Characteristic, Regulation and Targeting Strategies of Cancer Stem Cells and Their Niche in Digestive System Tumors
ABSTRACT
Digestive system tumor, including esophageal tumor, gastric tumor, intestinal tumor, liver tumor, pancreatic tumor, and cholangiocarcinoma, are the most common tumors worldwide and serve as a major cause of tumor-related death. Cancer stem cells (CSCs) are a small group of cells in tumors that harbor self-renewal, differentiation abilities, playing a crucial role in tumor initiation, progression, metastasis, and are supposed to be the fundamental cause of tumor recurrence after conventional treatment. A comprehensive understanding and targeting of CSCs is the key to overcoming tumors. In this review, focusing on digestive system tumors, we summarize the characteristics of CSCs, review the intracellular mechanisms that regulate self-renewal and functional maintenance of CSCs, including stemness pathways, transcription and epigenetic regulation, metabolic regulation, and noncoding RNAs, and demonstrate microenvironmental regulation and systemic regulation of CSCs at molecular and cellular levels. Finally, we summarize recent advances in tumor therapy with CSC targeting and their niche remodeling. These research progress on CSCs in digestive system tumors provide crucial insights into the occurrence, development, drug resistance, recurrence and metastasis of tumors, and offers new targeted treatment strategies for defeating tumors.
Graphical Abstract
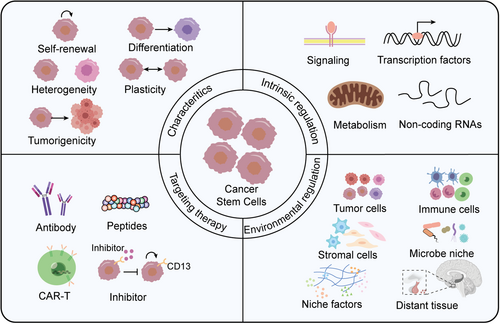
Cancer stem cells, characterized by self-renewal, differentiation, heterogeneity, plasticity and tumorigenicity, are regulated by intrinsic factors such as signaling pathways, transcription factors, metabolism and noncoding RNAs, as well as environmental cells (including tumor cells, immune cells and stromal cells), environmental factors and distant tissues. CSCs and their niche serve as promising target for tumor elimination.
1 Introduction
Tumor is a serious disease that extensively disturbs human health. The latest statistics show that in 2022, there were 19.96 million new cancer cases and 9.7 million deaths worldwide, with 4.82 million new cases and 2.57 million deaths in China. Digestive system is the most vulnerable to tumor, and there are five digestive system tumors among the top 10 tumor types in the world, including colorectal cancer (the second), liver cancer (the third), gastric cancer (the fifth), pancreatic cancer (the sixth), and esophageal cancer (the seventh). Among the five most deadly tumors in China, four belong to the digestive system, including liver cancer (the second), gastric cancer (the third), colorectal cancer (the fourth), and esophageal cancer (the fifth) [1, 2]. The main treatment methods for digestive system tumors include surgery, radiotherapy, chemotherapy, targeted therapy, and immunotherapy. However, the treatment outcome for digestive system tumors is generally limited, and the proportion of patients suitable for surgery is low, with high rates of drug resistance, recurrence, immune escape, and distant metastasis [3, 4]. Minimal residual disease (MRD) was first discovered in hematological tumors and were recently found to emerge after a variety of therapies, including digestive system tumors, which largely accounts for drug resistance and recurrence after tumor treatment [5, 6].
Recently, accumulating studies have demonstrated significant heterogeneity of tumor cells, and a small group of cells namely CSCs, is crucial for tumor progression. The substantial development of CSC theory explains most of the above mentioned clinical problems [7, 8]. CSCs have self-renewal and differentiation abilities, which is not only an important cause factor for tumor heterogeneity, but also plays a key role in tumor initiation, progression, metastasis, and recurrence. Accordingly, CSCs are known as “seeds” of tumor occurrence and progression, and their roles in digestive system tumors have been thoroughly and extensively explored. There are several CSC-related theories, including hierarchical theory, clonal evolution, and atavistic theory, among that “hierarchical theory” describes the most classical tissue framework of CSCs. There are hierarchical structures within the tumor tissue, in which CSCs are located at the top layer and generate various cell types in the tumor through self-renewal and differentiation [9]. Clonal evolution highlights the genetic heterogeneity and environmental adaptability of tumor cells, arguing that tumors evolve in the microenvironment through genetic mutations and natural selection in the environment, in which CSCs area subsets of cells containing specific mutations and that are more adapted to the environment [10]. In recent years, it has been found that CSCs are the result of the regression of tumors under environmental pressure, that is, the tumor cells restore the characteristics of their ancestor (stem cells) [11].
Digestive system tumors have brought enormous medical and economic burdens to human society, among which CSCs are a major challenge in treating digestive system tumors and have become a research hotspot. Developing new therapeutic strategies targeting CSCs is expected to provide new breakthroughs for the treatment of digestive system tumors. In this review, we summarize the research progress of CSCs in digestive system tumors, including characteristics, regulators, and targeting therapy, which will provide a comprehensive understanding on CSCs, and offer crucial insights for tumor treatment.
2 Characteristics of CSCs in Digestive System
2.1 Cellular Origin of CSCs
As the seed of a tumor, the source of CSCs has attracted long-term attention. Based on the previous research results, CSC can come from at least one of the following cell types: tissue stem cells, progenitor cells and mature cells [12]. In established tumor, CSCs can also be derived from differentiated tumor cells through plasticity.
2.1.1 Tissue Stem Cells
CSCs have many similarities with embryonic stem cells and tissue stem cells, with the abilities of self-renewal and differentiation, sharing critical signaling pathways and functional transcription factors. Epithelial cells of the digestive system renew rapidly, for example, intestinal epithelium is the fastest renewing tissue in mammals, updating approximately every 3 days, gastric epithelial cells renew approximately every 7 days. These rapid tissue updates are supported by specific adult stem cells. Tissue stem cells in the gastrointestinal epithelium are the earliest and most deeply studied adult stem cell types. Lineage tracing experiments have shown that LGR5+ stem cells, a tissue stem cell essential for intestinal maintenance, regeneration and repair, overactivation during adenomatous polyposis coli (APC) deletion, which lead to the formation of adenocarcinoma, while loss of APC in villous cells could not form tumors, indicating that LGR5+ intestinal stem cells (ISCs) are the source of tumor cells [13, 14]. Similarly, AQP5+ gastric stem cells are responsible for gastric self-renewal, and lineage tracing has shown them to be the origin for gastric carcinogenesis [15]. Of note, LGR5 is highly expressed in AQP5+ pyloric stomach cells, indicating a similar cell-of-origin among CSCs across digestive system tumors. Carcinogenesis of tissue stem cells is also recognized as the main source of esophageal cancers [16].
2.1.2 Tissue Precursor Cells
The liver is slowly renewed during homeostatic state, and Axin2+ cells generate expanding clones in a long-term (1 year) lineage tracing assay [17]. However, after damage, the liver exhibits vigorous plasticity and regenerative capacities, which is mainly due to differentiated hepatocytes re-entering the cell cycle. The activation of hepatic progenitor cells is frequently observed in chronic liver injury, such as hepatitis virus infection, fatty liver, cirrhosis, and so forth, which are the disease characteristics of early liver cancer. Michael Karin's team found hepatocellular carcinoma (HCC) progenitor cells, with CSC-like features, aggregate in the area of premalignant liver lesions 4-5 months before hepatocarcinogenesis. These progenitor cells, transform into CSCs through activating of stat3 signaling induced by autocrine IL-6, demonstrating tissue precursor cells are the source of CSCs [18].
2.1.3 Mature Cells
In addition to stem cells and progenitors, a variety of mature differentiated cells also form tumors under special circumstances, even in the intestine, where ISC exhibit vigorous and rapid self-renewal. Loss of APC in Paneth cells lead to the revival of stem-like cells upon stimulation from dextran sulfate sodium (DSS) or a high-fat diet, which mimicking approximately 25% of human colorectal cancers (CRCs), a much larger subset of colorectal tumors than inflammatory bowel disease (IBD)-type CRCs [19]. Similarly, villus cells from APCKO/IkBa−/− or APCKO/KrasMut generate LGR5+ stem cells through cell-fate reprogramming, ultimately driving colorectal tumorigenesis [20]. Unlike the universal expression of EpCAM in the intestinal epithelium, EpCAM is specifically expressed in proliferating ductal cells (PDCs). In liver tumorigenesis model involving combined chemically induced liver injury and cytidine deaminase-induced mutagenesis, lineage tracing showed that EpCAM+ PDCs differentiated only into cholangiocytes, not hepatocytes during liver injury. However, EpCAM+ PDCs with gene mutations transform into CSCs, supporting EpCAM+ PDCs as the source of CSCs in HCC [21]. LGR5 are expressed in long-lived hepatocytes near the central vein, contributing to postnatal liver development, homeostatic maintenance, and rapid regeneration after 2/3 partial hepatectomy. Furthermore, these cells are the main cellular source of liver cancer induced by diethylnitrosamine (DEN) [22]. An additional independent study demonstrated that the clearance of LGR5+ cells inhibited tumor growth in a DEN-induced liver cancer model, confirming the critical role of LGR5+ cells in HCC tumorigenesis and expansion [23]. These results indicated mature differentiated cells have the potential to transform into CSCs and promote the occurrence and development of tumors.
2.1.4 Tumor Cells
After tumor generation, the cell-fate plasticity of tumor non-stem cells provides an important source of CSCs. Tissue damage and inflammatory response, as hallmarks of colorectal tumorigenesis, induce the reduction of LGR5+ cells. Subsequently, regenerative stem cells (RSCs), characterized by an IFN-γ/TGF-β/Yap-induced fetal-like state and lacking LGR5 expression, contribute to tumor stemness [24, 25]. In addition to tumorigenesis, the transition of LGR5− tumor cells to LGR5+ CSCs also exists during tumor metastasis, LGR5− tumor cells are the metastatic cells of intestinal cancer, involving detachment from the primary site, entry into blood circulation, and colonization of the metastatic site. However, the expansion of tumors at the metastatic site requires the emergence of LGR5+ CSCs [26]. Targeted therapy aimed at CSCs often results in a more frequent transition from tumor non-stem cells to CSCs, in the CRC case where LGR5+ CSCs are cleared, moderate tumor suppression is followed by rapid relapse due to the emergence of a large number of new LGR5+ CSCs. Lineage tracing shows that differential CK20+ cells can dedifferentiate to form new LGR5+ CRCs [27, 28]. These results prove tumor non-stem cells have the ability to dedifferentiate into CSCs, thereby affecting the therapeutic effect of CSC targeting.
2.1.5 Other Cells
Some research showed that non-digest system cells transform into CSCs and lead to the occurrence of digestive system tumors, such as bone marrow-derived cells (BMDCs). Timothy C. Wang's team found that chronic infection with Helicobacter triggers gastric tumorigenesis from BMDCs, indicating that epithelial tumors can be generated from marrow cells [29]. BMDCs might be the most primitive uncommitted adult stem cells and are readily transformed into CSCs during chronic inflammation, but not in acute infection or transient cell loss, probably via inflammation factors/chemokine-induced cell mimicry or fusion [30]. Actually, cell fusion of BMDCs with mature tissue cells also drives tumorigenesis in liver cancer and colorectal cancer [31, 32].
2.2 Self-Renewal and Differentiation of CSCs
Self-renewal is a characteristic of cell populations that enables them to maintain their own stability, and different stem cells maintain self-renewal through different modes of cell division. Here, symmetric division of stem cells produces two stem cells or two non-stem cells, while asymmetric division produces one stem cell and one non-stem cell. A large number of lineage tracing results showed that LGR5+ ISCs mainly self-renewal through symmetric division, and the maintenance of the overall number of stem cells is determined by the specific microenvironment [33, 34]. CSCs and tissue stem cells have similar signaling pathways involved in self-renewal, such as the Wnt/β-catenin, Notch, and sonic hedgehog (Hh) pathways. However, CSCs may have different cell division mechanisms with tissue stem cells, the self-renewal of normal gastric tissue mainly depends on asymmetric division, which means that the two daughter cells are positioned differently relative to the basement membrane. In contrast, gastric stem cells change to symmetric division to generate more stem cells, during tumorigenesis. This process is influenced by intracellular factors such as SOX9, coregulation of tumor-inducing factors such as N-nitroso-methylurea, and microenvironmental factors such as gastrin generated by G cells [35, 36]. Nevertheless, the asymmetric division also exists in CSCs, which are considered an intrinsic property of CSCs due to the asymmetric distribution of their microenvironment [37]. We will elaborate on the intracellular and microenvironmental factors that regulate self-renewal of CSCs in subsequent sections.
Tumors harbor multiple cell types, and in addition to their self-renewal capacity, CSCs have the ability to differentiate into different cell types within tumors, and stemness of tumor is significantly positively correlated with heterogeneity [38, 39]. The multidirectional differentiation capacity of CSCs has been confirmed by multiple lineage tracing experiments in contrast to CD9low cells that produce only CK19+ cells, CD9high CSCs produce both CK19− and CK19+ cells, indicating that CD9high CSCs have multiple lineage differentiation capacity [40]. Transplanted CD133+ liver CSCs produce cells with different differentiated lineages, indicating that Prom1/CD133+ CSCs have differentiation or transdifferentiation potential [41]. LGR5+ CSCs in CRC are able to produce both LGR5+ cells and all adenoma cells, including Paneth cells, indicating that LGR5+ CSCs possess the ability of self-renewal and differentiation [42]. In addition to differentiating into tumor cells, CSCs are also able to transdifferentiate into other cell types to regulate tumorigenesis [43]. Transdifferentiation of CSCs into vascular endothelial cells (ECs) and promoting angiogenesis has been found in a variety of cancers, such as liver cancer [44, 45]. Unexpectedly, the CSCs of solid tumors also exhibit hematopoietic properties and are one of the sources of blood cells in tumors [46]. The multidirectional differentiation capacity of CSCs generates and maintains the diversification of cancer cells, playing a key role in shaping tumor composition, disease progression, and therapeutic resistance.
2.3 Heterogeneity of CSCs
Tumors are a mixture of cells with different molecular characteristics and medicine sensitivities, and the difference among different cells within a tumor is called intratumoral heterogeneity. Heterogeneity is the key cause of tumor drug resistance, recurrence, and difficulty in cure, which has a profound impact on tumor prevention, diagnosis, and treatment. Heterogeneity originates from the emergence and accumulation of events including genetic mutations and epigenetic modification's changes in different cells, as well as differences in the tumor microenvironment (TME). Increasing evidence shows that tumor heterogeneity is driven by CSCs [47]. In fact, the existence of CSCs is an important manifestation of tumor heterogeneity. Li and colleagues, using single-cell RNA sequencing and spatial transcriptomic analyses between colorectal cancer liver metastasis (metastases in the liver, LCRC) and ovarian metastasis (metastases in the ovary, OCRC), revealed the role of CSCs in heterogeneity of metastatic tumors [48].
Recently, an increasing number of studies have found that CSCs themselves are also heterogeneous. Single-cell omics, such as the emergence of single-cell transcriptome sequencing and single-cell ATAC sequencing, provide an ideal strategy for defining the heterogeneity of tumors and CSCs. Two studies utilized single-cell transcriptomic analysis of HCC formed by cell lines, freshly excised HCC samples, and HCC PDX to investigate CSC heterogeneity in HCC, and identified heterogeneity of HCC stem cells, with CSC subsets expressing different markers having different molecular characteristics and prognostic correlations [49, 50]. In the process of CRC development and treatment, CSCs and their microenvironment undergo dynamic changes, resulting in two types of CSCs in CRC, proliferating CSCs and resurrecting CSCs, which have significant differences in the regulatory levels of intracellular and microenvironmental factors [51, 52]. In addition, some new types of CSCs have been defined in digestive system tumors, which are described in detail below.
2.3.1 Circulating CSCs
Circulating CSCs refer to tumor cells with stem-like characteristics in the blood circulation system and are crucial for tumor metastasis. They can be used for early diagnosis, prognosis prediction, and treatment response evaluation for tumors. Nine CSCs biomarkers of liver cancer, including EpCAM, CD90, CD133, and ABCG2, are detected in blood samples, are closely related to HCC stage and have prognostic significance [53]. CD90+ circulating CSCs can be used to distinguish between normal and tumor condition, with 90% detection in HCC patients but not in healthy and cirrhotic individuals [54]. The increased number of ICAM1+ CSCs in the blood is positively correlated with a poor prognosis of HCC [55]. EpCAM+ CSCs in the blood can be used for the diagnosis of relapse [56]. The presence of circulating CSCs in the blood of intestinal and gastric cancer patients is also of important diagnostic and therapeutic value [57, 58].
2.3.2 Proliferating CSCs
Proliferating CSCs exhibit high self-renewal and differentiation potential, and these cells play a critical role in tumor growth and progression. In HCC, prom1+ CSCs exhibit high proliferation and stem cell characteristics [41]. In CD73+ CSCs, CD73 can promote the stemness of CSCs through abnormal activation of AKT and upregulation of SOX9 [59, 60]. In CRC, the subpopulation of receptor for hyaluronic acid-mediated motility (RHAMM+) CD44+ CSCs exhibits high proliferation characteristics and self-renewal ability, and RHAMM+ cells play an important role in liver metastasis of pancreatic ductal adenocarcinoma [61, 62].
2.3.3 Quiescent CSCs
Tissue stem cells are in different proliferative states, such as LGR5+ ISCs are proliferative, while LGR5+ liver stem cells are generally thought to be quiescent and activated when tissue damage, such as after CCl4 treatment [63]. Intravital tracing of LGR5+ CSCs in CRC demonstrates that 20% of LGR5+ cells are dormant, with high expression of P27 and COL17A1 but low activity of YAP1. These dormant cells are resistant to chemotherapy and drive regrowth by switching to p27-COL17A1-YAP1-activated cycling cells [64]. Similar to the retention capacity of DNA labeling commonly used in normal stem cells, quiescent features were used for the isolation of CSCs, and cells with longer retained labeled nucleotides were considered quiescent and led to several marker retention techniques, such as nuclear-localized fluorescent H2B-YFP [65-67]. Quiescent CSCs are responsible for the tumor resistance to chemotherapy or radiation therapy, which generally targets cells with active division [68]. Deterioration of cultivation conditions induces Mex3a+ quiescent CSCs, which have the ability of resisting chemotherapy and metastasis [69].
2.3.4 Slow-Cycling CSCs
Slow-cycling CSCs are a relatively quiescent subpopulation of CSCs with extended G0/G1 cycle and remain in a temporarily low metabolic activity state, resulting in inefficient drug therapy targeting proliferating tumor cells [70, 71]. In CRC, TCF1 (a Wnt-responsive transcription factor) activates PROX-1 (Wnt target gene) to induce CDKN1C (a cyclin-dependent kinase inhibitor) expression, leading cells into a slow-cycling state; DPPA3+ CSCSs possess low levels of forkhead box M1 (FOXM1) through the DPPA3–HIF1α (hypoxia-inducible factor 1-α) feedback loop to delay the cell cycle [72].
2.3.5 Drug-Tolerant Persister (DTP) CSCs
DTP CSCs shows strong resistance when exposed to anticancer drugs, and this phenotype can be temporary, it can also be caused by genomic changes to acquire irreversible drug resistance [73, 74]. These drug-resistant cells all showed high expression of aldehyde dehydrogenase (ALDH) and ABC (ATP binding cassette transporter) [75-77]. In CRC, DTP CSCs showed chromatin repression, especially H3K9me3 on long interspersed repeat element 1 (LINE-1); Mex3a+ CSCs downregulate the Wnt target genes after chemotherapy and enter a transient YAP+ fetal intestinal progenitor state to resist chemotherapy [69, 78].
2.4 Plasticity of CSCs
The conventional view is that CSCs are a type of rare subset of cells with expression of specific markers. However, an increasing number of studies have found that CSCs are not constant, and under specific circumstances or stimuli, differentiated tumor cells are able to de-differentiate into stem-like cells, which indicates that CSCs present a state rather than a fixed condition [79]. The latest version of “Hallmarks of Cancer: New Dimensions” is proposed, and the “unlocking phenotypic plasticity” is the emerging hallmark of tumor, and the plasticity of CSCs is a significant embodiment of tumor plasticity. Plasticity is a key component of cancer pathogenesis and can manifest in various ways, including differentiation, dedifferentiation and transdifferentiation. It is also an important trigger for tumor metastasis, recurrence and drug resistance [80]. For example, the plastic transition of intestinal cancer cells to intestinal CSCs reactivates the Wnt pathway, promoting the clonogenic ability and metastasis of tumors [20, 81].
The plasticity of CSCs may originate from normal stem cells. CSCs and normal stem cells share the same surface marker, and both exhibit significant plasticity. Similar to normal stem cells, the loss of ISCs leads to various types of cells dedifferentiating into stem cells to replenish the stem cell pool, including absorptive progenitor cells, secretory progenitor cells, and Paneth cells [82, 83]. Correspondingly, when LGR5+ CSCs are cleared using DT, LGR5− cells can dedifferentiate to form new CSCs, which subsequently establish a new tumor hierarchy and further drive tumor drug resistance and relapse. Moreover, similar mechanism is observed in the initiation process in which DSS and inflammation induced a depletion of LGR5+ cells, as well as in metastasis process in which the conservation from LGR5− to LGR5+ cells plays an essential role in the expansion of metastatic loci [49, 50]. Plasticity plays a key role in the repair process following tissue homeostasis maintenance and homeostasis dysregulation, and accordingly, tumor cells increase their ability to resist multiple environmental perturbations and targeted therapies through the inheritance of normal tissue plasticity [38].
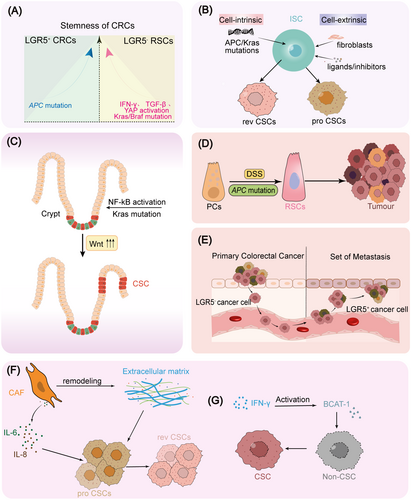
The plasticity of tumors and CSCs is regulated by both intracellular and microenvironmental factors, reflecting the ability of tumor cells to transition between stem cell and differentiated states. Similar to tissue stem cells, the chromatin structure and epigenetic similarities among tumor cells are prerequisites for cell transdifferentiation and plasticity [84]. Under environmental stress (such as tumor occurrence, tumor metastasis, tumor treatment, nutrient deficiency, etc.), tumor cells and CSCs exhibit different cellular and molecular characteristics through chromatin and epigenetic remodeling, thereby offsetting these stresses and showing a high ability to adapt to the environment. Tumor develop with sustained inflammatory responses such as IFN-γ and regenerative pathway YAP1 activation, which generally induces a reduction or differentiation of LGR5+ cells and emerging of fetal-like cells [85]. Accordingly, Vasquez and colleagues demonstrated that the stemness of CRC is attributed to LGR5+ crypt base columnar cell (CBCs) and LGR5− RSCs. Stemness of LGR5+ CBCs is enriched by APC mutation, whereas LGR5− RSCs stemness is enhanced by IFN-γ, TGF-β or Kras or YAP activation induced by Kras/Braf mutation (Figure 1A) [25]. Combining scRNA-seq and in vitro organoid formation with various mutant ISCs or cocultured with various niche cells or niche factors, Qin and colleagues also reveal the continuous polarization of CSCs between proliferative colonic stem cells and revival stem cells, which are driven by niche fibroblasts or cell-intrinsic mutation such as APC or Kras (Figure 1B) [86]. IkBa deletion-induced NF-kB activation or Kras mutation trigger hyperactivation of Wnt/β-catenin in APC-mutant background, driving the dedifferentiation of non-CSCs to CSCs (Figure 1C) [20]. Some differentiated cells, such as Paneth cells, can dedifferentiate in an inflammatory environment (for instance DSS treatment) to form revival stem cells to compensate the loss of LGR5+ cells, which in turn drive intestinal carcinogenesis. About 25% of CRC patients suffer from this disease, which is much higher than IBD-type CRC (accounting for 1%–2%) (Figure 1D) [19]. During metastasis of CRC, metastatic cells leave the primary LGR5− cells spread in the blood, and experience the fate transformation from LGR5- cells to LGR5+ cells in the destination of tumor metastasis, indicating that cancer cells are able to transit between stemness and non-stemness states (Figure 1E) [26]. Chemotherapy is one of the most deeply studied predisposing factors of cellular plasticity. Sublethal doses (IC20-IC30) of 5-FU+ Iri treatment induce colorectal cells into persistent quiescent-like (PQL) state in P53- and YAP1-dependent manners, and this reprogramming enhances fetal-ISC-like characteristics and tumor initiating capacity [87]. Zapatero and colleagues analyzed drug responses in patient-derived organoids and revealed cancer-associated fibroblasts (CAFs)-mediated remodeling of CSCs during drug treatment from proliferative colon stem cells to chemotherapy-resistant resuscitating stem cells (Figure 1F) [51]. A similar phenomenon was found during breast cancer treatment, NF-kB-activated CAFs boost CSCs enrichment and chemoresistance via IL-6 and IL-8 [88]. The IFN-γ produced by immunotherapy can directly deliver non-CSC to CSCs through branched-chain amino acid transaminase1 (BCAT-1), resulting in the enrichment of CSCs after immunotherapy. On this basis, a combination treatment of BCAT-1 inhibitor and immunotherapy could inhibit the increase of CSCs after immunotherapy and ultimately promoted the tumor treatment effect (Figure 1G) [89]. In addition, type I interferons produced by tumor immunotherapy can promote the number and function of CSCs through KDM1B-mediated chromatin remodeling [90]. In the absence of ideal culture conditions, a population of MEX3A+ CSCs with slow proliferation and chemotherapy resistance emerges in tumor organoids. Moreover, the conversion from MEX3A- LGR5+ cells to MEX3A+ LGR5+ cells in suboptimal conditions drives the acquirement of revival fetal-like state, also indicating that the regulation of CSCs plasticity mediated by environmental adverse factors promotes the adaptability of tumor cells to the harsh environment [69]. Upon isolation-induced stress among tumor initiation and metastatic seeding, therapeutic pressures induced by massive death of vulnerable cells, lacking of oxygenation and nutrients, pancreatic cancer cells were able to promote LPAR4 expression through downregulating miR-139-5p, further promoting fibronectin production through the LPAR4/AKT/CREB pathway, and finally enabling cells to cope with isolation stress by remodeling their own microenvironment and enabling LPAR4− cells to acquire stress tolerance [91]. In conclusion, environmental and intracellular factors jointly drive the plasticity of CSCs, which adapt to the intracellular state and extracellular environment, thereby acquiring the ability of self-renewal, drug resistance, proliferation, and metastasis.
Recent cancer stemness theory holds the idea that CSCs are a mimicry adopted by cells to adapt to environmental changes when signaling pathways are destroyed by environmental factors such as therapeutic stress, including typical viral-like mimicry, vascular mimicry (VM), and immune cell mimicry [92]. In fact, CSCs themselves can be regarded as the ability of tumor cells to mimic stem cells, and thus have the intrinsic properties of stem cells, including increased autophagic activity, reduced iron death activity, induced dormancy, low expression of major histocompatibility complex (MHC), upregulation of immune evasion molecules, dysregulation of costimulatory signaling, and so on [68, 93-98].
2.5 CSCs and Tumorigenesis
CSCs are often referred to as the seeds of tumorigenesis, serving as the initial cells that give rise to tumors. Based on this, the “cancer stem cell” theory of tumorigenesis has been established. Together with the tumor mutation theory, it has become an important theory of tumorigenesis. Based on this concept, the “cancer stem cell” theory of tumorigenesis has been developed, standing alongside the tumor mutation theory as a major framework for understanding how tumors form. While the tumor mutation theory emphasizes the molecular timeline of tumorigenesis, the CSC theory focuses on the cellular events that drive this process. Recent lineage tracing studies have shown that only APC mutations in ISCs can lead to the formation of adenocarcinoma, whereas mutations in differentiated cells do not result in tumor formation [14]. The primary reason for this may be that these differentiated cells typically have a lifespan of only 3–5 days, which is insufficient for tumor development [99]. Although some recent studies have found that tumor cells can be derived from non-stem cells, such as papillary epithelial cells and Paneth cells, all these cells are transformed into long-term cells such as LGR5+ cells or proliferating stem cells, indicating the requirement of long-term mutant cells for tumorigenesis [19, 20]. Cancer stem cell-driven tumorigenesis is a complex process, involving the dynamic changes of various intracellular and microenvironments and the regulation of cell status, including the acquisition of competitive advantages of cancerous cells, the escape of immune surveillance, and the formation of immunosuppressive microenvironment. The detailed regulatory mechanism will be discussed in parts 3–5.
2.6 Therapeutic Resistance of CSCs
Drug resistance and recurrence after tumor treatment are almost inevitable, occurring universally across chemotherapy, radiotherapy, targeted therapy and immunotherapy. Under therapeutic stress, CSCs can evade treatment-induced cell death through various pathways [100, 101]. Additionally, CSCs possess strong tumor-initiating capabilities, allowing a small number of surviving CSCs to rebuild treatment-resistant tumors within just a few days to months. This makes them a key factor in tumor resistance and recurrence. Even after long-term treatment without any visible tumor, some called persister cells persist and acquire proliferative capacity, making the tumor increasingly resistant, and these persister cells prove to be a specific cancer stem cell type or CSCs state [102].
2.6.1 Chemotherapy Resistance
CSCs highly express the ABC transporter, such as ABCG2, with a stronger drug efflux capacity, by protecting genomic stability and inducing drug resistance (Figure 2A) [103, 104]. In fact, ABCG2 has been regarded as one of the classical markers of CSCs [105]. Due to the high expression of drug resistance molecules such as ABCG2 and the drug resistance of CSCs, researchers have also established a classic research system for CSCs, termed “side population” (SP) cells, a subpopulation of cells that resist chemotherapy in digest system tumors [106, 107]. On the other hand, the existing chemotherapeutic drugs mainly target rapidly proliferating cells, while CSCs are relatively resting and can escape the killing of chemotherapeutic drugs, and the resting nature of CSCs is widely considered the source of tumor drug resistance. Recently, resting LGR5+p27+ CSCs were found to resist chemotherapy and promote tumor recurrence in CRC (Figure 2B) [64]. Similarly, LGR5+p57+ slowly cycling CSCs was also in the resting state and promoted tumor resistance and recurrence, whereas DT treatment for deletion of p57-DTR cells leads to impaired therapy resistance [108]. Due to the drug-resistance properties of CSCs, drug treatment will be accompanied by the enrichment of CSCs [109].
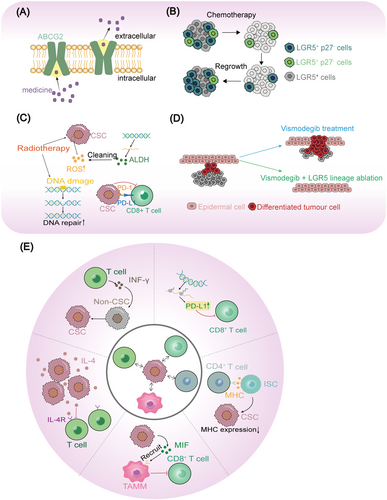
2.6.2 Radiotherapy Resistance
The CSCs are resistant to radiation killing through multiple pathways (Figure 2C). (1) CSC has high expression of ALDH, a marker for multiple CSCs with the capacity to eliminate radiation-induced reactive oxygen species (ROS), so CSCs have stronger ability to remove oxidative stress and have stronger resistance to radiotherapy and some chemotherapeutic drugs [109, 110]; (2) CSCs protect genomic stability during radiotherapy by activating DNA repair capacity, protecting cells from apoptosis [104]; (3) CSC are resistant to radiation-induced immune killing [111]. In fact, radiotherapy combined with hypoxic culture can also be used to enrich CSCs [112].
2.6.3 Targeted Therapy Resistance
CSC is highly plastic, making them prone to evading targeted therapies and leading to tumor recurrence. When specific signaling pathways are targeted, the stress on CSCs triggers the activation of multiple related stemness pathways, allowing them to escape targeted therapies through cell fate remodeling. Vismodegib, an inhibitor of SMO and Hh signaling, mediates differentiation of basal cell carcinoma, however, Wnt-driven LGR5+ slow-cycling persisting cells lead to relapse after therapy. Consequently, combining vismodegib with a Wnt inhibitor or LGR5 deletion effectively prevents relapse (Figure 2D) [113]. Similarly, Lenvatinib, an inhibitor of multiple kinases including VEGFR, PDGFR, RET, FGFR, and KIT, serves as a typical drug for liver cancer. However, CSCs in HCC can develop resistance to lenvatinib by activating the Wnt/β-catenin and Hippo signaling pathways [114-116].
2.6.4 Immunotherapy Resistance
Tumor immunotherapy become a new strategy of tumor treatment, in a variety of digestive system tumor, including liver cancer, bowel cancer, pancreatic cancer, gastric cancer and other digestive system tumor, but tumor immunotherapy leads to resistance and relapse frequently. More and more experiments reveal the cross-task between CSCs and tumor immunotherapy [117]. CSCs employ various strategies to resist immunotherapy. For example, they exhibit low expression of MHC molecules, production of the immunosuppressive factor IL-4, recruitment of inhibitory cells such as tumor-associated monocytes and macrophages (TAMM), and immunomodulatory remodeling of CSCs (Figure 2E) [89, 90, 118-120].
2.7 The Role of CSCs in Tumor Metastasis
Tumor metastasis is a complex process that involves the invasion of primary tumor cells, their spread to distant sites, seeding at these locations, clonal expansion at the distant site, and the transitions between different tumor cell states, such as epithelial-mesenchymal transition (EMT) and mesenchymal-epithelial transition (MET) [121]. CSCs have multiple cellular differentiation capacity and high plasticity, can shuttle between different cell states to meet the needs of the metastatic process, for example, CD26+ CD44v6+ CSCs has stronger metastatic potential [81, 122]. The animal model data also demonstrated that the clearance of LGR5+ CSCs was able to inhibit intestinal cancer metastasis, confirming the essential role of LGR5+ CSCs in CRC metastasis [26, 27, 123].
Molecularly, the genes driving tumor stemness and tumor metastasis interact to a large extent, leading to the stronger tumor metastasis ability of CSCs. First, certain gene or stimulation regulates both tumor metastasis and tumor stemness [124]. As one of the most important transcription factors for tumor metastasis, ZEB1 regulates the self-renewal of CSCs in multiple tumor types [125]. In turn, multiple genes specifically expressed in CSCs also have important regulatory effects on tumor metastasis, such as MAFF, cis-HOX, mcPGK1, rtcisE2F, and so forth [126-129]. Second, the genes controlling stemness and transfer have the same upstream regulators [130]. Sublethal chemotherapy-induced YAP1-dependent fetal reprograming drives tumor initiating and metastasis [87]. These cross-coupling of stemness and metastasis-related pathways drive the highly metastatic signature of CSCs.
Although the tumor stemness pathways promote tumor metastasis in various ways, it is not true that CSCs themselves establish tumor metastasis because CSCs can't cover every step in complicated cascade progress during metastasis. Metastasis-initiating cells (MICs) seeded as transfer are able to undergo a drastic phenotypic transition, adapt to the transcriptional cascade and the static metastasis [131]. Some studies have shown that metastatic stem cells are special types of CSCs with the same gene expression characteristics and markers, but they may be different cell types. Using emerging technologies such as lineage tracing and in situ imaging in vivo, Rheenen found that LGR5− cells are more diffuse compared with LGR5+ CSCs, and the tumor cells in blood are also LGR5− cells, indicating the inconsistency of MICs and LGR5+ CSCs [26]. In bowel cancer metastases, the surface markers of disseminated cells are mainly L1CAM and EMP1, instead of the marker LGR5 of the CSCs [132, 133]. In fact, in essence, distant tumor colonization and expansion are also a tumor initiation process, which also requires the tumor initiation ability of CSCs, so they need to reacquire the CSCs characteristics [26, 134].
2.8 Biomarkers for CSCs
The concept of CSCs emerged since 19 century, through an intensive investigation of hematological malignancy stem cells, when Dick and colleagues, among others, confirmed the tumor cell heterogeneity [135], and the first identification of CD34+CD38− CSCs in acute myeloid leukemia (AML), marked a major breakthrough in CSC's research [136]. These findings in hematologic malignancies provide a paradigm for research of CSCs: whether a specific cell is a CSC can be evaluated by sorting based on specific markers and tumor initiation experiments. In fact, the markers of CSCs can be divided into cell surface markers and intracellular markers, and have received more attention because they facilitate cell type identification and sorting, and hold more potential in clinical diagnosis and treatment [137].
Using the fluorescence-activated cell sorting/magnetic-activated cell separation (FACS/MACS) and gradient dilution tumor formation experiments, many subsequent studies have defined the surface markers of CSCs in digestive system tumors. In liver cancer, CD133, CD13, CD90, and EpCAM are a common surface marker of liver CSCs, and CD133+CD13+ CSCs have a stronger tumor-initiating capacity [138-142]. For colorectal cancer, CD133 is an excellent marker for human cancer but not for mouse tumor [143, 144]. CD44 is another marker for colorectal CSCs which contributes to cancer metastasis [81]. Interestingly, it seems that CD133 and CD44 are universal markers for digestive system tumors, as they also serve as surface markers of gastric cancer, esophageal cancer, and pancreatic cancer [145-149]. The similarity of CSC markers between various tumor types indicates the shared signaling pathways and expression landscapes between these tumors.
Due to the role of CSCs in tumor therapy resistance, in addition to conventional surface molecules such as CD44 and CD133, attention has been paid to factors related to clinical features, such as drug pump molecules directly related to drug resistance, and immune checkpoint factors related to tumor immunity. ABCG2 is a key drug transporter molecule that has been identified as a surface marker for a variety of tumors, including pancreatic cancer, liver cancer, intestinal cancer and other digestive system tumors [150]. PD-L1 is an important receptor protein for tumor escape immune killing in intestinal cancer, liver cancer, and gastric cancer. The high expression of ABCG2 and PD-L1 is closely related to the tolerance of CSCs to chemotherapy and immunotherapy [151-153].
Identification of CSCs markers is not only fundamental for the identification of CSCs, but also provides the possibility for specific targeting of CSCs, such as antibodies targeting α2δ1 that can target HCC stem cells [154]. However, there are great similarities between existing CSCs markers and normal stem cells, such as CD133, which is highly expressed in normal ISCs [155]. Therefore, a comprehensive understanding of the differences between CSCs, non-stem cells, and normal stem cells is of great significance [156]. We will address this issue in more detail in Section 5.
2.9 Methods for CSC Investigation
As we know, there are multiple methods to evaluate certain specific biological characteristics of CSCs, such as “sphere formation” for self-renewal, “lateral population cells” for resistance, “trans-well” for metastasis. However, “gold standard” assay is FACS and xenotransplantation [80]. For example, the earliest identified CD34+ CD38+ CSC were able to engraft into immunodeficient NOD/SCID mice to form heterogeneous tumors [135, 136]. Although this approach advances our understanding of CSCs, the method has some technical and scientific problems that may obscure the accurate understanding of CSCs, including (1) variations such as xenograft sites, dilution reagent and recipient mice leading to conflicting results; (2) this assay actually detect the ability of tumor cells to survive in harsh processing steps and established tumors in recipient mice; (3) the cell–cell and cell–niche interactions are ignored and CSC niche are totally different from the naive niche.
Genetic markers, lineage tracing, and elimination become key technologies for the identification of CSCs, allowing the self-renewal and differentiation capacity of CSCs, both of which are important features of stemness. Through intraperitoneal injection or oral administration, Hans Clevers team achieved fine control of Cre activity in Ahcre; APCflox/flox and found transformation of non-stem cell rarely drives neoplasia, which further proved that APC mutation in LGR5+ cells was the source of tumorigenesis [14]. CD133-Cre-LacZ; Rosa26-YFP mouse model proved that CD133+ stem cells could form all neoplastic cells in the tumor and retain a subpopulation of CD133+ cells, indicating that CD133 is also the marker of intestinal CSCs [144]. Of note, CD133 and LGR5 have similar tissue localization, both located at the base of crypts. In 2012, the Hans Clevers team used the “lineage retracing” technology to prove that the intestinal LGR5+ cells in mouse intestinal adenomas possess the ability of self-renewal (generating other LGR5+ cells) and differentiation (generating all other adenoma cell types), which firstly demonstrated the self-renewal and differentiation characteristics of CSCs in an in situ environment [42, 157]. On this basis, several studies utilize suicide genes such as DTR or thymidine kinase to deplete specific population cells and then determine the importance of this group of cells in tumorigenesis. For instance, iCaspas9-mediated LGR5+ cell ablation leads to tumor regression and subsequent reversion from KRT20+ cells [28]. Similarly, depletion of CSCs or CSC subsets reduces tumorigenesis and progress, such as Dclk1 in intestine tumor, LGR5 in gastric cancer, Prom1 in liver cancer [41, 123, 158]. To date, lineage tracing and lineage clearance have become the core technology for the study of CSCs maintaining the naive niche of tumor.
To detect CSCs behavior and characteristics in intact tumors, lineage tracing has been widely used for CSCs research. However, the technical requirements for lineage tracing are relatively high, particularly since it can only be performed on animals such as mice, which limits the study of human CSCs. In recent years, emerging organoid technology has addressed this limitation, becoming a key method for studying human CSCs and also serving as a major approach for mouse CSC research [28, 64]. Advantageously, tumors of the digestive tract and digestive system such as esophagus, stomach, and intestine can easily form organoids [159]. Significant discoveries have been made in the field of CSCs through the organoid research system, such as the resistance to chemotherapy of p57+ resting CSCs in intestinal cancer, effect of LGR5− cells on intestinal cancer metastasis, effect of AQP5+ CSCs on gastric cancer formation, and role of MSI2+ pancreatic ductal adenocarcinoma on tumor initiation [15, 26, 108, 160, 161]. Of note, organoids are capable of performing functional studies of single factors in vitro, for example, investigating the regulatory effects of specific signaling pathways or genes on stem cell self-renewal and differentiation [99]. For example, Tape group utilized organoid or tumor treatment approaches to analyze the impact of various microenvironmental cells and factors on the cellular composition and states of tumor organoids during tumorigenesis, and found dynamic changes in proCSC and revCSC during tumorigenesis and tumor treatment [51, 86]. Along with multidisciplinary and standardized next-generation cancer organoids, organoids will play an increasingly important role in cancer and CSCs [162-164].
Traditional research is very focused on specific surface markers, and more and more people study CSCs as a state with CSC functionalities and molecular characteristics, so it is very useful to study the characteristics of CSCs through molecular profiling. Stem cell characteristics and stemness indices can be obtained by machine-learning methods or through the established markers from related stem cells [25, 165]. In recent years, single-cell sequencing technology has become a great tool for cancer stem cell omics research, which can realize the identification and functional characterization of subsets of CSCs, and largely avoid the inconsistent results caused by different single markers. For example, PTPRO/ASCL2 overexpressing CSC subset drives metastasis of CRC cells, and-specific subsets are also able to be associated with organ-specific metastasis of bowel cancer, LEPR+ CSCs interact with the microenvironment to drive squamous cell carcinomas, colorectal adenomas are generated from stem cells but serrated polyps are not [48, 166-168]. The development of single-cell sequencing technologies and the enrichment of analytical measures, such as RNA velocity, CytoTRACE, CellChat and CellPhoneDB, single-cell sequencing will surely deepen our understanding of CSCs [169-172].
3 Intrinsic Regulation of CSCs in Digestive System
Compared with ordinary cancer cells, CSCs have significant tumor initiation ability, meaning that a very small number of CSCs can initiate tumor formation upon transplantation. At the same time, CSCs have the common characteristics of all stem cells: the ability for self-renewal and the capacity for multi-directional differentiation. In an excellent review, Stephanie Ma and colleagues define tumor initiation, self-renewal and multipotency as defining hallmarks of CSCs, plasticity as an emerging hallmark of CSCs, metastasis and therapy resistance as enabling attributes of CSCs, niche crosstalk and immune escape as associated features of CSCs [80]. All these characteristics are regulated by various intrinsic and niche factors. Genetic changes are the basis for the acquisition of tumor and CSC functions and are crucial for maintaining the quantity and functionality of CSCs, these changes represent the most concerning regulatory mechanisms, for example, APC mutations and Kras mutations confer significant competitive advantages on CSCs [181-183]. Innate immune signaling pathways within tumor cells, including DNA sensor AIM2, TLR4/MYD88 and RIG-I RNA sensor, are gaining more attention for regulating cancer stemness [176-178]. Here we focus on the regulatory roles of signaling pathways, transcriptional regulation, epigenetics, metabolism, and noncoding RNAs in CSCs (Table 1).
| Regulatory factor | Type | Target gene pathways | Type of tumor | Description | Ref. |
|---|---|---|---|---|---|
| YTHDF2 | RNA binding protein | Wnt/β-catenin and Hippo signaling pathway | Liver cancer | As a reader of m6A, YTHDF2 combines and stabilizes FZD10 mRNA in liver CSCs, and then activates Wnt/β-catenin and Hippo signaling pathway to initiate the stemness and Lenvatinib-resistance. | [114] |
| IGF2BP2 | Transcription factors | Wnt/β-catenin signaling pathway | Liver cancer | IGF2BP2, another m6A reader, preferentially binds to E2F6 and E2F3 mRNA, and inhibits their association with YTHDF2, further activates Wnt/β-catenin through inhibiting E2F6/E2F3 mRNA decay in CSCs. | [129] |
| cia-MAFF | circRNA | Wnt signaling pathway | Liver cancer | Circular RNA cia-MAF promotes self-renewal and metastasis of liver tumor-initiating cells through the transcription factor MAFF. | [153] |
| βII-Spectrin | Cytoskeletal proteins | Wnt/β-catenin signaling pathway | HCC | βII-Spectrin suppresses progression of HCC and Wnt signaling by regulation of Wnt inhibitor kallistatin. | [179] |
| SCD | Endoplasmic reticulum enzymes | Wnt/β-catenin signaling pathway | HCC | Stearoyl-CoA desaturase (SCD) promotes liver fibrosis and tumor development in mice via a Wnt positive-signaling loop by stabilization of low-density lipoprotein-receptor-related proteins 5 and 6 | [180] |
| GSK3β | Serine/threonine kinases | Wnt/β-catenin signaling pathway | HCC | GSK-3β activity is suppressed, which enhances the tumor stem cell characteristics of HCC and indicates a poor prognosis for patients. | [181] |
| DLL4 | Cycle gene | The Notch and β-catenin signaling pathways | Gastric cancer | Delta-like ligand 4 (DLL4) is highly expressed in gastric CSCs and enhances tumor angiogenesis and metastatic ability in CSCs. | [182] |
| SPOP | E3 ubiquitin ligase | Hh signaling pathway | Gastric cancer | Speckle-type POZ protein (SPOP) was reported to mediate the degradation of Gli2, thereby inhibiting the Hh signaling pathway and gastric cancer developments. | [183] |
| Zic2 | Transcription factors | Oct4 pathway | Liver cancer | Zic2 is among the most highly expressed transcription factors in liver CSCs, which drives liver CSC self-renewal via the Oct4 pathway. | [184] |
| PBX3 | Transcription factors | A wide variety of miRNA targets | Liver cancer | PBX3, a target of tumor suppressor miRNAs including let-7c, miR-200b, miR-222 and miR-424, is critical for the stemness maintenance of α2δ1+ liver CSCs, regulating several key stemness genes, including SOX2, Notch3 and EPCAM. | [185] |
| SOX9 | Transcription factors | Notch Signaling pathway | Liver cancer | SOX9 is highly expressed in liver CSCs and promotes symmetrical cell division of CSCs through negative regulation of Numb, which is critical for liver CSC self-renewal and tumorigenesis. | [186] |
| C8orf4 | Gene | The Notch signaling pathway | Liver cancer | Low expression of C8orf4 in HCC stem cells relieves its inhibitory effect on Notch pathway, thereby activating the Notch pathway in CSCs. | [187] |
| SALL4 | zinc finger transcription factor | Wnt/β-catenin signaling pathway | Liver cancer | Sal-like 4 (SALL4) enhances the self-renewal and tumor-initiating potential of NSCs by promoting the protein expression levels of several stemness genes, including EpCAM, ABCG2, and CD44. | [188] |
| FOXM1 | Transcription factors | Wnt signaling pathway | Liver cancer | FOXM1 promotes the self-renewal of liver CSCs by regulating ROS and activating CD44 expression. | [189] |
| HOXA5 | Transcription factors | Wnt signaling pathway | Digestive system neoplasm | HOXA5 induces differentiation of colorectal CSCs by inhibiting the Wnt signaling pathway. | [190] |
| BEX | Gene | β-Catenin pathway | Liver cancer | Due to the breakdown of DNMT1-mediated epigenetic silencing, brain-expressed X-linked (BEX) is highly expressed in liver CSCs, which promotes the activation of the β-catenin pathway, CSC stemness, and drug resistance. | [191] |
| DNMT1 | Five-fold transmembrane protein | Liver cancer | DNA methyltransferases 1 (DNMT1) modulates the activities of liver cancer CSCs and the expression of the marker CD133, and is itself regulated by TGF-β. | [192, 193] | |
| LSD1 | Lysine-specific demethylase | Wnt/β-catenin signaling pathway | Liver cancer | Lysine specific demethylase 1 (LSD1) inhibits APC and PRICKLE1 expression by regulating H3K4me1 and H3K4me2 at the promoters, thereby promoting Wnt/β-catenin activation, and ultimately promoting the stemness and drug resistance of LGR5+ liver CSCs. | [194] |
| MLL1 | Histone lysine methyltransferase | Wnt signaling pathway | Liver cancer | Mixed lineage leukemia 1 (MLL1) is an important factor for the stemness maintenance of colorectal CSCs and is critical for Wnt-mediated colorectal tumorigenesis. | [195] |
| RALYL | Genes encoding RNA-binding proteins | The TGF-β signaling pathway | Liver cancer | RALY RNA binding protein-like (RALYL)-mediated reduction of m6A modification can promote TGF-β signaling and downstream PI3K/AKT and STAT3 signaling to promote the stemness of liver CSCs. | [196] |
| TRMT6/TRMT61A | m1A methyltransferase | Hh signaling pathway | Liver cancer | m1A methyltransferase TRMT6/TRMT61A are highly expressed in liver cancer and CSCs, and involved in PPARδ translation and cholesterol synthesis via m1A modification of tRNA, promotes Hh signaling for liver CSC self-renewal and finally leads to poor survival. | [197] |
| miR-130b | miRNA | The PI3K/AKT signaling pathway | Liver cancer | Mir-130b enhances the proliferation and self-renewal of CD133+ liver tumor-initiating cells by targeting tumor protein 53-induced nuclear protein. | [198] |
| miR-148a | miRNA | Wnt signaling pathway | Liver cancer | Regulatory MiR-148a-ACVR1/BMP circuit delineates a cancer stem cell-like aggressive subtype of HCC. | [199] |
| miR-192-5p | miRNA | The IRAK 1/NF- κ B signaling pathway | Liver cancer | Genetic aberrations leading to the silencing of miR-192-5p play a crucial role in the development of HCCs characterized by cancer stem cell properties. | [200] |
| miR-429 | miRNA | The PTEN/PI3K/AKT signaling pathway | Liver cancer | Epigenetic modification of MiR-429 enhances liver tumor-initiating cell properties through targeting Rb binding protein 4. | [201] |
| lncBRM | lncRNA | Wnt/β-catenin signaling pathway | Liver cancer | LncBRM triggers the activation of Yap1 signaling to promote the self-renewal of liver CSCs. | [202] |
| Lnc-β-Catm | lncRNA | Wnt/β-catenin signaling pathway | Liver cancer | Lnc-β-Catm induces EZH2-mediated β-catenin stabilization and maintains liver cancer stem cell self-renewal. | [203] |
| lncDANCR | lncRNA | The Keap 1-Nrf 2/ARE signaling pathway | Liver cancer | DANCR enhances stemness characteristics in HCC through the derepression of Ctnnb1. | [204] |
| PVT1 | lncRNA | Wnt/β-catenin signaling pathway | Liver cancer | Oncofetal long noncoding RNA PVT1 enhances the proliferation and stem cell-like characteristics of HCC cells by stabilizing NOP2. | [205] |
| rtcisE2FF | circRNA | Wnt/β-catenin signaling pathway | Liver cancer | E2F transcription factor E2F1 (E2F1) promotes the self-renewal and metastasis of liver tumor-initiating cells through N6-methyladenosine-dependent stabilization of E2F3/E2F6 mRNA. | [206] |
3.1 Signaling Pathways in CSCs
The unique stem characteristics of CSCs are determined by various specific signaling pathways within the cells. The number and function of CSCs are precisely regulated by multiple signaling pathways, among which the Wnt/β-catenin, Notch and Hh signaling pathways are the most central. Recently, the importance of Hippo/Yap, PI3K/AKT, NF-κB, and JAK/STAT pathways in CSCs has also received increasing attention [207].
Wnt signaling is a conserved and complex pathway, consisting of 19 Wnt ligands and more than 15 receptors [208]. An elegant study by Hans Clevers' team found that Wnt3a is mainly distributed on the cell membrane of intestinal crypt stem cells, binds to Fzd molecules on the stem cell surface, travels away from its source in a cell-bound manner through cell division rather than through diffusion, since cell division is an important factor in Wnt diffusion [209]. Wnt signaling pathway can be divided into the β-catenin-centered canonical Wnt signal transduction and the noncanonical Wnt signal transduction involving downstream effectors such as PCP/RTK/Ca2+ [210]. Aberrant Wnt signaling activation is present in almost all cancers of digestive system and CSCs [211, 212]. The Wnt signaling can be activated in multiple ways, including constitutive activation due to gene mutation and dynamic activation caused by molecular network, epigenetics and microenvironment. The Wnt component mutations include APC mutation in CRC, β-catenin mutation in stomach and liver cancer and AXIN mutations in gastrointestinal cancers [213-216]. In terms of genetic regulation within the signaling network, activation of Wnt/β-catenin can be driven by loss of βII-Spectrin, high expression of SCD and inhibition of GSK3β [179-181]. In recent years, noncoding RNAs and epigenetic factors have become important regulators of Wnt/β-catenin via modulating the expression of Wnt/β-catenin components APC, TCF, β-catenin [142, 203]. In terms of environmental factors, stemness factors such as Wnt and Rspo, and even immune factors and neurotransmitters in the microenvironment can promote self-renewal of CSCs by regulating the Wnt/β-catenin signaling pathway [217-220]. That supported the importance of cell-type plasticity in the occurrence of intestinal tumors [20]. Even in colorectal cancer with APC mutations, Wnt/β-catenin can still be regulated by microenvironment cells such as myofibroblast [221]. These different levels of regulatory mechanisms reveal the complexity of Wnt/β-catenin signaling pathway.
Like Wnt/β-catenin, the Notch signaling pathway is also a conserved signaling pathway in evolution and development, consisting of Notch receptors, Notch ligands, CSL, DNA-binding molecules and other regulatory molecules. There are four Notch receptors, namely Notch1–4, and five Notch ligands, namely DLL1, DLL3, DLL4, Jagged 1, and Jagged 2 in human beings [222]. The activation of Notch requires tight cellular connections, therefore, an artificial Notch reporter system can assess direct cell–cell contact [223]. The engagement of receptors and ligands causes the cleavage of the Notch receptor, leading to the expression of target genes such as HES and HEY by the Notch intracellular domain [224]. Due to the different expression patterns of Notch ligands and receptors in specific tumor types, Notch signaling pathway exerts protumorigenic or tumor suppressor roles via tumor-type dependent manner. Notch-related genes are highly expressed in gastric and colon cancers, promoting tumorigenesis, while their expression is low in liver cancer [225-227]. However, Notch-signaling pathway is generally recognized as a positive modulator in stemness regulation in CSCs, via promoting CSC survival, self-renewal and metastasis. DLL4 is highly expressed in gastric CSCs and enhances tumor angiogenesis and metastatic ability in CSCs [182]. The expression of Notch1 and Jagged1 in colorectal CSCs is essential for their self-renewal [228]. Even in HCC stem cells, Notch2, a special Notch molecule, is elevated and drives the expression of downstream HES1, HEY1, and NRARP, ultimately promoting the self-renewal of HCC stem cells [187]. Of note, Notch pathway activation also exists for HES1–ATOH1-mediated lateral inhibition, which plays a central role in the quantitative maintenance of intestinal absorptive cell-secretory cells, the similar mechanism may also be used by CSCs for quantitative control [229].
The Hh-signaling regulatory network includes the extracellular Hh ligand, the transmembrane Hh receptor PTCH, the transmembrane protein SMO and the downstream molecule GLI1 [230]. When there is no Hh ligand, PTCH induces the degradation of SMO, thereby inhibiting its function, while Hh ligand is present, the binding of Hh to PTCH leads to the degradation of PTCH, resulting in the activation of SMO, this ultimately activates the Hh signaling pathway through downstream signaling molecules such as GLI1. Many target genes of Hh pathway are markers of CSCs, such as Bmi-1, ALDH1, CD44, Nanog, Oct4, and Snail. Therefore, Hh pathway is a critical pathway for promoting the self-renewal and metastasis of CSCs [231-233]. In turn, the activation of Hh signal is also regulated by other genes, SPOP was reported to mediate the degradation of GLI2, thereby inhibiting the Hh signaling pathway and gastric cancer developments [183]. Aberrant activation of the Hh signaling pathway is a common feature of various tumors, including pancreatic cancer, liver cancer and gastric cancer [231, 234-236].
The above signals are not isolated; instead, the signaling pathways regulating CSCs involve complex interactions, with different signaling pathways sharing the same transcriptional regulatory elements [20]. Whether crosstalk between signaling pathways promotes or inhibits often depends on the environment, such as the interactions between Wnt/β-catenin and NF-κB, Wnt/β-catenin and YAP, and Wnt/β-catenin and Notch.
Wnt/β-catenin and NF-κB are both critical modulators in CSC survival and proliferation, and their crosstalk is intensively investigated. (1) TNFRSF19 is a target gene of β-catenin. Its variant, TNFRSF19.2, can regulate NF-κB activity and colorectal carcinogenesis by encoding the protein containing a TRAF-binding site, indicating the regulatory role of Wnt/β-catenin on NF-κB [237]. On the contrary, NF-κB can also regulate the activity of Wnt/β-catenin pathway, P65 and TCF have the same transcriptional activating element, CBP. Abnormal activation of P65 can amplify the Wnt/β-catenin signal activated by APC loss, resulting in dedifferentiation of non-stem cell regions and “top-down” colorectal carcinogenesis [20]. At the same time, NF-κB/P65 can regulate the expression of GSK3β and promote the activation of Wnt/β-catenin by stabilizing β-catenin and facilitating its nuclear translocation [238]. Additionally, there are negative regulatory relationships between Wnt/β-catenin and NF-κB, for example, in liver and colon cancer cells, β-catenin negatively regulates NF-κB activity, while activation of NF-κB can also inhibit β-catenin/TCF activity [239, 240].
There is also crosstalk between Shh and Wnt/β-catenin signaling pathways. The Wnt/β-catenin target gene CRD-BP associates with the mRNA of GLI1, the most important component for Hh activation. CRD-BP enhances the expression and transcriptional activity of GLI1 by stabilizing GLI1 mRNA, and ultimately promotes the self-renewal of colorectal CSCs [241]. In turn, Hh signaling can positively regulate the Wnt signaling pathway and promote the self-renewal and survival of colon CSCs [242]. In addition to crosstalk with Wnt/β -catenin signaling, there is also crosstalk between the Hh pathway and PI3K/AKT/mTOR, which synergistically regulate the survival, self-renewal and metastasis of pancreatic CSCs [243].
3.2 Metabolic Characteristics of CSCs and Their Roles in Maintaining Stem Cell Properties
CSCs are a type of cells within a tumor that possess special functions, characterized by unique microenvironmental and intracellular features, including metabolic features. Tumor metabolism and metabolites play a crucial role in the quantitative maintenance and functional regulation of CSCs. Accumulating evidence demonstrates the relationship between CSC self-renewal and metabolism. Studies have shown that normal stem cells mainly use glycolysis rather than oxidative phosphorylation (OXPHOS) to reduce ROS production, thereby maintaining their stem cell state, while CSCs rely on glucose consumption function [244]. Compared with non-CSCs, CSCs also exhibit enhanced lipid metabolism and iron metabolism, maintaining their stemness [245, 246]. Of note, the metabolite ceramide is increased in CSCs, inhibits tumor microimmune activity and plays a role in driving the immune evasion of CSCs and tumor progression [247].
The Warburg effect is the main feature of metabolic reprogramming in tumor cells, and the energy source of most cancer cells is mainly aerobic glycolysis rather than OXPHOS [248]. However, several studies have shown that CSCs prefer OXPHOS [249]. The stemness characteristics of Nanog+ liver CSCs depend on the activity of complex-I and the enhancement of mitochondrial function, which are further regulated by the SIRT1/MRPS5 pathway [250]. In another study, it was found that CD133+ CSCs in HCC exhibit impaired mitochondrial OXPHOS and enhanced mitochondrial fatty acid oxidation, which is further induced by TLR4-E2F1 signaling [251]. Liver CSCs are also associated with metabolic reprogramming involving ANGPTL4 and mitochondrial PDK4. Restoring mitochondrial pyruvate oxidation can enhance the effectiveness of chemotherapeutic drugs targeting liver CSCs [252]. Pancreatic CSCs preferentially use OXPHOS rather than glycolysis to consume galactose. Activation of OXPHOS promotes stemness features of CSCs, including elevated marker expression, enhanced tumorigenicity and immune evasion [253]. Cholangiocarcinoma stem cells enriched by sphere culture also exhibit enhanced OXPHOS levels [254]. The above studies have shown that OXPHOS is commonly activated in CSCs, but its function varies among different tumors and different CSC types.
Multiple studies have demonstrated that amino acids enhance and maintain stemness properties in CSCs [255]. In Krasmut colorectal cancer cells, epigenetic remodeling induced by SLC25A22-dependent glutamine metabolism promotes WNT/β-catenin pathway and expression of colorectal CSC marker LGR5 [256]. CSCs in APCMin/+ CRC also show increased expression of adenosylhomocysteinase (AHCY), an enzyme involved in the methionine cycle. Therefore, there was a significant co-expression of LGR5 and AHCY, and inhibition of AHCY y is able to reduce LGR5+ CSCs [257]. Xanthine oxidoreductase is lost in CD13+ CD133+ liver CSCs, and its loss blocks the accumulation of ROS in liver CSCs for CSC propagation [258]. ASCT2 is essential for the intracellular transport of alanine, serine, and cysteine. The interaction between ASCT2 and CD9 can promote the cellular uptake of glutamine and drive the malignant propagation of CD9+ PDAC CSCs [40].
The adipogenic pathway is activated in drug-resistant CSCs, and among these, the research on SCD is the most extensive. The expression of SCD is increased in EpCAM+ liver CSCs. SCD induces the transition from liver fibrosis to HCC via a Wnt positive-signaling loop by stabilizing lipoprotein-receptor-related proteins 5 and 6 [180, 259]. At the same time, SCD-mediated ER stress can also promote function of CSCs on sorafenib resistance [260]. High-fat diet plays a key role in maintaining the stemness of colorectal CSCs and is an important risk factor for CRC [261].
In CRC, LGR5+ CSCs can be divided into an active cycle cell population and a quiescent cell population according to their states, and the quiescent cell population has been found to specifically express p57, which plays an important role in maintaining the stability of the tissue stem cell population. When the p57 gene is deleted, the quiescent state of normal tissue stem cells will be disrupted, and their differentiation ability will also be impaired. In colorectal cancer, LGR5+ colorectal CSCs expressing p57 have little effect on tumor growth during the tumor stable period. However, chemotherapy will activate these cells, and they will greatly promote tumor regrowth [108].
3.3 The Role of Transcription Factors in the Self-Renewal and Stemness Maintenance of CSCs
As mentioned above, CSCs are a cellular state, and transcriptional regulation plays a central role in cell fate state regulation. OCT4, SOX2, Nanog, KLF4, c-MYC and other core transcription factors of embryonic stem cells and IPSCs have been extensively studied in the regulation of CSC stemness [262, 263]. The role of other types of transcription factors in the stemness regulation of CSCs has also been reported.
Highly expressed in liver cancer and liver CSCs, OCT4 is essential for the self-renewal and tumor-initiating capacity of liver CSCs. Interestingly, the activation of OCT4 is mediated by ZIC2, a transcription factor that binds to the promoter region of OCT4. ZIC2 recruits the NURF complex to initiate transcription of Oct4 and ultimately drive self-renewal of liver CSCs [184]. c-MYC is one of the most frequently activated oncogenes in human tumors. Highly expressed in various cancers, c-MYC is one of the most potent oncogenes and plays an important role in maintaining the population of CSCs [264]. c-MYC deletion induced the differentiation of liver CSCs and the formation of bile duct structures, accompanied by the loss of α-fetoprotein and increased expression of CK19 [265]. Similarly, the expression of MYCN was positively correlated with the activation level of the Wnt/β-catenin pathway and markers of liver CSCs (AFP, EpCAM, CD133), but negatively correlated with differentiation markers (including those of mature hepatocytes and bile duct epithelial cells). This indicates the relationship between MYCN and liver CSCs [266]. Of note, c-MYC is not only an important driver of tumorigenesis, but its activation in normal cells can also lead to cell proliferation arrest, senescence and apoptosis, indicating its role as the proliferation-promoting factor or apoptosis-promoting factor [267]. Nanog is highly expressed in HCC cells and is directly related to tumor metastasis and staging, meanwhile, Nanog is associated with the survival of gastric and pancreatic cancer, and patients with high expression of Nanog tend to have a poor prognosis [268-270]. The role of KLF4 in tumors and CSCs remains unclear, KLF4 can function as either an oncogene or a tumor suppressor depending on the tumor type, serving as a positive or negative modulator of transcription. Although KLF4 may act as an oncogene in certain types of tumors, it generally functions as a tumor suppressor in the digestive system, including cancers such as colorectal, gastric, liver, and esophageal cancer [271-274].
Besides, some less-studied transcription factors also play roles in maintaining the self-renewal of CSCs. Through transcriptome sequencing, Zhu and colleagues reveal that ZIC2 is among the most highly expressed transcription factors in liver CSCs, which drives liver CSC self-renewal via the OCT4 pathway [184]. PBX3, a target of tumor suppressor miRNAs including let-7c, miR-200b, miR-222, and miR-424 is critical for the stemness maintenance of α2δ1+ liver CSCsr through regulating several key stemness genes, including SOX2, Notch3, and EpCAM [185]. SOX9 is highly expressed in liver CSCs and promotes symmetrical cell division of CSCs through negative regulation of Numb, which is critical for liver CSC self-renewal and tumorigenesis. Meanwhile, low expression of Numb promotes Notch pathway activity, which further enhances the self-renewal of CSCs [186]. Notch pathway activation in liver CSCs also requires the inactivation of certain tumor suppressor genes, such as C8orf4. C8orf4 interacts with the Notch2 intracellular segment to block its nuclear localization and Notch pathway activation. Low expression of C8orf4 in HCC stem cells relieves its inhibitory effect on Notch pathway, thereby activating the Notch pathway in CSCs [187]. SALL4, a member of the zinc finger transcription factor family, is highly expressed in liver cancer and liver CSCs. SALL4 enhances the self-renewal and tumor-initiation potential of CSCs by driving the protein expression levels of several stemness-associated genes such as EpCAM, ABCG2, and CD44 [188]. In turn, SALL4 itself is regulated by OCT4 at transcriptional level. SALL4 promoter is demethylated in HBV+ HCC, and the binding of OCT4 and STAT3 to SALL4 promoter is enhanced, driving SALL4 transcription [275]. Besides liver cancer, in other types of digestive system tumors, the role of transcription factors in regulating CSCs is also crucial. For example, FOXP4, as a downstream target of the Hippo-YAP signaling pathway, can upregulate the expression of SOX12, thereby enhancing the stemness of CSCs in gastric cancer [276]. FOXO activates ST3GAL3/4/5 and ST6GALNAC6, thereby increasing the sialylation level in CCKBR+ CSCs to maintain their stemness and promote the occurrence of gastric cancer. (CCKBR+ cancer cells contribute to the intratumor heterogeneity of gastric cancer and confer sensitivity to FOXO inhibition) BATF2 enhances 5-Fu reactivity by inhibiting ABCG2 drug transporter protein and promoting PTEN stability, reduced expression of stem cell markers CD44, SOX2, and Nanog, and ultimately reducing the drug resistance and stem cell-like characteristics of gastric cancer cells [277]. HOXA 5 induces differentiation of colorectal CSCs by inhibiting the Wnt signaling pathway [190].
3.4 The Key Impacts of Epigenetic Regulation Alterations on the Number, Function, and Related Pathways of CSCs
Like genetic variants, alterations in epigenetic regulation are also important for the number and function of CSCs and are key regulators of the self-renewal and differentiation potential of CSCs. Multiple studies have shown that aberrant expression of epigenetic factors is common in CSCs. For example, BPTF, a core component of NURF complex, is highly expressed in liver CSCs and colon CSCs [184, 218, 236], EZH2, a component of the PRC complex, is highly expressed in liver CSCs and promotes the methylation of β-catenin [203]; SWI/SNF is also highly expressed in CSCs [142], more importantly, there is a shift of epigenetic complex types in CSCs, such as a transition from BRM-type SWI/SNF complex to BRG1-type SWI/SNF complex [202]. Besides abnormalities in chromatin remodeling complexes, dysregulation of epigenetic regulatory factors is also common in CSCs, including the posttranslational protein methyltransferase PRMT6, histone deacetylase SIRT1 and histone variant macroH2A1 [278-280]. Therefore, aberrant expression of epigenetic factors is a hallmark of CSCs, indicating their central roles in CSC function.
Epigenetic factors in CSCs not only have abnormal expression, but also exhibit abnormal binding sites in the genome, altered chromatin structure, and misregulation of target genes, which ultimately leads to the activation of oncogenic, self-renewal and antiapoptosis pathways, including MEK-ERK, PARP, NF-κB-/p65 and OCT4 signaling pathways. Due to the breakdown of DNMT1-mediated epigenetic silencing, BEX is highly expressed in liver CSCs, which promotes the activation of the β-catenin pathway, CSC stemness, and drug resistance [191]. Another independent study demonstrated that DNMT1, which can be inhibited by TGF-β, regulates CSC function and CD133 expression. Epigenetic reprogramming induced by a transient DNMT1 inhibition generates long-lasting cell context-dependent memory effects and influences the malignant properties of liver CSCs [192, 193]. LSD1 inhibits APC and PRICKLE1 expression by regulating H3K4me1 and H3K4me2 at the promoters, thereby promotes Wnt/β-catenin activation, and ultimately promotes the stemness and drug resistance of LGR5+ liver CSCs [194]. On the other hand, BMI1 is a crucial factor in maintaining drug-resistant SP cells in liver CSCs, and thus is essential for the stemness and drug resistance of liver cancer. Targeting BMI1 can inhibit the tumor-initiating ability of liver CSCs [281, 282]. Besides HCC, epigenetic regulation also plays a key role in CSCs of other digest system tumors, for example, MLL1 is an important factor for the stemness maintenance of colorectal CSCs and is critical for Wnt-mediated colorectal tumorigenesis [195]. BRD9 and SMAD2/3 jointly regulate the stemness of pancreatic CSCs, and the dysregulation of methylation can activate a fetal-like state and complement the stemness of CSCs [283].
M6A modification is a methyl group addition to the carbon-6 position of adenine in mRNA, and its role in mRNA stability, translation efficiency, and splicing has been widely reported [284, 285]. Recent studies have found that nascent RNAs in the nucleus can be modified by m6A, and these newly modified m6A can have a profound impact on chromatin structure and transcriptional activity. Therefore, m6A-mediated RNA epigenetics has become a new research field of epigenetics [286]. As a reader of m6A, YTHDF2 combines and stabilizes FZD10 mRNA in liver CSCs, and then activates Wnt/β-catenin and Hippo signaling pathway to initiate the stemness and Lenvatinib-resistance [114]. In addition, YTHDF2 promoted CSCs stemness through stabilizing OCT4 mRNA [287]. Unlike FZD10 and OCT4, binding of E2F6 and E2F3 mRNA to YTHDF2 results in mRNA degradation. IGF2BP2, another m6A reader, preferentially binds to E2F6 and E2F3 mRNA, and inhibits their association with YTHDF2, further activates Wnt/β-catenin through inhibiting E2F6/E2F3 mRNA decay in CSCs [129]. Similarly, m6A modification of TGF-β mRNA also inhibits its stability, therefore, RALYL-mediated reduction of m6A modification can promote TGF-β signaling and downstream PI3K/AKT and STAT3 signaling to promote the stemness of liver CSCs [196]. The above studies indicate that the same epigenetic factor has different regulatory effects on different target genes, even in the same CSC pool.
Besides N6-methyladenosine, N1-methyladenosine and other RNA modifications are also involved in the self-renewal of CSCs. m1A methyltransferase TRMT6/TRMT61A are highly expressed in liver cancer and CSCs, and involved in PPARδ translation and cholesterol synthesis via m1A modification of tRNA, promotes Hh signaling and liver CSC self-renewal of CSCs finally leads to poor survival [197]. Xinyuan Guan and colleagues demonstrate that ADAR1-mediated GLI1R701G drives its cytoplasmic-to-nuclear translocation to drive self-renewal of liver CSCs [288]. Similarly, ADAR1-mediated A-to-I editing of SCD1 on 3'-UTR promotes mRNA stability to initiate gastric CSCs [100].
3.5 Diverse Regulatory Mechanisms of Noncoding RNAs in the Self-Renewal of Liver CSCs
With the development of RNA sequencing technology and the diversification of RNA-related research methods, a variety of functional noncoding RNAs have been identified in CSCs, among which microRNA, lncRNA and circRNA have been widely studied [289].
Hepatocellular carcinoma CSC is one of the most intensively studied types of noncoding RNA regulation. Multiple miRNAs regulate self-renewal of CSCs, including miR-130b, miR-148a, miR-192-5p, miR-429, and miR-1246 [198-201, 290]. These microRNAs function by regulating the stability of receptors on CSC surface or key genes in CSC signaling pathways, including miR155 for EpCAM, miR-1246 for AXIN2 and GSK3β, miR-181 for NLK, miR-148a for ACVR1 and miR-125b for SMAD, or by regulating the transcription of genes involved in differentiation or cell cycle. LncRNA are also important regulators in HCC stem cells, including lncTCF7, lncBRMlnca2, Lnc-β-Catm, lncDANCR, LncSOX4, PVT1 [142, 202-205, 236, 291]. CircRNAs involved in the self-renewal of HCC stem cells included circ-MALAT1, circIPO11, cia-MAFF, mcPGK1, and rtcisE2FF [126, 128, 129, 206, 292]. These lncRNAs and circRNAs exert their functions through various mechanisms, such as lncTCF7 recruiting SWI/SNF to the TCF7 promoter region, which promotes self-renewal of HCC stem cells through chromatin remodeling of TCF7 promoter, mcPGK1 encoded by mitochondria recruits PGK1 into the mitochondria and promotes the metabolic remodeling of liver CSCs, which further promotes the self-renewal of liver CSCs through the transcriptional regulation and protein stability regulation of β-catenin [142, 236]. Chromosome 8q24 region, including c-MYC and a non-coding RNA PVT1, frequently undergoes copy number amplification in tumorigenesis, PVT1 can maintain the stability of c-MYC, promote tumorigenesis and self-renewal of CSCs [293]. Of note, these noncoding RNAs may act together on the same signaling pathway, for example, lnncβ-CATM, lncTCF7, lncDANCR, mcPGCK1 and miR-1246 are all involved in regulating Wnt signaling pathway in HCC stem cells, illustrating the redundancy and complexity of noncoding RNA regulatory mechanisms.
4 Environmental Regulation of CSCs in Digestive System
CSCs exist in specific microenvironments, which are critical for their population and functional maintenance. The carcinogenic mutations that occur in the body exist in every cell of specific tissues, such as the APC mutation for colorectal cancer, however, only a small percentage of cells become CSCs, indicating the key regulatory role of the microenvironment for CSC. The microenvironment of CSCs is composed of multiple cells, including epithelial cells, stromal cells, and immune cells, as well as noncellular factors, including nutrients and oxygen, which together shape the stem characteristics of the tumor [294, 295]. Besides, CSCs have a significant remodeling effect on the microenvironment, the CSC-microenvironment interaction is considered to be an important driving force for malignant progression of tumors. In this section, we discuss recent advances in tumor CSC-microenvironment interactions, including autocrine regulation, CSC and microenvironmental cells interactions (including non-CSC epithelial cells, immune cells, nerve cells, ECs, fibroblasts, myofibroblasts, and adipocytes), microbial regulation of CSCs (intestinal flora, intracellular bacteria, and pathogenic microorganisms such as hepatitis B virus, Helicobacter pylori and Fusobacterium nucleatum) (Figure 3A), and the regulatory effect of microenvironmental factors on CSCs (e.g. hypoxia, nutrient deficiency, extracellular matrix, acidic microenvironment, immune factors, and neurotransmitters) (Figure 3B). We also expound the regulatory effects of CSC on the microenvironment and the latest progress in the regulatory stemness of CSC-microenvironment interaction (Figure 4). Altogether, these observations demonstrate that their niche, but not the CSC themselves, determines the fate of CSCs.
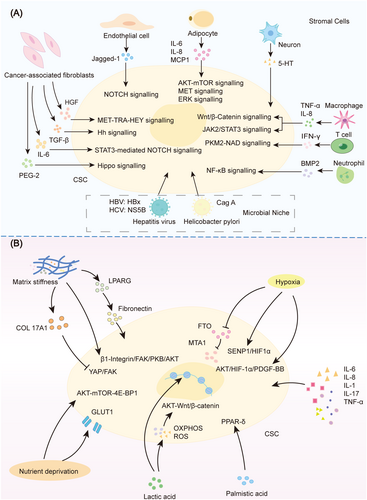
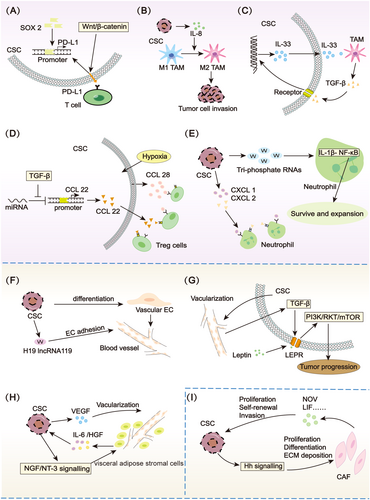
4.1 Autocrine Regulation of CSCs
CSCs have a unique gene expression landscape and secretion characteristics. CD133+ liver CSCs express higher amounts of secretory proteins IL-8 and ANXA3 than CD133− cells, and IL-8 efficiently promotes angiogenesis and self-renewal of CSCs. ANXA3 can activate Notch and JNK pathways, promoting self-renewal of liver CSCs [296, 297]. The above studies revealed that CSCs regulate their stemness in a positive feedback manner via secretory factors such as ANXA3. In fact, an ANXA3-neutralizing antibody has been developed, which shows an inhibitory effect on the propagation of HCC. Moreover, ANXA3 antibody increases the sensitivity of HCC to chemotherapy drugs such as cisplatin, sorafenib, and regorafenib via depletion of liver CSCs, indicating the clinical significance of blocking CSC autocrine regulation [298, 299]. Similarly, HCC progenitor cells, emerging several months before tumorigenesis, secrete IL-6 for malignant progression. IL-6 accumulation is induced by increased LIN28 expression, and activates JAK1/2–STAT3 signaling to further drive LIN28 transcription, forming a positive loop [18].
Autocrine mediates not only positive feedback regulation but also negative feedback regulation, which generally occurs in various cells. IFN-γ, secreted through immune cell activation, is the most important in vivo signal to initiate PD-L1, which is used to terminate or eliminate immune response [300]. Similarly, due to multiple Wnt inhibitory factors in Wnt target genes, activation of the Wnt pathway is accompanied by negative feedback regulation, for example, Wnt target genes RNF43 and AXIN2, which induce ubiquitination-induced degradation of FZDs (Wnt receptor) and β-catenin, respectively, therefore inhibit the Wnt pathway. As the most critical regulatory signal in CSCs, the Wnt pathway is also regulated by autocrine-mediated feedback inhibition loop. Notum, one of Wnt target genes, has recently been identified as a secretory feedback inhibitor of Wnt/β-catenin, when Wnt pathway is activated, high expression of Notum can deacylate Wnt ligand to diminish Wnt activation [301]. In tumorigenesis, gene or environmental disturbance may cause the failure of negative feedback regulation. For example, APC mutation, the most common type in colorectal cancer, causes deficiency of the β-catenin-degrading pathway, thus inducing constructive activation of Wnt/β-catenin signaling and subsequent Notum expression. Extracellular Notum deacylates Wnt ligands, which has little effect on APCmut cells but inhibit Wnt/β-catenin activation in APCWT cells. Accordingly, APCmut cells obtain advantages in clonal expansion compared to APCWT cells [173, 174]. A similar situation also exists in the mechanism of obtaining competitive advantage in KrasG12D and PIK3CAH1047R cells, in which BMPs secreted by LGR5+ cells drive the clonal advantage [175]. These studies suggest that autocrine is a key driver of CSC self-renewal and tumorigenesis.
4.2 Interaction Between CSCs and Tumor Non-Stem Cells
In addition to CSCs, tumor tissues contain a large number of non-stem cells. These non-stem cells are distributed around CSCs and play important roles in regulating CSCs. In fact, the ratio between CSCs and non-stem cells is always maintained at a certain level. When CSCs are depleted, tumor cells can dedifferentiate into CSCs, while tumor cells are cleared, CSCs can differentiate into tumor cells, indicating the inherent fate exchange mechanism between CSCs and non-stem cells [302].
Tumor non-stem cells reshape CSCs through direct contact or secrete signal factors. The initiation of Wnt signaling pathway in liver CSCs requires Wnt ligands in the environment and Wnt receptors on the surface of CSCs. FZD6 is highly expressed in CSCs, while Wnt5A is secreted from non-CSCs, the Wnt5A-FZD6 engagement drives Wnt/β-catenin activation and self-renewal of CSCs [217]. In homeostasis-state intestinal tissue, Paneth cells, a type of differentiated cell, provide factors such as Wnt ligands, EGF, and pyruvate for the self-renewal of ISCs [303, 304]. Besides, enteroendocrine and tuft cells serve as niche cells for stem cells when Paneth cells are absent [305]. Intestinal adenomas are reminiscent of normal crypt, and stem cells are adjacent to differentiated cells such as Paneth cells and KRT20+ epithelial cells [28, 42]. Therefore, it is expected that the maintenance of KIT+ stem cells in colorectal cancer requires stem cell factors produced by tumor non-stem cells [306]. The death of non-stem cells, generally induced by chemotherapy, radiotherapy and immunotherapy, significantly regulates the fate of stem cells via CSC-direct targeting or niche-dependent manner, for example, chemotherapy-induced cell death promotes the emergence of CD10+GPR77+ CAFs through activating NF-κB/P65 signaling, which further drives CSC self-renewal via IL-6 and IL-8 [88].
Cell competition occurs between CSCs and non-CSCs, or different CSCs with various intrinsic changes, such as mutations. CSCs often have a stronger competitive advantage than non-stem cells due to their greater environmental adaptability and plasticity. APC mutant CSCs maintain the advantage of self-renewal and clonal expansion by secreting Notum to inhibit the growth of surrounding normal cells [173, 174]. Similarly, KrasG12D or PIK3CAH1047R stem cells also outcompete neighboring cells via secreting BMP [175]. The surviving cells of gastric cancer after treatment usually have CSC capability, and the expression of SLC6A6 is upregulated, which escapes the killing of immune cells through taurine absorption, eventually leading to drug resistance and recurrence [307, 308].
4.3 Endothelial Cells
Lee M. Ellis and colleagues reveal that CD133+ liver CSCs and CD31+ ECs locate adjacently but without direct cell–cell contact, and ECs secreted soluble Jagged-1 to activate Notch signaling in CRC cells to promote CSC phenotype [309]. EC also secretes LRG1, which directly binds to and activates HER3, and activates protein synthesis through the PI3K–PDK1–RSK–eIF4B axis, thereby promoting CRC growth [310]. Vascular ECs promote CSCs to escape from radio-/chemo-therapy and promote, invasion and metastasis of CSCs [311, 312]. Some studies have shown that CSCs can differentiate into vascular ECs [313, 314]. This pattern of differentiation demonstrates the plasticity of CSCs and echoes the mimicry theory of CSCs as maintained above [92]. Interestingly, a recent lineage tracing study found that newly generated vascular ECs can be produced from within the tumor to the outside, indicating the tumor-intrinsic generation of ECs [223].
4.4 Cancer-Associated Fibroblasts
Activation and mass enrichment of fibroblasts are common features of various tumors, especially liver cancer, because most HCC develop in the context of cirrhosis [315]. α-SMA+ CAFs are directly related to the prognosis of HCC. CAFs secrete liver growth factor (HGF) to activate the MET–FRA1–HEY1 pathway, ultimately promoting the tumor initiation ability of hepatic CSCs and cirrhosis-dependent liver tumorigenesis [316]. Similarly, HGF produced by CAFs also promotes the expression of CK19, a marker of hepatic stem/progenitor cells and related to poor prognosis [317]. In addition to HGF, CAFs also secrete high levels of IL-6, which drives liver CSC self-renewal through IL-6–STAT3 pathway [318]. CAF-produced CLCF1 induces tumor cells to release TGF-β and CXCL6, further promotes tumor stemness via autosecretion and TAN-mediated paracrine [319]. CAFs also drive self-renewal of liver CSCs in Notch3-dependent manner through activating LSD1 [320]. Not only tumor-locating CAF cells, but the fibroblasts of adjacent tissues can secrete a series of cytokines, including HGF, IL-6, CCL2, CXCL1, CXCL8, and VEGF to recruit EpCAM+ liver CSCs for HCC metastasis [321]. Myofibroblasts deliver extracellular vesicles (EVs) to HCC cells, inducing the acquisition of tumor stem-like characteristics in HCC cells [322]. Hepatic astrocytes, which are fibroblasts located in the liver, can interact with monocyte, jointly maintain the immunosuppressed microenvironment and further promote tumor cell migration and globular formation [323].
In addition to liver cancer, the enrichment of fibrocytes is also reported in other tumors. Medema and colleagues found that Wnt/β-catenin activation was heterogeneous even in the context of APCmin, and tumor cells close to stromal myofibroblasts showed stronger Wnt signaling and stemness. Secretion of HGF by stromal myofibroblasts promotes Wnt/β-catenin activation to initiate CSC self-renewal, indicating a more complicated regulation network for Wnt/β-catenin activation [221]. The single-cell sequencing of mouse colorectal cancer revealed the presence of Ptges+ CAFs, which drives the expansion of Sca-1+ reserve-like stem cells and colorectal tumorigenesis via PGE2–Ptger4 engagement and subsequent YAP activation [324]. CAFs in CRC secrete TGF-β2, which together with HIF-1α, promotes the expression of GLI2 in CSCs, and further promotes the self-renewal and drug resistance of colorectal CSCs through the Hh pathway [325]. Contrary to the opinion that colorectal CSCs are located inside the tumor near blood vessels, Louis Vermeulen group reveals that functional CSCs are located at the edge of the tumor via constructing a marker-free lineage tracing assay. These marginal stem cells cannot be distinguished by traditional labeling systems such as LGR5 and are important drivers for tumor expansion [309]. Moreover, these CSCs located close to CAFs, which secrete osteopontin to drive clonogenicity and tumor expansion [326]. Treatment of pancreatic cancer cells with conditioned media from CAFs but not normal human fibroblasts increases tumor-sphere formation and CD44+ CSCs through SPP1 [327]. The regulative effect of CAFs on CSCs has also been validated in breast and pancreatic cancers [88, 328].
Multiple studies have revealed the key role of CAFs in tumors and tumor drug resistance. The drug resistance of CSC is partly driven by CAFs in the microenvironment. Christopher J. Tape's team used single-cell sequencing to analyze cell types in patient derived orgnoids (PDOs) coculture with CAFs or in response to multiple therapies, and identify the changes in the type of CSCs, with cell fate transition from MKI67+ proliferative CSCs to MKI67- revival CSCs [51]. The damage response of chemotherapy and alteration of TME such as CAFs are vigorous inducer for stemness. For example, chemotherapy for breast cancer induces the activation of NF-κB pathway in CAFs, leading to the emergence of CD10+GPR77+ cells, which further drives CSC expansion [88].
4.5 Neuron
Due to the promotion of anxiety and other neurological factors contributing to a variety of tumors, the neuroregulation of tumors has received much attention from researchers.
In recent years, an increasing body of evidence showed that nerve cells are one of the most important microenvironment cells in CSCs [329]. Gastric and colorectal CSCs have the potential to differentiate into neuronal cells that form the nervous system in tumor tissues, and it has been shown that knocking down the neurogenic capacity of CSCs reduces tumor growth [330]. Dclk1+ tuft cells and nerve cells in gastric cancer secrete acetylcholine (ACh), which drives epithelial proliferation and tumorigenesis via the ACh receptor M3R. At the same time, Ach treatment can also stimulate gastric cancer cells to produce nerve growth factors, therefore accelerating nerve expansion and forming ACh-NGF feedforward [331]. In pancreatic cancer, enhanced cholinergic signals inhibit self-renewal of CSCs and tumorigenesis of pancreatic carcinoma through CHRM1 and subsequent inhibition of MAPK/EGFR and PI3K/AKT [332]. The controversial functions of cholinergic signals in gastric and pancreatic cancer may be due to their different receptors and downstream transmission of activation/inhibition signals. The undecapeptide substance P promotes AKT activation and inhibits apoptosis upon binding to the neurokinin-1 (NK-1) receptor, which is highly expressed in gastric and intestinal cancers. And NK-1 receptor antagonist exerts antitumor effects on colon CSCs [333]. In colorectal cancer, the content of 5-HT significantly increased due to the blocking of 5-HT degradation pathway. At the same time, the 5-HT receptors HTR1B, HTR1D and HTR1F are highly expressed in colorectal CSCs which can bind to enriched 5-HT in bowel cancer tissues to promote the activation of Wnt/β-catenin signals and self-renewal of colorectal CSCs [334].
4.6 Adipocytes
Metabolic abnormalities are typical features of tumors, meanwhile, obesity and fat cells have promoting effects on a variety of tumors including liver cancer, bowel cancer and pancreatic cancer. Fatty liver is one of the most primary risk factors in liver cancer, which is mainly characterized by the accumulation of fat cells. Lipids and immune factors secreted by adipocytes, including IL-6, IL-8, and MCP1, can promote the expansion, metastasis and resistance to sorafenib of liver CSCs [335]. High-fat diets also facilitate TWIST1 expression via TLR4-Nanog and LEPR-Stat3 signaling in CSCs [336]. Palmitic acid from high-fat diet also activates the PPAR-δ and Wnt/β-catenin signals of ISCs to accelerate the numbers and functions of ISCs and progenitor cells, further promoting the carcinogenesis of CSCs [261]. Visceral adipose stromal cells (V-ASCs) locating near tumor cells secrete IL-6 and HGF to promote the expansion and metastasis of CD44V6+ colorectal CSCs while CSCs in turn secrete NGF and NT-3 to recruit adipose stem cells which forms an ASC-CSC feedback loop [337].
4.7 Immune Cells Modulate CSCs
Immune cells in tumors are an important part of TME. CSCs have stronger tumor initiation abilities and their ability to escape immune killing is a key step in tumorigenesis, so CSCs have a unique advantage in escaping immune system monitoring. More and more studies have proved that immune cells have become an important microenvironment cell type in CSCs.
4.7.1 Lymphocytes
CSCs evade the killing of immune cells through diversified mechanisms, including high expression of PD-L1, low expression of MHC molecules, secretion of immunosuppressive factors, and so forth. In consequence, the traditional view is that CSCs escape immune cells to survive upon immunotherapy and then drive tumor recurrence [117]. For example, galactose culture-induced CD133+ and CD24+ CSCs highly expressed multiple immune escape markers such as PD-L1, CD47, and CD206 [253]. Some immune checkpoint molecules, such as PD-L1, serve as target genes of the Wnt/β-catenin pathway and are therefore constructively highly expressed in CSCs, further illustrating the molecular basis of CSCs on immune evasion.
Several recent studies have found that immune cells significantly improve tumor stemness via plasticity transformation from non-CSCs to CSCs. The tumor immunotherapy represented by immune checkpoint inhibitors has achieved great success in clinical practice, however, some patients may experience hyperprogression. Recent studies by Zou Weiping's team have shown that IFN-γ produced by T cells can act on FGF2+ tumor cells to promote the activation of β-catenin and the expansion of CSCs through the PKM2–NAD pathway, while FGF2-tumor cells are killed [338]. Two similar independent studies have also shown the regulatory effects of interferons on CSCs. The epigenetic factors KDM1B induced by Type I interferons promote tumor stemness and immune escape, so KDM1B inhibition prevents CSC enrichment caused by immunotherapy [90]. IFN-γ can transform non-CSC into CSC through BCAT-1 and therefore increases the number of CSC after immunotherapy, while blocking BCAT-1 enhances the therapeutic effects of tumor immunity [89]. In fact, high immune features of tumors, such as increased infiltration of T cells, are associated with low differentiation of tumor cells, further demonstrating the critical roles of lymphocytes in the regulation of CSCs [339]. However, unlike the acceleration roles of CSC in tumors, T cells kill and wound various tissue stem cells especially proliferative stem cells, however, quiescent tissue stem cells can escape immune surveillance [340].
Ectopic lymphoid structures (ELS) are masses of white blood cells, which are similar to lymphatic organs and are common in chronic inflammatory tumors. ELS contain adaptive immune cells and are thought to be associated with optimistic survival rates in certain tumor types. The relatively low risk of replacement in patients with ELS+ liver cancer confirms that ELS may have antitumor effects and some studies have revealed the promoting effects of ELS on CSCs [341]. The prognosis of ELS+ patients is poor, NF-κB stimulated ELS may serve as a niche for hepatic CSC expansion and liver tumorigenesis [342]. At the same time, the appearance of ELS is related to the expression of genes which is relevant to immunosuppression and immune depletion, further certificating the tumor promotion of ELS in liver cancer [343].
4.7.2 Tumor-Associated Macrophages
Tumor-associated macrophages (TAM) is the most abundant cell type in various tumors, its regulatory functions on CSC have been extensively studied. TAM not only expedites tumor progression but is also capable of phagocytosis on immunosuppressive TME. The marker gene CD47, considered as a “don't eat me” signal, is highly expressed in the liver CSCs, which confers CSCs resistance to TAMs. Thus, blocking CD47-SIRRP-α provides an additional strategy for targeted therapy of CSCs [344, 345]. Meanwhile, CD24 is also highly expressed in liver CSCs and it is likewise a “do-not-eat-me” signal, so CSCs can also resist macrophages in CD24-dependent way [346].
TAM also accelerates self-renewal of CSCs through directly contacting or secreting multitudinous cytokines including TNF-α, TGF-β, IL-8 and exosomal microRNA and the like. TAM transforms its contacted non-stem cells into stem cells immediately through Notch-Jagged pathway [347]. TNF-α that is produced by TAM promotes the activation of Wnt/β-catenin pathway in liver cancer cells, thereby facilitating the EMT and stemness of tumors [348]. TAM also produces TGF-β that is a key mediator of EMT which activates HCC EMT and stemness [349]. IL-8, another secreting factor released by TAM, triggers EMT by activating the JAK2/STAT3/Snail pathway [350]. Meanwhile, exosomes secreted by TAM can expedite tumor cells proliferation and stemness, microRNAs in exosomes may play a regulatory role in this process [351].
4.7.3 Other Immune Cells
Neutrophils as the richest white blood cells in the blood, play significant roles in anti-infection and inflammatory response and its effects on the occurrence and progression of tumors have received more and more attention. After coculture of tumor-associated neutrophils (TAN) and HCC cells, the stemness of HCC cells was enhanced. BMP2 and TGF-β2 secreted by TAN promote the proliferation of CSCs through LSAMP/CyLD-mediated NF-κB activation, and the activated NF-κB in turn promotes the release of CXCL15 from liver CSCs to recruit more TAN which creates a positive feedback [352]. NK cells are another class of cytotoxic cells and have recently been recognized as a subgroup of innate lymphoid cells (ILCs). High levels of CEACAM1 or the granulin-epithelin precursor (GEP) in EpCAM+ liver CSCs can resist cell death caused by NK cells, thus blocking antibodies to GEP or CEACAM1 can make liver cancer cells more sensitive to NK cells [353, 354].
4.8 Microbial Niche Modulate CSCs
The TME contains not only a variety of cells but also a variety of types of microorganisms, some of which play crucial regulatory roles in the process of tumor development. In this part, we summarized the microbial signals that modulate stemness in the TME, including hepatitis viruses for HCC, Helicobacter pylori for gastric cancer, Clostridium nucleatum for colorectal cancer, and intramural microorganisms.
4.8.1 Hepatitis Virus
Hepatitis virus is a major risk factor for liver neoplasms, and its regulated role on CSCs has been extensively studied. HBV encodes multiple factors while HBV X oncoprotein (HBx) largely promotes HBV-induced HCC. Clinical data from patients with HBV-induced liver cancer showed that HBx and markers of CSCs are closely related [355]. HBx induces multiple CSC-related genes expression including OCT4, NANOG, KLF4, and EpCAM. HBx can remove inhibiting effects of PRC2 and HDAC1 on EpCAM promoter to facilitate the binding ability of NF-κB and EpCAM promoter, ultimately, facilitating EpCAM expression by remodeling chromatin structure [356]. Simultaneously, HBx can also accelerate the emergence of OV6+ liver CSCs and subsequent tumorigenesis through the MDM2/CXCL12/CXCR4/β-catenin pathway [357]. In addition to HBx, PreS1, another protein encoded by HBV can activate biomarkers of CSCs such as CD133, CD117 and CD90 and further accelerate hepatocarcinogenesis [358].
HCV-infected liver cells showed greater sphere formation ability which indicated stimulation of CSCs by HCV. HCV core protein significantly improves c-Kit transcription levels to promote EMT and hepatocarcinogenesis [359]. HCV factor NS5B was also associated with CSC phenotypes including increased expression of LGR5, CD133, AFP, and MYC [360].
4.8.2 Helicobacter pylori
The CagA oncoprotein of H. pylori induces a “hummingbird” phenotype which is similar to EMT and upregulates expression of the CSC marker CD44. Thus, H. pylori infection leads to EMT-like phenotypes and CSC characteristics [361]. A recent study found that gastric cancer cells infected with H. pylori exhibit CSC-like characteristics and express CSC marker CD44. CagA activates AKT/FOXO3a pathway which is essential for H. pylori-induced CSCs [362]. Another toxin produced by H. pylori, tumor necrosis factor-α-inducing protein, enhances the expression levels of CSC markers such as CD44, OCT4, and Nanog by activating the Wnt/β-catenin signaling pathway and thus promotes the self-renewal of gastric CSCs and the progression of gastric cancer [363]. Manuel R. Amieva et al. found that H. pylori localized in the regions of gastric stem cells in mice and patients which facilitated the numbers and functions of LGR5+ cells and finally accelerated the proliferation and hyperplasia as early as 2 weeks [364]. H. pylori infections activate LGR4-induced NF-κB activation and further expedite stem cell expansion and chemokine expression to promote proliferation and inflammation [365]. Altogether, H. pylori drives gastric CSCs via multiple pathways.
4.8.3 Fusobacterium nucleatum
Fusobacterium nucleatum (Fn) is an important hazard for the development and drug resistance of colorectal cancer. A retrospective study on 280 stage III/IV colorectal patients showed that Fn is a prognostic factor that influences patient survival rate and is related to EMT and CSCs. Fn level is significantly correlated with EMT markers such as E-cadherin and N-cadherin and CSC marker Nanog [366]. Fn expedites the expression of EMT markers Snail1, Vimentin, ZEB1 and CSC marker CD44 by activating the IL-6/STAT3 pathway [367]. Wnt/β-catenin, the most fatal signal channels in CSCs, can be regulated by Fn. Fn preferentially bonds and infects Annexin A1 positive CRC cells by forming the FadA/E-cadherin/Annexin A1/β-catenin complex, further promotes Annexin A1 expression and Wnt/β-catenin activation. A “two-hit” model is proposed in which genetic mutations act as the first hit and cancer-promoting factors (such as Fn) act as the second hit [368]. Similarly, another study showed that the aggregation of FadA promotes the E-Cadherin/β-Catenin signal pathway [369]. Fang Jing-Yuan et al. found that autophagy increased after Fn infection in large amounts of recurrent colorectal cancer samples. Fn functions on TLR4/MYD88 in tumor to inhibit miRNA-18a*/4802 and ultimately inhibits drug-induced apoptosis by suppressing autophagy [178]. Pengcheng Bu group revealed an unexpected CSC regulatory pathway of Fn through Notch activation which was mediated by lipid drops. Fn promotes self-renewal of colorectal CSCs by upregulating fatty acid oxidation while Fn facilitates lipid accumulation and lipid drop formation in non-CSCs. Both NumB and MDM2 are recruited into lipid droplets where NumB is degraded by MDM2, which cause Notch pathway activation and CSC self-renewal [370]. Interestingly, Fn infection gives rise to DNA damage and high levels of γ-H2AX staining, in consequence, high Fn loads are correlation to MSI-high [371].
In addition to direct regulations of CSC, Fn can also reshape the tumor immune microenvironment and is a precipitating factor for the immunosuppressed microenvironment in intestinal cancer. Fap2 of Fn recognizes the highly expressed Gal-GalNAc in colorectal cancer and facilitates its colonization in colorectal cancer. Targeting Fap2/GalNAc pathway can obstruct carcinogenesis of Fn [372]. Fap2 combined with TIGIT which is on the surface of NK and T cells to activate TIGIT-mediated inhibitory signals and promoted immune evasion during tumorigenesis [373]. An independent study showed that Fn treatment with APCmin/+ mice could facilitate selective recruitment of tumor-infiltrating myeloid cells and accelerate tumorigenesis by reshaping the tumor immune microenvironment [374].
4.8.4 Intratumoral Microbes
Using 1526 tumor/peri-tumor samples from seven cancer types, Ravid Straussman's team found that bacteria exist in tumor cells and microenvironmental immune cells. Interestingly, different tumor types contain different microbes. Susan Bullman and colleagues studied the intratumoral microbiota of colorectal cancer and oral squamous cell carcinoma and found that the bacterial population was located in the vascularization presenting, immunosuppressed and Ki67 low level region to participate in regulation of inflammation, metastasis, dormancy and DNA repair. Cells infected with bacteria recruit more neutrophils and modulate NF-κB and ECM remodeling pathways through promoting individual tumor cells to leave the tumor tissue [375].
4.9 Impact of Niche Factors on CSCs Regulation
Besides wide diversity of cells, the TME also includes diverse noncellular characteristics, which involves extracellular matrix, hypoxia, nutrient deficiency, acid, inflammatory environment, and so forth. These microenvironmental factors modulate tumor stemness in a variety of ways (Figure 3B).
4.9.1 Extracellular Matrix
Solid tumors generally show enhanced hardness because of increased extracellular matrix in the TME. The extracellular matrix is generated by fibroblasts, stromal cells, and some immune cells such as macrophages. Physical characteristics such as tumor hardness and microenvironmental stress are crucial for the regulation of self-renewal and differentiation of CSCs, but the positive or negative impacts of mechanical stress have not been clarified, both soft and hard matrix both induce CSC. Because cirrhosis is a critical step in the development of liver cancer, the regulation roles of mechanical pressure in liver cancer are relatively well studied. Some studies have found that matrix hardness facilitates stemness of liver CSCs, for example, low-shear stress (2 dyne/cm2) restrains the expression of β-catenin and significantly suppress the sphere formation ability and expression level of CSC's markers to increase the sensitivity to chemotherapy. Matrix stiffness accelerates liver cancer self-renewal and oxaliplatin resistance through integrin β1/AKT/mTOR/SOX2 [376, 377]. SP+ hepatoma cells express high levels of ECM Ln-332, which further promotes state of quiescence, drug resistance, and stemness but reduces proliferation [378]. These studies have verified that hardness expedites the stemness of liver cancer while other studies have demonstrated the opposite results. Schrader and colleagues treated liver cancer cell lines using polyacrylamide gels with different physical properties and found that matrix stiffness promotes cell proliferation and cisplatin resistance through β1-Integrin/FAK/PKB/AKT pathway while soft matrix enhances cell stemness and dormancy with enhanced expression of CSC's markers CD44, CD133, CXCR4, and NANOG [379]. A similar study also elucidated that the soft matrix improves sphere-forming capacity of tumor cells and proportion of SP cells with upregulation of CSC markers CD133 and CD90 [380]. THBS2-deficient CD133+ CSCs that is induced by chemotherapy reshape ECM through collagen degradation to form local soft spots, which further drive stemness, metastasis and drug-resistance through histone H3 modification, which regulates THBS2 and PROM1 promoters. Powerful mechanisms restrain CD133 promoter activation and reduce CD133+ liver CSCs [381]. Similarly, CD133+ CSCs produce metalloproteinases to degrade ECM and expedite soft microenvironments formation [382].
Colorectal cancer cells that were cultured in the matrix express low levels of CRAD and further restrain YAP in the cytoplasm to relieve the inhibitory effect of YAP on the stemness genes Nanog and OCT4. Ultimately, soft substrate mediated stemness and metastasis [383]. COL17A1, a structural element of hemidesmosome that mediates adhesion between epithelial cells and the basement membrane/matrix, is highly expressed in quiescent CSCs (LGR5+p27+ cells) and brings about relapse after chemotherapy. COL17A1 inhibits the activation of FAK and YAP and maintains the dormancy of LGR5+p27+ cells and therefore driving chemotherapy resistance. Chemotherapy accelerates degradation of COL17A1 by MMPs and activates FAK/YAP to promote cell cycle re-entering. Therefore, the extracellular matrix and COL17A1 mediate the quiescent state of CSCs which drives drug resistance and relapse [64]. Lysophosphatidic acid receptor 4 (LPAR4) is highly expressed in pancreatic cancer cells. Fibronectin is upregulated through the LPAR4/AKT/CREB pathway in response to environmental stress or chemotherapy. This fibronectin-rich autonomous matrix promotes CSCs survival and tumorigenesis [91].
4.9.2 Hypoxia
Hypoxia is a common feature of almost all solid tumors, and HIFs are the most critical transcription factors in cellular response to hypoxia. Hypoxia has long been considered a factor that promotes tumor stemness through various mechanisms [384]. In liver cancer, hypoxia promotes tumor growth and cisplatin resistance by activating the AKT/HIF1α/PDGF-BB autocrine signaling loop in hepatic progenitor cells [385]. Hypoxia also promotes the stability of HIF-1α through the deSUMOylation of HIF-1α mediated by SENP1, which is also the target gene of HIF-1/2α. The positive feedback between SENP1 and HIF-1α activates self-renewal of liver CSCs [386]. Hypoxia in colorectal cancer reduces FTO expression by increasing FTO ubiquitination. In turn, FTO inhibits the expression of MTA1 and IGF2BP2 in an m6A-dependent manner. Hypoxia promotes metastasis and xerogenicity of bowel cancer through FTO and MTA1 [387].
4.9.3 Nutrient Deprivation
Due to rapid growth and inadequate blood vessel supply, tumor cells face a long-term lack of nutrients. CSCs have the ability to reshape their own state in difficult situations, and nutrient deprivation induces the CSC characteristics of liver cancer stem cell CSCs. In liver cancer, glucose deprivation activates the expression of FUT1 through the activation of PERK/eIF2α/ATF4 and subsequent binding of ATF4 to FUT1 promoter. FUT1 can drive fucosylation of several membrane proteins, such as CD147, ICAM-1, EGFR, and EPHA2, and ultimately activate AKT–mTOR–4E-BP1 to promote the stemness and expansion of CD133+ CSCs [387]. CD133+ liver CSCs increase the expression of GLUT1 and GLUT3 to absorb more glucose in an environment with insufficient glucose supply, and GLUT1/GLUT3 was further stimulated by IL-6/STAT3 [388]. Similarly, IL-8 promoted GLUT3 expression and glucose influx and O-GlcNAcylation in colorectal CSCs, which are essential for generation and maintenance of colorectal CSCs [389].
4.9.4 Acid
Due to the large amount of lactic acid production, solid tumors show a certain weak acidity. In nutrient-deficient TME, lactic acid provides energy for a variety of cells and regulates multiple intracellular signals, promoting the activation of tumor cells and inhibitory immune cells in the microenvironment, and ultimately promoting tumor stemness and tumor initiation [390]. Colorectal CSCs and non-stem cells employ different metabolic pathways, glycolysis in cancer non-stem cells and OXPHOS in CSCs. Lactic acid produced by cancer non-stem cells acts on CSCs to promote the production of OXPHOS and ROS in CSCs, while ROS further promotes the stemness of colorectal CSCs through the AKT–Wnt/β-catenin pathway [391]. Meanwhile, lactic acid can also promote the metastasis of colorectal CSCs through PGC-1α [392]. Lactic acid also acts on histones, mediating histone lactylation modification and regulating chromatin regulation [393]. Recently, accumulating studies revealed the lactylation of non-histone proteins in tumorigenesis, including P53 lactylation for tumorigenesis, NBS1 lactylation and MRE11 lactylation for chemoresistance [394-396].
Addition to lactic acid, several other acids also regulate CSCs, such as palmitic acid, butyric acid, and bile acid. Palmitic acid is the most abundant component of the high-fat diet, which can significantly activate the PPAR-δ pathway to promote the self-renewal of colorectal CSCs and the occurrence of colorectal cancer [261]. Butyrate, as an important component of food and a metabolite of bacterial flora, can induce the expression of c-Fos, thus promoting the sensitivity of CSCs to ferroptosis. Therefore, the combined use of butyrate and oxaliplatin can enhance the ferroptosis of oxaliplatin [397]. Like H. pylori infection, bile acid reflux is also a cause of abnormal differentiation of gastric cancer progenitor cells and gastric intestinal metaplasia, which plays a significant role in promoting gastric cancer [398]. Bile acid receptors such as FXR, PXR, VDR and TGR5, FXR and TGR5 have been studied intensively in promoting the self-renewal of colorectal CSCs and the occurrence of colorectal cancer [399]. Tauro-β-muricholic acid and deoxycholic acid can antagonize the function of FXR and induce cell proliferation and genomic instability in LGR5+ cells, while selective activation of FXR can restrict the proliferation of LGR5+ cells and inhibit the occurrence of intestinal cancer. Therefore, bile acid promotes the proliferation of intestinal CSCs and the occurrence of intestinal cancer by inhibiting FXR [400]. Bile acids can also activate Tgr5, a receptor, and then promote the regeneration of intestinal epithelium by activating SRC and YAP [401]. However, in colorectal CSCs, ursodeoxycholic acid can inhibit the YAP pathway and the proliferation of CSCs via TGR5 [402]. These differences may be due to different types of bile acids and different cell types.
4.9.5 Inflammation
Inflammation is a hallmark of a variety of tumors and a key factor in tumorigenesis, such as acid reflux-induced Barrett's esophagus for esophageal cancer, H. pylori-induced gastric ulcer for gastric cancer, hepatitis virus-induced hepatitis for liver cancer, IBD for bowel cancer, and so on. Among them, inflammatory factors produced by stromal cells, immune cells or epithelial cells can trigger the signal transduction of CSCs, thereby regulating the self-renewal of CSCs. Mist1+ stem cells in gastric corpus isthmus, serving as the origin of intestinal-type gastric cancer and diffuse-type gastric cancer, are supported by inflammation nich generated by CXCL12+ ECs and CXCR4+ ILCs. Wnt5a produced by ILCs promotes the self-renewal of gastric CSCs [403]. A similar study also shows that Mist1+ stem cells respond to injury and inflammation and are modulated by ILCs-generated Wnt5a [404]. As a major inflammation factor, IL-8 is highly expressed in the blood and Barrett's esophagus of mice during a high-fat diet, with increasing myeloid cells and LGR5+ stem cells [405]. IL-6-mediated chronic inflammation is a key factor in the transformation of normal liver cells into liver CSCs [406]. In addition to IL-8 and IL-6, various inflammatory factors can stimulate the production and self-renewal of CSCs, including TNF-α, IL-1, and IL-17 [407-409]. These studies illustrate the important regulatory role of the inflammatory microenvironment on CSCs.
4.10 CSCs Remodel Niche
Tumor is a complex cell population, and there are many complex relationships between tumor cells and microenvironment cells, including parasitism, symbiosis, predation and mimicry. Tumor cells not only passively receive environmental signals, but also deeply reshape the microenvironment to create an environment suitable for their own survival, and eventually evade immune surveillance and gain growth advantages (Figure 4). The microenvironment can be regulated by CSCs through direct contact, paracrine or exosomes [410].
4.10.1 Immune Remodeling
Although type I interferon produced by tumor treatment can induce more CSCs, clinical data show that tumor stemness in most tumors is negatively correlated with the number of CD8+ cells and type I interferon, indicating that tumor stemness may have an inhibitory effect on immunity [39]. In fact, stemness signals can activate immunosuppressive pathways and participate in the formation of tumor immunosuppressive microenvironments, facilitating the immune escape of CSCs.
CSCs reshape the tumor immune microenvironment through multiple pathways. (1) Direct inhibition of T cells. The activated Wnt/β-catenin signaling pathway in CSCs is upstream of multiple immune checkpoint molecules, and therefore immune checkpoint molecules are constitutionally highly expressed in CSCs. Some stemness factors, such as SOX2, can also bind to the promoter of PD-L1 and promote its transcription (Figure 4A) [411]. In addition, CSCs are able to reshape the microenvironment through the secretion of multiple immunomodulatory factors. (2) The polarization of TAM. CD133+ liver CSCs secrete IL-8 to promote the polarization of TAMs from M1 to M2, thus promoting HCC invasion (Figure 4B) [296, 412]. TAMs, in turn, promote the expansion of CSCs via IL-6–STAT3 pathway. IL-33–TGF-β interaction mechanism also present in CSCs and FcεRIa+ macrophages. CSCs highly express IL-33, promote the secretion of TGF-β by FcεRIa+ macrophages, while TGF-β acts on CSCs, further promote drugs resistance of CSCs and IL-33 expression, forming a positive feedback loop (Figure 4C) [413]. (3) Recruitment of Treg cells. CSCs can also secrete CCL28 in response to hypoxia signals, promote the recruitment of Treg cells, and form an immunosuppressive niche [414]. Similarly, TGF-β signaling from CSCs promotes the production of CCL22 via miR-a blockade, and CCL22 recruits Treg cells to promote tumor immune escape (Figure 4D) [415]. (4) Neutrophils. Exosomes rich in tri-phosphate RNAs secreted by CSCs induce activation of the IL-1β-NF-κB pathway of neutrophils in the bone marrow, promoting the survival and expansion of neutrophils. At the same time, CSCs can also produce CXCL1 and CXCL2 to promote the recruitment of CSC-primed neutrophils (Figure 4E) [416]. Of note, when adverse factors such as drug therapy exist, CSCs can produce secretory factors similar to pathogen infection to improve the adaptability of CSCs, including IL-1b, IL-6, IL-4, IL-8, granulocyte-CSF, MF inhibitory cytokine-1 (MIC-1) and TGF-β [417]. These cytokines reduce T cell infiltration and block the antitumor immune response.
4.10.2 Vascularization
CSCs can promote blood vessel survival via various mechanisms in order to supply oxygen and nutrients to meet the rapid growth of tumors. CSCs can differentiate into endothelium-like cells and coordinate angiogenesis [418, 419]. CD90+ liver CSCs can release exosomes containing H19 lncRNA119 to reshape the cell adhesion and angiogenesis of vascular ECs (Figure 4F) [420]. CSCs in squamous cell carcinoma can promote vascular survival, which in turn promotes LEPR expression through TGF-β pathway, at the same time, the TME can also produce a large amount of Leptin to initiate PI3K/AKT/mTOR pathway in CSCs, and finally driving SCC expansion (Figure 4G) [166]. Colorectal CSCs promote vascular survival by producing VEGF, where visceral adipose stromal cells can induce the expression of CD44v6 through IL-6 and HGF. CD44v6+ colorectal cancer CSCs stimulate visceral adipose stromal cells via NGF and NT-3 signaling, which in turn stimulates visceral adipose stromal cells, resulting in a CSC-vesicle positive cycle (Figure 4H) [337].
4.10.3 Stromal and Matrix Remodeling
CSCs can activate CAFs via Hh signaling, and further, CAFs promote CSCs expansion, self-renewal, and invasion through secretory pathways (Figure 4I) [421]. Inhibition of proliferating CAFs and inflammatory factors can normalize the TME by inhibiting Hh signaling. In contrast, abnormal activation of the Hh signaling is beneficial to the proliferation of CSCs and the drug resistance of gastrointestinal tumors [421]. In a recent study, SETD2 deficiency in pancreatic cancer cells induces the appearance of ABCA8a+ lipid-laden CAFs in the microenvironment via secreting BMP2, and lipid-laden CAF can provide lipids to pancreatic cancer cells via ABCA8a, and then promote tumor progression [328]. During tumor therapy, quiescent CSCs incite hemidesmosome component COL17A1 through highly expressed MMP molecules to further activate the FAK/YAP pathway and mediate the activation of dormant CSCs and tumor recurrence [64].
4.11 Systemic Regulation
For the past several years, the mutual interactions of cancer (stem) cells and systemic factors are intensively investigated. Here, we address the systemic regulations of tumors, including the interaction of distal tissues, endocrine factors, lifestyle and other diseases and tumors.
4.11.1 Impact of Distal Tissues on Tumor Regulation
Tumor cells and CSCs not only remodel the microenvironment, but also have a profound remodeling effect on the distal tissues of the individual, where remodeling of the pre-metastatic microenvironment by cancer (stem) cells is a prerequisite for tumor metastasis [121, 422]. Tumor cells undergo deep remodeling of distant organs through the secretome before reaching the metastatic site. The exosomes produced by CSCs contain special microRNAs and mRNAs before they reach the metastasis site, which have a stronger advantage in vascular growth and tumor metastasis [423]. In recent years, there has been increasing attention to the effects of tumors on the systemic immune system. Exosomes produced by cancer cells express high levels of PD-L1 on the surface, inhibiting the function of CD8+ T cells and promoting tumor growth [424]. For more details, you can refer to the relevant overview [425].
The intestine is an extension of the external environment to the body, colonizing a large number of microbes for normal physiological functions such as digestion and absorption. Recent studies have found that intestinal microbiota can affect tumors and immunity throughout the body, ultimately regulating tumorigenesis and CSCs, suggesting that gut microbiota acts as tissue-like components in tumors regulation. Formic acid, a metabolite produced by F. aggregatum, can promote the stemness and invasion of colorectal cancer cells via activating the AhR signaling pathway. Formic acid and Fn treatment induce more ALDH+ stem cells, with enhanced SOX2 expression. At the same time, formic acid treatment can also promote the expansion of Th17 cells (Figure 5A) [426]. Intestinal bacteria Klebsiella spp. produces DNA alkylating metabolite tilimycin, which induces DNA damage of circulating stem cells, inducing apoptosis of intestinal epithelium and colorectal colitis, which contributes to colorectal tumorigenesis [427]. In addition to inflammatory response and DNA damage, gut microbiota can also produce metabolites such as butyrate, which promotes hyperproliferation of MSH2 mutant colorectal cancer, while DNA-mismatch pathway can reregulate β-catenin activity and TA cell differentiation, indicating that intestinal flora also plays a key role in the regulation of Wnt pathway [428].
CSCs are highly expressed hormonal receptors to response systemic factor derived from distant tissues. Insulin, an endocrine factor produced by pancreatic β cells, plays a key role in blood glucose regulation, and is able to promote the self-renewal of CSCs through multiple mechanisms. CSCs express high levels of insulin-like growth factor (IGF1R) and insulin receptor (IR), which have a high corresponding effect on insulin-like growth factor and insulin [429]. Interestingly, insulin acts on the IR of CSCs, inhibits the activity of GSK3β through IRS2–PI3K pathway, prevents phosphorylation and ubiquitination of c-MYC, which maintains the stability and activity of c-MYC, and finally promotes the self-renewal of CSCs (Figure 5B) [430]. A variety of tumors exhibit sex differences, suggesting that CSCs are also regulated by sex hormones, including androgens and estrogens [431]. Adipocytes secrete adipokine adipsin, a complement factor for activation of alternative pathway, to promote the secretion of HGF, mediate the interaction between “adipose and cancer stem cells,” and ultimately promote the self-renewal of CSCs (Figure 5B) [432].
4.11.2 Impact of Behaviors on Tumor Regulation
The most essential function of the digestive system is digestion and absorption. Dietary components, such as high-fat diets, calorie restriction, high-sugar diets, and ketogenic diets, deeply regulate the self-renewal and developmental differentiation of normal stem cells [261, 433-435]. Similarly, CSCs are affected by a variety of dietary factors. For example, high-fat diet promotes the expansion and inflammation of esophageal CSCs by stimulating the production of IL-8 and changes in the gut microbiota, ultimately promoting esophageal cancer tumorigenesis (Figure 5C) [405]. Similar studies have also found that cholesterol in food can remodel gut microbiota and metabolites, and promote the occurrence of fatty liver-mediated liver cancer [436]. Interestingly, calorie restriction (CR) promotes the function of stem cells in normal tissues and generally inhibits tumorigenesis [433]. However, a recent study found that CR activates early tumor suppression but induces the emergence of CSC's phenotype through LSD1 to promote tumor progression. Thus, the combination of LSD1 inhibitors and CR can suppress tumors more fundamentally (Figure 5D) [437]. Similarly, fasting and post-fast refeeding induced robust activation of mTORC1 pathway in ISCs. It further promotes protein synthesis through polyamine metabolism and drives tumorigenesis, which can be diminished by blockade of mTORC1–polyamine metabolite production–protein synthesis pathway [438].
Colorectal cancer shows a trend of younger age, which is related to irregular lifestyle. More and more studies have found that rhythm disorders are the key cause of tissue homeostasis disorders. Per2 deficiency induced increased the expression of β-catenin and CCND, thus driving CRC tumorigenesis. After Per2 KO, the proportion of tumors in APCmin mice was twofold higher, indicating that the loss of rhythm increased tumorigenesis [439]. Recent kinetic studies have found that many behaviors of tumors are rhythmic, including the spread of tumor cells to distant tissues which is controlled by melatonin, testosterone, and glucocorticoids. And the infiltration of immune cells regulated by the circadian rhythm of vascular ECs [440-442]. More importantly, CSCs and their microenvironment are also rhythmic, and readers could read the excellent reviews [443].
In recent years, the regulation of mood and stress on tumorigenesis and CSCs has received more and more attention. In the stress model, local and systemic immunity of the tumor was inhibited via increased corticosterone. Corticosterone promotes the expression of Tsc22d3 in dendritic cells (DCs), downregulates the expression of MHC antigen presentation pathway-related molecules, and further promotes tumor immunosuppression (Figure 5E) [444]. Similarly, stress-induced norepinephrine promotes pancreatic carcinogenesis and the activation of CSC-related genes through crosstalk with neurotrophins [332].
4.11.3 Impact of Comorbidities on Tumor Regulation
Epidemiological data show that some diseases often occur at the same time with other diseases, and this phenomenon is called “comorbidities.” The incidence of tumors is high in patients with some diseases, such as obesity, fatty liver, chronic inflammation, diabetes, cardiovascular diseases and so on, indicating that there is a common mechanism between tumors and these diseases. The disease state of the body can affect tumorigenesis in a variety of ways. For example, the expression of Rab27a is elevated in fatty liver cells to facilitate production of extracellular vesicles, which inhibit the YAP pathway in tumor cells via microRNAs. The activation of Yap in liver metastasis cells of colorectal cancer can further promote the formation of immunosuppressive microenvironment and the growth of tumor at the site of metastasis (Figure 5F) [445]. The pathogenesis of other diseases and tumors has been discussed in detail in other reviews, such as the comorbidities of cardiovascular disease and cancer, the comorbidities of obesity and diabetes and cancer [446, 447]. However, relevant studies often focus only on the impact of tumor populations. Considering the low proportion of CSCs in tumors and the important role of CSCs, it is imperative to investigate CSC specifically in the context of comorbidities.
Although the environmental regulation of CSCs in digestive system tumors has received extensive attention from researchers, due to technological limitations, existing studies only investigate a single, known microenvironmental cell type, and are unable to identify all the microenvironmental cell types of CSCs at the same time, thus lack a comprehensive understanding on the microenvironmental cells of CSCs; furthermore, existing studies on environmental regulation of CSCs only focus on the environment of CSCs at a specific point in time, and lack dynamic studies during tumorigenesis and tumor progression. For CRC, the typical events during tumorigenesis are the LGR5+ ISCs detach from their original niche and function as CSCs in a new environment, indicated that the environment of CSCs may vary greatly at different stages of tumorigenesis. Therefore, developing new research systems for the comprehensive and dynamic understanding of CSCs' microenvironment is a huge challenge and future development direction in the field of CSCs, which is of great significance for understanding the pathogenesis of digestive tract tumors and developing appropriate intervention strategies.
5 Targeted Therapy in Digestive System
CSCs are resistant to most of the existing therapies, including radiotherapy, chemotherapy, and immunotherapy, resulting in drug resistance and recurrence. Therefore, CSCs have become a new target for cancer. The combined targeting of CSCs and non-stem cells has a good antitumor effect [448, 449]. In addition, it is also important for the regulation of the immune microenvironment to target both CSCs and non-CSCs [450]. Here, we review the recent advances in targeting of CSCs, including CSC-targeting and niche-targeting.
5.1 Targeted Therapy for CSCs
After years of exploration and research, researchers have developed multiple methods to target CSCs, including targeting surface markers and intracellular factors including stem cell signaling pathways, epigenetics, stem cell regulators, kinases and phosphatases (Table 2), the drugs undergoing clinical trials for therapy targeted to the CSC showing in Table 3.
| Drug | Type of drug | Target type | Target name | Action | Ref. |
|---|---|---|---|---|---|
| Salinomycin | Other | NA | HOXC10 | Inhibiting the activation of the Wnt/β-catenin pathway and ultimately inhibiting self-renewal of liver CSCs. | [127] |
| CD13 inhibitors | Inhibitor | Surface marker | CD13 | Combined with 5-FU to reduce tumor volume. | [139] |
| 1B50-1 | Antibody | Surface marker | Calcium channel α2δ1 subunit | Inhibiting self-renewal and tumor formation capacity of CSCs and inducing apoptosis of CSCs. | [154] |
| Acyclic retinoid | Other | NA | MYCN | Inhibiting the stemness of MYCN+ HCC by targeting MYCN. | [266] |
| RG7356 | Antibody | Surface marker | CD44 | Preventing the activation of CD44-mediated signaling pathway. | [451] |
| Cartuxomab | Antibody | Surface marker | EPCAM/T cell CD3 | Targeting T cells to the tumor site. | [452] |
| CD133 and CD3 bispecific antibodies | Antibody | Surface marker | CD133/CD3 | Promoting the binding of CSCs and T cells, serving as promising strategy to target colorectal cancer, pancreatic cancer and liver cancer. | [453, 454] |
| Antibodies to Rspo3 | Antibody | Surface marker | Rspo3-PTPRK | Inducing differentiation and loss of stem cell properties in CRC fused with Rspo3-PTPRK. | [455] |
| anti-LGR5-vc-MMAE, anti-LGR5-NMS818 | Antibody | Surface marker | LGR5 | Significant effect in the treatment of colorectal cancer, and anti-LGR5-vc-MMAE has no side effects. | [456] |
| CD133-SAL-NP nanoparticles | Antibody | Surface marker | CD133 | Significantly reducing the proportion of CD133+ CSCs, with enhanced efficiency than SAL-NPs. | [457] |
| LS7 | Peptide | Surface marker | CD133 | Used for the detection of CSCs after combining with 1,4,7,20-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) and isotope labeling. | [458] |
| NGR-LDP-AE | Peptide | Surface marker | CD13 | Targeting CSCs and inhibits angiogenesis. | [459] |
| CD133 CAR-T | CAR-T | Surface marker | CD133 | Targeting glioblastoma without affecting normal hematopoietic stem cells. | [460] |
| CWP232228 | Inhibitor | Signaling pathway | β-catenin/TCF | Inhibiting the activation of Wnt/β-catenin signaling pathway and the maintenance of liver CSC via inducing CSC apoptosis. | [461] |
| Vismodegib | Inhibitor | Signaling pathway | Hh | Inhibition of Hh signaling pathway, used to regulate basal cell carcinoma. | [462] |
| Curcuminy | Inhibitor | Signaling pathway | NF-kB | Inhibiting the stemness of liver CSCs. | [463] |
| ALDH1 prodrug | Proportion | Enzyme | ALDH1 | For detection and enrichment of CSC. | [464] |
| SCD1 inhibitors | Inhibitor | Enzyme | SCD1 | Mediating the stemness and sorafenib resistance of liver cancer through the wnt pathway. | [465] |
| SHP099 | Inhibitors | Enzyme | SHP2 | Inhibiting the expansion of liver cancer when combined with sorafenib in HCC-derived organoid and xenograft model. | [466] |
| Olaparib | Inhibitor | Enzyme | PARP1 | Altering the stem-like transcriptome of HCC cells and overcoming the resistance of sorafenib. | [467] |
| All-trans retinoic acid | Other | NA | NA | Increasing the therapeutic effect of cisplatin by inducing the differentiation of liver CSCs. | [468] |
| PI-103/oligomycin | Other | NA | NA | Acting on OXPHOS to reduce the activity of liver CSCs. | [469] |
| Lup-20(29)-en-3 beta-OL (lupeol) | Other | Signaling pathway | PTEN–AKT–ABCG2 | Inhibiting the self-renewal and drug resistance of liver CSCs through PTEN–AKT–ABCG2 signaling pathway. | [470] |
| Baicalein | Other | NA | SAR1B | Making tumor cells more sensitive to mTORC1 inhibition by inhibiting autophagy. | [471] |
| Target | Drug | Clinical trial title | Action | Ref. |
|---|---|---|---|---|
| CD44 | RG7356 | A Study of RO5429083 in patients with metastatic and/or locally advanced, CD44-expressing, malignant solid tumors (NCT01358903) | Blocking the CD44 signaling pathway can impede the migration and invasion of CSCs, reducing tumor growth and metastasis. | [451] |
| EpCAM | EpCAM-targeting CAR-T cell immunotherapy | EpCAM CAR-T for treatment of advanced solid tumors (NCT02915445) | CAR-T cells can specifically recognize and kill epithelial tumor cells expressing EpCAM, activating the immune system to combat tumors. | [472] |
| CD133 | CD133-directed CAR T cells | Treatment of relapsed and/or chemotherapy refractory advanced malignancies by CART133 (NCT02541370) | CAR-T cells can specifically target and kill CSCs expressing CD133, thereby inhibiting tumor growth. | [473] |
| SLAMF7/CS1 | ABBV-838 | Dose-escalation study of ABBV-838, an antibody drug conjugate, in subjects with relapsed and refractory multiple myeloma (NCT02462525) | The antibody–drug conjugate can deliver cytotoxins to SLAMF7/CS1+ CSCs, inducing apoptosis and suppressing tumor growth. | [474] |
| HIF-2α | Belzutifan | A phase 2 study of Belzutifan (PT2977, MK-6482) for the treatment of von Hippel Lindau (VHL) disease-associated renal cell carcinoma (RCC) (MK-6482-004) (NCT03401788) | By suppressing HIF-2α, the occurrence and progression of von Hippel-Lindau disease-related pancreatic lesions can be attenuated, thereby impeding tumor growth. | [475] |
| TERT | hTERT (vx-001) cancer vaccine | Efficacy study of Vx001 vaccine in NSCLC patients (NCT01935154) | Via the activation of a specific T-cell immune response against human Telomerase Reverse Transcriptase (hTERT) by vaccination, the recurrence of tumors after chemotherapy can be prevented, and the progression-free survival period can be prolonged. | [476] |
| Cytotoxic T cells | Shared neoantigen vaccine | A study of a personalized cancer vaccine targeting shared neoantigens (NCT03953235) | In combination with the shared neoantigen vaccine and immune checkpoint inhibitor, the specific T-cell immune response against neoantigens can be elicited, enhancing the anti-tumor efficacy. | [477] |
| WT1 | Dendritic cells pulsed with multifunctional Wilms' tumor 1 (WT1) peptides | Treatment with chemotherapy and dendritic cells pulsed with WT1 peptides for pancreatic cancer. (WT1-based chemoimmunotherapy) (jRCTc030190195) | Through the presentation of WT1 peptide by dendritic cells, the specific T-cell immune response against WT1 can be activated, augmenting the antitumor effect. | [478] |
| Aminopeptidase N | NGR-hTNF | Study of NGR-hTNF as single agent in patients affected by advanced or metastatic HCC (NCT00484211) | By selectively binding to aminopeptidase N on tumor blood vessels, tumor necrosis can be induced, thereby inhibiting tumor growth. | [479] |
| PD-L1 | Adebrelimab | SHR-1316 combined with chemotherapy and chest radiotherapy in ES-SCLC (NCT04562337) | The PD-L1 antibody can enhance the immune system's ability to combat tumors. When combined with chemotherapy and sequential thoracic radiotherapy, a further improvement in the anti-tumor effect can be achieved. | [480] |
| VEGFR-2 | BC001 | Phase I clinical study of BC001 in Chinese subjects with advanced solid tumors (ChiCTR2300069367) | Blockade of the VEGFR-2 signaling pathway can suppress tumor angiogenesis, reducing the blood supply to tumors and consequently inhibiting tumor growth. | [481] |
| CD166 | Praluzatamab Ravtansine (CX-2009) | PROCLAIM-CX-2009: a trial to find safe and active doses of an investigational drug CX-2009 for patients with selected solid tumors (NCT03149549) | The Probody drug conjugate, when activated within the TME, can minimize the impact on healthy tissues and deliver cytotoxins to CD166-positive cells, thereby suppressing tumor growth. | [482] |
| mKras | Lymph-node-targeted, mKras-specific amphiphile vaccine | A study of ELI-002 in subjects with Kras mutated pancreatic ductal adenocarcinoma (PDAC) and other solid tumors (NCT04853017) | Through the targeted delivery of mKras-specific vaccine to lymph nodes, the T cell immune response against mKras mutation can be activated, inhibiting tumor growth. | [483] |
- Data sources: ClinicalTrials.gov; Chinese Clinical Trial Registry; Japan Registry of Clinical Trials.
5.1.1 Surface Marker
The identification of CSC's surface markers is crucial for the diagnosis and treatment of cancer. The development of antibody and aptamer technology, as well as the improvement of functionalization and delivery methods, has made it possible to use surface mark-based targeting strategies in the diagnosis and treatment of a variety of tumors [484]. For example, RG7356, a humanized monoclonal antibody to CD44, was able to block CD44-mediated signaling activation, and the therapeutic effect of RG7356 was closely related to the expression level of CD44 isoforms, especially CD44s. In addition, bis-specific antibodies against CD133 and CD3 can promote the binding of CSCs to T cells, providing promising strategies for the treatment of colorectal cancer, pancreatic cancer and liver cancer [451, 453, 454]. Advances in antibody delivery techniques, such as CD133-SAL-NP nanoparticles, have also enhanced the therapeutic efficacy of antibodies [457]. Peptides play a unique advantage in tumor therapy due to their tissue penetration ability. For example, LS7 is a peptide targeting CD133, which can be used for the detection of CSCs [458].
The targeting strategy based on surface of CSCs markers is very dependent on the identification of markers. Due to the similar gene expression profiles and signal pathway activation between CSCs and non-stem cells, markers of CSCs still face the problems of poor specificity and low expression level at present. Many markers of CSCs also mark normal stem cells. For example, CD133, Bmi1 and LGR5 are among the most classical markers of normal stem cells [13, 144, 485].
5.1.2 Intrinsic Factors
As the key target of CSC, the intrinsic factors of CSCs cover many aspects. At the signaling pathway level, Wnt/β-catenin, Hh, and NF-kB pathways are closely related to the maintenance of CSC's characteristics. For example, CWP232228 inhibits Wnt/β-catenin pathway and hinders the maintenance of liver CSC. Curcuminy inhibits the NF-kB pathway and reduces the stemness of liver CSCs [461-463]. These related drugs have been comprehensively described in other reviews [486].
Enzymes are also the focus of research. SCD1 mediates liver cancer stemness and sorafenib resistance, and its inhibitors can target CSCs [180, 260]. SHP2 activates related pathways to promote the proliferation of liver CSCs [465], SHP099 as an inhibitor combined with sorafenib can effectively inhibit the proliferation of liver cancer [466]. PARP1, a DNA repair enzyme highly expressed in ESCs and downregulation in normal tissue, is highly expressed in HCC tissues and induces lineage plasticity in tumors. The inhibitor of PARP1 olaparib could alter the stem-like transcriptome of HCC cells and overcome the resistance of sorafenib [467].
Similar to the problems faced by signaling surface receptors, the targeting of endogenous factors also faces the problem of specificity, including signaling pathways and enzymes. Most of the identified targets are tumor-associated antigen (TAAs) but not tumor-specific antigen (TSAs). Therefore, it is important to identify new specific antigens in tumors and CSCs.
5.2 Targeted Therapy for Niche of CSCs
The microenvironment plays a key role in the self-renewal and tumorigenesis of CSCs and is becoming a new target for tumor intervention. Tumor immunotherapy, which is essentially the remodeling of the immune microenvironment of CSCs, has achieved great success in clinical practice, suggesting that targeting the TME is an effective strategy. Here, we focus on recent advances in targeting the microenvironment of CSCs (Table 4), the drugs undergoing clinical trials for therapy targeted to the niche of CSC shown in Table 3.
| Drug | Type of drug | Target type | Target name | Action | Ref. |
|---|---|---|---|---|---|
| DC vaccine | Vaccine | Niche targeting | T cells | Activating T cells by stimulating DCS with tumor lysates or by transfection of DCS with tumor antigen mRNA. | [487] |
| CD133+ cell RNA | Vaccine | Niche targeting | DC cells | Stimulated with RNA from CD133+ cells have stronger tumor inhibition ability, accompanied by the elimination of CSCs. | [488] |
| CD44 and EpCAM polypeptide | Vaccine | Niche targeting | T cells | Stimulating the maturation of dendritic cells and producing a stronger tumor inhibitory effect by enhancing the killing of T cells against CSCs. | [489] |
| CD133 CAR-T | CAR-T | Niche targeting | CD133 | In the treatment of relapsed/drug-resistant CD133+ liver cancer, more than half of patients were stable and a few patients were in complete remission. These changes were accompanied by decreases in hemoglobin, platelet, and lymphocyte counts. | [490] |
| EpCAM CAR-T | CAR-T | Niche targeting | EpCAM | Significantly reducing tumor development in xenograft tumor models with few diminished effects. | [472] |
| IL-1α blocks antibodies | antibody | Niche targeting | IL-1α | Inhibiting il-1α-induced caf remodeling, alleviating tumor progression and reducing radiation resistance. | [491] |
| All-trans retinoic acid | Other | Niche targeting | Stromal cells of PDAC | Acting on the stromal cells of PDAC, inducing quiescence of stromal cells, and inhibiting tumor cell proliferation and stemness. | [492] |
| Clostridium butyricum | Bacteria | Niche targeting | c-MYC | Promoting the ubiquitination degradation of c-MYC, and inhibiting tumor proliferation and stemness. | [493] |
| Colistin-LipoFM | Other | Niche targeting | Tumor cells | Mimicking fn's ability to target tumors, eliminating tumor bacteria, thereby overcoming the chemotherapy-resistance of breast cancer. | [494] |
5.2.1 Immunotherapy in the Microenvironment
In recent years, tumor vaccines and chimeric antigen receptor T (CAR-T) cell therapy have become hot spots in tumor immunotherapy. In terms of tumor vaccines, DC vaccines produce peptides that activate T cells by transfection of DC with tumor lysates or antigen mRNA. Some CSC targeting tests based on this are in progress, with stimulation modalities including CSC lysate stimulation, Nanog peptide stimulation or ALDH+ CSCs stimulation [487]. The efficacy of traditional tumor vaccines is limited, but the tumor inhibition of DC cells stimulated by RNA from CD133+ cells is enhanced and the CSCs can be eliminated [488]. For example, CD44 and EpCAM peptides can stimulate DCs and enhance the killing effect of T cells on CSCs [489]. However, only 15% of patients clinically respond to DC vaccine, mainly due to the overexpression of immune checkpoint molecules, which can be improved by blocking PD-L1/CTLA-4 [495].
In terms of CAR-T cell therapy, at present, more than a dozen CAR-T cells targeting CSC markers are in clinical trials, mostly focusing on hematological tumors. In solid tumors, CAR-T cells targeting CD133 and EpCAM have made prominent progress. For example, CAR-T targeting autologous CD133 in the treatment of recurrent/drug-resistant CD133+ liver cancer, more than half of patients have stable disease, and a few have complete remission, but hemoglobin and other indicators will decrease [490]. The median PFS of phase II clinical trial is 5 months [473]. CAR-T targeting EpCAM in the treatment of related tumors can significantly inhibit the growth of xenograft tumors with few side effects [472].
5.2.2 Other Microenvironmental Targeting Strategies
In addition to the immune microenvironment, targeting other microenvironments is also an ideal strategy for CSCs. For example, targeting the stromal microenvironment promotes CSC quiescence and reduces radiation-induced inflammation. Targeting TGF-β, Hh, and MMP inhibits stemness, invasion, and metastasis of tumor by inhibiting the promotion of CAF to CSC [496]. IL-1α induced by radiation can induce CAFs to an inflammatory phenotype (iCAFs) and induce tumor progression and drug resistance. While IL-1α blocking antibody can inhibit IL-1α-induced CAF remodeling, alleviate tumor progression and reduce radiation resistance [491]. All-trans retinoic acid acts on the stromal cells of PDAC, induces quiescence of stromal cells, and inhibits tumor cell proliferation and stemness [492]. As the regulators of TME, gut microbiota can reshape the microenvironment of tumor and CSCs. Clostridium butyricum can directly promote the ubiquitination degradation of c-MYC, and inhibit tumor proliferation and stemness. At the same time, it can promote the infiltration of T cells and modify the immunosuppression of tumors [493]. Some new strategies have been used to target CSCs and their microenvironment, such as synthetic biology and nanomaterials [497-500].
6 Conclusion and Prospect
Digestive system tumors are difficult to cure with high rates of drug resistance, recurrence, immune escape, and distant metastasis. CSCs are the cause of tumor development, drug resistance, recurrence, and metastasis. Therefore, it is valuable to have a comprehensive understanding of CSCs and develop intervention strategies for digestive system tumor. Based on years of research accumulation in the field, we have summarized the progress of fundamental and targeted research of CSCs. In summary, the heterogeneity and plasticity of CSCs determine that to effectively target CSCs for cancer treatment, we need to conduct more comprehensive and in-depth research on their sources, markers, features, functions, destinations, and environments [49, 50].
The heterogeneity of CSCs includes genetic heterogeneity and phenotypic heterogeneity. CSCs can originate from nontumor cells, tumor cells, or other cells outside the tissue. To understand the occurrence process of specific digestive system tumors, more in-depth analysis of the sources for specific-type tumors in specific tissue are required. A tumor tissue simultaneously possessing diversified CSCs which have different surface marker genes, features, and functions, proliferating CSCs with high self-renewal and differentiation ability promote tumor progression, quiescent CSCs endow tumors with the potential for recurrence after adversity, DTP CSCs are the main culprit for relapse after drug treatment. Moreover, new subpopulations of CSCs are constantly being discovered in different tumors, comprehensive understanding of the phenotypic heterogeneity of CSCs still requires more research accumulation.
CSCs are capable of self-renewal and differentiation, maintaining the CSC pool and producing various types of tumor non-stem cells to promote tumor progression. Besides, tumor non-stem cells can also acquire the characteristics of CSCs. The plasticity of CSCs is mainly determined by the environment in which they are located. The types of microenvironment cells of CSCs are easily obtainable through single-cell sequencing, however, the influence extent of different microenvironment cells on the fate determination of CSCs still needs to be analyzed specifically for specific digestive tract tumors under specific conditions. In addition, the fate of CSCs is regulated by systemic factors such as diet, emotions, and behavior. How these macroenvironmental factors regulate CSCs and their microenvironment requires more attention.
Although some breakthroughs have been made in targeted therapy of CSCs, there are still significant challenges to its clinical application. First, CSCs and normal tissue stem cells share the same surface marker genes and similar regulatory pathways, drugs targeting CSCs need to be carefully evaluated their impact on normal stem cells [501]. Second, CSCs have heterogeneity and plasticity, diversified CSCs with different characteristics and biomarkers coexist in the same tumor and transform into each other under specific conditions, making CSC difficult to fully target. Even if CSCs are cleared, non-stem tumor cells will dedifferentiate into CSCs, affecting the effectiveness of CSC-targeted therapy for tumors. Therefore, targeted therapy based on CSCs requires collaboration among researchers in biology, medicine, materials science, and other fields. Due to the crucial role of microenvironment in determining the fate of CSCs, the complete elimination of tumor stem cells can be achieved by destroying the soil on which CSCs rely for survival, targeting research on microenvironment of CSCs should be given more attention.
Author Contributions
Zhenzhen Chen: resources (lead), writing – original draft (lead), writing – review and editing (equal). Huanle Qi: resources (equal), visualization (equal), writing – review and editing (equal). Yapeng Xue: resources (equal), visualization (equal). Yaqi Zhang: resources (equal), visualization (equal). Zhuo Zhang: resources (equal), visualization (equal). Shun Xu: resources (equal), visualization (equal). Shixin Liao: resources (equal), writing – review and editing (equal). Xiaoyu Zeng: resources (equal), writing – review and editing (equal). Jiayi Wu: resources (equal), writing – review and editing (equal). Xinrui Lv: resources (equal), writing – review and editing (equal). Qiankun He: resources (equal), writing – original draft (equal), writing – review and editing (lead). Pingping Zhu: resources (equal), writing – original draft (lead), writing – review and editing (lead). All authors read and approved the final manuscript.
Acknowledgments
The graphical abstract image and the figures, were created with BioRender (https://www.biorender.com/). We thank the supporting grants from Zhengzhou University to P.Z. This work was supported by grants from Joint Funding of Henan Provincial Science and Technology R&D Plan (222301420016 to P.Z., 232301420010 to Z.C.), Henan Province Outstanding Youth Science Foundation (242300421016 to Z.C.), National Natural Science Foundation of China (U23A20459 to P.Z., 32200652 to Q.H.), Natural Science Foundation of Henan Province (242300421310 to Q.H.).
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
No data was used for the research described in the article.