Mitochondrial Ribosomal Protein Family in Cancers: Mechanistic Insights and Therapeutic Implications
ABSTRACT
Mitochondria, as the main site for aerobic respiration in cells, are indispensable participants in the reprogrammed metabolic activities of tumor cells. Mitochondrial ribosomal proteins (MRPs), essential components of the mitochondrial ribosome (mitoribosome), play a critical role in maintaining mitochondrial function and regulating oncogenic signaling. Their molecular mechanisms and biological functions make MRPs key regulators of tumorigenesis, drug resistance, and tumor immune escape. MRPs are abnormally expressed in various cancer types and are linked to the prognosis of cancer patients. However, a thorough grasp of the specific mechanisms and a holistic analysis of the relationship between MRPs and different cancers are lacking. This review highlights the specific regulatory roles of MRPs, including MRPS5, MRPS29, MRPL9, MRPL12, MRPL13, MRPL33, MRPL58, and MRPL59, in cancer. Additionally, we examine the potential of MRPs as prospective clinical biomarkers and discuss their relationship with clinical prognosis and treatment response. We further explore the underlying reasons for the diverse functions of MRPs, their implications in cellular signaling and tumor immunity, and consider the prospects for developing MRP inhibitors as therapeutic strategies. Our comprehensive analysis deepens the understanding of MRPs complex biological functions and emphasizes their promising potential as therapeutic targets in cancer treatment.
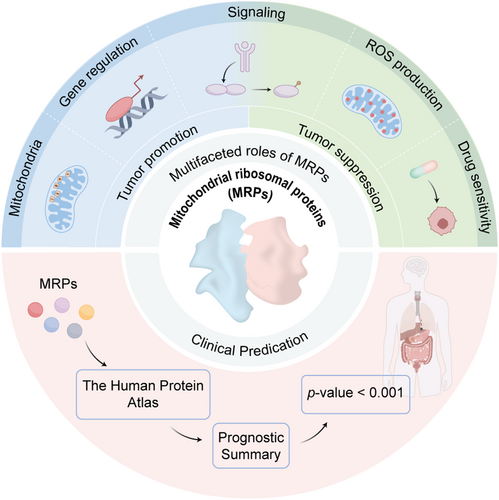
Graphical Abstract
Mitochondrial ribosomal proteins (MRPs) are the important components of mitoribosome. They are aberrantly expressed in various human cancers. In this review, we introduce the roles of MRPs in tumorigenesis and development by regulating OXPHOS and other mechanisms. Furthermore, to confirm the potential of MRPs as biomarkers, we predict the relevance of MRPs family with prognosis and immunotherapy in different cancers.
1 Introduction
Mitochondria, the powerhouses of eukaryotic cells, govern diverse cellular processes under both physiological and pathological conditions, such as energy production, programmed cell death, and redox signaling pathway [1, 2]. Beyond their intracellular roles, emerging evidence highlights intercellular or horizontal mitochondria transfer which supports the metabolism of recipient cells and facilitates mitochondrial quality control in donor cells [3]. Notably, cancer cells can exploit this process to acquire mitochondria from immune cells, driving tumor growth while impairing antitumor immunity [4, 5]. Mitochondria profoundly influence tumor progression by modulating metabolism, invasion, treatment response, and immune cell function [6, 7]. Mitochondria-driven metabolic changes enhance tumor cell plasticity [8-10], enabling adaptation to diverse environments [11, 12]. Mitochondria are highly dynamic organelles that undergo processes such as fission, fusion, and mitophagy [13, 14]. Cancer cells alter mitochondrial dynamic homeostasis to promote proliferation [15, 16]. These multifaceted roles position mitochondria as promising therapeutic targets in cancer.
Different from other organelles, mitochondria retain the remnants of their original genome and the corresponding whole gene expression mechanisms [17]. The human mitochondrial genome encodes 13 essential proteins of the oxidative phosphorylation (OXPHOS) complex, which are translated by mitoribosome [18-21]. Mammalian mitoribosome is composed of two subunits: the small 28S subunit (mt-SSU), which provides a binding and decoding platform for mRNA, and the large 39S subunit (mt-LSU), which contains a peptidyl transferase center (PTC) responsible for catalyzing the formation of peptide bonds [22, 23]. mt-SSU and mt-LSU consist of RNA and proteins, with RNA primarily positioned internally and proteins on the surface [24]. Mammalian mitoribosomes have a similar mass to bacterial ribosomes but contain scarcely half as much rRNA and over twice as much protein [23, 25]. This suggests that MRPs may have replaced some of the functions of vital rRNA and play a crucial role in mammals. MRPs are categorized into two specific groups: small mitochondrial ribosomal proteins (MRPS) and large mitochondrial ribosomal proteins (MRPL), corresponding to proteins of mt-SSU and mt-LSU, respectively. Identification of protein components of mt-SSU was completed in 2001 by liquid chromatography and tandem mass spectrometry [26]. In general, mt-SSU contains 12S rRNA encoded by mitochondrial DNA (mtDNA) and about 30 MRPs encoded by nuclear DNA (nDNA). mt-LSU is composed of 16S rRNA and approximately 52 MRPs encoded by nDNA.
MRPs are pivotal for mitochondrial integrity and function. Dysfunction in MRPs impairs mitochondrial structure and energy production [27, 28]. ADPGK-AS1 binds to and upregulates MRPL35, increasing mitochondrial fusion [29]. MRPL59 silencing induced excessive mitochondrial fission [30], indicating the importance of MRPs in mitochondrial dynamics. Beyond mitochondria, MRPs also regulate multiple cellular processes and signaling, such as cell cycle, immune response within tumors [31]. Consequently, mutations in MRPs can result in a range of effects from lethality to marginally impaired energy metabolism. For example, bi-allelic mutations of MRPS2 caused sensorineural hearing loss, hypoglycemia, and OXPHOS complex deficiencies [32]. MRPL4 regulates Notch signaling to function during animal development [33]. MRPL12 dysregulation is implicated in a variety of diseases such as breast cancer, neurodegenerative disease, and diabetic kidney disease [34]. This underscores the significance of MRPs in human health and disease.
In recent years, MRPs have garnered significant attention in cancer research due to their multiple functions in mitochondria and the nucleus. Most studies have primarily focused on MRPS5, MRPS29, MRPL9, MRPL12, MRPL13, MRPL33, MRPL58, and MRPL59. In this review, we will summarize the function and mechanisms of these MRPs in regulating tumorigenesis in different cancer types. Furthermore, given the absence of a holistic discussion on the clinical implications of MRPs, we intend to predict their clinical value based on data from The Human Protein Atlas (HPA) (www.proteinatlas.org) to explore the correlation between all members of MRPs and the prognosis of patients with cancer [35]. Finally, their implications are also discussed to provide a deeper understanding of MRPs and to help better explore their potential clinical application value.
2 Roles and Mechanisms of Mitochondrial Ribosomal Proteins (MRPs) in Cancer
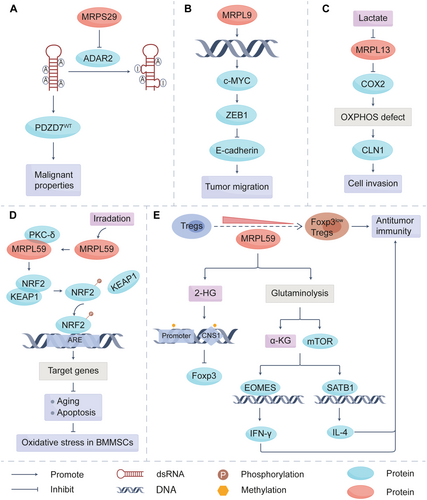
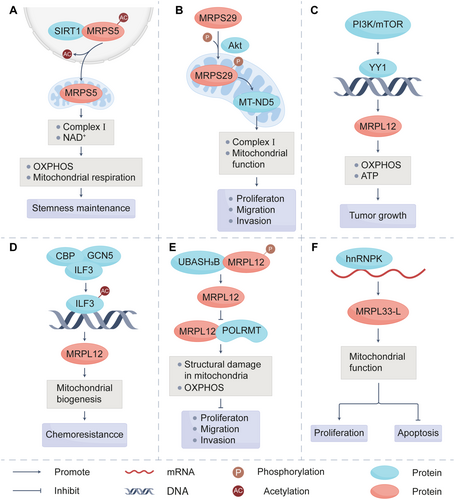
As integral components of the mitoribosome, MRPs are widely distributed within mitochondria of various cells [36] and tissues, including cancer tissues [37-39]. They have been proven by numerous studies to be closely associated with the prognosis of patients with cancer. The classic function of MRPs is to ensure the proper conduct of mitochondrial translation. MRPs regulate the expression of oncogenic or tumor suppressor genes by affecting the activity of RNA editing-related enzymes, such as the adenosine deaminase acting on RNA (ADAR) family (Figure 1). Except for the effects on tumor cells themselves, MRPs play an important role in the metabolic reprogramming of immune cells within the tumor microenvironment (TME). These actions are tightly linked to tumor growth, migration, invasion, and chemoresistance, which highlights the pivotal role of MRPs in tumor initiation and progression. In addition, MRP family members are critical downstream targets of signaling pathways essential for cancer cell survival, such as sirtuin-1 (SIRT1), UBASH3B, PI3K/Akt/mTOR, and interleukin enhancer-binding factor 3 (ILF3) (Figure 2). This section will address the roles of some MRP members in tumor initiation and progression to enhance our understanding of tumor biology and clarify how MRPs are regulated in this process.
2.1 MRPS5
MRPS5, a component of the mt-SSU, mainly participates in the synthesis of proteins related to the mitochondrial respiratory chain and is closely related to the function of mitochondrial complex I. MRPS5 contributes to various diseases and physiological processes. First of all, MRPS5 is a metabolic and longevity regulator [40]. Its knockdown led to an imbalance of mitochondrial-nuclear proteins, reduction of mitochondrial respiration, and activation of mitochondrial unfolded protein response, all of which were beneficial for prolonging lifespan [41]. Then, MRPS5/ATF4 is known to play a critical role in cardiomyocytes. The depletion of MRPS5 activated mitochondrial stress response and ATF4 signaling, which finally promoted cardiomyocyte proliferation [37]. MRPS5 also altered cardiac metabolism and mitochondrial-nuclear communication in the heart, with Kruppel-like factor 15 (Klf15) identified as a downstream target of it [42]. The deficiency of MRPS5 inhibits the expression of Klf15, a kind of transcription factor associated with various malignancies [43]. In breast cancer, Klf15 upregulated p21 expression to induce cell cycle arrest [44]. Klf15 also acts as an upstream regulatory factor of TFAP2A-AS1, suppressing the proliferation and migration of gastric cancer (GC) cells [45], which indicates that MRPS5 may inhibit the proliferation and metastasis of cancer cells through Klf15. However, Yang et al. discovered from patient-derived xenograft models that MRPS5 exhibited high expression in pancreatic cancer, and the knockdown of it inhibited the proliferation of PaCa-2, PANC-1, and BxPC-3 cells [46]. All these results demonstrated that MRPS5 may function as a suppressor in breast cancer and GC, but in pancreatic cancer, MRPS5 functions as a promoter.
Further experiments confirmed that MRPS5 knockdown mice have decreased size and weight of tumors compared with the control group, while also demonstrating markedly suppressed tumor initiation in vivo (Table 1). MRPS5 could maintain the stemness properties of cancer cells through promoting the production of NAD+. In liver cancer stem cells (CSCs), the knockout of MRPS5 resulted in some mitochondria appearing punctate, with disordered cristae [72]. This suggests that MRPS5 is essential for maintaining mitochondrial function in liver CSCs. Recent studies have shown that the acetylation of specific proteins can facilitate their nuclear translocation [73]. Furthermore, MRPS5 has been demonstrated to interact with SIRT1 and undergo deacetylation, which regulates the mito-nuclear translocation of MRPS5 [39]. The deacetylated MRPS5 then resided in mitochondria and enhanced mitochondrial respiration, which is beneficial for supporting CSC stemness, tumor metastasis, and chemotherapy resistance (Figure 2A).
| Name | Cancer types | Model | Animal | Cell line | Refs. |
|---|---|---|---|---|---|
| MRPS5 | Liver cancer | Subcutaneous xenograft | NOD/SCID mice | PLC/PRF/5 | [39] |
| MRPS16 | Lung adenocarcinoma | Subcutaneous xenograft | Nude mice | H23 H2030 |
[47] |
| Glioma | Brain orthotopic xenograft | Nude mice | U-138 MG U-87 MG |
[48] | |
| MRPS18B | NA | Subcutaneous xenograft | SCID mice | MEF | [49] |
| MRPS23 | Breast cancer | Intravenous injection model of lung metastasis | Nude mice | MCF7 | [50] |
| MRPS27 | Triple-negative breast cancer | Breast orthotopic xenograft PDX |
Nude mice | MDA-MB-231 | [51] |
| MRPS29 | Esophageal cancer | Subcutaneous xenograft | NOD/SCID mice NSG mice |
EC109 | [52-54] |
| Hepatocellular carcinoma | DEN-induced hepatocarcinogenesis Spontaneous mouse model Subcutaneous xenograft Peritoneal xenograft |
C57BL/6 mice Nude mice |
Huh7 | [55] | |
| MRPS38 | Triple-negative breast cancer | Subcutaneous xenograft | Nude mice | MDA-MB-231 BT 549 |
[56] |
| MRPL9 | Papillary thyroid cancer | Subcutaneous xenograft Intravenous injection model of lung metastasis |
Nude mice | K1 | [57] |
| MRPL12 | Lung adenocarcinoma | Genetically engineered mouse model Intravenous injection model of lung metastasis |
MRPL12flox/flox mice Nude mice |
A549 | [58] |
| Hepatocellular carcinoma | Subcutaneous xenograft Spontaneous mouse model |
Nude mice C57BL/6 mice |
HepG2 MHCC97H |
[59, 60] | |
| MRPL13 | Non-small lung cancer | Subcutaneous xenograft | Nude mice | A549 H1299 |
[61] |
| MRPL33 | Colon cancer | Subcutaneous xenograft | Nude mice | RKO | [62] |
| Gastric cancer | Subcutaneous xenograft | Nude mice | HGC27 | [63] | |
| MRPL35 | Lung cancer | Subcutaneous xenograft | NSG mice | A549 | [29] |
| Colorectal cancer | Subcutaneous xenograft | Nude mice | HCT116 | [64] | |
| Gastric cancer | Subcutaneous xenograft | Nude mice | BGC-823 MKN-45 |
[65, 66] | |
| MRPL52 | Breast cancer | Orthotopic xenograft | BALB/c mice | 4T1 | [27] |
| MRPL58 | Hepatocellular carcinoma | Subcutaneous xenograft | Nude mice | HepG2 | [67] |
| MRPL59 | Breast cancer | Subcutaneous xenograft | Nude mice | MCF-7 | [30] |
| Osteosarcoma | Subcutaneous xenograft | Nude mice | U2OS | [68] | |
Colorectal cancer Lung cancer |
Subcutaneous xenograft | C57BL/6 mice MRPL59flox/flox mice |
MC38 TC-1 |
[69] | |
| Liver cancer | DEN-induced hepatocarcinogenesis | Liver-specific MRPL59 KO mice | NA | [70] | |
| Hepatocellular carcinoma | Subcutaneous xenograft Intravenous injection model of lung metastasis |
Nude mice | SNU-368 | [71] |
2.2 MRPS29
Similar to MRPS5, MRPS29, also known as death-associated protein 3 (DAP3), serves as a prognostic marker in various cancers. Pancreatic cancer patients exhibiting elevated MRPS29 expression demonstrated a significantly reduced survival time compared to those with low expression levels, decreasing from 35 months to 21 months [74]. Moreover, MRPS29 expression in stage Ⅳ thymoma was significantly higher than that in stage Ⅰ thymoma [75]. Conversely, in breast cancer, the expression of MRPS29 correlates with better survival [76]. Thus, the prognostic value of MRPS29 is tumor-type dependent. Additionally, MRPS29 can predict not only the survival rate but also the possibility of metastasis [77].
2.2.1 Impact of MRPS29 on RNA Processing and Regulation
MRPS29 is overexpressed in almost 17 types of cancer and functions as a strong oncogenic gene in most cancer cells. The suppression of adenosine-to-inosine (A-to-I) RNA editing by MRPS29 may be a mechanism leading to its carcinogenic effect. A-to-I RNA editing is a posttranscriptional mechanism, whose dysregulation is a key driver in the pathogenesis of various cancers [78]. Editing of A-to-I is catalyzed by the ADAR enzymes. In vertebrates, the ADAR family consists of three members: ADAR1, ADAR2, and ADAR3 [79]. Han et al. showed that MRPS29 interacted with the deaminase domain of ADAR2 in nucleus and functioned as a repressor of A-to-I RNA editing in cancer cells through disrupting the association of ADAR2 with transcripts (Figure 1A) [52]. Then, cancer cells can acquire malignant properties through this for survival advantages. N6-methyladenosine (m6A) RNA methylation, the most prevalent modification on RNA [80], is also one of the important pathways through which MRPS29 exerts its function. m6A modification can facilitate rapid transcriptome turnover to suppress cell differentiation, which is associated with the stem-like properties of cancer cells and conducive to accelerating proliferation and invasion [81]. Methyltransferase-like 3 (METTL3) is the enzyme responsible for catalyzing m6A modification. MRPS29 promoted the binding of METTL3 to target RNAs for m6A modification [53]. Moreover, intracellular S-adenosylmethionine (SAM) levels emerge as another critical determinant for m6A modification, as SAM serves as the methyl donor substrate during the methylation of adenine [82, 83]. Methionine adenosyl transferase 2 alpha (MAT2A) catalyzes the synthesis of SAM. MRPS29 interacted with NUDT21 and promoted the splicing of MAT2A to increase its protein expression [53]. This regulatory action ensured the cellular SAM levels and contributed significantly to tumorigenesis. Besides, MRPS29 could modulate widespread alternative splicing changes in cancers, which facilitates the expression of oncogenic genes, such as WD repeat and SOCS box-containing protein 1 (WSB1), to promote tumorigenesis [54]. These findings highlight the therapeutic potential of targeting RNA modification and splicing mediated by MRPS29 in cancer treatment.
2.2.2 Impact of MRPS29 on Apoptosis
MRPS29 was initially identified in 1995 and found to be positively correlated with interferons-γ (IFN-γ) [84]. It has been implicated in programmed cell death, particularly apoptosis [85]. When treating the human glioma (GBM) cell line T98G with Camptothecin, a stark difference in survival rates was observed. The cells with high expression of MRPS29 had a survival rate exceeding 95% after 24 h, whereas the survival rate of those with low expression only reached 70% [86]. The resistance of cells to apoptosis was partially reversed when the expression of MRPS29 was blocked. Moreover, MRPS29 was significantly overexpressed in invasive GBM cells compared to cells residing in the tumor core [87]. This indicates that MRPS29 can enhance the resistance of migrating GBM cells to apoptosis, with decreased sensitivity of tumor cells to chemotherapy drugs, and suggests a role for MRPS29 in the aggressive behavior of GBM cells. However, previous studies showed that MRPS29 appears to have an opposite effect on other types of cancer. MRPS29 interacted with pro-caspase-8-binding adapter protein Fas-associated death domain (FADD) and induced cell apoptosis through binding FADD to TRAIL receptors DR4 and DR5 [88]. Compared with the cells in the control group, the cells with MRPS29 knockdown exhibited a more rapid cell proliferation rate and displayed heightened aggression under chemotherapy drug treatment [89]. This implies that MRPS29 induces apoptosis to inhibit proliferation and enhances the sensitivity of cancer cells to chemotherapy in GC and Osteosarcoma (OS). In other words, MRPS29 plays a tumor-promoting role in metastatic GBM cells by inhibiting apoptosis, but in GC and OS, MRPS29 functions as a tumor suppressor by inducing apoptosis in tumor cells. This contradictory phenomenon may be caused by the mutation of MRPS29. The proapoptotic function of MRPS29 depends on its P-loop motif [90]. The P-loop mutant of MRPS29 abolished the death-inducing capacity [91], confirming that the structural integrity of MRPS29 is essential for inducing apoptosis. These findings suggest that MRPS29 mutations may confer a dominant-negative effect in metastatic GBM cells, potentially enabling evasion of programmed cell death, though their clinical relevance in cancer remains to be validated.
2.2.3 Regulatory Mechanisms of MRPS29
Emerging evidence highlights the critical role of MRPS29 in tumor progression, underscoring the need to elucidate its regulatory mechanisms. Current research indicates that the expression of MRPS29 is primarily regulated by microRNA (miRNAs) and posttranslational modifications. miRNAs are a type of small RNA regulating posttranscriptional gene expression by degrading mRNA or inhibiting protein translation [92]. miR-365-1, a member of the miRNAs, bound to the promoter region of MRPS29 to inhibit its transcription [93]. Additionally, MRPS29 is important for the maintenance of the invasive and antisenescence phenotype of hepatocellular carcinoma (HCC) cells. Protein kinase B (Akt) phosphorylates MRPS29 at Ser185. Then phosphorylated MRPS29 translocated into mitochondria to increase MT-ND5 expression and enhance mitochondrial complex Ⅰ activity, which finally drove the antisenescence phenotype of HCC cells [55] (Figure 2B). It provides a novel strategy for cancer treatment by targeting MRPS29.
2.3 MRPL9
The survival time and recurrence-free survival time of HCC patients with MRPL9 high expression were significantly shorter than those of patients with low expression [94]. In vitro CCK-8 and transwell assays showed that MRPL9 depletion had a significant inhibitory effect on the proliferation and migration of SK-HEP1 cells and LM3 cells [95], further demonstrating the value of MRPL9 in predicting the prognosis of HCC. The transcriptional sequencing results revealed that MRPL9 was upregulated in persistent triple-negative breast cancer (TNBC) cells. MRPL9 knockdown inhibited the colony formation potential of TNBC cells and enhanced their sensitivity to paclitaxel [96]. In addition, our previous research found that MRPL9 expression was increased in lung cancer tissues and associated with poor overall survival and recurrence-free survival. Mechanistic studies unveiled that MRPL9 affected the transcription of c-MYC, thereby regulating the expression of E-cadherin and zinc finger E-box binding homeobox 1 (ZEB1) [97], ultimately promoting the metastasis of lung cancer (Figure 1B). These indicate that MRPL9 could be a potential therapeutic target in cancers, especially lung cancer.
2.4 MRPL12
MRPL12 is intricately linked to both cellular growth and mitochondrial adenosine triphosphate (ATP) synthesis. MRPL12 might be a potential therapeutic target for complications of diabetes, including diabetic cardiomyopathy [34] or diabetic kidney disease [98], given its capacity to mitigate the impairment of OXPHOS induced by long-term high glucose. MRPL12 also promotes tumor progression, especially those primarily driven by OXPHOS. There are two forms of MRPL12 in mammalian mitochondria: mature “long” and “short” forms. The precursors of MRPL12 containing mitochondrial targeting sequence (MTS) in the N-terminal region are first cleaved by mitochondrial processing protease to generate mature “long” forms. The mature “short” forms are then produced through inefficient or regulated cleavage by mitochondrial intermediate protease [99]. The existence of these two forms contributes to the multiple roles of MRPL12.
Previous analysis of MRPL12 expression in a clinical cohort suggested that MRPL12 was highly expressed in the clinical tissues of various cancers and significantly associated with poor prognosis and shorter survival [60, 100]. The knockdown of MRPL12 constrained the growth of lung adenocarcinoma (LUAD) organoids. Furthermore, MRPL12-knockout extended the median survival time of mice from 115 days to 135 days [58], suggesting that the depletion of MRPL12 not only reduced tumor burden but also improved survival time.
2.4.1 Impact of MRPL12 on Cancer
Recent studies highlight the multifaceted roles of MRPL12. As a critical contributor to mitochondrial translation, MRPL12 governs mitochondrial metabolism and OXPHOS. MRPL12 also binds to RNA polymerase mitochondria (POLRMT), a core component of the mitochondrial transcription mechanism [101], to activate mtDNA transcription in a ribosome-free form. The knockdown of MRPL12 led to the instability of POLRMT and reduced mitochondrial transcriptional efficiency [99]. Upon MRPL12 knockdown, mitochondrial structural changes were observed in HCC cells, including the expansion of matrix space, reduction of staining intensity, destruction of cristae, and rupture of the outer membrane [60]. Conversely, the overexpression of MRPL12 enhanced intracellular ATP production and induced proliferative and migratory effects. This phenomenon was inhibited in HCC cells treated with an aerobic respiration inhibitor, while the glycolysis inhibitor had no effect [102]. This suggests that MRPL12 activates OXPHOS and promotes mitochondrial biosynthesis to enhance carcinogenesis in HCC. Additionally, as a member of mt-LSU, the mutation of MRPL12 led to structural abnormalities in mt-LSU, resulting in a significant reduction of COX Ⅰ, Ⅱ, and Ⅲ subunits [98]. In other words, the oncogenic effects of MRPL12 are achieved by acting as a pivotal regulator of mitochondrial function. Collectively, these underscore the involvement of MRPL12 in maintaining normal structure and function of mitochondria.
2.4.2 Upstream Modulation of MRPL12
As previously established, the expression of MRPL12 is critical for the survival of tumor cells. Importantly, MRPL12 expression is dynamically regulated by upstream signaling pathways and interacting proteins. Targeting these regulators represents a promising therapeutic strategy to inhibit tumor progression. Nuclear factor erythroid 2-related factor 2 (NRF2) is a transcription factor that functions as the master regulator of redox and metabolic homeostasis and is frequently activated in cancer cells [103-105]. Wan et al. demonstrated that NRF2 regulated the transcription of MRPL12. NRF2 directly bound to the -509 to -519 bp region in the promoter of MRPL12, thereby activating the transcription of MRPL12 to promote OXPHOS [98]. Apart from NRF2, the knockdown of MRPL12 partially reversed the effects of PI3K agonist in promoting proliferation and metastasis in HCC cells (Figure 2C) [60]. This indicates that the PI3K/mTOR axis is also one of the upstream regulators of MRPL12. The ILF3-MRPL12 axis also plays a key role in HCC drug resistance. Lefamulin targets ILF3 to inhibit its acetylation, thereby inhibiting the transcription of MRPL12 (Figure 2D) [59]. Then, mitochondrial homeostasis is disrupted to reverse drug resistance. In addition to the effects on transcriptional levels, posttranscriptional modification is also an important way to regulate MRPL12 expression. UBASH3B, a member of the T cell ubiquitin ligand (TULA) family [106], was shown to bind to MRPL12 and dephosphorylate MRPL12 at residue Y60 to reduce the binding of MRPL12 and POLRMT, which attenuates mitochondrial biosynthesis to inhibit lung tumorigenesis [58] (Figure 2E). This discovery provides a theoretical basis for the development of small-molecule compounds targeting MRPL12 phosphorylation, potentially serving as a novel therapeutic strategy for cancer treatment.
2.5 MRPL13
Increased expression of MRPL13 is also associated with poor prognosis in patients with non-small lung cancer (NSCLC), breast cancer [107], or GC. In LUAD, knocking down of MRPL13 decreases the survival of cancer cells, delays tumor division and migration, and increases apoptosis [108]. Besides, Cai et al. demonstrated that MRPL13 increased phosphorylated mTOR and Akt levels. The knockdown of MRPL13 in breast cancer cells resulted in the loss of E-cadherin and upregulation of vimentin [109], indicating that MRPL13 activates PI3K-Akt-mTOR pathway to promote cell proliferation, migration, and EMT.
Many cancer cells rely on glycolysis for ATP production, which supports their proliferation and survival. This process results in the secretion of lactic acid and the formation of an acidic TME [110, 111]. It has been reported that in human liver cancer cell SNU387, exposure to 20 mM lactic acid significantly decreased the expression level of MRPL13. Low expression of MRPL13 then reduced complex IV activity, leading to OXPHOS defect and higher claudin-1 (CLN1) expression, which is crucial for the invasion process of liver cancer cells (Figure 1C) [112]. That's to say, the acidic tumor environment in vivo may inhibit MRPL13, thereby inducing OXPHOS deficiency and enhancing the invasive activity of liver cancer cells through CLN1 expression.
2.6 MRPL33
Similarly to MRPL12, MRPL33 also exists in two isoforms, MRPL33-long (L) and MRPL33-short (S), which are generated by the alternative splicing of MRPL33 pre-mRNA. MRPL33-L can promote proliferation in breast cancer [113]. Furthermore, the knockdown of MRPL33-L significantly induced apoptosis in colon cancer, indicating its role as a tumor promoter (Figure 2F). Conversely, MRPL33-S does not affect the proliferation of cancer cells [62]. According to this, MRPL33-L and MRPL33-S may play opposite roles in tumor progression.
Subsequent research showed that the overexpression of MRPL33-S decreased the level of p-Akt and p-CREB in GC, while MRPL33-L upregulated them [114]. Evasion of apoptosis is an important mechanism leading to chemoresistance. According to the research of You et al., trefoil factor 3 (TFF3) significantly increased the phosphorylation of Akt and decreased the sensitivity of HCC cells to doxorubicin [115]. This implies that p-Akt is highly involved in the chemoresistance of cancer cells. Besides, activated CREB could bind to the ITGA10 promoter, increasing the expression of ITGA10 and contributing to chemoresistance in OS cells [116]. Therefore, MRPL33-S may enhance cancer cell sensitivity to chemotherapy by deactivating Akt/CREB signaling pathway, while MRPL33-L contributes to chemoresistance. Exon 3 of MRPL33 is subject to the regulation of splicing regulators, including hnRNPK. hnRNPK is a member of RNA binding proteins (RBPs) family. RBPs possess the ability to influence the structure of RNA and are essential for RNA biogenesis, stability, and cellular localization [117]. Liu et al. observed that hnRNPK promoted the production of MRPL33-L variant through activating the inclusion of MRPL33 exon 3 in colon cancer [62] (Figure 2F). This indicates that targeting hnRNPK to reduce the expression of MRPL33-L could be an important approach for the treatment of colon cancer.
2.7 MRPL58
MRPL58, also known as immature colon carcinoma transcript 1 (ICT1), mL62, or DS-1, was initially identified in colon carcinoma cells [118]. MRPL58 possesses peptidyl-tRNA hydrolase activity [119], allowing it to hydrolyze the ester bond between the polypeptide and tRNA, thereby releasing tRNA from the peptidyl-tRNA. This activity is mainly due to the peptidyl-tRNA transferase (PTH) domain of MRPL58, which contains a highly conserved Gly-Gly-Gln (GGQ) motif essential for hydrolysis [1]. In 2010, MRPL58 was identified as a member of human mt-LSU by Richter et al. [120]. Subsequent research shows that apart from PTH domain, there is also a positively charged C-terminal domain and an N-terminal domain containing an MTS within MRPL58. The C-terminal domain is initially unstructured or flexible due to the high content of basic residues. But it becomes structured upon binding to ribosomes to facilitate function [121]. The PTH activity requires MRPL58 entry into the PTC, but the localization of MRPL58 in the mitoribosome is distant from the PTC, suggesting that MRPL58 might primarily serve as a structural protein in the mitoribosome while performing PTH functions elsewhere.
Clinically, the overexpression of MRPL58 is associated with advanced TNM tumor stage and metastasis. Patients exhibiting elevated levels of MRPL58 were found to have shorter overall and recurrence-free survival times, indicating a poor prognosis for MRPL58 in GC [122]. Additionally, the knockdown of MRPL58 by siRNA or shRNA was observed to inhibit proliferation of tumor cells [67], likely through cell cycle arrest. This arrest occurs at different stages in various cancers: G2/M phase in breast cancer cells, ZR-75-30 and T-47D [123], and S phase in lymphoma cells, U937 [124]. The reason for this phenomenon may be that MRPL58 affects the expression of different cell cycle proteins in different cancers. Furthermore, MRPL58 is targeted by specific miRNAs. The overexpression of miR-1301-3p induced G0/G1 phase arrest, apoptosis, and suppressed colony formation by inhibiting the expression of MRPL58 in breast cancer cells [125]. Besides, Tao et al. also identified MRPL58 as the direct target of miR-205. miR-205 inhibited GC cell migration and invasion by downregulating MRPL58 [122]. These findings indicate that MRPL58 influences cell cycle proteins, and miRNAs serve as a crucial regulator for regulating the expression of MRPL58.
2.8 MRPL59
MRPL59, also referred to as CR6-interacting factor 1 (CRIF1), GADD45GIP1, or CKBBP2, was initially identified as a nuclear protein that specifically binds to the GADD45 family protein, functioning as a key modulator of cell cycle progression [126]. However, subsequent research identified MRPL59 as a component of mt-LSU [127]. There is evidence that MRPL59 is essential for the maintenance of normal physiological functions and is associated with the occurrence and development of various diseases [128]. For example, MRPL59 homodeficiency in proopiomelanocortin (POMC) induced severe mitoribosomal stress and led to obesity in mice [129]. There was earlier islet failure in MRPL59beta+/− mice in response to high-fat feeding [130, 131], suggesting the role of MRPL59 in diabetes. In addition, MRPL59 deletion caused early-onset Parkinson's disease and induced vascular endothelial cell senescence which is a reason for cardiac-related diseases [132, 133]. MRPL59 is also critical for the prevention of autoimmune diseases. It maintained B cell self-tolerance to inhibit the excessive activation of follicular helper T cells [134]. Furthermore, MRPL59 exhibits aberrant expression across a spectrum of cancers, including lung cancer, HCC, and OS, with a strong correlation to patient survival and prognosis [135]. MRPL59 was involved in regulating the synthesis of OXPHOS peptides and played an important role in their insertion into the mitochondrial inner membrane [136]. Protein sequence analysis predicted the presence of MTS in the N-terminal region of MRPL59. Kim et al. found there was also a nuclear localization sequence (NLS) in the C-terminal region [136], suggesting that MRPL59 has functional roles in both the nucleus and mitochondria.
2.8.1 Role of MRPL59 in Tumor Cells
MRPL59 promoted the expression of SIRT3, which was linked to the malignant properties of NSCLC [137]. The SIRT3 induced by MRPL59 then facilitated the deacetylation of pyrroline-5-carboxylate reductase 1 (PYCR1) to increase its activity [138]. PYCR1 augmented proline production, which subsequently promoted collagen production and metastatic spread in vivo [139]. The deficiency of MRPL59 significantly elevated the levels of p53 and p21 at both the mRNA and protein levels in MCF-7, a breast cancer cell line, resulting in a notable rise in the number of cells in G1 phase compared to controls [30]. This indicates that MRPL59 knockdown may inhibit cell proliferation and migration by activating p53 to induce G0/G1 cell cycle arrest in breast cancer cells.
MRPL59 also has an impact on cancer radiotherapy. It could interact with CDK2 to influence G1 phase arrest [140]. MRPL59 enhanced the nuclear translocation and phosphorylation on T14/T160 of CDK2, activating the G1/S checkpoint and facilitating DNA damage repair, which contributed to radioresistance in OS [68]. While radiation therapy offers significant benefits, high doses can damage normal tissues, with bone being particularly susceptible [141]. The differentiation of bone marrow mesenchymal stem cells (BM-MSCs) into adipocytes rather than osteoblasts is the main cause of radiation-induced bone loss. MRPL59 promoted the phosphorylation of CREB, a transcriptional activator of adipocyte differentiation, and led to the adipogenic differentiation of BM-MSCs [142, 143]. This process impairs proper bone formation and contributes to the development of radiation-induced osteoporosis. That's to say, MRPL59 not only reduced the sensitivity of OS patients to ionizing radiation (IR) but was also involved in radiation-induced osteoporosis. Conversely, Chen et al. showed that the expression of MRPL59 was upregulated in response to radiation exposure. Then MRPL59 co-activated protein kinase C-δ (PKC-δ) to phosphorylate NRF2 [144]. This helped maintain redox homeostasis in BM-MSCs and supported hematopoietic recovery after high radiation exposure (Figure 1D).
Analogous to MRPL58, the expression of MRPL59 is also regulated by miRNA. The downregulation of miR-497-5p, a known tumor suppressor in various cancers [145], upregulated MRPL59. Then, elevated levels of MRPL59 significantly increased intracellular reactive oxygen species (ROS) and activated NF-κB signal transduction [71], which enhanced the proliferative, migratory, and invasive capabilities of HCC cells.
2.8.2 Impact of MRPL59 on T Cells
In addition to direct effects on tumor cells, MRPL59 also influences T cell function and tumorigenesis. In regulatory T (Treg) cells, MRPL59 deficiency induced mitochondrial dysfunction and increased the production of FOXP3low CD45RA− Treg cells [69]. These FOXP3low CD45RA− Treg cells could secrete pro-inflammatory cytokines and diminish the suppressive microenvironment [146]. It indicates that MRPL59 deficiency may enhance antitumor immunity through FOXP3low CD45RA− Tregs (Figure 1E). However, MRPL59 deficiency increased expression of programmed death receptor 1(PD-1) on T cells in HCC progression. Besides, MRPL59 elevated ROS and lactate levels, which induced a less activated and exhausted phenotype of tumor-infiltrating T cells [70]. This suggests that the overexpression of MRPL59 is beneficial in maintaining the function of T cells to inhibit HCC.
3 MRPs as Clinical Biomarkers
The increase in cancer mortality is associated with advanced-stage and widespread metastasis [147]. Reliable biomarkers can accurately predict the survival and treatment response of patients with cancer to further improve the overall treatment effects [148]. As discussed in the previous section, many MRPs are known to be closely associated with the survival and recurrence rates of patients with cancer [64, 149]. For instance, the majority (> 90%) of patients with breast cancer showed positive expression of MRPS27. High expression of MRPS27 was positively correlated with stage, poorer overall survival, and progression-free survival in TNBC patients [51]. It proves that models based on MRPs have great potential in predicting the prognosis, disease progression, and treatment response of cancer patients.
3.1 MRPs as Prognostic Biomarkers
Current research on the clinical value of MRPs mainly focuses on exploring the relationship between specific MRPs and different cancers. There is a lack of holistic analysis of all MRPs in cancers. To better explore the clinical value of MRPs, we use the HPA (www.proteinatlas.org) to analyze the relationship between MRPs and the survival of patients in different cancers [150]. First, the members of MRPs are imported into the website to obtain the Kaplan–Meier plots. Then the MRPs closely related to prognosis are screened and summarized by the condition of p-value < 0.001.
According to the results presented in Figures 3 and 4, we can find that the majority of MRPs are closely associated with the prognosis of cancer, especially renal cancer and liver cancer. The prediction reveals that the number of MRP members linked to good and poor prognosis is roughly the same. Notably, some MRPs, such as MRPS5, MRPL14, and MRPL37, exhibit a dual effect on prognosis prediction, aligning with the aforementioned conclusion (Figure 3). In contrast to their dual role in renal cancer, MRPs in liver cancer are only associated with poor prognosis. Furthermore, the mutation rates of MRPS21, MRPL9, and MRPS29 genes in HCC patients are approximately 11%–12%. These suggest that MRPs may be effective targets for HCC treatment. Additionally, a previous study showed that the attenuation of MRPL13 inhibited the proliferation and migration of breast cancer cells via PI3K/Akt/mTOR signaling pathway, which was consistent with our prediction that high expression of MRPL13 is significantly associated with poor prognosis in breast cancer patients. Collectively, these observations indicate that MRPs can be used as biomarkers in clinics to better guide clinical treatment. Furthermore, unlike other members of MRPs, MRPL9, MRPL12, and MRPL58 promote the development of tumors, and no research has shown them to have opposite functions. Our prediction further highlights that MRPL9 is specifically related to poor prognosis in renal, liver, and lung cancer (Figure 4). Therefore, it can be inferred that these three proteins are important for tumor growth and possess greater potential as targets for tumor treatment due to their single, consistent effect.
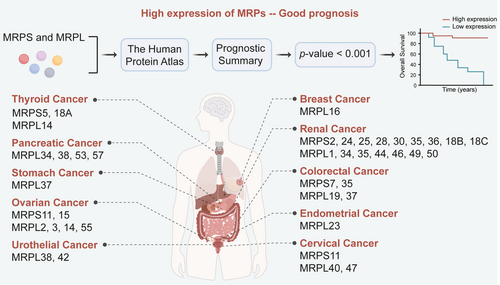
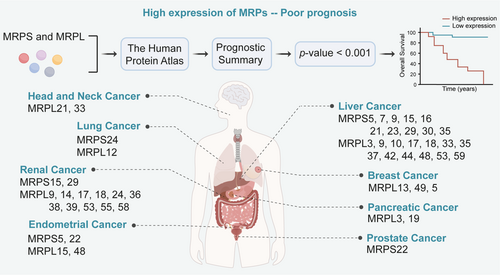
Based on existing research and analysis results, it is speculated that MRPs are of high importance in liver cancer. Overexpression of MRPS5, MRPS29, MRPS31, MRPL9, MRPL12, MRPL13, MRPL58, and MRPL59 has been shown to promote the development of liver cancer [151, 152]. In our analysis results (Figures 3 and 4), more than 20 members of the MRP family were associated with the prognosis of liver cancer, which ranks among the top cancer types. Current systemic treatments often exhibit highly heterogeneous efficacy in patients with advanced HCC, highlighting the urgent need to identify biomarkers for response prediction and patient selection [153]. MRPs, especially MRPS31, hold great potential in addressing this issue. MRPS31 is located in the 13q locus, which is recurrently deleted in HCC [154, 155]. This suggests that the loss of MRPS31 is likely to occur in the early stages of cancer, highlighting the potential of MRPS31 as an effective diagnostic marker for HCC. In addition, Gene Expression Omnibus (GEO) analysis revealed that the expression of MRPL12 is significantly upregulated in tumors of HCC patients who do not respond to sorafenib [59], making MRPL12 a promising biomarker to predict the efficacy of sorafenib in HCC patients. In summary, MRPs are crucial targets for cancer treatment, prognosis assessment, and treatment response prediction, especially in the context of liver cancer.
3.2 MRPs as Biomarkers of Treatment Response
In addition to being biomarkers for cancer patient prognosis, MRPs can also serve as biomarkers for cancer patient response to drugs. GEO analysis revealed that the expression of MRPL12 is significantly upregulated in tumors of HCC patients who do not respond to sorafenib [59], making MRPL12 a promising biomarker to predict the efficacy of sorafenib in HCC patients.
Over the past decade, immunotherapy with immune checkpoint inhibitors (ICIs) has revolutionized cancer treatments, bringing new hope to cancer patients [156-158]. A successful antitumor immune response relies on the presence and activation of immune cells within the TME, indicating that the response of immunotherapy is heavily influenced by immune cell infiltration [159]. Tumor cells accelerate their metabolism due to high energy demands, which leads to the depletion of nutrients in the TME and the release of numerous metabolites [160, 161]. This forces immune cells within the TME to deal with high concentrations of metabolites, especially lactate [162, 163], in a nutrient-deprived environment, which leads to immunosuppression and favors tumor growth [164, 165]. MRPs regulate the metabolism of tumor cells, suggesting that they may also regulate immune cells in the TME by altering metabolite secretion. Furthermore, MRPs are functionally expressed in immune cells and play critical roles in their biological processes. A compelling example is MRPL59, whose depletion has been shown to reshape the immune landscape by regulating the conversion of Treg cell subtypes, thereby promoting an antitumor immune microenvironment (Figure 1E). Mitochondrial translation is also associated with the polarization of macrophages. MRPL13 induced by Elp3 promotes M2 polarization [166]. These evidence stress the important role of MRPs in immune cells. However, there are currently few studies reporting the relationship between MRPs and tumor immunity. Therefore, we now analyze the correlation of MRPs and immune cell infiltration in the TME with BEST (https://rookieutopia.hiplot.com.cn/app_direct/BEST/) [167]. First, we focus on MRPS5, MRPS29, MRPL9, MRPL12, MRL13, MRPL33, MRPL58, and MRPL59, which have been described above. From Figures 3 and 4, we identified the cancer types significantly affected by these MRPs and then analyzed them on the BEST website. The results showed that MRPS5, MRPS29, and MRPL9 significantly promoted the infiltration of neutrophils, dendritic cells, CD8+ T cells, macrophages, CD4+ T cells, and B cells in the TME (Figure 5A). In contrast, MRPL12 and MRPL59 reduced the infiltration of these cells.
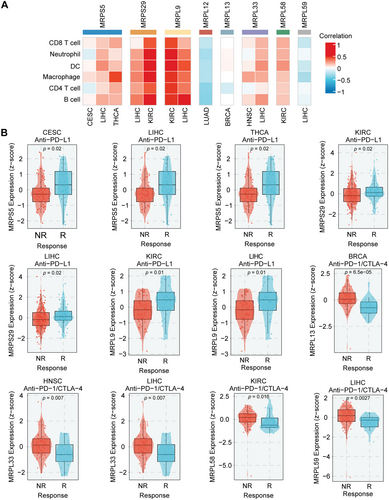
For most advanced solid tumors, therapies that block the binding of PD-1 and programmed death ligand 1 (PD-L1) have become the mainstay [168, 169]. Unfortunately, clinical studies have shown that some patients still exhibit congenital nonresponse to PD-1/PD-L1 blockade [170, 171]. The infiltration degree of CD8+ T cells and CD4+ T cells affects the role of PD-1/PD-L1 inhibitors [172]. MRPs are closely associated with the infiltration of immune cells, as previously mentioned. Therefore, we further evaluated whether MRPs play a role in the response of cancer patients to immunotherapy. From the results, we found that the expression of MRPS5, MRPS29, and MRPL9 was significantly higher in patients who responded to immunotherapy compared to non-responders (Figure 5B). Conversely, the expression of MRPL33, MRPL58, and MRPL59 was lower in the responders. These findings suggest that these MRPs can serve as biomarkers to predict patient response to immunotherapy, aiding in the optimization of clinical treatment plans. Additionally, inhibiting MRPL33, MRPL58, and MRPL59 in combination may potentially enhance the effectiveness of immunotherapy. High levels of lactate in the TME can hinder lactate efflux in T cells, leading to reduced cytokine production and decreased cytotoxic activity [173]. Lactate also prevents the activation of nuclear factors of activated T cells (NFAT) in T cells and NK cells, resulting in lower IFN-γ production [174]. It can be inferred from this that the role of MRPs in promoting immune cell infiltration may be due to their enhancement of OXPHOS in tumor cells. This will reduce the production and secretion of lactate by tumor cells, thereby restoring the function of CD8+ T cells and enhancing the efficacy of ICIs in cancer patients. However, the mechanisms by which MRPs inhibit immune cell infiltration remain unknown, and future research is required to fill this gap.
4 Therapeutic Implications of MRPs
As our understanding of the mitoribosome continues to deepen, the importance of MRPs has become increasingly apparent. So far, approximately 80% of MRPs in mt-SSU and about 70% of those in mt-LSU have been linked to the occurrence and development of cancer. Given the critical and severe upward trend in the latest statistics of cancer incidence and mortality [175, 176], the key role these MRPs play in tumorigenesis emphasizes their importance, with profound implications for advancing cancer research and treatment strategies. MRPs are a diverse family whose primary task is to maintain mitochondrial translation. Metabolic reprogramming is an important consequence of inhibiting mitochondrial translation [177, 178], suggesting the key role of MRPs in cancers by regulating intracellular metabolism [179]. Beyond their canonical roles in mitochondrial translation, accumulating evidence positions MRPs as multifaceted regulators of oncogenic signaling, including SIRT1, PI3K/Akt/mTOR, and DNA damage response. For example, MRPL59 functions as a regulator of the cell cycle. It is involved in the regulation of cell cycle checkpoints through binding to GADD45 family and interactions with DNA damage response proteins such as p53, p21, and CDK2. These noncanonical functions, intertwined with their immunometabolic reprogramming capacity in the TME, position MRPs as key regulators of cancer progression and treatment.
4.1 Insights Into Broader Regulators of MRPs
The regulation of MRPs can be categorized into three types: transcriptional regulation, posttranscriptional regulation, and posttranslational modifications. Several transcription factors, including Yin Yang 1 (YY1), NRF2, ILF3, and HIF-1, have been shown to bind to the promoter regions of specific MRP members to regulate their transcription. miRNAs are abnormally expressed in cancer and closely related to tumor progression. They act as gene switches in cancer cells, directly or indirectly controlling various cellular signaling pathways, including Notch, Jak/STAT, and Wnt signaling pathways [180, 181]. Some MRPs, such as MRPL58, MRPL59, etc., have been identified as direct targets of miRNAs. miRNAs bind to the 3'-UTR regions of the mRNA of MRPs, leading to mRNA degradation and ultimately resulting in reduced protein levels. In addition, a few studies have indicated that the alternative splicing of precursor mRNAs of MRPs is subject to the regulation of hnRNPK to form different splicing variants. It is beneficial for the diverse functions of MRPs.
Posttranslational modifications also have a significant impact on the function of MRPs. Phosphorylation and acetylation are two modifications that have been reported to regulate the intracellular localization of MRPs. For instance, acetylated MRPS5 is predominantly localized in the nucleus, and upon deacetylation, MRPS5 translocates to the mitochondria and enhances mitochondrial respiration (Figure 2A). Similarly, Akt induces the phosphorylation of MRPS29, promoting the translocation of MRPS29 to the mitochondria. Most MRPs are encoded by nDNA and synthesized in the cytoplasm, after which they are selectively and efficiently transported to their destinations where they exert their physiological functions [182]. Studies have shown that MRPs are present in both the nucleus and mitochondria. From the above results, it seems that posttranslational modifications are likely to be a key factor in determining their intracellular localization. MRPs enhance the function of ETC in mitochondria, which is beneficial to tumor growth. This phenomenon suggests that targeting the posttranslational modifications of MRPs may prevent them from exerting their functions in mitochondria, thereby achieving therapeutic effects against tumors. In addition to phosphorylation and acetylation, there are various types of protein posttranslational modifications, including methylation, glycosylation, palmitoylation, and lactylation [183]. Due to the Warburg effect, tumor cells produce a large amount of lactic acid even under aerobic conditions. Recent studies have highlighted that lactylation modification plays an important role in tumor development and immune response. For example, GTPCS interacts with p300 in the nucleus and induces histone lactylation, which promotes glioma growth and radioresistance [184]. Lactylated histones also induce the expression of YTHDC1, ultimately activating stearoyl-CoA desaturase-related lipid metabolism processes that promote the progression of HCC [185]. Current research indicates that exogenous lactate treatment can inhibit the expression of intracellular MRPs, including MRPS15, MRPS29, MRPL13, and MRPL39. It can be speculated that the expression of MRPs may be controlled by lactylation regulators. Moreover, lactylation can affect the intracellular localization of proteins [186], indicating that MRPs may also undergo lactylation modification, enabling their shuttling between the nucleus and mitochondria.
In summary, the expression of MRPs is regulated by a multilayered regulatory network encompassing transcription factors, miRNAs, and posttranslational modifications. These mechanisms collectively offer multiple druggable nodes for developing MRP-directed therapeutic strategies.
4.2 Multifaceted Roles of MRPs in Cancer
The multifaceted roles of MRPs in cancer are indeed intriguing and complex. A noteworthy phenomenon is that certain MRPs exhibit opposite effects in different cancer types (Table 2), such as MRPS29, MRPL13, MRPL33, and MRPL59. We speculate that MRPs are bifunctional proteins, although their role in promoting tumor growth still accounts for the majority. First, subcellular localization appears crucial. Several members of MRPs have been demonstrated to contain NLS. And MRPL59 was initially discovered as a nuclear protein. Accordingly, it can be inferred that MRPs are present in both mitochondria and nucleus, enabling functional diversification. Additionally, posttranslational modifications have been proven to be widely present in MRPs, including MRPS5, MRPS29, and MRPL12, and influence the localization of MRPs. For example, acetylated MRPS5 predominantly localizes to the nucleus, whereas its deacetylated form translocates to mitochondria to enhance mitochondrial respiration. Clinical analysis showed that the SIRT1High/Cytoplasmic-MRPS5High profile was associated with poor prognosis in HCC, indicating the pathophysiological significance of localization shifts of MRPs.
| Name | Other names | Cancer types | Status of MRPs | Effects | Assays | Refs. |
|---|---|---|---|---|---|---|
| MRPS5 | uS5m | Liver cancer | Upregulation | Maintain stemness properties Enhance metabolic plasticity through SIRT1/MRPS5 axis |
shRNA | [39] |
| MRPS29 | DAP3 mS29 Bmrp-10 |
Esophageal carcinoma Hepatocellular carcinoma |
Upregulation | Promote tumorigenesis through suppression of A-to-I RNA editome | shRNA CRISPR/Cas9 |
[52] |
| Esophageal carcinoma | Upregulation | Promote tumorigenesis by increasing WSB1 expression via repressing AS-NMD of WSB1 | shRNA CRISPR/Cas9 |
[54] | ||
| Gastric tumors | Downregulation | Promote cell migration and enhance resistance to chemotherapy by inhibiting apoptosis | DNA Plasmid | [89] | ||
| Lung adenocarcinoma | Upregulation | Involve in modulation of cellular radiation response by RIG-I-like receptor agonist | siRNA | [187] | ||
| MRPL9 | bL9m | Lung cancer | Upregulation | Promote cell proliferation, migration, and invasion through regulating c-MYC | siRNA | [97] |
| Thyroid cancer | Upregulation | Promote cell proliferation and migration through interacting with GGCT | shRNA | [57] | ||
| MRPL12 | bL12m | Hepatocellular carcinoma | Upregulation | Promote HCC tumorigenicity by regulating mitochondrial metabolism | DNA Plasmid | [60] |
| Lung adenocarcinoma | Upregulation | Promote cell proliferation and invasion | shRNA | [100] | ||
| MRPL13 | uL13m | Breast cancer | Upregulation | Promote cell invasion by regulating MMPs and VEGFA expression | siRNA | [107] |
| Breast cancer | Upregulation | Promote cell proliferation and migration Induce EMT through the Akt/mTOR pathway |
siRNA | [109] | ||
| Hepatoma | Downregulation | Promote cell invasiveness by inducing mitoribosomal defect and claudin-1 induction | siRNA | [112] | ||
| Non-small cell lung cancer | Upregulation | Promote cell proliferation Inhibit apoptosis |
siRNA shRNA |
[61] | ||
| MRPL33 | bL33m | Colon cancer | Upregulation | Promote cell proliferation Repress apoptosis by preserving mitochondrial function |
siRNA shRNA DNA plasmid |
[62] |
| Gastric cancer | Downregulation | Promote chemoresponse through PI3K/Akt signaling pathway | DNA plasmid | [114] | ||
| MRPL58 | ICT1 mL62 |
Gastric cancer | Upregulation | Promote migration and invasion through the regulation of miR-205 | shRNA DNA plasmid |
[122] |
| Breast cancer | Upregulation | Promote cell proliferation by inhibiting apoptosis | shRNA | [123] | ||
| Colorectal cancer | Upregulation | Promote tumor growth through AMPK, SAPK/JNK, and PARP pathways | shRNA | [188] | ||
| MRPL59 | CRIF1 mL64 GADD45γ CKBBP2 |
Breast cancer | Upregulation | Promote cell proliferation and migration | siRNA | [30] |
| Non-small cell lung cancer | Upregulation | Promote cell proliferation by accelerating the progression of cell cycle at G1/S phase Inhibit mitochondrial apoptosis Promote tumor growth by SIRT3-mediated deacetylation of PYCR1 |
shRNA DNA plasmid |
[138] | ||
| Hepatocellular carcinoma | Upregulation | Exacerbate cancer progression by inducing mitoribosomal dysfunction Promote tumor growth and metastasis by activating the ROS/NFκB signaling pathway Promote tumor growth by inducing G1-S cell cycle transition and suppressing cell apoptosis/necrosis Enhance cell invasion and migration by inducing EMT |
Cre-loxP siRNA shRNA |
[70] [71] |
||
| Colon cancer | Downregulation | Inhibit tumor growth by enhancing the expression of p53 and interacting with SNF5 to induce G1/S cell cycle arrest | shRNA | [189] | ||
| Leukemia | Downregulation | Promote cell cycle progress | siRNA DNA plasmid |
[190] |
- Abbreviations: AMPK, AMP-activated protein kinase; AS-NMD, alternative splicing coupled with nonsense-mediated mRNA decay; EMT, epithelial-to-mesenchymal transition; GGCT, γ- glutamylcyclotransferase; JNK, c-Jun N-terminal kinase; MMP, matrix metalloproteinase; PARP, poly(ADP-ribose) polymerase; PYCR1, pyrroline-5-carboxylate reductase 1; RIG-I, retinoic acid-inducible gene-I; SAPK, stress-activated protein kinase; VEGFA, vascular endothelial growth factor A.
Second, alternative splicing generates functionally distinct isoforms. Both MRPL33 and MRPL12 have been shown to exist in “long” and “short” forms with distinct properties and functions, suggesting splice variants may contribute to their functional diversity. Third, gene mutations in MRPs may be another important cause. A typical example is MRPS29, as mentioned above. Other MRPs may also have corresponding mutations, which may impair their ability to induce apoptosis or promote tumor development through other pathways. The functional complexity of MRPs presents a significant challenge for therapeutic applications, necessitating strategies that specifically exploit their tumor-suppressive functions. Developing isoform-selective inhibitors or agents targeting distinct subcellular MRP populations may enable precise therapeutic intervention.
4.3 Prospects of Druggability
Currently, there is a lack of targeted inhibitors for MRPs, which limits the clinical application. This emphasizes the need to explore the prospects of developing inhibitors for MRPs.
Gene therapy is emerging as a powerful tool to modulate gene expression for cancer treatment [191, 192]. Various tools for gene therapy have been developed, including CRISPR/Cas, DNA plasmids, siRNA, or shRNA, to facilitate in vivo gene manipulation [193-195]. However, the intracellular localization of MRPs in the mitochondrial matrix poses a significant challenge for drug delivery. Targeting MRP requires crossing three layers of membrane, making precise drug delivery difficult. Nanoparticles are expected to solve this problem. Encapsulation of nanomaterials can effectively protect plasmids from degradation before they reach their destination. In addition, surface modification of nanoparticles using organelle-targeted elements can achieve specific delivery to mitochondria [196]. For example, lipophilic cationic molecules can penetrate the negatively charged inner and outer membranes of mitochondria. Some peptides, such as octa-arginine and cationic peptides, can also specifically accumulate in mitochondria to achieve mitochondria-targeting delivery [197].
Another feasible targeting strategy is targeted protein degradation (TPD), with a typical example being proteolysis targeting chimeric (PROTAC) molecules. PROTAC can bind to the target protein in the form of a ternary complex and recruit ubiquitin ligases to promote the degradation of the target proteins [198, 199]. PROTAC can block all the functions of the target protein and has high selectivity, which helps avoid the off-target effects that may be caused by CRISPR/Cas 9 and siRNA [200]. The function of PROTAC depends on the ubiquitin-proteasome system, allowing it to effectively target MRPs in the nucleus.
Additionally, it is promising to target MRPs in mitochondria through mitochondrial protease targeting chimera (MtPTAC). MtPTAC utilizes mitochondrial caseinolytic protease P (ClpP), a protein that is widely distributed in mitochondria, to hydrolyze proteins, enabling mtPTAC to function even in mitochondria lacking proteasomes and lysosomes [201]. In vitro and in vivo results have shown that MtPTAC can completely block the function of POLRMT, which is only located inside mitochondria, demonstrating the great application potential of MtPTAC in targeting proteins in the mitochondrial matrix. These findings suggest that it is entirely possible to target MRPs through some effective carriers. Therefore, targeting MRPs is truly a practical approach for treating cancers.
5 Conclusion and Future Perspective
In general, MRPs function in both the nucleus and mitochondria. They are mostly overexpressed in tumor cells and act as oncogenes, participating in the occurrence and development of tumors. They affect the sensitivity of tumor cells to drugs by regulating mitochondrial metabolism and accelerate the proliferation of tumor cells by promoting the transition of the main phases of the cell cycle and enhancing the expression of cyclins and CDK proteins in the nucleus. Moreover, based on existing literature and our analysis results, MRPs play a significant role in liver cancer, which may provide some direction for future research.
Although MRPs are promising potential therapeutic targets for cancer treatment, our current understanding of their roles in cancer is limited, and there are still some limitations that need to be addressed. First, current in vitro studies of MRPs in cancer are conducted on cell lines, where MRPs are knocked down, knocked out, or overexpressed to validate their roles. However, in vivo validation of MRPs is mostly limited to subcutaneous or orthotopic xenograft models (Table 1), except for specific members. Few studies have used spontaneous tumor models or genetically engineered mouse models to study the role of MRPs in cancer. In addition to their effects on tumor cells themselves, MRPs can also regulate the immune microenvironment and mediate metabolic changes in immune cells. Therefore, 3D in vitro models and humanized mouse models that can reproduce the TME and tumor-immune interactions are urgently needed to better evaluate the role of MRPs in tumorigenesis, progression, and tumor immunity [202]. Furthermore, posttranslational modifications play an important role in regulating the functions of MRPs. It is necessary to detect different posttranslational modifications using mass spectrometry or protein microarray-based techniques.
Some specific MRPs, such as MRPS29, MRPL13, MRPL33, and MRPL59, have been shown to have opposite functions in certain other cancers; in other words, they act as tumor suppressors. These two opposite phenomena may be caused by the multiple modes of action of MRPs. Current research on MRPs rarely distinguishes these subtypes, underscoring a critical need for future studies to focus on this aspect. This will also help classify cancer patients with different subtypes, allowing for better formulation of treatment plans. However, this kind of distinction is difficult to achieve in cells or cell-line-derived tumor xenografts, which are currently the most commonly used in MRP studies. Therefore, future basic research on MRPs should strengthen cooperation with clinicians to better explore the role of different MRP subtypes and clarify their roles in tumor development. Furthermore, techniques that modulate exon usage should be applied in the studies of MRPs, such as antisense oligonucleotides [203]. This is beneficial to offer clearer insight into individual isoform function.
Author Contributions
Qian Chen: visualization (lead), writing – original draft (lead), writing – review and editing (lead). Yingli Zhang: writing – review and editing (equal). Jin-Jian Lu: funding acquisition (equal), supervision (equal), writing – review and editing (equal). Ting Li: funding acquisition (equal), supervision (equal), writing – review and editing (equal). All authors have read and approved the final manuscript.
Acknowledgments
We thank the Human Protein Atlas (www.proteinatlas.org) and BEST (https://rookieutopia.hiplot.com.cn/app_direct/BEST/) for the valuable information. This study was supported by the Science and Technology Development Fund, Macau SAR (File No. 0015-2022-A1 and 005/2023/SKL), University of Macau (File No. MYRG-GRG2024-00113-ICMS-UMDF), and the Internal Research Grant of the State Key Laboratory of Quality Research in Chinese Medicine, University of Macau (File No. SKL-QRCM-IRG2023-011) awarded to Jin-Jian Lu. Additionally, this study was supported by the National Natural Science Foundation of China (File No. 82403357) awarded to Ting Li.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.