Phase Separation: A New Dimension to Understanding Tumor Biology and Therapy
Xingwen Wang and Minqiao Lu contributed equally to this work.
ABSTRACT
Liquid–liquid phase separation (LLPS) plays a critical role in orchestrating various cellular processes, such as gene expression, signal transduction, and protein synthesis, by compartmentalizing cellular components without membrane boundaries. Emerging research has illuminated how dysregulated LLPS is integral to cancer development by influencing tumorigenesis, metastasis, immune system evasion, and resistance to therapy. The subtle differences in LLPS are crucial for understanding cancer progression and finding new treatments. However, despite its significant implications in oncology, the potential of specifically targeting LLPS in cancer therapy has not been thoroughly investigated. This review delves into the mechanisms of LLPS, exploring physiological triggers and their consequences in cancer biology. We discuss the profound impact of LLPS on the hallmarks of cancer and outline innovative strategies aimed at targeting LLPS. These strategies include the direct inhibition of phase condensate formation and the modulation of related signaling pathways. Although targeting LLPS poses several challenges, such as specificity and delivery methods, it represents a promising frontier in cancer treatment, potentially revolutionizing how we approach cancer therapy. This review emphasizes the academic and therapeutic importance of LLPS, advocating for it as an exciting and valuable target for future cancer treatment strategies.
Graphical Abstract
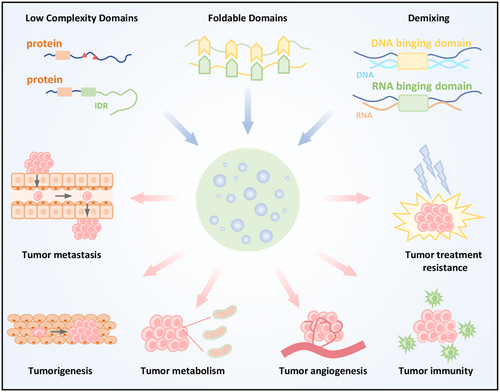
Liquid–liquid phase separation (LLPS) facilitates the assembly of biomolecular condensates by leveraging weak multivalent interactions. The low-complexity domains, foldable domains of proteins, and nucleic acids provide multivalent interaction sites among different molecules and contribute to the formation of condensates. Dysregulation of condensates contributes to cancer hallmarks, including tumorigenesis, metastasis, angiogenesis, metabolism, drug resistance, and tumor immunity. Targeting biomolecular condensates offers new therapeutic opportunities for advancing cancer treatment.
1 Introduction
Coordinating complex biochemical reactions in both time and space is critical for normal cell function. Any disruption in this balance can have serious consequences, including cancer [1]. To ensure proper progression of these processes, cells have developed distinct regions with specialized roles. Examples of such regions include membrane-enclosed organelles like the nucleus, endoplasmic reticulum, Golgi apparatus, mitochondria, and lysosomes [2]. The lipid bilayer membranes of these organelles act as physical barriers, separating their internal environments from the external surroundings.
In addition to classic membrane-bound organelles, a variety of membrane-less organelles, also known as biomolecular condensates, have been discovered. Examples include nucleoli, nuclear speckles, paraspeckles, heterochromatin, promyelocytic leukemia (PML) bodies, super-enhancers (SEs), P granules, and stress granules (SGs) [3]. Biomolecular condensates form via weak interactions between macromolecules like proteins and nucleic acids, with sizes typically ranging from micrometers [4]. These condensates are commonly found in the cytoplasm, nucleus, and membrane-associated organelles.
Biomolecular condensates are dynamically and reversibly formed via liquid–liquid phase separation (LLPS) [3]. This process involves the spontaneous demixing of a liquid solution containing biomacromolecules into two distinct phases: light phase and the dense phase. Besides exhibiting liquid characteristics, biomolecular condensates can also exhibit gel-like and solid-like properties [5]. As research advances, the diverse regulatory roles of biomolecular condensates have become increasingly evident; these aggregates are involved in regulating chromosome stability, RNA transcription, protein translation, and posttranslational modifications [6]. Recent studies have identified that abnormalities in the formation and regulation of these aggregates are associated with tumorigenesis, metastasis, drug resistance and tumor immunity [7, 8]. Therefore, targeting biomolecular condensates could be a promising avenue for cancer therapy.
In this review, we describe the history of LLPS, its mechanisms of formation, and its role in regulating intracellular molecular changes and signaling pathways. We provide an introduction in detail of how LLPS influences cancer hallmarks. Additionally, we discuss current strategies for targeting LLPS in cancer treatment and outline the challenges that remain in this field. In summary, targeting LLPS represents a promising new approach for cancer therapy.
2 History of Liquid–Liquid Phase Separation Research
Not all organelles are membrane structures. Over a century ago, the nucleolus was identified as the first membrane-less organelle and recognized for its role in cell division. This discovery was systematically described in the 1830s [9, 10]. However, at that time, the understanding of membrane-less organelles remained limited.
In 1899, Edmund Beecher Wilsonin and others proposed that the cytoplasm was composed of a mixture of different chemically suspended drops [11, 12]. Edmund Wilson summarized a series of research results on how cell cytoplasm is composed of phase-separated mixtures of different spherical objects, which could not be well explained at that time [12, 13]. Early researchers believed that more than 50% of protein–protein interactions were “noise,” meaning that these interactions had no apparent function. Later, it was shown that these protein interactions may be involved in the phase separation process [14, 15].
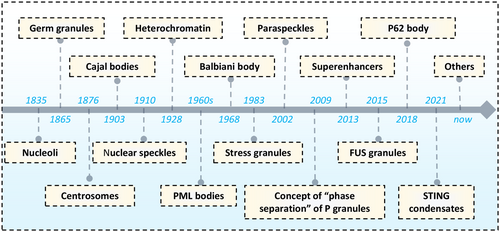
With the development of experimental methods and techniques, about a decade ago, research found that protein and protein or nucleic acid mixtures could undergo LLPS to form droplets or so-called “nonmembrane organelles,” where specific molecules were concentrated in liquid-like compartments that stably coexisted with the surrounding liquid environment, exhibiting liquid like behavior [2, 16-18]. In 2009, Brangwynne et al. studied embryonic cells of Caenorhabditis elegans and observed many small droplet-like structures composed of RNA and proteins inside the cells under a microscope. These structures can exhibit liquid behaviors such as fusion, separation, and flow in cells, and quickly coalesce or disintegrate with changes in the concentration of related components. They named it P granules, and for the first time introduced the physicochemical concept of “phase separation” into biological research, suggesting that cells can determine cell differentiation fate through the asymmetric distribution of P granules mediated by “phase separation” [19]. Subsequently, Rosen and McKnight respectively studied the phase separation phenomenon of proteins and RNA in 2012. Rosen discovered that when various synthetic, multivalent macromolecules (such as multi-domain proteins and RNA) interact, they rapidly shift from small complexes to larger polymeric structures as protein concentration increases, a process accompanied by macroscopic LLPS [20]. McKnight and his team demonstrated that LLPS governs the structure of RNA granules in a cell-free in vitro system [21]. These two studies indicated that phase separation could be easily induced in test tubes through straightforward methods, facilitating LLPS research. Since then, the field has grown, with numerous groups exploring LLPS [5].
As research has advanced, it has been confirmed that earlier discovered condensates, such as Balbiani body (1986) [22], Cajal bodies (1903) [23], centrosomes (1876) [24], germ granules (1865) [25], heterochromatin (1928) [26], nucleoli (1835) [9], nuclear speckles (1910) [27], paraspeckles (2002) [28], P granules (2009), PML (1990) [29], SGs (1983) [30], SE (2013) [31], fused in sarcoma (FUS) (2015) [32], P62 body (2018) [33], and stimulator of interferon genes (STING) (2021) [34] are also formed through LLPS [35] (Figure 1).
3 Molecular Structural Features of Driving Phase Separation
Within cells, biomacromolecules do not exist in isolation; they engage in a variety of multivalent interactions, such as electrostatic interactions, hydrophobic interactions, π–cation, cation–cation, and π–π interactions, which maintain the cell's ability to perform various functions. Current research indicates that multivalent interactions are the primary drivers of phase separation [3]. The foldable domains of proteins, low-complexity domains, intrinsically disordered regions, and nucleic acids can all lead to intra- or intermolecular interactions within the cell (Figure 2).
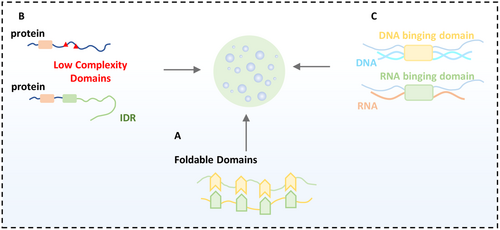
3.1 Foldable Domain-Driven Phase Separation
Concatenation of multiple folding domains can drive phase separation. In 2012, Li et al. discovered the interaction between the Src homology 3 domain (SH3) domain and its proline-rich motif (PRM), and purified proteins consisting of repeats of the SH3 domain and repeats of the PRM ligand domain in vitro. The results indicated that phase separation occurred as the concentration increased. They also looked at the number of tandem sequences of SH3 and PRM and found that the more tandem the repeat sequences, the more likely phase separation was to occur [20]. For example, after T cell receptor activation, growth factor receptor-bound protein 2 (Grb2) interacts with linker for activation of T cells family member (LAT) and son of sevenless (SOS) via its SH2 and SH3 domains, LAT–Grb2–SOS complex undergo phase transitions, and ultimately assembling on the membrane. This phase separation promotes the reorganization of actin, a cytoskeletal protein, and triggers the T cell receptor signaling pathway, which in turn activates the RAS pathway [36, 37]. SH2-domain-containing leukocyte protein of 65kDa (SLP65) is the scaffold protein that drives LLPS in the B cell receptors (BCR) signaling pathway. SLP65 forms liquid-like condensates with cbl-interacting protein of 85 kDa (CIN85) via multivalent interactions between the SH3 domains of trimeric CIN85 and the proline-rich motifs of SLP65. Nephrin is crucial for maintaining the glomerular filtration barrier, and its phosphorylated form binds to the SH2 domain of the NCK adaptor protein 1 (NCK). The three SH3 domains of NCK can interact with N-WASP. Through the multivalent interaction mediated by the SH domain, nephrin proteins form phase-separated particles with NCK and N-WASP to guard the skeleton [38]. In addition to the above signaling systems, Ras-GTPase-activating protein (GAP)-binding protein 1 (G3BP1), PML, Nucleophosmin (NPM1), the P-body components decapping mRNA 2 (DCP2) and enhancer of mRNA decapping 3 (EDC3), as well as speckle type BTB/POZ protein (SPOP) and TAR DNA binding protein-43 (TDP43), can be separated by the interaction between foldable domains [39-42].
3.2 Intrinsically Disordered Region-Driven Phase Separation
Proteins without a defined three-dimensional structure are known as intrinsically disordered proteins (IDPs). The intrinsically disordered region (IDR) of a protein plays a key role in promoting phase separation. A protein's primary sequence can be used to predict the presence of an IDR, and thus its potential to undergo phase separation. IDR increase a protein's propensity for phase separation, with certain key amino acids in these regions playing a pivotal role in driving the process. For example, Sui et al. found that the transcriptional activation domain of Yin-Yang 1 (YY1) contains IDRs, in which the H cluster of histidine and the E/D cluster of glutamate/aspartate can drive phase separation [43]. FUS is a typical phase separation protein, and its IDR region has very high content of glycine, serine, glutamine and tyrosine, which is difficult to fold into a three-dimensional structure, which can promote its phase separation [44]. Lin-28 homolog A (LIN28A) is a marker protein for nucleolar integrity. The R192 arginine residue in its IDR, which is associated with Parkinson's disease, is crucial for the phase separation of LIN28A and the nucleolus [45]. Shen et al. found that the IDR sequence of NOD-like receptor family pyrin domain containing protein 6 (NLRP6) contains a high frequency of positively charged lysine, which is the key to its phase separation mediated by dsRNA [46]. Many IDRs contain repetitive sequences, known as low complexity domains (LCDs), which also contribute to phase separation. Research by Kwon et al. demonstrated that the LCD of the FUS protein can fuse with the DNA binding domain of certain transcription factors, and this fusion protein undergoes phase separation [47]. TAR DNA binding protein-43 (TDP-43) is a typical protein that forms functional RNA particles in cells and liquid-like droplet in vitro through its LCD phase separation function. At the same time, abnormal TDP-43 LCD interactions can also lead to neurodegenerative diseases [48]. There is a LCD in the middle region of hematopoietic progenitor kinase 1–interacting protein of 55 kDa (HIP55), which can promote the formation of phase separation particles [49].
3.3 Nucleic Acids-Driven Phase Separation
Nucleic acids are abundant in cells and come in many forms, participating in the formation of condensates. Some nucleic acid molecules have an intrinsic ability to undergo phase separation, while most nucleic acids influence phase separation by interacting with proteins. These interactions occur through multivalent binding sites on nucleic acids, which bind to the RNA or DNA binding domains of proteins, thereby affecting phase separation.
For example, when double-stranded DNA (dsDNA) breaks, poly(ADP-Ribose) polymerase 1 (PARP1) is recruited to the damage site, forming phase-separated particles with other repair molecules to maintain the proximity of the broken DNA ends and initiate the repair pathway. It was also found that dsDNA of different lengths can bind varying amounts of PARP1, with a positive correlation between DNA length and the number of PARP1 molecules bound [50].
G3BP1 is a core component of SGs and acts as a molecular switch that triggers RNA-dependent phase separation. In the absence of stress, G3BP1 remains in a closed and compact structural state. Upon stress, translation is inhibited, leading to an increase in free RNA in the cytoplasm. This RNA binds to the RNA-binding region of G3BP1, causing a loosening of its structure and triggering phase separation [51, 52].
In the cytoplasm, cyclic GMP-AMP synthase (cGAS) detects abnormal cytosolic dsDNA from pathogens or cellular damage. Exogenous DNA can trigger phase separation of cGAS, facilitating the production of cGAMP. The LLPS of cGAS is highly sensitive to DNA concentration, indicating that cGAS is activated only when cytoplasmic DNA reaches a critical level [34, 53]. Additionally, YTH N6-methyladenosine RNA binding protein F2 (YTHDF2), an m6A modification reader, was found to undergo enhanced phase separation when bound to m6A-modified RNA of around 50 nucleotides in length [54]. Liu et al. further discovered that single-stranded DNA (ssDNA) with a parallel G-quadruplex structure can selectively induce the phase separation of the G-quadruplex binding protein SERBP1 [55].
4 Physiologically Relevant Triggers of Phase Separation
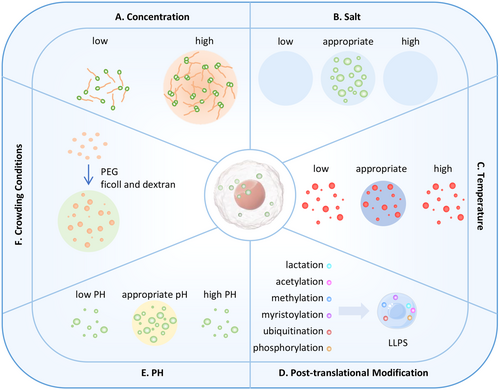
Most protein molecules can undergo phase separation under non-physiological conditions; therefore, the key question is whether phase separation occurs under physiological conditions. At the same time, changes in physiological conditions will affect phase separation and cause changes in the state of protein molecule aggregates (Figure 3), thereby changing downstream biological effects. Therefore, it is of great significance to study the physiological factors that affect phase separation.
4.1 Thermodynamic Conditions of Phase Separation
From a thermodynamic perspective, a decrease in the Gibbs free energy (g) of the system is conducive to the occurrence of phase separation [56]. The Gibbs free energy can be decomposed into an entropy change (Δs) and an enthalpy change (Δh). Among them, the enthalpy change includes the variations in potential energy between biomolecules and the solvent, between biomolecules, and between solvent molecules, while the entropy change measures the change in the degree of freedom of the system. In a binary system, it can be analyzed using the formula Δg = Δh − TΔs [57].
① When Δh > 0 and Δs < 0, Δg is always greater than 0, and phase separation cannot occur; the system remains in a mixed state.
② When Δh < 0 and Δs > 0, Δg is always less than 0, and phase separation occurs.
③ When Δh < 0 and Δs < 0, the higher the temperature (T), the more negative Δg becomes, and phase separation is more likely to occur. It should be noted that when the temperature is high enough, the orderliness of the binary system disappears, which affects the occurrence of phase separation due to temperature changes.
Hence, certain proteins suppress phase separation upon temperature elevation, such as heterogeneous nuclear ribonucleoprotein A1 (hnRNPA1), DEAD-Box helicase 4 (DDX4), and TIA1 [58]. whereas others enhance phase separation in response to temperature elevation, as seen in proteins like ubiquilin-2 (UBQLN2) and poly(A) binding protein cytoplasmic 1 (Pab1) [59, 60].
4.2 Concentration and Physicochemical Properties of Biomolecules
Biomacromolecules in solution will only phase separate at a specific concentration. When the concentration of biomolecules is lower than the threshold for phase separation, phase separation will not occur regardless of the temperature and pH. When the concentration of macromolecules in the system reaches a certain condition, under appropriate temperature and pH conditions, the mutual attraction between biomolecules will lead to the formation of condensates [61]. At this time, the concentration of biomacromolecules dispersed in the solution is low, while the concentration of macromolecules in the condensate is high, and the two states can be converted into each other under appropriate environmental conditions. As the concentration of macromolecules increases, phase separation will occur more and more, and will not change after reaching a certain threshold. Currently, the biomacromolecule condensates that are studied more exist in the form of LLPS, but there are still some condensates that exist in colloidal or solid forms. They can be transformed from condensates in the form of droplets, and this transformation is often irreversible.
The concentration of salt ions can also affect the occurrence of phase separation. Metal salt ions such as Na+ and K+ can promote or inhibit phase separation due to their electrostatic effects [62]. Generally, low-salt-induced LLPS is primarily driven by multivalent cation-π interactions, while high-salt-induced LLPS is thought to be stabilized by hydrophobic and nonionic interactions. FUS protein can form phase separation in both high and low salt, maintain phase separation at a salt concentration of 125 mM, form a well-mixed phase between 125 mM and 1.5 M, but re-enter the phase separation state when the potassium chloride concentration is above 1.5 M. At high and low transition concentrations of salt, FUS behaves as a two-phase boundary, respectively [63].
4.3 Temperature
Temperature is also an important factor affecting the occurrence of phase separation. According to the typical phase diagram of a binary mixture [57], we know that the influence of temperature on phase separation is not a simple linear relationship. Temperature shows different regulations on the phase separation of different substances. Sometimes, temperature promotes the occurrence of phase separation, while at other times, it inhibits it. Biology-related experiments have also confirmed this point.
For example, under heat stress conditions, mTORC1 phosphorylates P granule components PGL-1/PGL-3, promoting its ability to phase change [64]. Zhang et al. have found that under physiological conditions, heat shock transcription factor 1 (HSF1) and heat shock proteins exist in the form of a complex. After cells are subjected to heat stimulation, heat shock induces HSF1 to form SGs/bodies in the cell nucleus, indicating that changes in temperature play an important role in the formation of HSF1 phase separation [65]. UBQLN2 and Pab1 also promote the occurrence of phase separation when the temperature is increased [59, 60]. However, the protein whose phase separation is inhibited by increasing temperature included hnRNPA1, DDX4, and TIA1 [58].
4.4 Posttranslational Modification
Posttranslational modification of proteins involves the addition or removal of specific chemical groups at defined positions after protein synthesis, altering the structure and function of the proteins. Many phase-separating proteins IDRs that contain posttranslational modification sites, which influence the protein's activity, stability, and interactions with other biomacromolecules. Various posttranslational modifications, such as acetylation, phosphorylation, methylation, ubiquitination, myristoylation, and lactylation, can regulate phase transitions in proteins.
K(Lysine) acetyltransferase 8 (KAT8) is an intracellular acetyltransferase. Kang et al. found that under IFN-γ stimulation, KAT8 in tumor cells can acetylate the K78 site of interferon regulatory factor 1 (IRF1), promoting KAT8/IRF1 to form a condensate with the function of promoting transcription [66]. Zhou et al.'s study found that the enzymatic activity of acetylase sirtuin-1 (SIRT1) plays a decisive regulatory role in the phase separation of IRF3/IRF7. Acetylation modification prevents the phase separation of IRF3/IRF7 and ISRE-DNA [67]. The autophagy-related 1 (Atg1) kinase complex (including: Atg1, Atg13, Atg17, Atg29, and Atg31) play a critical role in the formation of the early pre-autophagosomal structure (PAS) and the subsequent formation of autophagosome. During autophagy, Atg1 is activated and undergoes autophosphorylation, which significantly weakens the phase separation of the Atg1 complex. The presence of protein phosphatase type 2C (Ptc2) in the body can dephosphorylate the Atg1 complex and maintain it in a phase-separated state [68]. The formation of phase separation of the transmembrane protein Syndecan 4 (SDC4) greatly enhances the recruitment of syntenin protein in the cytoplasm to the plasma membrane. The phosphorylation of serine (Ser 179) at position 179 of SDC4-CD can regulate the formation of SDC4 phase separation [69]. Phosphorylation of S120 and S200 serine residues in the LIN28A IDR promotes the phase separation of LIN28A and nucleolus [45]. In addition, the arginine–glycine–glycine (RGG) motif of FUS plays an important role in its phase separation. Studies by Mario et al. have shown that arginine methylation of the RGG motif inhibits the phase separation of FUS [70].
Ubiquitination is a crucial posttranslational modification involved in regulating various cellular processes. While p62 cannot undergo phase separation in vitro on its own, the addition of polyubiquitin chains to its K63 site induces phase separation. This allows p62 to recognize polyubiquitin chains on target proteins and form p62 bodies through phase separation, mediating the formation of autophagosomes [33]. Yang et al. discovered that the ubiquitin ligase tripartite motif containing 21 (TRIM21) catalyzes polyubiquitination at the K63 site of the SG core protein G3BP1, which inhibits G3BP1 self-aggregation and, consequently, the formation of SGs [71]. Additionally, myristoylation at the N-terminus of FSP1 is essential for the phase separation of FSP1 induced by the ferroptosis promoter 3-phenylquinazolinones (icFSP1) [72]. Lactylation of p53 reduces its DNA-binding ability and LLPS, thus diminishing its tumor-suppressing role [73].
4.5 Other Factors Regulating Phase Separation
The pH value affects the protonation state of proteins involved in phase separation. Studies have shown that in Atg1-mediated autophagy, phase separation is most pronounced at pH 6.0, while at pH levels above 7.0, phase separation is significantly weakened. This suggests that in vitro experiments can effectively simulate the in vivo environment, and that pH changes regulate the formation of the PAS mediated by Atg1 phase separation [68]. In the nucleolus, a pH gradient exists, with pH levels gradually decreasing from the nucleoplasm to the nucleolus, influencing both the phase separation and distribution of proteins. Kiyoto Kamagata et al. found that at pH 7.0, p53 forms spherical droplets with a cross-sectional area of 0.2–4 μm², while at pH 5.5, larger, nonspherical clusters dominate. In contrast, no p53 aggregates are observed at pH 8.0 [74]. These findings demonstrate that pH plays a key regulatory role in protein phase separation.
The degree of protein crowding significantly influences the occurrence of phase separation. To simulate the crowded environment of cells, crowding agents such as polyethylene glycol (PEG), ficoll, and dextran are often added in vitro. For some proteins, the presence of these agents increases the local concentration, promoting phase separation. For some proteins, the addition of crowding agents will increase the local concentration and promote phase separation. A large number of proteins do not phase separate in vitro without crowding agents, but will phase separate after adding crowding agents. For example, in vitro p53 protein did not detect phase separation in the absence of crowding, but when ficoll and dextran were used at the same time, p53 was detected to have phase separation [74].
In summary, the phase separation of biomacromolecules is usually not determined by a single factor, but the interaction of various factors promotes the occurrence of phase separation.
5 Regulation of Biomolecule Expression and Signal Transduction by Phase Separation
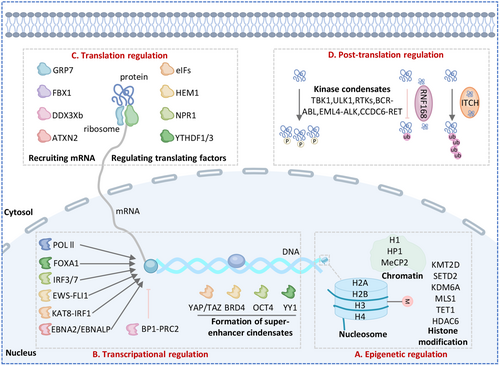
The expression of biomolecules and the orchestration of signal transduction pathways constitute the core regulatory networks governing cellular physiological activities. The emergence of LLPS theory has provided a transformative perspective for understanding these complex processes. By forming distinct compartments, LLPS creates efficient and highly specific platforms for biomolecular reactions, enabling precise spatial and temporal regulation of cellular functions. In this section, we will explore the roles of LLPS in epigenetic regulation, transcriptional control, translational modulation, posttranslational modifications, and the regulation of key signaling pathways.
5.1 Phase Separation in Epigenetic Regulation
Epigenetics involves alterations in gene expression without changes to the genetic sequence, including processes like chromatin remodeling, histone modifications, and DNA methylation. The dynamic regulation of chromatin remodeling is essential for controlling DNA accessibility in time and space, ensuring proper gene expression. Histones and heterochromatin protein 1 (HP1) are integral to nucleosome-mediated chromatin condensates and play a key role in chromatin compartmentalization [75]. Histones are classified into linker histone H1 and core histones (H2A, H2B, H3, and H4). H1 undergoes LLPS with DNA and nucleosomes of different lengths but does not phase separate without DNA, which leads to chromatin condensation [76]. H2A also forms droplets in the presence of DNA and nucleosome [77]. However, the tails of histones H3 and H4 are directly involved in phase separation, independent of histone-binding proteins [78]. HP1 is known to promote the formation of constitutive heterochromatin through phase separation. In vitro studies showed that HP1α triggers phase separation when phosphorylated or upon interaction with DNA. HP1α binds to the H3K9me3 mark on a nucleosome through its chromodomain (CD), bridging two nucleosomes. Furthermore, as a reader of H3K9me2/3, the interaction mechanism of HP1α is dependent on epigenetic modifications [79]. Histone is an important component of nucleosome. HP1 condensates promote conformational changes in nucleosomes, which can promote heterochromatin compression. Methyl-CpG-binding protein 2 (MeCP2), an intrinsically disordered protein, is a key regulator of gene expression. The condensates formed by MeCP2 specifically gather and concentrate heterochromatin cofactors, while excluding components of euchromatic transcriptional condensates. Li et al. suggest that MeCP2 promotes the segregation of heterochromatin and euchromatin through its condensate partitioning ability (Figure 4 and Table 1) [80].
| Liquid–liquid phase separation in regulation of biomolecule expression | |||
|---|---|---|---|
| Biological process | Key protein | Function | References |
| Epigenetic | HP1 | Formation of constitutive heterochromatin | [75, 79] |
| histone H1 | Formation of nucleosome | [76] | |
| H2A | Formation of nucleosome | [77] | |
| H3/H4 | Formation of nucleosome | [78] | |
| MeCP2 | Enhances the separation of heterochromatin | [80] | |
| KMT2D | Methyltransferases for H3K4 | [81] | |
| SETD2 | H3K36 methyltransferases | [82] | |
| KDM6A | H3K4 methylation | [83] | |
| MSL1 | Acetylates H4K16ac | [84] | |
| HDAC6 | Deacetylase | [85] | |
| TET1 | H3K27me3 and H2AK119 ubiquitylation | [86] | |
| Transcription | polymerase (Pol) II | Activate transcription | [87] |
| TAF15 | Activate transcription | [88] | |
| OCT4 | Activate transcription | [49, 89] | |
| BRD4 | Activate transcription | [90] | |
| YAP | Activate transcription | [91, 92] | |
| EBNALP | Activate transcription | [93] | |
| BP1 | Transcriptional repression | [94] | |
| EWS-FLI1 | Activate transcription | [95] | |
| KAT8-IRF1 | Activate transcription | [66] | |
| IRF3/7 | Activate transcription | [67] | |
| FOXA1 | Activate transcription | [96] | |
| Translation | HEM1 | Inhibited translation | [97] |
| GRP7 | Inhibited translation | [98] | |
| NPR1 | Enhances translation | [99] | |
| FXR1 | Enhances translation | [100] | |
| FBL | Enhances translation | [101] | |
| Ddx3xb | Enhance translation | [102] | |
| YTHDF1/3 | Inhibited translation | [103] | |
| ATXN2 | Enhance translation | [104] | |
| PTM | TBK1 | Promoted phosphorylation | [105] |
| ULK1 | Promoted phosphorylation | [106] | |
| BCR-Abl | Promoted phosphorylation | [107] | |
| EML4-ALK | Promoted phosphorylation | [107] | |
| CCDC6-RET | Promoted phosphorylation | [49] | |
| Itch | Promoted ubiquitination | [108] | |
| RNF168 | Inhibited ubiquitination | [109] | |
| Liquid–liquid phase separation in signaling pathway | |||
|---|---|---|---|
| Pathway | Key protein | Function | References |
| DNA damage repair signaling pathway | γ-H2A | Active | [110] |
| KU70/80 | Active | [111] | |
| P53 | Active | [112, 113] | |
| FUS | Active | [114] | |
| RNF168 | Active | [109] | |
| RAP80 | Active | [115] | |
| CycT1 | Inhibit | [110] | |
| BRD4 | Active | [111] | |
| MDC | active | [98] | |
| DDX3X | Active | [102] | |
| RAD52 | Active | [98] | |
| NONO | Active | [116] | |
| MRNIP | Active | [98] | |
| MtSUVR2 | Active | [98] | |
| RAS signaling pathway | SOS | Active | [36] |
| SHP2 | Active | [111] | |
| CCDC6-RET | Active | [49] | |
| EML4-ALK | Active | [107] | |
| Autophagy signaling pathway | P62 | Active | [33] |
| FIP200 | Active | [117] | |
| PGL | Active | [118] | |
| ATG1/13/17 | Active | [68] | |
| ATG8e | Active | [119] | |
| P53 pathway | P53 | Regulate | [120] |
| Immune signaling | LAT | TCR microclusters | [121] |
| SLP65 | BCR microclusters | [122] | |
| cGAS | Active | [53] | |
| STING | Inhibit | [34] | |
| MAVS | Active | [123] | |
| IFI16 | Active | [124] | |
| RIG-I | Active | [125] | |
| p65 | Inhibit | [126] | |
| IRF3/IRF7 | Active | [127] | |
| NLRP6 | Active | [46] | |
- Abbreviations: BCR, B cell receptors; BCR-Abl, breakpoint cluster region-abelson murine leukemia viral oncogene homolog 1; CCDC6-RET, coiled coil domain containing 6-rearranged during transfection; EML4-ALK, echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase; H2AK119, histone H2A lysine K119; H3K27me3, trimethylation of histone H3 at lysine 27; H3K36, histone H3 lysine K36; H3K4, histone H3 lysine K4; H4K16ac, histone H4 acetylation at lysine 16; MeCP2, methyl-CpG-binding protein 2; MRNIP, MRN complex interacting protein; MtSUVR2, Medicago truncatula; SUVR2; TCR, T cell receptors.
Histone modifications, such as methylation and acetylation, regulate DNA-templated processes by inducing local chromatin structural changes or by binding to effector proteins or chromatin remodeling complexes. SET domain containing 2 (SETD2) and lysine methyltransferase 2D (KMT2D) have been shown to form condensates. KMT2D is a methyltransferase for H3K4, while SETD2 is a key methyltransferase for H3K36 [82]. The lysine-specific demethylase 2A (KDM2A) contains LCD and IDRs, interacted with PHD finger protein 2 (PHF2), KDM2A, and KDM4B facilitate their functions by phase separation [81]. KDM6A forms condensates through its core IDR, bringing KDM2B along and facilitating MLL4-mediated H3K4 methylation [83]. Male-specific lethal 1 homolog (MSL1), which exhibits LLPS properties, is part of a histone acetyltransferase complex that acetylates H4K16ac, promoting gene activation [84]. Recently, Lu et al. found that phosphorylated histone deacetylase 6(p-HDAC6) forms phase separation in the transcriptionally activated region of chromatin in the nucleus of triple-negative breast cancer cell, thereby affecting chromatin organization and promoting tumor growth [85].
Dysfunction of DNA methylation modifiers can lead to disrupted gene expression and compromised chromosome stability. Tet methylcytosine dioxygenase 1 (TET1) is crucial for the deposition of 5hmC, H3K27me3, and H2AK119 ubiquitylation at pericentric heterochromatin. TET1 condensates recruit E3 ubiquitin-protein ligase RING2 to pericentric heterochromatin, influencing chromocenter clustering (Figure 4 and Table 1) [86].
5.2 Phase Separation in Transcription and Translation Regulation
Dysregulated transcription and translation, key steps in gene expression, can trigger uncontrolled cancer cell proliferation. Recent studies show that LLPS is crucial in regulating the progression of both transcription and translation (Figure 4 and Table 1).
The C-terminal domain (CTD) of the RPB1 subunit in human RNA polymerase (Pol) II consists of a repetitive, unstructured, low-complexity protein region [87]. The FUS, EWS, and TAF15 proteins can undergo phase separation, bind directly to the CTD of RNA Pol II, and activate transcription [88]. Recent studies also show that transcription factors (TFs), RNA Pol II, chromatin regulators, and coactivators form condensates (known as SEs) through phase separation at highly transcribed genes. TFs like OCT4 and GCN4 have been observed to form phase-separated condensates with the mediator complex, a crucial coactivator [46, 89]. Sabari et al. demonstrated that transcriptional coactivators, such as bromodomain-containing protein 4 (BRD4) and mediator complex subunit 1 (MED1), are concentrated in nuclear puncta and exhibit liquid-like properties [90]. Furthermore, strong evidence shows that coactivators like Yes-associated protein (YAP) and PDZ-binding motif (TAZ) organize cofactors to enhance target gene expression via LLPS [91]. YAP associates with the transcription factor TEAD4, histone acetyltransferase EP300, and Mediator1 to form transcriptional hubs that promote target gene transcription [92]. Studies have shown that Epstein–Barr virus (EBV) nuclear antigen 2 (EBNA2) and its coactivator EBNALP undergo LLPS through their IDRs at active SEs, interacting with several TFs to enhance their own transcription [93]. BP1, a reader of H3K27me3, has two IDRs and forms dynamic nuclear puncta that undergo LLPS. IDR2 in BP1 plays a key role in droplet formation, contributing to liquid-like properties and condensate formation. Phase separation of BP1 regulates its interaction with PRC2 and recognition of H3K27me3, ensuring transcriptional repression [94]. Recently, Du et al. revealed the relationship between transcriptional condensates and gene locus distance regulation of gene expression. Transcriptional condensates Pol Ⅱ enhances burst size and frequency when in proximity of SRY-Box transcription factor 2 (SOX2) gene [128]. EWS-FLI1 condensates bind to the protein chaperone network and regulates the transcription gene targets [95]. IRFs is a large family of transcription factors, and phase separation of IRFs family can activate downstream immune-related genes, such as KAT8-IRF1 activates PD-L1 transcription via LLPS [66], and deacetylation increases IRF3/7 phase separation activate IFN-stimulated genes (ISGs) transcription [67]. Ji et al. found that FOXA1 forms biomolecular condensates, acting as a pioneer transcription factor that binds to condensed chromatin and facilitates local chromatin opening for gene expression [96].
The translation machinery controls the quality and quantity of newly synthesized peptides, influencing cellular adaptation. P bodies and SGs have long been recognized for their role in regulating mRNA translation. 5-aminolevulinate synthase (HEM1) condensates are formed through the interaction of plant-specific LCD domain with a large number of translation factors (eIF family), and the formation of HEM1 condensates effectively inhibits over translation during immune activation [97]. Plant exposure to warm conditions can rapidly induce GRP7 condensates, contributing to the formation of stress particles that recruit RNA, and translation mechanism components eIf4e and mRNA chaperone cold shock protein 1 (CSP1) and CPS3, which inhibiting translation [98]. Under stress conditions, translation factors (TrFs, including eIFs, eEFs, and eRFs) are absorbed into natriuretic peptide receptor 1 (NPR1) condensates after salicylic acid treatment. However, it remains unproven whether NPR1 condensates with TrFs affect their availability. It is likely that TrFs in NPR1 condensates influence their availability for translation [99]. Lin et al. revealed that FMR1 autosomal homolog 1 (FXR1) expression increases in the late stage of spermatogenesis, phase separation occurs to form FXR1 particle enrichment target mRNA, and then interacts with translation initiation factors such as EIF4G3 to recruit translation machines to activate mRNA translation stored in FXR1 granules [100]. Yang et al. revealed that fibrillarin (FBL) undergoes LLPS to form agglomerates through GAR and RNA binding domains, coordinates nucleolar formation and early processing of pre-RNA, and ultimately enhancing the translation of myc proto-oncogene protein (MYC) and other oncogenes [101]. In zebrafish, Ddx3xb undergoes LLPS through its N-terminal IDR, and increased ATP content promotes Ddx3xb condensates during the maternal-to-zygotic transition. Phase separation of Ddx3xb helps unwind the 5'UTR of maternal mRNAs, enhancing translation [102]. Chen et al. found that high levels of O-GlcNAc modification on YTHDF1/3 improved its dynamics in phase separation, accelerated depolymerization, interfered with the recruitment of translation-promoting factors, and inhibited its translation-promoting function on m6A-RNA [103]. Zhuang et al. revealed the role of ataxin 2(ATXN2) and ATXN2L-mediated phase separation in the regulation of circadian oscillation translation. As RNA-binding proteins (RBPs), ATXN2 and ATXN2L coordinate the spatiotemporal rhythmic recruitment of translation mechanisms and specific mRNA, thereby acting as major regulators of mammalian circadian translation [104].
5.3 Phase Separation in Posttranslation Regulation
Posttranscriptional modification is one of the key factors affecting LLPS, and LLPS can also regulate the process of posttranscriptional modification (Figure 4 and Table 1).
Phosphorylation is the most widely distributed posttranslational modification known. It is estimated that nearly one-third of the proteins in eukaryotes can be phosphorylated at any given time. Kinases are key executive factors in phosphorylation. Early studies show how kinases recognize LLPS events in ubiquitin condensates and SGs, either directly or indirectly. TANK binding kinase 1 (TBK1) and unc-51 like autophagy activating kinase 1 (ULK1) detect ubiquitin aggregates, maintain their kinase activity, and initiate downstream signaling while recruiting aggregates [105, 106]. SGs and P-bodies recruit several kinases, such as S6K, PKCα, PKR, Rio2, Sky1, DYRK3, and CK2. These kinases are highly active within the condensates, phosphorylating proteins and regulating the assembly and disassembly of aggregates [107]. Kinases themselves act as a scaffold for LLPS to occur, including PLK4, PKA, FAK, LCK, RTKs and oncogenic kinase fusion proteins (EML4-ALK, BCR-Abl, CCDC6-RET) [107]. The dynamic, multivalent nature of condensates may broaden the substrate repertoire of kinases by providing a wider variety of binding sites, beyond what traditional sequence analysis predicts. Therefore, by means of phase separation, kinases can achieve excessive activation of kinases and increase the frequency of phosphorylation modification.
Protein ubiquitination is an important process of protein quality control and signal transduction. Polyubiquitin chains have also been shown to drive LLPS. Li et al. found that spartin promotes the formation of Itch condensates [108] by activating Itch through its PPAY motif platform, which is created by self-oligomerization. This targets the WW12 domains of Itch, releasing its autoinhibition. Spartin-induced activation and autoubiquitination of Itch lead to LLPS of polyubiquitinated Itch, along with Spartin and E2, forming a positive feedback loop. The catalytic HECT domain of Itch is sufficient for phase separation with poly-, but not oligo-, ubiquitin chains. Other HECT E3 ligases also exhibit LLPS-mediated promotion of ligase activity. Wei et al. found that RNF168 undergoes LLPS, which reduces its recruitment to DNA damage sites and interferes with mono-ubiquitination of H2A [109]. Therefore, LLPS regulates the ligase activity of the E3 ligases.
5.4 Phase Separation Regulates Signaling Pathway
Cellular signal transduction is a complex and highly efficient biological process, and LLPS plays unique roles in signal initiation, transmission, and amplification. Key pathways such as DNA damage repair, Ras, autophagy, p53, and immune signaling are critical for regulating cell fate in cancer. In the following sections, we will elaborate on these aspects in detail.
5.4.1 Phase Separation in DNA Damage Repair Signaling Pathway
DNA damage repair (DDR) ensures genomic integrity and stability, and defects in DDR can lead to oncogenic mutations. In response to DNA damage, LLPS promotes the formation of repair compartments through two distinct mechanisms involving various DDR mediators (Figure 5 and Table 1). Upon DNA damage, MRE11–RAD50–NBS1 complexes bind to DNA damage sites, recruiting damage-induced lncRNAs (dilncRNAs), which then attract p53-binding protein 1 (53BP1) to facilitate DNA repair foci formation via LLPS [112, 113]. This repair mechanism, common in cancer cells, helps avoid apoptosis. Another pathway begins with the nucleation of PAR with intrinsically disordered proteins like FUS at DDR sites [114]. FUS is directed to these sites by the formation of long, branched PAR130 chains. After PAR signaling ends, 53BP1 accesses the damage sites, forming liquid-like compartments to facilitate further signaling and repair. Wei et al. show that SUMOylated RNF168 undergoes LLPS, which limits its recruitment to DNA damage sites, reduces H2A ubiquitination, and impairs nonhomologous end joining repair by restraining 53BP1 in nuclear condensates [129]. Qin et al. found that RAP80's LLPS potential is necessary for its accumulation at DNA double-strand break (DSB) sites and recruitment of BRCA1. PARylation-dependent inhibition of CycT1 phase separation promotes DNA repair and cell survival after DNA damage [115]. Bao et al. report that the mitotic deacetylase complex (MiDAC) regulates DNA end synapsis during nonhomologous end-joining (NHEJ) repair by removing acetyl marks on H2A near DSBs, preventing excessive accumulation of BRD4, which could otherwise undergo LLPS with KU80 and interfere with LIG4-XRCC4-XLF function at DSB ends [110]. In addition, wang et al. also reviewed the function of proteins, including MDC, DDX3X, RAD52, NONO, MRNIP and MtSUVR2, in the formation of DNA damage repair foci [98].
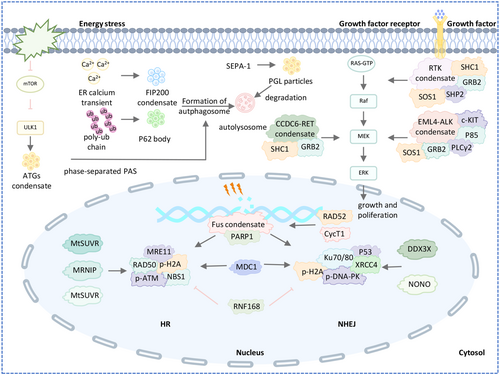
5.4.2 Phase Separation in Ras Signaling Pathway
SOS, a key Ras activator, is autoinhibited in the cytosol and activated upon membrane recruitment. Huang et al. showed that the LAT–Grb2–SOS phase transition at the membrane prolongs SOS dwell times and boosts Ras activation [36]. SHP2, a non-receptor protein tyrosine phosphatase, is crucial for Ras-MAPK signaling. Zhu et al. identified a common LLPS behavior in disease-associated SHP2 mutants, where LLPS is mediated by the PTP domain through multivalent electrostatic interactions and regulated by autoinhibition. These mutants activate wild-type SHP2 in LLPS, promoting Ras/MAPK activation [111]. Qiu et al. revealed that CCDC6-RET undergoes LLPS, highlighting the link between LLPS and kinase activity. RET fusion protein constitutively activates the Ras/MAPK pathway [49]. Tulpule et al. found that EML4-ALK forms cytoplasmic condensates that activate Ras and MAPK signaling (Figure 5 and Table 1) [130].
5.4.3 Phase Separation in Autophagy Signaling Pathway
Autophagy refers to the process by which eukaryotes transport malfunctioning organelles or biomacromolecules through bilayer vesicles to vacuoles or lysosomes for degradation to promote recycling. Recent studies have shown that autophagy can degrade liquid condensates, and the PAS is also undergoing LLPS to regulate autophagosomal formation (Figure 5 and Table 1). In the process of autophagy, there are several important ubiquitin-like conjugation systems, which play a key role in autophagy. p62 recognizes polyubiquitin chains on target proteins and forms p62 bodies through phase separation mechanism, thereby mediating the formation of autophagosomes [33]. The calcium transient on the surface of the endoplasmic reticulum under autophagy induction is a key signal that determines the formation of autophagosomes on the endoplasmic reticulum. The calcium transient on the surface of the endoplasmic reticulum caused the LLPS of FIP200 complex, and the formed FIP200 condensate bound to the endoplasmic reticulum protein and became the initiation site of the autophagosome [117].
In addition to regulating the phase separation of autophagy initiation, some biological macromolecular agglutinates can be degraded through the autophagolysosomal pathway (Figure 5 and Table 1). Zhang et al. found that PGL particles are assembled by LLPS, and the size and biophysical properties of PGL particles determine their autophagy degradation efficiency. The receptor protein SEPA-1 promotes the phase transition of PGL-1/3 [118]. The scaffold protein EPG-2 controls the size of PGL-1/-3 particles and promotes their conversion from liquid to hydrogel state. At elevated temperatures, mTOR-mediated phosphorylation of PGL-1/-3 is enhanced, accelerating the phase transition of PGL-1/3 and preventing their degradation by autophagy. The accumulated PGL particles are necessary for the normal development of nematode embryos under heat-stress conditions. Zhang et al. reveal a novel mechanism by which mTOR, as a heat stress receptor, regulates the phase transition of PGL particles to control their autophagy degradation and protect nematodes from heat stress during embryonic development [64]. Fujioka et al. showed that Atg1-complex droplets can be tethered to membranes via specific protein–protein interactions, explaining the vacuolar membrane localization of the PAS [68]. Guan et al. proposed that phase separation of ATG8e in Arabidopsis regulates autophagy and demonstrated the important role of ATG3 in enhancing LLPS of ATG8e [119].
5.4.4 Phase Separation in P53 Signaling Pathway
p53 is a transcription factor and guardian of the genome, preventing malignant transformation. Beyond apoptosis, senescence, and cell cycle arrest, p53 plays a growing role in tumor suppression. However, destabilized p53 mutations lose these functions, leading to malignancy. Mutant p53 is found in 50% of tumors and often forms amyloid-like aggregates (Figure 5 and Table 1) [120]. DNA binding domain (DBD) contributes to phase separation of p53 [131]. Deletion of DBD region inhibited p53 phase separation ability. Some mutants in DBD domain of p53 affect p53 phase separation. For example, the R248Q, R248W, R175H, and Y220C mutants are more prone to aggregation than others. p53 droplet formation is regulated by crowding, nucleic acids, and posttranslational modifications. Zong et al. showed that site-specific lactylation of p53 reduces its DNA binding and LLPS, thereby impairing its tumor-suppressive function [73]. In DNA damage repair, P53 droplets depend on HP1a to form and locate in heterochromatin. Cellular senescence is regulated by P53 accumulation in the nuclear foci and phase separation [132]. In addition, p53 is still important in neurodegenerative diseases [133]. The aggregates of various neurodegenerative diseases, including prions, α-synuclein, Aβ peptide, and tau, interact with p53 to influence the development of the disease.
5.4.5 Phase Separation in Immune Signaling
Immune signaling pathways transform pathogenic stimuli into cytosolic events that resolve infections. Recent studies on phase separation in immunology offer new insights into immune responses (Figure 6 and Table 1).
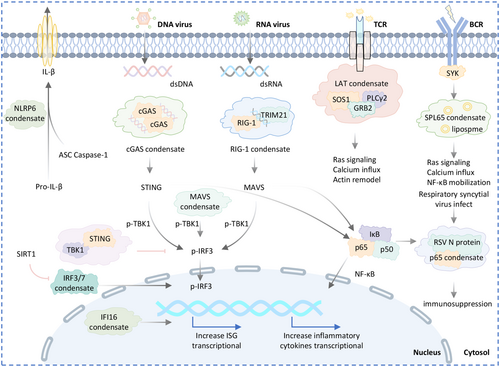
Immune cell surface receptors, along with ligands and downstream binding partners, can form clusters ranging from nanometers to micrometers on the plasma membrane, including T cell receptors (TCR) and BCR [121]. LAT, GRB2, and SOS1 are key proteins that form oligomers in solution, contributing to T cell microclusters. LAT condensates promote tyrosine phosphorylation, a key activation marker for TCR signaling [122]. SLP65 condensates enhance calcium signaling and other downstream pathways, potentially utilizing vesicle-associated membrane protein 7 (VAMP7)-positive vesicle trafficking to efficiently deliver BCR signaling components to the plasma membrane for signal transduction [134].
Phase separation regulates both plasma membrane and intracellular immune signaling. DNA binding to cGAS triggers the formation of liquid-like condensates [53], which protects DNA from degradation by three prime repair exonuclease 1 (TREX1) and activates STING, leading to type I interferon and pro-inflammatory cytokine expression [135]. Yu et al. found that STING condensates restrain STING and TBK1 to prevent overactivation of innate immunity [34]. Additionally, interferon-gamma inducible protein 16 (IFI16) binding to viral DNA initiates LLPS and cytokine production during herpes simplex virus infection [124]. About initiation of the immune response against RNA viruses. Haubrich et al. showed that RNA binding triggers TRIM25 LLPS, which recruits RNA sensor RIG-I (RIG-I) and enhances its ubiquitylation by TRIM25. TRIM25 catalyzes K63-linked ubiquitylation of RIG-I, enabling its interaction with mitochondrial antiviral-signaling protein (MAVS) and promoting antiviral signaling and interferon production [125]. PIAS3-induced poly-SUMOylation promotes polyubiquitination of lysine 63 junctions and formation of MAVS condensates after viral infection [123]. In RSV-infected cells, the NF-κB subunit p65 is sequestered into perinuclear puncta, along with MAVS and MDA5, which are recruited to inclusion bodies to suppress interferon β expression [126]. The acetylation of two lysine residues in theDBD of IRF3/IRF7 prevents their phase separation. Zhou et al. showed that SIRT1-mediated deacetylation of these lysine promotes LLPS, which is essential for interferon transactivation and antiviral immunity activation [127].
Inflammasomes are protein complexes that activate caspases and release pro-inflammatory cytokines during infection or cellular damage. They consist of a sensor protein, an effector caspase, and often an adaptor protein. NLRP6, a sensor of inflammasomes, undergoes LLPS, which is crucial for inflammasome assembly and responses to viruses or intestinal microbiota [46].
6 Phase Separation and Tumor Hallmarks
LLPS has emerged as a fundamental process driving tumor initiation and progression. Over the past decade, numerous LLPS-driven events have been identified as key regulators of tumor cell fate, influencing oncogenesis, metastatic potential, metabolic adaptation, therapeutic resistance, and immune evasion. Our review comprehensively summarizes the critical phase-separating proteins and their mechanistic roles across various tumor types (Table 1). These findings highlight the therapeutic potential of targeting LLPS-related molecules for cancer treatment.
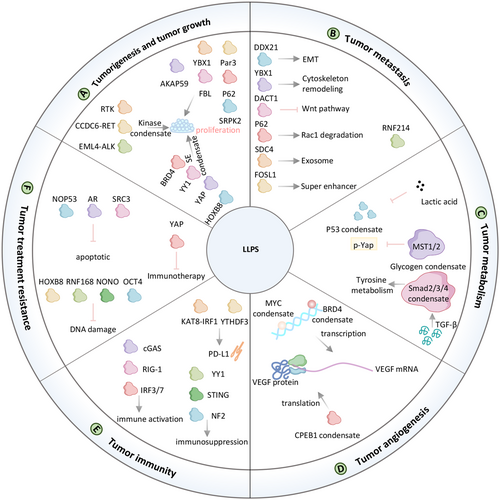
6.1 Phase Separation in Tumorigenesis and Tumor Growth
Kinase-mediated regulatory pathways are central to tumorigenesis and development [136]. Among these, the receptor tyrosine kinase (RTK) pathway is a critical driver of tumor cell proliferation and survival. These growth factor receptors can rapidly activate their downstream growth signals, including MEK/MAPK, through intracellular domain-driven LLPS (Figure 7 and Table 2) [107]. Fusion gene expression caused by chromosome rearrangement can also promote the development of tumors, such as EML4-ALK and CCDC6-RET. The EML4-ALK fusion, first identified in non-small cell lung cancer (NSCLC) patients by Soda et al. in 2007, is present in approximately 3%–7% of NSCLC cases. This fusion protein activates key signaling pathways, including MAPK, PI3K, and STAT3, to promote tumor growth. Ji et al. demonstrated that EML4-ALK condensates facilitate downstream pathway activation [130]. Recently, Qiu et al. found that fusion protein CCDC6-RET form condensates and activate Ras/MAPK signaling pathways to promote tumorigenesis in thyroid papillary cancer [49].
| Liquid–liquid phase separation in tumor biology | ||||||||
|---|---|---|---|---|---|---|---|---|
| Key protein | Cancer type | Function | References | |||||
| RTK | Pan cancer | Activate downstream growth signals promoting proliferation | [107, 136] | |||||
| CCDC6-RET | Thyroid papillary cancer | Enhance Ras/MAPK signaling promoting tumorigenesis | [49] | |||||
| EML4–ALK | Lung cancer | Activate Ras downstream signaling pathways promoting proliferation | [107] | |||||
| BRD4 | Pan cancer | Active oncogene transcription promoting proliferation | [137, 138] | |||||
| β-catenin | Pan cancer | Transcriptional co-regulator and increased cell proliferation | [139] | |||||
| YY1 | Pan cancer | Forming an enhancer cluster or super-enhancer and promoting tumor growth | [43] | |||||
| YAP | Pan cancer | Form a transcriptional hub and promote tumor cell growth | [92, 140-142] | |||||
| AKAP95 | Breast cancer | Regulates transcription and RNA splicing, then supports tumorigenesis | [143] | |||||
| FBL | Acute Myelocytic Leukemia | Enhances the translation of MYC and promotes tumorigenesis | [101] | |||||
| P62 | Prostate cancer | Active the NRF2 pathway promoting tumor growth | [144] | |||||
| HOXB8/FOSL1 | Osteosarcoma | Activate abnormal tumor transcription program promoting tumor growth | [145] | |||||
| YBX1 | Lung cancer | Activate ANXA8 to regulate tumorigenesis | [146] | |||||
| Par3 | Breast cancer | Regulates the polarity establishment mediates tumorigenesis | [117, 147] | |||||
| SRPK2 | Pancreatic cancer | Mediated tumor growth by activated IGF1/PI3K/mTOR/S6K1 pa | [148] | |||||
| IGF2BP1 | Lung cancer | Promotes the progression of lung cancer mediated by c-myc signal | [127] | |||||
| DDX21 | Colorectal cancer | Regulates EMT pathway promotes metastasis | [129] | |||||
| DAZAP1 | Oral squamous cell carcinoma | Regulates EMT pathway promotes metastasis | [149] | |||||
| circRNA-YBX1 | liver cancer | Suppresses metastasis by cytoskeleton remodeling | [150] | |||||
| DACT1 | Bone metastasis | Repress Wnt signaling to promote bone metastasis | [151] | |||||
| FOSL1 | Head and neck cancer | Formation of super enhancers to drive metastasis | [152] | |||||
| RNF214 | Liver cancer | Promotes proliferation and metastasis | [153] | |||||
| NBR1/p62 | Lung cancer | promoting Rac1 degradation limited cancer metastasis | [154] | |||||
| SDC4 | Cervical cancer | Promotes the biogenesis of exosomes and cell migration | [69] | |||||
| P53 | Breast cancer | Lactylation of p53 reducing p53 tumor-suppressing roles | [73] | |||||
| Mst1/2 | Liver cancer | Phosphorylates YAP and promotes cancer progression | [155] | |||||
| Smad2/3/4 | Liver cancer | Promote the expression of TAT and inhibit apoptosis of cancer cell | [156] | |||||
| CPEB1 | Liver cancer | Regulating VEGF translation affects angiogenesis | [157] | |||||
| MYC | Pan cancer | Increase VEGF transcription promoting angiogenesis | [158] | |||||
| BRD4 | NA | Inhibiting CCNA1 expression and decreasing angiogenesis | [159] | |||||
| KAT8–IRF1 | Pan cancer | Promote transcription of PD-L1 mRNA and inhibit antitumor immunity | [66] | |||||
| YY1 | Prostate cancer | Upregulated IL-6 in M2 macrophages increasing prostate cancer progression | [160] | |||||
| NF2-IRF3 | Pan cancer | Suppressing inhibits the STING-initiated antitumor immunity | [161] | |||||
| YTHDF3 | Renal cancer | Downregulation of the downstream immune checkpoint PD-L1 and | [162] | |||||
| HOXB8/FOSL1 | Osteosarcoma | Regulates chromatin accessibility and regulates drug resistance | [145] | |||||
| OCT4/NRF1 | Prostate cancer | Activate the AR/FOXA1 axis and drug resistance | [163] | |||||
| RNF168 | Colorectal adenocarcinoma | Resistance to DNA-damaging agents | [129] | |||||
| Androgen receptor(AR) | Prostate cancer | Aberrant activation of ARE reporter activity and drug-resistance | [164] | |||||
| YAP | Lung cancer | Leading to tumor survival and immunotherapy resistance | [92] | |||||
| SRC3 | Multiple myeloma | Enhanced the expression of antiapoptotic gene BCL2 and resisted bortezomib | [165] | |||||
| NOP53 | Colorectal cancer | Inhibiting p53 activation enhances radio-resistant | [166] | |||||
| NONO | Pan cancer | Accelerating DNA repair and radio-resistance in tumor cell | [116] | |||||
| Liquid–liquid phase separation in tumor therapy | |||||||
|---|---|---|---|---|---|---|---|
| Targeting strategy | Representative drugs | Drug targets | Goal of drug targeting | References | |||
| Direct | Targeting of IDRs | PRIMA-1; MQ, ReACp53, Polyarginine | p53 mutants | Inhibited aggregated mutant p53 proteins induced cancer cell growth | [167, 168] | ||
| Elvitegravir | SRC-1 | Inhibits YAP oncogene transcription by disrupting SRC1 LLPS in SRC1/YAP/TEAD condensates | [169] | ||||
| PCG | BRD4 | Suppression of BRD4 LLPS-dependent gene transcription | [170] | ||||
| ET516 | AR | Disrupts AR condensates, and inhibits the proliferation and tumor growth of prostate cancer cells expressing AR-resistant mutants | [164] | ||||
| CIP-12 | CNBP | Blocking phase separation and interaction of CNBP with SMARCC2 inhibited ribosome biogenesis and NB progression | [95] | ||||
| CGX-635 | FBL | Elimination of AML by targeting FBL | [101] | ||||
| Targeting other domains | 2142-R8 | KAT8-IRF1 | Disrupts KAT8-IRF1 condensate formation and consequently inhibits PD-L1 expression and enhances antitumor immunity | [66] | |||
| ZZW-115 | NUPR1 | Inhibit NUPR1-dependent stress granules (SGs) and leading to cell death | [171] | ||||
| SHP099 and ET070 | SHP2 | Blockade of SHP2 phosphatase activity and LLPS, suppressed malignant transformation of SHP2E76K mesenchymal stem cells (MSCs) | [172] | ||||
| Targeting RNA-mediated LLPS | RNA silencing technology | RNA | RNA mediated LLPS | [152] | |||
| Alteration of the drug partitioning process | Cisplatin; mitoxantrone; tamoxifen, FLTX1, THZ1, JQ1 | SEs | Actively partition into SEs and enhance the therapeutic activity | [146] | |||
| Decondensation or recondensation | Oleic acid | cGAS | Dissolves the cGAS-DNA PS and inhibits immune surveillance of DNA | [173] | |||
| LY2835219 | FUS–ERG | Dissolved FUS–ERG condensates in reporter cell lines expressing Ewing sarcoma fusions | [174] | ||||
| Re-form | All-trans retinoic acid, Arsenic trioxide | PML-RARA | Recover PML bodies in acute promyelocytic leukemia | [175] | |||
| icFSP1 | FSP1 | IcFSP1-induced FSP1 condensates and promoted ferroptosis | [72] | ||||
| Indirect | RNA silencing technology | RNAs | Inhibit protein expression and LLPS | [129, 166] | |||
| SI-2 | SRC-3 | Reduces protein concentrations of SRC-3, sensitizes BTZ treatment and overcomes drug resistance | [165] | ||||
| JQ1 | BRD4 | Blocking condensate-mediated transcriptional co-activator BRD4 activity inhibits tumorigenesis | [141] | ||||
| TBCA | CK2 | Promote SG disassembly via inhibited phosphorylation of G3BP1 | [176] | ||||
- Abbreviations: AKAP95, a-kinase anchoring protein 95; CNBP, cellular nucleic acid-binding protein; EMT, epithelial-mesenchymal transition; HOXB8/FOSL1, homeobox B8/fos-like antigen 1; MQ, methylene-3-quinuclidinone; NUPR1, nuclear Protein 1; PML-RARA, promyelocytic leukemia-retinoic acid receptor alpha; PRIMA-1, p53 reactivation and induction of massive apoptosis; RTK, receptor tyrosine kinase; SE, super-enhancer; SI-2, EPH 116 hydrochloride; TBCA, tetrabromocinnamic acid.
Dysregulated gene transcription, driven by genetic and epigenetic alterations, underpins uncontrolled cancer cell proliferation SEs, which comprise clusters of enhancer elements, transcription factors, and associated modifications, play a pivotal role in this process. SE-regulated genes are strongly associated with tumorigenesis, including the Wnt/β-catenin pathway, which forms condensates at SEs in a Wnt-dependent manner [139]. Key transcriptional coactivators such as BRD4 and MED1 undergo phase separation at SEs, organizing the transcriptional machinery into functional condensates [137, 138]. YY1 facilitates enhancer-promoter interactions through phase-separated condensates, further driving tumor growth [43, 177]. Additionally, YAP and TAZ, downstream effectors of the Hippo pathway, form transcriptional hubs with TEAD4, EP300, and other components, amplifying oncogenic gene expression and contributing to cancer pathophysiology [92, 140-142]. Intracellular phase-separated structures also regulate mRNA processing to promote tumorigenesis. For instance, AKAP95, a nuclear protein involved in RNA splicing, forms dynamic condensates that support oncogenic transformation [143]. Yang et al. revealed that FBL undergoes LLPS to enhance the translation of MYC and other oncogenes to promote tumorigenesis [101].
Nuclear factor erythroid 2-related factor 2 (NRF2) is the master regulator of the cellular antioxidant response. NRF2, a master regulator of antioxidant responses, is activated in prostate cancer through SPOP mutant-mediated p62 LLPS, reducing reactive oxygen species and promoting tumor growth [144]. Disruption of HOXB8 and FOSL1 phase separation inhibits SE-driven chromatin accessibility and RNA polymerase II release, significantly attenuating osteosarcoma growth [145]. MELTF-AS1 regulates tumorigenesis by driving phase separation of YBX1 to activate ANXA8 [146]. Par3 condensates act as a mechanical mediator of breast cancer tumorigenesis [117, 147]. SRPK2 mediates SG formation mediated tumor growth through overactivation driven by the IGF1/PI3K/mTOR/S6K1 pathway [148]. LLPS of IGF2BP1 is regulated by MNX1-AS1, which promotes the growth of lung cancer mediated by stabilizing c-myc and E2F1 mRNA [127].
6.2 Phase Separation in Tumor Metastasis
The activation of epithelial–mesenchymal transition (EMT) is a critical mechanism in cancer metastasis, enabling epithelial cells to adopt mesenchymal traits that enhance motility and invasiveness. The review by Guo et al. provides a detailed summary of reports of phase separation on EMT (Figure 7 and Table 2) [178]. Recently, Gao et al. reported that DDX21 phase separation promotes metastasis of colorectal cancer by MCM5-dependent EMT pathway [129]. Zhang et al. found that DAZAP1 was elevated in oral squamous cell carcinoma(OSCC) and enhanced COX16 expression by liquid–liquid separation, promoting tumor growth and metastasis via EMT [149]. Phase separation of circRNA-YBX1 mediates cytoskeleton remodeling, which suppresses liver cancer metastasis [150]. The Wnt signaling pathway, is a well-organized cascade that has been reported to play important roles in cancer metastasis. Esposito et al. demonstrated that transforming growth factor-β (TGF-β) induced dishevelled binding antagonist of beta catenin 1 (DACT1) biomolecular condensates repress Wnt signaling to promote bone metastasis [151]. FOS Like 1 (FOSL1) phase separation with CYTOR and formation of SEs to drive metastasis in head and neck squamous cell carcinoma [152]. He et al. revealed that ring finger protein 214 (RNF214) functions as an oncogenic protein, driving cancer metastasis through phase separation [153]. P62 body limited cancer metastasis by promoting Rac family small GTPase 1 (Rac1) degradation [154]. During metastasis, cancer cells navigate the tumor microenvironment under physical constraints. Das et al. highlighted Syntenin as a key regulator of prostate cancer metastasis, suggesting its potential as a therapeutic target [179]. Zhao et al. further elucidated the role of SDC4, whose cytoplasmic domain undergoes phase separation to form condensates on the cytomembrane. These condensates recruit syntenin via interactions between the SDC4 C-terminal motif and syntenin's PDZ domain. Dephosphorylation of SDC4 at Ser179 drives a phase transition essential for syntenin secretion within exosomes, with functional experiments confirming that inhibiting SDC4 phosphorylation reduces cell migration [69].
6.3 Phase Separation in Tumor Metabolism
Compared to normal tissue, tumor cells tend to undergo metabolic reprogramming. Many groups have identified that LLPS proteins regulate different metabolic processes (Figure 7 and Table 2). For example, CDC19 condensates regulates glycolysis [180], CTP filament regulates pyrimidine metabolism [181], reversible aggregation of ACC1 regulates fatty acid metabolism [182], and mTOR regulates energy stress [183]. Recent findings highlight the involvement of LLPS in tumor-specific metabolic adaptations. In liver cancer, glycogen accumulation through LLPS inhibits Mst1/2-mediated YAP phosphorylation, promoting tumor progression. Under hypoxic conditions or increased energy demand, glycolysis-related enzymes form glycolytic bodies (G bodies) via LLPS, accelerating glycolysis to meet energy requirements [155]. Mitochondrial protein tyrosine aminotransferase TAT is involved in the breakdown of tyrosine and the conversion of tyrosine to p-hydroxyphenylpyruvate. Activation of TGF-β signaling pathway can promote the formation of condensates of smad2/3/4 complexes, which increase TAT expression and break down tyrosine into p-hydroxyphenylpyruvate, thereby increasing tumor cell apoptosis [156]. The tumor cell metabolite lactic acid lactated p53 via alanine-tRNA ligase (AARS1). Lactylation of p53 attenuates their DNA-binding and LLPS, thereby reducing p53 tumor-suppressing roles [73].
6.4 Phase Separation in Tumor Angiogenesis
Angiogenesis is a hallmark of various malignancies, including melanoma, breast cancer, colorectal cancer, non-small cell lung cancer, and renal cell carcinoma. A complex interplay of pro- and antiangiogenic biomolecules regulates this process [184]. Various biomolecules that promote or inhibit angiogenesis constitute a complex and dynamic angiogenic system (Figure 7 and Table 2). Vascular endothelial growth factor A (VEGF) is an important factor regulating angiogenesis. VEGF mRNA is reported to be post-transcriptionally regulated by cytoplasmic polyadenylation element-binding proteins (CPEB1). Vittorio et al. found that VEGF 3′-UTR contains various CPE elements, making it susceptible to being regulated by CPEBs [157]. Liu et al. uncovered that CPEB1 interaction with target messenger RNA in the liquid-like condensates increased the translation of target genes [95]. Therefore, CPEB1 condensates may affect angiogenesis by regulating VEGF translation. In metastatic cells, MYC phase-separated transcription condensates drive VEGF transcription, promoting angiogenesis [158]. Conversely, 1,6-hexanediol, an LLPS inhibitor, disrupts BRD4 phase separation in HVUECs, reducing CCNA1 expression and inhibiting angiogenesis [159].
6.5 Phase Separation in Tumor Immunity
The immune system plays a pivotal role in cancer initiation and progression, LLPS is characterized in the context of both the cell surface receptor pathways including the TCR, BCR signal and the cytosolic signaling pathways including the cGAS-STING, RIG-I, and NFκB pathway (Figure 7 and Table 2) [121]. On the one hand, the inflammatory response caused by the immune system will induce carcinogenesis, on the other hand, cancer cells will escape the attack of the immune system. For instance, Wu et al. demonstrated that KAT8–IRF1 condensates promote IRF1 K48 acetylation, enhancing its binding to the CD247 (PD-L1) promoter and recruiting transcriptional machinery to upregulate PD-L1 expression, thereby suppressing antitumor immunity [66]. Tumor-associated macrophages (TAMs), particularly the M2 phenotype, remodel the tumor microenvironment by secreting cytokines that facilitate tumor progression. In M2 macrophages, phase separation of the YY1 complex upregulates IL-6 by enhancing enhancer-promoter interactions, thereby promoting prostate cancer progression [160].
The immune system relies on the RLR-MAVS and cGAS-STING pathways to detect microbial invasion and support tumor immune surveillance. However, tumors frequently evade immune clearance. Meng et al. revealed that neurofibromin 2 (NF2) enhances innate immunity by activating TBK1, whereas missense mutations in the FERM domain of NF2 (NF2m) form NF2m-IRF3 condensates that suppress TBK1 activation, thereby inhibiting STING-mediated antitumor immunity [161]. YTHDF3 was found to have phase separation characteristics and enhanced the degradation of its target mRNA HSPA13 by phase separation, which resulted downregulation of the downstream immune checkpoint PD-L1 and promoted ccRCC progression and immune evasion [162].
6.6 Phase Separation in Treatment Resistance
Given its central role in tumorigenesis, LLPS also influences tumor drug resistance (Figure 7 and Table 2). In the past, LLPS involvement in drug resistance has been partially reported. HOXB8/FOSL1 [145], OCT4/NRF1 [163], YAP/TAZ/SRC1 [169] condensates complex regulate drug resistant.
Recent research has further elucidated these mechanisms. Wei et al. demonstrated that RNF168 undergoes LLPS to regulate DNA damage repair. SUMO-specific peptidase 1 (SENP1), a deSUMOylase highly expressed in colorectal adenocarcinoma, reduces RNF168 SUMOylation in response to DNA damage, preventing RNF168 condensate formation and enhancing repair efficiency, thereby conferring resistance to DNA-damaging agents [129]. In castration-resistant prostate cancer, mutations in the androgen receptor (AR), such as AR(W742C), convert the antagonist bicalutamide into an agonist [164]. AR(W742C) exhibits increased binding affinity for bicalutamide, promoting phase separation and transcriptional activation, which underlies resistance to antiandrogen therapies. Yu et al. described a resistance mechanism of anti-PD-1 immunotherapy. Yu et al. identified a resistance mechanism to anti-PD-1 immunotherapy, where IFN-γ induces YAP nuclear phase separation and transcriptional activation, enabling tumor survival and immunotherapy resistance independent of canonical ISGs [92]. In multiple myeloma, Nuclear receptor binding SET domain protein 2 (NSD2) interacts with SRC3 to promote its phase separation. SRC3 condensates enhanced the expression of antiapoptotic gene BCL2 and resisted bortezomib on tumor [165]. NOP53 ribosome biogenesis factor (NOP53) condensates inhibit p53 activation enhances radio-resistance in colorectal cancer [166]. Elevated non-POU domain containing octamer binding (NONO) expression in colorectal cancer tissues facilitates LLPS-mediated recruitment of DNA-dependent protein kinase and nuclear EGFR, promoting their phosphorylation and accelerating DNA repair, thereby contributing to radio-resistance [116].
7 Phase Separation in Cancer Therapy
As the critical role of LLPS in tumor biology becomes increasingly understood, various strategies for targeting LLPS in cancer treatment have been proposed. Despite existing challenges, the potential for developing new therapeutic approaches remains promising. There are two primary strategies for targeting condensates in cancer therapy (Table 2). The first involves directly targeting the core molecules of the condensates or altering their physical and chemical properties to inhibit condensate formation. The second approach focuses on indirectly preventing condensate formation by inhibiting the expression of related proteins or interfering with upstream and downstream regulatory molecules.
7.1 Directly Targeting Phase Separation Formation
Direct targeting strategies predominantly rely on small molecule compounds or peptide-derived agents to engage with specific biomolecular targets, effectively interfering with the formation of the structural underpinnings necessary for phase separation. The selection of targets for these interventions should focus on key scaffold proteins, which play a pivotal role in orchestrating the formation and stability of liquid–liquid phase-separated condensates.
7.1.1 Targeting Intrinsically Disordered Region
In traditional targeted therapies, the focus is typically on protein regions with well-defined secondary and tertiary structures. However, IDRs, which are prevalent in oncoproteins and transcription factors, have historically been considered challenging therapeutic targets due to their lack of stable structures [185]. Recent research has demonstrated that small molecule compounds BI-3802 has been identified as a selective inhibitor that specifically binds to the IDR in the TAF2 subunit of TFIID, thereby modulating TFIID activity and impacting transcription initiation [186]. Similarly, YK-4-279 binds to the IDR of EWS-FLI1, inhibits the interaction between EWS-FLI1 and RNA helicase A, and thus delays the growth of EWS cells [187]. Additionally, IIA4B20, IIA6B17, and mycmycin-1/2 bind to disordered MYC with high specificity and can effectively inhibit MYC-induced cellular phenotypes [188, 189].
Recent studies have demonstrated that small molecule inhibitors can bind to the IDRs of tumor-related proteins, thereby regulating tumor progression by affecting the formation of biomolecular condensates (Table 2). For instance, PRIMA-1 and 2-methylene-3-quinuclidinone (MQ) inhibit p53 aggregation and restore its activity, offering a potential strategy for cancer treatment. Similarly, the cell-penetrating peptide ReACp53, along with polyarginine analogs like polyornithine, canavanine, and citrulline, inhibits mutant p53 aggregation and suppresses the proliferation of cancer cells harboring p53 mutations [167, 168].
Elvitegravir (EVG), an anti-HIV drug, directly binds to the disordered SRC1, disrupting SRC-1/YAP/TEAD condensates and limiting cancer cell growth in a YAP-dependent manner [169]. Moreover, PCG, a natural compound found in Chinese herbal medicine, specifically binds to the IDR of BRD4, converting phase-separated BRD4 into static aggregates, which leads to the inhibition of BRD4-dependent gene transcription in tumor cells [170].
In castration-resistant prostate cancer, AR mutations or splice variants play a critical role. ET516 specifically targets disordered AR transcriptional condensates, inhibiting AR activity and suppressing the proliferation of prostate cancer cells expressing AR resistance mutants [164].
Cellular nucleic acid-binding protein (CNBP) forms liquid condensates that inhibit SWI/SNF core subunits (SMARCC2/SMARCC1/SMARCA4) by interacting with SMARCC2, promoting 18S rRNA processing in tumor cells. The cell-penetrating peptide CIP-12 binds to disordered CNBP, blocking its phase separation and inhibiting ribosome biogenesis, thereby suppressing neuroblastoma progression [95].
FBL is a key nuclear protein whose phase separation regulates acute myeloid leukemia (AML) cell survival. CGX-635 targets FBL's phase separation ability, leading to the elimination of AML cells [101].
7.1.2 Targeting Other Domains
Targeting IDRs is highly challenging, and existing studies have reported that phase separation can also be disrupted by targeting core protein interactions or other ordered domains. For example, PD-L1, a critical immune checkpoint, can be inhibited by the 2142-R8 peptide, which disrupts KAT8-IRF1 binding and condensate formation, suppressing PD-L1 transcription and enhancing antitumor immunity [66]. Nuclear Protein 1 (NUPR1) can induce the production of SGs. ZZW-115 binds to NUPR1's nuclear localization signal (NLS), inhibiting its nuclear translocation [171], reducing NUPR1-dependent SG formation, and triggering cell death in cells expressing KRAS-G12D [190]. SHP2 mutations can induce LLPS, and its allosteric inhibitors, SHP099 and ET070, can inhibit SHP2 phosphorylation, block LLPS, inhibit the overactivation of complexes I and III, and prevent the malignant transformation of SHP2E76K mesenchymal stem cells (MSCs) [172].
7.1.3 Targeting RNA Mediated Phase Separation
As highlighted above, RNA-driven biomolecular condensates play a significant role in cancer pathogenesis. Recently, RNA silencing technologies, including antisense oligonucleotides (ASO), RNA interference (RNAi), and CRISPR/Cas systems have been employed to regulate RNA-driven phase separation [191]. For example, lncRNA CYTOR promotes tumorigenicity and metastasis by facilitating FOSL1 phase separation and SE formation. ASO-mediated CYTOR loss disrupts FOSL1-dependent SEs, thereby inhibiting the growth and metastasis of head and neck squamous cgeneell carcinoma [152]. MNX1-AS1 and MTAR1 [192] promote IGF2BP1 protein-associated phase separation, while MELTF-AS1 [146] promotes YBX1 phase separation. RNA interference-mediated degradation of MNX1-AS1, MTAR1, and MELTF-AS1 inhibits tumor growth. Additionally, SNHG9, a tumor-promoting lncRNA, drives LATS1 droplet formation and inhibits the Hippo pathway. CRISPR/Cas-mediated SNHG9 knockout suppresses LATS1 phase separation and breast tumor growth [193].
7.1.4 Targeting Drug Partitioning
Traditional methods typically screen small molecule inhibitors that disrupt LLPS by directly interacting with condensate components. However, a pioneering study has revealed that the concentration and partitioning of anticancer drugs within condensates can significantly affect their therapeutic efficacy. Klein et al. discovered that anticancer drugs, including the broad-spectrum chemotherapeutic agent cisplatin, the topoisomerase inhibitor mitoxantrone, the estrogen antagonist tamoxifen, the cyclin-dependent kinase 7 (CDK7) inhibitor THZ1, and the BRD4 inhibitor JQ1, can enrichment in specific protein condensates (e.g., SGs) through physicochemical properties independent of the drug target. π–π or π–cation interactions are among the physicochemical properties that facilitate this molecular partitioning. The selective partitioning and concentration of small molecules in condensates contribute to the pharmacodynamics and therapeutic effects of these drugs. Interestingly, cisplatin exerts its anticancer activity by dissolving SEs, and altering the properties of the condensate can influence the concentration and activity of the drug. A deeper understanding of this phenomenon could enhance cancer treatment strategies [194].
7.1.5 Decondensation or Recondensation
Several compounds that affect phase separation by inducing decondensation or recondensation have been discovered. For instance, the fatty alcohol 1,6-hexanediol (HDO) can dissolve liquid-like condensates by disrupting weak hydrophobic protein–protein or protein–RNA interactions. However, the non-selectivity and cytotoxicity of HDO limit its application [195]. Microtubules are essential for SG formation, and microtubule-depolymerizing drugs such as nocodazole and vinblastine inhibit arsenate-induced SG formation [196]. Additionally, antioxidants like lipoamide and lipoic acid promote the dissolution of FUS-associated SGs. Bis-ANS and similar compounds are modulators of TDP43 condensates. At low concentrations, they strongly promote molecular LLPS, while at high concentrations, their negatively charged parts disrupt droplets through decondensation driven by electrostatic repulsion [197]. Wippich et al. discovered that the kinase tyrosine-phosphorylation-regulated kinase 3 (DYRK3) can drive the dissolution of SGs to release mTORC1. GSK-626616 inhibits DYRK3 activity, promotes SG reaggregation, and inhibits mTORC1 signaling [198].
Small molecule compounds that regulate tumor-related phase separation through decondensation or recondensation have been discovered. For instance, oleic acid (OA) dissolves the phase separation of cGAS-DNA, thereby weakening cGAS-mediated antiviral and anticancer immune surveillance [173]. The expression of many pathogenic fusion proteins is closely related to cancer progression. In Ewing sarcoma, the FUS-ERG fusion protein drives phase separation and is associated with the abnormal expression of target genes. High-throughput screening has identified LY2835219 as an effective compound that dissolves FUS-ERG condensates in Ewing sarcoma, partially rescuing the abnormal expression of target genes [174].
Traditionally, the primary strategy for tumor treatment has been the destruction of condensates. However, recent studies have shown that promoting the formation of condensates can also be an effective anticancer strategy. For example, the production of the PML/RARA fusion protein is a key driver of acute promyelocytic leukemia (APL). PML/RARA disrupts PML nuclear bodies (NBs) and leads to the transcriptional repression of differentiation genes. The restoration of PML nuclear bodies by retinoic acid (RA) and arsenic has proven to be an effective treatment for APL [175]. Additionally, ferroptosis is a newly discovered form of programmed cell death, and FSP1 is known as a ferroptosis inhibitor. Through small molecule library screening, icFSP1 were identified as inhibitors of FSP1. icFSP1 induces the formation of FSP1 condensates, thereby impairing tumor growth [72].
7.2 Indirect Targeting Phase Separation
The therapeutic effects can also be achieved by altering the concentration of RNA, and protein, or inhibiting upstream and downstream molecules of the phase separation protein. For instance, DDX21 [129] and NOP53 [166] inhibits tumor phenotype by reducing the concentration of LLPS proteins using techniques like RNA interference (RNAi). SRC-3 can undergo phase separation, and SI-2 can selectively reduce the transcriptional activity of SRC-3, decrease its protein concentration and phase separation, sensitize cells to bortezomib (BTZ) treatment, and overcome drug resistance [165]. The HIPPO-YAP fusion protein is associated with the development of ependymoma. LLPS of YAP fusions YAP-MAMLD1 and C11ORF95-YAP from relapsed patients underlies the development of neural progenitor ependymomas. BRD4 is a member of the YAP transcription complex, and the BRD4 inhibitor JQ1 can inhibit YAP fusion protein-mediated tumorigenesis [141]. Casein kinase 2 (CK2) promotes the dynamic changes of SGs by phosphorylating G3BP1. The CK2 inhibitor TBCA can reverse this process [176].
7.3 Other Therapeutic Strategies Dependent on Phase Separation
In the development of therapeutic strategies, leveraging the intrinsic properties of phase separation offers innovative approaches beyond merely targeting its occurrence. Klein et al. demonstrated that the nuclear phase separation component MED1 can significantly enrich the chemotherapy drug cisplatin [194]. This enrichment occurs independently of cisplatin's original molecular targets, with the concentration of cisplatin within MED1 condensates reaching up to 600 times that of the external environment, thereby markedly enhancing its cytotoxic effects. Building on this, Liang et al. utilized the dynamic nature of phase separation to regulate in situ condensate formation through highly reactive and selective bioorthogonal chemistry, enabling sustained drug retention [199]. By employing the inverse electron-demand Diels–Alder (IEDDA) reaction between trans-cyclooctene (TCO) and tetrazine (Tz), they linked positively charged histones with DNA strands capable of phase separation. The resulting protein-DNA conjugates readily formed biomolecular condensates within cells, efficiently recruiting the anticancer drug doxorubicin, delaying its release, and enhancing its therapeutic efficacy.
Furthermore, phase separation has been creatively applied in cell therapy. Chen et al. developed a cell surface engineering strategy using phase-separating peptides to modify immune cells [200]. hey designed a tripeptide, Fmoc-Lys-Gly-Dopa-OH (KGδ), which undergoes phase separation in aqueous solutions to form condensates. With the assistance of Fe³⁺ ions, these condensates coat the surface of natural killer (NK) cells, creating an outer layer capable of encapsulating monoclonal antibodies (mAbs), such as trastuzumab. This antibody-embedded condensate layer enhances antibody-dependent cellular cytotoxicity (ADCC), enabling NK cells to more effectively recognize and eliminate cancer cells.
8 Conclusion
The formation of biomolecular condensates mediated by LLPS is essential for regulating various fundamental cellular processes, including biomolecule expression and signal transduction [201]. LLPS regulates gene expression through epigenetic modification, transcription, translation, and posttranslational modification, and is involved in nucleosome structure, chromatin regulation, and histone modification. By forming SEs, transcription condensates, and translation condensates, LLPS regulates gene transcription and mRNA translation and ultimately regulates protein function through posttranslational modification. LLPS regulates many signaling pathways, such as growth factor receptor signaling, Ras, Wnt, YAP, and other pathways, as well as biological processes including DNA damage and repair, autophagy, immune response. These processes are involved in key cancer characteristics, including tumorigenesis, angiogenesis, metastasis, tumor immunity, and drug resistance. Building on these insights, significant progress has been made in developing targeted therapies that exploit phase separation mechanisms (Table 2).
Despite substantial advancements in understanding phase separation phenomena within cells, several challenges persist that require attention. Firstly, while current research establishes that intrinsically disordered protein regions drive most instances of LLPS within cells, these regions remain elusive targets for small molecule drug development due to their IDR. Additionally, IDRs are often overlooked by structural biologists. Secondly, given that LLPS-driven biological condensates represent highly dynamic systems within cells, traditional experimental approaches face limitations when directly elucidating the regulatory mechanisms of such processes. Although super-resolution imaging techniques have partially addressed this issue, future efforts aim to develop integrated platforms that combine super-resolution imaging with high-throughput and automated capabilities, thereby facilitating large-scale drug screening initiatives targeting aberrant LLPS events within cells. Thirdly, despite sporadic reports detailing interactions between specific membrane-bound organelles and biomolecular condensates, comprehensive investigations into these relationships remain scarce in the literature. It is therefore imperative that researchers focus on unraveling reciprocal regulations between condensates and other organelles. Lastly, while certain groups have used proteomic analyses posttreatment with agents like 1,6-hexanediol to identify components involved in LLPS from whole-cell proteomes, concerns arise regarding potential losses during sample preparation, especially of unstable components associated with LLPS. There is a need to develop more effective tools to describe the overall phase separation process. In addition, many current studies on phase separation are mainly carried out by means of exogenous overexpression. Therefore, in addition to focusing on the functions of this ectopic expression, the phase separation characteristics of endogenous molecules also need to be paid attention to. Thus, it is crucial to depict the phase separation characteristics of molecules at the endogenous expression level. The methods for characterizing the phase separation of endogenous molecules are as follows: ① Knock-in a fluorescent protein into the genome of the target protein, and express the endogenous fusion protein to track of endogenous protein condensates [202]. ② Develop live-cell probes to visualize endogenous biomolecular condensates in real time [150]. ③ Use molecular probes targeting IDRs to isolate and analyze phase-separated components [203].
Understanding the role of LLPS in tumor initiation and progression is critical for developing effective therapeutic strategies. While numerous phase separation phenomena have been linked to cancer, fundamental questions remain. For example, why do SEs formed by BRD4 and YY1 preferentially promote oncogene expression [43, 90] rather than tumor suppressor genes? During RAS signal transduction, kinase condensates facilitate rapid phosphorylation [107], how do phosphorylated proteins exit the condensates and transfer to the next signaling switch? Proteins within membrane-bound organelles, such as endoplasmic reticulum-associated proteins, have signaling peptides that guide protein localization during synthesis. whether proteins in biomolecular condensates that regulate tumor development have sequences that facilitate specialized localization? These are theoretical questions requiring in-depth study. Additionally, Tumor condensates undergo reprogramming in response to different therapies, such as DNA-damaging agents, tyrosine kinase inhibitors, and immunotherapies. Identifying key condensate reprogramming events associated with specific drug mechanisms may provide new strategies to overcome drug resistance.
From the discovery of phase separation to tumor therapy, there are currently no effective therapeutic methods that can be applied clinically. At present, in the research stage, it mainly includes gene silencing, small-molecule drugs or small peptides used in experimental studies. Therefore, the research on developing tumor treatment methods using phase separation should be emphasized. For example, Son et al. used a live-cell probe with low cytotoxicity of phase separation peptides [204]. Whether it can be transformed into a drug with strong cell-killing ability to kill tumors. Xu and others used engineered sequences to fuse with CARs, promoting the formation of CAR condensates, thereby enhancing the antitumor effect of CAR-T therapy [205]. Ma and others developed nanoparticles modified with d- and l-pen that can affect the assembly of SGs [206]. Key condensates related to cancer can also use these strategies to develop new methods. In the field of drug screening, Wang et al. conducted a comprehensive investigation of oncogenic fusion proteins and identified 1500 potential phase separation-DNA binding domain fusion proteins. Building on this, they developed a computer vision-based high-throughput screening method called DropScan, which holds promise for discovering new drugs targeting phase separation [207].
In conclusion, the critical role of phase separation in tumor regulation and the understanding of its mechanisms make it a new target for cancer therapy. The current direction in drug development is to create new drugs that directly target functional phase separation components or enhance drug activity based on phase separation properties. In summary, targeting phase separation offers promising opportunities for therapeutic interventions in cancer.
Author Contributions
Xingwen Wang: resources (equal), writing – original draft (equal), visualization (equal), writing – review and editing (equal). Minqiao Lu: resources (equal), visualization (equal), writing – review and editing (equal). Yi Zhang: resources (equal), visualization (equal), writing – review and editing (equal). Jianngwen Ma: resources (equal), writing – review and editing (equal). Ying Hu: resources (lead), writing – original draft (equal), writing – review and editing (equal). All authors read and approved the final manuscript.
Acknowledgments
All images were created with BioRender (http://www.biorender.com/). The work was funded by the National Nature Science Foundation (Nos. 82025027 and 82472678), China Postdoctoral Science Foundation (Nos. 2022TQ0093, 2023M730871, 2023M740931, and BX20240479), the Nature Science Foundation of Heilongjiang Province (No. LH2023C070), and the Postdoc Foundation of Heilongjiang Province (No. LBH-Z22176), the Heilongjiang Province Youth Talent Support Project (No. 2023QNTJ003).
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.