatic cancer mouse models to study the influence of senescent cells on the initial stage of tumors.
Senescent tumor cells also seem to play different roles in different tumor types [102]. In hepatocellular carcinoma, the induction of p53-mediated senescence in tumor cells increases the number of macrophages and lymphocytes, and significantly remodels the surface proteome of senescent cells, which results in decreased sensitivity of senescent cells to type II interferon signaling due to induction of IFN-γ receptor IFNGR1. Senescent cells enhance the expression of MHC-I molecules, and IFN-γ signals further promote this expression, thereby improving their antigen presentation ability [103]. In addition, it has been found that senescent melanoma cells can also effectively activate dendritic cells and antigen-specific CD8+ T cells to promote tumor regression by activating interferon signaling, enhancing MHC Class I molecules, and presenting senescence-specific autopeptides that can activate CD8+ T cells [104]. On the contrary, for breast cancer patients, the survival rate of patients with p53 wild-type tumors is lower than that of patients with p53 mutation tumors, and patients with p53 wild-type tumors have poor response to chemotherapy. Chemotherapy of such tumors will activate p53-dependent senescence response and simultaneously express powerful immunosuppressive signals, such as PD-L1 and CD80, which will inhibit T cell activity after binding to PD-1 and CTLA-4 on T cells [105].
Cellular senescence plays context-dependent roles in tumor development, with its effects varying across different stages and tumor types. While senescence can suppress tumor initiation through immune-mediated clearance of pre-malignant cells, it may later promote tumor progression by recruiting immunosuppressive cells. This duality underscores the complexity of senescence in cancer biology. Future studies should utilize spontaneous tumor models to better understand the dynamic roles of senescent cells and identify therapeutic strategies that can selectively harness their tumor-suppressive functions.
4 Functional Reprogramming of Senescence
Cellular senescence is a multifaceted biological process that plays a dual role in tumorigenesis, acting as both a tumor-suppressive mechanism and a potential driver of cancer progression. In recent years, significant progress has been made in understanding the functional reprogramming of senescence, particularly in the context of TME modulation and cancer stemness. This section provides an overview of the key mechanisms and functional reprogramming associated with cellular senescence, highlighting their impact on tumorigenesis and therapeutic resistance.
4.1 Reprogramming Mechanisms
SASP production, metabolic reprogramming, and enhanced stemness are the most prevalent phenotypic alterations observed in senescent cells. These changes have garnered significant attention in recent research, revealing complex underlying mechanisms that contribute to the diverse roles of senescent cells in various biological contexts [106].
The p53-p21 and p16-Rb pathways are central to the formation of stable proliferative arrest in senescent cells and represent key drivers of cellular reprogramming. These pathways not only establish the hallmark growth-inhibitory characteristics of senescent cells but also promote widespread transcriptional and phenotypic changes. Specifically, they activate retinoblastoma protein (Rb)-dependent transcription and interact with STAT and SMAD transcription factors to orchestrate a complex bioactive secretome [107, 108]. This interaction enables the production of a wide array of cytokines, chemokines, and growth factors characteristic of the SASP. Moreover, p53 drives the biosynthesis of hexosamine and de novo UDP-GlcNAc production by upregulating GFPT2, thereby influencing cellular metabolism [109]. In parallel, p16 regulates lipid peroxidation and cellular sensitivity to ferroptosis by modulating glioblastoma (GBM) lipid metabolism [110], highlighting the multifaceted regulatory roles of these pathways in senescence.
Another critical pathway involves the DDR and NF-κB signaling. The NF-κB subunit p65 accumulates on senescent cell chromatin and serves as a primary regulator of SASP [48]. DNA damage rapidly activates NF-κB, which subsequently modulates the expression of pro-inflammatory SASP components during the secondary phase of activation. This includes a wide range of cytokines such as IL-1α, IL-1β, IL-6, and IL-8, chemokines like CXCL1, CCL2, and CCL5, as well as matrix metalloproteinases (MMPs) [106, 111]. These factors collectively contribute to the inflammatory and tissue-remodeling properties of senescent cells.
Additionally, the cytosolic DNA-cGAS-STING pathway plays a crucial role in senescence, particularly when induced by DNA damage. cGAMP synthase (cGAS) recognizes cytoplasmic chromatin fragments in senescent cells, and its activation triggers interferon signaling via the stimulator of interferon genes (STING) [112-114]. This pathway not only induces the production of SASP factors but also regulates cellular metabolism. Specifically, STING controls the balance between glycolysis and oxidative metabolism by modulating the expression and localization of hexokinase 2 (HK2) [115].
The p38 MAPK and mTOR pathways further contribute to the reprogramming of senescent cells by regulating SASP secretion. p38 MAPK is activated with slow kinetics during genotoxic stress-induced senescence, with p38 MAPKα being the primary functional subtype [116]. It induces the secretion of NF-κB-associated SASP by modulating NF-κB activity. Conversely, rapamycin, an mTOR inhibitor, reduces SASP translation by suppressing NF-κB transcriptional activity and inhibiting IL1A translation [117]. This highlights the intricate interplay between these pathways in shaping the secretory phenotype of senescent cells.
Together, these pathways underscore the complexity of senescence reprogramming, involving interconnected networks that regulate cell cycle arrest, inflammation, metabolism, and stemness. Understanding these mechanisms is essential for elucidating the dual roles of senescent cells in tissue homeostasis and disease progression.
4.2 Senescence and Tumor Stemness
Stemness, characterized by the functional attributes of stem cells, endows tumor cells with heightened invasiveness, drug resistance, and propensity for relapse. Emerging evidence suggests that stemness and senescence are intricately linked, regulated by overlapping signaling networks. Key senescence-associated signaling components, including p16INK4a, p21CIP1, and p53, play significant roles in modulating both processes. Previous studies have demonstrated that senescent cells exhibit significant upregulation of stem cell markers, conferring greater tumor-initiating potential compared to their non-senescent counterparts [20, 80, 118]. This phenotypic shift also contributes to chemoresistance in tumors [100], highlighting the complex interplay between senescence, stemness, and therapeutic response.
4.3 Secretome and Metabolome Reprogramming
Although senescent cells are characterized by irreversible cell cycle arrest and low proliferative potential, they can also display distinctive traits associated with apoptotic or necrotic cells, such as secretome reprogramming (Figure 3A) and metabolome reprogramming (Figure 3B) [119-122]. Such unique functional reprogramming allows senescent cells to play a significant role in tumorigenesis. Here, we discuss reprogramming of the secretome and metabolome of senescent cells, and how this contributes to their context-dependent beneficial or detrimental roles in the TME.
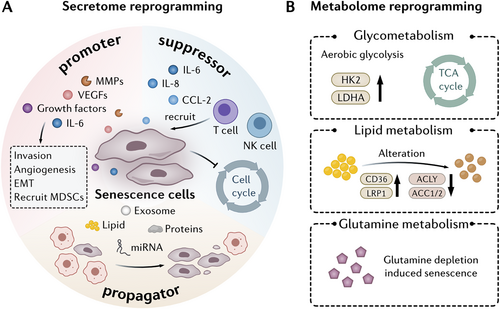
4.3.1 Reprogramming of the Secretome
The concept of SASP was first reported by Chaib and colleagues [123], and was used to describe a characteristic of senescent cells, namely, that senescent cells secrete substantially higher levels of specific proteins than non-senescent cells. SASP factors include pro-inflammatory cytokines, chemokines, growth factors, and proteases, such as IL-6, IL-1α, IL-1β, matrix metalloproteinase-3 (MMP-3), chemokine (C-C motif) ligand 2 (CCL2), CXC motif chemokine ligand 1 (CXCL1), vascular endothelial growth factor (VEGF), and fibronectin [124]. Among them, IL-6, IL-1α, and IL-1β are the most common SASP factors in the TME and are secreted by multiple types of senescent cells. The composition and duration of SASP are influenced by the nature of senescence inducers and senescent cell types, making it highly variable and broad-ranging during tumor development [125]. Evidence of SASP secretion through exosomes has recently emerged. Extracellular vesicles derived from senescent cells carry bioactive factors such as proteins, lipids and miRNAs, which promote a pro-inflammatory environment and regulate the communication between senescent and neighboring cells [126-128].
SASP plays a dual and complex role in the context of tumors, depending on the TME and SASP profile. With respect to the development of hepatocellular carcinoma (HCC), IL-6 has been shown to inhibit the occurrence of tumors in chronic liver injury, but increase the risk of liver cancer in acute liver injury [129]. Galectin-9, which is secreted by senescent melanoma cells, has also been shown to have a paradoxical role. Galectin-9 not only promoted apoptosis of T cells and monocytes to induce an immunosuppressive TME [130], but also inhibited melanoma metastasis [131]. SASP factors can also act in a paracrine manner by inducing senescence in nearby non-senescent cells to accelerate senescence propagation [132], which can inhibit tumorigenesis by halting cell proliferation and promote tumorigenesis by creating a pro-inflammatory microenvironment. Overall, as a tumor suppressor, SASP can reinforce cell cycle arrest [76] and induce immune clearance of tumor cells [133, 134], while as a tumor promoter, SASP can stimulate cancer cell proliferation, invasion, metastasis [135-139] and angiogenesis [140, 141]. The complex paradoxical role of SASP makes it a key player in shaping the landscapes of cancer progression and therapy response.
4.3.2 Reprogramming of the Metabolome
Senescent cells undergo distinct metabolic changes including enhanced aerobic glycolysis, increased fatty acid oxidation (FAO), and a reliance on glutaminolysis. These metabolic adaptations enable senescent cells to maintain their energy demands, biosynthetic processes, and the pro-inflammatory SASP, contributing to their role in tumorigenesis.
4.3.2.1 Aerobic Glycolysis
Senescent cells display increased expression of glycolysis-associated gene profiles, including hexokinase 2 (HK2) and lactate dehydrogenase A (LDHA) [142, 143], suggesting that glucose metabolism is increased. Senescent cells have been shown to increase glucose uptake and consumption accompanied by enhanced aerobic glycolysis [144]. Recently, Dou et al. demonstrated that pyruvate dehydrogenase kinase 4 (PDK4) plays a crucial role in senescence-associated glucose reprogramming in tumors [145]. PDK4+ stromal cells were shown to display a senescent phenotype, as well as increased aerobic glycolysis. Furthermore, increased production of lactate in these cells was found to promote cancer cell proliferation, migration, invasion and drug resistance. In addition, inhibition of PDK4 was found to partially correct altered glucose metabolism in senescent cells, as well as suppress SASP secretion, indicating that PDK4 could be a potential target for senotherapy.
Although glycolysis is upregulated, mitochondrial respiration remains active in senescent cells, allowing the tricarboxylic acid (TCA) cycle to continue producing energy, which is distinct from the Warburg effect in tumor cells [142, 146]. Changes in mitochondrial homeostasis, as well as alterations in the activities of enzymes regulating energy metabolism in senescent cells may contribute to these metabolic alterations [142, 147, 148]. Thus, targeting abnormal metabolic processes in senescent cells may be a specific target to induce their clearance. Consistent with this, treatment with a pyruvate dehydrogenase (PDH) inhibitor has been shown to reduce mitochondrial metabolism and mitochondrial electron transport chain (ETC) complex I, thereby inhibiting SASP secretion [149], and specifically eliminating the effects of senescent cells.
4.3.2.2 Lipid Metabolism
Senescent cells exhibit distinct changes in lipid metabolism, which are characterized by alterations in lipid uptake and fatty acid synthesis. A decline in FAO levels has been shown to impair stem cell activity and drive stem cell senescence [150]. Studies have also shown that H2O2-induced senescence can be reversed by inhibiting lipogenesis with agents such as fatty acid synthase (FAS) inhibitors. The results of these studies highlight the close correlation between lipid metabolism and senescence [151].
Alterations in lipid metabolism in senescent cells are complex, and include the upregulation of lipid importers, such as cluster of differentiation 36 (CD36) and low-density lipoprotein receptor–related protein-1 (LRP1), and downregulation of lipid synthesis enzymes, such as ATP citrate lyase (ACLY), acetyl-CoA carboxylase 1 (ACC1) and ACC2 [152, 153]. The balance of lipid uptake and de novo synthesis during cellular senescence regulates the cellular lipid metabolic profile. For example, cholesterol levels increase during senescence due to cholesterol import and de novo synthesis [23]. In addition, levels of the sphingolipid, ceramide, increase in senescent cells and promote p21 expression [154, 155]. These findings indicate that lipid metabolism is elevated in cellular senescence.
In contrast, other studies have shown that lipid metabolism is reduced in senescent cells. Carnitine palmitoyltransferase 1 (CPT1) and acyl-CoA oxidase 1 (ACOX1) are rate-limiting enzymes in fatty acid catabolism. Studies have shown that deletion of CPT1C, an isoform of CPT1, induced mitochondrial dysfunction and cellular senescence in different types of tumor cells and inhibited cancer growth [156]. A reduction in ACOX1 levels was observed in aged rats and age-related disease [157, 158]. Peroxisome proliferator-activated receptor α (PPARα) is a transcriptional activator of ACOX1 and CPT1. Fenofibrate, an agonist of PPARα, was found to not only reduce the number of senescent cells through apoptosis, but also increase autophagic flux [159].
The role of FAO in senescence requires further examination. Some studies have suggested that FAO generates ROS as a by-product [160], resulting in oxidative stress and damage to cellular components, which in turn contribute to senescence and tumorigenesis. In addition, some lipids such as ceramides, S1P, and prostaglandins, can modulate key senescence-associated genes, such as p53, NF-κB, p38 MAPK, JNK, and mTOR, which may also regulate secretion of SASP factors [2, 161, 162]. In conclusion, although senescent cells display dysfunctional lipid metabolism, the role of lipid metabolism in senescence requires further elucidation.
4.3.2.3 Glutamine Metabolism
Glutaminolysis is crucial for the survival of senescent cells and glutaminase has been shown to be upregulated in senescent cells [163]. Depletion of glutamine in tumor cells inhibits senescence and reduces the stem cell subpopulation [164], while an increase in glutamine promotes the induction of senescence. Furthermore, glutamine has been identified in senescence-associated nutrient consumption profiles [165]. Above all, inhibition of glutamine metabolism has been shown to sensitize senescent cells to apoptosis, making it a potential target for therapeutic interventions aimed at mitigating the harmful effects of cellular senescence.
5 Senescence in Nontumor Cells of the Tumor Microenvironment
Nontumor cells, including cancer-associated fibroblasts (CAFs), immune cells, and endothelial cells, are involved in various processes including promoting tumor growth, facilitating metastasis, and modulating immune responses [166-169]. The senescent microenvironment is considered to be a common feature in human neoplastic tissues, including prostate cancer, CRC and neurofibroma [44, 170-174]. Thus, it is important to understand the unique senescent features of each cell subtype within the TME (Table 1).
| Cell category | Cell type | Causes | Biomarkers | Impact on TME | Reference |
|---|---|---|---|---|---|
| Lymphoid cells | T cells | Metabolic Pressure; Metabolic Competition with Treg cells, oxidative stress | CD28 ↓, CD27 ↓; KLRG-1 ↑, CD57 ↑, Tim-3 ↑, TIGIT ↑, CTLA-4 ↑ | Immunosuppression, proliferation ↓; monocyte activation | [175-182] |
| NK cells | Aging, oxidative stress | NKp30 ↓, NKp46 ↓; inhibitory receptors↑ | Immune surveillance ↓, antitumor immunity ↓, tumor growth↑ | [183-185] | |
| B cells | Aging, inflammation | Tbx21 ↑, IL-4 ↑, IL-10 ↑ | Humoral immunity ↓, chronic inflammation ↑; disrupts immune homeostasis | [186, 187] | |
| Myeloid cells | Macrophages | SASP signals, oxidative stress | CD38 ↑, SPP1↑ | Angiogenesis ↑ immune evasion ↑; antitumor immunity↓ | [187, 188] |
| Neutrophils | Aging, oxidative stress | TREM2 ↑; VEGF ↑; MMP-9 ↑; NETs↑ | Tumor metastasis ↑; tumor growth↑ | [189] | |
| Dendritic cells | Aging, oxidative stress | Costimulatory molecules ↓; mitochondrial damage ↑; | Adaptive immune responses ↓, chronic inflammation ↑; immunotherapy efficacy ↓, tumor immune response↓ | [190] | |
| Stromal cells | Cancer-associated fibroblasts | Chronic inflammation metabolic pressure | IL-6 ↑, CXCL1 ↑, CXCL12 ↑; PD-L1 ↑ | Immunosuppression; tumor invasion ↑; tumor cell growth | [191-195] |
| Endothelial cells | Chronic inflammation, surgery-induced stress responses | CXCL11 ↑, CXCL12 ↑, VCAM1 ↑ | Tumor metastasis ↑; immunosuppression | [196-199] |
- Abbreviations: CTLA-4, cytotoxic T-lymphocyte-associated protein 4; CXCL1, CXC motif chemokine ligand 1; IL, interleukin; MMP-9, matrix metalloproteinases; PD-L1, programmed cell death 1 ligand; TME, tumor microenvironment; TREM2, triggering receptor expressed on myeloid cells 2; VCAM1, vascular cell adhesion molecule 1; VEGF, vascular endothelial growth factor.
5.1 Senescence in Stromal Cells
Senescent stromal cells, including fibroblasts, endothelial cells, and other components of the TME, exhibit distinct characteristics that contribute to tumor initiation and progression. Understanding the role of senescent stromal cells could provide critical insights into their impact on tumor development and resistance to therapy.
5.1.1 Cancer-Associated Fibroblast Senescence
CAFs are stromal cells within the TME that promote malignancy and tumor progression. Senescent CAFs, representing the majority of aged stromal cells, accumulate with advanced PDAC [200], as well as with breast cancer, where a strong correlation between senescent CAFs and cancer recurrence has been reported [201]. In lung cancer [202], the accumulation of ROS in senescent fibroblasts has been shown to activate the signal transducer and activator of transcription 3 (STAT3) signaling pathway, which mediates CAF functions that significantly impact lung cancer cell migration and invasion.
Senescent CAFs contribute to the shaping of an immunosuppressive microenvironment by secreting various factors [193, 203]. For example, in gastric cancer, fibroblasts with a higher senescent score were found to secrete more cytokines and chemokines, including IL-6, CXCL1, and CXCL12, to form an immunosuppressive microenvironment [194]. In skin cancer, senescent CAF-derived IL-6 orchestrates the expansion of immunosuppressive myeloid cells and enhances their ability to inhibit antitumor T cell responses [193]. In breast cancer, the extracellular matrix secreted by senescent CAFs specifically impairs the cytotoxic function of NK cells, thereby facilitating tumor growth [192]. Senescent CAFs have also been shown to promote tumor cell proliferation and invasiveness through the secretion of SASP factors [23, 195]. Furthermore, heterogeneous upregulation of PD-L1 has been reported in senescent fibroblasts. Interestingly, neutralizing PD-L1 in fibroblasts using anti-PD-1 has been shown to enhance the cytotoxicity of CD8+ T cells [191].
Senescent CAFs also undergo metabolic reprogramming. For example, senescent CAFs can regulate aerobic glycolysis through mitochondrial oxidative stress, leading to lactate production that promotes breast cancer growth [204]. Alicea et al. also found that senescent fibroblasts increase the secretion of neutral lipids and increase the uptake of lipids of melanoma cells [205]. Recent studies have identified a unique senescent subpopulation of myofibroblasts located near tumor ducts that accumulate with the progression of PDAC [200] and suppress immune cells in the TME through the secretion of SASP factors.
5.1.2 Endothelial Cell Senescence
Endothelial cell senescence plays a critical role in tumor progression and responses to therapy. A machine learning model based on endothelial cell senescence signatures (EC.SENESCENCE.SIG) has been shown to more accurately predict immunotherapy responses across multiple cancers than previous transcriptomic models [198]. In addition, TIS in endothelial cells leads to the secretion of CXCL11, which enhances breast cancer cell metastasis [197]. The same phenomenon has also been observed in uveal melanoma, where senescent endothelial cells promote tumor cell migration through CXCL12 secretion and contribute to the formation of an immunosuppressive microenvironment [196]. Furthermore, sustained activation of neurogenic locus notch homolog protein 1 (NOTCH1) in endothelial cells promotes senescence, resulting in the expression of chemokines and vascular cell adhesion molecule 1 (VCAM1), which drive neutrophil infiltration, tumor cell adhesion, and metastasis, particularly to the lungs, following surgery [199].
5.2 Senescence in Immune Cells
Lymphocyte senescence is characterized by functional decline and phenotypic alterations, including reduced proliferative capacity, diminished effector functions, weakened antigen-specific responses, and altered cytokine secretion profiles. This process not only impairs immune surveillance but may also contribute to tumor initiation and progression through modulation of the immune microenvironment.
5.2.1 T Cell Senescence
T cells are critical components of the immune system, playing a pivotal role in tumor initiation and progression. T cell senescence is irreversible and is an indicator of T cell dysfunction in cancer [179]. Senescent T cells exhibit numerous molecular alterations. For example, costimulatory molecules CD28 and CD27 are downregulated in senescent T cells, while the killer cell lectin-like receptor subfamily G (KLRG-1) and CD57 are expressed [157, 178, 206-208]. Moreover, the expression levels of immune checkpoint molecules, including Tim-3, TIGIT, and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), are elevated with aging [178]. In addition, senescent CD8+ T cells primarily rely on anaerobic glycolysis for energy production instead of a flexible utilization of both glycolysis and oxidative phosphorylation, which leads to mitochondrial dysfunction and increased ROS production [209].
The TME can induce T cell senescence. For example, an increased number of senescent cells was observed in the blood, lymph nodes, spleens, and tumors derived- from B16F0 tumor–bearing mice [210]. Both cancer cells and Treg cells can induce responder T cell senescence [177]. Furthermore, tumor-derived ILT4/PIR-B induces T cell senescence by binding to the HLA-G ligands on the surface of T cells [211]. Treg cells induce DNA damage in effector T cells through metabolic competition during their interaction, which leads to senescence and functional alterations that are molecularly distinct from anergy and exhaustion [176]. In addition, MDSCs can induce CD8+ T cell senescence and attenuate an antitumor response via the exosomal transfer of GPR84 from MDSCs to CD8+ T cells [175]. Mechanistically, the MAPK signaling pathway plays a significant role in regulating T cell senescence [212-215]. The senescent T cell population can restore proliferation and telomerase activity by inhibiting AMP-activated protein kinase (AMPK), TAB1, and/or p38 activation [215]. Treg cells can compete with effector T cells for glucose, leading to AMPK activation and ATM-associated DNA damage response, which subsequently induces senescence in T cells [214, 216]. Moreover, ERK1/2 and p38 are selectively phosphorylated and activated during Treg cell-mediated T-cell senescence [214, 215, 217].
Senescent T cells have a crucial role in the TME. Increasing evidence indicates that senescent T cells are critical players in immune suppression and tumor progression [180, 181]. Senescent CD8+ and CD4+ T cells have been shown to suppress the proliferation of normal T cells [180]. Thus, the development of intervention strategies that impede the progression of T cell senescence and rejuvenate the functionality of T cells are pivotal for the enhancement of antitumor immune responses [181]. Furthermore, senescent T cells promote CD16 expression and reduce CD14 expression in monocytes, thereby mediating monocyte activation and changes in secretion profiles to promote tumor progression [182]. Despite the notion that senescent T cells may exhibit reduced functionality, there is substantial evidence suggesting that these cells play a pivotal role in antitumor immune responses. Higashiguchi et al. showed that the absence or reduced expression of p21 in CD4+ T cells correlated with enhanced tumorigenesis in animal models [23]. Similarly, low p21 expression levels in the CD4+ T cells of CRC patients was associated with poorer outcomes in terms of cancer-specific survival than patients with CD4+ T cells expressing high levels of p21. Although costimulatory factors are downregulated in senescent T cells, it has been reported that senescent T cells express a protein complex containing the agonistic NK receptor NKG2D and the NK adaptor molecule DAP12, which may contribute to tumor immunity by acquiring NK-like innate immune functions [218, 219].
5.2.2 NK Cell Senescence
NK cells are key components of the immune system, possessing both cytotoxic and immunoregulatory functions, and playing a crucial role in regulating malignancies and infections. As individuals age, the number of NK cells may increase, but their functional capacity tends to decline [184]. NK cell senescence is characterized by a decline in cytotoxic function and proliferative capacity, together with altered expression of surface markers, specifically, a decrease in activating receptors, including NKp30, NKp46 and DNAM-1 and an increase in inhibitory receptors [220]. Studies have shown that in the elderly, the NK cellular compartment undergoes restructuring, marked by a decrease in the production of immature CD56bright cells and an accumulation of highly differentiated CD56dim NK cells [221, 222]. These changes may affect the immune monitoring function of NK cells.
Senescent NK cells exhibit metabolic dysfunction, including mitochondrial impairment and increased production of ROS, which contribute to their functional decline [183], particularly with respect to antitumor immunity. In addition, senescent NK cells contribute to weakened immune surveillance in the TME, leading to reduced antitumor immunity and the promotion of cancer progression [185].
5.2.3 B Cell Senescence
Senescent B cells are characterized by reduced production of new B cells, impaired antibody generation [186], and a diminished B cell receptor repertoire [223], which limits their ability to respond to new antigens. In addition, senescent B cells adopt a more pro-inflammatory phenotype [187] and show an accumulation of memory B cells with lower functionality [224]. These changes contribute to a decline in humoral immunity.
Aged B cells in the spleen and bone marrow undergo significant phenotypic and functional changes, including the expansion of B cell subsets distinct from conventional naive and memory B cells, known as “age-associated B cells” (ABCs) [225]. These cells express Tbx21, resembling B cells that contribute to lupus-like autoimmunity in mice. The development and survival of ABCs depends on IFN-γ signaling. Upon activation, ABCs secrete IL-4 and IL-10, which enable ABCs to present antigens to T cells, potentially promoting inflammation by altering immune homeostasis [186, 226].
5.2.4 Macrophage Senescence
Senescent macrophages have a dual role in tumors, which is influenced by the TME. Senescent macrophages can adopt an M2-like phenotype, promoting tumor growth by secreting anti-inflammatory cytokines, supporting angiogenesis, and facilitating immune evasion [227, 228]. However, under certain conditions, senescent macrophages exhibit an M1-like phenotype, enhancing antitumor immunity by producing pro-inflammatory cytokines [188]. SPP1+ macrophages secrete SASP factors and display a senescent phenotype when surrounded by a large number of senescent tumor cells in high-grade tumors [229]. This complex behavior makes their role in tumors highly context dependent. Senescence-like macrophages are characterized by high expression of CD38, a nicotinamide adenine dinucleotide (NAD) glycohydrolase, which is linked to NAD depletion in senescent cells. NAD depletion impacts cellular metabolism and function, contributing to the altered phenotype of these macrophages in the aging process and in diseases such as cancer [230].
Recently, alveolar macrophages (AMs), which are tissue-resident macrophages in the lung, have been shown to be highly prone to senescence in Kras-mutant mice [231]. A novel cluster of senescent AMs, characterized by high expression of sialic acid binding Ig-like lectin (siglec-F) and C-X-C motif chemokine receptor 1 (CXCR1), was identified by single-cell RNA sequencing. These cells express several SASP factors, including Spp1, Igf1, and Ctsb, as well as genes involved in negatively regulating cell proliferation, such as Ctnnb1 and Ctsl. Furthermore, these senescent CXCR1High AMs were found to accumulate with age, as well as inhibit cytotoxic T cell infiltration during lung tumorigenesis. The harmful role of senescent macrophages in lung cancer was also highlighted in a study by Haston et al. [232], who reported that senescent macrophages constituted the majority of senescent cells in early pre-malignant lesions, which were previously thought to originate mainly from tumor or epithelial cells. Together, the findings of these studies demonstrate that senescent macrophages in pre-malignant lung lesions promote tumor development. Thus, the removal of these senescent macrophages through senolytic treatments could prevent malignant progression. Interestingly, the senolytic drug ABT263 (Navitoclax) has been shown to reverse the exacerbated immunosuppressive profile of macrophages in the TME and enhance sensitivity to PD-1 therapy [233].
5.2.5 Neutrophil Senescence
Senescent neutrophils play a complex role in the TME. Their impaired phagocytic ability and reduced cytotoxic function limit their capacity to eliminate tumor cells effectively [234]. Furthermore, senescent neutrophils produce higher levels of ROS and pro-inflammatory cytokines, which can promote tumor progression by inducing chronic inflammation [235]. Senescent neutrophils may also exert their immunosuppressive effects by interacting with other immune cells to create an environment that allows tumors to evade immune surveillance. Overall, senescent neutrophils may shift from an antitumor role to one that supports tumor growth and progression [189]. Senescent neutrophils express higher levels of triggering receptor expressed on myeloid cells 2 (TREM2) and display stronger immunosuppressive and tumor-promoting effects than normal neutrophils [236]. Senescent neutrophils more effectively facilitate tumor migration and support metastasis by releasing higher levels of metastasis-promoting factors, such as NETs, ROS, VEGF, and MMP-9 [65]. In breast cancer, senescent neutrophils exhibit distinct cell markers and possess distinct morphological features such as over-segmented nuclei [237]. Senescent neutrophils use sirtuin-1 (SIRT1) transcription to release mitochondrial DNA, which leads to the formation of mitochondria-dependent vital NETs. Senescent neutrophils accumulate in the lung premetastatic niche during the early stages of breast tumorigenesis and promote tumor metastasis [237], as well as cancer cell proliferation through the release of neutrophil elastase (NE) [238]. In addition, senescent neutrophils have been shown to produce an increased number of exosomes. Exosomal piRNA-17560 derived from senescent neutrophils promotes the expression of fat mass and obesity-associated protein (FTO) in breast cancer cells [239]. Upregulation of FTO enhances the stability and expression of zinc finger E-box binding homeobox 1 (ZEB1) transcripts by reducing N6-methyladenosine (m6A) RNA methylation. This process leads to chemoresistance and facilitates EMT in tumor cells.
5.2.6 Dendritic Cell Senescence
DCs play a crucial role in initiating adaptive immune responses. Senescent DCs exhibit diminished antigen uptake, processing, and presentation, which reduces their effectiveness in initiating adaptive immune responses [240, 241]. Senescent DCs also display impaired migration to lymph nodes, decreased expression of costimulatory molecules, and an increased secretion of pro-inflammatory cytokines, contributing to chronic inflammation. In addition, senescent DCs have reduced responsiveness to external stimuli and display metabolic dysfunction, such as mitochondrial impairment and heightened oxidative stress, further compromising immune surveillance and function [146]. Recently, Zhivaki et al. found that the weakened migratory activity of DCs in elderly mice decreased the effects of PD-1 and CTLA4-based cancer immunotherapies on tumors [190], suggesting that targeting DCs could be a potential strategy to enhance immune therapy.
Overall, the functions of senescent nontumor cells are complex and diverse. While the majority of current studies focus on their tumor-promoting roles, their tumor-suppressive functions remain to be further explored.
6 Senescence-Targeted Therapy
Recently, there has been an emergence of therapeutic strategies that target senescent cells either by inducing senescence to halt tumor growth or eliminating senescent cells to counteract their harmful effects. Approaches like the “one-two punch” therapy, which combines senescence induction with senolytic clearance, along with innovations such as nanoparticle delivery systems and senolytic chimeric antigen receptor (CAR)-T cells, are being developed to enhance cancer treatment and manage age-related diseases. Here, we highlight these strategies, their mechanisms, and their potential contribution to improving cancer therapy (Figure 4).
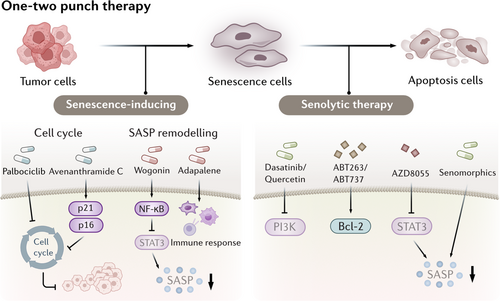
6.1 Senescence-Inducing Therapy
The concept of promoting cellular senescence in tumor cells to induce cell cycle arrest and promote tumor regression has led to the emergence of senescence-inducing therapies. These therapeutic strategies leverage the biological mechanisms underlying senescence to inhibit tumor growth and enhance the efficacy of conventional treatments. Palbociclib, which inhibits cell proliferation in human liver cancer cell lines by inducing a reversible cell cycle arrest [242], has shown promising outcomes in preclinical models of HCC and represents a novel therapeutic strategy for HCC treatment, when used either alone or in combination with sorafenib. Avenanthramide C (Avn-C), a polyphenol compound found predominantly in oats, has been shown to enhance the expression of p21 and p16, acting as an adjunctive agent in the treatment of CRC [243]. Wogonin, a natural flavonoid extracted from the honeysuckle plant, can induce cellular senescence and activate NF-κB, thereby inhibiting STAT3, which regulates SASP factors. The SASP produced by these senescent cells inhibits tumor cell proliferation, promotes M1 macrophage polarization In Vitro, and enhances immune cell infiltration in xenografted tumors in vivo [244]. Adapalene, as a retinoic-acid-receptor agonist, has been shown to trigger a robust senescence response and tumor-suppressive SASP [245]. Using a combination of adapalene with the chemotherapy drug docetaxel may enhance tumor-suppressing senescence and activate an antitumor immune response through NK cells. In conclusion, cellular senescence constitutes a promising component of the anticancer therapeutic repertoire.
6.2 Senolytic Therapy
While senescence-inducing therapies can result in cell cycle arrest in tumor cells, the majority of factors associated with the SASP promote tumor progression. Furthermore, the senescence of stromal and immune cells within the TME generally facilitates tumor progression. Therefore, targeting the clearance of senescent cells may also serve as a potential therapeutic strategy for treating tumors.
Senolytics are a class of drugs specifically designed to selectively eliminate senescent cells, thereby reducing their negative effects [246]. Senolytics operate through various mechanisms that disrupt the survival signaling pathways of these cells, with the primary mechanism involving the inhibition of antiapoptotic pathways. For example, previous In Vitro screening studies have demonstrated that both dasatinib and quercetin can reduce the viability of senescent cells [247]. Dasatinib, a tyrosine kinase inhibitor, reduces the viability of senescent cells by interfering with the EFNB (ephrin B)-dependent apoptotic signaling pathway [248], while quercetin, a natural flavonoid, has been shown to decrease senescent cell viability by inhibiting kinases such as PI3K, as well as serpins [249]. Studies have shown that the combined use of dasatinib and quercetin (D + Q) effectively reduced the number of senescent cells, and subsequently peritoneal and adipose tissue metastasis of ovarian cancer [250, 251]. Currently, the combination of D + Q has shown promising results in clinical trials [252-254], although its safety and efficacy require further investigation and validation. Another senolytic agent, ABT263, a specific inhibitor targeting the antiapoptotic proteins BCL-2 and BCL-xL, has been shown to selectively induce apoptosis in senescent cells [255]. In a mouse model of total body irradiation at sub-lethal doses, ABT263 effectively cleared senescent cells [256]. Similar to ABT263, ABT737 can also induce apoptosis by specifically targeting and inhibiting BCL-W and BCL-xL [257]. Activation of the BCL protein family is a core molecular mechanism that grants senescent cells enhanced antiapoptotic capabilities, and blocking this pathway often yields favorable outcomes. For example, the removal of p16Ink4a-expressing cells by AP20187 was found to delay tumor formation and mitigate age-related decline in multiple organs, such as the kidney, heart, and fat, without noticeable adverse effects [258, 259]. In addition, Mcl-1, which is found to be highly expressed in senescent cells, has become a new target for senolytic therapy [185]. For example, treatment with S63845, an inhibitor of Mcl-1, led to total eradication of senescent cancer cells and metastatic growths, effectively surpassing BCL2 inhibitors [259].
SASP is a key factor for senescent cells to promote tumorigenesis [260]. Senomorphic drugs work by suppressing the harmful effects of SASP without necessarily killing the senescent cells. Senomorphic therapies offer a safer alternative to senolytics by modulating the harmful effects of senescent cells without killing them or affecting normal cells. For example, AZD8055 was found to effectively reduce the expression of SASP-related genes by inhibiting the mTOR pathway [261]. Indeed, AZD8055 treatment has been shown to decrease the expression of SASP factors in senescent cells In Vitro [262]. In Vivo, combined therapy using AZD8055 and XL413 significantly reduced tumor volume and decreased the number of SASP-positive cells, demonstrating substantial therapeutic potential in cancer treatment [261]. Recently, Lui et al. reported preliminary outputs from the screening of a natural medicinal agent library for senotherapeutic candidates and validated several agents with prominent potential as senomorphics, such as Rutin [263]. Moreover, the effects of Senolytic therapy has been demonstrated in multiple preclinical animal experiments and clinical trials in various cancer types (Table 2).
| Agents | Drug mechanisms | Preclinical evidence | Clinical studies | Reference |
|---|---|---|---|---|
| D + Q | Inhibits tyrosine kinase, PI3K and serpins | With Carboplatin or Olaparib in murine ovarian cancer lines | Phase Ⅱ in Head and Neck Squamous Cell Carcinomas (NCT05724329) Phase Ⅱ in Childhood Cancer (NCT04733534) Phase Ⅱ in TNBC (NCT06355037) | [251] |
| ABT263 | Inhibits BCL-2 and BCL-xL | With ionizing radiation in breast and lung cancer lines | Phase Ⅱ in Ovarian cancer (NCT02591095), Small Cell Lung Cancer (NCT00445198) and Prostate Cancer (NCT01828476) Phase Ⅰin Ovarian Cancer and TNBC (NCT05358639) | [264] |
| ABT737 | Inhibits BCL-W and BCL-xL | With ionizing radiation in breast cancer cell, as single agent in small cell lung cancer lines and xenografts models | With Platinum in Ovarian Cancer in an observation study (NCT01440504) | [265, 266] |
| AP20187 | Induces dimerization of FK506 binding protein | Leading to a reduction in tumor incidence among mice | N/A | [267] |
| S63845 | Inhibits Mcl-1 | With trametenib in breast cancer line, with tarceva in NSCLC cell line, with trametinib in melanoma cell line | N/A | [268] |
| AZD8055 | Inhibits mTOR | With XL413 in liver cancer cell lines and xenografts models | PhaseⅠin advanced solid tumors (NCT00973076, NCT00999882, NCT00731263) | [261] |
- Abbreviations: D + Q, dasatinib + quercetin; N/A, not applicable; TNBC, triple-negative breast cancer.
- Sources: https://clinicaltrials.gov/.
6.3 One-Two Punch Therapy
In the context of senescence therapy, the “one-two punch” approach has emerged as an innovative strategy to effectively combat cancer by first inducing tumor cell senescence and then selectively eliminating these senescent cells [260, 269]. This concept recognizes that while inducing senescence can halt tumor growth, the SASP often promotes tumor invasion and metastasis. The first step involves the use of therapeutics that trigger cellular senescence in tumor cells, leading to a temporary cessation of tumor growth. The second step employs senolytic agents to clear the accumulated senescent cells, thereby mitigating the potential pro-tumor effects of SASP. The selective lysine-specific demethylase 4 C (KDM4C) inhibitor QC6352 has been shown to effectively induce cellular senescence in gastric cancer cells harboring TP53 mutations. When combined with the senolytic agent SSK1, this “one-two punch” therapy successfully eliminated TP53-mutant tumor cells [270]. Similarly, methionine adenosyltransferase II alpha (MAT2A) inhibitors have been shown to promote senescence in liver cancer cells. Furthermore, when used in conjunction with GSK3 inhibitors, which have been identified as senolytics, MAT2A inhibitors selectively killed senescent liver cancer cells [271]. In addition, the antidepressant sertraline has been shown to kill liver cancer cells induced to senescence by the CDC7 inhibitor [261]. Sertraline functions by inhibiting the mTOR signaling pathway, which is reactivated in cells treated with the CDC7 inhibitor, leading to sustained mTOR suppression and subsequent cell death. Overall, the “one-two punch” approach in senescence therapy harnesses the dual strategies of inducing senescence followed by targeted clearance, presenting a promising avenue for improving therapeutic outcomes in cancer treatment. What's more, the latest study shows that clinical trials combining senescence inducing drugs with BCL-2 inhibitors are being conducted in small cell lung cancer, triple negative breast cancer, high-grade ovarian cancer, and recurrent acute myeloid leukemia [272]. These preclinical animal experiments and clinical trails all demonstrated that the one-two punch therapy could be a promising strategy to cancer treatment.
6.4 Other Treatments Targeting Senescent Cells
Recent advances in targeting senescent cells have introduced innovative strategies for cancer treatment and age-related disease management. These approaches include nanoparticle delivery systems that induce senescence and enhance immunotherapy [273], combination of senescence-inducing agents with nanoparticle delivery of innate immune agonists that promote NKT and CD8+ T cells' functions [234], piezoelectric catalysis that triggers tumor cell senescence while generating ROS [274], dual inhibition of CDK4/6 and exportin 1 (XPO1) to promote senescence in cancer cells with enhanced sensitivity to targeted therapies [275]. Moreover, using the nanoparticulate crystals composed of Aurora kinase inhibitor alisertib and JAK2 inhibitor ruxolitinib that induce senescence of tumor cells may remodel the immune microenvironment and enhance immunotherapy [235]. In addition, the development of senolytic CAR-T cells that selectively eliminate senescent cells has shown promise in reversing tissue damage in cancer and fibrosis [276, 277]. Since the senescence tumor cells have been identified as capable of recruiting and regulating immune cells, A increasing number of studies focus on the efficacy of the combination of senescence-inducing agents and checkpoint blockade. Ruscetti et al. demonstrated that CDK4/6 inhibitors may cause senescence-induced vascular remodeling and enhancing the efficacy of anti-PD-1 [74]. Similarly, Jerby-Arnon et al. showed that the resistance of ICB could be reversed by CDK4/6 Inhibition [278]. Recently, a multifunctional nanodrug carrying several CDK 1/2/5/9 inhibitor and ICB was revealed to creates therapeutic chance against liver metastasis [279]. These strategies highlight the therapeutic potential of harnessing cellular senescence to treat resistant caners and age-related conditions.
7 Conclusion and Prospects
Developing treatments that selectively induce or clear senescent cells in a tumor-specific manner remains a critical challenge. While pro-senescence therapies effectively halt cancer cell proliferation, the SASP produced by senescent cells also fuel an immunosuppressive TME that potentially supports tumor progression. The “one-two punch” strategy, which involves inducing senescence followed by senolytic clearance, offers a promising solution but requires precise timing and selection of agents to avoid SASP-driven inflammation and metabolic reprogramming that could lead to tumor recurrence.
Senescent cells exhibit metabolic shifts similar to cancer cells, including increased glycolysis, altered lipid metabolism, and dependency on glutaminolysis. These metabolic dependencies represent therapeutic vulnerabilities that may be exploited through metabolic senotherapy, targeting senescence-specific pathways to reduce the SASP and inhibit tumor-promoting effects. Future research should focus on differentiating the distinct metabolic vulnerabilities of senescent versus cancer cells to develop highly selective therapies with minimal off-target effects.
The impact of SASP varies widely depending on tumor type and microenvironment. Fine-tuning SASP to retain tumor-suppressive functions while reducing pro-tumor signals, such as immunosuppressive cytokines, could offer new therapeutic avenues. Achieving this requires a comprehensive understanding of the molecular composition of SASP, as well as its regulatory mechanisms, and interactions with surrounding cells across various tumor contexts.
Beyond cancer cells, other components of the TME, such as CAFs, immune cells, and endothelial cells, can also undergo senescence, often supporting tumor progression through immunosuppression or SASP production. Innovative therapeutic strategies must consider these noncancerous cell populations, potentially through senolytics or senomorphics that modulate SASP without causing cell death, thereby preserving tissue integrity while minimizing tumor-promoting signaling.
As senescence-targeting therapies evolve, identifying biomarkers specific to senescent cells across various cancer stages will be essential for patient stratification and personalized medicine. Biomarkers can enable more precise senescence-targeted treatments, track treatment efficacy, and predict potential adverse effects. Such approaches may also help define optimal therapeutic windows for senescence-targeting interventions.
In summary, while cellular senescence offers significant potential as a therapeutic target in cancer, its dual role in tumor biology necessitates a sophisticated approach that capitalizes on its antitumor functions while mitigating its tumor-promoting aspects. Continued scientific advances in these areas could lead to the development of refined strategies, positioning senescence-targeted therapies as a novel, impactful approach in cancer treatment.
Author Contributions
Jingrui Yan: conceptualization, writing – original draft. Yu Zhang and Guohua Mao: data curation and Investigation. Jihui Hao, Jun Yu, and Tianxing Zhou: writing – review and editing. All authors have read and approved the final manuscript.
Acknowledgments
The figures in this manuscript were created using Adobe Illustrator software (version 29.4) and Affinity Designer (version 2). We acknowledge the use of these software tools in the preparation of the visual content. This study was sponsored by National Key Research and Development Program of China (2021YFA1201100), the National Natural Science Foundation of China (Grant No. 82403245) and the Science, Technology Development Fund of the State Administration of Traditional Chinese medicine in Hebei Province (Grant No. T2025063).
Ethics Statement
The authors have nothing to report.
Consent
The publication of the article was made by all authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.