Xanomeline-Trospium Chloride (Cobenfy): A New Era in Managing Schizophrenia—Comparative Effectiveness and Economic Challenges
Funding: The authors received no specific funding for this work.
Graphical Abstract
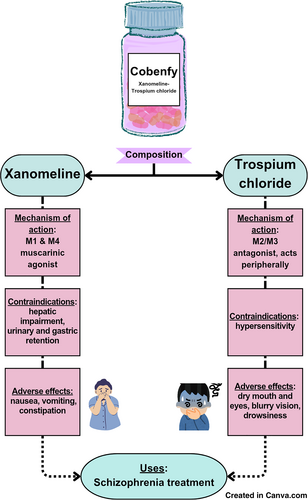
Xanomeline-trospium chloride (cobenfy) is a muscarinic agonist used to treat hallucinations and psychotic illnesses such as Alzheimer's and Schizophrenia. It has been constructed for patients who are unable to tolerate dopamine-targeting therapies and a comparison is drawn against other similar drugs with variables like cost, mechanism of action, efficacy and contraindications taken into account. Specifically effective for schizophrenia, it is safer with fewer side effects and efficient with stronger success rates, offering pharmacists and physicians an alternative to mainstream antipsychotic drugs.
Abbreviations
-
- AE
-
- adverse effect
-
- PANSS
-
- positive and negative syndrome scale
-
- TEAE
-
- treatment-emergent adverse event
-
- XTC
-
- Xanomeline-Trospium chloride
1 Introduction
Cobenfy, the brand name for the novel antipsychotic Xanomeline-Trospium chloride (XTC), functions as a muscarinic agonist to help manage symptoms of schizophrenia. XTC is intended for use in patients presenting with psychotic symptoms such as hallucinations, disordered speech and behavior, and paranoia. Xanomeline was first developed alongside other muscarinic agonists to reduce the effects of Alzheimer's disease [1]. When paired with xanomeline, the hydrophilic muscarinic antagonist trospium dramatically reduces cholinergic adverse effects (AEs). This combination drives the therapeutic potential of XTC without blocking the dopamine D2 receptor, the primary mechanism of conventional antipsychotics [2]. Although blocking these receptors reduces psychotic symptoms, it has not been demonstrated that this blockage and symptom reduction are directly correlated. The purpose of this paper is to assess the potential of XTC as a novel therapeutic approach in the treatment of schizophrenia, to highlight its efficacy compared with other antipsychotics, and to examine the economic obstacles that might limit patient access to this new treatment.
2 Molecular Mechanism of Xanomeline-Trospium Chloride
XTC functions by acting at muscarinic receptors rather than by antagonizing dopamine D2 receptors. This has a profound impact on central intracellular pathways related to cognition and dopamine regulation. Xanomeline is a selective M1 and M4 muscarinic agonist. Activation of Gq protein-coupled M1 receptors in the prefrontal cortex and hippocampus by xanomeline results in phospholipase C activation. This process leads to the hydrolysis of phosphatidylinositol 4,5-bisphosphate, forming inositol triphosphate and diacylglycerol. These molecules increase the concentration of intracellular calcium and activate protein kinase C. These molecular events enhance glutamate N-methyl-D-aspartate NMDA receptor function, synaptic plasticity, and cognitive processing through the mitogen-activated protein kinase/cAMP response element binding protein MAPK/CREB pathway. Meanwhile, agonism of M4 receptors in the striatum and ventral tegmental area (VTA) activates Gi/o proteins that inhibit adenylyl cyclase and reduce the activity of cyclic AMP and protein kinase A. This suppresses excessive dopamine release and thus helps to control psychotic symptoms without affecting normal motor function; this is an important advantage over traditional dopamine-blocking antipsychotics, which commonly cause movement disorders. However, broad muscarinic activation by xanomeline activates M1, M2, and M3 receptors in peripheral tissues, causing side effects such as nausea, diarrhea, and bradycardia. To counteract this, XTC includes trospium, a peripherally acting muscarinic antagonist that blocks M2 and M3 receptors in the gut, heart, and glands but does not cross the blood-brain barrier. This limits the activity of xanomeline to central areas and helps prevent unwanted gastrointestinal and cardiovascular side effects. By combining targeted dopamine modulation, cognitive enhancement, and reduced side effects, XTC offers a new approach for the treatment of schizophrenia, avoiding the complications and metabolic AEs associated with traditional dopamine-blocking antipsychotics [3]. The molecular mechanism of XTC is illustrated in Figure 1.
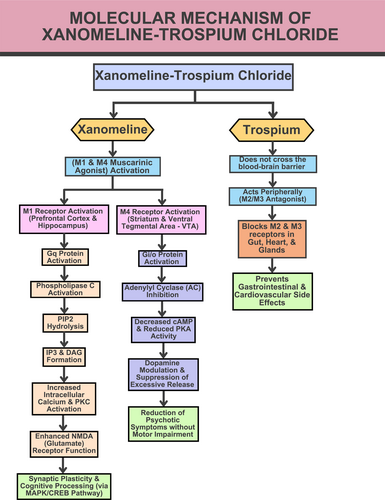
The molecular mechanism of xanomeline-trospium chloride.
3 Efficacy and Side Effects of Xanomeline-Trospium Chloride
In terms of side effects, XTC is superior to dopamine-targeting antipsychotics, which are often linked to weight gain and extrapyramidal symptoms. Its approval was supported by data from the EMERGENT program's five clinical trials, three of which were placebo-controlled. Following a screening of 431 people, 256 participants were randomly assigned to receive either a placebo (131 participants) or XTC (125 participants). XTC outperformed the placebo in the EMERGENT-3 trial by lowering the overall positive and negative syndrome scale (PANSS) score by 8.4 points in terms of symptom severity. Improvements in the PANSS total score were observed from the second week of XTC treatment and were maintained until the end of the trial (5 weeks) [2]. After 52 weeks, the average weight loss was 2.56 kg, with no change in HbA1c levels. HbA1c measures the percentage of hemoglobin in the blood that is coated with glucose (glycated). It reflects the average blood sugar levels over the previous 2–3 months. This was notable, as long-term studies have indicated a low risk to blood sugar and cholesterol levels, demonstrating long-term overall symptom relief without adverse metabolic changes [4]. The most commonly reported side effects included nausea (19.2%), vomiting (16%), diarrhea (5.6%), hypertension (6.4%), constipation (12.8%), increased body weight (6.4%), and gastroesophageal reflux disease. The treatment-emergent adverse events (TEAEs) were mainly gastrointestinal, were mild to moderate in severity, and resolved over the first two to 3 weeks of the experiment. Eight subjects (6.4%) suffered hypertension with XTC compared with two participants (1.6%) in the placebo group. A total of 46 participants (36.8%) in the XTC group and 38 participants (29.0%) in the placebo group discontinued the trial early, primarily due to AEs. However, the proportion of participants who specifically discontinued treatment due to a TEAEs was similar between the groups—6.4% in the XTC group and 5.5% in the placebo group [2]. Although the withdrawal rate was low, 3.2% of participants experienced symptoms of akathisia, some of which were unresolved at the end of the treatment. The trials also established an association between XTC and an increase in supine heart rate and elevated blood pressure compared with placebo; however, these effects were resolved by the end of the trial. However, the blood pressure-related effects of XTC are still being addressed in real-world research [5]. Angioedema of the face and lips and urinary retention were also noted.
4 Comparative Analysis
Dopamine receptor antagonists are associated with many AEs, including metabolic syndrome and weight gain. For instance, olanzapine and clozapine are known to cause hyperlipidemia and insulin resistance in many patients [6]. Meanwhile, XTC produced an average weight loss of 2.56 kg. Another dopaminergic antipsychotic, aripiprazole, causes numerous neurological side effects such as headache (27%), anxiety (17%), insomnia (16%), and akathisia (13%), along with gastrointestinal symptoms including nausea (13%), vomiting (11%) and constipation (10%) reported in clinical studies [7]. In older adults with dementia-related psychosis, aripiprazole is contraindicated. XTC has little effect on weight and metabolism, differentiating it from other dopaminergic antipsychotics such as aripirazole, brexpiprazole, cariprazine, lurasidone, olanzapine and clozapine. Although it has a lower risk of weight gain compared with other antipsychotics, XTC may still cause some metabolic problems, including dyslipidemia and hyperglycemia. Overall, the favorable side effect profile of XTC may improve patient adherence. Likewise, the risk of weight gain with aripiprazole is lower than that with other antipsychotics, which is also beneficial for adherence. However, the response of individual patients may be different.
A study conducted in patients with more severe symptoms of schizophrenia supported the use of brexpiprazole as being safe and well-tolerated. There was little sign of the motor-related AEs typically associated with the antipsychotics cariprazine and lurasidone [8]. Brexpiprazole is associated with weight gain (5%), constipation (2%), diarrhea (3%), nausea (3%), akathisia (7%), headache (9%), dizziness (3%), extrapyramidal disorder (6%), anxiety (2%), and insomnia (8%). Additionally, it also has lower akathisia rates than aripiprazole. Brexpiprazole produced a large decrease in the Montgomery–Asberg Depression Rating Scale (3.2, compared with 3.0 after aripiprazole treatment) when used as an adjuvant treatment for serious depression. However, confidence intervals for the medications like aripirazole, brexpiprazole and cariprazine suggest similar efficacy for the same indication. Brexpiprazole had the lowest rate of discontinuation, followed by aripiprazole and cariprazine, which had the highest rates. For all three atypical antipsychotics, akathisia was the most frequent AE resulting in discontinuation [7]. Cariprazine causes dizziness (5%), extrapyramidal disorder (21%), parkinsonism (18%), akathisia (14%), headache (12%), hypertension (4%), constipation (8%), diarrhea (4%), nausea (8%), vomiting (6%), and insomnia (8%) [7]. Brexpiprazole and cariprazine are contraindicated if the patient is hypertensive. A lower rate of discontinuation was reported for XTC related to the reduction of AEs compared with traditional antipsychotics; discontinuation is often caused by severe extrapyramidal symptoms or metabolic complications. Such tolerability makes XTC an appropriate alternative to conventional treatments, especially among patients who are intolerant or at high risk for metabolic syndrome. XTC shows comparable PANSS score reduction (8.4 points) to other atypical antipsychotics, such as aripiprazole (12.7 points), brexpiprazole (8.7 points), and cariprazine (10.4 points) [7]. These findings indicate that XTC efficacy is comparable to, or in some cases exceeds, that of existing atypical antipsychotics.
When it comes to treating the negative symptoms of schizophrenia, XTC and lurasidone are both good choices. According to the long-term data, there is a small chance of lurasidone treatment eliciting a negative impact on weight or metabolic parameters, with changes from normal to high values versus high to normal values observed for LDL cholesterol (9.6% vs. 6.8%), triglycerides (8.2% vs. 9.1%), and HbA1c (6.1% vs. 4.7%) [9]. These mixed shifts suggest variable effects across different metabolic markers [9]. AEs include parkinsonism (6.4%), akathisia (10.8%), nausea (5.2%), vomiting (6.3%), and anxiety (4.7%). Lurasidone has shown efficacy in treating negative symptoms, supported by multiple analyses [10], but some patients are hypersensitive, for whom the drug is contraindicated. Both drugs have favorable safety profiles. XTC lacks significant metabolic side effects, while lurasidone minimally affects weight and metabolic parameters, making both drugs good choices for patients concerned about these issues. These traits may contribute to improved adherence and long-term success in schizophrenia treatment. The comparative analysis of the efficacy, side effects, and contraindications of XTC with other antipsychotic drugs is illustrated in Table 1.
| Parameter | Xanomeline-trospium chloride | Aripiprazole | Brexpiprazole | Cariprazine | Lurasidone |
|---|---|---|---|---|---|
| Efficacy (PANSS score reduction) | Lowering the overall PANSS score by 8.4 points, in terms of symptom severity | 12.7 points | 8.7 points | 10.4 points | Improvement in mean PANSS total score in the group randomized to lurasidone (27.0) |
| Side effects | Nausea (19.2%), vomiting (16%), diarrhea (5.6%), hypertension (6.4%), constipation (12.8%), akathisia (3.2%) elevated heart rate, angioedema of the face and lips and urinary retention | Headache (27%), anxiety (17%), insomnia (16%), and akathisia (13%), nausea (13%), vomiting (11%), constipation (10%), and dizziness (9%), EPD (4%) | Constipation (2%), diarrhea (3%), nausea (3%), akathisia (7%), headache (9%) dizziness (3%), EPD (6%), anxiety (2%), insomnia (8%) | Dizziness (5%), EPD (21%), parkinsonism (18%), akathisia (14%), headache (12%), hypertension (4%), constipation (8%), diarrhea (4%), nausea (8%), vomiting (6%), insomnia (8%) |
Parkinsonism (6.4%) and akathisia (10.8%) nausea (5.2%), vomiting (6.3%), anxiety (4.7%) |
| Metabolic impact | Increase body weight (6.4%) and GERD | Dyslipidemia and hyperglycemia | Moderate adverse metabolic and weight gain (5%) effects | Weight gain (2%), glucose intolerance, hyperlipidemia and increased triglyceride levels |
Normal to high versus high to normal LDL cholesterol: 9.6% vs. 6.8%; triglycerides: 8.2% vs. 9.1%; HbA1c: 6.1% vs. 4.7% |
| Contraindication | In patients with renal failure, untreated narrow-angle glaucoma, moderate or severe hepatic impairment, gastric retention, and hypersensitivity to trospium chloride | In older adults with dementia-related psychosis | Hypersensitivity to REXULTI or any of its components | Hypersensitivity to cariprazine | Hypersensitivity to lurasidone |
- Abbreviations: EPD, extrapyramidal disorder; GERD, gastroesophageal reflux disease; LDL, low-density lipoprotein; PANSS, positive and negative syndrome scale.
5 Metabolism of Xanomeline-Trospium Chloride
XTC is mainly metabolized by the liver, which may contribute to increased liver enzyme levels associated with liver dysfunction. Furthermore, the cholinergic activation of muscarinic receptors that regulate bile secretion may contribute to gallstone formation and biliary stasis, which are linked to serious liver disorders. XTC is therefore contraindicated in cases of moderate to severe hepatic impairment and in patients with untreated narrow-angle glaucoma, gastric retention, or hypersensitivity to trospium chloride. Pupillary dilation caused by anticholinergic effects may trigger acute angle-closure glaucoma in patients with anatomically narrow angles. Therefore, patients should be closely monitored for urinary retention and other central anticholinergic effects, such as dizziness, confusion, hallucinations, and somnolence, specifically during initiation or increases in dosage [11].
Consistent liver enzyme monitoring is therefore essential for preventing detrimental reactions when treating individuals with compromised liver function with XTC. In patients with compromised renal function, trospium chloride may cause drug buildup and raise the risk of nephrotoxicity because it is mostly eliminated via the kidneys. The anticholinergic effects of trospium may also contribute to chronic urinary retention and increase the risk of urinary tract infection, which may worsen renal complications. Thus, caution is also advised when prescribing XTC to individuals with renal impairment, with close monitoring required to prevent adverse renal outcomes. These mechanisms emphasize the risks linked to XTC use in patients with kidney and liver dysfunction.
6 Economic Challenges
XTC is priced at around $1850 for a 30-day course, totaling an annual expenditure of $22,500; therefore, it is more expensive than generic antipsychotics [12]. In comparison, generic medications such as aripiprazole average around $540 annually. XTC has a novel mechanism of action and promising efficacy; however, the price is a prohibitive factor for most patients, especially those in developing countries with limited healthcare budgets. To address the issue of affordability, pharmaceutical companies should implement tiered pricing strategies, and reduce prices for low- and middle-income countries. Collaborating with generic drug companies and international health organizations might also contribute to the development and distribution of budget-friendly alternatives. Aside from these measures, governments and non-governmental organizations may also help subsidize the cost or negotiate global purchase contracts to improve access. Such interventions would go a long way toward ensuring equitable access to XTC, thereby bridging the gap that exists for patients in poorly served regions.
Many patients who are unable to afford XTC based on the cost may decide to take alternative antipsychotics, such as aripiprazole, that offer effective and cheaper treatment. A systematic review and network meta-analysis found that aripiprazole exhibited more favorable metabolic profiles than other antipsychotics, showing minimal changes in related parameters compared with placebo [13]. However, while aripiprazole possesses a good safety profile and is cost-effective, it does not completely replicate the therapeutic advantages of XTC, which are principally built upon its innovative mechanism that minimizes metabolic disturbances and enhances symptom control. While alternatives such as aripiprazole are indeed helpful for underprivileged patients, XTC remains the strongest choice, owing to its novel approach and related benefits.
7 Future Recommendations
Future studies on XTC should focus on establishing a comprehensive study of its safety and efficacy within a wide cross-section of the patient population. In particular, this sample should include those who suffer from comorbid diseases such as diabetes, which are common in people with schizophrenia. Studies ought to emphasize how such comorbid diseases interact with the mechanisms of XTC through cholinergic action. In addition, the cognitive benefits of XTC should be a target of research to provide comparisons with traditional dopaminergic antipsychotics. In addition, AEs related to gastrointestinal and cardiovascular health noted in earlier formulations need to be addressed. Trials should also examine the potential of avoiding these effects through dose adjustments, adjunctive therapies, or more refined formulations.
Advanced monitoring techniques, such as ambulatory blood pressure monitoring, should be integrated into ongoing and future trials to verify the effects of XTC on cardiovascular health in real-life settings. This will facilitate effective patient selection and monitoring protocols that maximize the therapeutic use of XTC while improving safety. The research priorities outlined above can establish XTC in research and the clinic as a versatile and accessible treatment for schizophrenia. The article has some limitations. While the article does mention the price of XTC, it does not place its economic cost in perspective alongside other antipsychotics, other than aripiprazole. A cost-effectiveness analysis may provide a more comprehensive economic picture. While the article mentions efficacy and side effects, it lacks additional discussion of the differences in mechanisms between XTC and other muscarinic or non-dopaminergic drugs.
8 Conclusion
Findings relating to the novel drug XTC suggest a promising alternative for schizophrenia treatment due to its unique mechanism. Its efficacy in reducing both positive and negative symptoms with a favorable metabolic safety profile is broadly encouraging. However, the available data represent early research stages, and analysis relating to comorbid disease, in particular, is lacking. The reduced side effects on weight, glucose control, and cholesterol levels may provide a possible solution for patients' intolerant to common dopamine receptor antagonists. However, additional research and clinical trials based on larger study samples are required to draw more substantial conclusions. Risks concerning the gastrointestinal and cardiovascular systems, as well as its high price, remain concerns that could hinder access and compliance to the drug. If these challenges are overcome, XTC has the potential to be a useful addition to the current options for schizophrenia treatment, and to enhance the quality of life of patients suffering from the condition.
Author Contributions
Jawairya Muhammad Hussain: conceptualization (lead), formal analysis (lead), investigation (lead), project administration (lead), software (lead), supervision (lead), visualization (lead), writing – original draft (lead), writing – review and editing (lead). Wajiha Arif: data curation (supporting), formal analysis (supporting), investigation (supporting), software (equal), validation (supporting), writing – original draft (supporting), writing – review and editing (supporting). Sharjeel Humayun: data curation (supporting), investigation (supporting), methodology (supporting), software (supporting), validation (supporting), writing – original draft (supporting). Nida Shoaib: data curation (supporting), formal analysis (supporting), investigation (supporting), methodology (supporting), software (supporting), validation (supporting), writing – original draft (supporting), writing – review and editing (supporting). Saim Mahmood Khan: conceptualization (equal), data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), software (equal), validation (equal), writing – original draft (equal), writing – review and editing (equal).
Acknowledgments
The authors have nothing to report.
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this article.