Oxidative Stress in Antigen Processing and Presentation
Qinxia Chang and Yaying Zhang contributed equally to this study.
ABSTRACT
Antigen processing and presentation are fundamental for connecting innate and adaptive immune responses in combating cancers and infections. Reactive oxygen species (ROS), serving as second messengers in various physiological processes, play a vital role in modulating antigen processing and presentation. However, oxidative stress due to an imbalance characterized by excessive accumulation of ROS or inadequate antioxidant defenses can severely impair antigen-specific immune responses, contributing to the pathophysiology of multiple health conditions, notably including various cancers, cancer-associated infections and autoimmune diseases. This review comprehensively investigates the multifaceted effects of ROS on antigen processing and presentation, encompassing immunopeptide generation, the functionality of antigen-presentation machinery (APM), and the interactions of antigen-presenting cells and antigen-specific effector cells. It emphasizes the critical pathophysiological roles of oxidative stress in diseases such as cancers, cancer-associated infections and autoimmune diseases. Moreover, we delve into the therapeutic potential of targeting redox homeostasis to enhance antitumor immune responses. By illuminating the intricate interplay between ROS and immune functionality, this review provides an essential theoretical framework for developing innovative immunotherapy strategies aimed at restoring immune competency and improving clinical outcomes in patients with immune-related diseases.
Graphical Abstract
Reactive oxygen species (ROS) play a crucial role in antigen processing and presentation, essential for linking innate and adaptive immunity. While balanced ROS levels promote immune function, excess ROS can disrupt antigen recognition, resulting in immune dysfunction. Targeted therapies to regulate ROS present new avenues for enhancing immunotherapy.
1 Introduction
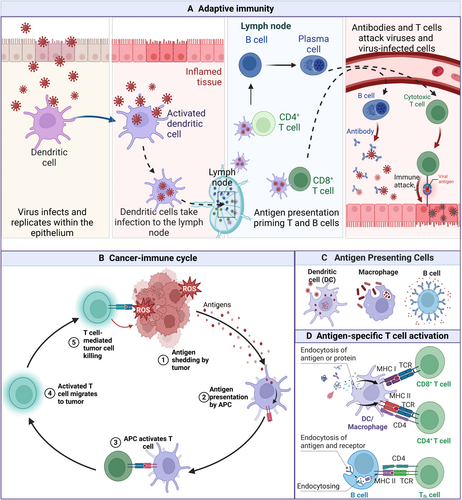
Innate and adaptive immunity are important barriers for organisms to resist infection. Parallelly, anticancer immune responses have been triggered by malignant cells that harbor tumor-specific antigens (TSA) owing to the accumulation of DNA damage [1, 2]. These immune responses have driven the development of diverse immunotherapies, including antibody-based treatments, vaccines and adoptive cell therapies (ACT) [3]. The critical prerequisite of immune response is that antigens are correctly recognized and processed, then presented to immune effector cells, including T and B cells. Then, the successful completion of the APM initiates and regulates the immune response [4-6]. Normally, a robust immune response can be elicited by professional antigen-presenting cells (APCs), like dendritic cells (DCs), macrophages and B lymphocytes, activating naive CD8+ or CD4+ T cells (Figure 1). The critical function of the finely tuned antigen presentation process in initiating and modulating immune responses, particularly within the context of cancer immunotherapy, underscores the need for a comprehensive understanding of these mechanisms to optimize the therapeutic efficacy and improve outcomes for immunocompromised patients.
Oxidative stress arises from a disruption in the equilibrium between the generation of reactive ROS and the inherent ability of the antioxidant defense mechanisms [7-9]. Physiologically, intracellularly generated ROS have a role in physiological processes, including cellular signaling, cell metabolism, proliferation, and differentiation [10, 11], they also serve as critical signaling mediators within the immune system [12-14]. However, excessive concentrations of ROS can severely compromise cellular integrity by damaging nucleic acid structures, inactivating proteins, and inducing lipid peroxidation, which ultimately leads to ferroptosis [15]. ROS function as both cytotoxic agents against pathogens and malignant cells and as modulators of cytokine and antibody expression during immune responses [16, 17]. The detrimental effects of ROS are typically kept in check by the antioxidant defense system, encompassing ROS-scavenging enzymes, proteins, and reducing agents [18, 19]. However, excessive ROS production can overwhelm the antioxidant defense system, causing irreversible oxidative damage to DNA, RNA, proteins, and lipids [8, 15, 20]. Immune cell membranes exhibit greater vulnerability to oxidative stress damage owing to their delicate composition relative to other cells. Furthermore, excessive production of oxygen free radicals can provoke inflammation through both pattern-recognition receptor (PRR)-dependent and PRR-independent mechanisms.
The intricate interplay between oxidative stress and immune function is profoundly influenced by the concentration of ROS. A nuanced, context-dependent relationship exists, wherein moderate ROS levels can be beneficial, while excessive ROS is generally detrimental to immune homeostasis [10]. Firstly, oxidative stress significantly modulates the immunopeptidome. It alters antigen generation through mechanisms such as genomic instability, aberrant mRNA splicing, and oxidative posttranslational modifications (oxPTMs), leading to the generation of neoantigens [21]. These neoantigens may serve as targets for immunotherapeutic interventions, but their dysregulated production can also contribute to autoimmunity [22]. Furthermore, oxidative stress affects the efficiency of both proteasomal and endosomal/lysosomal antigen processing pathways, influencing major histocompatibility complex (MHC) I and II presentation [23]. Secondly, oxidative stress exhibits a biphasic effect on lymphocyte activation. Moderate ROS levels promote T cell and B cell activation by upregulating costimulatory molecules (e.g., CD80/CD86, CD40) and influencing metabolic reprogramming [24]. Conversely, excessive ROS contributes to T cell exhaustion and apoptosis, particularly within the immunosuppressive milieu of the tumor microenvironment (TME), and impairs B cell differentiation and regulatory B cell function [25]. In conclusion, the immune response to oxidative stress is finely tuned by ROS concentration. A comprehensive understanding of these complex interactions is paramount for the development of targeted immunotherapies capable of precisely modulating immune responses.
Here, we will discuss how oxidative stress regulates antigen processing and presentation by altering the generation of peptide epitopes, modulating the APM and manipulating antigen-specific immune cell activation and function. We also explore the role of oxidative stress-modulated immune response in the pathophysiologic processes, like cancers, cancer-associated infections, and autoimmune diseases. Additionally, we propose several potential novel therapeutic strategies for improving immune response through targeting oxidative stress, particularly in their modulation of antigen presentation processes.
2 Impact of Oxidative Stress on Antigen Generation and Processing
Oxidative stress affects the composition of the immunopeptidome by altering the processes of antigen generation and processing. The DNA damage and genomic mutations caused by oxidative stress lead to the formation of new or altered proteins, which in turn change the antigenic epitopes [26]. These altered cellular proteins are then broken down into peptides through the cytosolic proteasome pathway as well as phagosomal, lysosomal, and autophagic vesicle pathways. By impacting the processing of antigens derived from both external and internal sources, such as viral or intracellular bacterial proteins, oxidative stress plays a role in modulating the immunopeptidome composition [27].
2.1 Influence on the Peptide Epitope Generation
Oxidative stress can increase the peptide epitope generation through various mechanisms, including the induction of genomic instability, dysregulated alternative splicing processes, and alterations in protein posttranslational modifications (PTMs) [21, 28, 29] (Figure 2).
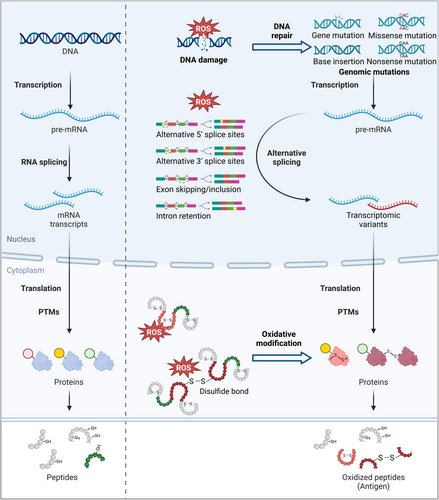
2.1.1 Oxidative Stress Creates Mutational Antigens
Oxidative stress, a hallmark of tumors, causes a higher antigen burden by inducing DNA damage and genetic mutations [30]. To date, over 100 types of oxidative base lesions have been reported, with 8-hydroxyguanine (8-oxoG) being the most extensively studied [31-33]. These DNA damages can prompt cells to activate the mismatch repair (MMR) system, which is responsible for identifying and rectifying base mismatches, as well as correcting insertions and deletions that occur during DNA replication [34, 35]. Notably, oxidative DNA lesions typically require the base excision repair/single-strand break repair (BER/SSBR) [36]. Under oxidative conditions, mutations in BER/SSBR lead to a corresponding high frequency of microsatellite instability (MSI) [37]. Tumors with DNA MMR defects tend to accumulate high levels of mutations such as frameshift mutations and single nucleotide variations (SNVs), characterized by a high tumor mutation burden (TMB), which is associated with rapid tumor progression [38]. The peptides generated from mutations differ significantly from wild-type peptides, thus potentially possessing high immune antigenicity [39]. Therefore, DNA damage induced by oxidative stress may generate a large number of highly antigenic mutated peptides, providing additional sources of immune peptide repertoire and neoantigenic epitopes [40].
When cells experience oxidative stress, abnormal transcriptional and posttranscriptional events may occur, potentially expanding the antigen repertoire. For instance, oxidative stress depleted Brahma-related gene 1 (BRG1)-associated factor helicase ATPase of mammalian switch/sucrose non-fermentable complexes (SWI/SNF) complexes (BRAHMA), a core ATPase subunit of SWI/SNF chromatin-remodeling complex, thereby shifting the alternative splicing towards the inclusion of the distal terminal exon [41]. Another mechanism involves the Hu antigen R (HuR), which modulates cellular stress responses by stabilizing the translation of targeted mRNAs. Under oxidative stress, phosphorylated HuR could bind with the proximal region of TRA2β exon 2, leading to the production of transcription containing exon 2 with several premature stop codons that increase the likelihood of antigen formation [42]. Additionally, oxidative modifications of the Ro ribonucleoprotein particles (Ro-RNPs), which is essential in RNA processing, have been observed in patients with systemic lupus erythematosus (SLE), potentially triggering autoimmunity or promoting epitope spreading. The oxidative-modified ribonucleoproteins are more readily internalized than the unmodified ones by APCs, on account of the new conformation after oxidation. Consequently, new self-peptides are presented to T cells, thereby facilitating intramolecular spreading for autoreactive B cells [43-45]. These changes in alternative splicing and mRNA processing may result in aberrant gene expression or the generation of novel RNA splice forms, enhancing the diversity of neoantigens [46].
Thus, genomic mutation and transcriptional dysregulation mediated by oxidative stress can lead to the production of neoantigens, which may be monitored by the immune system and potentially used to facilitate targeted immune responses against the tumor cells.
2.1.2 Oxidative Posttranslational Modifications Enhance Non-Mutational Neo-Antigenicity
Oxidative modifications of proteins can lead to the posttranslational formation of non-mutational neoantigens. Specific PTMs such as glycosylation, phosphorylation, and O-linked β-N-acetylglucosamine (O-GlcNAc) create distinct antigenic determinants and corresponding T cell receptor (TCR) specificities [47-49]. Neoantigens derived from glycosylated mucin 1 (MUC1) exhibited an approximately 100-fold enhancement in binding affinity for MHC I relative to their unmodified counterparts [50]. Furthermore, O-GlcNAc-modified peptides elicit memory T cell responses, providing a highly immunogenic therapeutic target for leukemias with low mutational load [51]. Dysregulated phosphorylation, by modulating epitope-MHC interactions or altering antigenic properties, can generate immunogenic neoantigens, as exemplified by phosphopeptides derived from insulin receptor substrate 2 (pIRS2) and breast cancer antiestrogen resistance 3 (BCAR3) [52-54]. Furthermore, enzymatic and nonenzymatic PTMs of proteins alter self-epitopes, for instance, deamidation in RA, phosphorylation in T1Ds, and oxidation in atherosclerosis, rendering them targets for autoimmune reactions, and due to their unconventional translation, they possess immunogenicity when highly expressed [55]. Collectively, these findings highlight the significance of PTM-derived neoantigens as a novel class of potential targets for immunotherapeutic strategies.
Oxidative modification of proteins, hereafter called oxPTM, represents another source for posttranslational antigenic epitopes [56, 57]. Oxidative alteration of proteins can result in enhanced recognition by autoantibodies in various disorders, including diabetes mellitus (DM), alcoholic liver disease, rheumatoid arthritis (RA) and SLE [58]. An illustrative instance is the coexistence of autoantibodies to normal insulin and oxidized insulin in 50% of type 1 diabetes mellitus (T1D) patients. Most individuals with T1Ds or children at risk of diabetes have autoantibodies to oxidative post-translationally modified insulin (oxPTM-insulin). Physically, the separation of C-peptide from pro-insulin, results in the formation of functional insulin wherein the A and B chains are interconnected through disulfide bonds. Oxidative processes lead to specific modifications in insulin structure, including oxidation of amino acids such as histidine 5, cysteine 7, and phenylalanine 24, along with glycation events at lysine 29 and phenylalanine one within the B chain [59]. Exposure to reactive oxidants can induce oxPTM-insulin, resulting in the generation of oxPTM-insulin neoantigenic peptides with the ability to elicit T cell responses [56]. Notably, oxidant-modified insulin has a higher affinity than native insulin, increasing the risk of going on to develop T1D [60-62]. Similarly, the oxidation of human serum albumin (HSA) mediated by hydroxyl radicals (•OH) elevates its immunogenicity, leading to increased recognition by circulating autoantibodies [63].
Highly reactive metabolite binds to proteins to form immunogenic neoantigens upon exposure to oxidative stress. Increased levels of oxidative stress led to greater lipid peroxidation in tissues, resulting in the production of malondialdehyde (MDA). MDA subsequently breaks down into acetaldehyde (AA), which then combines with proteins to create the more stable protein adduct known as malondialdehyde-acetaldehyde (MAA). Patients suffering from various systemic inflammatory diseases, including RA, have serum containing MAA-adducted proteins that are highly immunogenic. In a subset of patients from a neuropsychiatric systemic lupus erythematosus (NPSLE) cohort, detectable serum autoantibodies were found that target proteins modified by MAA, advanced glycation end-products (AGEs), and carbamylation (CarP) [64]. Additionally, 3-hydroxypropionate (3-HPA), a metabolite derived from gut microbiota, forms thioether bonds with cysteine residues in integrin alpha-2B (ITGA2B), a process catalyzed by cystathionine β-synthase (CBS). The resulting carboxyethylated peptide, ITGA2B-96Cys, acts as a neoantigen, eliciting CD4+ T cell responses and human leukocyte antigen (HLA)-restricted autoimmunity [22, 65]. These studies further highlight that oxidative stress-induced protein modifications can generate distinct neoantigens, thereby enhancing the immune recognition of target cells [66].
Oxidative modifications may alter the MHC-binding motifs within peptide antigens or the contact residues on the TCR, thereby influencing the effectiveness of the immune response [67]. For example, oxidation of methionine residues within the human cytomegalovirus pp65495–503 (NLVPMVATV) immunodominant peptide diminishes its antigenicity by impairing TCR binding [68]. This differential impact of oxidative modification on antigenicity may be attributed to the location of the modified residue within the MHC binding interface. Specifically, PTMs at residues directly involved in MHC interaction interfere with peptide-MHC binding, while those distal to the interface predominantly modulate TCR recognition via altered TCR binding modes [69].
Collectively, these findings highlight the critical interplay between oxidative stress, PTMs, and altered antigenicity in immunopathogenesis. Further investigation into the impact of oxidative stress on immunopeptidome alterations is crucial for understanding the complex interplay between disease and the immune system, and for informing the development of personalized immunotherapeutic approaches.
2.2 Effects on Antigen Processing
Oxidative stress impacts antigen processing and presentation by altering the efficiency of proteasome-mediated degradation of intracellular proteins and endosomal degradation of extracellular proteins [70]. All nucleated cells utilize the proteasome system for endogenous antigen processing, enabling MHC I antigen presentation. APCs with phagocytic capacity utilize endosomal pathways for exogenous antigen processing, promoting MHC II antigen presentation [71-73].
2.2.1 Effects on Proteasome-Based Antigen Processing
Antigen processing in nonprofessional cells begins with proteasome-mediated degradation of endogenous misfolded, damaged, or aberrant proteins [74]. The widely distributed and highly adaptable proteasome system is the 26S ubiquitin-proteasome system, composed of a 20S core particle (CP) and a 19S regulatory particle (RP), responsible for ubiquitin/ATP-dependent protein degradation [75-77]. The 19S RP is essential for identifying ubiquitinated proteins, removing their ubiquitin chains, and unfolding them before they are degraded by the 20S CP. The 20S CP, a cylindrical structure comprising α and β subunits, possesses catalytic activity via the β1, β2, and β5 subunits. Under specific conditions, inducible β1i, β2i, and β5i subunits are capable of substituting for the constitutive catalytic subunits, forming the so-called immunoproteasome (IP) [78]. In response to cellular oxidative stress, the IP can associate with 19S and proteasome activator 28α/β (PA28α/β) regulatory factors to form hybrid proteasomes (HP, 19S-20S-PA28α/β), promoting the rapid, ubiquitin-dependent degradation of oxidatively damaged proteins [79, 80]. Furthermore, both standard proteasomes (SP) and IP can generate neo-epitopes composed of two distinct fragments from noncontiguous peptide sequences within the parent antigen. This occurs through a process known as proteasome-catalyzed peptide splicing (PCPS), where proteasomal cleavage products are fused together, forming peptides that serve as MHC I ligands on the cell surface [81, 82]. Constitutively expressed in immune cells, IP is inducible and expressed in nonimmune tissues by type I and/or II interferons (IFNs). Upon IFN-γ stimulation, the IP, with its distinct proteolytic subunits and cap structures, enhances the processing of proteins into HLA-I compatible peptides. The IP exhibits higher cleavage rates than SP, displaying a preference for cleaving hydrophobic and basic amino acids, thus facilitating peptide binding to MHC I. Additionally, the replacement of 19S caps by PA28 in IP accelerates antigen peptide release [83-85]. Upon IFN-γ stimulation, the IP, with its distinct proteolytic subunits and cap structures, enhances the processing of proteins into HLA-I compatible peptides by generating peptides with hydrophobic C-terminal residues, thereby initiating cytotoxic T lymphocytes (CTL) responses against viral and bacterial antigens [83, 86].
Proteasomes are essential for regulating the peptide and antigen repertoire during adaptation to oxidative stress [76]. Normally, proteasomes, particularly the 20S complex, maintain cellular redox balance by identifying and eliminating oxidatively modified or damaged proteins. Exposure to H2O2 increases proteasome-mediated degradation by over tenfold, while proteasome inhibitor treatment intensifies oxidative damage, highlighting the critical regulatory function of the proteasome system in maintaining cellular homeostasis [87, 88]. However, elevated oxidative stress disrupts the function of the proteasome system and subsequent protein degradation. Acute H2O2 exposure compromises the structural integrity of the 26S proteasome complex, causing 20S core-19S particle dissociation, reducing 26S proteasome proteolytic activity, and leading to ubiquitinated protein accumulation [89] (Figure 3). Additionally, oxidative stress induces modifications of 19S and 20S proteasome subunits, such as 4-hydroxynonenal (4-HNE) modification, S-glutathionylation, and carbonylation [90-92]. The proteolytic function of the 20S proteasome is inhibited by carbonylation or HNE modification [93]. S-glutathionylation, another oxidative modification, enhances proteasome activity at low concentrations and inhibits it at high concentrations. The biphasic effect of S-glutathionylation on cysteine residues potentially acts as a signal transduction mechanism to modulate proteasome activity in response to cellular redox status [90, 94]. These findings suggest that oxidative stress can influence peptide/antigen repertoire regulation by altering proteasomal degradation.
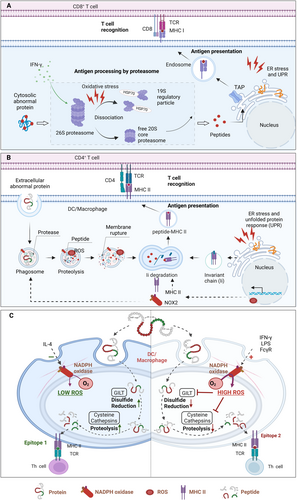
2.2.2 Regulations on Antigen Processing in Vacuolar Pathway
APCs primarily recognize exogenous antigens via pattern recognition receptors and internalize them through various endocytic mechanisms, including pinocytosis, macropinocytosis, phagocytosis, and receptor-mediated endocytosis. Following internalization, receptor-mediated endocytosis, and pinocytosis result in the formation of endosomes including exogenous antigens and phagosome-enclosed particulate antigens, such as bacteria. Similarly, autophagosomes sequestered cellular damaged components and proteins. Endosomes, phagosomes, and autophagosomes subsequently fuse with lysosomes, forming endolysosomes, phagolysosomes, or amphisomes, respectively. These vesicle organelles execute the degradation of antigen proteins into peptides of 10-30 amino acids suitable for MHC I/II binding. ROS produced by NADPH oxidases (NOXs) in these organelles influences the antigen (cross) presentation capacity of APCs by changing in the endo/phagosomal properties.
ROS generated by NOXs tightly controls phagosome maturation, a crucial step in the cross-presentation process [95]. The NOX2 complex, consisting of cytosolic proteins (p47phox, p40phox, and p67phox) and integral membrane proteins (p22phox and gp91phox), is vital for the processing of ingested pathogens within phagocytes. Similar to neutrophils and macrophages, monocyte-derived DCs assemble the NOX2 complex at the nascent phagosome, enabling rapid ROS production upon pathogen encounter [96]. As the phagosome develops, cytochrome b558—a stable heterodimer made up of gp91phox and p22phox—is recruited from the plasma membrane to the phagocytic cup and is internalized along with bacterial antigens [97, 98]. After antigen uptake, NOX2 is removed because the ROS produced by NOX2 leads to the auto-oxidation of cytochrome b558. Subsequently, cytochrome b558 can be sourced from a lysosomal reservoir and transported to the phagosomes to replace the oxidatively damaged components of NOX2 [98, 99]. This replenishment of NOX2 within the phagosome maintains ROS production for several hours following antigen uptake, which is crucial for efficient antigen processing and presentation [100, 101].
A moderate and sustained level of ROS enhances the antigen processing and presentation capabilities of APCs. Following pathogen encounter, APCs induce ROS generation within minutes by rapidly upregulating NOXs in response to innate immune signaling, such as Toll-like receptor (TLR) signaling in DCs [100, 102]. Neutrophils rapidly produce high levels of ROS within phagosomes (mM/s) after engulfing pathogens. This “oxidative burst” kills the pathogen, but often also kills the neutrophil due to the extensive oxidative damage [103]. Optimal antigen processing requires a delicate redox balance, as excessive oxidative stress leads to excessive degradation of the antigen proteins and impaired antigen-presenting capacity. Therefore, unlike neutrophils, DCs exhibit a much lower, but sustained, rate of ROS production (approximately 0.5 mM/s for at least 10 h). This prolonged, low-level ROS production is essential for DC function, supporting MHC I antigen presentation to CTLs and influencing the range of epitopes presented via MHC II to helper T cells [100]. Moderate NOX2-derived ROS production induces the alkalinization of the endo/phagosomal lumen, thereby inactivating lysosomal proteases with acidic pH optima and inhibiting excessive degradation of antigen peptides [104, 105]. In contrast, NOX2-deficient DCs exhibit lower endosomal and phagosomal pH, resulting in the destruction of ingested antigen and impaired cross-presentation [104]. NOX2-derived ROS also promotes antigen translocation from endo/phagosomal lumen to the cytosol for cross-presentation through mechanisms involving phagosomal alkalinization, lipid peroxidation, and membrane disruption. Furthermore, this controlled ROS generation may contribute to antigen preservation during DC migration from the site of infection or tumor to draining lymph nodes, thereby optimizing T cell antigen presentation [106-108]. NOX2 deficiency in bone marrow-derived macrophages (BMDMs) decreases processing and presentation of the myelin oligodendrocyte glycoprotein (MOG) I-Ab-dominant peptide, leading to reduced CD4+ T cell responses [109]. Collectively, these findings demonstrate a selective role for ROS in antigen processing and cross-presentation.
The lysosomal redox microenvironment is a critical determinant of antigen processing and presentation. The degradation activity of lysosomes is required for antigen processing and presentation, mediating the proteolytic processing of intracellular materials via autophagy pathway and extracellular substances through endocytosis, phagocytosis, and micropinocytosis [110]. Oxidative stress compromises lysosomal activity, which in turn disrupts DC endocytic compartments and MHC class II epitope production [111, 112]. The dysfunction is partly attributed to oxidative stress-mediated inhibition of cathepsins B, L, and S, crucial lysosomal proteases for antigen processing in phagocytic cells [113, 114]. Cathepsin expression is tightly coupled to cellular redox status; for instance, reducing cellular antioxidant capacity by depleting nuclear factor erythroid 2-related factor 2 (Nrf2) elevates the transcription of cathepsin S and MHC II [115, 116]. Furthermore, enzymatic activity of cathepsins is sensitive to redox balance. NOX2-generated superoxide shifts the phagolysosomal lumen towards an oxidative environment, inhibiting the activity of cathepsins S and L, which require reducing conditions for optimal function [117]. Conversely, the overactivation of cathepsin due to NOX2 deficiency leads to excessive peptide cleavage and impaired production of antigen peptides for presentation in MHC I and II [109, 118]. The IFN-γ-inducible lysosomal thiol reductase (GILT) counteracts the oxidative damage to cysteine cathepsins by maintaining redox homeostasis within the phagolysosomal compartment [119].
ROS can also directly oxidize antigens within endo/phagosomal lumens. The oxidization of antigens, such as disulfide bond formation, potentially alters their susceptibility to proteolytic degradation. Reduction of disulfide bond in antigenic proteins is another key step in antigen processing regulated by the redox potential within the phagosome. Lysosomal reduction of disulfide bonds is essential for unfolding protein antigens, modulating proteolytic processing, and exposing T cell epitopes containing free cysteine residues [120]. Phagosomal NOX2-derived ROS would constrain the denaturation of antigens with disulfide linkages, restricting the presentation of certain antigen proteins [120]. The lack of ROS due to mutation of Ncf1, a component of the NOX2 complex, alters T cell responses to peptides containing cysteine [121]. Similarly, the reduced production of ROS in NCF4-mutated APCs inhibited the cross-linking of cysteine residues within the antigen peptides, thereby facilitating their presentation to T cells [122]. Effective cross-presentation of disulfide-bonded antigens requires GILT activity, which reduces disulfide bonds, promoting antigen unfolding in the acidic APC phagolysosome [123]. These unfolded antigens then translocate to the cytoplasm, potentially via the chaperone heat shock protein 90 (HSP90) [124]. The alkaline, oxidizing environment generated by ROS inhibits GILT activity, thereby impairing cross-presentation of disulfide-rich antigens. GILT expression is increased in macrophages of NOX2-deficient (Ncf1m1J/m1J mutant) mice, promoting the reductive processing of cysteinylated peptides [121]. On the other hand, the lack of GILT in class II+ melanomas impairs the processing of cysteinylated peptides, while its expression in malignancies may enable T cell recognition of oxidized peptides [125]. Collectively, ROS-mediated endo/phagosomal alkalinization, altered protease activity, and antigen structural modification collectively modulate antigen presentation.
Redox-regulated autophagy is essential for the thymic self-antigen presentation in CD4+ T cell tolerance, as well as MHC I cross-presentation of viral, tumor antigens in APCs. Thymic stromal cells (TSCs) exhibit high H2O2 levels due to low catalase expression [126, 127]. High constitutive autophagy enables the take up and degradation of intracellular proteins, facilitating endogenous MHC class II loading. This autophagic-lysosomal pathway significantly expands the range of self-peptides presented to T cells, promoting positive selection of a more diverse T cell repertoire [128]. Autophagy inactivation in cortical thymic epithelial cells disrupts MHC class II processing, substantially reducing CD4+ T cell receptor repertoire diversity [129, 130]. Transgenic mice expressing mitochondria-targeted human catalase showed reduced mitochondrial H2O2 levels, decreased autophagy, and impaired thymocyte clonal deletion; these defects were partially rescued by Becn1F121A/F121A expression. These findings suggest that the redox status of TSCs regulates autophagic antigen processing, playing a critical role in central T cell tolerance [126]. Autophagy has also been involved in the cross-presentation of exogenous antigens by MHC I to CD8+ T cells and a route for MHC II mediated presentation of cytoplasmic and nuclear antigens to CD4+ T cells [131]. ROS can initiate autophagy by modulating autophagy-related genes (e.g., ATG4), transcription factors (e.g., HIF-1α, TFEB), and signaling pathways (e.g., mTOR, AMPK, MAPK) [132]. The formation of autophagosomes serve as antigen sources for DCs, promoting MHC class I-mediated cross-presentation of antigens to specific CD8+ T cells [133].
Additionally, autophagy is involved in removing oxidatively damaged NOX2 from the phagosomal membrane. Ubiquitinated, oxidatively damaged gp91phox is recognized by p62, leading to LC3 recruitment and subsequent encapsulation within LC3-positive autophagosomes. This process shares mechanistic similarities with LC3-associated phagocytosis (LAP), a distinct immune regulatory pathway. NOX2-produced ROS promotes LC3 recruitment, a process inhibited by α-tocopherol, potentially through ROS-mediated oxidative inactivation of ATG4, enhancing LC3 lipidation. Then, NOX2-derived ROS stabilize LAPosome formation, prolonging MHC class II antigen presentation [134]. This ROS-dependent modulation of autophagy fine-tunes antigen presentation, as exemplified by the presentation of citrullinated peptides to CD4+ T cells and the stabilization of phagosomes for sustained MHC class II presentation in human macrophages [135].
Collectively, oxidative stress modulates antigen processing by influencing the function of intracellular vesicular protein degradation pathways, including phagosomal, lysosomal, and autophagic processes.
3 Effects of Oxidative Stress on Antigen Presentation
Oxidative stress significantly impacts antigen presentation, the crucial first step in T cell activation. This process involves TCR engagement with peptide-MHC complexes (pMHC) on APCs, providing the initial activation signal. Full T cell activation, however, requires additional costimulatory signals and subsequent cytokine-dependent proliferation and differentiation. Oxidative stress regulates the expression of MHC molecules, costimulatory molecules, and cytokines, thereby modulating this intricate signaling cascade for complete T cell activation.
3.1 Modulating Antigen Presentation by Oxidative Stress
Oxidative stress exerts a complex regulatory effect on antigen presentation, influencing MHC expression, pMHC formation and transport, and ultimately, the efficiency and specificity of T cell activation.
3.1.1 Regulation on the Expression and Stability of Major Histocompatibility Complex Molecules
When it comes to antigen presentation, we cannot avoid MHC that is mainly divided into MHC I and MHC II with different coding genes and structures [136]. MHC I exists in nucleated cells and presents a peptide ranging in length from 8 to 11 amino acids to the TCR of CD8+ T cells. MHC II that exists in APCs and hematopoietic cells binds relatively longer peptides (10–16 amino acids), mainly activating CD4+ Th cells [137-139]. Oxidative stress increases the expression of MHC molecules, which are central to antigen presentation. ROS produced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) enhances MHC I expression and triggers autoimmune responses, leaving dopaminergic neurons susceptible to immune cell attack and degeneration [140]. In neutrophils, the activity of NOXs, a ROS-generating complex, correlates with MHC II molecule expression [109]. Moreover, specific cytokines such as IFN-γ and GM-CSF increase MHC II expression in human neutrophils, a process that correlates with higher intracellular ROS levels [141-143]. In patients with chronic granulomatous disease (CGD), neutrophils that lack NOX activity fail to respond to cytokines or express MHC II, leading to immune dysfunction [144].
Oxidative stress affects the appropriate positioning of the cell membrane and the stability of MHC complexes. Although the stability of MHC I molecules largely determines the rate of loss after assembly and export from ER, all forms of assembled MHC I molecules on the cell membrane ultimately undergo translocation to late endosomes or lysosomes for digestion, a process referred to as MHC I molecule recycling. This recycling process depends on the activity of several GTPases, such as Rab5 (an early endosomal marker), Rab7 (a late endosomal marker), Rab11a (a marker for recycling endosomes), Rab22a, and Arf6. The recycled MHC I molecules are then reassembled with endogenous and exogenous antigens in endosomes for presentation, thus completing a cycle [145]. The internalization and subsequent degradation of membrane proteins are orchestrated by a cascade of oxidative stress signaling events triggered by ischemia/reperfusion. Specifically, superoxide radicals regulate the colocalization of target proteins with Rab5 and Rab7, but not Rab11 [146]. It is believed that oxidative stress can mediate the internalization and degradation of MHC complexes on the surface, although further studies are needed to fully characterize it.
3.1.2 Modulating the Formation and Transportation of Peptide-Major Histocompatibility Complexes
The transportation process of pMHC complexes from the ER to the plasma membrane is influenced by oxidative stress. Once pMHC complexes are presented on the membrane, they can be identified by the TCRs, eliciting an immune response. Oxidative stress impacts the transportation of mature pMHC I complexes from the ER to the Golgi apparatus, a process that requires the proper function of the ER and Golgi apparatus [147]. For example, the activating transcription factor 6α (ATF6α) was activated under the influence of alcohol, accelerating oxidative stress in the ER and causing Golgi apparatus fragmentation. The disruption of Golgi inhibited the presentation of hepatitis B virus (HBV) pMHC I complex on the cellular surface of HBV+ hepatocytes, thereby affecting CTL recognition and leading to the persistence of HBV infection in hepatocytes [148]. The lipid redox homeostasis may also play a role in the transport of pMHC complexes. The deficiency of glutathione peroxidase 4 (GPX4), which is crucial for sustaining cellular redox equilibrium. This accumulation of lipid peroxides disrupts the conveyance of cargo from the ER to the Golgi apparatus [149, 150]. Furthermore, oxidative lipids activate the ER stress response factor X-box binding protein 1 (XBP1), inducing lipid synthesis and abnormal lipid droplet aggregation. Lipid bodies (LB) containing electrophilic lipids with oxidative truncation, but not in control LBs with typical lipid profiles, form covalent bonds with the chaperone HSP70. This isolation of HSP70 hinders the positioning of pMHC on the plasma membrane, resulting in the retention of pMHC within late endosomes/lysosomes in DCs of tumor-bearing hosts [4, 151, 152]. Therefore, the defect in trafficking of pMHC complexes to the cell surface is largely associated with impaired cross-presentation in DCs.
Oxidative stress can also modulate the accurate localization of pMHC by inducing alterations in the structural integrity and functional properties of the cell membrane. It has been demonstrated that a subset of MHC II molecules is situated within detergent-resistant membrane microdomains, commonly known as lipid rafts, on the surface of APCs [153]. The concentration of MHC II molecules within microdomains facilitates efficient antigen presentation even at low ligand concentrations [154, 155]. It has also found that oxidative stress can mediate changes in the physical and biochemical properties of lipid rafts [156]. Palmitoylation, membrane/lipid raft and n-3 polyunsaturated fatty acid (PUFA) are vulnerable to the corruptive effects of oxidative and nitrosative stress [157]. Oxidative stress repositions membrane receptors into lipid rafts within the plasma membrane by modulating receptor exocytosis [158]. It seems that oxidative stress prompts the recruitment process by aggregating soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) within the lipid raft domains, causing vesicles to directly translocate to the plasmalemma rafts [159, 160]. Thus, membrane disruption in reaction to oxidative stress may regulate the proper localization of pMHC complexes, creating a positive feedback loop that exacerbates disease progression and challenges the immune system.
3.2 Impact on Costimulatory Molecules
Oxidative stress influences the antigen-specific immune response by modulating the expression and function of key costimulatory and immune checkpoint molecules. A costimulatory signal (e.g., CD28 with CD80/CD86, CD40 with CD40L, 4-1BB with 4-1BBL) is essential for complete T cell activation following priming by the antigen-specific signal [161]. The absence of costimulation results in activation-induced cell death (AICD) and/or energy, leading to immune tolerance [162]. Immune checkpoint pathways, such as those involving cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed cell death protein-1 (PD-1), exert immunosuppressive effects in contrast to the positive immunoregulatory roles of costimulatory signals [163]. Moderate levels of ROS can enhance immune activation through upregulation of costimulatory molecules, whereas excessive oxidative stress promotes immune evasion by upregulating immune checkpoint molecules, indicating a biphasic role of oxidative stress in immune function [164, 165].
Moderate ROS enhances costimulatory molecules (e.g., CD80/CD86), promoting T cell activation. The accumulation of ROS in the bone marrow (BM) after ovariectomy prompts DCs to enhance the production of CD80, a costimulatory molecule that boosts T cell activation [166]. The ability of DCs to stimulate autologous naive CD4+ T cells is enhanced during parasite infection due to the upregulation of CD80 surface expression caused by heightened ROS produced by xanthine oxidase [167]. Elevated ROS levels correlate with increased CD80 and CD86 mRNA expression in macrophages in a murine model of heart failure with preserved ejection fraction (HFpEF) [168]. Moreover, ROS coordinate immune monitoring of colon preneoplastic lesions by promoting CD80 expression in colon cancer epithelial cells via the JNK/p38 MAPK–STAT3 pathways [169].
Oxidative stress also modulates the expression of immune checkpoint molecules, including PD-L1, which interacts with PD-1 to suppress immune activation [170, 171]. Increased ROS production due to KRAS-G12V activation promotes PD-L1 expression through fibroblast growth factor receptor 1 (FGFR1) pathway, promoting cancer evasion of the immune surveillance [172]. Enhanced PD-L1 expression in tumor-associated macrophages (TAMs) has been consistently observed following exposure to various ROS inducers, such as buthionine sulfoximine and paclitaxel [173]. Multiple mechanisms underlying ROS-mediated PD-L1 regulation have been reported [174], including iron-mediated oxidative stress-induced c-Myc activation in lung cancer [175], ROS-mediated activation of the JAK/STAT3 pathway [176], accelerating expression of the transcription factor NRF2 [170], and nuclear factor κB (NF-κB) signaling activation [173]. Furthermore, solute carrier family 7 member 11 (SLC7A11) inhibition-induced ROS upregulation promotes PD-L1 expression in melanoma cells via the IRF4/EGR1 pathway. The increased PD-L1 were transported by exosome to drive M2 macrophage polarization, resulting anti-PD-1/PD-L1 therapy resistance [177]. ROS regulates PD-L1 protein levels; for example, IL-17A-induced ROS production increases Nrf2 and p62 expression, thus inhibiting autophagic PD-L1 degradation [178]. Therefore, modulating ROS levels offers a potential novel approach to overcoming PD-L1-mediated immunosuppression and enhancing antigen-specific immune responses.
Moderate ROS levels boost T cell activation by increasing costimulatory molecules (like CD80/CD86). However, high ROS levels increase immune checkpoint molecules (like PD-L1), suppressing the immune response and promoting immune evasion. Therefore, controlled ROS induction may enhance antigen-specific signaling and overcome resistance to checkpoint inhibitors such as anti-PD-1.
3.3 Influence on Antigen-Specific Response of Immune Cells
Oxidative stress significantly influences the function of antigen-specific immune cells, including B cells and T cells. By influencing the activation and differentiation of immune cells, oxidative stress affect the efficacy of the immune system in monitoring and combating pathogens and diseased cells.
3.3.1 Antigen-Specific T Cells
The local concentration of ROS rises quickly during T-cell activation as a byproduct of oxidative metabolism due to the increased need for energy metabolism [179]. The engagement of pMHC complex on APC with TCRs initiates T cell activation, driving adaptive immune responses. Upon antigenic stimulation, quiescent naive T cells undergo metabolic reprogramming to meet the heightened energy demands of activation. Glycolysis, amino acid metabolism and fatty acid synthesis were upregulated to promote cell proliferation and cytokine secretion in effector CD8+ T cells, whereas the tricarboxylic acid cycle (TCA) and fatty acid oxidation (FAO) were essential for the lifespan maintenance of memory CD8+ T cells [180]. CD4+ T-cell development primarily relies on aerobic glycolysis as its main energy source, whereas FAO is the primary energy source for regulatory T (Treg) metabolism. The overactivation of these metabolic pathways is accompanied by the production of ROS, which serve as messenger to initiate the proliferation, activation, and effector functions of antigen-specific T cells.
The cellular redox state is crucial for the antigen-specific response of T cells. The stimulation of CD28 and CD3 receptors, which collaborate with the TCR to activate T cells, induces the generation of mitochondrial ROS by CD3-initiated calcium signaling. The mitochondrial ROS is necessary for the activation of nuclear factor of activated T cells (NFAT) and subsequent expression of IL-2, thereby facilitating the antigen-specific expansion of T cells in vivo [181]. Following TCR and CD28 costimulation, the production of ROS leads to the stabilization and elevation of SUMO-specific protease 3 (SENP3). The accumulation of SENP3 facilitates the deSUMOylation of BTB and CNC homology 1, basic leucine zipper transcription factor 2 (BACH2), which in turn preserved the stability and functionality of Treg cells [182]. Similarly, TCR stimulation-induced ROS promote the cytosolic translocation of SUMO-specific peptidase 7 (SENP7), which mediates the deSUMOylation and degradation of phosphatase and tensin homolog (PTEN), thereby maintaining the metabolic status and antitumor function of CD8+ T cells [183]. Conversely, oxidative stress due to elevated ROS production promotes T cell exhaustion and terminal differentiation, which can be hampered by the neutralization of ROS. Oxidative stress work in tandem with endosome recycling to reorganize the TCR signaling complex at the cell surface [184, 185]. Chronic oxidative stress, driven by diminished antioxidant glutathione (GSH) levels, causes the membrane displacement of linker for activation of T cells (LAT), a key adapter protein in the TCR-mediated signaling pathways. This disruption of TCR-mediated signaling due to a conformational change in redox-sensitive cysteine residues of LAT ultimately reduces T lymphocyte responsiveness [186]. Extracellular/microenvironmental redox state can also determine the activity of T cells. Adoptively transferred T cells, following chronic activation and expansion, exhibit increased vulnerability to cell death within oxidative TMEs [187]. Survival within these oxidative TMEs requires enhanced antioxidant capacity and elevated levels of reduced thiol groups (–SH) on T cell membranes, facilitated by reduced thioredoxin (Trx) and CD4 molecules [188, 189]. Therefore, central memory-like (TCM) T cells demonstrate superior survival compared to effector memory-like (TEM) cells in oxidative TMEs, attributable to their superior antioxidant capacity and higher surface thiol levels. Enhancing surface thiol levels through treatments like N-acetylcysteine (NAC) or rapamycin extends in vivo lifespan and improves tumor suppression in TCR-transduced CD8+ T cells [190]. Importantly, the surface redox status of T cells is primarily dictated by the local oxidative environment rather than by intracellular redox regulation systems. Reducing extracellular ROS improves T cell responsiveness and mitigates immunosuppressive cell death by increasing surface thiol groups [191]. Additionally, hypoxia, prevalent in the TME across various cancer types, intensifies T cell exhaustion through impaired mitochondrial function and consequent ROS accumulation [192]. The persistent production of ROS in the mitochondria under low oxygen conditions is enough to induce a state of exhaustion in CD8+ T lymphocytes. This is partially achieved by increasing the levels of phosphotyrosine signaling and the nuclear location of NFAT, a known transcriptional circuit associated with T cell exhaustion [193]. Therefore, modulating oxidative stress factor could offer a viable approach for regulating T cell function (Figure 4) [183].
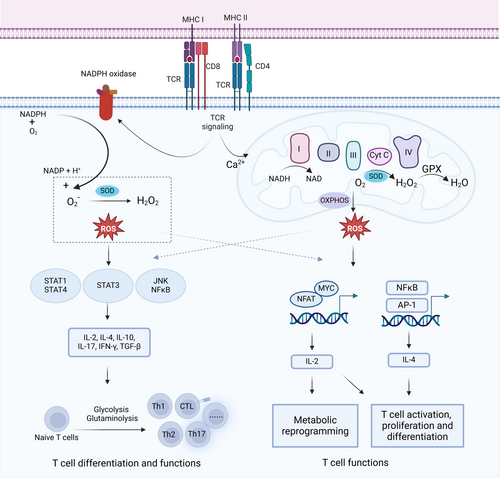
In summary, both intracellular and extracellular redox conditions are critical determinants of T cell activity and function, suggesting avenues for targeted therapeutic intervention.
3.3.2 Antigen-Specific B Cells
Maintaining redox balance is crucial for the survival, antibody secretion, and immune homeostasis of antigen-specific B cells. B cell activation occurs through two primary pathways: T-dependent (TD) antigen stimulation via the B cell receptor (BCR), and T-independent (TI) antigen stimulation via the BCR or TLRs. Following activation, once activated, B cells differentiate into plasma cells (also known as plasmacytes or effector cells), memory B cells, and cytokine-secreting cells. Plasma cells mediate humoral immunity through antibody production, contributing to complement-dependent cytolysis and bactericidal activity, as well as antibody-dependent cell-mediated cytotoxicity (ADCC) via Fc receptor engagement on effector cells such as macrophages and natural killer (NK) cells. Memory B cells, characterized by extended lifespans, retain immunological memory of the initiating antigen, facilitating enhanced secondary immune responses upon subsequent antigen exposure [194-196].
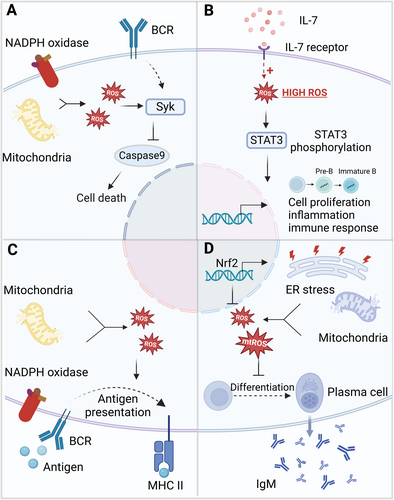
Physiological levels of ROS are crucial for B cell activation and proliferation by enhancing BCR signaling. Ligation of the BCR in splenic B cells initiates biphasic ROS production: an initial, short-lived burst generated by NOX2, followed by a sustained increase mediated by NOX3 or mitochondrial sources. This later phase of ROS generation is vital for enhancing BCR signaling and facilitating B cell activation and growth [197, 198]. When B cells are exposed to H2O2, the redox factor APE/Ref- [1] rapidly translocate to the nucleus, increasing the DNA-binding activity of Pax5a, a transcription factor that regulates early B cell development and maturation [199]. Investigations into MHC II-mediated activation of resting B cells have highlighted the role of ROS in pro-survival signaling pathways, such as RAS/ERK/AP-1, PI3K/AKT/mTOR, ERK/JNK/P38 MAPK, and PKC/NF-κB [200, 201]. Conversely, oxidative stress induced by antigens can lead to apoptosis in regulatory B cells (Bregs) [202]. Treatment with hydrogen peroxide activates spleen tyrosine kinase (Syk), which can trigger pro-survival Akt pathways, inhibiting caspase-9 and offering protection against apoptosis induced by oxidative stress. In contrast, activation of PLC-γ2 through Syk promotes apoptosis in response to oxidative stress. The interplay between these proapoptotic and pro-survival pathways ultimately determines the fate of B cells under different levels of oxidative stress [203].
The differentiation of B cells is significantly influenced by the homeostatic levels of ROS. Redox alterations have been shown to play a role in the physiological activation and terminal differentiation of B cells in peripheral tissues [204]. A modest increase in ROS levels, induced by 1 μM CpGC, enhances the frequency of IL-10+ Breg cells [205]. ROS may also facilitate the differentiation of B cells into plasma cells and boost antibody production. In mice experiencing chronic oxidative stress, those lacking the antioxidant protein Tumor Protein 53-Induced Nuclear Protein 1 (TP53INP1) demonstrate an increase in plasma cell generation and greater antibody secretion [206]. Conversely, under conditions of pathological severe oxidative stress, the activation and differentiation of B lymphocytes are often suppressed [207]. Higher ROS concentrations significantly decrease the frequencies of IL-10+ Breg cells, without affecting the overall frequencies of transitional (CD24hiCD38hi), mature (CD24intCD38int), memory (CD24+CD38lo) B cells, or plasmablasts (CD24lo/−CD38hi Blimp1+). Reducing ROS levels to “physiological” ranges through the action of Trx, an antioxidant enzyme, promotes the induction of Breg cells while suppressing effector B cells. The inactivation of Trx in B cells leads to mitochondrial outer membrane depolarization and elevated ROS levels, which impair IL-10 expression and suppressive functionality, resulting in an increase in B cells that produce pro-inflammatory cytokines. Trx deficiency contributes to Breg cell dysfunction in patients with SLE, while exogenous stimulation with Trx can restore Breg cells and mitochondrial membrane polarization in SLE to levels typical of healthy B cells [205].
Oxidative stress influences the antibody synthesis and storage functions of plasma cells, thereby impacting the humoral immune response. In a group of patients with non-alcoholic fatty liver disease (NAFLD), there was an observed increase in IgG antibody levels targeting oxidative damage molecules, referred to as oxidative stress-derived epitopes (OSE) [208]. Notably, the elevated levels of anti-OSE antibodies in these patients were positively associated with liver inflammation, suggesting an antibody-mediated response to oxidative stress linked to disease progression [209]. Treatment with Ginsenoside Rb1, an antioxidant compound, significantly alleviated immune damage caused by deoxynivalenol (DON) in mice, as indicated by a substantial rise in serum levels of IgA, IgG, and IgM [210, 211].
In general, physiological ROS produced by mitochondria or NOXs can boost the function and activity of APCs, whereas pathophysiological oxidative stress tends to suppress the antigen-specific immune response (Figure 5). Gaining a thorough understanding of how oxidative stress affects the antigen-specific immune responses of T cells and B cells could have important implications for the development of new immunotherapeutic strategies.
4 The Pathophysiologic Role of Oxidative Stress-Regulated Immune Responses in Diseases
Oxidative stress in tumor cells raises the antigen burden and feeds the cancer immunity cycle. However, oxidative stress in immune cells propels pathogenesis by altering antigen-specific immune responses. On the one hand, moderate ROS levels can enhance antigen presentation and boost the ability of immune cells to eliminate cancer cells and infections. Excessive oxidative stress, on the other hand, can impair immune cell function, increasing the release of inflammatory mediators and exacerbating inflammatory reactions in organs and tissues like the liver and skin. In this section, we will discuss the interplay of oxidative stress and antigen presentation in the context of cancer immunology, infectious illnesses, and autoimmune disorders.
4.1 Oxidative Stress in Cancer: Dual Immune Activation and Suppression
The redox balance within cancer cells and the TME profoundly influences antitumor immune responses. ROS can enhance antitumor immunity through three mechanisms: promoting antigen generation, activating antigen presentation pathways, and stimulating immune cell functions. Oxidative stress-induced DNA damage and protein oxidative modifications produce TSAs or neoantigens, which activate T cells to recognize and attack tumors. At moderate levels, ROS enhance the antigen presentation activity of APCs—such as DCs and macrophages, and boost the antitumor activity of T cells and B cells, strengthening the cancer-immune cycle. However, excessive ROS inhibits antitumor immunity by causing dysfunction in antigen presentation, immune cell exhaustion and apoptosis, and the formation of an immunosuppressive microenvironment.
Physiologic ROS levels promote antitumor immunity by enhancing the cross-presentation capacity of DCs. DCs internalize tumor-derived microparticles (T-MPs) into lysosomes, where increased NOX2 leads to elevated ROS production and pH. This higher pH facilitates the generation and presentation of tumor antigenic peptides to CD8⁺ T cells. Simultaneously, elevated ROS levels upregulate CD80 and CD86 expression through lysosomal Ca²⁺ pathways and downstream transcription factor EB (TFEB), providing costimulatory signals that enhance T cell activation [212]. As a metabolic regulator, oxidative stress promotes STING-mediated DC antitumor immune responses. DC-generated ROS facilitates the interaction between SENP3 and interferon-induced protein 204 (IFI204), leading to IFI204 deSUMOylation. This activates the stimulator of interferon genes (STING) pathway, amplifying the type I interferon response and strengthening DCs' ability to present antigens and activate T cells [213]. Unlike conventional DC1s, plasmacytoid DCs (pDCs) rely on mitochondrial ROS (mtROS) rather than NOX2 for cross-presentation. Studies using mCAT transgenic mice, which express human catalase in mitochondria, show that reduced mtROS in pDCs significantly impairs CD8⁺ T cell responses, highlighting mtROS's critical role in pDC cross-presentation [95]. The ROS-responsive factor Nrf2 plays a key role in CD8⁺ T and chimeric antigen receptor (CAR) T cell antitumor responses within the ROS-rich tumor microenvironment. T cells lacking Nrf2 show enhanced antitumor responses due to ROS resistance and sustained effector functions. In human CAR-T cells, Nrf2 knockdown improves intratumoral survival and function in solid tumor xenograft models, effectively controlling tumor growth. Consequently, tumor growth in Nrf2−/− mice is significantly suppressed and can be reversed by T cell depletion [214]. However, NRF2 in cancer cells inhibits MHC-I complex expression and NK cell-activating ligands. This reduced antigen presentation enables cancer cells to evade immune surveillance and progress to malignant tumors [215].
The oxidative stress within the TME fosters an immunosuppressive milieu that facilitates immune evasion and resistance to immunotherapy. Tumor cell metabolic activity and dysregulated antioxidant defenses contribute to a ROS-rich TME. High ROS levels inhibit antigen processing, blocking antigen cross-presentation, and the effectiveness of CD8⁺ T cells. Ethanol-induced oxidative stress impairs antigen-processing enzymes, such as 20S and 26S proteasomes, limiting antigenic peptide processing [216]. In an ovarian cancer model, DCs produce oxidized lipids upon ROS activation, which block the antigen cross-presentation by sequestering partner HSP70 and preventing the translocation of pMHC complexes to the surface of mouse cells, thereby inhibiting the occurrence of immune responses [151]. Specifically, peroxynitrite (PNT), a highly reactive oxidant synthesized by myeloid cells within the TME, hinders the proteasome activity of tumor cells. This impaired antigen processing reduces the presentation of PNT modified peptides (PNT-S peptides) on MHC I molecules, characterized by increased peptide dissociation rates. Consequently, CD8⁺ T cell antitumor responses are diminished, negatively impacting immunotherapy efficacy [217, 218]. Oxidative stress, particularly under nutrient restriction, promotes CAR T cell exhaustion and apoptosis. Decreasing levels of ROS by isocitrate dehydrogenase 2 (IDH2) ablation or NAC, a cell-permeable antioxidant, mitigate CAR T cell exhaustion and apoptosis, improve the anticancer effect of CAR T cells [219]. T cell–targeting fusogenic liposomes equipped with 2,2,6,6-tetramethylpiperidine, which protected T cells from oxidation-induced activity loss and efficiently inhibited tumor growth in multiple mouse tumor models [191]. Finally, ROS promotes the polarization of macrophages toward an M2 phenotype, which drives tumor progression in colorectal cancer through increased angiogenesis, tissue remodeling, and immune tolerance [220, 221].
The ability to modulate redox balance offers a promising strategy for cancer therapy. A deeper understanding of how oxidative stress and immune regulation interact will be essential for developing more effective cancer immunotherapies.
4.2 Oxidative Stress in Infection-Related Cancers: Immune Defense and Oncogenesis
Oxidative stress plays a complex and multifaceted role in infectious diseases caused by pathogenic microorganisms such as bacteria, viruses, fungi, or parasites. The ROS generated by phagocytes during respiratory bursts via NOXs are crucial for pathogen killing and aid the host antigen-specific immune response by enhancing antigen processing and presentation. Furthermore, ROS triggered by bacterial lipopolysaccharides (LPS), such as those from Escherichia coli (E. coli) and Salmonella, enhances antigen presentation and pathogen clearance [222-224]. This occurs because oxidative molecules (like H₂O₂) promote maturation of APCs, increasing expression of MHC II and costimulatory molecules (CD80, CD86) via NF-κB signaling [225]. This enhanced immune response is observed in both Gram-negative bacterial infections and Mycobacterium tuberculosis, where ROS production activates macrophages and upregulates costimulatory molecules (CD80, CD86, and CD40), improving antigen presentation to T cells and facilitating pathogen elimination. The increased expression of MHC molecules and costimulatory molecules, particularly CD40, on APCs strengthens T cell interactions and improves antigen presentation efficiency [70, 226, 227]. Similarly, ROS are essential for effective antiviral immunity, especially against respiratory viruses like influenza A and SARS-CoV-2. These viral infections trigger a respiratory burst, increasing ROS levels and activating CD4+ and CD8+ T cells through direct interactions or cytokine signaling [228-230]. This ROS-dependent T cell activation is demonstrated by reduced T cell expansion following treatment with the antioxidant Mn(III) tetrakis (4-benzoic acid) porphyrin chloride (MnTBAP) [231].
Oxidative stress-mediated immune evasion plays a crucial role in viral-induced cancer development. This is particularly evident in cancers like hepatocellular carcinoma (HCC) and cervical cancer. In HCC, Hepatitis B virus (HBV) infection elevates ROS levels, which silences the SOCS3 gene and activates the IL-6/STAT3 pathway, promoting cancer development [232]. Additionally, HBV-related HCC patients with low antioxidant levels show poorer prognosis [233]. In cervical cancer, human papillomavirus (HPV) 16/18 E7 protein generates ROS, which modifies lactate dehydrogenase A (LDHA) and increases nuclear α-hydroxybutyrate (α-HB). This α-HB protects cancer cells from oxidative damage through Wnt signaling pathway activation via epigenetic modifications [234]. Excessive oxidative stress in hepatitis C virus (HCV) infected cells disrupts immune responses by impairing proteasomal function, reducing MHC I presentation, and altering APC function. This hinders pathogen clearance and can worsen disease severity. In HCV-positive hepatocytes exposed to ethanol, oxidative stress disrupts proteasomal composition, compromising viral antigen processing. This leads to reduced presentation of viral peptides on the cell surface by pMHC-I [235]. While CTLs typically target hepatocytes, the limited antigen peptides from impaired proteasomal activity reduce CTL recognition, potentially allowing HCV to persist in hepatocytes [236]. HPV-related cancers provide another example of oxidative stress disrupting immune responses. HPV-induced ROS accumulation facilitates HPV16 DNA integration into human keratinocytes, promoting cancer development [237, 238]. Oxidative stress weakens NK cell cytotoxicity by reducing perforin and granzyme B expression, diminishing their ability to eliminate HPV-infected cells [239]. Furthermore, ROS-induced DC dysfunction reduces HPV antigen presentation efficiency and creates a Th1/Th2 immune imbalance that favors Th2-type responses (primarily IL-4 and IL-10), suppressing cellular immune responses [240].
Collectively, ROS play a crucial role in pathogen clearance by enhancing APC maturation and antigen presentation. However, in viral-related cancers, virus-induced ROS contributes to immune evasion through multiple mechanisms: it impairs antigen processing and presentation, reduces NK cell activity, and disrupts the Th1/Th2 balance. These findings suggest that modulating redox balance could improve cancer immunotherapy outcomes.
4.3 Oxidative Stress in Autoimmune Disorders: From Epitope Spreading to Immune Tolerance Breakdown
Autoimmune diseases are characterized by the immune system's inappropriate and excessive attacks on the body's own cells. These responses typically involve abnormal presentation of self-antigens, loss of immune tolerance, and the production of autoantibodies or autoreactive T cells that target self-antigens, resulting in detrimental effects on healthy tissues. Under normal physiological conditions, self-tolerance is preserved by removing self-reactive lymphocytes in the thymus during immune development and by inducing energy in self-reactive T cells in the peripheral tissues. When this self-tolerance is disrupted, the immune response can generalize and extend to various epitopes derived from the whole complex containing the original antigenic epitopes or other structural/functional proteins, causing these epitopes to be unrecognized as self by the immune system. The aberrant presentation of these self-antigens leads to the development of systemic autoimmune diseases such as SLE, RA, and autoimmune hemolytic anemia, as well as tissue-specific autoimmune diseases, including diabetes mellitus, ulcerative colitis, Graves' disease, and myasthenia gravis. Oxidative stress-mediated oxidative PTMs cause the formation of neoepitopes and gives rise to autoantibodies in autoimmune disease, providing new insights for uncovering the mechanisms underlying these disorders.
Oxidative stress results in a loss of tolerance and epitope spreading within antigen-specific autoimmune responses. This epitope spreading can lead to the diversification and amplification of autoimmunity in an individual. Several studies on the immunization of non-autoimmune mice with self-peptides suggest that the wide range of B and T cell responses seen in SLE could stem from a single protein or even a particular cryptic self-epitope. A notable example is the modified Ro RNP complex, composed of a 60-kDa protein noncovalently associated with human cytoplasmic RNA, which is targeted by antibodies in 25%–40% of lupus patients. The modification of the lupus-associated 60 kDa Ro protein by HNE, a common reactive lipid oxidation product, significantly enhance its antigenicity and promoting epitope spreading to nuclear antigens such as La, double-stranded DNA, and snRNP [45, 241]. Another instance of oxidative PTMs leading to the breakdown of immune tolerance involves the complement component 1, q subcomponent (C1q), which features a collagen-like domain. Antibodies against C1q are recognized as clinically valuable for diagnosing nephritis in SLE. Recently, it has been shown that the oxidative modification of C1q enhances its antigenicity in an animal model and when analyzing serum from SLE patients [242]. Additionally, NOX-derived intracellular ROS in macrophages play a critical role in antigen presentation related to autoimmune diseases. The single-nucleotide polymorphism (SNP) rs201802880 in the human neutrophil cytosolic factor 1 (NCF1) gene, which encodes an essential component of the inducible NOX2 complex, is associated with autoimmune diseases like SLE [243]. A deficiency in NCF4 leads to reduced intracellular ROS production, impairing macrophages' ability to process and present antigenic peptides. Specifically, macrophages lacking NCF4 are unable to effectively oxidize and cross-link cysteine-containing antigenic peptides (such as GPI [244-258]), making these peptides more prone to processing and presentation to T cells. This autoantigen presentation subsequently activates autoreactive T cells and triggers arthritis [259, 260]. These findings indicate that oxidative stress plays a vital role in antigen-specific autoimmune responses by regulating immune tolerance and promoting epitope spreading.
Increased levels of free radicals can induce modifications to biomolecules, resulting in the production of neo-epitopes that sustain antigen-driven autoimmune responses. For example, the conversion of aspartic acid to isoaspartate derivatives or arginine to citrulline leads to the generation of autoantibodies in RA models. These autoantibodies often cross-react with native antigens, perpetuating the autoimmune response [261]. Similarly, isoaspartyl modifications of the lupus autoantigen Sm snRNP enhance lupus autoimmunity by increasing interaction with autoantibodies in SLE patients [262]. Furthermore, oxidative modifications of amino acids by reactive nitrogen species (RNS) and ROS increase the antigenicity of both DNA and immunoglobulin G (IgG), leading to the production of autoantibodies with higher affinity ligands [263]. Elevated levels of autoantibodies against oxidized low-density lipoprotein (LDL) have been observed in both SLE and RA patients compared to healthy controls. Additionally, RA patients with cardiovascular complications show increased autoantibodies targeting nitrated LDL, which is more easily taken up by macrophages than native or oxidized LDL. The clearance of oxidized or nitrated LDL via scavenger receptors, such as CD36 on macrophages, may contribute to the higher incidence of cardiovascular issues commonly seen in RA patients [264]. In addition to its effect on modified proteins, oxidative stress also influences the production of autoantibodies against other macromolecules, like DNA. Oxidized DNA antigens are more easily recognized by antibodies present in the serum of SLE patients compared to native DNA [265].
Oxidative stress also contributes to the autoimmune-mediated destruction and dysfunction of pancreatic β-cells in insulin-dependent diabetes mellitus. The autoimmune etiology of T1D is well-defined, with CD4+ and CD8+ T cells capable of recognizing various β-cell-derived antigenic epitopes, like oxPTM-insulin [266]. In individuals with diabetes, hyperglycemia and insulin resistance lead to oxidative stress, resulting from both elevated ROS production and a weakened antioxidant defense system [267]. Oxidative processes lead to specific modifications to insulins, resulting in the generation of oxPTM-insulin neoantigenic peptides with a higher ability to elicit T cell responses [56]. In addition to nonenzymatic processes implicated in neoepitope generation, oxidative stress also leads to enzymatic modifications of specific residues, thereby increasing the immunogenicity of β-cell peptides [268]. Oxidative or cytokine stress enhances the activity of tissue transglutaminase 2 (TTG2) in β-cells, which results in the production of deamidated neoepitopes. Deamidated neoepitopes from insulin, zinc transporter 8 (ZnT8), insulinoma-associated antigen 2 (IA-2), and glutamic acid decarboxylase 65 (GAD65) are more efficiently presented by disease-susceptible HLA-DQ proteins in APCs [268-270].
In conclusion, abnormal antigen presentation triggered by oxidative stress may play a key role in the development of autoimmune diseases. Further research is needed to clarify the exact causal relationship between these two processes.
5 Potential Therapeutic Strategies and Implications From Redox Regulation of Antigen-Specific Immune Response
An understanding of how oxidative stress affects immune responses has led to various strategies being investigated to modulate redox processes as potential therapeutic methods to enhance immunotherapy (Figure 6). This section explores ways to adjust oxidative stress to improve antigen presentation and immune responses, and summarizes the corresponding therapeutic strategies in Table 2.
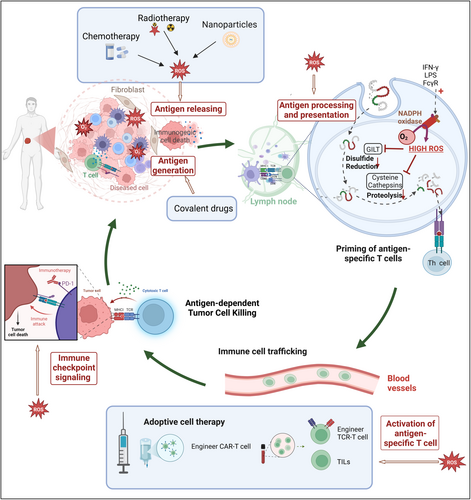
5.1 Covalent Modification Induces Neoantigen Generation and Immune Responses
Covalent drugs can induce covalent modifications in abnormal proteins, leading to the production of neoantigens and enhancing T cell immune responses. One of the antigens presented by MHC is a relatively unique hapten that possesses immune reactivity but lacks immunogenicity, such as polysaccharides, lipids, and certain drugs. However, a hapten can transform into a complete antigen when it binds to a carrier protein. This hapten-protein complex is recognized as a foreign entity and is presented by MHC on APCs. Following this principle, numerous covalent drugs have been developed to modify specific “undruggable” proteins, facilitating their presentation to T cells and B cells and triggering an adaptive immune response.
The notable example of covalent drugs is targeting the KRAS mutation, a prevalent genetic alteration in the initial identified oncogene RAS in humans. The G12C mutation is a common genetic alteration in the KRAS gene, where the amino acid glycine is substituted with cysteine. This mutation is known to contribute to the development of tumors [271, 272]. Targeted covalent inhibitors (TCI), like ARS1620, permanently alter the mutant cysteine in KRAS-G12C and produce changed peptides that can be processed and displayed on the cell surface by MHC I. This leads to the creation of novel hapten epitopes, which stimulate T cell responses and activate cytotoxic T cells [273-275]. Similar covalent inhibitors like sotorasib (AMG510) [276], adagrasib (MRTX849) [277-279], JNJ-74699157 [280], LY3499446 [281], have been proven to react with cysteine residue and drive tumor regression. Building on this principle, a novel class of molecules can target mutated tumor suppressor proteins, covalently modifying hotspot residues to generate neoantigens and elicit specific immune responses such as p53 Y220C and p53 R273C [282, 283]. In recent times, many covalent drugs have been developed for the treatment of cancer or HCV [284, 285]. By incorporating a bio-reactive amino acid-fluorosulfate-l-tyrosine (FSY) into human PD-1, the resulting covalent PD-1 (FSY) significantly augments antitumor activity in mice [286].
Furthermore, the direct covalent interaction between drugs and HLA can alter the peptide repertoire presented by HLA, thereby inducing T cell proliferation. It has been demonstrated that abacavir, covalently bound to amino acids, binds to HLA-B*57:01 and triggers a hypersensitivity reaction [287, 288]. Therefore, haptens that selectively modify mutant oncoproteins, rather than merely acting as pharmacological inhibitors, could considerably broaden the spectrum of tumor-specific neoantigens available for therapeutic targeting. By selectively covalently modifying specific amino acid residues on receptors or enzymes, these drugs offer enhanced biochemical potency and selectivity [289]. These tumor-specific PTMs involve the covalent alkylation of mutated cysteine residues on oncoproteins by drugs, creating novel neoantigens that immunotherapy can effectively target and providing a promising solution for drug resistance.
5.2 Reactive Oxygen Species Formation and Elimination in Immune Response
The generation of ROS such as superoxide anion (•O2−), hydrogen peroxide (H2O2), hydroxyl radical (•OH), and singlet oxygen (1O2) in immune cells occurs through various pathways [290]. NOXs, especially within macrophages and neutrophils, are pivotal enzymes that convert oxygen into superoxide anion, the primary form of ROS [291]. Mitochondria also generate ROS (•O2− and H2O2) during electron transport [292]; however, mitochondrial dysfunctions, like mtDNA mutations and reduced membrane potential, can lead to increased ROS production [293]. Moreover, other enzymes, including cyclooxygenases (COXs), lipoxygenases (LOXs), and xanthine oxidase, further contribute to ROS generation [294]. To combat ROS, cells employ antioxidant enzymes such as superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPx), along with antioxidants like GSH and vitamins C and E [295]. Given their short lifespan (Table 1), ROS play crucial roles in signaling and metabolism. Balanced levels of ROS are essential for normal immune functions, acting as second messengers in TCR signaling, which regulates T cell activation and differentiation, stimulates the release of inflammatory cytokines (e.g., IL-6, TNF-α), and modulates autophagy [298]. Conversely, excessive ROS can lead to cellular damage and dysfunction, such as T cell apoptosis through the upregulation of Fas and downregulation of Bcl2 [299]. Therefore, maintaining ROS homeostasis is vital for health. Because of the dual nature of ROS, both antioxidant and pro-oxidant approaches are being actively pursued in drug development for various diseases related to oxidative stress.
| ROS Type | Chemical formula/characteristics | Generation pathway | Half-life | Clearance mechanism | Reference |
|---|---|---|---|---|---|
| Superoxide anion | ·O₂− | NOX enzyme, mitochondrial ETC, xanthine oxidase | 10⁻⁶ s (μs range) | Dismutation to H₂O₂ by SODs | [296, 297] |
| Hydrogen peroxide | H₂O₂ | SOD-catalyzed conversion of ·O₂−, direct enzymatic generation | 1 ms to several minutes | Catalysis by peroxidases (catalase, glutathione peroxidase) | |
| Hydroxyl radical | ·OH | Fenton reaction (H₂O₂ + Fe²⁺) | 10⁻⁹ s (ns range) | Rapid reaction with nearby molecules (e.g., lipids, proteins) | |
| Singlet oxygen | ¹O₂ | Photosensitized reactions, lipid peroxidation by-products | 1–4 μs | Quenchers (carotenoids, vitamin E) | |
| Peroxynitrite | ONOO− | Reaction of ·O₂− with nitric oxide (NO) radical | 0.05–1 s | Decomposition or reaction with bicarbonate |
Specific inhibitors targeting ROS sources, including NOX inhibitors and mtROS inhibitors, improve the antigen-specific immune response in TME. NOX inhibitors, such as GKT137831, effectively prevent hypertensive cardiac remodeling in mice [300], reduce oxidative stress and proteinuria in T1D mouse models [301]. In in vivo experiments, GKT137831 reduced the levels of cancer-associated fibroblasts (CAFs), and the accumulation of CD8+ T cells at the tumor edge was no longer apparent, while the infiltration of CD8+ T cells into the tumor significantly increased. Therefore, targeting CAFs specifically with GKT137831 can reshape the immune microenvironment [302]. NOS31, a selective NOX1 inhibitor derived from Streptomyces, inhibits NOX1 activity and cell growth in multiple cancer cell lines, including gastric and colorectal cancers [303]. Other NOX inhibitors, including GKT136901, VAS2870, APX-115, CPP11G, and CPP11H, have shown promising clinical efficacy by indirectly reducing ROS production [304-306]. NOX inhibitors may mitigate oxidative stress within the tumor microenvironment and rejuvenate the functionality of immune cells by diminishing the production of ROS [307]. Furthermore, NOX inhibitors facilitate the polarization towards the M1 phenotype by reducing ROS levels, thereby enhancing antigen presentation and the secretion of inflammatory cytokines [308]. Mitoquinone, a ubiquinone derivative with selective mtROS-scavenging activity, inhibits lipid peroxidation and prevents apoptosis induced by oxidative stress [309]. Lipid peroxidation is a chain reaction triggered by oxygen free radicals attacking cell membrane lipids, which can lead to biomembrane damage. Oxidized lipids induce maturation phenotype characteristics in DCs, upregulating CD86 while suppressing phagocytic activity, thereby reducing the phagocytic efficiency and antigen-processing capacity of APCs. The antioxidant effects of mitoxantrone can mitigate such damage [310].
It is important to note that once cells progress to a precancerous state, antioxidants are no longer sufficient to halt cancer development, and apoptosis-inducing agents are required. In the regulation of cell fate, ROS levels exhibit a threshold effect. When redox balance is disrupted, the accumulation of ROS can damage biomolecules and promote malignant transformation. Elevated levels of ROS beyond this threshold can induce cancer cell death [311]. Therefore, employing pro-oxidant strategies to enhance ROS generation in cancer cells or to weaken their antioxidant defenses can disrupt the oxidative balance in tumor cells. This, in turn, activates various cell death pathways, including apoptosis, necrosis, and autophagy, ultimately restraining cancer progression [312]. One widely used small molecule drug in clinical practice, Elesclomol (formerly STA-4783), has been shown to elevate intracellular ROS levels, selectively inducing apoptosis in melanoma cells (NCT00879866) [313]. As a metal chelator, Elesclomol forms a stable complex with copper ions (Cu²⁺) and participates in the Fenton reaction within cells, leading to the generation of ·O₂− and H₂O₂. This oxidative stress disrupts the balance of Bcl-2 family proteins, promoting the activation of proapoptotic proteins such as Bax, which increases mitochondrial membrane permeability and selectively induces apoptosis in melanoma cells [314]. During the process of apoptosis, cells release tumor-associated antigens (TAAs) and damage-associated molecular patterns (DAMPs), such as HSP. These antigens and DAMPs can be taken up by APCs, thereby initiating an immune response [310]. A research team developed a ROS-sensitive polymer (PHPM) that co-encapsulates Elesclomol (ES) and Cu to form nanoparticles (NP@ESCu). The combination of ES and Cu works synergistically to induce ROS and copper-mediated cell death, effectively killing bladder cancer cells. Additionally, Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis revealed that protein processing in the endoplasmic reticulum, antigen processing, and presentation are activated in cells treated with NP@ESCu, inducing a significant immune response [315]. Nitrovin (difurazone), an antibacterial nitrofuran drug, can induce paraptosis-like cell death in glioblastoma multiforme cells independent of caspase activation by targeting thioredoxin reductase 1 (TrxR1), accompanied by ROS generation and endoplasmic reticulum stress activation. This confirms the cytotoxic effects of Nitrovin on cancer cells [316]. ROS and ER stress may enhance the efficiency of antigen presentation by upregulating the expression of MHC molecules through the activation of NF-κB signaling pathway [317]. In fact, anthracycline-based anticancer drugs, such as doxorubicin, can chelate intracellular iron, leading to the accumulation of •OH and ultimately resulting in cell death [318]. These drugs are effective against breast cancer and various solid tumors [319]. This process not only directly kills tumor cells but may also enhance its anticancer effects by activating the immune response. Specifically, anthracycline drugs can induce immunogenic cell death and the release of DAMPs in tumor cells, which in turn helps to trigger tumor-specific CD8+ T cell infiltration through cross-presentation by DCs [320]. Cisplatin, by cross-linking DNA purine bases and disrupting DNA repair mechanisms in cancer cells, damages mitochondrial DNA (mtDNA) and causes ETC injury, thereby inducing oxidative stress to target cancer cells [321]. mtDNA stress is involved in cytoplasmic antiviral signaling, enhancing the expression of a subset of interferon-stimulated genes, and plays an important role in antiviral signaling and type I interferon responses during infection [322]. Detailed information regarding some drugs that regulate ROS levels and have entered clinical trials or are in the clinical phase has been summarized in Table 2.
| Compound/treatment | Main mechanism | Disease types | NCT number | Reference |
|---|---|---|---|---|
| Small-molecule drugs | ||||
| ARS1620 | Covalent KRAS-G12C inhibitors Giving rise to chemically modified cancer neoepitopes. |
Non-small cell lung cancers (NSCLCs); pancreatic adenocarcinoma | — | [323, 324] |
| Sotorasib (AMG510) | Covalent KRAS-G12C Inhibitors. Induced a pro-inflammatory TME. | NSCLC | NCT05578859 | [276] |
| Adagrasib (MRTX849) | Covalent KRAS-G12C inhibitors. Enhancement of tumor antigen presentation through increased ICD. | Colorectal cancer | NCT04330664 | [325] |
| D3S-001 | Inhibiting the activity of KRAS-G12C protein, releasing tumor-associated antigens. | Solid tumors like NSCLC | NCT05410145 | [326] |
| Triptolide | Covalent p53 Y220C inhibitors. increasing the transcriptional activity of HSP70, degrading unfolded mutp53 proteins. | HIV-infection/AIDS | NCT03403569 | [327] |
| APR-246 | APR-246 converts to MQ, binding TP53 Y220C cysteines, activating p53. MQ also boosts TrxR1, increasing ROS for cytotoxicity. | Oesophageal carcinoma | NCT02999893 | [328] |
| SLMP53-2 | Mutp53 reactivators boost mutp53-Y220C/HSP70 interaction, restoring p53 function, triggering ER stress. | Hepatocellular carcinoma (HCC) | — | [329] |
| Acalabrutinib | Binds Cys481, inhibits BTK, blocks B cell signaling, triggers ER stress and ROS. | Chronic lymphocytic leukemia | NCT02717611 | [330] |
| BTKB66 | Covalent BTK inhibitors. BTKB66 dose-dependently inhibits IgM-activated B cell activation and LPS-induced proliferation. | Rheumatoid arthritis (RA) | — | [331] |
| Itaconate and its derivatives | Itaconate covalently binds to cysteine residues, inhibits SDH, reduces ROS generation. | Atopic dermatitis | NCT05455060 | [332] |
| Antioxidant strategies | ||||
| Catalase | Catalyzes the decomposition of H2O2 into water and oxygen, thereby reducing the accumulation of ROS. | Advanced malignant neoplasm | NCT05985278 | [333] |
| Peroxiredoxins (PRXs) | PRXs detoxify peroxides to water/alcohols via cysteine, curbing ROS accumulation. | Parkinson's disease (PD) | — | [334] |
| Glutathione Peroxidases (GPXs) | GPX reduces H2O2/hydroperoxides to water/alcohols with GSH/TRX. | Pancreatic cancer, lung cancer, melanoma | NCT04513015 | [335] |
| Dismutases | Converts superoxide (•O₂−) to O₂ and H₂O₂, reducing ROS levels. | Alzheimer's disease (AD) | — | [336] |
| Glutaredoxins (GRXs) | GRXs form mixed disulfides with GSH, reducing disulfides and mediating protein glutathionylation to regulate redox balance. | Chronic obstructive pulmonary disease (COPD) | — | [337] |
| Glutathione reductases (GR) | Catalyzing the reduction of GSSG to GSH, supporting antioxidants system. | Diabetes | — | [338] |
| GSH | Reacting with ROS, generating GSSG, maintaining GSH/GSSG balance via GPX and GR cycling. | PD | NCT01398748 | [339] |
| Thioredoxins (TRXs) | Participating in redox reactions, TRXs reduce peroxides directly and scavenge ROS indirectly via cysteine residues. | NSCLC | — | [244] |
| Sulfiredoxins (SRXs) | Acting on peroxidases like PRXs, enhancing cellular antioxidant capacity. | Cervical cancer | — | [245] |
| Thioredoxin reductases | Reducing oxidized thioredoxin to its reduced form, maintaining TRXs' antioxidant function. | Benign neoplasm of lung | NCT01985113 | [246] |
| Methionine sulfoxide reductases (MSR) | Reducing oxidized methionine, scavenging ROS via methionine's antioxidant properties. | Breast cancer | — | [247] |
| Vitamins A/C/E | Directly scavenge free radicals (such as singlet oxygen and peroxyl radicals). | Diabetes | NCT01691365 | [13] |
| N-acetylcysteine (NAC) | Directly scavenging of ROS, promoting GSH synthesis, reducing GSSG and inhibiting ROS generation. | Carcinoma Hepatocellular |
NCT01394497 | [248] |
| Mitoquinone | A derivative of coenzyme Q10. Accepting electrons to remove •O₂− and hydrogen peroxide H₂O₂ from mitochondria. | SARS-CoV infection (COVID-19) | NCT05886816 | [249] |
| Nitrones | Reacting with free radicals, forming stable nitroxide free radical adducts. | Acute ischemic stroke | NCT04951440 | [250] |
| Edaravone | Ingesting active groups neutralizing hydroxyl, peroxyl, nitric oxide radicals, reducing ROS accumulation. | Amyotrophic lateral sclerosis (ALS) | NCT00424463 | [251] |
| GKT137831 | Dual NADPH oxidase (NOX1/NOX4) inhibitor. | Liver, kidney, and pulmonary fibrosis | — | [252] |
| GKT136901 | Dual NADPH oxidase (NOX1/NOX4) inhibitor. | Diabetic nephropathy | — | [304] |
| VAS2870 | Inhibits multiple NOX isoforms (e.g., NOX1, NOX2, and NOX4), broadly reducing ROS production. | Acute respiratory distress syndrome (ARDS) | — | [305] |
| APX-115 | Broadly inhibiting the activity of the NOX family. | Type 2 diabetes (T2D) | NCT04534439 | [306] |
| Pyruvate | Reacting non-enzymatically with ROS (e.g., H₂O₂) to yield nontoxic byproducts (acetic acid, H₂O). | — | — | [13] |
| α-ketoglutarate | Activating Nrf2 pathway, upregulating antioxidant genes, enhancing cellular antioxidant capacity. | Myocardial infarction (MI) | — | [253] |
| Oxaloacetate | Intermediate of the TCA cycle. Optimizing mitochondrial function, supporting antioxidants, reducing electron leak, and lowering ROS levels. | Post-COVID-19 syndrome | NCT05840237 | [254] |
| Difurazone | Directly reacting with ROS, neutralizing free radicals, reducing ROS accumulation. | Glioblastoma | — | [316] |
| Berberis fruit extract | Directly removing ROS, enhancing endogenous antioxidant system, and inhibiting ROS generation pathway. | T2D | NCT03299153 | [255] |
| Chlorogenic acid | A powerful natural antioxidant. Directly scavenging ROS, inhibiting ROS-generating enzymes. | Glioblastoma | NCT02728349 | [256] |
| Phenethyl isothiocyanate (PEITC) | Activating the Nrf2/ARE pathway, promoting the expression of antioxidant enzymes and enhancing the antioxidant capacity of cells. | Lung cancer | NCT00691132 | [257] |
| Catechin | A powerful natural antioxidant. Directly scavenging of ROS, chelating metal ions, inhibiting ROS-generating enzymes. | Influenza infection | NCT00239213 | [258] |
| Rutin | Directly scavenging ROS and upregulating antioxidant enzymes. | T2D | NCT03437902 | [340] |
| Quercetin | Inhibiting ROS-generating enzymes, activating the Nrf2 pathway, and regulating mitochondrial function. | COPD | NCT01708278 | [341] |
| Berberine | Activating AMPK, inhibiting NOX, upregulating antioxidant enzymes, and scavenging free radicals. | COVID-19 | NCT04479202 | [342] |
| Magnoflorine | Inhibiting ROS-generating enzymes, scavenging free radicals, and regulating mitochondrial function. | AD | — | [343] |
| Carbon monoxide-releasing molecule-2 (CORM-2) | Suppression of intracellular ROS production, effectively curbing T cell proliferation. | Autoimmune hepatitis | — | [344] |
| Cerium oxide nanoparticles | ROS scavenger, further suppressing APC activation and enhancing antigen-specific immune tolerance. | Multiple sclerosis (MS) | — | [345] |
| Melanin nanoparticles (MNPs) | Interacting with ROS to generate O2 while simultaneously scavenging ROS. | MI | — | [346] |
| Pro-oxidant strategies | ||||
| Elesclomol | A pro-oxidant that binds to copper ions and catalyzes the generation of ROS. | Melanoma | NCT00522834 | [313] |
| Doxorubicin | Doxorubicin can bind to Fe²⁺ in cells to form a complex, catalyzing the reduction of O₂ to •O₂− and H₂O₂. | Ovarian cancer | NCT01227941 | [318] |
| Cisplatin | Inducing DNA damage, activating NOX enzymes, and inhibiting antioxidant defense systems. | Ovarian and prostate cancers | NCT01611727 | [321] |
| Auranofin (AF) | Inhibiting TrxR and glutathione system, increasing ROS, leading to oxidative stress and cell damage. | Human immunodeficiency virus (HIV) | NCT02176135 | [347] |
| Piperlongumine | Inhibiting antioxidant enzymes, depleting GSH, and activating ROS pathways. | Glioblastoma | — | [348] |
| Piperacillin | A β-lactam antibiotic. Inducing mitochondrial dysfunction and oxidative damage. | Bacterial infections | NCT03738683 | [349] |
| Acetaminophen-quinone imine | Consuming the antioxidant GSH in cells, causing the redox balance to be disrupted. | Acute liver failure | — | [350] |
| Afatinib | Activating REDD1-TSC1-mTORC1 axis and inducing pro-survival autophagy via ROS. | head and neck squamous cell carcinoma (HNSCC) | NCT04640870 | [351] |
| Sulfamethoxazole (SMX) | Contributing to the formation of ROS, leading to cell damage and apoptosis. | Methicillin-resistant Staphylococcus aureus | NCT03637400 | [352] |
| Nonsteroidal anti-inflammatory drugs (NSAIDs) | Generating ROS and activating the apoptosis pathway. | RA | NCT03112213 | [353] |
| Lapatinib | A dual tyrosine kinase inhibitor. Increasing ROS via cystine transport inhibition, Siramesin and Lapatinib induce cell death. | Prostate cancer | NCT00246753 | [354] |
| Sorafenib | Inhibiting mitochondrial respiration, enhancing ROS production, suppressing PD-L1 expression, and inducing ICD. | NSCLC | NCT00098254 | [355] |
| Biomineralized nano-vaccine, NVscp | Release ROS and promoting proteasome activity. | Mouse melanoma | — | [356] |
| ROS-inducing all-trans-retinoic acid into pH-sensitive polysaccharide-based nanodelivery systems | Augmenting proteasomal activity and MHC I antigen presentation. | T lymphoma | — | [357] |
| ROS-responsive nanomaterials modified with HA, PHO NPs | Promoting DC maturation, antigen uptake, and presentation. | — | — | [358] |
| Cationic liposomes | Effective ROS inducers. | Human papillomavirus (HPV) | — | [359] |
| Liposomal nanomedicines encapsulating Doxy and Ce6 | Inducing ROS and amplifying PDT-induced ICD. | Mouse melanoma | — | [360] |
- Data sources: https://clinicaltrials.gov/.
5.3 Promoting the Antigen-Presentation Machinery by Redox Regulation
Oxidative stress directly influences the activity and functionality of APCs by either modulating signaling pathways or altering the expression levels of proteins involved in antigen presentation, suggesting that redox regulation could be used as a therapeutic strategy to boost immune response.
A potent antioxidant of itaconate, a potent electrophile capable of alkylating cysteine residues in various target proteins, has been linked to several antigen presentation conditions due to its ability to alleviate oxidative stress by inducing the expression of the transcription factor Nrf2.
The cell-permeable itaconic acid derivative, 4-octyl itaconate (4-OI), acts as an immunomodulator by alleviating pathogenic inflammation and oxidative stress. It achieves this by modifying cysteine residues in target proteins, such as the major antioxidant transcription factor Nrf2 and the critical metabolic regulator glyceraldehyde 3-phosphate dehydrogenase (GAPDH) [361-363]. These compounds also inhibit the JAK1/STAT6 signaling pathway, which leads to reduced succinate dehydrogenase (SDH) activity in macrophages, thereby decreasing ROS production and preventing M2 macrophage polarization [364]. Given in antigen presentation, itaconate and its derivative dimethyl fumarate (DMF) exhibit therapeutic potential for the treatment of autoimmune diseases (such as psoriasis and multiple sclerosis), allergic conditions (such as asthma), and infections (including SARS-CoV-2) [361, 364, 365]. Furthermore, the cross-presentation ability of mesenchymal stem cells (MSCs) treated with the pyrimidinyl-indole derivative UM171a is dependent on mitochondrial ROS production. These treated MSCs show increased MHC I expression and elevated levels of several genes associated with antigen presentation. This increase allows MSCs to effectively cross-present immunogenic peptides derived from exogenous soluble proteins, thereby enhancing T cell responses [366].
Bruton's tyrosine kinase (BTK) is a crucial enzyme in the BCR signaling pathway and is known to be involved in inflammatory and oxidative signaling across various immune cells. When activated, BTK triggers the upregulation of NOX-2 and iNOS, contributing to oxidative stress in immune cells such as DCs, neutrophils, and B cells [367]. This oxidative stress can be alleviated by BTK inhibitors, as indicated by reduced levels of markers such as iNOS, NOX2, lipid peroxides, nitrotyrosine, and protein carbonyls. Inhibition of BTK via BIIB091 has also been found to dampen B cell-mediated antigen presentation to T cells [368]. Therefore, highly selective BTK inhibitors offer safer therapeutic alternatives for cancer patients and autoimmune diseases which exhibits disturbed antigen presentation. Covalent BTK inhibitors (acalabrutinib, ibrutinib, orelabrutinib, zanubrutinib, BGB-8035, tirabrutinib) are clinically validated for various cancers [284, 369, 370]. Furthermore, several other selective BTK inhibitors (evobrutinib, bribrutinib, spebrutinib, remibrutinib, rilzabrutinib, fenebrutinib) are in clinical trials for autoimmune diseases such as SLE, RA, multiple sclerosis (MS), and chronic spontaneous urticaria (CSU) [371-373].
5.4 Nanotechnology-Based Regulation of Antigen Processing and Presentation
Nanoparticle technology is currently attracting much attention due to its potential in redox regulation and promoting antigen presentation [374, 375]. A biomineralized nano-vaccine, NVscp, which is made by combining a self-assembled nano-vaccine with calcium peroxide, generates significant amount of ROS in response to the acidic environment of lysosomes. The accumulation of ROS promotes proteasome activity and the cross-antigen presentation by DCs, leading to the activation of tumor-specific Th cells and CTLs for tumor immunotherapy [356]. Incorporating of ROS-inducing all-trans-retinoic acid into pH-sensitive polysaccharide-based nanodelivery systems has been found to boost proteasome activity and significantly enhance ovalbumin (OVA) antigen presentation in mice [375]. Since optimal ROS levels promote DC maturation, ROS induction nanoparticles may also serve as a potential cancer vaccine platform [108]. Nanogel-induced lysosomal rupture directly stimulates ROS production within DCs, further augmenting proteasomal activity and subsequent MHC I antigen presentation. This controlled intracellular ROS elevation facilitates better cross-presentation of antigens, thereby improving the efficacy of cancer vaccines [357]. Furthermore, ROS-responsive nanomaterials modified with hyaluronic acid (HA, a ligand for CD44 receptors) such as PHO NPs, could serve as effective antigen delivery systems that promote DC maturation, antigen uptake, and presentation [185]. The optimal liposomal formulation containing cationic lipids has been shown to induce appropriate ROS levels in APCs, effectively controlling tumor growth [359, 376].
Simultaneously, certain nanomedicines can enhance antigen presentation through oxidative stress-induced antigen release [377-379]. For instance, liposomal nanomedicines encapsulating doxycycline hydrochloride (Doxy) and chlorin e6 (Ce6) generate ROS through dual pathways: Ce6 directly produces ROS upon near-infrared laser irradiation, while Doxy-induced mitochondrial damage synergistically increases ROS production, thereby amplifying photodynamic therapy (PDT)-induced immunogenic cell death (ICD) [360]. Additionally, Doxy enhances antigen presentation by inhibiting autophagy, leading to upregulated MHC I expression on the tumor cell surface [380]. By integrating the photosensitizer AIE with conversion nanoparticles, a robust ROS-dependent immune activation mechanism is achieved, which induces ICD and subsequently releases antigens, thereby facilitating a potent antitumor immune response [381]. Albumin-based NPs combined with indocyanine green, gold nanorods, catalase, and KDEL peptides increase ER stress and oxidative stress, leading to ICD and damaging tumor cells. This approach enhances antigen presentation and stimulates tumor-specific immune responses [382]. Moreover, MPS-PVP, polyvinylpyrrolidone (PVP)-modified two-dimensional manganese thiophosphate (MnPSe3, MPS) nanosheets, promotes antigen release by generating ROS from disrupted biofilms in the infectious microenvironment (IME). The subsequent rebalance of immunosuppressive signals, such as CD206, and costimulatory signals, including CD40 and CD80, enhances APC functionality and antigen presentation, triggering robust antibacterial immunity [383].
Interestingly, nanoparticle formulations with ROS scavengers regulate antigen-specific immune tolerance, playing a pivotal role in treating autoimmune diseases [384-387]. For instance, lignin-based nanoparticles, synthesized from plant-derived natural products with inherent ROS-scavenging properties, have been conjugated with self-antigens to facilitate antigen presentation to tolerogenic DCs. This approach reinstates immune tolerance by inhibiting autoreactive Th1 and Th17 cells, making it a promising tolerogenic vaccine platform for treating MS [388]. The introduction of cerium oxide nanoparticles, another potent ROS scavenger, can further suppress APC activation and enhance antigen-specific immune tolerance. This approach has been shown to induce a significant recovery in the late chronic phase of experimental autoimmune encephalomyelitis (EAE) in mice [345].
Collectively, redox-active nanomaterials can regulate the generation and clearance of ROS, thus showing potential applications in treating diseases related to antigen-specific immune disorders.
5.5 Improving the Function of Antigen-Specific Immune Cells
Establishing effective antitumor immunity depends on the activation, proliferation, and differentiation of lymphocytes that are specific to the tumor antigen [389]. Most cancer immunotherapies rely on the activation and infiltration of immune cells to generate immune response effects, such as cellular immunotherapy, including CAR-T and engineered T cells [390, 391]. This process largely depends on the interaction between T cells and APCs. However, recent studies have shown that only 10 percent or even fewer tumor-infiltrating CD8+ T cells have identifiable cytotoxic effects on tumors [392-394]. One of the challenges of tumor epitope-reactive T cells is oxidative stress in the TME, which causes T cell death [395]. Researchers hypothesize that reducing the oxidative stress by enhancing Trx could sustain the antitumor function of T cells [396, 397]. Trx1-overexpressing T cells from transgenic mice have lower levels of ROS and diminished vulnerability to TCR restimulation or oxidation-induced cell death. These T cells showed a central memory-like phenotype, characterized by decreased glucose uptake and diminished effector functions. Additionally, culturing T cells with recombinant Trx improved their mitochondrial metabolism and tumor control ability [189]. Another method by scavenging ROS to protect T cells from oxidative stress damage is using a nanomaterial delivery system. Researchers have utilized T-cell-targeted liposomes composed of 2,2,6,6-tetramethylpiperidine fused with anti-CD3 F(ab')2 antibodies (T-Fulips) to regulate the redox state of T cells and control their activity. This liposome protects T cell activity from being lost due to oxidative stress by neutralizing the levels of ROS, thereby maintaining their antigen presentation, recognition, and processing capabilities [191]. Thus, enhancing the antioxidant capacity of antitumor T cells can reshape their immunometabolic phenotype and enhance their tumor control capacity.
Although excessive amounts of ROS can be harmful, moderate levels act as crucial signaling molecules in T-cell activation and differentiation [398]. Studies indicate that appropriate ROS elevation partially activates T lymphocytes [244, 399, 400]. ROS is significant in the activating signaling pathways initiated by the TCR and CD28. Particularly, mitochondrial complex III-derived ROS production is essential for T cell activation by activating NFAT. Activation of NFAT increase the production of IL-2 and leading to antigen-specific T cell activation [181]. T cells isolated from patients on treatment of Venetoclax, a Bcl-2 inhibitor, have shown increased ROS levels in vitro. Studies indicate that Venetoclax, when administered at safe levels, enhances ROS production, leading to improved T-cell efficacy without harming cell survival [181, 401]. Additionally, certain small-molecule drugs can modulate the activity of immune cells. The inhibitory mechanism triggered by carbon monoxide-releasing molecule-2 (CORM-2) involves the suppression of intracellular ROS production, effectively curbing T cell proliferation. This inhibition parallels the effects seen with the antioxidant NAC, ultimately leading to a reduction in autoimmune hepatitis severity [344].
ROS has the ability to modify the functionality of Tregs. Studies have found that SENP3 is rapidly stabilized under the influence of ROS generated by TCR and CD28 stimulation, leading to the desumoylation of BACH2 and thereby maintaining the sustained functionality of Treg cells [182]. These molecular mechanisms underlying the interaction between ROS and Treg cell-mediated tumor immunosuppression indicate that targeting ROS within Treg cells may improve tumor immune tolerance. The fruits of the Berberis genus are a significant source of bioactive compounds, such as chlorogenic acid, catechin, rutin, quercetin, 3-d-galactoside, berberine, and magnoflorine, which can be leveraged in the development of alternative therapeutic strategies to alleviate inflammation and oxidative stress [258, 340-343, 402, 403]. Studies have demonstrated that cells treated with Berberis fruit extracts exhibited a reduction in ROS levels, which can be partly attributed to the activation of the heme oxygenase-1 (HO-1) pathway [404]. Moreover, Berberis fruit extract appears to positively influence adaptive immune cells by controlling concanavalin A-mediated Treg cell proliferation [255, 405]. Additionally, the inhibition of the NF-κB, MAPK inflammatory pathways by Berberis fruit extract further contributed to the mitigation of ROS accumulation and the suppression of the oxidative-inflammatory cycle [403].
Beyond its regulatory effects on DCs and T cells, ROS also plays a crucial role in modulating macrophage activity [406-408]. Melanin nanoparticles (MNPs), derived from the ink of marine organisms like cuttlefish, are incorporated into hydrogels synthesized through the reaction with alginates extracted from seaweed. These MNPs can interact with ROS to generate O2 while simultaneously scavenging ROS, thereby mitigating oxidative stress-induced damage in cardiomyocytes. This dual action fosters the M1 macrophages to the reparative M2 phenotype [346]. Additionally, high iron loads induce elevated ROS levels, which enhance the activity of p300/CREB-binding protein (p300/CBP) acetyltransferases and promote the acetylation of p53, facilitating the conversion of macrophages towards the M1 phenotype [409]. Moreover, a deficiency in the GTPase family M protein (IRGM) in macrophages inhibits ROS production, and the removal of ROS effectively suppresses IRGM overexpression-induced macrophage apoptosis [410]. The Irg1/itaconate axis enhances macrophage phagocytosis by modulating the Keap1/Nrf2/CD36 molecular mechanism. This process effectively reduces bacterial load and offers therapeutic potential for treating bacterial infectious peritonitis [411]. These the regulatory role of oxidative stress in influencing the functionality of antigen-specific immune cells and suggest a potential therapeutic strategy for antigen-specific immune disorders.
6 Conclusion
In summary, the immune system's capability to eliminate malignant and abnormal cells is dependent on its ability to recognize foreign antigens. Proper processing and presentation of antigens are crucial for eliciting antigen-specific cellular and humoral responses. ROS, acting as second messengers that influence various physiological processes, affect multiple stages of antigen processing and presentation, including the proteasomal or lysosomal processing of antigenic proteins and their presentation by MHC complexes. Furthermore, maintaining redox homeostasis is vital for regulating the activity and function of antigen-specific immune cells. As a result, managing redox status presents a practical approach for modulating the function of antigen-specific immune responses.
ROS produced from various sources, such as NOXs and mitochondria, can modulate immune responses by regulating APM, albeit through slightly different mechanisms. Firstly, ROS originating from NOX are among the most crucial signals for regulating the processing of antigens within phagosome/lysosome vesicles. They also impact the structure and function of MHC molecules, which facilitates antigen cross-presentation and activation of T cells. NOX-derived ROS also affect the expression of costimulatory molecules on APCs, such as CD80 and CD86, thereby enhancing the activation of antigen-specific T cells. In contrast, mitochondria represent another significant source of ROS, which is closely tied to the overall metabolic state of the cell and can indirectly influence antigen processing. While NOX-derived ROS tend to exert a more direct effect on the enzymes responsible for antigen degradation and peptide loading, mitochondrial ROS primarily impact the cellular environment and metabolic conditions, affecting the efficiency of antigen processing. It is known that mtROS can regulate the unfolded protein response (UPR), which influences the ability of endoplasmic reticulum to process antigenic peptides and load them onto MHC molecules. Elevated ROS levels can lead to oxidative stress, a common feature in immune disorders, including infections, autoimmune diseases, and cancers. On one hand, oxidative stress can generate new antigenic epitopes through mechanisms such as genomic damage, transcriptional dysregulation, and PTMs, thereby expanding the antigen repertoire. On the other hand, oxidative stress can impair antigen processing and presentation by causing oxidative damage to the involved components. Given that ROS generation and clearance during immune responses are dynamic processes, the precise control of ROS levels and their localization to ensure effective immune responses while preventing excessive activation remains a significant challenge.
Oxidative stress exhibits a complex and context-dependent influence on antigen-specific immune responses. Moderate levels of ROS are essential for effective immunity against pathogens and cancer, enhancing antigen presentation, and activating antigen-specific immune cells. This includes promoting DC cross-presentation and T cell activation through mechanisms such as increased lysosomal pH and upregulation of costimulatory molecules. Conversely, excessive ROS production is detrimental, impairing immune function, increasing inflammation, and promoting immunosuppression. High ROS levels can directly inhibit proteasomes, block antigen presentation, and modify immune checkpoint proteins (e.g., PD-L1), leading to immune evasion and resistance to immunotherapy. In autoimmune diseases, excessive ROS contributes to the pathogenesis by generating neo-epitopes, disrupting antigen presentation, and promoting autoantibody production, ultimately causing tissue damage.
This dual role of ROS highlights the therapeutic potential of redox modulation. In infectious diseases, controlled increases in ROS can enhance pathogen clearance; however, excessive ROS must be avoided due to the risk of severe complications. In autoimmune diseases and cancer, reducing excessive ROS can suppress immune overactivation and improve therapeutic outcomes. Specifically in cancer, reducing ROS levels can improve CAR T-cell function and counteract immune evasion mechanisms. Targeting redox-sensitive pathways (e.g., NF-κB, JAK/STAT) and employing redox-modulating agents (e.g., itaconate derivatives, dimethyl fumarate) represent promising therapeutic avenues. Moreover, the development of covalent drugs that generate neoantigens from otherwise “undruggable” proteins offers a novel immunotherapy strategy. Therefore, precise modulation of the redox balance is crucial for optimizing immune responses and improving therapeutic efficacy in various immune-related disorders.
Furthermore, manipulating oxidative stress through nanotechnology can significantly enhance this process. Nanoparticles can either increase antigen presentation by inducing ROS to boost proteasome activity or suppress it using ROS scavengers to induce immune tolerance. This dual approach allows for targeted modulation of immune responses, potentially beneficial in both cancer and autoimmune diseases. Ultimately, effective antitumor immunity depends on the activation of antigen-specific lymphocytes, and these methods aim to robustly stimulate these cells. Although there has been notable advancement in recent times, there are still a number of obstacles that continue to exist, resulting in a largely disjointed profession. Undoubtedly, further in-depth research is essential to unravel the multifaceted dynamics of antigen presentation, ensuring that immunotherapies can be applied with greater precision in clinical settings.
Author Contributions
Conceptualization: Na Xie and Guobo Shen. Supervision: Na Xie and Guobo Shen. Writing – original draft: Qinxia Chang and Yaying Zhang. Writing – review and editing: Na Xie, Guobo Shen and Qinxia Chang. Validation: Wenbing Pu, Shanshan Liu, Jing Zhang and Yuan Tian. Visualization: Qinxia Chang, Yaying Zhang, Peng Miao, and Xiaojun Liu. All authors have read and approved the final manuscript.
Acknowledgments
BioRender was used to create the figures. This study was supported by grants from the National Key R&D Program of China (2023YFC3402100 and 2020YFA0509400), the National Natural Science Foundation of China (82130082 and 82341004), and 1·3·5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYGD22007 and ZYJC21004).
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.





