Cryoprotectants for red blood cells: Evaluate safety and effectiveness by in vitro measures
Yuying Hu and Xiangjian Liu contributed equally to this study.
Abstract
The transfusion of red blood cells (RBCs) is crucial in current medicinal research. The shelf-life of donated RBCs preserved by the normal method is very short, limiting their clinical application. Cryopreservation is a reliable and effective technology for the long-term storage of RBCs. During the process, ice formation will cause irreversible injuries to RBCs. Glycerol is used as a cryoprotectant (CPA) for RBCs to mitigate cryoinjuries. But it severely induces RBC hemolysis and deformation. This review introduces some biocompatible and effective CPAs used for RBC cryopreservation, outlining recent advances. Currently, there is no uniform standard to determine whether a CPA is suitable for RBCs. According to previous studies, we summarize for the first time comprehensive methods to evaluate the performance of CPAs by ensuring their safety and effectiveness. The safety of CPAs is defined as the degree of damage to RBCs, while the effectiveness is demonstrated by the properties of thawed RBCs, including membrane properties, protein activities, rheological properties, and metabolites levels. A novel CPA that is confirmed suitable for RBCs by these methods may be applied to other cells. We believe comprehensive methods can thoroughly evaluate the performance of CPAs and promote the development of transfusion therapy in clinic.
1 INTRODUCTION
As early as the 17th century, experiments with blood transfusion were conducted. Transfusion medicine was greatly advanced by the discovery of the ABO blood groups in the early 1900s.1 Since then, the safety of the transfusion of blood products has significantly increased, and refrigerated stored red blood cells (RBCs) are now frequently infused to make up for excessive blood loss or to treat moderate-to-severe anemia, traumas, leukemia, and so forth.2 According to the American Red Cross, 29,000 units of RBCs are needed each day in the United States.3 However, like with any medical procedure, the patients may be at risk for the adverse reactions of RBC transfusion, including immunological and nonimmune-mediated complications.4 Effective methods for the preservation of RBCs are important to maintain the quality and safety of RBCs provided to the patient as well as the overall clinical usage of blood products.
The use of RBCs during hypothermic storage in transfusion medicine has been extensively studied and evaluated. To date, RBCs are routinely stored at 2–6°C ranging from 21 to 42 days, depending on the used preservation solutions, including acid-citrate-dextrose, citrate-phosphate-dextrose-adenine, saline-adenine-glucose-mannitol (SAGM), and so forth.5, 6 Although RBC biochemical processes are slowed down at 4°C, cellular metabolism is not completely suppressed during storage.7 A variety of metabolic, biochemical, and morphologic changes are observed, known as the storage lesion, which could affect the RBC quality after infusion.8 Cryopreservation is an advanced technology for the long-term storage of cells.9 Similar to hypothermic storage, cryopreservation employs the advantage of low temperature to reduce molecular motion and halt metabolic and biochemical processes. Under ultra-low temperatures (−80 or −196°C), biological samples would maintain a state of “suspended animation” and the physiological activity would almost stop.10 Compared to hypothermic storage, RBC physiology, including hemoglobin (Hb) structure and membrane and cellular energetics, is not affected by long-term storage in the frozen state.6 Therefore, RBCs can be stored even for decades under a suitable cryopreservation protocol.11
However, the cells inevitably suffer from cryoinjuries during cryopreservation. First, the ice formation inside the extracellular matrix increases the concentration of solutes and leads to severe cell osmotic dehydration. Second, the ice can directly cause mechanical injury to cells.12, 13 Furthermore, the overproduction of reactive oxygen species (ROS) induced by low temperature can cause lipid peroxidation, DNA damage, and protein oxidation.14-17 To decrease cryoinjuries, cryoprotectants (CPAs) should be used in the cryopreservation process.18
For RBCs, glycerol is regarded as the current state-of-the-art CPA in many clinical settings.6, 19, 20 During cryopreservation, glycerol can permeate through RBCs to reduce intracellular ice formation and osmotic shock.21 Currently, two methods for the cryopreservation of RBCs are developed accordingly for clinical use, which employ glycerol at concentrations of 20% and 40% (w/v) termed the Low Glycerol Method (LGM) and the High Glycerol Method (HGM), respectively. In the first one (used in Europe), RBCs are cryopreserved and stored at −196°C using 20% w/v glycerol with rapid freezing rates (~100°C/min).22-24 The HGM protocol (used in the United States) is based on the slow freezing (~1°C/min) of RBCs at −80°C in 40% w/v glycerol.25-27 However, the optimal concentration of glycerol is up to 40%w/v, which may lead to hemolysis and RBC morphology alteration.28, 29 Therefore, the search for safe and effective CPAs is ongoing to promote cell cryopreservation. Many CPAs, such as hydroxyethyl starch (HES),30 trehalose,31 block copolymer worms,32 proline,33 and so forth, have been explored as CPAs for RBC cryopreservation.
However, there is no uniform standard to evaluate the performance of CPAs for RBCs. In earlier articles, the protective effects of CPAs were evaluated only by RBC recovery due to the limitations of technologies.34, 35 But it is insufficient and inaccurate. Although some researchers have added functional indicators of thawed RBCs to evaluate CPAs, it is still not enough to fully determine their performance and may be an obstacle for the cryopreservation of RBCs and transfusion therapy. According to previous studies, we have summarized for the first time comprehensive methods to evaluate the performance of CPAs based on their safety and effectiveness. First, the toxicity of conventional CPAs is a main challenge for cell cryopreservation. Therefore, it is necessary to use biocompatible CPAs to ensure the safety. Moreover, the effectiveness of CPAs can be determined by the properties of thawed RBCs, which are elucidated from membrane properties, protein activities, rheological properties, and metabolites levels.
In this review, we summarize the current development of CPAs for RBC cryopreservation, describing their advantages and disadvantages and the impact on the RBCs being frozen, which can help researchers fully understand the latest advances. We highlight the safety and effectiveness of CPAs evaluated by comprehensive methods. Further, we discuss specific parameters that need to be considered before CPAs progress to clinical transfusion therapy. Consequently, this review provides a new perspective to comprehensively evaluate CPAs for RBC cryopreservation, and novel CPAs confirmed by the methods may also be applied to other cells.
2 DEVELOPMENT OF CPAS FOR RBC CRYOPRESERVATION
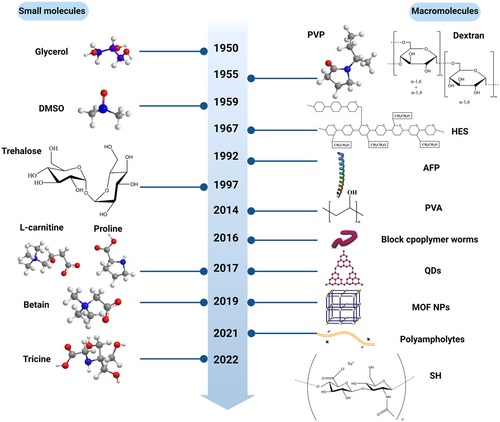
Based on the cryoinjuries during cryopreservation, it is obvious that the addition of CPAs can weaken the effects of cryoinjuries and improve the efficiency of RBC cryopreservation. With the rapid development of materials and chemistry, a series of CPAs have been explored and used in the process of cell cryopreservation for a long time. Herein, we briefly sum up the CPAs for RBC cryopreservation, which can be broadly classified into two categories: small molecule- and macromolecule-based CPAs (Figure 1). Additionally, more details about various CPAs used for the cryopreservation of RBC, including RBC species, the volumes of the RBC suspensions, CPA concentration, the recovery of cells after thawing or washing, and the advantages and disadvantages of different CPAs have been further summarized in Table 1.
| CPAs | RBC species | CPA concentrations | Outcomes | The advantages of CPAs | The disadvantages of CPAs | References | |
|---|---|---|---|---|---|---|---|
| Small molecule-based CPAs | Glycerol | Human RBCs | 20% (w/v) (LGM) 40% (w/v) (HGM) |
Postdeglycerolized recovery: ≥80%a | High-thawed RBC recovery, the only clinically licensed CPA for cryopreserving RBCs | Time-consuming and expensive deglycerolization process | [6, 36] |
| DMSO | Human RBCs | 10 wt % | Postthaw recovery: ~95% | Great membrane permeability, high thawed RBC recovery | Cytotoxicity | [37, 38] | |
| Trehalose | 0.5–1 mL human RBCs | 1 M | Postthaw recovery: ~81% | Good biocompatibility, high thawed RBC recovery | Trehalose cannot provide sufficient intracellular protection due to its impermeability | [31, 39] | |
| l-carnitine | Sheep RBCs | 8 wt % | Postthaw recovery: 83.99% | Good biocompatibility, high thawed RBC recovery | More experiments are needed to prove the effectiveness and safety of l-carnitine on a large volume of human RBCs | [40] | |
| Proline | 100 μL sheep RBCs | 4.5 wt % | Postthaw recovery: ~72% | Good biocompatibility | Low efficiency when used alone | [33] | |
| Betain | 40 μL sheep RBCs | 6 wt % | Postthaw recovery: ~80%; postthaw-wash recovery: ~70% |
Good biocompatibility, easy removal via membrane transport proteins | More experiments are needed to prove the effectiveness of betain on a large volume of human RBCs | [41] | |
| Tricine | 100 μL sheep RBCs | 6 wt % | Postthaw recovery: 81.2 ± 8.5% | Low toxicity, high thawed RBC recovery | Hard to be removed from cells | [42] | |
| Macromolecule-based CPAs | PVP | Sheep RBCs (1 × 109/mL) | 10% PVP Kb 15 MWc 10000 | Postthaw-wash recovery: 83.7% | Great biocompatibility, directly transfusable CPA | Increased hemolysis after transfusion | [43-45] |
| Dextran | 0.5-1 mL human RBCs | 30% (w/v) | Postthaw recovery: ~90% | Great biocompatibility, directly transfusable CPA | Increased hemolysis after transfusion | [31, 43, 46] | |
| HES | 450 mL Human RBCs | 11.5 wt % | Postthaw viability in terms of saline stabilityd: 92 ± 1% | Great biocompatibility, directly transfusable CPA | Increased hemolysis after transfusion | [43, 47, 48] | |
| AFP | Human RBCs | 30% (w/v) HES; 62 μg mL−1 type I AFP + 30% (w/v) HES |
Postthaw recovery (slow warminge): ~55%; postthaw recovery (slow warming): ~87.5% |
The low concentration of AFPs, a much higher IRI activity | Toxicological and immunological issues, high cost, and scale-up restrictions | [34, 49, 50] | |
| PVA | 500 μL ovine and human RBCs | 0.1 wt % 9 kDa PVA | Postthaw recovery: >40% | Good biocompatibility, low concentration | Low efficiency when used alone | [51] | |
| Block copolymer worms | 250 μL ovine RBCs | 5 wt % worms; 5 wt % worms +1 mg mL−1 PVA |
Postthaw recovery: 20%; postthaw recovery: 68% |
Worms enable postthaw gelation by warming to 20°C, thus providing a new approach for RBC storage and transport and a convenient matrix for 3D cell cultures. | Low efficiency when used alone | [32] | |
| QDs | 50 μL sheep RBCs | 10.0 mg mL−1 OQCNs-180-3 | Postthaw recovery: ∼55% | Good biocompatibility, simple and up-scalable synthetization compared to AFPs | Low efficiency when used alone | [52] | |
| MOF NPs | human RBCs | 0.5 mg mL–1 UiO-66-OH MOF NPs | Postthaw recovery: ∼40% | Good biocompatibility | Low efficiency when used alone | [53] | |
| Polyampholytes | 500 μL sheep RBCs | 100 mg mL–1 | Post-thaw recovery: >60% | Less processing challenges and easy removal | Low efficiency when used alone | [51] | |
| SH | 100 μL sheep RBCs | 1 mg/mL MSH | Post-thaw recovery: 63.2 ± 3.5%; post-thaw-wash recovery: 60.7 ± 2.3% |
Good biocompatibility, easy removal by direct washing | Low efficiency when used alone | [54] |
- a AABB guidelines: American Association of Blood Banks.
- b K = relative viscosities.
- c MW = average molecular weights.
- d Saline stability [%]=(1–HbS/HbT) × 100, where HbT corresponds to the total hemoglobin and HbS to the hemoglobin in the supernatant.
- e Slow warming: the sample was thawed in the air at room temperature.
2.1 Small molecule-based CPAs
Small molecule-based CPAs comprise glycerol,19 dimethyl sulfoxide (DMSO),37 trehalose,31 and so forth, which have been assessed for their cryoprotective activities in RBCs.
CPAs have received many researchers' attention since glycerol was first discovered in 1949.55 Freezing RBCs with glycerol as a CPA dates back to 1950. Smith19 reported an approach for freezing whole blood to −79°C with the addition of glycerol, which prevented hemolysis and did not alter the normal morphology of RBCs. At present, glycerol is the only CPA used for RBC transfusion products.6 Although glycerol is often regarded as having low toxicity, the working concentrations during cryopreservation are up to 20%–40%, which require the washing process after thawing to prevent RBC death or subsequent side effects.56 Moreover, cryopreserved RBCs in glycerol must meet certain guidelines (Table 2). After cryopreservation, thawed RBCs require a deglycerolization process to reduce the residual glycerol concentration to below 1%. According to international guidelines, thawed deglycerolized RBC recovery needs to be at least 80%, and the hemolysis in the RBC units should not exceed 0.8% in Europe and 1% in the United States. Additionally, at least 75% of transfused RBCs should still circulate 24 h after infusion.36, 57, 58
| Variable | European guidelines57 | AABB guidelines36 |
|---|---|---|
| Hemolysisa | < 0.8% | <1.0% |
| Postthaw recovery | - | ≥80% |
| In vivo RBC viability after 24 h transfusion | - | ≥75% |
| Volume | >185 mL | - |
| Hb content | >36 g/unit | - |
| Hematocrit | 0.65%–0.75% | - |
| Leukocytes | <1 × 106/U | - |
| Osmolarity | <340 mOsm/L | - |
- Abbreviations: AABB guidelines, American Association of Blood Banks; Hb, hemoglobin; RBCs, red blood cells.
- a Maximum allowable hemolysis at the time of infusion.
Subsequently, Lovelock and Bishop37 reported the use of DMSO for RBC cryopreservation in 1959. The advantage of DMSO is its more rapid penetration into cells as a CPA, but it is more toxic compared to glycerol.59 Other CPAs have been researched as substitutes for glycerol. Trehalose, a nonreducing disaccharide, could protect RBCs against cryoinjuries during cryopreservation owing to its exceptional cryoprotective properties.31 The nontoxicity of trehalose allows it to not be removed from cells after thawing. However, trehalose cannot be produced by mammalian cells and cannot penetrate the cell membrane, thus its cryoprotective activity is limited.60 Currently, a variety of methods have been researched to transport trehalose into cells for effective cryopreservation, including phospholipid phase transition, fluid-phase endocytosis, microinjection, and so forth, which could significantly promote the application of trehalose in the cryopreservation of cells.39 Moreover, Yang et al.33 compared the abilities of three biocompatible osmoprotectants (proline, glycine, and taurine) to inhibit osmotic injury and reduce ice formation and explored their potential as CPAs. Proline demonstrated the strongest capacity to prevent cryoinjuries. As a result, it also achieved the highest RBC recovery during the freezing and thawing process. Another study by Zhai et al.40 showed that natural zwitterionic l-carnitine could effectively cryopreserve RBCs by reducing osmotic and mechanical injury. The thawed sheep RBC recovery was up to 83.99% at 8% l-carnitine, demonstrating an obvious advantage over the other CPAs. Additionally, betaine41 and N-[Tri(hydroxymethyl)methyl]glycine (tricine)42 have also been explored as novel CPAs for RBC cryopreservation.
2.2 Macromolecule-based CPAs
The main benefit of using macromolecular CPAs like polyvinyl pyrrolidone (PVP),61 HES,30 antifreeze protein (AFP),34 and so forth is that they do not penetrate the cells. This property greatly facilitates their removal after thawing.62 Moreover, some nonpermeating CPAs that are biodegradable and well tolerated by the patient do not even need to be removed after thawing.63
In 1955, Bricka and Bessis61 demonstrated that RBCs suspended in high concentrations of dextran or PVP would survive at an extremely low temperature. In 1967, human RBCs were successfully cryopreserved using HES and liquid nitrogen (LN2) for the first time by Knorpp et al.30 Compared to dextran, HES is more stable during the freezing and thawing processes and long-term storage and is inexpensive to produce. HES is also as effective as PVP in the cryopreservation of RBCs, but it has the advantage over PVP of being metabolized and consequently not retained in the recipient, which would eliminate the need for laborious blood processing before transfusion.
AFPs have potent ice recrystallization inhibition (IRI) activity in frozen solutions.64, 65 Carpenter et al.34 found that the addition of relatively low concentrations (5–160 μg/mL) of (Pseudopleuronectes americanus) AFP greatly improved the survival of RBCs that were cryopreserved in HES. However, clinical use of AFPs in cryopreservation is challenging because of toxicological and immunological issues, high cost, and scale-up restrictions.49 Thus, synthetic AFP mimics were developed. Polyvinyl alcohol (PVA) has been explored as an AFP mimic and possesses the strong ability to inhibit ice recrystallization.66-68 Deller et al.69 reported that the RBC recovery in a 0.1 wt% PVA solution was more than 40%, indicating PVA provided protection to the RBCs by inhibiting ice crystal growth during thawing.
Nanomaterials with ice-regulation function show great potential in the cryopreservation of RBCs. Zhu et al.53 used zirconium (Zr)-based metal-organic framework (MOF) nanoparticles (NPs) for the cryopreservation of RBCs. The thawed human RBC recovery could reach ~40%, which was higher than that obtained using HES. Bai et al.52 found that oxidized quasi-carbon nitride quantum dots (OQCNs), which exhibited thermal-hysteresis activity, an ice-crystal shaping effect, and IRI activity, had good protective effects on sheep RBCs and could improve RBC recovery to 55% during cryopreservation.
Polymeric CPAs, such as block copolymer worms and polyampholytes, have demonstrated promising applications as cell cryopreservation enhancers.32, 49 Highly hydroxylated block copolymer worms are demonstrated to be a viable alternative to HES as an extracellular matrix for RBCs. When used alone, the worms are not an especially efficient preservative. However, when complemented by poly(vinyl alcohol), an ice-inhibiting polymer, the thawed RBC recovery was significantly increased from 20% to 68%.32 For polyampholytes, there is a study conducted by Bailey et al.51 wherein a polyampholyte synergistically enhanced sheep RBC cryopreservation and achieved >60% thawed RBC recovery. The polymer has the advantages of fewer processing challenges and easy removal due to its nonpermeability compared to glycerol.
Moreover, the natural macromolecule sodium hyaluronate (SH) has been reported as a biocompatible CPA for RBC cryopreservation. The addition of SH greatly improved RBC survival, up to 63%, which was higher than in the control group (33%). And the properties of thawed RBCs were well maintained.54
3 THE SAFETY OF CPAS
The safety of CPAs can be represented by their biocompatibility to RBCs, which refers to interactions occurring between them and RBCs.70 Most of the organic solvent CPAs show poor biocompatibility, which can lead to severe side effects.71, 72 Therefore, it is necessary to evaluate the biocompatibility of CPAs, which can be determined by the hemolysis test and RBC morphology observation.
MOF NPs could be excellent candidates for cryopreservation applications due to their good biocompatibility. Hemolysis of RBCs after incubation with MOF NPs at different concentrations was lower than 8%. To understand the underlying reason for low hemolysis, the interaction between the RBC membrane and UiO-66 MOF NPs that showed the highest hemolytic activity was evaluated through scanning electron microscopy (SEM) analysis. The results displayed that UiO-66 MOF NPs were flatly orientated on the surface of the RBCs along their planes with no evident membrane deformation, which explained the reason for their good biocompatibility.53 Besides this, Stefanic et al.29 first reported that colloidal bioinspired apatite NP could assist in delivering nonpermeable trehalose into RBCs through improved membrane permeation, thus increasing thawed RBC recovery, up to 91% (42% improvement over the control group without NP assistance). Incubation of RBCs with apatite NP at pH 6.5 and 7.4 for 7 h resulted in low levels of hemolysis (<8%), indicating its good biocompatibility.
After cryopreservation, the residual CPAs pose a risk for thawed RBCs. If CPAs exhibit toxicity or poor biocompatibility, they must be completely removed before clinical use. One-step and multistep methods are usually utilized to remove CPAs from cryopreserved cells, whose processes often require cell washing and centrifugation.73-75 For glycerol-based freezing methods, intracellular glycerol must be washed by the multi-step method to avoid posttransfusion intravascular hemolysis.76 After the deglycerolization process, ~15% of the RBCs are hemolyzed, the RBC morphology alters, and there is ~1% Gly residue in the cells.11, 77, 78 The residual concentration of glycerol can be determined by measuring the osmolarity of the final resuspension fluid.57 However, CPAs with excellent biocompatibility are usually well-tolerated and do not need to be removed. Janis et al.79 delivered trehalose into RBCs by sonoporation without removal steps, and the thawed RBCs can be directly used for clinical applications without any adverse transfusion reactions. Some previous studies have declared that HES is somewhat special. Due to its low toxicity, it is unnecessary to be removed under certain circumstances.80 Therefore, nontoxic CPAs are more attractive because they are not removed in some clinical applications, but whether they can fully replace glycerol as a CPA has to be further explored.
Moreover, it must be noted that free hemoglobin released from damaged RBC needs to be removed before transfusion. Due to the toxicity of free hemoglobin, the final washing steps restored a hemolysis level below 1% (United States) or 0.8% (Europe).81
As a result, it is important to overcome the biocompatibility problem when designing CPAs. For intracellular CPA, it should be nontoxic to the cryopreserved cells at the concentration used in the study and be easily removed from cells. The zwitterionic molecule, betaine, can provide both intracellular and extracellular protection to cells as a natural osmoprotectant. During cryopreservation and the removal of betain, RBC integrity was well maintained and hemolysis was avoided, indicating that it had good biocompatibility and could be easily removed from cells via transport proteins.41 Similarly, it is equally crucial to assess the cytotoxicity of extracellular CPAs for cells. Cryoprotective extracellular macromolecules should be biocompatible, biodegradable, and well tolerated by the patient, like some polymers, sugars, and starches. Because they do not penetrate the cell membrane, the osmotic problems would be avoided, and the removal process of CPA would be easier than that of glycerol.6
4 THE EFFECTIVENESS OF CPAS
The effectiveness of CPAs is traditionally evaluated by thawed cell recovery because it is the key indicator of in vitro quality required by current regulatory bodies in blood transfusion therapy.82, 83 But the protective effects of CPAs need to be estimated further by considering thawed cell functional properties. Therefore, the four aspects of thawed RBCs: membrane properties, protein activities, rheological properties, and metabolites levels are chosen to evaluate the effectiveness of CPAs. First, the integrity of the RBC membrane, which maintains normal cell shape, deformability, and mechanical stability, is crucial for RBC functionality and viability.84 Second, proteins in RBCs are involved in most biochemical reactions. For example, the ATPases can maintain intracellular gradients of ions.85 Third, the rheological properties of RBCs play a vital role in ensuring oxygen transport in the microcirculation.86 Fourth, the metabolites levels can indicate the physiological properties of RBCs.87 These must be taken seriously because transfusion of impaired RBCs can cause infection, multiple organ dysfunction, and delirium.88
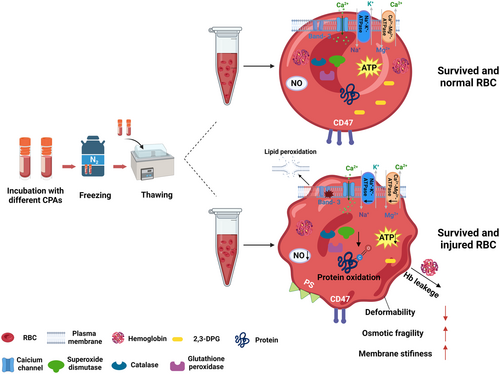
Herein, Figure 2 clearly shows the protective effects of different CPAs on RBCs, which are reflected by thawed RBC properties. Additionally, all the detailed parameters and detection methods for cell properties are summarized in Table 3. It should be noted that the detection methods are a summary of techniques that have been used by other researchers and are not necessarily the gold standard.
| Properties of RBCs | Parameters | Detection methods | References |
|---|---|---|---|
| Membrane properties | Osmotic fragility | Spectrophotometry | [85] |
| Morphology | SEM, flow cytometry | [11, 76] | |
| Membrane lipid peroxidation | MDA level, mass spectrometry | [89, 90] | |
| Membrane stiffness | AFM | [91] | |
| PS exposure and CD47 expression | Flow cytometry | [92] | |
| Protein activities | Hb content | HiCN, proteomics, and metabolomics | [93, 94] |
| Band-3 phosphorylation | Immunofluorescence staining, proteomics, and metabolomics | [95, 96] | |
| ATPase activitya | Inorganic phosphorus method | [54] | |
| Protein oxidation | Carbonylation assay | [89] | |
| Antioxidant enzyme activity | SOD: xanthine oxidase method | [97] | |
| CAT: ammonium molybdate colorimetry | |||
| GSH-PX: DTNB chromogenic reaction | |||
| Rheological properties | Aggregation | Automated hemorheology analyzer | [98] |
| Deformability | Ektacytometry, AFM, microfluidics | [99-101] | |
| Metabolites levels | ATP level | Firefly luciferase method | [102] |
| 2,3-DPG level | NADH oxidation method | [102] | |
| NO content | Photolysis/Chemiluminescence | [103] |
- Abbreviations: 2,3-DPG, 2,3-diphosphoglycerate; AFM, atomic force microscopy; ATP, adenosine triphosphate; CAT, catalase; CPAs; cryoprotectants; DTNB, 5′-dithiobis (2-nitrobenzoic acid); GSH-PX, glutathione peroxidase; Hb, hemoglobin; HiCN, hemoglobin cyanide method; MDA, malondialdehyde; NADH, nicotinamide adenine dinucleotide; NO, nitric oxide; PS, phosphatidylserine; RBCs, red blood cells, SEM, scanning electron microscopy; SOD, superoxide dismutase.
- a Including Na+/K+-ATPase and Ca2+/Mg2+-ATPase.
4.1 Membrane properties
Maintaining the integrity of the membrane is the basis for the functions of RBCs. If there is not a selectively permeable barrier to stop the loss of its contents, the cell will die.104, 105 Osmotic fragility, morphology, membrane lipid peroxidation, membrane stiffness, phosphatidylserine (PS) exposure, and CD47 expression can be evaluation criteria for the membrane properties of thawed RBCs.
4.1.1 Osmotic fragility
Osmotic fragility is the resistance of RBC hemolysis to osmotic changes.106 It is an efficient way of showing how susceptible RBCs are to osmotic stress.107, 108 The osmotic fragility is measured by stepwise diluting graded NaCl solutions ranging from 0.1% to 1.0%.85 The osmolarity at half-maximal hemolysis is defined as the osmotic fragility index. According to this approach, a higher osmotic fragility index indicates that the cellular ability to resist osmotic stress is weaker.109 Dou et al.85 found that RBCs cryopreserved in l-proline and trehalose showed a higher osmotic fragility index compared to fresh RBCs, which indicated they could not maintain normal membrane stability. Dextran was evaluated as a glycerol substitute for human RBC cryopreservation by Pellerin-Mendes et al.31 90% cryopreserved RBCs in 30% (w/v) dextran in LN2 were recovered. The osmotic fragility of RBCs was not changed.
4.1.2 Morphology
The normal morphology of RBCs plays a key role in their survival and oxygen-carrying capacity.110 Healthy RBCs are unique biconcave discocytes, which makes RBCs more flexible to move across the microvasculature and aids in enhancing their oxygen-delivering ability.111, 112 The mature RBC is about 7.2 μm in diameter, 1.5–2.5 μm thick, and has a 90 fL mean volume.113 SEM analysis is utilized to investigate the morphology of the RBCs.11 Graham et al.114 compared the effectiveness of HES and DMSO solutions for cryopreservation of avian RBCs. SEM revealed that RBCs cryopreserved in a freezing solution containing 10% DMSO remained in their regular shape with no observable cell deformation. Conversely, many RBCs that were frozen in a 20% HES solution presented severe membrane changes, including deformation and hemispheric protrusions. Another type of analysis of cell morphology is flow cytometry. The size of the forward scatter would increase in swollen RBCs, while the size of the side scatter would increase in shrunken cells. These analyses would demonstrate if cells suffer damage in a manner that alters the shape of their surface membrane.38 Yao et al.76 explored the hydrogel microencapsulation method combined with 0.7 M trehalose to cryopreserve RBCs. Compared to fresh RBCs, cryopreserved RBCs using the above method displayed similar characteristics after freeze-thaw-wash, with no significant alterations in either forward scatter or side scatter.
4.1.3 Membrane lipid peroxidation
When RBCs are subjected to extreme environmental stress, such as freezing, oxidative injury leads to a level of membrane lipid peroxidation, measured as malondialdehyde (MDA) content. Alotaibi et al.89 investigated a protective agent called salidroside (Sal) to promote the cryopreservation of RBCs. The addition of Sal to the cryo-solutions containing glycerol or trehalose improved RBC survival. Sal is also an antioxidant, which could reduce oxidative injury during cryopreservation. The supplementation of Sal decreased the lipid peroxidation of thawed RBCs that were cryopreserved using glycerol or trehalose.
4.1.4 PS exposure and CD47 expression
PS exposure onto the outer leaflet of the RBC membrane and loss of the cell adhesion molecule glycoprotein CD47 expression, which leads to RBCs being recognized and phagocytosed by macrophages, can act as potentially important indicators of RBC membrane damage.94, 112 Flow cytometry was used to measure RBC membrane PS externalization and CD47 expression.92 Holovati et al.115 studied the use of liposomes, which are regarded as microscopic vesicles, to deliver trehalose to mammalian cells. This study demonstrated that trehalose-containing liposomes greatly enhance human RBC recovery and membrane quality after cryopreservation. Flow cytometry analysis of RBC PS externalization showed that freezing greatly damaged the membrane asymmetry of both l-RBCs (liposome-treated RBCs) and c-RBCs (control RBCs, not treated by liposomes). But the PS expression of l-RBCs was much lower than that of c-RBCs after cryopreservation in terms of RBC mean fluorescence intensities. For RBC expression of CD47 antigen, thawed l-RBCs produced tremendously more CD47 antigen on the membranes than thawed c-RBCs.
4.2 Protein activities
Proteins in RBCs serve an important role in most biochemical reactions, such as Hb, Band-3, ATPases, and so forth. Therefore, being aware of their changes could be used as indicators to evaluate thawed RBC properties.
4.2.1 Hb content
Hb is the main component of RBCs and constitutes up to 95% of the cell's total protein.116 This protein comprises four globular subunits, each having a heme group with a central molecule of iron in the ferrous state. Each heme group can bind one oxygen molecule.117 The main function of Hb is to carry O2 from the lungs to the tissues, where it returns CO2 to the lungs.118 Hb can be estimated by the hemoglobincyanide method (HiCN). The number of RBCs per milliliter is determined using a hemocytometer.93, 119 Consequently, the Hb content of RBCs could be obtained. Liu et al.42 explored the potential of tricine as a CPA for RBC cryopreservation. With the aid of tricine, the Hb of thawed RBCs is comparable to that of fresh RBCs during the freezing and thawing process.
4.2.2 Band 3 phosphorylation
Band 3 is a crucial protein that maintains the flexibility and stability of the RBC membrane, as it is a transporter that mediates electroneutral anion exchange across the plasma membrane and a structural protein for the cytoskeleton network.112, 120 Changes in band 3 phosphorylation levels have been related to the increased rigidity of RBCs, which reduces their perfusion to host organs and their lifespan in the circulation.121 The band 3 proteins on the RBC membrane can be measured by immunofluorescence staining.122 Shen et al.95 demonstrated a practical and simple approach for improving the efficacy of RBC cryopreservation and examined their functional expressions, including RBC band 3 proteins after cryopreservation. By choosing 5 × 6 mm polytetrafluoroethylene (PTFE) tubes with superior cooling capabilities and using the trehalose dehydration technique, the results showed that most band 3 proteins were evenly distributed and clearly outlined on the RBC membrane in the 5% Gly and 0.4 M trehalose as well as the fresh group.
4.2.3 ATPase activity
ATPases can maintain intracellular gradients of ions.85 And the stability of ion concentration in cells plays a key role in maintaining signal transduction and regulating cell metabolism.123 Na+/K+-ATPase and Ca2+/Mg2+-ATPase are two ATP-hydrolyzing enzymes that are determined by the inorganic phosphorus method.54 Dou et al.85 explored the combination of l-proline and trehalose as a cryoprotective protocol in the cryopreservation of RBCs. As a result, the postthawing hemolysis rate decreased, and the activities of Na+/K+-ATPase and Ca2+/Mg2+-ATPase were not altered by using this protocol.
4.2.4 Protein oxidation
Protein oxidation may occur in RBCs due to excessive ROS, which can exacerbate metabolic impairments.88, 124 For proteins, the measurement of protein carbonyl content (PCC) is the most reliable and widely used marker for protein oxidation.125 Protein carbonylation occurs when protein molecules are attacked by ROS, altering the side chains of amine groups such as lysine, arginine, and proline into carbonyl groups.126 There are several methods used to detect and quantify protein carbonylation, and the most common one is based on the derivatization of carbonylated proteins with 2,4-dinitrophenylhydrazine (2,4-DNPH).127 RBCs cryopreserved with glycerol or trehalose were discovered to exhibit increased ROS accumulation and protein oxidation by immunodetection of 2,4-DNPH derivatized proteins. Supplementation of the antioxidant Sal mitigated this effect.89, 128
4.2.5 Antioxidant enzyme activity
To prevent and alleviate intracellular oxidative stress, RBCs express several potent and efficient enzymatic antioxidant defense systems. Superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-PX) are part of them to counteract free radicals.129 SOD removes excess superoxide radicals (O2−) by transforming them into oxygen (O2) and hydrogen peroxide (H2O2). The latter is in turn converted by CAT to water and oxygen. Therefore, SOD and CAT in RBCs can defend Hb and other cellular components from free radicals to some extent. And GHS-PX eliminates peroxide metabolites of macromolecules.97, 130 Liu et al.42 assessed the antioxidant enzyme activities of RBCs cryopreserved in tricine after cryopreservation. With the aid of tricine, the activities of antioxidant enzymes were maintained well, indicating tricine has the ability to mitigate antioxidant damage.
4.3 Rheological properties
The rheological properties of RBCs (i.e., aggregation and deformability) are crucial for oxygen delivery in microcirculation.86, 131 Changes to the rheologic properties of RBCs may restrict or impede blood flow in the microcirculation, which can affect tissue perfusion and cause ischemia or even infarction.132, 133
4.3.1 Aggregation
RBC aggregation is an essential factor in the flow behavior of blood, which refers to the tendency of RBCs to stick together.110, 134 It is influenced by numerous factors, including shear stress, the tonicity of suspension solutions, and the presence of potentiators such as dextran that can induce RBC aggregation.135, 136 The aggregation ability of RBCs was measured by the aggregation index (AI) depending on the kinetics and extent of aggregation, where a larger AI indicates an enhanced ability to aggregate.109 Gao et al.98 synthesized a dynamic membrane-active glycopeptide called PL-g-(MT/BA) (PMB) to improve RBC cryopreservation, which could efficiently help nonpermeable trehalose enter human RBCs with low hemolysis. In addition to high trehalose uptake, maintaining the biofunctions of human RBCs is crucial during incubation and freeze-thaw. The aggregation index of PMB-treated RBCs after cryopreservation, determined by an automated hemorheology analyzer, was comparable to that of the fresh RBCs, suggesting the aggregability of thawed RBCs was maintained normally.
4.3.2 Deformability
The deformability of RBCs is mostly influenced by the membrane mechanical properties. Variations in mechanical properties, particularly stiffness and viscosity, often impair RBC deformability, which might restrict blood flow through the microvascular system140 and result in pathophysiological effects like anemia141 and sepsis.142
RBCs must maintain the proper stiffness range to go through capillaries in the microcirculation in the human body.143 Cell stiffness is assessed by determining the cells' elastic moduli using atomic force microscopy (AFM).144 The higher the cell's elastic modulus, the stiffer it is.145 El Assal et al.91 used a novel cryo-ink integrated with a bioprinting approach to preserve RBCs during nanoliter vitrification. Vitrification is the solidification process from liquid to glass.146 This cryo-ink includes ectoine, trehalose, and polyethylene glycol, which functions as a CPA to aid the cells in preventing freeze-thaw injury during cryopreservation. The results showed that the recovered RBCs after ectoine-based vitrification maintained their comparable stiffness to fresh RBCs.
Blood viscosity is affected by plasma viscosity, haematocrit, and the mechanical properties of the RBC.147 The effects of cryopreservation on RBC hemorheological properties and related metabolic parameters were explored by Bizjak et al.86 A sample of 10 healthy participants' blood was collected, glycerolized, and frozen at −80°C. Following 4, 8, and 12 weeks, aliquots were thawed and processed further, respectively. At these points in time, fresh blood samples were also taken from each participant. The influence on blood viscosity after cryopreservation was evaluated. The data demonstrated that the sample viscosity of stored blood was lower than the corresponding values for fresh blood, which was most likely caused by residual cellular glycerol. The possible consequences of the viscosity change need to be further studied.
4.4 Metabolites levels
Strong evidence suggests that the metabolites levels are crucial for determining post-transfusion RBC viability.86, 148 It includes adenosine triphosphate (ATP),84 2,3-diphosphoglycerate (2,3-DPG),149 and nitric oxide (NO).150
4.4.1 ATP level
ATP levels in RBCs are critical for maintaining the overall functioning of the RBC. ATP is the energy source for numerous biochemical reactions in RBCs, such as actively holding PS to the inner layer of the RBC membrane.143 A reduction in ATP levels of RBCs triggered by storage will impair all RBC metabolic activities, including glycolysis, the formation of cytosolic antioxidants, and the maintenance of membrane integrity.84
4.4.2 2,3-DPG level
The main function of RBCs is to carry oxygen to tissues and collect carbon dioxide. In this process, 2,3-DPG plays an essential role in regulating hemoglobin oxygen affinity.150 A decrease in 2,3-DPG during storage impairs RBCs ability to release oxygen and enhances rigid RBC adhesion to endothelial cells.86 As a result, stored RBC cannot provide the tissues with oxygen as well as fresh cells. After 6 to 8 h in circulation, this impairment is repaired.151 However, patients who require urgent oxygen transport to organs and tissues are nearly put at risk when using low 2,3-DPG blood.83 Moreover, many transfusion complications are related to the low 2,3-DPG levels of transfused RBCs.152
In the cryopreservation of RBCs, CPAs are added to help reduce the loss of ATP and 2,3-DPG.153 Shen et al.102 showed that when RBCs were cryopreserved with trehalose and glycerol, ATP and 2,3-DPG levels were not visibly affected after cryopreservation and met clinical standards for the safety of blood transfusion.
4.4.3 NO content
NO is a crucial signaling molecule that contributes to hypoxic vasodilation by acting as a strong vasodilator.154 Cellular NO, which is produced in RBCs by RBC-NO synthase activity, is essential to advantageously promote RBC deformability.155 Rogers et al.103 refined the glycerolization process by minimizing front-end processing time and optimizing a deglycerolization protocol. To assess the effect of RBC cryopreservation in the research-optimized process, the NO content of cryopreserved in 40% v/v glycerol or deglycerolized RBCs was compared to that of fresh RBCs. NO content was measured by photolysis/chemiluminescence. Total NO content was not significantly different between cryopreserved or deglycerolized RBCs and fresh RBCs, though freezing seemed to somewhat increase NO levels. The iron nitrosyl hemoglobin (HbFeNO) content stayed unchanged under all circumstances. Independent of the presence of glycerol, the content of S-nitrosohemoglobin (SNO-Hb) significantly increased after freezing. These findings suggest that the research-optimized process for RBC cryopreservation and deglycerolization in 40% v/v glycerol has no significant effect on the NO content.
In short, the effectiveness of CPAs can be evaluated by membrane properties, protein activities, rheological properties, and metabolites levels. Apart from these aspects, some indicators on RBCs that are not used in the cryopreservation field can be carried out to evaluate the quality of cryopreserved RBCs. For example, RBC microparticles (RMPs) that can be used as biomarkers of RBC quality are potentially immunogenic and inhibitory to NO regulation. The organization and integrity of the RBC membrane, which is made up of asymmetrically distributed phospholipids (PLs), cholesterol (C), and proteins, are crucial for in vivo RBC circulation and function. RBC quality can also be determined by the composition and quantity of PLs and C.7
5 DISCUSSION AND FUTURE PERSPECTIVES
There is an enormous demand for blood products in clinical medicine, particularly RBCs, which can significantly enhance survival. To reduce hypothermic storage lesions and extend the shelf life of RBCs, cryopreservation is a promising method to preserve cells. During the process, cells inevitably suffer from cryoinjuries. Therefore, many CPAs have been found and used in cryopreservation processes to protect cells. The recent development of CPAs for RBC cryopreservation has been outlined in this review. Currently, glycerol is the only CPA that is used clinically for cryopreservation of RBCs. Meanwhile, there are many more biomaterials that are worthy of further study, such as sugar derivatives, amino acids, AFP mimics, nanomaterials, and so forth.
Moreover, we have pointed out that biocompatible CPAs may have advantages in the cryopreservation of RBCs. Some articles have declared that the use of trehalose and HES in RBCs does not require removal steps because of their good biocompatibility, which can decrease the cost and the risk of hemolysis in the cryopreservation of RBCs. Conversely, CPAs with poor biocompatibility must be removed after thawing. For example, when a high concentration of glycerol is used as the CPA, the thawed RBCs need to be washed with a deglycerolization process before transfusion. The addition of other biocompatible CPAs can reduce the concentration of glycerol, which may be a more effective and less toxic method for RBC cryopreservation.
We have also discussed the effectiveness of CPAs based on RBC properties. Membrane properties, protein activities, rheological properties, and metabolites levels of RBCs can be used to judge the effectiveness of CPAs. Osmotic fragility, morphology, membrane lipid peroxidation, membrane stiffness, PS exposure, and CD47 expression are employed as indicators of RBC membrane properties. Protein activities of RBCs are elucidated by different aspects: Hb leakage, Band-3 phosphorylation, ATPase activity, protein oxidation, and antioxidant enzyme activity. The rheological properties of RBCs include aggregation and deformability. ATP, 2,3-DPG, and NO are several important metabolites of RBCs. It must be noted that all studies have only been done at the laboratory level, and more experiments are needed for the clinical applications of thawed RBCs.
However, it is unrealistic to measure all the in vitro tests mentioned for the evaluation of CPAs. The important parameters include the biocompatibility test of CPAs and postfreeze-thaw/wash cell recovery used for evaluating the safety and effectiveness of CPAs, respectively, which are really needed. In contrast, thawed cell functional properties, including membrane properties, protein activities, rheological properties, and metabolites levels, would be nice to know.
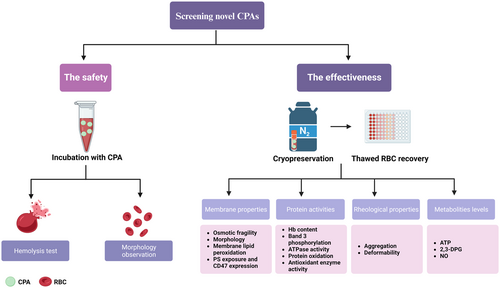
We believe that CPAs confirmed by comprehensive methods have the potential to be used for clinical transfusion therapy. In Figure 3, we provide a schematic figure to illustrate how to screen novel CPAs for the cryopreservation of RBCs. To further ensure CPA effectiveness in cryopreserving RBCs for clinical applications, more experiments are needed before transfusion. Currently, the RBCs cryopreserved in CPAs are from sheep or avian, which cannot simply be extrapolated to human RBCs. Apart from the RBC species, a volume of at least 150 mL of packed RBCs for transfusion is needed,156 while most CPAs are tested on very small volumes of RBCs. Therefore, further studies are required to prove the safety and effectiveness of CPAs on a large volume of human RBCs. Apart from the species and volume of RBCs, serologic compatibility between the recipient and the donor should be tested before transfusion. Pretransfusion testing of patients receiving allogeneic blood includes ABO and rhesus D (RhD) typing and antibody screens to detect unexpected antibodies to RBC antigens.5
6 CONCLUSION
In conclusion, this paper reviewed the recent advances in CPAs for RBC cryopreservation and summarized the methods for determining the safety and effectiveness of CPAs comprehensively. More importantly, RBC cryopreservation can be used as a simple model for novel CPA screening, and research results from in vitro measures can initially ensure the biocompatibility and effectiveness of CPAs before progressing to nucleated cell cryopreservation. We believe that the review can provide a new perspective for cryopreservation and promote the future development of biomedical research.
AUTHOR CONTRIBUTIONS
Yuying Hu: Writing—original draft (equal). Xiangjian Liu: Writing—original draft (equal). AKhlaq Ahmad: Writing—review & editing (supporting). Jiangming Chen: Writing—review & editing (supporting). Xiaoxiao Chen: Writing—review & editing (supporting). Wenqian Zhang: Writing—review & editing (supporting). All authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The authors have nothing to report.
Open Research
DATA AVAILABILITY STATEMENT
The authors have nothing to report.




