Gasdermin E plasmid DNA/indocyanine green coloaded hybrid nanoparticles with spatiotemporal controllability to induce pyroptosis for colon cancer treatment
Ailing Jiang and Mao Wang contributed equally to this study.
Abstract
Pyroptosis is an immunogenic cell death and would trigger robust antitumor immunity. However, due to cytoxicity of pyroptosis executors, Gasdermin family proteins, it is indispensable to construct tumor-specific vectors. Here, we report the development of a novel vector named lipid-coated poly(lactide-co-glycolide) (PLGA) nanoparticles coloaded with Gasdermin E expressing plasmid DNA (GSDME-pDNA) with a heat-inducible mouse heat shock protein 70 (mHSP70) as the promoter and a photosensitizer indocyanine green (ICG) to activate the mHSP70 element. The cellular internalization and transfection rate of the vector were remarkably enhanced by photothermal treatment. And the mHSP70 promoter further improved the gene transfection rate for about 15-fold. With the combination of oxaliplatin (OXA), the mechanism switch between apoptosis and pyroptosis was fulfilled by cleavage of GSDME through activated caspase-3, which promoted damage-associated molecular patterns (DAMPs) release and increased the infiltration of immune cells at the tumor site. This combination strategy not only prominently inhibited the growth of the treated tumor, but also exhibited a lethal effect on the distal tumors. Besides, mouse colon cancer cell CT26 overexpressing GSDME after OXA treatment had the potential to be the preventive tumor vaccine. This study provides a novel thought and feasible method for the clinical treatment of colon cancer.
Graphical Abstract
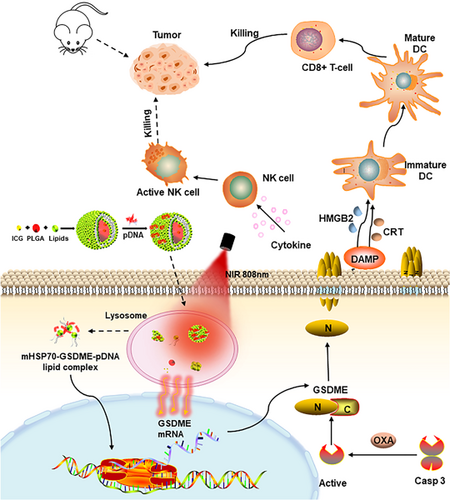
We develop lipid-coated poly(lactide-co-glycolide) nanoparticles coloaded with indocyanine green and Gasdermin E expressing plasmid DNA (GSDME-pDNA) to possess the spatiotemporal controllability of target gene expression induced by a heat-inducible mHSP70 promoter. Oxaliplatin is administered to activate caspase-3 to cleave GSDME to cause pyroptosis, which promotes the release of damage-associated molecular patterns molecules and causes an immunogenic inflammatory response and attendant strong antitumor effect.
1 INTRODUCTION
Colorectal cancer (CRC) is one of the most commonly diagnosed tumors of the gastrointestinal track, with high morbidity and mortality,1-3 which is prone to tumor metastasis and recurrence.4 Surgery shows favorable efficacy in the early stages of CRC. In the late stage of the tumor, chemotherapy is advantageous but with significant side effects and drug resistance.5
Pyroptosis is a programmed cell death caused by the cleavage of the key executive protein family Gasdermin through cysteine asparase on the self-repressing C-terminus end. And several N-terminal domains of Gasdermin localize to the cell membrane and form nanoscaled pores with a size of 10–15 nm by oligomerization.6 Subsequently, the cells continue to swell due to the osmotic pressure imbalance within and outside of the cell membrane until the membrane ruptures, showing a representative morphology of bubble blowing7 and accompanied by the release of pro-inflammatory factors including interleukin-1β (IL-1β) and IL-18, lactate dehydrogenase (LDH), high mobility group proteins, and adenosine triphosphate.7, 8 Due to distinct executive proteins between apoptosis and pyroptosis, both the morphology and the organisms' response after the cell death are widely divergent. Apoptosis is a clean cell death pathway, while pyroptosis releases the damage-associated molecular patterns (DAMPs) that activate innate immune cells through pattern-recognized receptors, which in turn activate NK cells and T cells and lead to a strong immune response.7, 8 Pyroptosis of only 15% of tumor cells might result in an intense immune response enough to eradicate the whole tumor tissue.8-10 GSDME is a member of the gasdermin family that is cleaved by caspase-3 to implement pyroptosis.7, 11 GSDME function is always deficient in tumor tissues owing to several factors including promoter methylation, absence of gene expression as well as gene mutation.11, 12 Therefore, we intend to import exogenous GSDME through a nonviral vector in combination with OXA as a first-line chemotherapy drug for colon cancer to evoke GSDME-related caspase-3-dependent tumor cell pyroptosis.
To avoid the potential risks associated with the unexpected expression of GSDME at normal tissue, high tumor selectivity, and sufficient transfection are indispensable. Photothermal therapy (PTT) has attracted wide attention for its unique advantages of good controllability, high selectivity, minimal side-effect, and noninvasiveness.13-15 PTT at moderate temperatures (40–43°C) enhances the fluidity of cell membranes so as to promote the uptake of nanoparticles by cells. PTT aids the escape of gene carriers from endosomes (key to gene transfection) by promoting the rupture of endosomal membranes. In addition, by means of breaking the chemical bond between nanoparticle and gene, PTT can facilitate cytoplasm release of gene to improve transfection efficiency.16-21 Indocyanine green is a Food and Drug Administration of USA (FDA) approved near-infrared light (NIR) fluorescent dye with high safety and adequate photothermal conversion efficiency.22, 23 HSP70 is a member of the heat shock protein family existing in the nucleolus, and can improve the stress reaction of the organism by preventing misfolding of proteins.24, 25 HSP70 is expressed extremely low under physiological conditions, of which the expression would be multiplied during hyperthermia or other stress.26 It was reported that integration of the HSP promoter into target genes would allow for their temperature-controlled gene expression.27, 28 Above all, HSP70 promoter will be utilized to achieve tumor-selective expression of GSDME gene, and the constructed plasmid vector is abbreviated as mHSP70-GSDME.29, 30
At present, gene delivery vectors include viral and nonviral vectors. Nonviral vectors with easy preparation process, low cost, and good compatibility can avoid the potential immunogenicity and carcinogenicity of viral vectors.31 Cationic liposome is an ideal nonviral vector; however, the cytotoxicity and low efficiency in vivo always restrict its application.32, 33 Inclusion of hydrophobic poly(lactide-co-glycolide) (PLGA) inner core inside of the lipid membrane can contribute to pack water-insoluble drug and avoid drug leakage.22, 34 And PEGylation of the nanoparticle with PEG-containing lipid membrane can prevent serum protein binding, prolong circulation time in vivo and shield the toxicity of cationic lipids to some extent.35
In this study, a photosensitizer-induced photothermal effect was exploited to enhance the expression of Gasdermin E expressing plasmid DNA (GSDME-pDNA) with a heat-inducible promoter HSP70, which facilitated the mechanism switch between apoptosis and pyroptosis triggered by OXA and caused potent inhibition of mouse colon cancer. Furthermore, a novel vector named lipid-coated PLGA nanoparticles coloaded with GSDME-pDNA and a photosensitizer ICG was constructed to achieve the tumor-specific expression of GSDME with spatiotemporal controllability to avoid harmness to normal tissues. We anticipate that this study would bring about novel thoughts and new methods for the treatment of colon cancer.
2 RESULT
2.1 Formulation screening for ICG-loaded nanoparticles
To develop an ideal gene delivery vector, formulations of the nanoparticles were optimized using a single-factor screening method with the particle size, encapsulation efficiency (EE), drug loading (DL) capacity, and lipid utilization efficiency (LUE) as indicators.
First, three methods were used to prepare ICG vectors. Nanoparticles1 loaded with ICG (NP1-ICG) (liposome) was prepared by the classical thin-film dispersion method. A two-step method was adopted to prepare lipid-coated ICG-loaded PLGA nanoparticles with shell-core structure as reported previoulsy36 (nanoparticles2 loaded with ICG [NP2-ICG]). Lipid-coated PLGA nanoparticles can also be prepared by one-step method.37
The prescriptions screened for NP1-ICG are shown in Supporting Information: Table S1. ICG is amphiphilic,36 and can be dissolved in both oil phase and aqueous phase during preparation. A higher DL of 8.5% was attained with ICG addition in the aqueous phase relative to the DL of 4.4% with ICG in oil phase, suggesting facile encapsulation of ICG in the aqueous phase of liposome (Supporting Information: Figure S1A,D). In prescription (2)–(5), as the nanoparticle surface charge changed from positive to negative, the EE of ICG decreased correspondingly implying that ICG encapsulation was implemented through electrostatic adsorption between ICG molecule and the phospholipids. In optimized formulation of NP1, the molar ratio of 1,2-dioleoyl-3-trimethylammoniumpropane (DOTAP)/L-α-phosphatidylcholine, hydrogenated (Soy) (HSPC)/cholesterol (Chol) was 1/2.8/1.2, and particle size, potential, EE and DL were 149.1 nm, 37.8 mV, 92.6%, and 8.5% (w/w), respectively.
The prescriptions screened for NP2-ICG are shown in Supporting Information: Table S2. With the increase of PLGA/lipid weight ratio, the nanoparticle particle size also increased probably ascribed to the increased size of PLGA inner core.38 The optimum particle size was 163.8 nm, but the EE and DL were only 52.3% and 5% (w/w), respectively. The low EE of ICG was probably ascribed to ultrasonication after ICG loading, which might lead to ICG leakage (Supporting Information: Figure S1B,E).
The prescriptions screened for nanoparticles 3 loaded with ICG (NP3-ICG) are shown in (Supporting Information: Table S3). Rise of polyvinyl alcohol (PVA) concentration resulted in increase of NP3-ICG particle size owing to a decreased shear force during the emulsification process (Supporting Information: Figure S1C,F).39 And higher sonication times might induce an increase in NP3-ICG particle size resulting from demulsification. Prescriptions (8), (9), (11), and (12) were identified as the better prescriptions. Compared with two-step method, NP3-ICG exhibited smaller particle size, higher lipid utilization (Supporting Information: Figure S2A) and DL. The optimal formulation for NP3-ICG was shown in Table 1. In conclusion, the one-step NP3 was selected for further study.
| Size (nm) | Zeta potential (mV) | EE (%) | DL (%, w/w) | |
|---|---|---|---|---|
| NP3-ICG | 185 ± 1.1 | 46.8 ± 1.3 | 83.6 ± 1.4 | 6.5 ± 1.3 |
- Abbreviations: DL, drug loading; EE, encapsulation efficiency; ICG, indocyanine green; NP3-ICG, nanoparticles3 loaded with ICG.
2.2 Characterization of pharmaceutical and biological properties of the nanoparticles
After obtaining the optimized prescription, the main properties of the nanoparticles including size, gene condensation rate. photothermal effect, cytotoxicity, tumor targeting effect in tumor-bearing mice as well as photothermal protransfection effect were comprehensively investigated.
NP3-ICG is a homogeneous sphere with a round-like shape examined by transmission electron microscopy (Figure 1A). NP3-ICG had good stability in terms of particle size, polydispersity coefficient (PDI), and potential measured at 4°C within 14 days (Supporting Information: Figure S2B). The maximum UV absorption peak of NP3-ICG at 805 nm (Supporting Information: Figure S2C) indicated that ICG was effectively loaded onto NP3. The binding ratio of NP3-ICG to red fluorescence protein (RFP) detected by agarose gel electrophoresis (Figure 1B, Supporting Information: Figure S2D) showed that all three nanoparticles completely condensed the plasmid DNA at N/P ratio > 4. Formulation 12 of NP3-ICG at N/P ratio of 10 displayed the best transfection efficiency on both CT26 and 293T (human embryonic kidney cells) cells in vitro relative to formulation 8/9 indicating that the molar ratio of DOTAP was positively correlated with the transfection rate (Supporting Information: Figure S3). And DOTAP might achieve a higher transfection efficiency through enhanced cellular uptake and lysosome escape mediated by cell membrane binding and the proton sponge effect of DOTAP.38 NP1-ICG and NP3-ICG at N/P ratio of 20 resulted in lower transfection efficiency probably imputed to high cytocotoxity (Supporting Information: Figure S3).
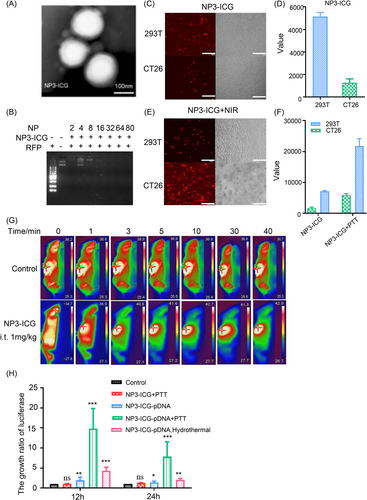
The MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide, Thiazolyl Blue Tetrazolium Bromide) result showed that the half maximal inhibitory concentration (IC50) of NP1-ICG and NP3-ICG were 33.3 and 50 μg/mL, respectively (Supporting Information: Figure S4A). The main organs had no obvious pathological lesions after the administration of NP3-ICG. Liver and kidney function-related indexes such as serum urea, creatinine, alanine transaminase, and glutathione transaminase showed no apparent change (Supporting Information: Figure S4B,C). NP3-ICG did no cause visible hemolysis (Supporting Information: Figure S4D). In summary, NP3-ICG had good biocompatibility and was used in the following research.
After administration of NP3-ICG-DID (1,1-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate as DID) to tumor-bearing mice, the highest accumulation in the tumor site was observed at 6 h, second only to that of liver, suggesting that the vector has tumor targeting capability (Supporting Information: Figure S5A–D). Besides, the fluorescence of DID was also detected in the tumor sections of these mice, of which the fluorescence strength at different time points was consistent with semiquantitative results of tumor nodules (Supporting Information: Figure S5E). The photothermal effect of NP1-ICG and NP3-ICG were comparable with peak temperature reached up to 48°C after PTT irradiation in vitro, which was higher than the free ICG (Supporting Information: Figure S6).40, 41 And the photothermal efficiency was augmented with increasing NIR power and ICG concentration. The photothermal effect of the nanoparticles in vivo was also examined (Figure 1G, Supporting Information: Figure S7). The nanoparticles administered intravenously at dose as high as 8 mg/kg of ICG made the temperature at the tumor site rise to around 41°C and lasted 40 min followed by 808 nm laser irradiation. And a lower dose (4 mg/kg ICG) barely caused temperature rise (Supporting Information: Figure S7). By contrast, NP3-ICG after intratumoral injection, exhibited a more significant photothermal effect, which was promoted with increasing both NIR power and ICG dose. And a low dose of 1 mg/kg of ICG with NIR power of 0.6 w/cm2 would make the tumor temperature rise to 42°C. As the optimal temperature range for inducing HSP70 expression was 39–43°C and temperature over 43°C might cause cell death,15 the final irradiation conditions were set as intratumoral administration of NP3-ICG (ICG at 1 mg/kg) with NIR power of 0.6 w/cm2.
In this paragraph, the promotion of NP3-ICG endocytosis and transfection in vitro by photothermal effect will be discussed. It has been reported in several studies that the photothermal effect exhibited pleiotropic effects on promoting transfection through enhanced cellular uptake of the vector, accelerated lysosome escape for gene cytoplasmic delivery, and induced gene release from the vector. To obtain the optimal photothermal effects, ICG concentration and irradiation time points after vector administration were optimized in 293T and CT26 cells. The screening of irradiation time point indicated that irradiation right after transfection gave the highest transfection efficiency, which was probably because the impact of the photothermal effect on nanoparticle endocytosis overwhelmed those on nanoparticle lysosome escape and gene cytoplasmic release. Both too high NIR power and ICG concentration would cause cell death, which explained the reduction of gene transfection efficiency when these two factors were at too high level. The optimal PTT condition after screening was irradiation at 0 h with ICG concentration of 6 μg/mL and NIR power of 0.6 W/cm2 (Supporting Information: Figure S8,9). Photothermal effect on the optimized irradiation condition remarkably enhanced vector endocytosis and RFP expression in both cell lines (Figure 1C–F, Supporting Information: Figure S10A). NP3-ICG+NIR group induced a 14.8-fold higher luciferase expression with an HSP70 promoter compared to the control group with NP3-ICG transfection only (Figure 1G,H, Supporting Information: Figure S10B).
It was worth noting that NP3-ICG+PTT group showed a 7.9-fold increase in luciferase expression relative to NP3-ICG+hyperthermia group. Compared with the hyperthermia effect of water bath treatment, PTT enabled superior gene expression through multiple effects as described above.
2.3 Antitumor effect in vitro
Chemotherapy drugs with a caspase-dependent apoptosis inducing effect can trigger pyroptosis by cleaving gasdermin proteins with activated caspase 3, thus converting apoptosis into pyroptosis. OXA can initiate apoptosis by activating caspase-3. Whether OXA can cleave GSDME in tumor cells and consequently induce pyroptosis is not determined, which will be investigated in this section.
The results showed that the infection rate of CT26 and HCT116 were 85.4% and 78.7%, respectively (Figure 2C, Supporting Information: Figure S11A,B). G-CT26 and G-HCT116 cell mortality rate after OXA treatment increased about 20.9% and 13.4% compared to control group (Figure 2C, Supporting Information: Figure S11B).
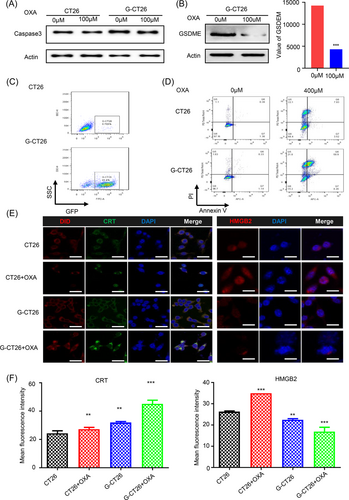
Cleavage of caspase-3 and GSDME occurred after OXA treatment in both CT26 and HCT116 cells with overexpression of GSDME, which explains the variation in total protein expression after OXA treatment (Figure 2A,B, Supporting Information: Figure S12A,B). The result was consistent with previous report that activated caspase-3 induced by chemotherapy drug like OXA would cleave GSDME, thereby realizing antitumor mechanism transformation from apoptosis to pyroptosis.
As calreticulin (CRT) and high mobility group box 2 (HMGB2) are representative DAMPs and in favor of antitumor immunity, their expression in tumor tissues were studied by immunohistochemistry assay (Figure 2E,F). CRT expression after OXA treatment was higher in G-CT26 than CT26; on the contrary, HMGB2 expression inside of G-CT26 was diminished more strikingly than that in CT-26. Studies have demonstrated that CRT is overexpressed on tumor cell surface after drug treatment such as anthracyclines. CRT can enhance antitumor immune responses by binding to CD91/LRP1, a receptor located on the surfaces of innate immune cell.42 Meanwhile, secreted CRT can not only control microvessel growth but also prevent tumor cell reproduction. In normal physiological conditions, HMGB2 is mainly located in nucleus, while it is secreted outside of the cells at the stage of necroptosis or even in the late stage of apoptosis causing inflammatory reactions. These results suggested that overexpression of GSDME would enhance the immunogenicity of CT26 cells after OXA treatment.
2.4 Antitumor effects in vivo and analysis of immune cell population variation in tumor microenvironment
As discussed above, OXA not only mediated caspase-3-dependent GSDME cleavage, but also induced DAMPs release. Whether OXA in combination with GSDME-pDNA loaded nanoparticles would play a tumor-suppressing effect in vivo and simultaneously activate intense antitumor immunity was discussed in this section using xenograft, orthotopic as well as distal tumor models. The feasibility of GSDME-overexpressing CT26 cells treated with OXA as a tumor vaccine were also explored.
In preliminary experiment, mG-NP3-ICG administration route and irradiation duration were first screened in preliminary experiments with tumor-bearing mice (Supporting Information: Figure S13A,B). The tumor suppression effect was superior when the irradiation lasted for 30 min. Although the tumor inhibitory rate was comparable between intratumoral and intravenous administration groups, the intratumoral group would not cause obvious body weight loss of mice. Based on these results, intratumoral administration and irradiation for 30 min were employed in the subsequent experiments.
In the xenograft colon-cancer model (Figure 3), the highest tumor inhibition ratio reached 77.7% in the mG-NP3-ICG+PTT+OXA group without significant changes in body weight of mice. And the survival period was also the longest compared with all other groups. As shown in hematoxylin and eosin staining sections (Supporting Information: Figure S14), no significant organ injury occurred in all groups, while apparent necrosis occurred at tumor site in the mG-NP3-ICG+PTT+OXA group. And this group showed a remarkable decrease in neovascularization (CD31) at tumor sites as well as the quantity of proliferative tumor cells (KI67). Besides, CRT was also conspicuously increased, while HMGB2 was decreased at the tissue level (Supporting Information: Figure S15–17).
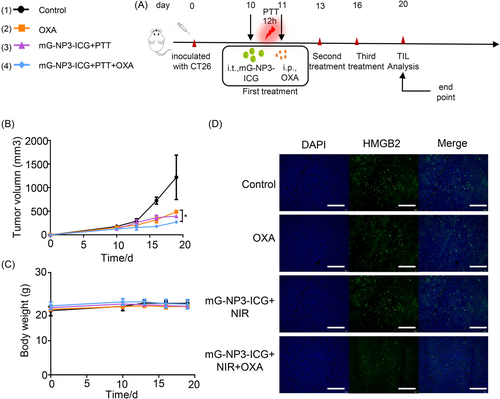
In the distal tumor model, although OXA has been reported to induce immunogenic cell death, the inhibitory effect of OXA or mG-NP3-ICG+PTT alone on either the primary or distal tumor was limited in the CT26 distal tumor model. In contrast, antitumor effect of the mG-NP3-ICG+PTT+OXA group was not affected by inoculation of distal tumors. And the tumor inhibition efficiency reached 84.5%, which is similar to the result of xenograft CT26 model (Figure 4). It deserves to be mentioned that mG-NP3-ICG+PTT+OXA group also displayed definite antitumor effect on distal untreated tumors, implying that the combination group indeed enhanced the antitumor immunity and thus remarkably inhibited the untreated tumors with the potential to arrest distal tumor metastasis. No visible weight loss of the mice was observed in all groups during the whole treatment process indicating good tolerance (Figure 4D).
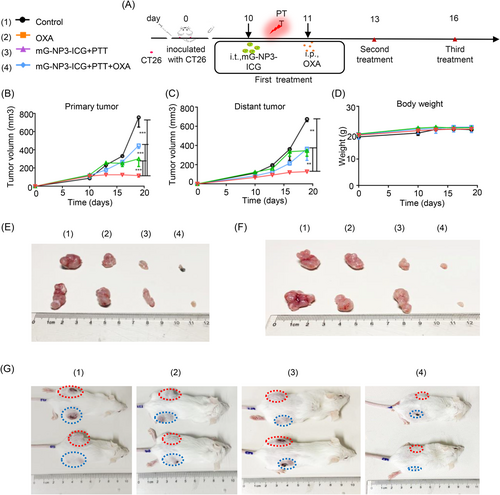
In the orthotopic CT26 tumor model (Supporting Information: Figure S18), the inhibition efficiency of mG-NP3-ICG+NIR+OXA group for the subcutaneous tumor was 85% and was comparable to those of the xenograft and distal tumor models, which was also not affected by the intraperitoneal inoculation of tumors. And tumor inhibition rates for the orthotopic tumors of OXA, mG-NP3-ICG+NIR, and mG-NP3-ICG+NIR+OXA groups were 40%, 5.4%, and 48.8%, respectively. In our experimental procedure, tumors were inoculated both subcutaneously and intraperitoneally at the same time. After tumor inoculation, the treatment was started on the 10th day when the subcutaneous tumor was big enough for the intratumoral injection and irradiation. Nevertheless, the peritoneal tumors growed so rapidly that it was difficult for the intervention started on 10th day to control their growths, which probably explained the low inhibition rate in our study.
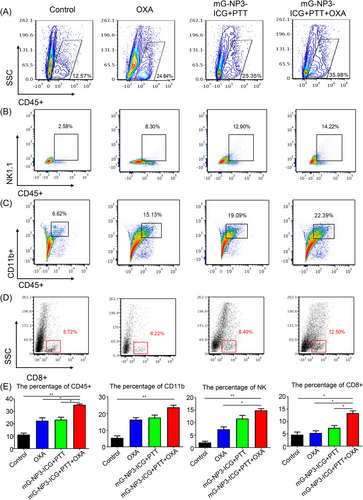
Insufficiency in lymphocyte infiltration at the tumor site in CRC is a major cause of poor antitumor efficacy for chemotherapeutic agents, so the effects of various treatments on tumor-infiltrating lymphocyte (TIL) subpopulations were systematically examined (Figure 5). We analyzed the change in proportions of NK cells, CD11b+ cells, and CD8+ T cells. Although the proportion of these subsets increased in all treatment groups, the change in the combination group was the most significant, with raised CD45+ cells, NK cells, CD11b+ cells, and CD8+ T cells of 23.41%, 11.64%, 15.77%, and 6.78%, respectively.
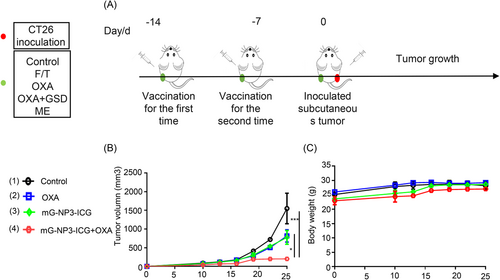
Antitumor vaccine is the gold standard for validating drug-induced immunogenic cell death. According to Figure 6, mice inoculated with G-CT26 after treatment with OXA was effective in preventing tumorigenesis with tumor suppression efficiency of 89% and had no apparent loss in body weight. These results were consistent with literature, suggesting that the combination group had the potential as an antitumor vaccine.43
3 DISCUSSION
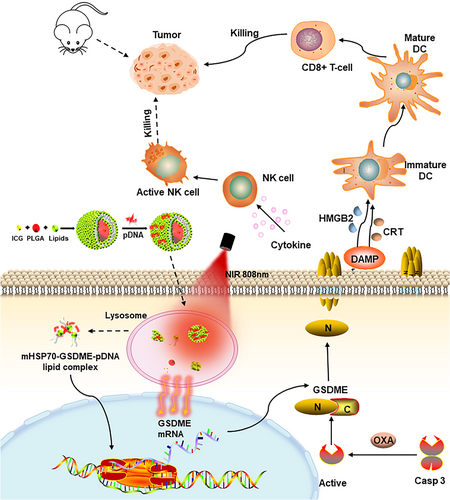
In this study, we construct a gene delivery system with an ICG-loaded PLGA inner-core and cationic lipid membranes as the outer shell loaded with the mHSP70-GSDME through electrostatic adsorption (Figure 7). Subcutaneous tumor nodule after injection of the gene vector will be irradiated with an 808 nm laser to cause local temperature rise to around 40–43°C in purpose of activating the HSP70 promoter and consequently inducing GSDME expression. This vector is expected to achieve the spatiotemporal controllability of target gene expression. OXA is administered to activate caspase-3 to cleave GSDME, which promotes the release of DAMPs molecules and causes an immunogenic inflammatory response and attendant strong antitumor effect.
Despite the problems with gene therapy, about 2000 clinical studies of gene therapy have been ongoing in more than 30 countries since 2012. Multiple gene therapy drugs have been approved for market in recent years such as Strimvelis, Kymriah, Yescarta, and Luxturna (trade names), two of which are used for cancer gene therapy. The drawback of the traditional gene therapy strategy for cancer is that it only acts on the cells with the introduced therapeutic gene, while other unaffected cells continue to proliferate. This phenomenon is particularly pronounced for nanoscaled gene delivery systems, as the nanoparticles cannot penetrate very deeply into the tumor tissues due to the elevated fluid pressure of the tumor stroma, which causes recurrence as the presence of many survived tumor cells. In our study, the GSDME-expressing gene not only eventuated in the death of transfected tumor cells directly, but also eliminated nontransfected tumor cells by activating a strong antitumor immune response, which is also known as the “Bystander effect.”8 Therefore, the GSDME-based gene therapy strategy is promising.
To avoid side effects of gene delivery vectors on nontarget sites, we utilized an inducible vector system for spatiotemporal-controllable gene expression. Available inducible gene expression systems include tetracycline-inducible systems and progesterone receptor/mifepristone (RU486)-inducible systems, which are activated by orally taken small molecules. However, the pharmacokinetics of small molecules after oral administration would have a prominent impact on the regulation of expression systems, probably resulting in slow action or short duration. The heat-inducible gene expression system is also widely studied, of which the protein expression is very low without induction and exhibits a burst growth under heat induction. Besides, hyperthermia therapy is also one of the strategies in oncology with well spatiotemporal controllability. Therefore, the gene expression vectors used in this study was constructed using the heat shock-responsive HSP70 as a promoter, which is one of the most successful heat-inducible gene expression systems.
The colon cancer model was investigated in our study due to the anatomical characteristic of colon. And photodynamic therapy (PDT) is quite beneficial in the diagnosis and prevention of CRC, such as detecting lesions that are hard to distinguish, allowing simultaneous inspection and treatment after intravenous injection of photosensitizer with high safety. There are phase I/II/III clinical trials of PDT applied to CRC.44 Although PDT is not a first-line treatment option for colon cancer at present, it is still clinically recognized by many doctors and patients owing to its minimally invasiveness and compliance. Thus, PTT for colon cancer has great potential for clinical translation.
There are some limitations of this study. First, though NIR irradiation can be localized to the site of tumor nodules, the photothermal effect would play a role in all the cells that have internalized the nanoparticles, including tumor cells as well as macrophages/dendritic cells in the tumor microenvironment. Thus, tumor-targeting ligand modification of the vector would further improve the specificity. Second, due to the limited penetrability of NIR, this strategy only applies for those superficial tumor types accessible to light, such as breast cancer, colon cancer, skin-related cancers, and so on.
4 CONCLUSION
In conclusion, lipid-coated PLGA nanoparticles co-loaded with photosensitizer ICG and heat-inducible mHSP70 promoter-regulated GSDME-pDNA to achieve tumor-specific expression of pyroptosis implementing protein GSDME is rationally designed. The mHSP70 promoter facilitates GSDME expression response to temperature rise at the tumor site mediated by ICG upon local irradiation. Activated caspase-3 initiated by chemotherapeutics OXA would cleave GSDME and induce pyroptosis followed by DAMPs release and boosting of adaptive immunity. It is speculated that inspired GSDME expression using spatiotemporal controllable heat-inducible promoter can be a smart strategy to trigger pyroptosis and augment antitumor efficiency.
5 MATERIALS AND METHODS
5.1 Reagents and plasmids
Indocyanine green was purchased from MedChemExpress. Oxaliplatin (OXA, 98%) was purchased from Shanghai Yuanye Bio-Technology Co., Ltd. DOTAP, 1,2-dimyristoy-sn-glycero-3-phosphocholine (DMPC), 1,2-dioleoyl-sn-glycero-3-phosphate (DOPA), HSPC, and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000) were purchased from Lipoid. Chol was purchased from Shanghai Bio Science & Technology. PLGA (lactic acid:glycolic acid = 50:50) was purchased from Shandong Institute of Medical Instruments. PVA (Molecular weight = 30,000–50,000, 87%) was purchased from Sigma-Aldrich. DID (>98%) was purchased from Biotium. Coumarin 6 (C6, >98%) was purchased from TCI Development Co., Ltd. 4′,6-diamidino-2-phenylindole (DAPI) was purchased from J&K Scientific. Propidium iodide (PI) was purchased from Shanghai Yuanye Bio-Technology Co., Ltd. Annexin V-APC (550475) was purchased from BD Pharmingen. CD31(GB11063-3) and KI67(GB111499) was purchased from Servicebio. CRT(12,238 s), caspase-3(9665), and p-p53(9284) were purchased from Cell Signaling Technology. HMGB2(ab67282) was purchased from Abcam. Anti-GAPDH(AF5718) was purchased from R&D. DFNA5(K2217) was purchased from Santa Cruz Biotechnology. FlowAB-AF555(A21429), FlowAB-AF488(A10667), and PEcy7-NK1.1(25-5941-82) were purchased from ThermoFisher. FITC-CD45.2(09806) and PB-CD11b(101224) was purchased from biolegend. PEcy7-CD8(25-0081-81) was purchased from Invitrogen.
RFP expressing reporter plasmid was purchased from Invitrogen. pDRIVELucia-mHSP70 was purchased from Invivogen. pcDNA3.1-mHSP70-mGSDME and pcDNA3.1-hHSP70- mGSDME were constructed by GENEWIZ.
5.2 Cell culture and animals
Mouse 293T cells were purchased from Nanjing Kebai Biotechnology Co., Ltd. and cultured in DMEM supplemented with 10% fetal bovine serum together with 1% penicillin/streptomycin and incubated at 37°C, 5% CO2. Mouse CT26 cells and human HCT116 cells were purchased from Nanjing Kebai Biotechnology Co., Ltd. and cultured in 1640 containing 10% fetal bovine serum and 1% penicillin/streptomycin, and incubated at 37°C, 5% CO2.
Male Balb/c mice (6–8 weeks old, 20 ± 2 g) were purchased from Sichuan University Experimental Animal Centre and fed in the purified air laminar flow frame of the Animal Center of the State Key Laboratory of Biotherapy, Sichuan University. All animal experiments involved in the present study were approved by the Animal Care and Use Committee of Sichuan University.
5.3 NP3-ICG preparation
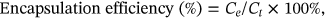
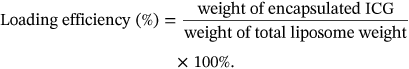
C6-NP3-ICG was prepared following the preparation of C6-NP2-ICG described in the attachment, and LUE was calculated accordingly.
5.4 Characterization of size, zeta potential, and morphology
The particle size, zeta potential, and PDI were determined by Malvern Nano-ZS 90. Then the morphology of the nanoparticles was visualized by transmission electron microscope (TEM) (H-600; Hitachi) following negative staining using 2% phosphotungstic acid. The stability of the nanoparticles stored at 4°C was determined after 14 days.
5.5 Integrity of pDNA
The nanoparticles dispersed in deionized water were incubated with the reporter gene at various N/P ratios from 1 to 40. And the binding ratio was evaluated by agarose gel electrophoresis at 120 V for 20 min.
5.6 Photothermal effect of the nanoparticles in vitro and in vivo
The nanoparticles were irradiated with an 808 nm laser (Changchun Leishi Photo-Electric Technology Co., Ltd.) and the temperature variation of the nanoparticle suspension within 40 min was measured consecutively.
Mouse was administered with the NP3-ICG intravenously. After 24 h, the xenograft tumors were irradiated with NIR (808 nm laser). The temperature change at the tumor site was measured consecutively over 40 min.
The temperature variation was visualized by an Infrared Thermal Imager (H36; Hikvision).
5.7 Transfection of the nanoparticles in vitro
293T or CT26 (5 × 104) cells were seeded in 24-well plates. After 24 h, the cells were transfected with NP3-ICG loaded with RFP-expressing pDNA or pDRIVE5Lucia-mHSP70 followed by irradiation with 808 nm laser and incubated with the transfection reagents for 4 h before the medium was replaced with fresh medium. At 48 h after transfection, RFP expression was observed by a fluorescent microscope (OLYMPUS). The secreted luciferase encoded by pDRIVE5Lucia-mHSP70 in the supernatant utilizes the coelenterazine as substrate for luciferase reaction. And the light signal produced can be quantified using a fluorescent plate reader (Biotek) and expressed as relative light units (RLU).
5.8 MTT assay
CT26 cells (1 × 104) were seeded in 96-well plates and cultured for 24 h. NP3-ICG with various concentrations of ICG in the media (5, 12.5, 25, 50, 125, 250, 500, 1000, and 2000 µg/mL) were used to treat the cells for 48 h. Then, 20 µL of MTT solution (5 mg/mL, pH 7.4) was added to each well and incubated for 4 h. The formazan crystals formed were dissolved in dimethyl sulfoxide (150 µL/well), and absorbance was read at 490 nm on a UV spectrophotometer. The cell viability was positively correlated with the amount of formazan crystals.
5.9 Biodistribution in vivo
NP3-ICG-DID (DID 0.5 mg/kg) was slowly infused via the tail vein and biodistribution of the nanoparticles in vivo was observed by an in vivo imaging system (Perkin Elmer).
5.10 Flow cytometry assay for analyzing cell viability and change of TIL subtypes
Tumor cells stably expressing human/mouse GSDME were treated with various antitumor drugs for 24 h, of which the apoptosis markers were detected by flow cytometry after staining with Annexin V/PI (BD Biosciences).
Tumors resected from the treated or control mice were digested with collagenase D (1 mg/mL; Sigma-Aldrich) and DNase I (0.05 mg/mL; Sigma-Aldrich) for 40 min at 37°C followed by passing through 70 μm cell strainer. The TILs were isolated by percoll and stained with anti-CD45-FITC, anti-CD11b-PB, anti-CD8-PEcy7, and anti-NK1.1-PEcy7 before subjecting to flow cytometry analysis.
5.11 Western blot
Cells or tissues were harvested and lysed in radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitor cocktails. Bicinchoninic acid (BCA) protein assay (Beyotime Biotechnology) was used to determine protein concentration. Then equal amounts of proteins were separated by 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and transferred onto the Nitrocellulose membrane. The transferred membranes were blocked with 5% skim milk at room temperature, then incubated with primary antibodies including anti-caspase-3 (9665; CST), anti-DFNA5 (sc-393162; SANTA), and anti-GAPDH (AF5718; R&D) at 4°C overnight. Subsequently, the membranes were incubated with the horseradish peroxidase secondary antibody at room temperature and developed by ECL (Beyotime Biotechnology).
5.12 Immunofluorescence
Treated cells were fixed in 4% paraformaldehyde solution for 10 min. After permeabilization with 0.1% Triton X-100 PBS for 10 min, they were washed three times with PBS and blocked with 5% bovine serum albumin for 30 min. Then, the cells were incubated with primary antibody like anti-CRT and anti-HMGB2 at 4°C overnight followed by incubation with fluorescent probe-labeled secondary antibodies and counterstaining with DAPI solution. Finally, the slides were visualized with a confocal microscope (Zeiss 880).
5.13 Immunohistochemistry
Frozen sections of the tumors were treated as described in Section 5.12 except for different primary antibodies used including anti-CD31, anti-KI67, anti-CRT, and anti-HMGB2. The sections were visualized with a fluorescent microscope (Olympus IX-70).
5.14 Xenograft colon cancer model
GSDME-pDNA-loaded NP3-ICG abbreviated as mG-NP3-ICG was prepared by incubating NP3-ICG with GSDME-pDNA at a N/P ratio of 10 and used for the following experiments. Female Balb/c mice were inoculated subcutaneously with CT26 (1 × 106 cells/each mouse) and randomly grouped on Day 10. The treatment groups included (a) model group without treatment (control group), (b) OXA (i.p., 5 mg/kg), (c) mG-NP3-ICG+NIR (i.t., ICG 1 mg/kg), (d) mG-NP3-ICG+NIR+OXA (mG-NP3-ICG, i.t., ICG 1 mg/kg; OXA, i.p., 5 mg/kg). At 12 h after intratumor administration of the nanoparticles, the tumors were irradiated with NIR followed by intraperitoneal administration of OXA after 24 h. The treatment was repeated on Day 13 and Day 16. The mice were killed on Day 20, then mice weight, volume (V = length × wenth2 × 0.5) and weight of tumor were measured. Main organs were resected for further study.
5.15 Distal colon cancer model
CT26 (2 × 106) was inoculated subcutaneously on both sides of hind limbs of mice. Tumor treatment and tumor inhibition study were performed as described in Section 5.14 on the 10th day.
5.16 Orthotopic colon cancer model
CT26 was inoculated both subcutaneously (2 × 106) and intraperitoneally (1 × 105) in mice. And only the subcutaneous tumors were treated. Tumor treatment and tumor inhibition study were performed as described in Section 5.14.
5.17 Antitumor vaccines
Mice were immunized twice with CT/G-CT26 after treatment of OXA for 24 h as vaccines on left hind limb on Days 14 and 7, and were rechallenged with CT26 (2 × 106) on right hind limb on Day 0. Tumor volume and mouse weight were assessed afterward.
AUTHOR CONTRIBUTIONS
Ailing Jiang is in charge of data curation. Mao Wang is responsible for review and editing of the manuscript. Huimin Liu, Simeng Liu, and Xiaoshuang Song undertake methodology. Yu Zou, Yuchuan Deng, and Qin Qin are in charge of formal analysis. Yiran Song is responsible for writing the original draft. Yu Zheng undertakes conceptualizaion. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
The GSDME expressing lentiviral vectors were gifts from Dr. Feng Shao (National Institute of Biological Sciences, Beijing, 100101, China). This work was supported by the Sichuan Science and Technology Program (2021YJ0193).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of Interest.
ETHICS STATEMENT
All animal experiments involved in the present study were approved by the Animal Care and Use Committee of Sichuan University (20190509023, Chengdu, Sichuan, China).
Open Research
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.