Development of covalent inhibitors: Principle, design, and application in cancer
Lang Zheng and Yang Li contributed equally to this work.
Abstract
Covalent inhibitors have been a rapidly growing field in drug discovery due to their therapeutic potential and unique advantages in cancer therapy. As opposed to noncovalent inhibitory drugs, covalent inhibitors reversibly or irreversibly modify proximal nucleophilic amino acid residues on proteins, aiming to selectively recognize and bind to protein targets and addressing some of the challenges faced by noncovalent drugs. Most successful targeted covalent inhibitors depend primarily on binding-site cysteine residues, but this has limitations for certain protein targets that lack targetable cysteine residues. Recently, the rational design of covalent inhibitors or covalent probes targeting other nucleophilic residues, such as lysine, tyrosine, serine, has turned out to be another promising strategy for cancer therapy. Thus, the development of novel strategies to extend the scope of covalent binding and improve the binding properties is required. This review gives a summary of the development of covalent inhibitors targeting noncysteine from different aspects, including target identification, structure–activity relationships, drug discovery strategies, and binding properties, in the hope of providing a scientific reference for future covalent drug discovery as a means of expanding research in cancer therapy.
Graphical Abstract
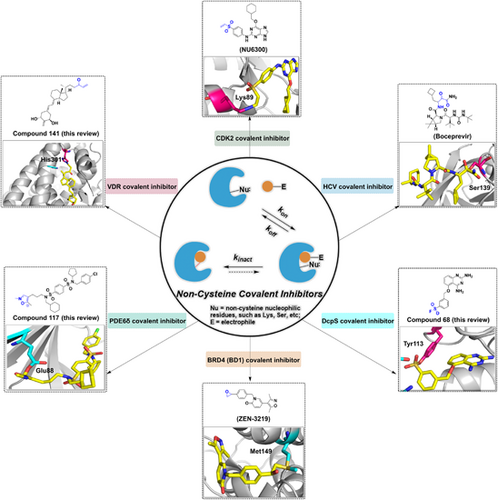
The noncysteine covalent inhibitors are a class of small molecule inhibitors that can bind irreversibly to their target enzymes through covalent bonding with noncysteine residues. They have shown promise as potential therapeutics for a variety of diseases, including cancer, infectious diseases, and autoimmune disorders. Examples of noncysteine covalent inhibitors. The illustration emphasizes the representative noncysteine covalent inhibitors in drug discovery and development.
1 INTRODUCTION
Substantial evidence shows that covalent inhibitors exerted therapeutic value for various human diseases, especially cancers.1-7 Functionally, covalent inhibitors are characterized by forming covalent complexes with their targets. With the development of structural biology and medicinal chemistry, important insights into the structural features of covalent inhibitors have been provided. Specifically, most of these inhibitors contain an electrophilic warhead (e.g., acrylamides, sulfonyl fluorides (SFs), epoxides, and β-lactams), which reacts with a nucleophilic amino acid (e.g., cysteine, serine, or threonine) in close proximity to the ligand binding site. Compared with conventional noncovalent reversible inhibitors, reversible and irreversible covalent targeting possessed several advantages,1, 8 including complete target blockage, superior selectivity, and longer duration of action. Compared with other nucleophilic amino acids, the thiol functional group of Cys exerts a high sensitivity to redox conversion, thereby converting into different states. Additionally, the sulfhydryl group of Cys is capable to bind to a weak electrophilic warhead, thus reducing the risk of off-target and nonspecific labeling. To date, many covalent inhibitors that attach to catalytic cysteine in the target have exhibited clinical benefits. Specifically, epidermal growth factor receptor (EGFR) inhibitors (e.g., afatinib,9 dacomitinib,10 osimertinib,11 neratinib,12 and mobocertinib13) and BTK inhibitors (e.g., ibrutinib,14 acalabrutinib,15 tirabrutinib,16 and zanubrutinib17), which contain acrylamide as Michael acceptors, have a good clinical therapeutic effect in cancers. Nirmatrelvir covalently targeting catalytic Cys145 of the SARS-CoV-2Mpro could inhibit the main viral protease of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).18, 19
Although the low abundance of cysteine allows for the development of selective covalent inhibitors, opportunities for the production of covalent protein regulators are limited when residues are unsuitable for Michael addition chemistry.20 Another challenge associated with irreversible cysteine-targeting inhibitors, particularly those that bind noncatalytic residues, is the susceptibility to cysteine-to-serine mutations that retain enzyme function while reducing drug efficacy.21 Furthermore, enzymes and proteins have disulfide bonds formed between the thiol groups of Cys residues,22 which makes covalent modification of such cysteine residues difficult.
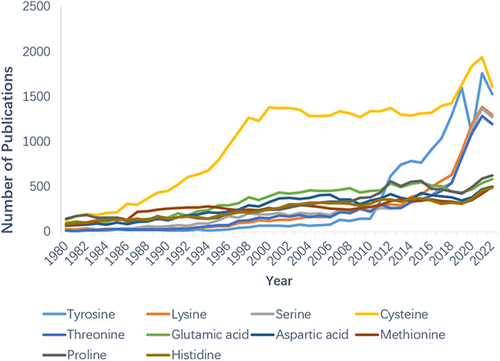
Therefore, there is an urgent need to broaden the targeting space for amino acids other than cysteine. To date, rational covalent inhibitors or covalent probes targeting other nucleophilic residues with better abundance in vivo, such as lysine, tyrosine, serine, and threonine, have been developed23, 24 (Figure 1). Furthermore, covalent modifications targeting aspartic acid, glutamic acid, methionine, histidine, and proline have also been reported. Covalent modifications targeting these noncysteine residues are mainly in the area of covalent inhibition, as well as most of them are used mainly for the treatment of tumors and a few for other diseases. These are discussed in detail in later chapters. And representative FDA-approved noncysteine covalent inhibitors and the ones in clinical trials are summarized in Supporting Information: Tables S1 and S2.
In this review, we focus on the recent findings on noncysteine covalent inhibitors including their drug discovery techniques, structural–activity relationships, and potential applications in cancer therapy. With this review, we hope to increase researchers' interest in noncysteine covalent inhibitors in an effort to exploit the great therapeutic potential of this class of compounds and expand the diversity of drug design approaches.
2 COVALENT MODIFICATION OF LYSINE RESIDUES
Lysine residues are usually located on proteins' surfaces, interfaces, and near ligand binding sites.24, 25 Additionally, lysine at the active site can assist catalysis by stabilizing reactive residues or activating them through hydrogen bonding.26 Compared to cysteine, lysine residues are more abundant at protein binding sites.27, 28 Comparatively, only about 260,000 cysteine residues are spread among about 20,000 human proteins, while there are 650,000 lysine residues.29 Lysine's ligandability essentially depends on two factors29: First, there is the proximity to the small-molecule binding sites. More than 500 human protein kinases have been structurally proven to contain lysine residues that can react with covalent inhibitors, since almost all catalytically active kinases have a catalytic lysine in the binding pocket.29 At least one lysine was found to be within 5 Å of 75% of the metabolites while analyzing 470 human proteins with different types of metabolites (such as enzyme substrates, cofactors, and metamorphic modulators), and these proximal lysine residues at the binding site are preferable for ligand binding.29 Second, pKa is partially governed by its intrinsic nucleophilicity, which is essential for lysine to become a nucleophile. Indeed, the nucleophilic primary amines in lysine residues can interact irreversibly with electrophilic inhibitors. Lysine's ε-amino group is estimated to have a pKa value in the region of 10.0–10.5.30 Under physiological settings (pH = 7.4), exposed lysine residues on the surface of target proteins are almost entirely protonated and cannot be employed as nucleophile agents. However, by internal lysine analysis at 25 positions of staphylococcal nucleases, Isom et al.31 found that Lys92 is entrenched in the staphylococcal nuclease and is disturbed by the interaction of other surrounding residues, and had the lowest pKa (5.3). One possible benefit of lysine over cysteine is that its neutral state is more amenable to increased nucleophilicity.
Since amino is a hard nucleophile, this highly abundant hard nucleophile prefers to react with hard electrophiles.32 Advanced chemical proteomics techniques could measure each lysine's relative reactivity to electrophilic probes,33 but it still presents challenges due to the abundance of lysine. Integrating lysine-targeting warheads into drug-like compounds that have the desired properties is another challenge. Currently, it seems more difficult to target lysine residues than cysteine residues, but if these challenges can be addressed, targeting lysine residues may offer significant potential in covalent drug development.
2.1 Covalent inhibitors targeting lysine residues
2.1.1 Covalent cyclin-dependent kinase 2 (CDK2) inhibitors
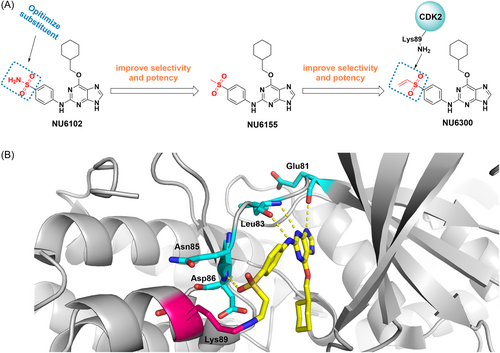
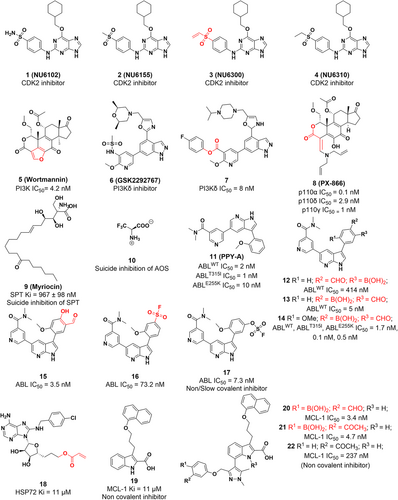
The CDK group is a group of serine/threonine protein kinases that play a role in transcription and cell cycle regulation in eukaryotes.34, 35 Most human malignancies exhibit cell cycle dysregulation and evidence has shown that CDK2, a member of CDKs, is highly expressed and activated inappropriately in many tumors, providing a potential therapeutic target for cancer.36 Initially, NU6102 was developed with a Ki of 6 nM.37 Further structural optimization resulted in a similar inhibitory activity of NU6155 with improved metabolic stability. NU6300, which is the intermediate of NU6155, is also a potent CDK2 inhibitor (IC50 = 0.16 μM) with increased cellular activity compared to its reversible counterpart (compound 4), which binds to CDK2 irreversibly with a vinyl sulfone warhead (Figure 2A). Mechanically, NU6300 selectively forms a covalent bond through a Michael addition reaction with nonconserved lysine (Lys89), which is outside the ATP binding cleft of CDK2 (Figure 2B).37, 38 These studies provide a novel strategy for treating tumors with well-defined genetic signatures.
2.1.2 Covalent Phosphatidylinositol 3-kinase (PI3K) inhibitors
PI3K is a signal transduction enzyme that promotes phosphorylation of the inositol ring of phosphatidylinositol,39 which is required for cell proliferation, apoptosis.40 PI3K is frequently mutated in cancer, making it a promising target for therapeutic intervention.41, 42 Antibiotic Wortmannin was first identified from Penicillium wortmannii in 1957.43 It is a broad-spectrum PI3K inhibitor with an IC50 value of 4.2 nM and exhibits antitumor activity in xenograft animals.44 Mechanically, it reacts with Lys80245 or Lys83332 of different isoforms by the furan ring at the C20 position and then opens the ring to produce a covalently bound enamine, which irreversibly binds to the ATP pocket of PI3K to inhibit kinase activity (Figure 3A,C).
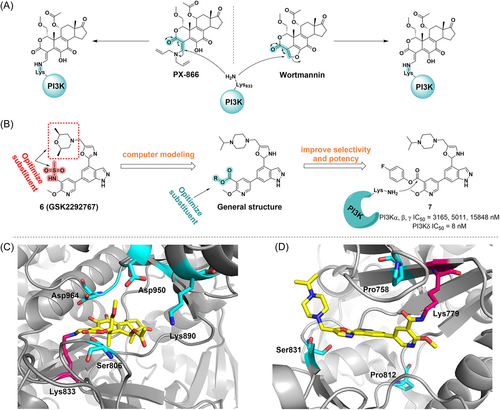
Recently, Dalton and his colleagues identified a selective PI3K covalent inhibitor that reacted to its highly conserved Lys779 (Figure 3B).46 With GSK2292767, they replaced the cis-dimethyl morpholine moiety in GSK2292767 with a more soluble piperazine moiety. Furthermore, activated phenolic esters are found to be tolerated in the enzyme–inhibitor complex, so a variety of activated phenolic ester compounds were synthesized. Biochemical and cellular experiments showed that the inhibitory potency against PI3Kδ increased with the electron-withdrawing capacity of the ester-bound R group, indicating that the electrophilicity of the carbonyl group is crucial for activity. By structural optimization, they introduced 4-trifluoromethylphenol ester, phenol, and NO2, CF3, F, and OMe substituted benzene rings on the R group, and found that compound 7 exhibited a potent inhibitory effect, with an IC50 value of 10 nM in inflammatory cytokine response assays of the hWB phenotype. A long duration of action (>48 h in CD4+ T cells) in cell washing experiments indicated the formation of amide adducts with Lys779 (Figure 3D). Furthermore, compound 7 was found to have minimal off-target effects in cells by kinetic measurements and was confirmed to have excellent PI3Kδ selectivity by chemoproteomics.24, 46 Therefore, compound 7 is considered the first highly selective PI3Kδ irreversible covalent inhibitor, which demonstrates a general strategy to develop selective, irreversible kinase inhibitors.
PX-866 (Sonolisib) is a stable oral pan-PI3K inhibitor, which is an analogue of Wortmannin with reduced reactivity.47 It opens the furan ring of Wortmannin to increase selectivity and off-target toxicity. PX-866 was used to treat a variety of solid tumors in clinical trials48, 49 and has more potency and stability than Wortmannin and exhibits considerable anticancer effects in xenograft models. PX-866 irreversibly inhibits PI3K activity by covalently binding to lysine residues in the catalytic site of PI3K and subsequently generating covalently bound enamine species (Figure 3A), leading to a decrease in Akt phosphorylation levels. Thus, PX-866 inhibits the growth and survival of tumor cells by modulating the PI3K/Akt signaling pathway.47 PX-866 can also inhibit temozolomide (TMZ)-induced autophagy50 and enhanced tumor growth inhibition induced by radiation, cisplatin, or gefitinib.47, 51
2.1.3 Covalent alpha-oxoamine synthases (AOS) inhibitors
AOS belong to a small subfamily of pyridoxal-5′-phosphate (PLP) dependent enzymes that catalyze decarboxylation and condensation between amino acids and substrates of acyl-CoA or acyl-acyl carrier protein (acyl-ACP) to form α-amino ketones.52, 53 And mutations in some AOS enzymes are associated with a variety of human diseases. Members of the AOS include the first enzyme identified for biotin biosynthesis, 8-amino-7-oxonononanoate synthase (AONS)54; as well as 5-aminolevulinate synthase (ALAS),55 which is related to heme biosynthesis; serine palmitoyltransferase (SPT), which catalyzes sphingolipid (SL) biosynthesis; and 2-amino-3-ketobutyrate CoA ligase (KBL), which is involved in the threonine degradation pathway.56 SPT is a key enzyme in the control of SL production, which ensures the right amount of SLs by catalyzing the first and decisive steps of SL synthesis. SLs play a vital role in membrane biology, acting as signaling molecules to mediate a variety of crucial cellular functions such as cell growth, adhesion, migration, and death. Regulatory processes of SL metabolism and signaling have made major advances in tumor therapy.57-59 SPT is particularly important for cellular function, and blocking its biological activity is a promising approach for the treatment of a wide range of diseases. In 1972, myriocin (ISP-1) was first isolated as an antifungal agent.60, 61 Wadsworth et al.62 revealed its molecular mechanism of SPT inhibition (Figure 4A). First, myriocin forms an external aldimine with PLP, which is then catalytically degraded to C18 aldehydes through a “retro-aldol-like” cleavage mechanism that, in turn, acts as a suicide inhibitor of SPT by covalent modification of the essential catalytic lysine. The extraordinarily effective and long-acting character of myriocin can be rationally explained by this unexpected dual inhibitory mechanism. Subsequently, the covalent adducts were found by protein mass spectrometry analysis of the reaction, and Lys265 was identified as the modification site by the peptide mass fingerprint. To date, myriocin continues to be the most valuable and popular chemical probe in the investigation of SL.63
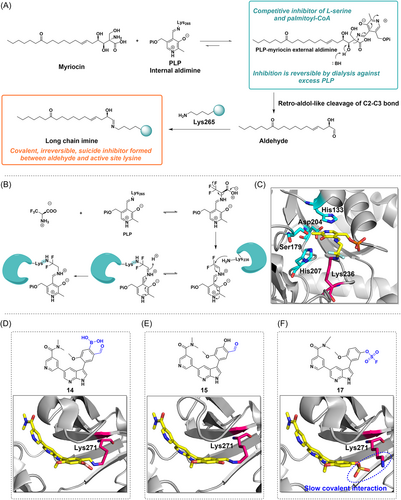
The Alexeev group also found the suicide inhibition mechanism of α-oxyamine synthase.64 In this process, trifluoro alanine (compound 7) forms a covalent adduct with a lysine residue at the active site of AONS, a mechanism that also involves the cofactor PLP (Figure 4B). After forming a covalent complex with PLP, trifluoro alanine undergoes decarboxylation and defluorination to form an imine intermediate, which then covalently binds to the amino group of Lys236 to form an imine ion, and can further hydrolyze HF to produce the 2-(pyridoxamine phosphate) acetyl protein adduct (Figure 4B,C). Due to the limited number of examples targeting AOS and to expand the library of warheads used for covalency, this study serves as an example of lysine-targeted covalency only, and may inform the development of lysine-targeted drugs, although no study has demonstrated a direct relationship between inhibition of AONS and cancer therapy.
2.1.4 Covalent BCR-ABL inhibitors
Breakpoint cluster region-abelson (BCR-ABL) fusion genes are typically generated by chromosomal translocations, and a reciprocal translocation fusion between the ABL tyrosine kinase gene on chromosome 9 and the BCR tyrosine kinase gene on chromosome 22 results in BCR-ABL fusion oncoproteins,65, 66 which in turn induce chronic myeloid leukemia (CML).67, 68 By activating downstream signaling pathways (such as the JAK/STAT, PI3K/AKT, and MAPK/ERK pathways), the BCR-ABL fusion protein promotes uncontrolled cell proliferation.69, 70 Therefore, blocking abnormal activation of the BCR-ABL fusion protein could be a promising strategy for cancer therapy. Carbonylboronic acid (CBA) is a low-reactivity electrophile that can reversibly and covalently modify amino groups in proteins.71 A series of BCR-ABL inhibitors have been reported in which CBA is used as an electrophilic warhead to reversibly and covalently target the catalytic lysine residue (K271) of the BCR-ABL kinase, resulting in potent and long-lasting inhibition of BCR-ABL wild-type and mutants.72 The CBA warhead was added to ABL1 inhibitor PPY-A and further structure–activity relationships (SAR) study demonstrates that the inhibitory activity decreased 20 times after the removal of the ortho-methoxy group, whereas significantly enhanced by the addition of the aldehyde group; the position of the aldehyde group is crucial to its effectiveness. For example, the inhibitory activity of compound 12 (aldehyde group in para position, IC50 = 52 nM) was approximately 10 times lower than that of compound 13 (aldehyde group in meta position, IC50 = 5 nM). After structural optimization, compound 14, containing the methoxy and aldehyde groups, exhibited the best inhibitory activity (IC50 = 1.7 nM) and selectivity for two ABL mutants, ABLT315I and ABLE255K (IC50 = 0.5 nM). The crystal structure of the kinase/inhibitor complex showed that iminoborate formed between the CBA moiety and the catalytic lysine K271 (Figure 4D). Unlike the kinase inhibitor imatinib, which cannot access the hydrophobic region on the back side of the kinase's ATP-binding site, compound 14 binds to the ABL kinase without steric conflicts, resulting in potent kinase inhibitory activity in vitro.
However, compound 14 has weak or no cellular activity. Therefore, the research team developed a covalent inhibitor using sulfur (VI)-fluoride exchange (SuFEx) and salicylaldehyde groups as warheads, which exhibits good cellular activity.73 SAR studies revealed that compound 15 was the first reversible covalent inhibitor of the catalytic lysine residue K271 with cellular activity targeting the BCR-ABL kinase (IC50 = 0.2 nM). Although compound 16 is an irreversible inhibitor, it lacks selectivity. Compound 15 prolongs residence time and exhibits a potent time-dependent cytostatic effect in BCR-ABL-positive K562 CML cells and Ba/F3BCR-ABL, with an IC50 value of 50 and 74 nM, respectively. Furthermore, most drug-resistant BCR-ABL mutants were efficiently inhibited by compound 15. The X-ray crystallography study confirmed binding to the catalytic lysine residue K271 in the ABL kinase domain (Figure 4E). An imine bond was successfully formed between the ε-NH2 in K271 of ABL and the CHO of compound 15. Compound 17 can get its weakly electrophilic OSO2F close to ABL's K271, leading to the conclusion that compound 17 is a slow covalent inhibitor (Figure 4F).
2.1.5 Covalent HSP72 inhibitors
Molecular chaperones and their regulators play a role in maintaining protein homeostasis through activities such as removing misfolding, promoting efficient folding, and preventing aggregation. Molecular chaperones are proteins that aid in the proper functioning and structural stability of other proteins,74, 75 and this process depends on the regulation of ATP and cofactors. Members of these protein families are generally called stress proteins or heat shock proteins (HSPs), with subfamilies classified according to molecular weight as HSP40, HSP60, HSP70, HSP90, HSP100, and small heat shock proteins (HSPB).76 Furthermore, numerous studies have demonstrated a strong connection between ferroptosis and HSP.77
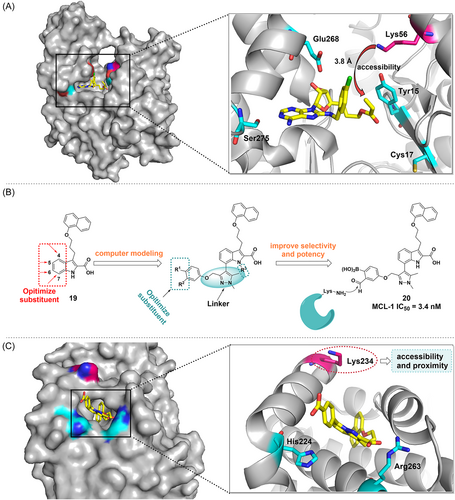
HSP72, which is overexpressed in cancer cells and stabilizes and folds substrate proteins to maintain cellular homeostasis, has been identified as an important target in cancer therapy.75, 78 In 2017, Cheeseman and colleagues reported a novel, irreversible covalent inhibitor 18 of HSP72 that targets Lys56.79 Initially, Cys17 in HSP72's nucleotide-binding structural domain (NBD) is expected to be a promising target since it is located at the bottom of the binding cleft with no steric conflicts and orients itself toward the 5′ position of the reversible binding ligand. Therefore, the design of appropriate 5′-adenosine derivatives could be an efficient strategy for the development of electrophilic reagents. Among them, compound 18 was identified as a covalent inhibitor of HSP72 by fluorescence polarization (FP) and protein mass spectrometry. It uses acrylate as a covalent warhead. Unexpectedly, crystallography demonstrated that Lys56 (located at a distance of 3.8 Å), a residue involved in a crucial salt bridge in HSP72, is the closest possible nucleophilic residue possible to the electrophilic warhead. Site-directed mutation studies demonstrated that the ATPase binding domain of HSP72 could be covalently modified by targeting noncatalytic Lys56 (Figure 5A), which provides an innovative strategy to target HSP72.
2.1.6 Covalent protein–protein interactions (PPI) inhibitors targeting lysine residues
PPI plays an important role in many physiological processes, including signal transduction, cell proliferation, differentiation, and apoptosis, and therefore are crucial in regulating cell function.80-82 Consequently, aberrant PPIs are associated with a wide variety of diseases. For example, p53, a tumor suppressor, loses its ability to inhibit tumor growth after interacting with MDM2 in tumor cells.83-85 However, protein binding interfaces are typically between 1500 and 3000 Å in size and lack well-defined deep binding pockets, making them challenging to target with small-molecule inhibitors, which are typically less than 1000 Å in size.86-89 Antibodies and peptide drugs have shown promising potential as PPI modulators and recent studies suggest that targeted covalent inhibition (TCI) may be an efficient way of blocking PPI.90-93
MCL-1 plays a critical role in the proliferation of many different types of tumor cells and is one of the most frequently amplified genes in malignant neoplasms.94-98 In 2016, Akçay and colleagues developed the first reversible covalent inhibitor of MCL-1 by covalently targeting its noncatalytic lysine side chain with an aryl boronic acid carbonyl warhead93 (Figure 5B). First, a previously reported MCL-1 inhibitor (compound 19) was attached with a carbonyl boronic acid warhead to covalently target Lys234 (Figure 5C). They synthesized a series of derivatives that could position their electrophilic warheads within ∼3 Å of Lys234. It was found that the efficacy improved upon the inclusion of the warheads into the indole acid backbone. In addition, the SAR revealed that the effectiveness of inhibitors was affected by the substituents in its phenyl group. More specifically, phenyl with two substituents is more potent than phenyl with one substituent, which is more potent than unsubstituted phenyl (compound 19). Subsequently, the effectiveness of the compounds was evaluated in the MCL-1-K234A cell line. In general, this study reports the first reversible covalent PPI inhibitor that targets MCL-1. Carbonyl boronic acid warheads selectively target lysine residues, paving the way for the discovery of small-molecule covalent inhibitors of high-value targets in MCL-1 and other proteins.
More interestingly, a novel lysine targeting strategy was recently reported by Chen et al.99 designed a series of irreversible covalent inhibitors targeting different protein kinases (e.g., ABL, EGFR) by using 2-ethynylbenzaldehyde (EBA) as a new covalent warhead, among which the first irreversible covalent inhibitor targeting MCL-1 was obtained, which is also a potent PPI inhibitor (compounds 23–25). Through EBA chemistry, ε-NH2 on the lysine residue firstly attacked the aldehyde group of benzaldehyde and dehydrated to form an imine, and then attacked the acetylenic group due to the lone pair of electrons of the N atom, and finally formed a stable isoquinoline through tandem cyclization, which ultimately led to the irreversible modification of the lysine residue. This imine-driven EBA has a low intrinsic reactivity and also reduces the reactivity of lysine, and only when bound to the kinase active site the EBA is locally concentrated and in close contact with the pKa-reduced ε-NH2 of lysine, resulting in selective and irreversible inhibition. This strategy is universal and can target different protein kinases and lysine residues (catalytic/noncatalytic, solvent exposure), and is a useful tool for designing lysine-targeted TCIs.
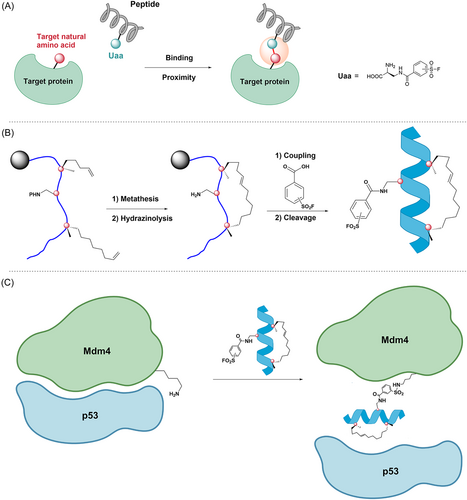
The p53 protein is encoded by the p53 gene, which is a tumor suppressor gene and plays an important role in cell cycle proliferation, apoptosis promotion, and genomic stability maintenance. Mdm2 and Mdm4 are very important negative regulators of the p53 protein, and they interact with the p53 protein to inactivate it.100 Inactivation of the p53 protein may result in malignant proliferation of tumor cells.101-103 Therefore, it is a promising approach to inhibit p53–Mdm2 or p53–Mdm4 interactions.101, 104 In 2016, Hoppmann et al.105 developed a covalent peptide inhibitor of p53–Mdm4 based on proximity-enabled bioreactivity. The method works by introducing chemically stable unnatural amino acids (Uaa) into the peptide, which react with the protein's target natural amino acid only when the peptide is bound to the protein and brings the Uaa close to the target natural amino acid. This novel approach facilitates the covalent binding of peptide inhibitors to target proteins under mild conditions106-108 (Figure 6A). First, based on the complex structure of Mdm2-SAHp53-8 and the structure of Mdm4 in complex with the transactivation peptide, Hoppmann et al. replace leucine at position 22 (L22) with an Uaa. Recently, SuFEx of SFs (–SO2F) has been highlighted as a good reaction for click chemistry.109 Furthermore, lysine residues and other nucleophiles can attack aryl-SF (Ar-SO2F) to generate stable sulfonamide bonds and are stable to hydrolysis over a wide range of pH. Thereby, they developed Uaa with an aryl-SF (Ar-SO2F) moiety and added it to the SAHp53-8 peptide (compound 26), which disrupted the interaction between p53 and Mdm2/4. When the peptides interacted with proteins, Uaa was close to the natural amino acid residues (lysine or histidine) in the Mdm4's p53 binding pocket and covalently bound to the target amino acid residues. This allowed Uaa to compete with Mdm4, successfully inhibiting the p53–Mdm4 interaction and releasing wild-type p53 (Figure 6C). Subsequent peptide cleavage and purification produced a large number of stapled peptides, and CD spectroscopy confirmed the α-helical conformation of the stapled peptide (Figure 6B). Subsequently, they access the inhibitory potency of several designed stapled peptides on p53–Mdm2/4 interactions using ReBiL (recombinase-enhanced bimolecular luciferase complementation). The ReBiL test demonstrated that compound 28 (Figure 7) had the most potent inhibitory effect on the p53–Mdm2 interaction and the p53–Mdm4 interaction, with IC50 values of 1.3, and 0.2 μM, respectively. Furthermore, the study of SAR also showed that positioning the –SO2F group in the meta-position of the benzene ring made compound 28 an order of magnitude more potent in inhibiting the p53–Mdm4 interaction (IC50 = 0.2 μM) than positioning it in the para-position of the benzene ring in compound 27 (IC50 = 3.5 μM) (Figure 8).
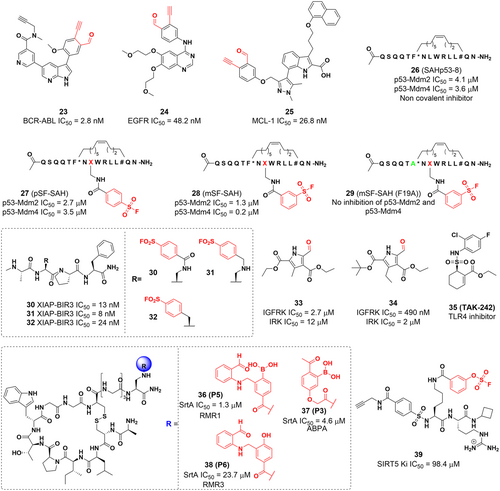
In 2019, Gambini reported a series of lysine-targeted covalent inhibitors targeting the BIR3 domain of the X-linked inhibitor of apoptosis protein (XIAP) using benzamide-SF as electrophilic warheads.110 They then demonstrated covalent interactions between ligands and proteins using the dissociation-enhanced lanthanide fluorescence immunoassay assay (DELFIA) platform. Further experiments demonstrated that compounds 30, 31, and 32 can form stable covalent adducts with Lys311 in the BIR3 domain of XIAP (Figure 8).
2.1.7 Covalent inhibitors targeting lysine residues in other biological targets
The insulin-like growth factor (IGF) signaling axis has been shown to play a key role in the development and progression of various tumors, and studies have shown that the use of modulators of IGF signaling can enhance the effectiveness of cancer therapy and hold promise for overcoming tumor drug resistance.111-114 The type 1 insulin-like growth factor receptor (IGF-1R) is a vital member of the IGF axis and regulates cell proliferation, transformation, and signal transduction, and is considered a potential target for tumor therapy.115-120 Bell and colleagues121 designed a group of ATP-competitive pyrrole-5-carbaldehyde derivatives (compounds 33 and 34), whose aldehyde moieties form a Schiff base with Lys1003 inside the IGF-1R ATP binding pocket to generate reversible covalent adducts.
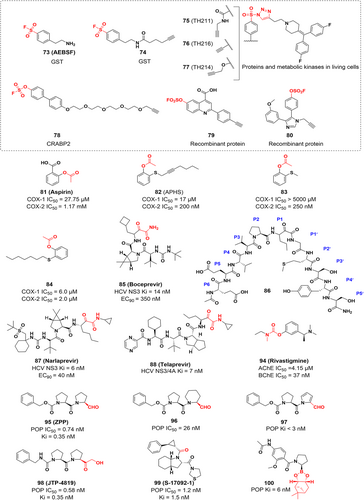
TAK-242 (Resatorvid) is a selective inhibitor of Toll-like receptor 4 (TLR4),122 which binds to the intracellular domain of TLR4 and inhibits the release of cytokines. Conjugation to human serum albumin (HSA) has become an effective method of extending the half-life of many small molecule and peptide/protein drugs in vivo. Studies have shown that the cyclohexene moiety of TAK-242 can tightly bind to endogenous macromolecules to form stable conjugates. This is a covalent binding mechanism based on the Michael addition with cysteine or lysine, followed by the elimination of the sulfonamide moiety by allylic rearrangement. And lys64 was experimentally identified as the major conjugation site and this conjugation strategy overcame the limitations of maleimide conjugation in the preparation of HSA conjugates and further improved the serum stability of HSA conjugates.123, 124 TLRs are commonly thought to play a role in the natural immune response and in inflammatory therapies, and it has recently been found that TLRs are also expressed in cancer cells, including cells from brain, liver, prostate, and ovarian tumors.125, 126 Activation of TLR signaling in tumor cells can inhibit apoptosis, promote tumor growth, and generate tumor resistance to host immune responses. Therefore, inhibition of TLR may be a potential approach for tumor therapy.
Reja et al. recently reported a lysine-targeted reversible covalent inhibitor, which has slower dissociation kinetics and longer residence durations than iminoborate, a lysine conjugation known to have fast dissociation kinetics.127 When comparing the inhibitory potency of peptide ligands (P1–P7) containing different warheads against staphylococcal sortase A (SrtA), the results showed that P5 (RMR1 warhead, a benzaldehyde derivative, compound 36) was the most potent inhibitor (IC50 = 1.3 μM). Subsequently, peptide mapping experiments and liquid chromatography-mass spectrometry (LC-MS) analysis showed that Lys173 is the conjugation site for P5, which is consistent with the results of covalent docking studies. Mechanistic studies have shown that AMPB adopts a closed conformation in neutral aqueous media due to the N–B coordination where the boron center is brought close to the aldehyde to promote RMR1 conjugation to lysine, resulting in a hydrated diazaborine. As a result, the RMR1 warhead can expand the warhead library, providing a new idea for designing lysine-targeted reversible covalent inhibitors. This particular study exclusively illustrates the concept of lysine-targeted covalency. Although no research has directly established a correlation between the inhibition of SrtA and cancer therapy, the findings presented here may contribute to the advancement of lysine-targeted drug development.
SIRT5 has been demonstrated to regulate gene transcription and metabolism, making it a valuable target for potential therapeutic interventions in cancer treatment. Given this, there is a growing demand for the development of novel SIRT5 inhibitors to effectively combat tumor growth and improve patient outcomes.128, 129 Bolding et al. reported a novel selective inhibitor (compound 39) of SIRT5 based on SuFEx chemistry,130 which uses an aryl fluorosulfide group as an electrophilic moiety that specifically recognizes a tyrosine residue (Tyr102) located within the SIRT5 substrate binding pocket. This compound with an aryl fluorosulfate group, offers enhanced stability compared to a SF, displaying reduced susceptibility to hydrolysis and limited off-target reactivity within the proteomic environment. Notably, it demonstrates selective covalent targeting of SIRT5 isoforms in a dose-dependent manner. These findings provide a solid foundation for the development of probes and drugs utilizing novel ligands, paving the way for further advancements in this field.131
2.2 Covalent probes targeting lysine residues
Due to its proper balance between biocompatibility and protein reactivity, sulphonyl fluorides (SFs), also known as a privileged warhead, can modify environmentally specific amino acid residues.132 SFs are widely used for four main reasons: (1) serine,133 lysine,134 cysteine135 (but the reaction product with cysteine is not very stable), threonine,136 tyrosine,137 and histidine residues138 can all be covalently modified under physiological circumstances; (2) SF containing ligands have balanced reactivity and moderate water stability and are easily introduced into complex chemical molecules; (3) the fluoride leaving-group has excellent biological properties109; (4) the reaction product sulfonate is stable with the sulfonamide bond.139 Additionally, some of these SFs have been explored as activity-based protein profiling (ABPP) probes, which are very crucial tools in chemical biology.133 Using SFs as warheads to develop selective covalent ligands is another strategy to develop Lys-targeted inhibitors. The most notable example in this field is Grimster et al.134 used SFs to target Lys15 of the transthyretin (TTR). TTR is a thyroid hormone (T4) transporter; two T4 binding sites are formed at the dimer-dimer interface of the TTR tetramer; and dissociation of the tetramer will lead to misfolding and aggregation of the protein. Choi et al.140 proposed a pharmacological strategy for covalent molecules targeting Lys15 as kinetic stabilizers of tetramers. Unlike SFs, aryl-fluoro sulfates (Ar-OSO2F) are very stable against hydrolysis and nucleophiles, and show minimal reactivity in the presence of nontarget amino acids.109, 139 Kelly et al.141 replaced the original SF probes (compounds 40, 41, and 42) with Ar-OSO2F and synthesized a series of 1,3,4-oxadiazole with aromatic SFs (compounds 43, 44, and 45), which stabilize the TTR and thus inhibit the formation of amyloid fibrils. It also shows that aryl-fluoro sulfates react three orders of magnitude faster than the previous Lys15 targeting series,134 making SFs an appealing option as warheads to target lysine residues.
One of the covalent chemical probes for FGFR1 is 5′-fluorosulfonylbenzoyl adenosine (FSBA). FSBA contains the functional group that replaces the phosphate group in ATP, and the peptide map indicates that conserved lysine in the kinase ATP binding site is targeted by FSBA.142, 143 Additionally, FSBA is also an active probe for recombinant kinases (ALK5 and CDK2).144 The electrophilicity of SF situated at different places of the warheads also affected the labeling potency of the biotinylated FSBA derivatives, with compounds 49 and 50 only faintly labeling the protein and compound 48 being more selective in labeling the kinase. Subsequent experiments confirmed that compound 51 could target Src family kinases as a probe. Lys514 in the ATP-binding pocket of FGFR1 was covalently modified by 5′-O-3-((fluorosulfonyl) benzoyl) adenosine (m-FSBA) (Figures 9A and 10), as detailed by Mukherjee et al., who recently examined the structure-reactivity relationship between SF and cysteine, tyrosine, lysine, and serine.139 The results of this study are useful in the rational design of new covalent probes and inhibitors that contain SF, especially in the absence of properly placed cysteine residues.
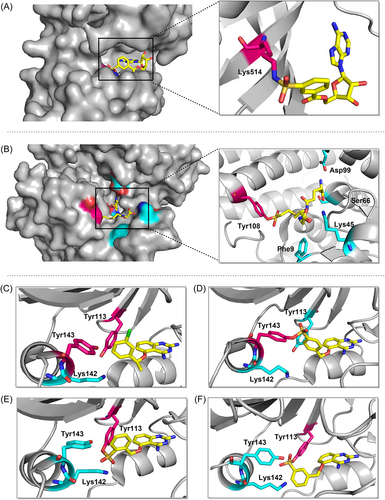
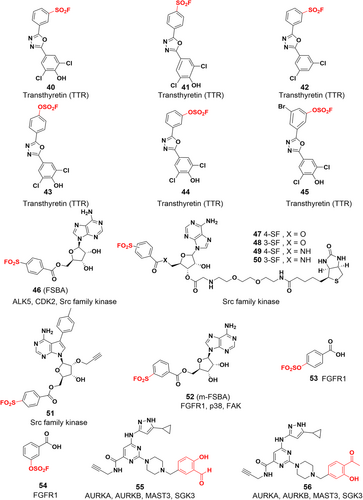
While most of the current covalent inhibitors targeting lysine are irreversible inhibitors,46, 110, 145, 146 there is growing interest in the development of reversible covalent inhibitors. Reversible inhibitors have the potential to provide several advantages, including increased proteomic selectivity and suitability for in vivo experiments. By exploring reversible covalent inhibitors, researchers can further expand the range of options available for lysine-targeted drug development and potentially enhance their therapeutic efficacy. Yang et al. designed a series of clickable benzaldehyde probes by attaching salicylaldehyde to a kinase noncovalent recognition scaffold (compounds 55 and 56).147 More than 200 kinases were experimentally identified, and it was shown that such probes efficiently and selectively bind to conserved catalytic lysine residues of protein kinases. The lysine side chain forms an imine with the aldehyde group, which in turn forms an intramolecular hydrogen bond with the o-phenol hydroxyl group, and this hydrogen bonding interaction enhances the hydrolytic stability of the imine. Different kinetics of imine hydrolysis can affect the residence time of the drug leading to differential and selective kinase inhibition. Kinases that bind tightly to such probes are key targets for cancer therapy, such as SGK3,148 AURKA and AURKB.149 Hence, the exploration and development of reversible covalent inhibitors utilizing salicylaldehydes hold great promise for targeting cancer therapy-relevant kinases that lack targetable cysteine residues. By harnessing the unique properties and interactions of salicylaldehydes, it becomes possible to design inhibitors that can efficiently and selectively bind to these kinases, providing a potential avenue for the development of novel and effective therapies for cancer treatment.
3 COVALENT MODIFICATION OF TYROSINE RESIDUES
The intrinsic nucleophilicity of the phenolic side chain of tyrosine residues is relatively weak compared to that of cysteine or unprotonated lysine. As a result, the development of covalent inhibitors that act on tyrosine residues has been quite challenging, resulting in relatively few covalent drugs targeting tyrosine residues. Several reported tyrosine covalent inhibitors as well as covalent probes are described below.
3.1 Covalent inhibitors targeting tyrosine residues
3.1.1 Covalent GSTP1-1 inhibitors
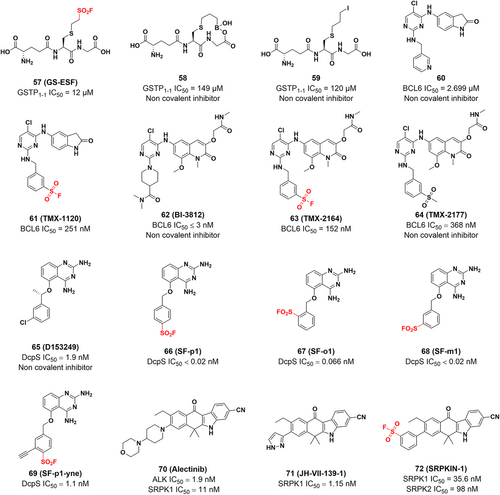
In 2017, Shishido et al.150 used SF warheads to target Tyr108 of GSTP1-1 and successfully designed the first irreversible covalent inhibitor of GSTP1-1, namely GS-ESF (compound 57), which binds to the GSH-binding site (G site) of GST. High expression of GSTP1-1 can inhibit apoptosis and promote tumor growth.151, 152 Generally, each GST subunit has two types of ligand binding sites.151, 153 In addition to GSH-binding sites (G-sites), there are hydrophobic substrate binding sites (H-sites) that can accommodate a wide range of compounds. It is challenging to develop inhibitors of the G site that must compete with GSH due to the high concentrations of GSH in cells.154 Since the active residue of GSTP1-1 at the G site is Tyr7, which absorbs the thiol proton of GSH, the researchers expected to form a covalent bond by placing a variety of electrophilic reactive groups around the thiol of GSH. As a result, they developed a series of compounds with different warheads (compounds 57–59). Compound 57 was the most potent inhibitor, with an IC50 value of 12 μM compared to 149 and 120 μM for compounds 58 and 59, respectively. Meanwhile, compound 57 also showed selectivity to other GST subtypes, with an IC50 value of 50 μM for GSTM2-2 and 12 μM for GSTA1-1. and exhibited appropriate stability. The sulfonyl group of compound 57 covalently binds to the Tyr108 hydroxyl group at the GSTP1-1 binding site, while compounds 58 and 59 were not (Figure 9B). To explain the selectivity of the reaction of compound 58 with Tyr7 and Tyr108, they predicted the pKa values of the two tyrosine phenolic hydroxyl groups using PROPKA and showed that the pKa for Tyr7 and Tyr108 were 14.10 and 11.36, respectively, indicating that Tyr108 tends to be more deprotonated than Tyr7 and therefore reacts selectively with the SF group, which further identifies the site of covalent modification as Tyr108.155, 156 This approach uses irreversible covalent binding to address the limitations associated with noncovalent competitive inhibitors, and it is the first GST covalent inhibitor capable of irreversibly inhibiting GSTP1-1 function.
3.1.2 Covalent B-cell lymphoma 6 (BCL6) inhibitors
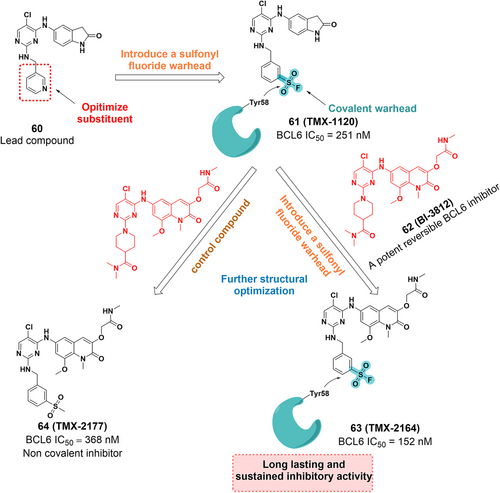
BCL6 is a potential therapeutic target for cancer157 and is abundantly expressed in many tumors, including diffuse large B-cell lymphoma (DLBCL),158 nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL), follicular lymphoma (FL) predominant with nodular lymphocytes,159, 160 and breast cancer.159, 161, 162 BTB domain-mediated dimerization of BCL6 generates two identical lateral grooves that can accommodate ligands and serve as binding sites for small molecule inhibitors.163, 164 The complex structure of BCL6 with its inhibitor 60 shows that Tyr58 of BCL6 is advantageously located within 4.2 Å of the pyridine ring of compound 60, suggesting that Tyr58 can be targeted covalently. Subsequently, the lead compound 60 was introduced with a SF warhead to afford the compound TMX-1120 (Figure 11). The crystal structure reveals that the original interaction between the hydroxyl group of Tyr58 and Arg28 was replaced by the oxygen of the sulfonyl moiety, which strongly indicates the potential for the covalent bond formation. Furthermore, they incorporated the SF warhead into BI-3812 (a potent reversible BCL6 inhibitor) to develop TMX-2164 and a control compound, TMX-2177 (Figure 11). TMX-2164 had a greater inhibitory effect with an IC50 value of 152 nM than TMX-2177 and exhibited enhanced antiproliferative activity. Therefore, TMX-2164 represents a new example of a tyrosine-targeted covalent inhibitor of BCL6 with a potent, long-lasting inhibitory effect against cancer cells.165
3.1.3 Covalent DcpS inhibitors
DcpS is a scavenger messenger RNA (mRNA) decapping enzyme that hydrolyzes residual cap structures after the 3′–5′ decay of mRNA.166 In addition to participating in the metabolic hydrolysis of mRNA, it protects cells from the potentially toxic accumulation of short-cap mRNA fragments and modulates the activity of other cap-binding proteins to prevent their activity from being inhibited. Additionally, spinal muscular atrophy (SMA) is an autosomal recessive genetic disorder caused by mutations in the SMN1 gene that results in the loss and reduction of the survival motor neuron protein (SMN). The SMN protein is required for neuronal survival, and its reduced abundance will lead to the degeneration of motor neurons and muscle atrophy.167 Although a series of small molecules, such as diaminoquinazoline (DAQ), have been identified that up-regulate SMN proteins,168 the biological connection between DcpS inhibitors and SMA regulation is not clear. Hett et al.169 rationally designed the DAQ-derived SF probe based on chemical proteomics to develop a covalent inhibitor targeting specific tyrosine residues of DcpS, confirming that DAQ is an inhibitor of DcpS. The reported crystal structures suggest that Tyr113, Lys142, and Tyr143 are close to the benzyl group of the ligand and could be covalently targeted (Figure 9C). Subsequently, three regioisomeric SF probes (SF-p1 66, SF-o1 67, and SF-m1 68) with inhibitory activities at the subnanomolar level based on the structure of D153249 were rationally designed. To investigate the mechanism of covalent interaction, the probe was incubated with recombinant DcpS, and LC-MS analysis and crystal structure showed that the para-isomer SF-p1 preferentially reacts with Tyr143, while the ortho-isomer SF-o1 and the meta-isomer SF-m1 react with Tyr113 (Figure 9). This suggests that subtle changes in the functional position of SFs determine which tyrosine residue reacts with the warhead. Analysis of reported proteomics studies by the authors suggests that tagged tyrosine residues proximal to basic residues account for the majority of cases and that proximal residues promote deprotonation of tyrosine-OH and therefore may give rise to some targetable tyrosine residues. Furthermore, they developed an intracellular occupancy biomarker technology to illustrate the utility of tyrosine-targeted probes and prepared an alkyne-labeled SF probe, namely SF-p1-yne (compound 69), which was a potent inhibitor of DcpS (IC50 = 1.1 nM). This study describes the first rational targeting of tyrosine residues using small-molecule covalent probes, which enables efficient protein capture from complex proteomes and facilitates the sensible development of tyrosine-targeted covalent inhibitors. Yamauchi et al. revealed by genome-wide CRISPR/Cas9 screening that DcpS scavenger decapase is essential for the survival of acute myeloid leukemia (AML) cells, revealing DcpS as a novel target for AML therapy and the potential repurposing of DcpS inhibitors used for the treatment of SMA as an antileukaemia drug.170, 171 Therefore, the development of novel covalent DcpS inhibitors may be a promising approach for the treatment of AML.
3.1.4 Covalent SRPK1/2 inhibitors
The SRPK kinase family plays a crucial role in pre-mRNA splicing. Due to their specificity for splicing factors and their role in tumorigenesis, SRPK kinases have been proposed as therapeutic targets for cancer.172, 173 Furthermore, activated Akt has also shown to act directly on SR proteins174, 175 or indirectly to transmit its signal to the nucleus through SR protein-specific kinases. SRPKs are central sensors of EGF signaling, suggesting that the Akt-SRPK-SR axis constitutes the major branch in the transduction of the EGF signaling pathway to regulate the splicing program in nucleus.176 Hatcher et al.177 reported the first selective irreversible SRPK1/2covalent inhibitor, namely SRPKIN-1 (Figure 12A). Using alectinib, an ALK inhibitor with good SRPK1 inhibitory activity, as a lead compound, they constructed a compound library and performed a SAR study. JH-VII-139-1 was constructed by replacing the 4-morpholine piperidine moiety in alectinib with a pyrazole ring, which has better inhibitory activity against SRPK1 (IC50 = 1.15 nM) than alectinib (Figure 12A). Since the extra helix αS1 from the spacer region of SRPK1 can form an extended molecular surface close to the ATP-binding pocket, the smaller pyrazole ring in JH-VII-139-1 does not interact with Tyr227 and Leu231, nor does it cause steric conflict with Tyr227, thus providing a framework for the subsequent design of irreversible covalent inhibitors targeting Tyr227 of the SRPK1 ATP-binding pocket. Benzene-SF would maintain the desired size for the solvent-exposed area, which would also put the warhead in the desired position to covalently modify Tyr227 (Figure 12A). Therefore, benzene-SF was used as the electrophilic warhead to replace the pyrazole ring in JH-VII-139-1, resulting in SRPKIN-1 (Figure 12A). SRPKIN-1 has a good inhibitory effect against SRPK1 and SRPK2 with IC50 values of 35.6 and 98 nM, respectively. And it was selective with reduced activity against ALK (IC50 = 195 nM). Furthermore, SRPKIN-1 was significantly selective for SRPK1 and SRPK2. Through the covalent interaction of its SF moiety with the phenolic hydroxyl group of the Tyr227 side chain at the entrance of the ATP binding pocket, SRPKIN-1 could inhibit the activity of serine protein kinases and consequently inhibit the tumor cell proliferation. Furthermore, SRPKIN-1 has a potent antiangiogenic effect, successfully converting VEGF from a proangiogenic subtype to an antiangiogenic subtype. Overall, this study develops the first covalent kinase inhibitor targeting tyrosine, providing additional possibilities for the design and development of novel covalent kinase inhibitors.
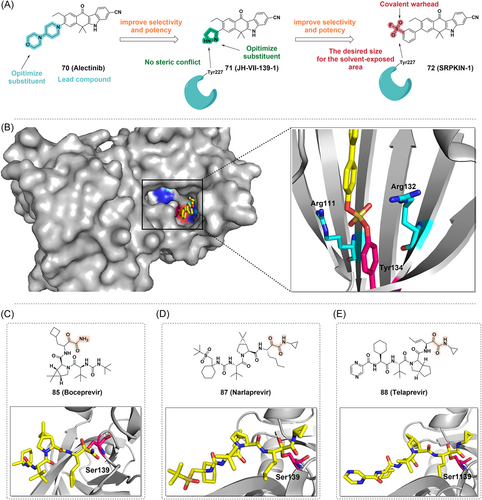
3.2 Covalent probes targeting tyrosine residues
Verhelst et al.137 designed a probe (compound 68) derived from AEBSF and proved it covalently targets the tyrosine residue (Figure 13). In the covalent labeling of GST, it was surprising that the labeled tyrosine residues clustered around the H site opposite the G site. Gu et al.178 identified functional tyrosines in proteins beyond GST, suggesting that SF probes are promising for identifying functional tyrosines in other protein families. Hahm group179 found that sulfur-triazole exchange (SuTEx) chemistry has a wide range of applications in chemical proteomics, which has great potential and application value for the development of covalent probes and protein ligands. SuTEx utilizes a triazole heterocycle as a leaving group (LG) capable of tuning the chemoselectivity of tyrosine in the proteome through covalent reactions with protein sites. This approach can be used to discover subsets of tyrosines with enhanced reactivity and to perform global phosphotyrosine analysis. By modifying the triazole LG, they developed a sulfonylated probe that had excellent selectivity for tyrosine residues. Subsequently, a series of functionalized SuTEx probes were synthesized180 (compounds 75–77) that covalently bind to tyrosine residues to discover ligandable tyrosines within the catalytic and regulatory domains of proteins and metabolic kinases in living cells, allowing the development of novel covalent tyrosine inhibitors targeting these sites.
Mortenson et al.181 developed several structurally unique aryl-fluoro sulfates that specifically bind to the tyrosine residue of cellular retinoic acid-binding protein 2 (CRABP2), a member of the family of modified intracellular lipid-binding proteins (iLBPs). Through catalysis of SuFEx with tyrosines at the binding site, iLBP activity can be suppressed and the expression of downstream genes attenuated.109 Since CRABP2 contains a conserved Arg-Tyr-Arg motif in its binding pocket, these two Arg side chains may reduce the pKa of phenol and may stabilize the reaction transition state of the SuFEx reaction, and thus the reactivity of aryl-fluoro sulfates towards the Tyr of CRABP2 may be determined from this.182 Meanwhile, tandem mass spectra showed that aryl-fluoro sulfate probes bind iLBP via conserved tyrosine residues at the fatty acid binding sites.183 Furthermore, the crystal structure reveals that probe 78 and Tyr134 form a diarylsulfate diester bond, and that the hydrophobic biaryl groups interact hydrophobically with several other residues at the retinoic acid (RA) binding site (Figure 12B). Additionally, covalent labeling of Tyr in recombinant proteins was also achieved with aryl-fluoro sulfate alkyne probes 79 and 80. These examples may help to design covalent inhibitors with specific targets, reduced proteome-wide reactivity, and amino acid labeling preferences. The robust addition of aryl-fluoro sulfates or SFs to the available warhead tool set promises more exciting developments for covalent probes and inhibitors.
4 COVALENT MODIFICATION OF SERINE AND THREONINE RESIDUES
The nucleophilic potential of amino acids depends on the microenvironment of the protein in which they are located. Both serine and threonine contain hydroxyl groups that can react with the electrophilic centers of inhibitors to form covalent bonds in biochemical reactions. However, unactivated serine and threonine residues have low side-chain hydroxyl reactivity. Besides, many electrophiles that can bind to them are susceptible to competitive hydrolysis under aqueous conditions. However, sulfur(VI) fluorides, oxophilic phosphorus (V) compounds and boron-based reagents are probably the most suitable for use as warheads to deal with such hydroxyl groups.184 There are many approved covalent drugs targeting serine and threonine residues, including aspirin, penicillin, telaprevir, boceprevir, avibactam, carfilzomib, bortezomib, rivastigmine, and saxagliptin.22, 23, 39 However, the weaker reactivity and higher abundance of these residues still make selective targeting more challenging.185
4.1 Covalent inhibitors targeting serine residues
4.1.1 Covalent cyclooxygenase (COX) inhibitors
Aspirin (Figure 14) is an irreversible COX inhibitor and acts by acetylating serine residues at the active site. COX is an enzyme that catalyzes the conversion of arachidonic acid to prostaglandins, which exists in three isoforms: COX-1, COX-2, and COX-3. COX-1 and COX-2 have been extensively studied to explore their connection with Alzheimer's disease (AD). Specifically, COX-2 inhibitors also have potential preventive properties against a variety of tumors, including colon, breast, and prostate cancers.186
As early as 1998, Kalgutkar187 reported the first novel inactivator for selective covalent modification of COX-2, namely 2-acetoxyphenylhept-2-ynyl sulfide (APHS), which selectively inactivates COX-2 by acetylating the same serine residues as aspirin acetylation. Subsequently, Wei et al.188 conducted an extensive SAR study of the initial lead compound, 2-acetoxyphenyl methyl sulfide (compound 83), with different acyl, alkyl, aryl, and heteroatom substitution patterns leading to the identification of the most effective inhibitor 82. The extension of the S-alkyl chain in 83 leads to a significant increase in inhibitory potency, the heptyl chain in 2-acetoxyphenyl heptanethioether (compound 84) is optimal for COX-2 inhibition and the introduction of a triple bond in the heptyl chain (compound 82) leads to a further increase in inhibitory potency and selectivity. Further, SAR studies have shown that the alkynyl analogues are more effective and selective than the corresponding alkyl congeners and that the sulfides are more effective and selective than the corresponding sulfites or sulfonic acids or other heteroatom-containing compounds. Recent findings suggest that APHS is more selective for COX-2 than COX-1 and it can inhibit the growth of COX-2-expressing colon cancer cells in vitro and in vivo.188 APHS has much better selectivity and activity against COX-2 than aspirin, which could lead to the development of aspirin-like drugs to treat or prevent inflammatory and proliferative diseases with few gastrointestinal or hematological side effects.187, 189
4.1.2 Covalent Hepatitis C virus (HCV) protease inhibitors
HCV infection is one of the most critical health problems that affect humans. HCV protease is an important target for the treatment of HCV infection. HCV protease inhibitors could inhibit the activity of HCV NS3 protease, which is essential for the posttranslational modification of viral polypeptides. Since HCV NS3/4A protease plays a crucial role in viral replication, it is considered an ideal target to develop new HCV therapeutics.190, 191 In addition, HCV infection is a major cause of cirrhosis and various tumors such as intrahepatic cholangiocarcinoma, pancreatic cancer and non-Hodgkin's lymphoma (NHL).192, 193 Therefore, HCV inhibition holds promise for reducing the risk of extrahepatic cancer development. Njoroge et al.194 used a structure-based drug design approach to develop boceprevir (Figure 14), a small molecule covalent inhibitor that targets the serine residue of the HCV NS3 protease with α-ketoamide as a warhead. The α-ketoamide fraction of boceprevir is essential for the inhibitory activity. The crystal structure indicates that Ser139 is located in the catalytic site of the NS3 protease and is highly nucleophilic, forming a stable, covalent and reversible complex with the enzyme when the α-ketoamide is attacked by Ser139 (Figure 12C). This study was guided by the lead compound 86 and further optimization gives the tripeptide inhibitor with the best fraction of each site (Figure 14). In addition, narlaprevir and telaprevir are also covalent inhibitors targeting serine proteases and have been approved by the FDA. Both contain α-ketoamine electrophilic warheads, and their primary target is the HCV NS3/4A protease. Telaprevir is covalently bound to Ser1139 (Figure 12E), while narlaprevir and boceprevir are covalently bound to Ser139 (Figure 12D,C).
Proteolysis targeting chimeras (PROTACs) improves selectivity and further extends the duration of action by overcoming the limitations of conventional medicinal chemistry's incomplete inhibition of target proteins.195 There are also some disadvantages to PROTAC, for example, the potential toxicity of PROTAC may be greater than that of traditional small molecule drugs. Therefore, the introduction of covalent chemistry to target protein degradation has the potential to both retain the benefits of PROTAC and TCI or overcome their limitations to further degrade more therapeutically challenging targets (e.g., KRAS G12C that lacks a druggable pocket on its surface).196, 197 This combined approach may lead to new discoveries, but the two approaches cannot simply be added together as there is the potential for positive or negative effects. When designing and developing covalent PROTACs, there are often many possibilities for the location of the covalent warhead: covalent POI ligands, covalent E3 ligase ligands and dual covalent POI and E3 ligase ligands. Although it is conceivable that bis-covalent PROTACs have advantages, no examples have been reported and so far covalent PROTACs based on covalent POI ligand designs are still in the majority. Like TCI, covalent PROTACs can be divided into reversible and irreversible PROTACs depending on the nature of the reactivity of the POI ligand warhead198 (Figure 15A,B). However, irreversible binding may reduce the effectiveness of PROTACs by negating their catalytic properties. There have been several reports suggesting that the effectiveness of PROTACs is diminished by the introduction of irreversible covalent bonding.199, 200 Theoretically, the potency, selectivity, and extended duration of action of reversible covalent PROTACs might be enhanced along with the covalent bond formation. Additionally, because reversible covalent warheads are quickly reversible, noncovalent interactions between small molecules and the intended target promote the formation of covalent bonds and prevent indiscriminate protein modifications, thus potentially lowering the risk of toxicity. Currently, most covalent PROTACs with POI-based TCIs are still targeting cysteine residues in target proteins, such as dacomitinib-based PROTACs to degrade EGFR, and covalent PROTACs to degrade BTK, ERK1/2.201-205 Therefore, the development of covalent PROTACs for amino acid residues beyond cysteine is an exciting and promising research field.
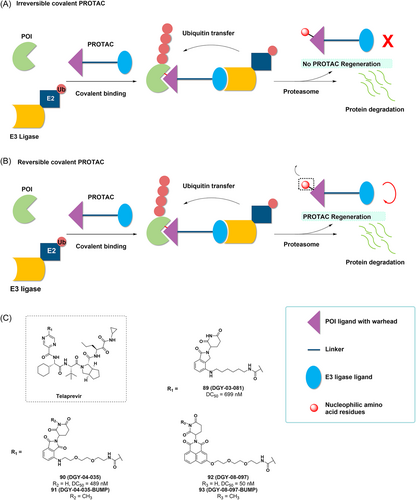
In 2019, Yang at al. synthesized a functional degradation agent targeting the HCV NS3/4A protease using telaprevir as a ligand for the HCV NS3/4A protease.206 The crystal structure reveals that the pyrazine ring of telaprevir is in the solvent exposed area and is suitable for ligating the ligand of E3 ubiquitin ligases with linker. Therefore, using lenalidomide and pomalidomide and a novel tricyclic imide fraction as CRBN ligands, they developed DGY-03-081, DGY-04-035, and DGY-08-097 and assessed their inhibitory activity in vitro (Figure 15C). The results showed that all three compounds inhibited HCV NS3/4A protease at submicromolar levels. Subsequently, they detected selective degradation of the target and found that the three candidate degradants reduced the intracellular NS3 protein in a concentration-dependent manner, DGY-08-097 exhibiting the best degrading effect (DC50 = 50 nM). Furthermore, the negative controls DGY-04-035-BUMP and DGY-08-097-BUMP, both analogues of CRBN that are unable to bind due to glutarimide methylation, both suppress NS3/4A enzymatic activity in vitro but do not promote the degradation of HCV NS3. Furthermore, HCV NS3 was rescued from degradation in the presence of DGY-03-081, DGY-04-035, and DGY-08-097 by excess of the CRBN ligand lenalidomide, this further demonstrated the dependency of these degraders on CRBN for mediated degradation of NS3. All three degraders showed antiviral activity and effective degradation of NS3, with DGY-08-097 remaining the most effective. The study developed a reversible covalent PROTAC strategy based on known TCIs, improving the inhibition of target proteins. In addition, there is exciting potential for this approach to develop degradants that utilize noncatalytic binding pockets. This would allow targets without conventional drug pockets to be degraded and allow degraders to be used in combination with conventional inhibitors to develop durable antiviral drugs.
4.1.3 Covalent acetylcholinesterase (AChE) inhibitors
Since endogenous acetylcholine is easily destroyed and inactivated in tissues by AChE, and reduced acetylcholine levels may be an indication of AD, drug design strategies against AD dementia symptoms should involve increasing acetylcholine levels at these synapses.207 To be precise, rivastigmine (Figure 14) is a covalent AChE inhibitor. Once bound to AChE, rivastigmine acylates Ser200 at the active site via its phenolic carbamate to inhibit the activity of AChE and reduce the cleavage of acetylcholine, further improving AD symptoms (Figure 16A). Furthermore, it inhibits AChE for up to 10 h and can be used to treat dementia associated with Alzheimer's disease and Parkinson's disease.207-209 Kandiah and his group210 revealed that rivastigmine also inhibited both AChE and butyrylcholinesterase (BChE) activities, and this dual inhibition improved the therapeutic effect in subcortical vascular dementia and Parkinson's disease dementia. In addition, it has been shown that neural–cancer interactions are increasingly recognized as critical for pancreatic cancer (PCa) development and progression.211, 212 Inhibition of AChE activity using AChE inhibitors such as pyridostigmine and physostigmine has been shown to effectively decrease PCa cell survival both in vivo and in vitro. This effect is achieved through the inhibition of pERK signaling. As a result, the design and development of AChE inhibitors present potential as a viable strategy in the treatment of PCa.
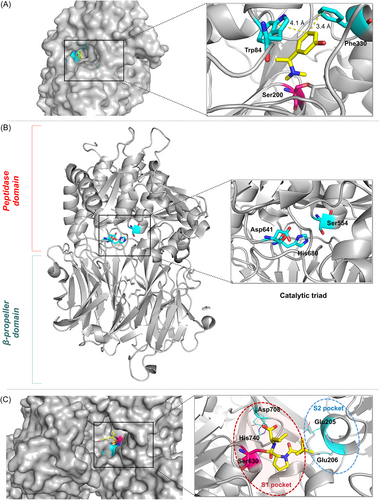
4.1.4 Covalent prolyl oligopeptidase (POP) inhibitors
POP is a monomeric serine protease213 and consists of two domains214 (Figure 16B): (1) the protease catalytic domain with an α/β hydrolase fold (consisting of residues 1–72 and 428–710) and (2) the noncatalytic domain forming a seven-bladed β-propeller (consisting of residues 72–437). The active site of human POP is called the catalytic triad and consists of Ser554, His680, and Asp641.215 Targeting POP is an emerging therapeutic strategy for treating diseases associated with cognitive impairment, such as Alzheimer's and Parkinson's diseases.216, 217 Furthermore, research has shown that the inhibition of POP strongly inhibits tumor cell growth and proliferation in vivo, making POP a potential target for antitumor drugs.218-222 Latest findings show that prolyl oligopeptidase is highly expressed in serum samples and biopsies from patients with glioblastoma (GBM) and that this upregulation negatively correlates with patient survival, as well as with the tumor suppressor PP2A.223 Thus, prolyl oligopeptidase has the potential to be a biomarker for the diagnosis and prognosis of GBM and may also serve as a therapeutic target for GBM. X-ray crystallography shows that POP inhibition can be achieved by the formation of a covalent bond between the ligand warhead and the catalytic serine (Ser554) in the active site.215
Z-Pro-Prolinal (ZPP) is a POP covalent inhibitor that covalently binds to Ser554 at the active site to form a reversible hemiacetal.215 Racys et al.224 developed a series of compounds derived from ZPP and found that compound 96 had better inhibitory activity (IC50 = 26 nM), while compound 97 had very low inhibitory activity and was degraded before co-crystallization with POP (Figure 14). The crystal structure showed that compound 96 covalently bound to Ser554 and the size of the piperidine ring was well suited to the hydrophobic pocket of POP. In conclusion, they confirmed that compounds containing piperidine rings can also act as effective substrate mimics of POP enzymes by synthesizing and characterizing two potential POP inhibitors 96 and 97, which play a leading role in the development of new POP inhibitors.
JTP-4819 is a highly specific covalent inhibitor of POP (Figure 14). It showed concentration-dependent and competitive reversible inhibition for cortical and hippocampal POP, with IC50 values of 0.58 and 0.61 nM, respectively, with high selectivity.225-228 Furthermore, JTP-4819 has shown potential to treat AD by improving memory deficits in animal models due to its effects on neuropeptides and cholinergic neurons.217 S-17092-1 (Figure 14) is also a potent and selective inhibitor of POP that covalently reacts with Ser554 with an IC50 value of 1.2 nM.229 The carbonyl group of S-17092-1 forms a hemiacetal adducts with the nucleophilic hydroxyl group of residue Ser554 in the active site. This covalent adduct mimics the tetrahedral transition state of the enzyme-catalyzed reaction and improves the affinity of the inhibitor for the enzyme.
Plescia et al.230 develop POP inhibitors (compounds 100 and 101) with nanomolar inhibitory potency in vitro based on computer-aided drug design (CADD) by using boronic acid esters as prodrugs for the release of boric acid, covalently bound to catalytic serine residues (Figures 14 and 17). Unlike previously identified inhibitors that use pseudopeptide scaffolds, these molecules employ reversible covalent electrophiles to effectively inhibit POP.222, 231 This work has excellent potential for future drug design because of the easy availability of this class of POP boronic ester inhibitors.
4.1.5 Covalent dipeptidyl peptidase-4 (DPP-4) inhibitors
DPP-4 is a serine protease responsible for the degradation of incretin hormones, a process that regulates the secretion of insulin and glucagon and contributes to the regulation of blood sugar levels. Consequently, DPP-4 has been identified as a target for the treatment of type 2 diabetes, but is also closely linked to the development of human malignancies.232, 233 Depending on the type of cancer, DPP-4 plays a different role (activation or inhibition). DPP-4 inhibition increases the metastatic potential of breast cancer,233 accelerates the progression of prostate cancer,234 and potentially increases the risk of cholangiocarcinoma in adults with type 2 diabetes.235 In contrast, DPP4 inhibitors have beneficial effects on progression-free survival (PFS) in diabetic patients with advanced colorectal and airway cancers.236 As well as a clinical study suggests that DPP-4 inhibitors prevent acute graft-versus-host disease, thereby preserving the graft-versus-tumor effect.237 In this section, we discuss the design and discovery process of covalent inhibitors only from a drug design perspective, with only a brief overview of their therapeutic effects. At its binding site, DPP-4 has two pockets (S1 and S2). The proline-specific pocket S1 has a catalytic triad of Ser630, Asp708, and His740; the pocket S2 contains Glu205 and Glu206, which can bind to any amino acid (Figure 16C).
Saxagliptin (Figure 17) is a covalent DPP-4 inhibitor that treats type 2 diabetes by forming an imidate with Ser630 of DPP-4 using nitrile as an electrophilic warhead.238, 239 To increase potency, noncovalent inhibitors are often extended further into the S2 pocket. Conversely, covalent inhibitor-enzyme complexes form reversible covalent bonds, which cause the drug to slowly dissociate from its binding pocket, resulting in a sustained inhibitory effect. Additionally, similar to saxagliptin's mechanism of action, vildagliptin (Figure 17) inhibits the DPP-4 by forming a covalent bond with the active site serine via the Pinner reaction.
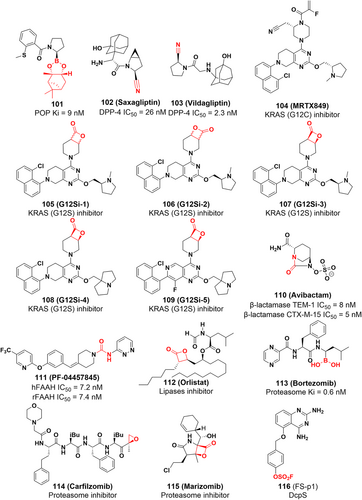
4.1.6 Covalent KRAS (G12S) inhibitors
KRAS gene mutations continuously stimulate cell growth, leading to tumorigenesis.240, 241 The KRAS hotspot mutant (G12S) is an oncogenic driver with GTPase activity and has a transformation potential similar to that of KRAS (G12C). Recently, Zhang et al. reported a study of small molecule inhibitors covalently targeting KRAS (G12S),242 which covalently target the G12S somatic mutation in KRAS and suppress its oncogenic signaling. They synthesized small-molecule compounds G12Si-1 and G12Si-2 (Figure 17) with β-lactone structure by linking β-lactone moieties to the tetrahydropyridopyrimidine moiety of MRTX849, a selective covalent inhibitor of KRAS (G12C). However, G12Si-2 did not form a covalent bond with serine residues. The crystal structure of the K-Ras (G12S)•GDP•G12Si-1 complex shows that G12Si-1 binds to noncatalytic Ser12 in S-IIP and adopts an orientation similar to that of the G12C ligand. Additionally, Ser12 has been acylated by G12Si-1, and the β-lactone has opened the ring to form an ester bond between the protein and the ligand. The crystal structure shows hydrogen bonding interactions between the carbonyl oxygen of the ester group of G12Si-1 and Lys16, and between the secondary alcohol produced by ring opening of the β-lactone and the backbone carbonyl of Gly 10. In addition, water molecules bridging the same secondary alcohol and the main chain N–H of Gly 10 were also observed. These anchoring interactions demonstrate that G12Si-1 is a good electrophilic reagent. To further improve the inhibitory potency of G12Si-1, they developed other inhibitors (G12Si-3 to G12Si-5) by modifying the N-methylprolinol substituent and the tetrahydropyridopyrimidine moiety (Figure 17). Among them, G12Si-5 exhibited the best activity and selectivity in treating A549 cells (homozygous KRAS p. G12S mutation) with a Ki value of 2.5 μM, and preferentially inhibited the expression of KRAS (G12S) in transduced cells with an IC50 value of 2.4 μM.
Together, G12Si-5 is the first potent covalent inhibitor of KRAS (G12S) and expands the scope of serine-targeted electrophilic reagents.
4.1.7 Covalent inhibitors targeting serine residues in other biological targets
Avibactam (Figure 17), a covalent inhibitor of β-lactamase, was approved for the treatment of diseases associated with Gram-negative bacterial infections.243 It differs from existing inhibitors with a β-lactam backbone in that the carbonyl of avibactam's five-membered ring is nucleophilically attacked by the catalytic serine residue of β-lactamase, opening the ring to form a reversible covalent bond that results in inhibition of the enzyme activity. Furthermore, avibactam can be recovered by reverse reaction and therefore exhibits a long-lasting inhibitory effect.243 As inhibitors of β-lactams have the high ring strain of a four-membered lactam ring, no such reversible inhibitor regeneration mechanism is observed, and therefore the recovery of the avibactam structure may be related to its own five-membered ring structure, which may have a lower intrinsic strain than that of β-lactams. This mechanism of reversible acylation and less susceptibility to hydrolysis is the unique inhibitory mechanism of avibactam. While β-lactamases are traditionally recognized as antimicrobial targets, their direct connection to cancer treatment remains unclear. Nonetheless, it is worth noting that avibactam is a covalent inhibitor of β-lactamases, which expands the repertoire of examples for covalent inhibitors targeting serine residues. Consequently, this study merits inclusion in this review as a valuable addition to the understanding and exploration of covalent inhibitors, despite its primary focus on antimicrobial applications. PF-04457845 is an orally covalent inhibitor of FAAH (fatty acid amide hydrolase), which irreversibly inhibits FAAH by covalently modifying the serine nucleophile of the active site244 (Figure 17). Furthermore, there has been a recent suggestion that combining FAAH inhibition with PPAR activation could offer a novel and promising approach to cancer therapy.245 FAAH inhibition shows antiproliferative activity in colorectal,246 human colon adenocarcinoma,247 prostate,248 and lung cancer cell lines.249 This effect is mediated through PPAR, and FAAH inhibitors can directly act as PPAR agonists to achieve anticancer effects; therefore, the development of novel FAAH inhibitors has good prospects for cancer therapy. Orlistat is a covalent inhibitor of pancreatic and gastric lipase that covalently reacts with serine residues at the catalytic site to combat obesity (Figure 17).39, 250, 251 Furthermore, studies have shown that orlistat reduces the expression of GPX4, a central ferroptosis regulator, and induces lipid peroxidation, ultimately leading to ferroptosis-like cell death of lung cancer cells.252 This noteworthy research not only expands the therapeutic applications of orlistat but also uncovers a novel mechanism underlying its anticancer activity.
4.2 Covalent inhibitors targeting threonine residues
4.2.1 Covalent proteasome inhibitors
The proteasome is a large protein complex that regulates cellular functions by catalyzing the ATP-dependent degradation of cellular proteins.253 Covalent binding of threonine residues usually existed in proteasome inhibitors, where the accessibility and reactivity of N-terminal threonine residues are fully exploited. Bortezomib was the first proteasome inhibitor approved by the FDA, and its success was the inspiration for the discovery of other proteasome inhibitors, including carfilzomib (Figure 17).254, 255 Bortezomib and carfilzomib can covalently bind to threonine at the active site via boronic acid and epoxide ketone as electrophilic warheads, respectively.253 Marizomib (Figure 17) is also a proteasome inhibitor that is now in phase III clinical study.253, 256 The inhibitory activity of marizomib in a variety of cancer cells, including NCI-H226 nonsmall-cell lung cancer cells, SF-539CNS cancer cells, SK-MEL-28 melanoma cells, and MDA-MB-435 thymoma cancer cells with IC50 value below 10 nM.253, 255 Therefore, the development of covalent inhibitors targeting the proteasome may be a therapeutic strategy with great potential in cancer therapy.
4.3 Covalent probes targeting serine residues and tyrosine residues
Hett et al.169 developed an SF probe (SF-p1-yne) in 2015 and verified that the target of DcpS inhibitors is the tyrosine residue (see Section 3.1.4 above). Because of the high reactivity of SFs, there are challenges in selectivity. To address this limitation, the group developed the aryl-fluoro sulfate probe (FS-p1) (Figure 18) as a new covalent probe with fluoro sulfate as the electrophilic warhead in an attempt to develop more selective covalent inhibitors.257 FS-p1 selectively labeled noncatalytic Ser272 at the DcpS binding site with excellent chemical and metabolic stability. Furthermore, a series of SF and fluoro sulfate probes that can be used to explore and validate serine or threonine was developed, demonstrating the utility of these warheads as reactive electrophile reagents.133
5 COVALENT MODIFICATION OF ASPARTIC AND GLUTAMIC ACIDS
Both aspartic and glutamic acids contain carboxyl groups, which completely lose their protons to carboxylate anions under physiological pH conditions. Because of the low nucleophilicity of the carboxylate anion, covalent modification of the aspartic and glutamic acid side chains presents certain challenges. Since the reactivity of electrophiles must be improved to compensate for the lack of reactivity of amino acid residues, most of the focus in that direction is on developing active covalent probes on protein surfaces. Electrophilic warheads used to target such amino acid residues include tosylates,258 fluorosugars,259 diazo compounds,260, 261 and tetrazoles under photolytic conditions.262 Therefore, it is necessary to develop new strategies for selective covalent modification of these carboxylic groups at binding sites, and some covalent inhibitors and probes targeting aspartic and glutamic acids are introduced in the following.
5.1 Covalent inhibitors targeting glutamic acids
5.1.1 Covalent inhibitors targeting the PDE6δ–KRAS interaction
PDE6δ, a δ isoform of phosphodiesterase 6, plays a key role in maintaining the dynamic distribution of KRAS in cells.263 Thus, PDE6δ is a potential target to block oncogenic KRAS signaling.264 The prenyl binding pocket of PDE6δ showed no cysteine or lysine residues that could be a target.265, 266 Instead, Glu88 is located at the upper end of the binding site near the outlet and is possibly a suitable site for covalent modification with reactive groups in the ligand. Surprisingly, Waldmann and colleagues267 reported a covalent inhibitor targeting glutamic acids on PDE6δ. Mass spectrometry revealed that the ligand can covalently bind to PDE6 and that bis-sulfonamide can label glutamic acids, but with low labeling efficiency.267 As the isoxazolium salts (such as WRK) opens its ring to form a ketimine under basic conditions, which can react with carboxylic acids to form enamides and finally rearrange to a more stable enol ester, making them a potentially better electrophilic warhead (Figure 19B). Molecular-docking calculations showed that replacing the benzene ring of compound 118 with an N-methylisoxazol-2-ium ring could covalently bind to Glu88, and the introduction of a 5-methyl substituent could improve the stability of the covalent adduct and improve the labeling efficiency. Consequently, the covalent inhibitor of PDE6δ (compound 117) was finally identified using isoxazolium salts as electrophilic warheads after a series of discussions on the SAR and structural optimization by introducing stable substituents267 (Figure 19A). The isoxazolium salt moiety in compound 117 can open the ring in the presence of a base to form a ketenimine, which reacts with the carboxyl group to form a covalent bond with Glu88 (Figure 19C).268
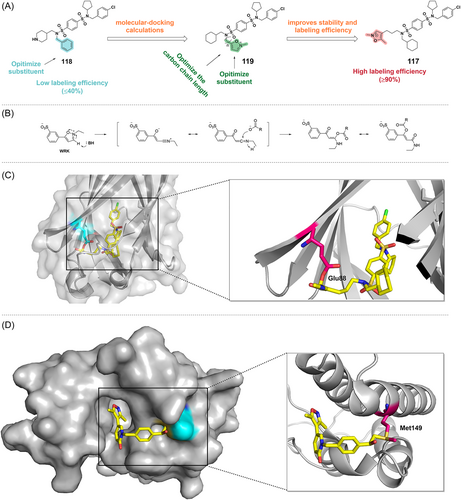
Despite the widespread presence of protein carboxyl groups, these ligands selectively target PDE6δ and are solely involved in the covalent modification of PDE6δ, GDPGP1, PSMG3, and PTGES2. In general, compound 117 can effectively inhibit the KRAS-PDE6δ interaction and thus suppress tumor growth driven by the KRAS oncogene. This new method of selective covalent labeling of less reactive amino acid residues may provide a new direction of thought for drug design studies. However, the stability of the WRK isoxazolium salts, incubation time and membrane permeability need to be further optimized in the scale-up of the method.
5.2 Covalent inhibitors targeting aspartic acids
In 2022, Uesugi's group reported a covalent inhibitor targeting aspartic acids.269 After conducting an activity-based proteomic screening on 1601 small compounds, they found an epoxide molecule (compound 120) with very high reactivity to glyceraldehyde-3-phosphate dehydrogenase probes (GAPDH). As the rate-limiting enzyme in glycolysis, GAPDH is considered a potential therapeutic target for cancer and other associated disorders.270 A detailed mechanistic analysis revealed that compound 120 inhibited GAPDH in a concentration-dependent way by competing with the enzyme cofactor NAD+ to create a covalent adduct with Asp35 at the active site.269 This study demonstrates a new class of epoxide-containing GAPDH inhibitors targeting aspartic acids, which further expands the scope of studies on the covalent targeting of low reactive nucleophilic amino acid residues.
Even though aspartic acid residues possess carboxyl side chains that are less nucleophilic and challenging to target, significant progress has been made in the development of small molecule inhibitors that can efficiently covalently modify these residues. Yu et al.271 reported the first novel dual covalent inhibitor capable of binding to KRAS (G12D) and KRAS (G12C) mutants (compound 121). In view of the previously reported feasibility of ethylene oxide for targeting aspartic acid residues269 and targeting the weakly nucleophilic amino acids of KRAS (G12R)272 and KRAS (G12S),242 the group used ethylene oxide as a reactive warhead that could modify both KRAS (G12D) and KRAS (G12C). And they were selectively targeted at different active sites, respectively. The Cys12 of KRAS (G12C) could selectively attack the position with smaller spatial site resistance of ethylene oxide, while the aspartic acid residue of KRAS (G12D) could selectively attack the position with larger spatial site resistance of ethylene oxide, which led to the effective covalent targeting of these two mutants. However, this inhibitor exhibit moderate activity in KRAS (G12D) mutant cells and further structural optimization is required. Overall, this strategy successfully developed the first covalent inhibitor targeting KRAS (G12D) and also demonstrated the feasibility of targeting weak nucleophilic residues, providing a basis for the development of covalent inhibitors for other residues such as Glu.
5.3 Covalent probes targeting aspartic and glutamic acids
McGregor et al.273 achieved a specific modification against oncogenic KRAS mutants (e.g., G12D) by expanding the types of amino acids targeted by covalent inhibitors. By optimizing and screening in a library of synthesized compounds, they discovered that aziridine and diazo compounds preferentially generate covalent adducts with carboxylates rather than thiols under physiological conditions (compounds 122–125), making them promising candidates for site-specific modification of Asp or Glu side chains in proteins.
6 COVALENT MODIFICATION OF METHIONINE RESIDUES
Although methionine also has an S-containing side chain, it is difficult to target because of its weak nucleophilicity and location in the core region.274 However, methionine may be targeted through a redox-based mechanism. Lin et al.275 develop a redox-activated chemical tagging (ReACT) method that enables the recognition and linkage of methionines in chemical proteomics. The method uses Oxaziridines (Ox) (compounds 126–129) as oxidizing agents to form sulfimide links (S = N), enabling highly selective, fast, and robust methionine labeling under a range of biocompatible reaction conditions.
In 2018, Kharenko's group276 first reported covalent inhibitors targeting methionine, ZEN-3219, ZEN-3862, and ZEN-3411 (Figure 18). They develop new bromodomain and extraterminal domain (BET) inhibitors by selectively targeting Met149 in the binding pocket of the BET protein BRD4 (BD1), providing a potential strategy for the design and development of antitumor drugs.276-278 They used the existing BET inhibitor compound 130 as a lead compound and showed by crystal structure that the dimethylisoxazole moiety of compound 130 forms hydrogen bonds with the amide nitrogen of Asn140 and water molecule-mediated hydrogen bonds with the hydroxyl group of Tyr97, respectively, thus participating in key interactions within the binding site. In addition, the epoxide has potential as a covalent warhead for targeting methionine due to its high reactivity towards methionine under physiological conditions. Therefore, they attached epoxide to compound 130 and designed a series of covalent inhibitors targeting methionine in the binding pocket. The inhibitors (ZEN-3219, ZEN-3862, and ZEN-3411) (Figure 18) were confirmed to selectively form covalent bonds with Met149 at the binding site of BRD4 (BD1) (Figure 19D). Covalent BET inhibitors have a prolonged duration of action and sustained target inhibition.
7 COVALENT MODIFICATION OF HISTIDINE RESIDUES
There are a few examples of covalent inhibitors that modify histidine, the majority of which have been characterized by accidental discovery. Based on the structure of fumagillin, a fungal metabolite, the researchers developed a small molecule compound called TNP-470 (Figure 18). The epoxide in TNP-470 serves as an electrophilic warhead that forms a covalent bond with His231 at the active site of methionyl aminopeptidase 2 (MetAP-2),279, 280 thereby inhibiting angiogenesis and tumor growth.281 Furthermore, Crews et al. reported the first covalent PROTAC (compound 136).282 This PROTAC consists of two structural domains, one containing an IκBα phosphopeptide recognized by the F-box protein β-TRCP, and the other consisting of ovalicin. Specifically, the ovalicin part covalently binds to His231 in methionine MetAP-2, thereby recruiting MetAP-2 to a specific class of E3 ligases, the heterotetrameric Skp1-Cullin-F box (SCF), and thereby promoting MetAP-2 ubiquitination and degradation. This research provides the first proof-of-concept study for targeting protein degradation and covalent PROTACs. As mentioned in Section 2.2 above, SFs have been found to covalently modify histidine residues in addition to targeting lysine residues, as well as acting as irreversible covalent inhibitors and reactive probes. By quickly localizing the reaction site of small molecule compounds with 5-phosphoribosyl-alpha-1-pyrophosphate (PRPP) synthase, Harlow and Switzer et al. discovered that 5′-fluorosulfonylbenzoyl adenosine (FSBA) (Figure 10) could covalently bind to PRPP synthase at His130.138 Furthermore, Bullough and colleagues283 investigated the reactivity of FSBA with bovine mitochondrial F1-ATPase and found that FSBA predominantly modified Tyr368 at pH = 8.0, while at pH = 6.0, FSBA reacted with His247. In 2020, Li et al.284 developed covalent protein drugs using proximity-enabled reactive therapeutics (PERx) (Figure 20A). In this study, the potentially bioreactive amino acid fluorosulfate-l-tyrosine (FSY) was incorporated into the programmed death receptor-1 (PD-1). When PD-1 interacts with the programmed death receptor ligand-1 (PD-L1) in tumor cells, FSY selectively reacts with the proximal histidine of PD-L1 (Figure 20B), resulting in irreversible binding of PD-1 (FSY) to PD-L1 both in vitro and in vivo with minimal off-target effects and blocking the interaction between PD-1 and PD-L1. Furthermore, covalent PD-1 (FSY) exhibited significantly stronger antitumor effects compared to wild-type PD-1 when administered to immunized humanized mice. This nonantibody therapy achieves efficacy equal to or better than anti-PD-L1 antibodies and has manufacturing advantages over antibodies.
Recently, Cruite et al.285 developed a rational CRBN inhibitor using sulfonyl exchange chemistry in the binding site for the first time to rationally target histidine residues in the binding pocket of immunomodulatory drugs (IMiD). Using structure-based drug design, they incorporated the SF and triazole reaction groups into the isoindolinone thalidomide homolog EM12 to obtain EM12-SO2F, a powerful covalent inhibitor of CRBN through binding at His353 (Figure 18). The fluorosulfate derivative EM12-FS is also covalently labeled with His353 and degrades the protein N-terminal glutamine amide hydrolase NTAQ1, which is essential for the N-terminal rule pathway and the DNA damage reaction. EM12-SO2F and EM12-FS are useful chemical probes to investigate the CRBN, NTAQ1, and Arg/N-terminal rule pathways, respectively. Modifying IMiDs was also shown to possibly tune novel substrates of CRBN and find new molecular gels.
Yoshizawa's group286 designed and synthesized vitamin D analogs that act as covalent modifiers of the vitamin D receptor (VDR). These novel vitamin D analogues 141–144 use the enone groups as electrophilic warheads and compounds 141–143 can covalently bind to His301 in the ligand binding domain (LBD) of VDR (Figure 20C–F). Although the covalent modifier showed agonistic rather than inhibitory activity, this study still exhibits the usefulness of histidine as a covalent modification target.
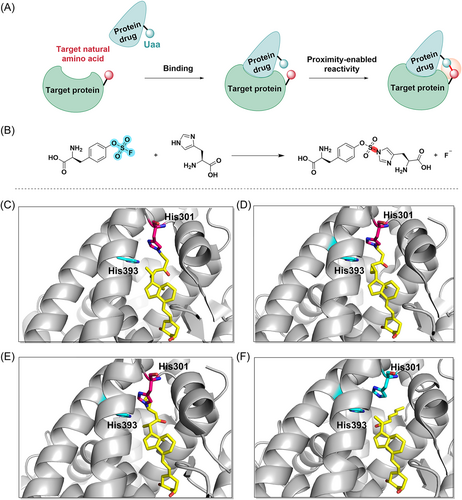
Gambini et al.110 inserted aryl-fluoro sulfate or aryl-SF as warheads into the binding peptides to target lysine, tyrosine, and histidine residues at the binding site, which provides some feasible strategies for the design and development of new covalent PPI inhibitors.
8 COVALENT MODIFICATION OF PROLINE RESIDUES
Crichlow et al.287 reported that the widely used serine protease inhibitor phenylmethylsulfonyl fluoride (PMSF) (Figure 18) forms a novel, stable nitrogen-sulfur bond with macrophage migration inhibitory factor (MIF). Structural analysis showed that PMSF covalently bonds to the catalytically active N-terminal proline, preventing MIF from performing its function. MIF is a proinflammatory cytokine that counteracts the anti-inflammatory activity of glucocorticoids288 and is also a potential target for the development of therapies for autoimmune diseases, cancer, and cardiovascular diseases.289-291
9 COVALENT DRUG DESIGN AND DISCOVERY PROCESS
Identifying and validating one (or more) biological targets is the first step in drug discovery. Following that is the optimization of a lead compound identified from the identification process. Many of the methods used in covalent drug discovery are similar to those used in traditional medicinal chemistry; examples include virtual screening and rational drug design. However, in the rational design of covalent drugs (especially covalent inhibitors), many places are unique (e.g., target selection, identification of appropriate warheads, and target residues). Therefore, the design of general covalent drugs must go through four processes8, 24 (Figure 21): target identification and selecting the residue; hit identification; binding characterization, and further optimization.
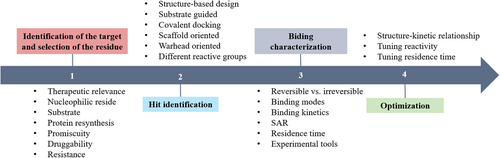
9.1 Identification of the target and selection of the residue
Identifying and characterizing potential therapeutic targets is often the first step in drug discovery. Target identification is the process of discovering specific molecular targets that small molecules will directly interact with, such as proteins or nucleic acids, employing methods that may be based on genetics, biophysics, chemical biology, or other principles. Generally, the disease target should be used as a guiding principle in drug design, and it is important to determine the strength of the disease-target association before initiating any drug discovery work. Prospectively searching for targets that can be covalently targeted is another strategy.
9.1.1 Methods in target identification
Currently, most studies are performed by ABPP using reactive covalent fragments to identify possible targets for covalent action. In addition, virtual screening is a useful tool in the target identification process. By adding ligand reactivity towards cysteine residues into AutoDock4, Scarpino et al. carried out a virtual screen utilizing WIDOCK, a reactive coupling protocol, to enable the selection of numerous electrophiles as covalent inhibitors.292 With high sensitivity and high binding efficiency in binding to active covalent fragments, this research shows the value of WIDOCK as a warhead-sensitive covalent virtual screening scheme.292, 293 However, there are several covalent virtual screening algorithms available, each with its own advantages and disadvantages, and appropriate choices need to be made when using them. In the search for protein kinases suitable for covalent modification, Cravatt's group investigated a series of electrophilic reactive fragments of covalently modified proteins using isotope tandem orthogonal proteolysis activity-based protein profiling (isoTOP-ABPP).293 The isoTOP-ABPP platform couples iodoacetamide-probe labeled lysates to isotopically differentiated azide-biotin tags, enabling selective enrichment, release and relative quantification by LC-MS of IA-labeled peptides from two samples.294 By using alkyne chemical probes, reactive amino acids and novel targets within the proteome can be labeled and identified. To identify covalently modified sites quickly and accurately, Browne et al. developed the covalent inhibitor target site identification (CITe-Id) platform using more complicated irreversible inhibitors in 2019.295 The platform allows for deeper coverage of cysteines modified by pharmacological CKI, as well as confirmation of covalent interactions and direct quantification of cysteine-thiol binding in the proteome. Using this method, protein kinase N3 (PKN3) was identified to be a novel target for covalent inhibition in prostate cancer. Furthermore, Zhang et al.296 detected covalent binding sites using the FTMap method and distance-based covalent fragment probing, and analysis of hydrogen bond interaction also guides covalent verification and identification. FTMap allows global sampling of small organic molecules in the form of probes on the surface of target proteins, finding favorable positions, clustering conformations and ranking the clusters (also known as “hot spots”) according to their average energy. It is very helpful and precise in determining the binding locations of user-selected compounds and determining if a compound is likely to bind in the hot spot region.297 Furthermore, several new “druggable” targets can be identified, such as cathepsin K (catK), which has also been discovered in the context of covalent inhibition as an emerging target for the treatment of osteoporosis.298, 299
9.1.2 Validation of drug targets
The term “validation of a drug target” refers to the scientific demonstration that the target (usually a protein) is regulated (typically inhibited) and has therapeutic utility. Target verification can be chemical or genetic verification, with the former meaning that therapeutic effect can be achieved through the regulation of target proteins by chemicals and the latter meaning that therapeutic effect can be produced by genetic techniques such as animal gene knockout or human gene mutation. These two validation methods are complementary. Additionally, genomic, transcriptomics, proteomics, and metabolomics-based technologies will provide a more detailed and comprehensive analysis as new target validation tools for drug discovery.300 For example, genome-wide association studies (GWAS) can identify frequently occurring genetic variants and targets with genetic associations have a high probability of success in clinical development.301 Existing computational techniques can indicate potential binding pockets in protein structures and also provide the basis for target selection and validation.302
9.1.3 Selection of the right residues
Covalent action can allow targets located at shallow binding sites to be targeted more effectively; it can also make certain undruggable targets modifiable.91 The half-life and physiological processes of a protein can determine whether the protein is suitable for covalent targeting,268 but it may also be the case that multiple residues are available for covalent modification in the target protein. Therefore, the rational selection of nucleophilic residues is particularly important. According to Hard–Soft–acid–base (HSAB) theory, nucleophile-electrophiles of different hardnesses are less reactive and have larger potential energy barriers because of their insufficient orbital overlap. Therefore, when designing covalent molecules, the reactivity of the warhead and residues must be carefully taken into account. Descriptors of the reactivity of covalent warheads and residues are important for predicting the rate and spontaneity of covalent modification of amino acids by inhibitors.303 The first step is to select the most reactive functional groups, which must be sufficiently reactive with the target to spontaneously form covalent bonds with amino acids. Second, amino acids within proteins differ in their reactivity due to electrostatic interactions, solvation and the degree of accessibility to the inhibitor warhead. It is therefore important to predict their descriptors of reactivity. The prediction of reactivity for covalent warheads is usually described by conventional physical organic parameters such as the Hammett parameter (σind or σmeta), which is obtained by modeling.304, 305 In addition, the reactivity of electrophiles towards nucleophiles can be described by calculated electronic structure properties such as the Fukui function and the local electrophilicity index. Specifically, the greater the positive value of the Fukui function at the position on the surface of the molecule, the more susceptible it is to attack by the nucleophiles.303 Furthermore, direct calculation of the kinetic energy of a reaction can be used to estimate the stability of species on the reaction profile by calculating the electronic structure of the species on the reaction profile. The stability of these species can be used as a descriptor for analogous species in a covalent modification reaction within a protein target. In general, the first step in covalent modification is the deprotonation of nucleophilic amino acid side chains in proteins, and the tendency of ionizable residues to be deprotonated in this way can be expressed in terms of pKa, which can be used to assess the nucleophilicity of amino acid residues as well as the stability of the deprotonated state.306 In addition, the orientation and accessibility of the amino acid side chain and the proximity to the binding site for small molecule inhibitors within the protein are also important factors in the selection of amino acid residues. However, single descriptors are less accurate predictors of cellular inhibition and the most immediate limitation to the development of more complete and accurate descriptors is the limited amount of data from certain quantitative experiments and the difficulty of validation. Therefore, predictions of covalent inhibitory activity require rigorous physically based models of the complete kinetics of binding and covalent bond formation. The hardness and softness of electrophiles and nucleophiles have not been quantified on a systematic scale, but several computational and quantum mechanical algorithms have been used to derive the nucleophilic (ω-) index based on hardness (η) or the electrophilic (ω) index based on softness (δ). The measurements of these metrics reflect electrophiles' propensity to form covalent adducts with nucleophiles, making them useful tools for selecting electrophile–nucleophile pairs. Furthermore, this pairing not only helps reduce the undesirable side effects, of off-target effects but also increases the potential for covalent modification of target proteins.24, 184, 307 Furthermore, the position of the residue is crucial; it must be in or near a pocket where the inhibitor might bind, and some computational techniques can indicate potential binding sites in the protein structure. However, recent studies in 2022 have discovered covalent inhibitors that can target distal (>10 Å) cysteine, further expanding the selection of nucleophilic amino acid residues.308 Developing a covalent inhibitor requires careful treatment and selection of several biological targets that have similar biological responses and toxicity. Furthermore, biological targets that are compatible with specific covalent mechanisms must be considered to avoid potential toxicity, while also achieving high selectivity to design safe and effective covalent inhibitors.8
9.2 Hit identification
Hit identification is the discovery of compounds that have the desired activity against a fully validated target. In this critical step, the identified small molecule will be shown to bind to a target (usually a protein), thereby altering its function. High-quality initiation hits make the drug discovery process more efficient, intending to obtain more selective covalent ligands.8 Hit identification methods generally include X-ray crystallography, mass spectrometry, virtual screening of structures, ligand-based virtual screening, pharmacophore-based screening, shape-based screening, similarity-based screening, covalent docking procedures, high-throughput screening, virtual screening and structure-based drug design, molecular fragment-based ligand discovery strategies, strategies to expand the size of covalent fragment libraries, nucleic acid-encoded libraries, CADD.1, 8, 296, 309-313
9.3 Binding characterization
Generally, the binding process between the covalent inhibitor and the target protein is divided into two steps (Figure 22). The first step is the reversible noncovalent binding of the inhibitor to the target, which makes the active functional group on the weak electrophilic ligand close to the specific nucleophilic residue on the protein. The second step is the reaction of the ligand with the nucleophilic amino acid residue involved in the protein to form a covalent bond, producing the inhibited complex.8 Depending on the various types of chemical reactions involved in the formation of covalent complexes, covalent inhibition can be subdivided into reversible and irreversible inhibition (Figure 22A,B). For example, the formation of hemiacetal or disulfides is a reversible reaction, and ketones, nitriles, boronic acids are commonly used as electrophilic warheads (Figure 22F). While other reactions that irreversibly bind inhibitors to target proteins such as alkylation, Michael addition reactions,314 these processes frequently employ electrophilic warheads such as epoxides, aziridines, halomethyl ketones, Michael acceptors, SFs314, 315 (Figure 22E). Given sufficient reaction time, irreversible covalent inhibitors will achieve complete and permanent inhibition of the target protein, providing the possibility of significantly prolonging drug efficacy when the pharmacokinetic half-life of the inhibitor is longer than the resynthesis rate of protein.316
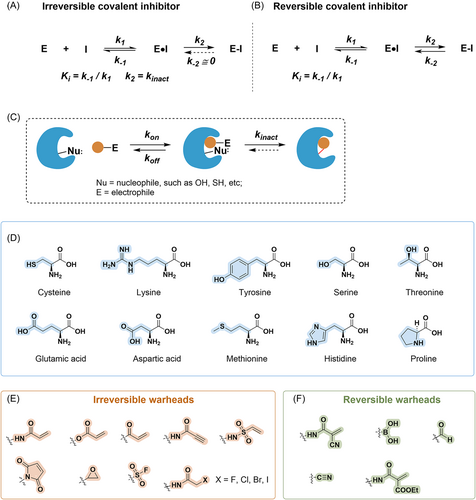
Characterization of inhibitors is a critical step in the drug discovery process. Since there are numerous characterization parameters to be selected, it is important to determine which parameters are evaluated. During drug discovery and design, affinity or dissociation constants (Ki, Kd, or IC50) are commonly used to characterize potential inhibitors.8 Ki, the dissociation constant, is typically defined as the ratio of the microscopic rate constants for the first step forward (k1) and reverse (k−1) binding processes. In this case, kon is equal to the forward rate constant (k1) and koff is equal to the reverse rate constant (k−1). And Kd is defined as the ratio of koff to kon (koff/kon).
The efficacy of the drug is usually directly related to koff, while there is little correlation with kon.24, 317 Typically, a tighter binary complex is obtained by this second step, which is slower than the first step. The second equilibrium kinetics is also defined as Ki* (k2/k−2). In irreversible covalent inhibition, k−2 is called as kinact since k−2 is near zero. Furthermore, IC50 values may be useful for ranking compounds with potency in a structural series, but since measured IC50 values are highly dependent on experimental conditions, the kinact/Ki ratio is an appropriate indicator of the potency of TCI. The kinact/Ki ratio, which is determined by calculating the total occupancy of the target over time at different inhibitor concentrations and is correlated with the free exposure of the targeted covalent inhibitor, provides a more accurate evaluation of the drug efficacy in vitro and in vivo.91 However, it is important to note that even if the protein target is irreversibly inhibited, the activity of the enzyme or receptor will be restored upon resynthesis once the unbound drug is cleared from the body. After the removal of small-molecule compounds, the time required to recover target protein activity depends on the residence time of the inhibitor and the rate of protein resynthesis.91 Therefore, explicit consideration of the time dependence of inhibition is essential to assess the absolute or relative activity of covalent inhibitors. In recent years, the inhibition kinetics of inhibitors, especially the target residence time, have also received increasing attention.
Residence time (Tr) is the reciprocal of the dissociation rate constant (Tr = 1/koff), which indicates the average time it takes for the ligand to remain bound at the binding site.92 Residence time has a large impact on the pharmacodynamics (PD) of drug candidates; the longer the residence time, the longer the compound will inhibit the target. For example, optimizing the interaction between the covalent inhibitor and the binding site can affect the residence time of the inhibitor, which usually results in a decrease in the dissociation rate, thus increasing the affinity constant and the intensity of the inhibitory effect on the target. This is the case for reversible inhibitors. In contrast, for irreversible inhibitors, a longer residence time will be achieved with sufficiently long exposure, and complete inhibition will be achieved regardless of any differences in the rate of mutation-induced formation.
The main methods for the kinetic characterization of covalent inhibitors are time-resolved fluorescence resonance energy transfer technology (TR-FRET), in vitro bioactivity recording, surface plasmon resonance (SPR) technology,318 mass spectrometry (Table 1).
| Method | Measured parameter | Throughput |
|---|---|---|
| Measurement of enzyme activity | IC50 | High to very high |
| In vitro drug washout experiments | Inhibition reversibility; koff | Low |
| In cellular drug washout experiments | Occupancy reversibility Time-dependent recovery of enzyme activity |
Low |
| Mass spectrometry | Modification site and stoichiometry | Low |
| Analysis of activity progress curves | kinact, Ki (or kinact/Ki) | Low |
| Nucleophilic residue mutant enzyme activity | IC50 | Low |
| Analogues of nonreactive inhibitors | IC50 | High |
To have higher biological effects, covalent drugs must undergo rigorous verification. For the validation of the binding mode, the structure of selected enzyme–inhibitor complexes can be determined by whole protein spectroscopy and X-ray crystallography.1 The inhibitory activity of the compounds can be measured by cell-free in vitro assays, cellular assays and in vivo animal experiments. Computer-aided covalent drug design can also be used to guide the covalent process. For example, molecular mechanics (MM) based simulations allow the evaluation of molecular conformations and binding modes. Due to chemical bonding, researchers frequently utilize quantum chemistry to model the mechanisms, dynamics, and structures associated with covalent modifications.320 Density functional theory (DFT) is extensively employed for modeling biological systems due to its capability to describe quantitatively accurate energies and structures in large chemical systems.321 The use of quantum mechanical (QM)/MM methods allows the simulation of chemical reactions and also the calculation of transition state stabilization energies of residues around the active site, allowing the further identification of electrophilic residues that covalently interact with the inhibitor.322, 323 The mixed QM/MM potentials provide a more complete description of covalent ligand binding. Furthermore, free energy perturbation (FEP) methods can calculate the calculation of relative binding affinities of novel compounds to protein binding pockets.324 An example is the mechanism of aspirin covalently modifying cyclooxygenase Ser530 by QM/MM modeling.325 The researchers constructed a QM/MM model in which aspirin and nearby amino acid residues in the enzyme active site were modeled using a semiempirical SCC-DFTB QM method, and the equilibrium of the system was modeled using a CHARMM force field. Finally, the mechanism of irreversible inhibition of cyclooxygenase by aspirin was revealed. In addition, a combination of molecular dynamics simulation methods and advanced QM/MM calculations have been studied to simulate all the steps involved in the covalent modification of Cys481 in BTK.326 They calculated the rigorous, complete free energy profile of chemical reactions, and this maturing computer modeling will help in the development of new covalent modifier drugs. However, in order for this method to more accurately understand and predict the mechanism of covalent modification, it is necessary to develop more accurate QM methods, improved algorithms to interface the QM and MM regions, and a broader method of configuration sampling.321 Additionally, any comprehensive validation process must include chemo proteomics analysis and cell-based target validation experiments, such as the use of gene knockdown and target overexpression to achieve confirmation of the target of action.1 To further determine the biological activity and selectivity of covalent inhibitors in vivo, a key approach to validate covalent ligands is to use cellular assays with mutation of key residues, which allows for the demonstration of targets and mechanisms compatible with the specific phenotypes induced by covalent inhibitors.
9.4 Optimization
9.4.1 The relationship between drug structure and activity
The structure of a covalent inhibitor affects its binding kinetics. Based on the SAR relationship and binding kinetics data, it can be specifically optimized for the interacting target to produce the desired biological effect. The correlation of ligand structure and intrinsic warhead reactivity with the stability of protein-ligand complex and residence time demonstrates new possibilities for fine-tuning covalent inhibition based on established modalities. Furthermore, the potency and selectivity of the inhibitor can be modulated by tuning k1 of the compound to optimize Ki,91 and also by monitoring the increase in k2 to reflect.
9.4.2 Optimizing warhead reactivity
Many weakly nucleophilic (e.g., unactivated serine or threonine) or electrophilic (e.g., tryptophan) amino acid residues are difficult to target efficiently and reliably by current electrophilic warheads, so it is necessary to expand the scope of research of ligandable residues. Electrophilic warheads can be nonspecifically bound to their targets, indicating one of the major risks of covalent inhibitors.91, 327 Covalent warheads can cause unpredictable toxic reactions if they bind to residues in nontarget proteins. Furthermore, like noncovalent compounds, some covalent drugs tend to generate reactive intermediates as a by-product of metabolism (such as paracetamol), which can result in DNA or protein damage. To avoid this limitation, less reactive covalent warheads should be preferred.268 When the reactivity of covalent warheads does not meet the appropriate reaction range, structural changes around the warhead or modification to the warhead itself can be used to adjust it. However, the more mature warheads that are widely used also have many unsolved issues, although they have shown advantages in targeting and selectivity. For example, the toxic potential of leaving groups in SFs, aryl-fluoro sulfates, or activated esters has not been studied recently, and metabolites of the drug can also cause a variety of toxic or allergic reactions.39 Furthermore, phenols and other alcohols eliminated from activated esters may have unique biological activities or toxicity. The nature of the warhead affects the general reactivity of the covalent ligand and has an impact on its selectivity, potency, tissue distribution, cellular localization, and metabolic stability. Therefore, the development of new warheads remains a direction to be explored.
Optimizing the reactivity of a covalent inhibitor warhead requires a balance between maximizing the reaction rate with the nucleophilic amino acid residues selected on the target protein and minimizing the reaction rate with nontarget nucleophilic residues. A common approach to achieving minimal reaction rates with nontarget residues is an in vitro benchmarking strategy using protein substitutes (e.g., GSH) to assess the intrinsic chemical reactivity of the warhead with thiols by LC-MS/MS or NMR techniques.91, 328, 329 Most recent studies on modulating warhead reactivity have focused on acrylamide, but it has only been used for targeting Cys and Lys. In addition, it has been suggested that N-hydroxysuccinimide esters (NHS-esters) may be widely used because of their ability to react with Cys, Lys, Ser, Tyr, Thr, and Arg residues.24 In addition, Byun330 as well as Galbiati et al.331 have reported a unique natural electrophile, 3-bromo-4,5-dihydroisoxazole (BDHI), a novel warhead that irreversibly covalently binds to catalytic Cys. BDHIs are capable of selectively engaging reactive cysteine residues in the human proteome and the selectivity landscape of cysteines liganded by BDHI is different from that of the haloacetamide electrophiles. This study further expands the covalent fragment library.
There are also reports of substituent effects for newer warheads such as aryl halides, which can be used to achieve the required reactivity of the warhead relying on steric or electronic substituents (EDG or EWG).332-334 In addition, some studies have used terminal alkyne groups, a potential class of electrophiles, as electrophilic warheads that can minimize off-target reactivity.1 In addition, a new library of ligands consisting of various carbon-based nucleophilic reagent fragments that selectively react with cysteine sulfinic acid formed in proteins via oxidation or hydrolysis reactions has also been reported.335 It is also possible to investigate the effects of warhead exchange through computational methods in addition to substituent effects. The matched molecule pair analysis (MMPA) analyzes s to determine how different electrophilic warheads affect scaffolds.336 In this method, warhead correction coefficients can be calculated between compounds containing similar noncovalently bound components but with different warheads, which is useful for estimating reactivity-specific parameters when different warheads are switched. Furthermore, Albeck et al.337 describe a new CADD tool, an enzyme mechanism-based method (EMBM), that allows the filtering of inhibitor warheads by activity and subsequent virtual screening by computer-aided molecular libraries to select out effective covalent inhibitors. The EMBMs derive covalent descriptors that distinguish the reactivity of covalent compounds toward serine and cysteine hydrolases and account for the energetic contribution of the covalent bonds formed in enzyme–inhibitor complexes.
9.4.3 Optimizing residence time
In recent decades, inhibitors based on structural design have become increasingly popular with the continuous development of structural biology. This approach allows medicinal chemists to visualize possible structural modifications of the ligand to optimize its interactions with target biomolecules. Optimization of residence time needs to be based on the properties of biochemical reactions. The long residence time of the covalent drugs may lead to favorable pharmacokinetics (PK) and more effective target inhibition, which may reduce drug dosage, reduce dosing frequency, and achieve a long-term effect. However, the longer residence time is not preferable since it will result in persistent inhibition of the target and may cause severe toxic side effects if off-target. To achieve the required level, it is also essential to optimize the residence time of the drug's binding kinetics, including PK, PD, and other characteristics. Moreover, Bradshaw and colleagues338 devised a new method for tuning residence time by manipulating the warhead's electronic environment and spatial configuration, they have therefore rationalized the design of novel and tunable electrophiles whose residence time can be varied extensively to change potency (Supporting Information: Figure S1). In addition, CADD can be used to rank the relative electrophilic reactivity, residence time, and covalent bonding energy of covalent ligands as well as to score and rank kinetic properties, and thus, predict more trends of covalent ligands. Docking algorithms are employed to rapidly screen extensive compound databases for their ability to bind proteins or nucleic acids that are intended to be inhibited.321, 339, 340 Common algorithms include CovDock, CovDock-VS, MacDOCK, FITTED, DOCKovalent, DOCKTITE and Two-point attractor/flexible side chain.341-346 Different covalent docking algorithms have different advantages and are described in detail in Awoonor-Williams et al.321
9.4.4 Optimizing selectivity
To reduce the risk of off-target toxicity, TCI requires high selectivity. Although the absolute specificity of TCI may be difficult to achieve, optimizing this property is essential in the design of covalent inhibitors. As previously described, covalent inhibitors have two drivers to achieve selectivity for protein targets: Ki in the first step and K2 in the second step.83 To achieve high selectivity, the noncovalent binding (Ki) of the inhibitor must be high enough to ensure that the compound selectively approaches the desired target and reaches a residence time sufficient for a covalent reaction. Selective formation of noncovalent complexes of the initial inhibitor-target protein is a key factor in achieving effective and selective covalent inhibition of conserved residues. Additionally, the reaction rate K2 of the inhibitor must be high enough to allow the reaction to occur over the lifetime of the noncovalent complex formed in the first step.
Highly reactive electrophiles must also be avoided during the reaction to achieve high selectivity for target reactions. However, even with an electrophile with low intrinsic reactivity, achieving selectivity is a challenge if the compound targets nucleophilic residues that are highly conserved throughout the protein family. An efficient method to assess the selectivity of covalent kinase inhibitors across the proteome was proposed by Lanning et al.,347 who combined ABPP and quantitative mass spectrometry to globally localize specific and nonspecific targets of covalent kinase inhibitors in human cells to achieve selectivity in kinase inhibition.
10 SUMMARY AND FUTURE PERSPECTIVES
In recent years, the application of targeted covalent inhibitors in drug discovery has increased rapidly. The development of structural bioinformatics, chemical proteomics, crystallography, and computational methods146, 268, 348 has allowed the development of highly selective covalent inhibitors targeting specific residues. Despite considerable progress in the development of covalent inhibitors, most TCIs still rely heavily on the proper localization of cysteine residues at or near the small molecule binding site, but this strategy does not apply to all protein targets. Indeed, other residues such as lysine, serine, and tyrosine are increasingly being considered suitable residues for covalent targeting in covalent drug design. Even residues that are weakly reactive or form unstable covalent complexes, such as aspartic acid, glutamic acid, and histidine, have received continued attention in the recent wave of covalent drugs. As we have seen, a variety of proteins can be made more susceptible to covalent modification by inhibitors by taking into consideration the chemical environment of the residues and the structure and reactivity of electrophilic warheads. This paper highlights the research advances and design strategies for noncysteine-targeted covalent inhibitors in the hope of expanding the diversity of drug design approaches.
Covalent inhibitors have many attractive features. For example, covalent interactions can enhance the efficacy of drug-target binding; the long residence times of covalent inhibitors may lead to favorable PK properties and potentially to slower metabolism and prolonged clearance of the drug in vivo. Additionally, covalent interactions prevent enzyme inhibitors from being displaced by natural substrates to achieve long-term efficacy, thus potentially reducing the frequency of dosing. Furthermore, covalent inhibitors are powerful tools for combating drug resistance. In the presence of covalent inhibitors, a whole new class of “drugable” targets has been developed that can potentially address targets with shallow, undruggable binding sites, such as those involved in protein–protein interactions.
Although covalent inhibitors have many attractive features, covalent inhibitors are still widely valued for potential nonspecific reactions, toxicity, off-target effects, and other issues. One of the main risks of targeted covalent inhibitor research is achieving the target selectivity of the reactive warhead, where unpredictable toxic events may occur if the covalent inhibitors bind to residues in nontarget proteins. Although sustained occupancy of the target can produce beneficial biological effects, a longer residence time can also lead to toxicity. Therefore, covalent inhibitors may not be suitable for mechanisms that require a short residence time and transient or partial inhibition. Furthermore, some covalent warheads tend to generate reactive intermediates as a by-product of metabolism that can result in DNA or protein damage, ultimately causing specific allergic reactions and toxic side effects.8 To overcome this challenge, the structure and reactivity of the electrophilic warhead should be rationally selected. Although electrophilic warheads that can target weakly reactive residues (e.g., lysine and tyrosine) have emerged, many problems remain.184 For example, vinyl sulfone and SF possess high intrinsic reactivity, SF has limited stability to hydrolysis, and activated esters are sensitive to metabolic inactivation. Therefore, there is an urgent need to develop new electrophilic warheads that can reliably and precisely modify the target residues without cross-reacting with other residues.
However, as the field of research matures, experimental and computational methods are available to assess the effects of warhead structure and reactivity on the selectivity, efficacy, and toxicity profiles of TCI.305, 349-351 As a result of problems with toxicity related to the permanent inhibition of cellular proteins, reversible covalent inhibitors have emerged as versatile alternatives with broad applications. They allow this specific molecule to be safely covalently targeted to the binding site through reasonable steric hindrance and broad reversibility, with the potential to achieve tunable subtype-selective reactivity while reducing unwanted off-target effects. In addition, reversible covalent PROTAC tends to be more prevalent in targeted protein degradation, as this process allows for the release of PROTAC molecules again for the next round of protein degradation. Reversible covalent chemistry combines the advantages of covalent binding and catalytic target turnover with higher cellular uptake, higher selectivity, and the possibility of better transport and delivery. Additionally, pharmacological properties and proteome selectivity can also be optimized by applying SuFEx, modulating warhead electrophilicity, and balancing binding interactions, while also maintaining high pharmacokinetic properties such as chemical and metabolic stability.109, 132, 179 Moreover, accurate scoring of different warhead classes and nucleophiles is vital in developing covalent inhibitors, allowing for the prediction of additional trends in covalent ligands. In addition, “reverse drug discovery” strategies are effective in discovering selective covalent probes of many human proteomes, which can link chemical structures not yet used in pharmaceutical chemistry to new or important protein targets.181 Although targeting cysteine residues in proteins represents a reasonable alternative for the development of covalent inhibitors, the lack of such residues shows limitations in the development of covalent inhibitors. Therefore, covalent targeting of noncysteine residues such as lysine, tyrosine, and serine would allow a real expansion of the range of proteins that can be targeted for covalent inhibition.
The design of targeted covalent inhibitors is a very promising field. Covalent inhibitors are becoming more diverse and numerous, and the field of knowledge related to their discovery, design, evaluation, and validation is continually advancing and improving. Many strategies involved in the covalent drug design process, such as chemical proteomics, computational chemistry techniques, and other biochemical analytical methods, can have a crucial influence on the design of covalent inhibitors. Although the design of covalent inhibitors remains a great challenge in terms of drug selectivity and specificity, with the rational selection of electrophilic residues and covalent ligands, it is reasonable to believe that targeted covalent inhibitors will also be used in a wider range of fields with the cross-cooperation of protein structural chemistry and computer science and other disciplines. For example, covalent inhibition strategies targeting distal cysteine residues (>10 Å) have been proposed, opening new avenues for designing and developing covalent inhibitors. And the use of covalent PROTACs, covalent stapled peptides, and other covalent strategies to target proteins that are difficult to target for the drug is on the rise and is showing promising results. However, covalent inhibition is not only limited to cysteine residues; covalent targeting of noncatalytic residues other than cysteine also holds good promise. The rational development of covalent inhibitors will continue to be a direction of interest for researchers.
AUTHOR CONTRIBUTIONS
Guan Wang and Qiu Sun conceived the project and supervised the project. Lang Zheng and Yang Li summed up the literature, drafted the manuscript and drew the figures. Qiu Sun, Defa Wu and Huan Xiao collected and organized the inhibitors. Lang Zheng and Yang Li proofread the structures and figures. Zhengshi Long reviewed and editing the revision. Guan Wang and Qiu Sun revised the manuscript. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (Grant 22377085, 22177083, China), Natural Science Foundation of Sichuan Province (Grants 2023NSFSC1839, 2022NSFSC1290), the Sichuan University Postdoctoral Interdisciplinary Innovation Fund (JCXK2221, China), the Sichuan Science and Technology Program (2023NSFSC1688, China), the Full-time Postdoctoral Research and Development Fund of West China Hospital, Sichuan University (2023HXBH057, China).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The authors have nothing to report.
Open Research
DATA AVAILABILITY STATEMENT
The authors have nothing to report.