Intercellular Mitochondrial Transfer Enhances the Antitumor Immunity of CD8+ T Cells
In their paper published in Cell [1], Baldwin et al. used advanced techniques such as single-cell RNA sequencing, field emission scanning electron microscopy (FESEM), and confocal microscopy to systematically investigate the process by which bone marrow stromal cells (BMSCs) transfer mitochondria to CD8+ T cells via tunneling nanotubes (TNTs). Through a series of experiments, they revealed how this process enhances T cell metabolic adaptability and antitumor efficacy, thus establishing mitochondrial transfer as an organelle transplantation strategy for significantly boosting T cell metabolic resilience and antitumor potential.
Adoptive T cell therapy (ACT) is a personalized immunotherapy; however, its efficacy against solid tumors is often limited because of the suppressive tumor microenvironment, which impairs T cell mitochondrial function, leading to T cell exhaustion and reduced antitumor immunity [2]. Recent research has demonstrated mitochondrial transfer across different cell types, which can repair damaged cells and in some cases, support tumor growth by providing mitochondria to tumor cells.
At present, tunneling nanotubes (TNTs) are recognized as a major pathway for mitochondrial transfer. These structures, supported by F-actin, span considerable distances between cells, facilitating the intercellular exchange of cytoplasmic materials and organelles [3]. However, whether mitochondrial transfer could restore mitochondrial function in exhausted T cells and present a new avenue for T cell–targeted solid tumor therapy remained unclear until Baldwin et al. provided crucial evidence supporting this model.
Within coculture systems, the researchers observed interactions between BMSCs and CD8+ T cells, with field emission scanning electron microscopy (FESEM) capturing the formation of nanotubes between the two cell types. These nanotubes created intercellular “bridges” that enabled the transfer of mitochondria and other organelles from BMSCs to T cells. Confocal imaging analysis revealed a significant increase in mtDNA content within CD8+ T cells (referred to as Mito+ T cells) that had received mitochondria, confirming the occurrence of mitochondrial transfer from BMSCs. Further mechanistic investigation using gene enrichment analysis and immunoprecipitation sequencing revealed that Talin 2 (TLN2) acted as a key mediator of mitochondrial transfer via TNTs from BMSCs to CD8+ T cells, highlighting its essential role in initiating nanotube formation in BMSCs and facilitating mitochondrial transfer (Figure 1).
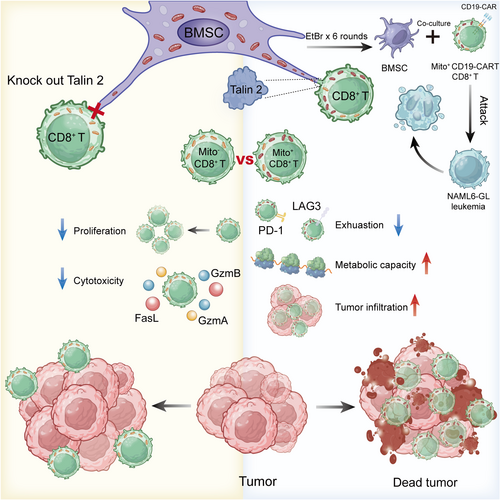
To assess how mitochondrial transfer influences the metabolic performance of T cells, the researchers analyzed the oxygen consumption rate (OCR) of CD8+ T cells, focusing on parameters such as basal respiration and spare respiratory capacity. The results indicated that Mito+ T cells exhibited significantly higher basal and spare respiration rates, demonstrating that the transferred mitochondria remained functional within T cells and effectively contributed to cellular energy production. Additionally, injection of Mito+ T cells into the tumor tissue in a mouse model resulted in prolonged survival, and quantification of Mito+ T cell numbers and localization within the tumor revealed enhanced survival of these cells in the tumor microenvironment (Figure 1).
To further clarify the impact of mitochondrial transfer on CD8+ T cell function, the researchers compared the distribution of Mito+ and Mito− cells within tumors and spleen-resident pmel-1 CD8+ T cells, revealing distinct subtype distributions between the two groups. Mito+ cells exhibited more effector-like characteristics, with significant differences in the expression levels of exhaustion markers (such as PD-1 and LAG3 [4]) and cytotoxic molecules (such as GzmB) across various groups (Figure 1). Using the SCENITH method, the metabolic pathways and energy levels in Mito+ cells were assessed, which showed that mitochondrial transfer enhanced T cell metabolic capacity within the tumor microenvironment. To further analyze the contribution of different metabolic pathways, the researchers evaluated the glycolytic capacity and fatty acid/amino acid oxidation (FAAO) capacity of Mito+ T cells. Their results indicated that Mito+ T cells within tumor tissues exhibited higher glycolytic capacity compared to that of spleen T cells. This characteristic, similar to the Warburg effect observed in cancer cells [5], supports rapid proliferation by providing essential energy and metabolic intermediates. Notably, oxidative phosphorylation (OXPHOS) showed no significant differences across different environments, suggesting that Mito+ T cells can flexibly adjust their metabolic pathways to adapt to the tumor microenvironment. In summary, these T cells that received mitochondrial transfer demonstrated enhanced proliferation, infiltration, and resistance to exhaustion within tumors, with significantly improved survival in the tumor microenvironment, highlighting the tumor-suppressive potential of Mito+ cells.
After describing the occurrence and mechanisms of mitochondrial transfer, along with the associated changes in recipient cells, the researchers found that mitochondrial transfer enhanced the antitumor immunity of human CD19-CAR CD8+ T cells against systemic leukemia in xenograft models. Cytotoxicity assays and in vivo experiments using the NALM6-GL leukemia mouse model further confirmed the ability of mitochondrial transfer to enhance CAR T cell antitumor activity in vivo (Figure 1). To investigate whether mitochondrial transfer could be applied to other T cell subsets, human TILs (MART-1 TILs) were cocultured with BMSCs, and their ability to exert cytotoxic effects on SK23-GFP melanoma cells was examined. The results showed that Mito+ TILs exhibited significantly improved target cell clearance in vitro compared to that of TILs that did not receive mitochondria. In summary, these findings demonstrate the broad applicability of mitochondrial transfer across various human immune cell types, including CD19-CAR T cells and TILs, and further reveal its enhanced antitumor efficacy across different in vivo and in vitro cancer models. Therefore, this study extends the potential of mitochondrial transfer technology in immunotherapy, highlighting its promise for advancing immune cell-based therapies.
In conclusion, through this series of experiments, Baldwin et al. demonstrated for the first time that mitochondrial transfer from BMSCs to CD8+ T cells can significantly enhance T cell metabolic adaptability, with Mito+ CD8+ T cells exhibiting greater antitumor activity, resistance to exhaustion, and improved survival. This enhanced antitumor potential is mediated by TNTs constructed by Talin 2 protein. This study thus provides a novel theoretical and technical foundation for applying organelle transplantation in immunotherapy, demonstrating the potential of mitochondrial transfer to improve the efficacy of T cell therapy. However, direct evidence regarding the precise mechanism by which Talin 2 facilitates mitochondrial transfer via TNTs remains limited. After conducting in-depth studies on the specific mechanisms of mitochondrial transfer, it may be worthwhile to further explore the duration of functional maintenance in T cells following mitochondrial transfer. Can mitochondrial transfer sustain the antitumor function of T cells over the long term and support their prolonged expansion within patients? This direction represents a critical question for future clinical applications. Additionally, as mitochondrial transfer can also occur in tumor cells receiving mitochondria from T cells [3], exploring mitochondrial transfer across other cell types in the tumor microenvironment and understanding its implications for antitumor immunity represent important avenues for future research.
Author Contributions
Ce Guo drafted the manuscript and created the figure. Qiqing Yang enhanced the image and contributed insightful discussions. Long Zhang reviewed and approved the final version of the manuscript. All authors have reviewed and consented to the final manuscript.
Acknowledgments
We acknowledge support from the platform of Life Sciences Institute and the Zhejiang University School of Medicine. The figure elements are sourced from BioRender.com and created with Adobe Illustrator. The current work was supported by the Chinese National Natural Science Funds (31925013 and W2411011), a Key R&D Program of Zhejiang Province (2024C03142), and the Joint Project of Pinnacle Disciplinary Group, the Second Affiliated Hospital of Chongqing Medical.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
Author Long Zhang is an Editorial board member of MedComm – Oncology. Author Long Zhang was not involved in the journal's review of or decisions related to this manuscript. The remaining authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.




