Relationships Between Serum Vitamin D, Inflammatory Markers, and Outcomes in Non-Critically Ill Patients With COVID-19: A Cross-Sectional Study
Funding: This work was supported by Mashhad University of Medical Sciences, Iran (Grant number: 981890).
Reza Rezvan and Hassan Vatanparast shared the co-corresponding authorship.
ABSTRACT
Backround
Treatment options for COVID-19 remain limited and are primarily focused on specific patient populations; accordingly, preventive measures continue to be a crucial aspect of effective management. There is evidence that vitamin D effectively prevents viral upper respiratory tract infections during epidemics. The aim of this study was to assess the association between serum vitamin D and inflammatory markers, mortality, and clinical symptoms in patients with COVID-19.
Methods
This cross-sectional study involved non-critically ill patients with COVID-19 in a provincial reference hospital in Mashhad, Iran. Demographic and clinical data were extracted from patient medical records. Serum vitamin D was measured for each patient within 12 h of admission. Data were analyzed using linear and logistic regression models.
Results
In total, 452 patients (mean age 63.87 ± 17.97 years) were included in this study during 2 months of data collection. The most common serum vitamin D status was sufficient (30.0%), followed by deficient (29.4%), insufficient (23.2%), and severely deficient (17.3%). Partial symptom improvement was observed in 326 (72.1%) patients after 22 days of hospitalization, disregarding the vitamin D status. The mortality rate was 22.6%. COVID-19 mortality was significantly related to serum urea (p = 0.002, OR = 1.020, 95% CI: 1.008–1.033), pulse rate (p = 0.015, OR = 1.047, 95% CI: 1.009–1.086), and age (p = 0.002, OR = 1.076, 95% CI: 1.027–1.127).
Conclusions
Among patients with COVID-19, serum vitamin D levels were linked to mortality and some clinical parameters, including urea and pulse rate. Further longitudinal studies should evaluate the relationship between serum vitamin D levels and COVID-19 outcomes.
Abbreviations
-
- ACE2
-
- angiotensin-converting enzyme 2
-
- NLR
-
- neutrophil-to-lymphocyte ratio
-
- PLR
-
- platelet-to-lymphocyte ratio
-
- SARS-COV-2
-
- severe acute respiratory syndrome coronavirus-2
-
- Th
-
- T helper
-
- TNF
-
- tumor necrosis factor
1 Introduction
Upper respiratory tract infections are very prevalent and are the most common infectious diseases in adulthood every year. These infections are mainly caused by viruses, although bacterial infections are possible [1]. Respiratory viral infections are acute and transmitted rapidly, resulting in epidemics and pandemics. Recently, severe acute respiratory syndrome due to coronavirus-2 (SARS-Cov-2), also called coronavirus virus 2019 (COVID-19), has presented a major public health challenge. The COVID-19 outbreak has become a disastrous global pandemic with immeasurable impacts on communities. The main cause of mortality from the SARS-CoV family to date is atypical pneumonia. As the disease progresses, pro-inflammatory cytokines are secreted; in particular, interleukin (IL)-1β and IL-18 are secreted by type 1 T helper (Th1) immune cells and macrophages and exacerbate lung inflammation, finally causing fibrosis [2].
Observational studies have indicated that increases in serum vitamin D levels are associated with decreases in inflammatory markers, including hs-CRP [3]. In a systematic review and meta-analysis, Martineau et al. analyzed 25 randomized controlled trials and demonstrated that vitamin D could have a protective effect against acute respiratory tract infections [4]. Furthermore, evidence suggests that vitamin D has therapeutic effects or prevents adverse outcomes in COVID-19. Vitamin D inhibits the expression of inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and IL-1β [5]. Vitamin D insufficiency is correlated with overproduction of Th1-derived cytokines [6]. Diminished Th1 proliferation could reduce serum levels of pro-inflammatory cytokines and adaptive immune responses. Furthermore, vitamin D can impact T cell maturation and could shift the maturation of inflammatory Th17 to anti-inflammatory regulatory T cells, thereby reducing serum levels of pro-inflammatory cytokines, including IL-6, IL-1, TNF-α, IL-12, and IL-17, while elevating levels of the anti-inflammatory IL-10 [7]. A potential mechanism underlying the protective effect of vitamin D against acute lung injury is the modulation of Angiotensin-converting enzyme 2 (ACE2) receptors in lung tissue [8]. This mechanism may also explain the relationship between vitamin D deficiency and COVID-19. However, data on the relationship between serum vitamin D levels and COVID-19 are limited [9]. A negative association has been reported between serum 25(OH)D and mortality and morbidity rates due to SARS-CoV-2 infection in 20 European countries, especially in Spain, Switzerland, and Italy (countries with a high rate of COVID-19) [10]. The prevalence of vitamin D deficiency is high in Iran [11], and this region has a particularly high prevalence of infection and mortality rate due to SARS-CoV-2 [12]. However, little is known about the nutritional causes of COVID-19 susceptibility in the Iranian population. We assessed the association between serum 25(OH)D and clinical symptoms, mortality, and inflammatory markers among patients admitted for COVID-19 in Mashhad, Iran.
2 Materials and Methods
2.1 Study Design and Data Collection
This study used a single-center cross-sectional design involving patients with COVID-19 admitted to the main COVID-19 center in Mashhad city, Iran, from March 20 to May 4, 2020. The COVID-19 center of the Imam Reza Hospital is a tertiary referral center. Among admitted patients with confirmed COVID-19, 452 patients over 15 years old were selected. The medical records of the patients were accessed to record relevant data. All data collection and biochemical assessments were performed in the same hospital.
SARS-CoV-2 infection was confirmed for all cases by an infectious disease specialist according to the WHO guideline [13]. A confirmed COVID-19 case was defined as a positive result for a nasal or pharyngeal specimen by real-time polymerase chain reaction (RT-PCR) and clinical and paraclinical manifestations of the disease, such as fever, myalgia, dyspnea, elevated C-reactive protein (CRP) levels, or reduced lymphocyte count as well as O2 saturation not compatible with any other disease. Only cases who were admitted to the wards (excluding ICUs) and did not require intubation or invasive ventilation were included in this study. All demographic characteristics, medical history, underlying diseases, vital signs, and blood samples in the first 12 h of the admission were collected from the patient files.
The study was conducted during the first peak of the COVID-19 pandemic. All participants who met the inclusion criteria were included; therefore, the global sampling method was used.
2.2 Demographic and Clinical Data
Demographic (age and sex) and clinical (past medical history of chronic obstructive pulmonary disease, cardiovascular disease, chronic kidney disease, diabetes, hypertension, any type of cancer, and history of addiction) data for the study patients were recorded in a researcher-made checklist from patient records. When patient records were incomplete, the patients were interviewed by the researcher to complete the checklists.
Clinical measurements included vital signs (axillary body temperature, recumbent blood pressure, pulse and respiratory rates, and O2 saturation at room air). Furthermore, the outcome of admission and admission duration to the end of data collection were recorded for each patient. For patients who were still admitted at the end of the study period, the outcome was recorded as the current condition compared with the condition at the time of admission.
2.3 Laboratory Assessments
Complete blood count (determined using the Sysmex K-21, Kobe, Japan), blood sugar (BS), kidney profile (urea and creatinine), liver profile (alanine aminotransferase, aspartate aminotransferase, total and direst bilirubin), serum electrolytes (sodium, potassium, calcium, and magnesium), serum albumin, prothrombin time (PT), partial thromboplastin time (PTT), international normalized ratio (INR), erythrocyte sedimentation rate, and CRP were measured through venous blood sampling, while arterial blood sampling was used to measure pH, HCO3−, PCO2, PaO2. The neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and red cell distribution width-to-platelet ratio (RPR = RDW/platelet) were also calculated.
Serum 25-hydroxy vitamin D, 25(OH)D, was measured using commercial ELISA kits (Pishgaman Sanjesh Company, Tehran, Iran) and a StatFax 2100 (Awareness Technology Inc, Palm City, USA) analyzer. The intra- and inter-assay coefficients of variation (CV) of the kit were 5.4%–6.1% and 5.7%–7.7%, respectively. Cut-off points for severe deficiency, moderate deficiency, insufficiency, and sufficiency were < 10, 10–20, 21–29, and ≥ 30 ng/mL, respectively, based on guidelines [14].
The primary outcomes were the relationships between serum vitamin D levels and inflammatory factors and other clinical and laboratory findings for patients admitted due to COVID-19. The secondary outcome was mortality among admitted patients with COVID-19. Owing to difficulties in performing follow-ups during the first two peaks of COVID-19, the timing of follow-ups varied, and only the disease outcome was evaluated. In cases where death occurred between follow-ups, the specific time of death was not recorded. Therefore, the actual time-to-event was unclear for patients who left the hospital.
The study was approved by the Ethical Committee of the Mashhad University of Medical Sciences (No. IR.MUMS.REC.1398.314). Informed consent was obtained from all participants.
2.4 Statistical Analysis
The normality of quantitative data was assessed using the Kolmogorov-Smirnov test. Data with a normal distribution are presented as the mean ± standard deviation and non-normally distributed data are presented as the median and interquartile range. Ordinal and categorical data are presented as percentages. Pairwise comparisons were performed using the Mann-Whitney test, Kruskal-Wallis test, independent t-test, or ANOVA depending on normality. Chi-squared tests were used to compare categorical variables. For laboratory parameters, adjusted values were determined using linear regression. Confounders were selected based on the comparisons of variables between vitamin D groups. Variables with p-values less than 0.500 were selected as confounders and comparisons of markers of inflammation and disease severity were performed using both adjusted and unadjusted variables. Finally, multivariate logistic regression models were developed to assess the relationship between the binary outcome (deceased or alive) as the dependent variable and other study parameters as independent variables. The final model was defined using the backward elimination method. Moreover, a classification tree was used to identify the predictors and cut-offs for mortality in the study cases based on the Chi-square Automatic Interaction Detection (CHAID) method using the rpart package in R. Classification trees divide a population into categories with the highest probability of predicting the outcome, visualized in the form of a tree structure with nodes indicating the variables (with a condition to predict the outcome) and branches directed to the next node based on the classification rules. The CHAID method is preferred for classification trees as it can be applied to continuous and categorical variables. The chi-squared test is performed for possible splitting points (all possible cut-offs for continuous variables and all categories for categorical variables) and the variable with the highest significance is chosen as the first node [15]. The final decision model is shown in the form of a tree, where nodes represent the variable and branches lead to child nodes based on the fulfillment of the rule [15]. A random sample of the data (80%) was used to train the model and the model was tested on the remaining 20% of data. The accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the trained and test models were determined. The Monte Carlo test was used to compare categorical variables between groups when more than 20% of the cells had less than five members.
Analyses were performed using the Statistical Package for Social Sciences (SPSS) version 18 (IBM Inc. Chicago, IL, USA) and rpart package in R version 4.1.2 (R Core Team. 2020). The threshold for statistical significance was 0.05.
3 Results
A total of 452 patients were evaluated. The mean age of the patients was 63.87 ± 17.97 years. Demographic characteristics of the study patients are presented in Table 1. The median and IQR for vitamin D levels were 20.60 and 20.88 ng/mL, respectively. Only 30% of patients had serum vitamin D levels of > 30 ng/mL. Severe vitamin D deficiency was observed in 78 patients (17.3%), and vitamin D deficiency (29.4%) and insufficiency (23.2%) were more prevalent.
| Variable | Total | Vitamin D status | Overall difference | ||||
|---|---|---|---|---|---|---|---|
| N = 452 | Severely deficient n = 78 | Deficient n = 133 | Insufficient n = 105 | Sufficient n = 136 | p | ||
| Age (years) | 67.00 (53.00–78.00) | 65.50 (44.00–79.00)a | 64.00 (51.00–77.00)b | 64.00 (50.00–75.50)c | 69.50 (60.00–80.00)a,b,c | 0.004*, † | |
| Gender n (%) | Male | 250 (55.3) | 46 (61.3) | 85 (64.9) | 54 (51.4) | 65 (48.1) | 0.025* |
| Female | 196 (43.4) | 29 (38.7) | 46 (35.1) | 51 (48.6) | 70 (51.9) | ||
| Cardiovascular disease n (%) | 253 (56.0) | 44 (56.4) | 74 (55.6) | 61 (58.1) | 74 (54.4) | 0.544 | |
| Diabetes mellitus n (%) | 43 (9.5) | 5 (6.4) | 8 (6.0) | 15 (14.3) | 15 (11.0) | 0.159 | |
| Hypertension n (%) | 43 (9.5) | 2 (2.5) | 10 (7.5) | 16 (15.2) | 15 (11.0) | 0.030*, ‡ | |
| Chronic kidney disease n (%) | 9 (2.0) | 1 (1.3) | 2 (1.5) | 2 (1.9) | 4 (2.9) | 0.801‡ | |
| Addiction n (%) | 2 (0.4) | 0 (0.0) | 1 (1.0) | 1 (1.0) | 0 (0.0) | 0.704‡ | |
- † Medians and interquartile ranges are reported; the Kruskal-Wallis test was used for comparisons.
- ‡ The Monte Carlo test was used to compare categorical variables between groups when more than 20% of the cells had less than five members. Pairwise comparisons were performed using the chi-square and Fisher exact tests except for age (Mann-Whitney test).
- * Significant difference: ap = 0.022, bp = 0.005, cp = 0.001.
Age (p = 0.004), sex distribution (p = 0.025), and hypertension (p = 0.030) differed significantly with respect to vitamin D status (Table 1). Among the blood gas indices, O2 saturation differed significantly among vitamin D categories (p = 0.013) (Table 2). Among the CBS indices, the platelet count (p = 0.018) and mixed cell percentage (p = 0.017) differed significantly among vitamin D categories (Table 2). There were also significant differences in terms of CRP (p < 0.001) and RPR (p = 0.014) among vitamin D categories (Table 2). After controlling for confounders, significant differences were observed in PR (p = 0.009), pH (p = 0.025), PaO2 (p = 0.005), O2 saturation (p = 0.006), admission duration (p = 0.004), CRP (p = 0.002), and INR (p = 0.027) among vitamin D categories (Table 2).
| Variable | Condition | Vitamin D status |
Overall difference p |
||||
|---|---|---|---|---|---|---|---|
| Total | Severely deficient | Deficient | Insufficient | Sufficient | |||
| N = 452 | n = 78 | n = 133 | n = 105 | N = 136 | |||
| Clinical findings | |||||||
| Pulse rate (/min) | Unadjusted | 92.50 (85.00–108.00) | 91.00 (82.75–107.00) | 98.00 (85.00–114.00) | 90.00 (82.75–104.75) | 93.00 (80.00–109.75) | 0.609Ɨ |
| Adjusted | 89.97 (87.4–91.96) | 90.79 ± 3.21a | 90.43 ± 2.53b | 89.82 (87.11–92.27) | 89.02 ± 2.81a,b | 0.009*, Ɨ | |
| Respiratory rate (/min) | Unadjusted | 18.00 (18.00–20.00) | 19.00 (18.00–20.00) | 18.00 (17.00–20.00) | 18.00 (16.75–20.00) | 18.00 (18.00–20.00) | 0.969Ɨ |
| Adjusted | 19.12 (18.64–19.40) | 18.96 ± 0.44 | 19.11 ± 0.52 | 18.98 ± 0.52 | 19.05 ± 0.48 | 0.310Ɨ | |
| Temperature (°C) | Unadjusted | 37.00 (36.60–37.60) | 37.00 (36.70–38.00) | 37.00 (36.50–37.00) | 37.00 (36.90–38.00) | 37.00 (36.55–37.60) | 0.111Ɨ |
| Adjusted | 36.51 (36.36–36.60) | 36.49 ± 0.16 | 36.53 (36.38–36.61) | 36.49 (36.34–36.56) | 36.51 (36.37–36.60) | 0.451Ɨ | |
| Systolic blood pressure | Unadjusted | 120.00 (111.00–133.50) | 125.00 ± 15.69 | 120.00 (110.75–133.50) | 122.50 (116.75–134.75) | 120.00 (111.00–132.00) | 0.998Ɨ |
| Adjusted | 121.08 ± 2.81 | 120.78 ± 3.07 | 121.32 ± 3.01 | 120.63 ± 2.66 | 121.40 ± 2.53 | 0.265† | |
| Diastolic blood pressure | Unadjusted | 80.00 (70.00–80.00) | 80.00 (70.00–85.00) | 80.00 (70.00–80.00) | 80.00 (72.00–80.00) | 80.00 (70.00–82.00) | 0.713Ɨ |
| Adjusted | 76.67 (75.62–77.48) | 76.43 (74.86–77.70) | 76.54 (75.57–77.26) | 76.54 (75.37–77.28) | 76.94 (76.20–77.51) | 0.076Ɨ | |
| PH | Unadjusted | 7.41 (7.36–7.46) | 7.41 (7.36–7.45) | 7.42 (7.35–7.46) | 7.41 ± 0.10 | 7.40 ± 0.10 | 0.883Ɨ |
| Adjusted | 7.38 (7.36–7.40) | 7.38 (7.37–7.40)c | 7.38 (7.34–7.40)d | 7.37 (7.34–7.39) | 7.37 (7.35–7.39)c,d | 0.025*, Ɨ | |
| PaO2 | Unadjusted | 42.30 (31.80–56.23) | 41.25 (30.03–52.25) | 41.80 (31.00–55.40) | 40.10 (28.50–52.10) | 46.40 (34.50–61.18) | 0.062Ɨ |
| Adjusted | 47.95 (43.22–48.78) | 43.35 (43.14–48.74)e,f | 43.35 (43.20–48.68)g,h | 48.59 (43.20–48.87)e,g | 48.63 (43.28–48.84)f,h | 0.005*, Ɨ | |
| PCO2 | Unadjusted | 38.55 (32.80–44.86) | 35.85 (31.75–43.13) | 38.80 (33.60–47.30) | 39.44 ± 11.70 | 39.83 ± 12.52 | 0.287Ɨ |
| Adjusted | 40.42 (37.38–40.87) | 40.40 (37.51–40.86) | 40.55 (37.80–40.91) | 40.41 (37.37–41.05) | 40.17 (37.30–40.73) | 0.151Ɨ | |
| HCO3 | Unadjusted | 24.55 ± 5.94 | 23.60 ± 5.64 | 24.87 ± 6.25 | 24.83 ± 5.72 | 24.62 ± 5.99 | 0.537† |
| Adjusted | 24.59 (23.63–25.43) | 24.56 ± 1.29 | 24.75 ± 1.19 | 24.68 ± 1.25 | 24.29 ± 1.26 | 0.100† | |
| O2 saturation | Unadjusted | 79.00 (65.00–89.50) | 76.90 (64.50–86.20)i | 73.89 ± 18.02j | 76.40 (56.70–88.70)k | 84.60 (69.20–92.23)i,j,k | 0.013*, Ɨ |
| Adjusted | 74.63 (72.41–78.16) | 74.07 (71.19–77.86)l | 74.31 ± 3.04m,n | 75.79 (72.26–78.42)m | 76.66 (73.26–78.95)m,n | 0.006*, Ɨ | |
| Admission duration (days) | Unadjusted | 7.00 (4.00–11.00) | 6.00 (4.00–10.50) | 6.00 (4.00–10.00) | 7.00 (4.00–12.00) | 8.00 (4.00–12.00) | 0.173Ɨ |
| Adjusted | 8.67 (7.16–10.19) | 8.02 ± 2.17z | 8.32 ± 1.92o | 8.95 ± 2.44 | 9.27 ± 2.18z,o | 0.004*, † | |
| Serum albumin | Unadjusted | 3.40 (3.08–3.80) | 3.47 ± 0.49 | 3.30 (2.90–3.68) | 3.70 (3.30–3.90) | 3.60 (9.93–3.80) | 0.157Ɨ |
| Adjusted | 3.47 (3.42–3.52) | 3.46 ± 0.09 | 3.47 ± 0.08 | 3.48 ± 0.09 | 9.19 (7.81–10.67) | 0.077Ɨ | |
| Inflammatory indices | |||||||
| ESR (1 h) | Unadjusted | 42.50 (24.00–69.00) | 51.20 ± 30.26 | 39.00 (16.00–69.00) | 45.50 (26.00–78.75) | 46.14 ± 32.31 | 0.367Ɨ |
| Adjusted | 48.17 (47.38–49.28) | 48.17 (47.14–49.28) | 47.96 (47.21–48.79) | 48.20 (47.26–49.59) | 48.52 (47.82–49.52) | 0.069Ɨ | |
| CRP | Unadjusted | 74.20 (28.80–140.50) | 135.86 ± 83.38p,q,r | 76.45 (32.18–145.50)p,s,t | 47.85 (18.55–115.85)q,s | 54.50 (21.00–119.85)r,t | < 0.001*, Ɨ |
| Adjusted | 100.06 (82.44–106.54) | 106.16 (82.53–106.59)u | 106.29 (82.55–106.67)u,v | 82.67 (82.13–106.31)u | 82.75 (82.44–106.58)v | 0.002*, Ɨ | |
| NLR | Unadjusted | 5.49 (3.56–8.91) | 5.62 (3.14–8.86) | 6.13 (3.93–9.81) | 4.79 (3.34–8.22) | 5.57 (3.53–8.91) | 0.394Ɨ |
| Adjusted | 7.29 (6.59–7.91) | 7.02 ± 1.05 | 7.23 ± 1.00 | 7.06 ± 0.94 | 7.38 ± 0.84 | 0.098† | |
| PLR | Unadjusted | 175.60 (127.70–245.02) | 208.89 (124–37–293.02) | 167.30 (131.13–226.77) | 168.04 (129.93–213.45) | 187.92 (126.51–266.83) | 0.254Ɨ |
| Adjusted | 203.79 ± 19.01 | 200.69 ± 20.94 | 202.04 ± 18.40 | 202.97 ± 19.22 | 207.97 ± 17.80 | 0.144Ɨ | |
| RPR | Unadjusted | 7.22 (5.42–9.61) | 7.45 (5.75–10.29)w | 7.51 (6.05–10.72)x | 7.17 (5.34–10.06) | 6.47 (4.95–8.71)w,x | 0.014*, Ɨ |
| Adjusted | 8.73 (8.11–9.32) | 8.68 ± 0.85 | 8.77 ± 0.83 | 8.38 ± 0.85 | 8.80 (8.18–9.42) | 0.043Ɨ | |
- Note: All variables were adjusted for age, gender, diabetes and hypertension history except for systolic and diastolic blood pressure that were adjusted for age, gender and diabetes history. Numbers with similar superscript letters were significantly different. The p values for the significant comparisons are as follows: ap = 0.015, bp = 0.023, cp = 0.003, dp = 0.030, ep = 0.003, fp = 0.029, gp = 0.001, hp = 0.003, Ip < 0.001, jp < 0.001, kp = 0.041, lp = 0.035, mp = 0.030, np = 0.002, op = 0.007, pp = 0.001, qp = 0.007, rp = 0.022, sp = 0.041, tp = 0.018, up = 0.016, vp = 0.003, wp = 0.012, xp = 0.044.
- Abbreviations: CRP, C-reactive protein; HCO3, bicarbonate; INR, International normalized ratio; NLR, neutrophil to lymphocyte ratio; PaO2, Arterial oxygen pressure; PCO2, Carbon dioxide pressure; PLR, platelet to lymphocyte ratio; RPR, red cell distribution width to platelet ratio.
- Ɨ Median and first and third quartiles were reported and the Kruskal–Wallis test was used for the comparison and pairwise comparisons were performed using the Mann–Whitney test for non-parametric tests.
- † Mean and standard deviation were reported and the one-way analysis of variance was used for the comparison and the Bonferroni test was used as post hoc test.
- * Significant difference.
Of the 452 patients, 326 (72.1%) had a partial improvement in symptoms (median and IQR for duration were 6 and 5 days, respectively), 102 (22.6%) died (median and IQR for duration were 9 and 9.5 days, respectively), 19 (4.2%) were discharged with consent, 3 (0.7%) had no improvement, and 1 (0.2%) was referred to another center. Serum vitamin D levels were similar between the groups (p = 0.310). Therefore, vitamin D was excluded from the model. Regression analysis was performed to assess the relationships between the study parameters and dichotomized outcome (deceased or not deceased, while excluding data for patients lost during follow up due to referral or discharge, n = 19) (Table 3). COVID-19 mortality was related to serum urea (p = 0.002, OR = 1.020, 95% CI: 1.008–1.033) among laboratory assessments, pulse rate (p = 0.015, OR = 1.047, 95% CI: 1.009–1.086) among clinical findings, and age (p = 0.002, OR = 1.076, 95% CI: 1.027–1.127) among clinical findings. A one unit increase in serum urea was related to 1.4% increase in the risk of death, while a 1-year increase in age and one beat/minute increase in pulse rate were related to 4.9% and 2.5% increases in the risk of death due to COVID-19, respectively.
| Variable | Wald | p | OR | 95% CI | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Urea (mg/dL) | 9.418 | 0.002* | 1.020 | 1.008 | 1.033 |
| Age (year) | 11.478 | 0.002* | 1.076 | 1.027 | 1.127 |
| Pulse rate (/min) | 5.687 | 0.015* | 1.047 | 1.009 | 1.086 |
- Note: Logistic regression model was used for the analysis.
- * Significant relationship.
The cut-offs and predictors of mortality in the study patients were determined using a classification tree. The accuracy, sensitivity, specificity, PPV, and NPV for the trained model (on 347 patients) were 86.2%, 93.2%, 63.4%, 89.2%, and 74.3%, respectively. The model was tested on data for 105 additional patients and the accuracy, sensitivity, specificity, and positive and negative predictive values were 80.2%, 87.9%, 55.0%, 86.6%, and 57.9%, respectively. The classification tree findings revealed that 10 variables were important determinants of mortality in patients with COVID-19 (Figure 1). Among the 10 variables, serum urea level below 83 mg/dL, admission duration of less than 22 days, serum vitamin D level higher than 6.9 ng/dL at admission, RDW less than 14, PLR less than 257, INR less than 1.3, and neutrophil count higher than 83 (× 1000/dL) were associated with survival, while pulse rate higher than 86/min, serum potassium less than 4.5 mg/dL, and PT higher than 15 were predictors for mortality.
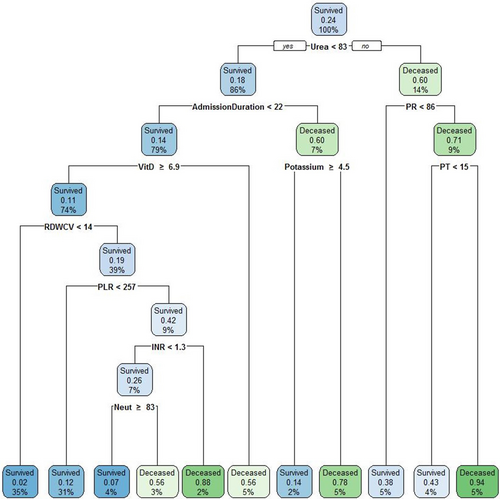
Classification tree for predictors of mortality in the study patients. The expected rate of the condition (survived or deceased) in the box is presented by a two decimal figure in each leaf. The percentage of the survived or deceased participants who met the criterion based on the cut-off is reported in the leaf. The cut-offs for each category in the leaf (survived or deceased) are presented in front of each variable under the leaf (e.g., 100% of patients who survived, which included 24% of the population, had urea levels below 83 mg/dL). INR, international normalized ratio; Neut, neutrophile percentage; PLR, platelet-to-lymphocyte ratio; PR, pulse rate; PT, prothrombin time; RDWCV, red cell distribution width—coefficient of variation; VitD, vitamin D.
4 Discussion
In this study of individuals who were hospitalized with confirmed SARS-Cov-2 infection, we detected significant differences in pulse rate, O2 saturation, PaO2, admission duration, pH, CRP, and RPR among vitamin D categories. Although the differences in pH and pulse rate were statistically significant, no significant changes in clinical findings were observed. Among the included patients, 102 (22.6%) died, 326 (72.1%) showed a partial improvement in symptoms, 19 (4.2%) were discharged with consent, 3 (0.7%) had no improvement, and 1 (0.2%) was referred to another center. Furthermore, we detected significant associations between mortality and urea, pulse rate, and age. A classification tree also revealed that serum urea, admission duration, serum vitamin D level, RDW, PLR, INR, and neutrophil count were related to survival, and serum potassium, pulse rate, and PT were related to mortality due to COVID-19.
The majority of clinical and laboratory parameters did not differ significantly among serum vitamin D categories. D'Avolio et al. observed lower serum vitamin D concentrations in patients with confirmed SARS-CoV-2 infection [16]. Radujkovic et al. showed a significant correlation between a vitamin D deficiency and the severity and mortality of COVID-19 [17]. It should be noted that the cut-off values for vitamin D deficiency or sufficiency are subject to debate. In our study, 17.3% of patients had serum vitamin D concentrations below 10 ng/mL and 29.4% had serum vitamin D concentrations between 10 and 20 ng/mL. The vitamin D status was defined based on a previous study in which the cut-offs were defined based on the impact on bone health [18].
Our results suggest that patients with a vitamin D deficiency have lower O2 saturation and PaO2 and therefore need more oxygen therapy. Similarly, Faul et al. reported that low serum vitamin D concentrations in patients with COVID-19 are related to a significantly elevated risk of invasive mechanical ventilation [19]. Furthermore, in a study of 185 patients with COVID-19, a vitamin D deficiency increased the risk of invasive mechanical ventilation and the need for more oxygen therapy after adjusting for sex, comorbidities, and age [17]. These findings appeared to be consistent with our observations. The observed relationship might be related to the cytokine storm observed in SARS-CoV-2 infection and the anti-inflammatory effects of vitamin D in this regard [20]. Potential mechanisms by which vitamin D controls inflammation are presented in the following paragraphs.
In this study, RPR was significantly lower among patients with sufficient levels of vitamin D than in those with < 30 ng/mL serum vitamin D. RPR has been identified as a novel indicator of inflammation severity based on the ratio of RDW to platelets [21]. The anti-inflammatory function of vitamin D could explain the protective role of 1,25(OH)D against cytokine storm in patients with COVID-19 [22]. Our results were consistent with the anti-inflammatory effects attributed to vitamin D. Recently, RPR was recognized as a marker of inflammation in acute pancreatitis and myocardial infarction and as a powerful predictor of hepatitis and hepatic fibrosis [23]. Patients with major burn injuries may exhibit a high RPR [24]. An elevated RPR is also associated with levels of inflammatory markers in patients with systemic lupus erythematosus [25]. Accordingly, RPR could be considered an indicator of inflammation. To the best of our knowledge, the relationship between the RPR and vitamin D status has not been assessed in COVID-19. The low serum vitamin D levels among patients with elevated RPR could be explained by the inflammation-modifying role of vitamin D via suppressing the production of IL-2, interferon, and TNF-α as well as through the induction of anti-inflammatory cytokines, such as IL-10 [26]. Low serum vitamin D concentrations were correlated with a rise in inflammatory markers and inhibition of RBC maturation. Moreover, pro-inflammatory cytokines deform RBC membranes and decrease the RBC half-life, along with increases in RDW and O2 capacity [27]. The proposed mechanism is summarized in Figure 2.
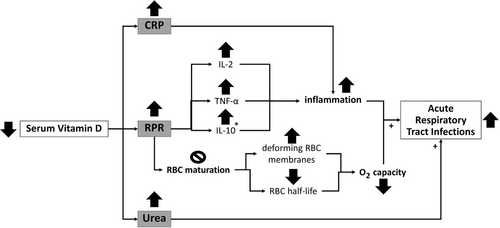
Potential mechanism for the relationship between vitamin D and acute respiratory tract infections in non-critically ill patients. * IL-10 is an anti-inflammatory factor. ↑ and + increase, ↓ decrease,  inhibit. CRP, C-reactive protein; IL, interleukin; RPR, red cell distribution width-to-platelet ratio; TNF-α, tumor necrosis factor alpha.
inhibit. CRP, C-reactive protein; IL, interleukin; RPR, red cell distribution width-to-platelet ratio; TNF-α, tumor necrosis factor alpha.
Many studies have evaluated the link between serum vitamin D and CRP. Li et al. analyzed laboratory data for 1873 individuals (NHANES, 2007–2008) and found a strong association between high CRP levels and severe vitamin D deficiency [28]. A study of COVID-19 showed that patients with severe vitamin D deficiency have a 1.4 times higher risk of high CRP levels [22]. Similarly, Daneshkhah et al. observed an inverse relationship between hs-CRP and serum vitamin D concentrations; they also reported that when compared with that for moderate COVID-19, severe COVID-19 could increase the risk of high CRP by 44.5% [29]. In this study, CRP levels were lower among patients with sufficient vitamin D than in those with less than 20 ng/mL serum vitamin D. This finding could be attributed to the anti-inflammatory effect of vitamin D, and this is supported by the low CRP levels observed in our study. Low serum vitamin D concentrations induce the production of IL-6, IL-1β, and TNF-α by means of intercellular calcium activity. As a result, cytokine storm and CRP elevation may occur in patients with COVID-19 [29, 30].
We detected a significant relationship between blood urea nitrogen (BUN) and COVID-19 mortality. BUN is one of the main indicators of renal function and neurohormonal activity [31]. Consistent with our findings, Cheng et al. reported that increased BUN was a good predictor of mortality in patients with COVID-19 [32]. Similarly, another study suggested that NLR and BUN/Cr could predict the severity of COVID-19 and COVID-19 -related mortality [33].
Coronavirus enters cells using ACE2 receptors, which are expressed in the kidney and lungs. SARS-CoV-2 interferes with kidney function in an ACE2-dependent manner. As a result, the renin-angiotensin-aldosterone system is activated and enhances water and sodium absorption in renal tubules, leading to the passive reabsorption of BUN [29]. Renin-angiotensin-aldosterone system activation also results in renal vasoconstriction and therefore glomerular filtration and BUN excretion [34]. While creatinine is an important marker for renal function, we did not observe a significant association between COVID-19 mortality and serum creatinine. Further studies are needed to explore the precise relationships between creatinine and BUN and COVID-19 mortality.
Overall, the study findings indicated that vitamin D has predictive value for the severity of COVID-19; however, its performance is negligible compared with those of more powerful predictors, such as BUN, age, and vital signs at admission. Our study had some limitations that should be noted. First, it was a single-center study although the hospital was one of the three centers for COVID-19 referrals in the province. Second, owing to the cross-sectional design, we could not establish causation. Finally, several unmeasured confounding factors were not recorded or could not be controlled. Therefore, larger, multi-center studies are needed to assess the relationship between severity and mortality due to COVID-19 and renal function tests, including possible confounders. Furthermore, our results indicate the need for cohort studies to assess the cause of the observed clinical and paraclinical findings in patients with COVID-19. Owing to the high admission load in the first two peaks of COVID-19 and the limitations set for external researchers in accessing patients and records, some variables could not be collected for all patients. Therefore, statistical analyses were performed on available variables, which might result in the exclusion of some patients in regression models. However, considering the unknown behavior of the disease, data imputation was not performed. Nevertheless, considering the number of patients in the models, the findings could still be considered valid. The authors also acknowledge that the amount of vitamin D can be affected by sun exposure; however, considering the high prevalence of vitamin D deficiency among the 20- to 50-year-old Iranian population (72%) and the reduced outdoor activities due to the COVID-19 quarantine and movement restrictions, the effect of sun exposure was considered minimal in this study [35].
A strength of the study is that it addressed the limited information on clinical, laboratory, and demographic characteristics of patients with COVID-19 in Iran and their associations with outcomes. Furthermore, owing to the large number of patients who were referred to this center, the findings could be generalized to the COVID-19 patient population of the city and even province. Another strength of our study is the inclusion of the newly proposed indices in the assessment of the outcome of COVID-19.
5 Conclusion
Serum urea was significantly associated with COVID-19 in-hospital mortality. Additionally, levels of inflammatory markers, including CRP and RPR, were higher in patients with vitamin D deficiency than in patients with normal serum vitamin D concentrations. Larger cross-sectional or cohort studies are necessary to further investigate the association between serum vitamin D levels and clinical outcomes in COVID-19 before proceeding to clinical trials. Furthermore, serum urea at admission may serve as a potential risk marker for in-hospital mortality among patients with COVID-19 in conjunction with general risk factors for disease severity; however, further studies are needed to confirm its predictive value. It may be useful to include serum urea and vitamin D levels in the assessment of all admitted patients with COVID-19.
Author Contributions
Zahra Khorasanchi: conceptualization (equal), project administration (equal), validation (equal), writing – review and editing (equal). Ali Jafazadeh Esfehani: data curation (equal), formal analysis (equal), methodology (equal), software (equal), visualization (equal). Mohammad Reza Shadmand Foumani Moghadam: project administration (equal), visualization (equal), writing – review and editing (equal). Sara Shojaei Saadatqoli: project administration (equal). Payam Sharifan: data curation (equal), writing – review and editing (equal). Majid Ghayour Mobarhan: conceptualization (equal), supervision (equal), validation (equal). Sajjad Arefinia: project administration (equal). Afshin Roghani: writing – original draft (equal), writing – review and editing (equal). Naghme Mirhossini: writing – original draft (equal), writing – review and editing (equal). Masoud Pezeshki Rad: investigation (equal), validation (equal). Saeid Eslami: data curation (equal), formal analysis (equal). Hamidreza Naderi: writing – original draft (equal), writing – review and editing (equal). Hassan Vatanparast: supervision (equal), validation (equal), writing – original draft (equal), writing – review and editing (equal). Reza Rezvani: conceptualization (equal), supervision (equal), writing – review and editing (equal).
Acknowledgments
We sincerely thank all patients participating in this study. We also express our appreciation to all hospital staff or colleagues in the department who helped us in conducting this study.
Ethics Statement
The study was approved by the Ethical Committee of the Mashhad University of Medical Sciences (No. IR.MUMS.REC.1398.314).
Consent
Informed consent was obtained from all participants.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.




