Label-free Raman spectroscopy for the assessment of liver fibrosis
Abstract
The enormous incidence and dire consequences of liver fibrosis highlight the need for biologically plausible markers as readouts of pathology and drug efficacy. The classic reference standard for diagnosis of liver fibrosis is histopathological examinations. However, it is burdened by time-consuming steps, subjectivity, and the static nature of information. Raman spectroscopy is a rapid, objective, and label-free analytical tool that provides numerous molecular information. Thus, we utilized Raman spectroscopy to characterize the pathological features of liver fibrosis and to evaluate the efficacy of drugs (obeticholic acid [OCA] and gigantol [YS30]). Principal component analysis with subsequent linear discriminant analysis could basically discriminate between fibrotic mice and healthy controls and showed that animals with OCA or YS30 treatment resembled those with vehicle treatment. Moreover, we identified several potential biomarkers, including cytochrome C, for liver fibrosis development based on their Raman “fingerprints.” Raman imaging provided quantitative and spatial distribution of collagen and cytochrome C in situ. In addition, we demonstrated the role of OCA or YS30 in revitalizing mitochondrial function to alleviate liver fibrosis using Raman spectroscopy. Collectively, Raman spectroscopy succeeded in tracking liver fibrosis progression, assessing drug efficacy, and yielding significant molecular information useful for quantitatively linking pathological assessment and mechanism of drug action.
1 INTRODUCTION
Biopsy is the current gold-standard technique for liver fibrosis assessment.1 Histopathology has been an essential tool in the diagnosis and management of liver fibrosis by monitoring the morphological changes that occur during the progression of the disease. Hematoxylin and eosin (H&E) staining is used most frequently for pathological sections. Although this approach can discriminate between healthy and diseased tissues, it has significant limitations. Histopathology is time-consuming and relies on the expertise of pathologists. Considerable intra- and interobserver variability cannot be excluded. In addition, it primarily provides morphological information rather than a myriad of quantifiable molecular data.2-4 Immunohistochemistry is restricted to a single specific molecular marker for specimens.5
As an emerging analytical tool, Raman spectroscopy enables a more rapid analysis of numerous different biological samples, such as cells, tissues, and biological fluids like serum and bile.6-9 It has the merits of high spatial resolution, excellent detection sensitivity, great molecular specificity, and good reproducibility.10 The diverse components from a biological sample that give rise to a unique Raman spectrum reflect its holistic biochemical state. Therefore, analyzing Raman spectral peaks based on changes in the overall biochemical composition of the sample during disease progression, combined with chemometric or machine learning methods for Raman data, can aid in disease diagnosis and drug discovery.11-13 In comparison with histopathology, “molecular fingerprint”-based Raman spectroscopy can serve as a label-free, objective, and semiquantitative tool for digital pathology. For instance, Raman spectroscopy has been applied to quantify the lipid content in steatotic livers.14, 15 Additionally, Raman imaging can not only perform qualitative and quantitative analysis but also provide morphological and material distribution information.16, 17
Raman spectroscopy has been used in several studies to diagnose liver fibrosis disease. In one study, principal component analysis (PCA) has been employed to differentiate Raman spectra of healthy and carbon tetrachloride (CCl4)-treated rat liver tissues.18 However, the study only adopted a single-point sampling mode for Raman signal acquisition, resulting in a rough and small number of single spectra. Similarly, Raman spectroscopy was performed to distinguish among healthy, CCl4-induced cirrhotic, and spontaneously recovered rat liver tissues.19 Nevertheless, the study was limited in its biological replicates (two rats per group), and no Raman analysis was carried out on the early stages of cirrhosis and its subsequent progression. Furthermore, neither study had yet undertaken an intensive quantitative investigation of the altered molecular states of liver fibrosis by Raman spectroscopy to unveil its etiological hallmarks and to furnish definitive markers for the surveillance of disease progression.
Raman spectroscopy has also not been used to measure the drug response of liver fibrosis. Obeticholic acid (OCA) is a Food and Drug Administration-approved farnesoid X receptor agonist for patients with primary biliary cholangitis.20, 21 OCA could also significantly ameliorate fibrosis in patients with nonalcoholic steatohepatitis22-25; thus, OCA has been used as a positive control in mouse models of liver fibrosis. Our previous studies demonstrated that gigantol (YS30) alleviated acute liver inflammatory damage in mice.26 However, its efficacy in chronic liver fibrosis injury remains unknown.
In this study, we investigated the capability of Raman spectroscopy to track the development along with the drug response in liver fibrosis. For the first time, we established a link between the quantitative information of Raman spectra and the molecular features during disease evolution and drug treatment of liver fibrosis.
2 RESULTS
2.1 Assessment of hepatic fibrosis progression in mice by Raman spectroscopy
To assess the capacity of Raman spectroscopy to monitor the development of the liver fibrosis process, we collected Raman spectra of liver tissues from mice at various disease progression phases (2, 4, and 8 weeks with CCl4 induction) and the corresponding vehicle-treatment controls. Figure 1A listed the averaged Raman spectra. As the duration of CCl4 injection increased, changes in overall Raman spectrum were observed in a time-dependent manner compared to normal mice, indicating constant changes in the biochemical status of livers from diseased mice. Consistently, serum levels of alanine transaminase (ALT) and aspartate transaminase (AST) rose substantially, indicating that the mice experienced liver injury (Supporting Information S1: Figure S1A). Col1a1, Col3a1, and Acta2 messenger RNA (mRNA) levels showed similarly significant elevations, indicating the emergence of fibrotic lesions in the mouse liver (Supporting Information S1: Figure S1B). In addition, as shown in Supporting Information S1: Figure S1C, the histological examination of Sirius red staining exhibited the same results as described above. PCA with subsequent linear discriminant analysis (LDA) was adopted to reduce dimensions and classify different groups of Raman data into clusters. LDA (Figure 1B) made it possible to discriminate, to some extent, between fibrotic mice and healthy controls, particularly between mice with CCl4 injection for 8 weeks and the ones with vehicle treatment for 8 weeks. Each cluster was comprised of at least 12,500 spectra from five mice. The higher number of Raman spectra and more biological replicates compared to previous studies guaranteed the reliability of Raman spectra analysis.
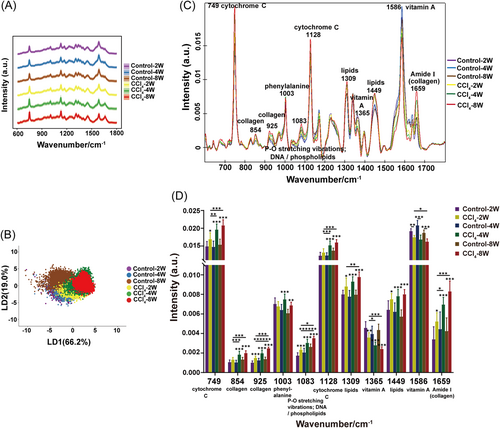
The vibrational peaks of Raman spectra (600–1800 cm–1) were then assigned to biomolecules (Figure 1C). Further semiquantitative analysis by integrating the intensities of individual Raman bands revealed significantly different peaks predominantly located at 749 and 1128 cm−1 (cytochrome C), 854, 925, and 1659 cm−1 (collagen), 1003 cm−1 (phenylalanine), 1083 cm−1 (P–O stretching vibrations; DNA/phospholipids), 1309 and 1449 cm−1 (lipids), and 1365 and 1586 cm−1 (vitamin A) (Figure 1D).27-33 It showed that with the prolongation of CCl4 dosing in mice, the Raman peaks assigned to collagen progressively intensified, which echoed the foregoing biochemical results, mirroring the cascading exacerbation of fibrotic injury. Analogous incremental enhancement was ascribed to the Raman peaks of lipids and cytochrome C, suggesting potential lipid accumulation and mitochondrial dysfunction in fibrotic livers. Raman peaks associated with vitamin A, on the other hand, exhibited a steady decline. Furthermore, an increased phenylalanine-related peak was evident. These results showed that as liver fibrosis progressed in mice, vitamin A and amino acid metabolism might be disturbed. Collectively, the findings suggested that Raman spectroscopy held promise for early diagnosis and tracking of disease progression, and offered promising biomarkers for liver fibrosis in mice.
2.2 Evaluation of drug efficacy using Raman spectroscopy
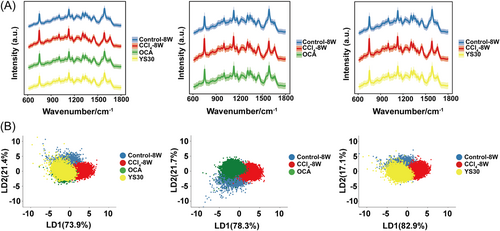
Having established the potential of Raman spectroscopy to follow disease progression, we aimed to explore its ability to assess the efficacy of drug treatment. Liver tissues from mice with OCA/YS30 treatment were taken for Raman measurement. The outstanding efficacy of OCA and YS30 in mitigating liver fibrosis following 4 weeks of gavage treatment was indicated by decreased serum liver enzyme concentrations, reduced collagen mRNA levels, and a lessened Sirius red staining response (Supporting Information S1: Figure S1). In the context of Raman spectroscopy, PCA-LDA clustering analysis permitted us to basically discern between healthy and fibrotic livers in mice. Of note, the clustering of Raman spectra for mice treated with OCA or YS30 was dispersed partially from the CCl4-alone group and partially overlapped with the distribution of the healthy group. The spectral clustering distributions of both drug administration groups were highly similar (Figure 2A,B). It was implied that both OCA and YS30 were potent drugs against liver fibrosis, sharing very similar downstream signaling detected by the overall Raman spectrum. These results underscored the potential application of Raman spectroscopy in pharmacodynamic studies.
2.3 Raman spectroscopy served as a molecular readout of OCA or YS30 treatment
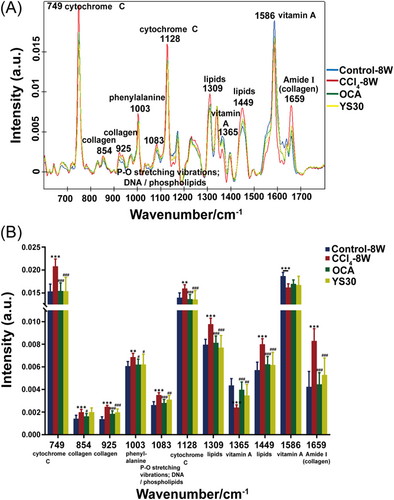
Quantitative analysis of individual Raman spectral peaks was then performed to explore whether OCA or YS30 had any impact on molecular pathological changes in the mouse liver (Figure 3A,B). We observed that the following peaks changed in the same way upon OCA or YS30 treatment, comparing with the vehicle treatment group, including higher intensities at 1365 and 1586 cm−1 (vitamin A) and lower intensities at 749 and 1128 cm−1 (cytochrome C), 925 and 1659 cm−1 (collagen), 1003 cm−1 (phenylalanine), 1083 cm−1 (P–O stretching vibrations; DNA/phospholipids), and 1309 and 1449 cm−1 (lipids). Taken together, these findings provided insight into the efficacy of OCA and YS30 against liver fibrosis and their potential shared actions on mitochondrial homeostasis, lipid, vitamin A, and phenylalanine metabolism. Meanwhile, OCA significantly weakened the vibration of the peak at 854 cm−1 (collagen), while YS30 had no significant effect on it. These data suggested that OCA and YS30 might have different roles in collagen metabolism in fibrotic livers.
2.4 Raman imaging visualized and quantified the spatial distribution of collagen and cytochrome C in fibrotic and healing livers
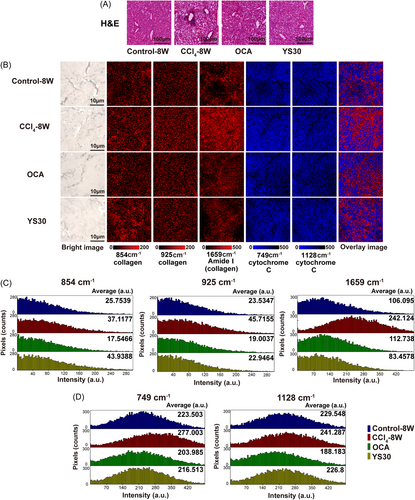
H&E staining only revealed morphological abnormalities in fibrosis, as well as morphological recovery induced by OCA or YS30 treatment (Figure 4A). Herein, Raman imaging was used to provide a comprehensive visual characterization of the content alterations in situ. The intensity distribution of Raman peaks attributed to collagen was analyzed, with the band at 1659 cm−1 showing a greater distribution in the liver than in the bands at 854 and 925 cm−1. The distribution regions of all three collagen peaks increased significantly in the fibrotic liver and decreased in the liver treated with OCA. YS30 treatment had no effect on the distribution area of the peak at 854 cm−1 but significantly reduced those of peaks at 925 and 1659 cm−1 (Figure 4B, columns in red). Additionally, statistical histogram analysis of collagen intensity per pixel within the liver demonstrated the onset and regression of fibrosis, indicating the efficacy of OCA and YS30 (Figure 4C). Raman peaks attributed to cytochrome C were also analyzed, with the bands at 749 and 1128 cm−1 showing similar intensity distributions in the liver. Their intensity distributions both increased in the fibrotic liver and decreased in the liver treated with OCA or YS30 (Figure 4B, columns in blue), as confirmed by histogram analysis (Figure 4D). The superimposed Raman image of the two molecules suggested that cytochrome C was predominantly distributed in hepatocytes, while collagen was mainly distributed around the hepatic vessels and presented a diffuse deposition and aberrant distribution in the fibrotic liver, which was restored after drug treatment (Figure 4B, last column). Collectively, the findings demonstrated that Raman imaging could provide quantitative and spatial distribution of relevant biomolecules in situ.
2.5 Change of Raman spectrum for cytochrome C was associated with hepatic mitochondrial homeostasis
In the liver fibrosis development and the recovery from this disease with the OCA or YS30 treatment, we found that several events related to hepatic mitochondrial homeostasis occurred: (1) The liver levels of nicotinamide adenine dinucleotide (NAD), a coenzyme of the mitochondrial electron transport respiratory chain, dramatically diminished in response to CCl4 delivery and rebounded substantially following 4 weeks of OCA or YS30 administration (Figure 5A). (2) A reduction of Sirt1, an enzyme that exerts a role in promoting mitochondrial homeostasis with NAD+ as a substrate, and an increase in Cd38 (NAD-degrading enzyme) were observed in mice with hepatic fibrosis. Also, both agents modulated the activity of the enzymes, suggesting their contribution to hepatic NAD homeostasis (Figure 5B). (3) Glutathione (GSH) is an enriched cellular antioxidant that acts to neutralize reactive oxygen species (ROS) generated by mitochondria. There was dramatic consumption of GSH and adenosine-triphosphate (ATP) in the mouse liver following CCl4 injection for 8 weeks, and replenishment occurred after the administration of the drugs (Figure 5C,D). Considering that cytochrome C is a crucial carrier of the mitochondrial electron transport chain, taking part in ATP generation for energy-supporting tasks,34 it is intimately intertwined with mitochondrial function and homeostasis. Correlations between Raman intensity at 749 cm−1 for cytochrome C and NAD, GSH, or ATP levels in liver tissues were analyzed, respectively. As liver fibrosis progressed in mice, the peak intensity at 749 cm−1 showed no correlation with GSH content and a significant negative correlation with NAD and ATP content (p = 0.0033, r = −0.7050; p = 0.0380, r = −0.5394; Figure 5E). In livers treated with OCA, the peak intensity at 749 cm−1 showed a prominent negative correlation with the concentrations of NAD, GSH, and ATP, respectively (p = 0.0031, r = −0.7082; p = 0.0035, r = −0.7026; p = 0.0006, r = −0.7779; Figure 5F). Similarly, in livers treated with YS30, the peak intensity at 749 cm−1 also showed a noteworthy negative correlation with the levels of NAD, GSH, and ATP, respectively (p < 0.0001, r = −0.8456; p = 0.01, r = −0.6411; p = 0.0004, r = −0.7984; Figure 5G). These results exemplified the credibility of quantitative indicators of Raman bands ascribed to cytochrome C.
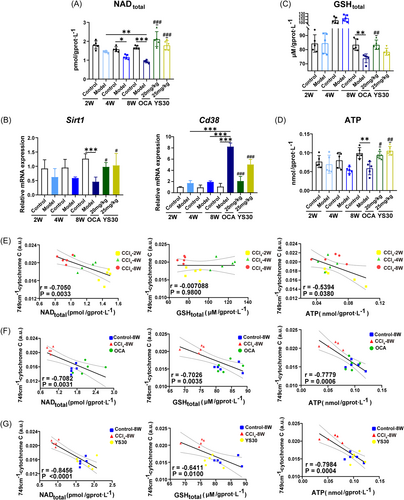
The expression levels of genes related to mitochondrial activity were further explored. Downregulation of Tfam and Ppargc1a mRNA expression indicated that prolonged liver exposure to CCl4 impeded mitochondrial biogenesis (Supporting Information S1: Figure S2A). Elevated expression of Fis1, which is involved in mitochondrial fission (Supporting Information S1: Figure S2B), along with decreased expression of genes that facilitate mitochondrial fusion, including Mfn1, Mfn2, and Opa1, were observed in fibrotic mouse liver (Supporting Information S1: Figure S2C). Uncoupling protein 2 (Ucp2) hinders the mitochondrial synthesis of ATP. It was found to increase with longer durations of CCl4 administration (Supporting Information S1: Figure S2D). OCA and YS30 may ameliorate mitochondrial dysfunction and liver fibrosis in mice by facilitating mitochondrial biogenesis, boosting energy supply, and managing mitochondrial dynamics, that is, suppressing excessive mitochondrial fission and raising mitochondrial fusion.
Triglyceride (TG) content in serum and liver was assayed and showed significant upregulation after 8 weeks of CCl4 injection, and treatment drugs substantially reduced TG accumulation (Supporting Information S1: Figure S3A,B). Correspondingly, Raman bands classified as lipids were observed to increase with prolongation of CCl4 dosing and decrease appreciably with drug treatment. Given that triglycerides are the predominant form of lipid storage and transport in the liver, the correlations between Raman intensity at 1449 cm−1 for lipids and TG content in liver tissues were analyzed. Due to the progression of fibrotic injury, there was no correlation between the peak intensity at 1449 cm−1 and the liver TG concentration. However, there was a significant positive association between the two variables for livers treated with OCA (p = 0.0021, r = 0.7279) or YS30 (p = 0.0065, r = 0.6676), respectively (Supporting Information S1: Figure S3C). The mRNA levels of genes associated with lipid metabolism pathways also reflected the deposition of lipids in fibrotic livers and the actions of drugs on lipid dynamics to mitigate liver fibrosis (Supporting Information S1: Figure S3D). Raman bands for vitamin A exhibited the opposite variations to that for lipids. Further investigation of the activity of genes responsible for the metabolism of retinol, retinyl ester, and retinoic acid suggested the disturbed vitamin A metabolism in fibrotic livers and the adjustive action of OCA and YS30 in this process (Supporting Information S1: Figure S4). Taken together, cytochrome C, lipid, and vitamin A Raman bands stand out as early biomarkers of liver fibrosis.
2.6 Machine learning classification model
Machine learning models facilitate the classification of large and complex Raman data sets conveniently and efficiently. Hence, the accessible data sets herein were split into two subsets: training (70%) and testing (30%) data sets, which served for model training and model evaluation, respectively. The LDA model was constructed by 10-fold cross-validation with five repetitions. Table 1 showed the relevant parameters of the classification test with an overall accuracy of 73.94% for mice at various disease progression phases. The sensitivity was 82.66%, 74.14%, 85.40%, 61.95%, 67.33%, and 75.98%, and specificity was 94.06%, 94.74%, 97.06%, 96.48%, 91.54%, and 95.16% for mice with vehicle treatment and CCl4 induction for 2, 4, and 8 weeks, respectively. In the group composed of healthy, fibrotic, and OCA- or YS30-treated mice, the performance of the classification test derived a sensitivity of 82.29%, 96.14%, 64.16%, and 65.27%, and a specificity of 91.32%, 97.76%, 92.27%, and 86.58%, respectively. The overall accuracy was 75.87% (Table 2). The overall accuracy was as high as 89.20% (Supporting Information S1: Table S1) and 86.83% (Supporting Information S1: Table S2) for mice treated with OCA or YS30, respectively. These results indicated the ability of Raman spectroscopy to classify liver tissues with various states.
| Reference | ||||||
|---|---|---|---|---|---|---|
| Control-2W | Control-4W | Control-8W | CCl4-2W | CCl4-4W | CCl4-8W | |
| Model prediction | ||||||
| Control-2W | 2559 | 428 | 382 | 327 | 20 | 5 |
| Control-4W | 334 | 2632 | 177 | 457 | 35 | 3 |
| Control-8W | 138 | 223 | 3469 | 99 | 76 | 11 |
| CCl4-2W | 58 | 173 | 18 | 3190 | 166 | 201 |
| CCl4-4W | 6 | 94 | 14 | 863 | 2051 | 683 |
| CCl4-8W | 1 | 0 | 2 | 213 | 698 | 2857 |
| Total number of spectra | 3096 | 3550 | 4062 | 5149 | 3046 | 3760 |
| Sensitivity (%) | 82.66 | 74.14 | 85.40 | 61.95 | 67.33 | 75.98 |
| Specificity (%) | 94.06 | 94.74 | 97.06 | 96.48 | 91.54 | 95.16 |
| Overall accuracy (%) | 73.94 | |||||
- Note: The entire Raman spectra (~75,000) were randomly split into two subsets: training (70%) and testing (30%) data sets, which served for model training and model evaluation, respectively. Control-2W, Control-4W, and Control-8W represented mice with vehicle treatment for 2, 4, and 8 weeks, respectively (n = 5). CCl4-2W, CCl4-4W, and CCl4-8W represented mice with CCl4 induction for 2, 4, and 8 weeks, respectively (n = 5).
- Abbreviations: CCl4, carbon tetrachloride; LDA, linear discriminant analysis.
| Reference | ||||
|---|---|---|---|---|
| Control-8W | CCl4-8W | OCA | YS30 | |
| Model prediction | ||||
| Control-8W | 4246 | 161 | 648 | 826 |
| CCl4-8W | 40 | 5453 | 275 | 96 |
| OCA | 249 | 23 | 4913 | 992 |
| YS30 | 625 | 35 | 1821 | 3597 |
| Total number of spectra | 5160 | 5672 | 7657 | 5511 |
| Sensitivity (%) | 82.29 | 96.14 | 64.16 | 65.27 |
| Specificity (%) | 91.32 | 97.76 | 92.27 | 86.58 |
| Overall accuracy (%) | 75.87 | |||
- Note: The entire Raman spectra (~80,000) were randomly split into two subsets: training (70%) and testing (30%) data sets, which served for model training and model evaluation, respectively. The sample size for each group of mice was 5.
- Abbreviations: CCl4, carbon tetrachloride; LDA, linear discriminant analysis; OCA, obeticholic acid; YS30, gigantol.
3 DISCUSSION
Hepatic fibrosis is a prominent contributor to morbidity and mortality worldwide.35, 36 Raman spectroscopy, a cutting-edge technology, shows promise in avoiding the dependence and variability in the expert judgment of traditional histopathology. Raman spectroscopy is label-free, efficient, objective, and informative. Previous research on using Raman spectroscopy to identify hepatic fibrosis focused on the differentiation of diseased livers by means of chemometrics. In our current work, we successfully evaluated the utility of Raman spectroscopy at the histopathological level to monitor hepatic fibrosis evolution, assess candidate therapeutic agents, and provide information on the quantification and spatial distribution of relevant biomolecules. The pathological characteristics of liver fibrosis and the underlying molecular actions of drugs were probed using Raman spectroscopy ulteriorly.
Raman spectroscopy can display extensive data at a single time with high reliability. It was found that the changes in cytochrome C Raman bands could signify mitochondrial function. They intensified with exacerbation of liver fibrosis, waned with drug treatment, and were negatively correlated with hepatic NAD, GSH, and ATP levels. Also, preliminary explorations of the impairment of mitochondrial biogenesis, dynamics, and energy metabolism in liver fibrosis and their modulation by drugs were undertaken. Consistently, it has been reported that Raman spectroscopy could give insights into the mitochondrial activity of cells or tissues by monitoring intensity fluctuations of cytochrome C peaks.37 Furthermore, Raman spectroscopy has been successfully applied to quantify the redox state of mitochondria for the diagnosis of diabetic cognitive impairment,38 sepsis,31 and cardiac arrest.39 However, our study was the first to showcase the value of Raman spectroscopy in assaying mitochondrial dysfunction in fibrotic livers and assessing the ameliorative actions of OCA and YS30 on this process. Also, beyond Raman technology, surveillance of mitochondrial respiration and other activities has been proposed to predict postliver transplant outcomes.40, 41 That is, it is possible and feasible to monitor mitochondrial activity to characterize liver function.
Raman lipid bands were more sensitive than biochemical assays of TG content in that they rose intensely at 2 weeks of liver injury, while the latter only increased prominently after 8 weeks. Lipid metabolism is dominated by the liver. In turn, lipid dysregulation mirrors liver derangements.42 For example, excessive aggregation of lipid droplets (LDs) in hepatocytes is a prominent symptom of nonalcoholic fatty liver disease, which can progressively deteriorate into liver fibrosis.43 Pnpla3 exerts hydrolase activity not only for TG but also for retinyl esters, a form of vitamin A storage in hepatic stellate cell (HSC) LDs. Considerable studies have been carried out to prove that Pnpla3 and its polymorphic variants impair lipid metabolism in hepatocytes and boost HSC activation.44-46 Activation of HSCs is accompanied by loss of vitamin A-containing LDs, which is a central event in liver fibrosis formation. This was consistent with the weakening of the vitamin A Raman bands that we observed. The attenuation of lipid Raman bands, potentiation of vitamin A Raman bands, and reduction of Pnpla3 (Figure S3D) after OCA and YS30 treatment implied that both drugs could substantially impact lipid and vitamin A metabolism, potentially counteracting liver fibrosis. The results were also supported by relevant genetic assays.
In this study, Raman imaging enabled the visualization of the spatial distribution of molecules in the liver and the quantification of changes in their abundance during the progression and regression of liver fibrosis. This showed its superiority over histopathological examinations, which provided relatively homogeneous information. However, this study had some limitations. First, Raman spectroscopy and imaging were captured from liver tissue sections, which was still an invasive procedure. Advances in Raman technology, such as spatially offset Raman spectroscopy (SORS) and fiber optic probes, might hopefully overcome this limitation.47 They will play an important role in the detection of biomarkers of liver fibrosis in vivo with no need for pathological sections. Second, we only adopted the PCA-LDA model for the analysis of Raman data. More machine learning approaches and multivariate statistical methods are needed to improve the classification of Raman spectral data, as well as to identify and quantify more molecules. Third, we only employed one mouse model of hepatotoxic injury-induced liver fibrosis by CCl4 administration. Given that hepatic fibrosis is a multietiological and intricate chronic disease, more efforts are needed to evaluate liver fibrosis in other mouse models and human patients.
In conclusion, this study demonstrated that Raman spectroscopy in combination with PCA-LDA analysis was anticipated to track the progression of liver fibrosis and assess the potency of drug candidates. The spectroscopic analysis identified biomarkers of liver fibrosis progression, including collagen, lipids, vitamin A, and cytochrome C, and their relevance to the effectiveness of OCA or YS30. Specifically, the volatility of cytochrome C bands in Raman spectroscopy offered potential new biomarkers for the management of liver fibrosis. In addition, the pathological features of liver fibrosis and the direct pharmacological actions of OCA and YS30 were quantified and interlinked. Above all, the effect of OCA and YS30 on mitochondrial homeostasis in liver fibrosis was explored with the help of Raman spectroscopy. Raman imaging could highlight new possibilities for digitization in pathological research on liver fibrosis by enabling the visualization and quantification of the spatial distribution of molecules in the liver, including collagen and cytochrome C.
4 MATERIALS AND METHODS
4.1 Animals
Permission for animal experiments was granted by the Institutional Animal Care and Use Committee of the Shanghai Institute of Materia Medica, Chinese Academy of Sciences. Male BALB/c mice (weight: 20–23 g) were supplied by Shanghai SLAC Laboratory Animal Co., Ltd. The animals were kept in an SPF-grade animal room that had a 12-h light/dark cycle, with a room temperature of 23 ± 1.5°C and a humidity of 70 ± 20%. Mice had unrestricted access to food and water.
4.2 CCl4-induced liver fibrosis
The mice were arbitrarily classified into the following six groups after a week of acclimatization: vehicle (olive oil); CCl4 (2, 4, and 8 weeks); CCl4 + OCA; and CCl4 + YS30. Mice received 2 mL/kg intraperitoneal injections of 20% CCl4 twice a week. Following 4 weeks of CCl4 injection, mice were gavaged with OCA (20 mg/kg) or YS30 (25 mg/kg) daily for four consecutive weeks while continuing the twice-weekly CCl4 administration. The mice were then euthanized. The serum derived from whole blood was centrifuged at 5000 rpm for 5 min and stored at –80°C. The liver tissues were frozen at −80°C for gene expression assay, or fixed in a formaldehyde solution for histological study, or embedded in optimal cutting temperature compound (OCT) for Raman spectroscopy.
4.3 Serum biochemical analysis
The enzymatic activities of ALT and AST in serum samples were analyzed with a cobas® 6000 automated biochemical analyzer (Roche).
4.4 Real-time quantitative PCR (RT-qPCR) analysis
The mouse liver tissues (~20 mg) were homogenized and lysed using TRIzol reagent (Life Technology), after which their total RNA was extracted and purified using EZ-10 spin columns and collection tubes (Sangon Biotech). One microgram of RNA was reverse-transcribed to complementary DNA (cDNA) using Hifair® III 1st Strand cDNA Synthesis SuperMix (Yeasen Biotech). Thereafter, cDNA was diluted and mixed with forward and reverse primers and Hieff® qPCR SYBR Green Master Mix (Yeasen Biotech). PCR was performed on an Applied Biosystems™ 7500 Fast Real-Time PCR System (Thermo Fisher Scientific). The expression level of each gene was normalized to glyceraldehyde 3-phosphate dehydrogenase. For all genes in this study, the primer sequences were listed in Supporting Information S1: Table S3.
4.5 TG measurements
Mouse liver tissues were homogenized in saline, and the supernatant was collected for analysis after centrifugation at 8000 rpm for 10 min. The triglyceride content of each sample was determined using a triglyceride assay kit following the manufacturer's instructions (Nanjing Jiancheng Bioengineering Institute) and normalized to the protein concentration measured with a BCA assay kit (Beyotime). Serum samples were assayed directly.
4.6 NAD and GSH assays
The NAD+/NADH assay kit (Beyotime) and the total glutathione assay kit (Beyotime) were used to assess NAD and GSH levels, respectively, in mouse liver tissues. In brief, supernatants were obtained by homogenizing and centrifuging liver tissues with the corresponding solutions from the kits. Each assay reagent was added sequentially to the supernatant for the reaction and incubation according to the manufacturer's instructions. The absorbance at the appropriate wavelength was measured using a microplate reader (BioTek). The results were normalized to the protein concentrations.
4.7 ATP determinations
Following the manufacturer's instructions, the ATP content of each mouse liver tissue was determined using the ATP assay kit (Beyotime), and the levels were normalized to the protein concentrations.
4.8 Raman spectroscopy study
Ten-micron-thick slices of mouse liver tissue embedded in OCT were obtained by frozen sectioning. The tissue sections were attached to a CaF2 Raman scattering microslide for Raman measurement. Raman imaging was performed with a confocal Raman microscope (alpha 300R; WITec) using a 532 nm laser and a 600 grooves/mm grating (blaze wavelength = 500 nm). Peak intensity and position (520.7 cm−1) were calibrated using the Raman scattering peak produced by a silicon plate. With a ×100 objective (numerical aperture = 0.9), 20 mW laser power, and 0.5 s integration time, Raman spectra within 600–1800 cm−1 were acquired. For each set of mouse liver tissues, the averaged Raman spectra were derived from tissue slices of at least five mice, and two to three regions in each section were randomly chosen for Raman spectra acquisition. For a high-resolution Raman image in Figure 4B, a 0.4 μm × 0.4 μm step size was taken with 2 h acquisition time for an ~1 cm × 1 cm liver section; while for a regular-resolution Raman image, a 1 μm × 1 μm step size was taken with 20 min acquisition time.
4.9 Histological analysis
Mouse liver tissues embedded in paraffin were sectioned and then stained with Sirius red for pathological assessments. Adjacent sections from liver tissues embedded in OCT were used for high-resolution Raman imaging and H&E staining, respectively.
4.10 Data processing and analysis for Raman spectroscopy
Raman spectral data were processed with Control FIVE 5.2.10.88 software (WiTec) to remove the cosmic ray influence and then employed an internal script in the R4.1.2 environment for baseline correction and area normalization. Since the acquired wave numbers in different batches may slightly shift (~±1 cm−1), linear interpolation was used to ensure that spectra data from different batches share the same values of Raman shifts. Then, the number of features of the spectra was reduced to 20 by PCA before they were fed into the LDA algorithm. The PCA and LDA analysis was performed by Python 3.8 with packages of numpy and sklearn. The spectra were split into a training set (70%) and a testing set (30%) to evaluate the performance of the LDA model. During model construction, 10-fold cross-validation with five repetitions was used.
For molecular profile analysis, the raw Raman spectra were preprocessed using Control FIVE 5.2.10.88 software for cosmic ray removal, fifth-order polynomial-based background subtraction to alleviate the contribution of fluorescence, and area normalization. Semiquantitative analysis of the biomolecules to which they were attributed was carried out by direct comparison of the peak intensities of the specific Raman bands.
4.11 Statistical analysis
Data were analyzed using GraphPad Prism 8.0.1 software, and results were shown as mean ± SD. One-way analysis of variance was adopted to calculate statistical significance. p < 0.05 was deemed statistically significant.
AUTHOR CONTRIBUTIONS
Huiying Cao: Conceptualization (lead); formal analysis (lead); investigation (lead); methodology (lead); validation (lead); writing—original draft (lead); writing—review and editing (lead). Chen Ma: Data curation (equal); formal analysis (equal); methodology (equal). Zhitao Wu: Investigation (equal). Lin Sun: Data curation (lead); formal analysis (equal); methodology (equal). Wenjing Liu: Investigation (equal). Chenhui Ma: Investigation (equal). Feihong Chen: Investigation (equal). Zhaoliang Peng: Investigation (equal). Lijiang Xuan: Resources (equal). Ruimin Huang: Conceptualization (lead); funding acquisition (lead); project administration (lead); supervision (lead); writing—original draft (lead); writing—review and editing (lead). Guoyu Pan: Conceptualization (lead); funding acquisition (lead); project administration (lead); supervision (lead); writing—original draft (lead); writing—review and editing (lead). All authors have read and approved the manuscript.
ACKNOWLEDGMENTS
We thank technical support from the Institutional Center for Shared Technologies and Facilities of Shanghai Institute of Materia Medica, Chinese Academy of Sciences. We also thank Biorender for providing some graphical elements. This study was supported by the National Science Foundation of China (Grant numbers 82173878 and 82172001), the Independent Deployment Program of the Bohai Rim (Yantai) Advanced Research Institute for Drug Discovery of the Chinese Academy of Sciences (Grant number LX211004), the “Organ Reconstruction and Manufacturing” Strategic Priority Research Program of the Chinese Academy of Sciences (Grant number XDA16020205), and Shanghai Municipal Science and Technology Major Project.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
All animal experiments were approved by the Institutional Animal Care and Use Committee of the Shanghai Institute of Materia Medica, Chinese Academy of Sciences (No.: 2021-11-PGY-44).
Open Research
DATA AVAILABILITY STATEMENT
All data and materials are available to the researchers once published.




