Lack of causal association between cheese intake and risk of peripheral vascular diseases: A two-sample Mendelian randomization study
Abstract
Background
Previous observational studies have yielded inconclusive findings regarding the potential association between cheese intake and peripheral vascular diseases (PVDs). We sought to systematically investigate the causal link between cheese intake and PVDs.
Methods
A two-sample Mendelian randomization (MR) investigation was undertaken to evaluate the causal link between cheese intake and PVDs. This MR analysis relied on summary statistics derived from published genome-wide associations studies (GWAS). GWAS summary statistics for cheese intake (N = 451,486), peripheral artery disease (PAD) (N = 361,194), PVD (N = 463,010), pulmonary embolism (PE) (N = 463,010), and deep vein thrombosis (DVT) (N = 6795) were derived from the UK Biobank. GWAS summary statistics for peripheral angiopathy (N = 162,394), aortic dissection (N = 218,792), aortic aneurysm (AA) (N = 218,792), other PVDs (N = 218,792), and arterial embolism and thrombosis of lower extremity artery (N = 218,792) were extracted from the FinnGen. Primary analysis was performed using an inverse variance weighted method. Sensitivity analyses included weighted median, MR Egger, and weighted mode analyses. Results are shown as odd ratio (OR) and 95% confidence interval (CI).
Results
Genetically predicted cheese intake was not associated with PAD (OR = 1.00, 95% CI: 1.00–1.00, p = 0.953), PVD (OR = 1.00, 95% CI: 0.99–1.00, p = 0.265), peripheral angiopathy (OR = 0.56, 95% CI: 0.09–3.66, p = 0.566), aortic dissection(OR = 0.69, 95% CI: 0.19–2.55, p = 0.583), AA(OR = 0.92, 95% CI: 0.46–1.82, p = 0.809), other PVDs (OR = 0.99, 95% CI: 0.44–2.21, p = 0.979), PE (OR = 1.00, 95% CI:1.00–1.00, p = 0.635), DVT (OR = 0.83, 95% CI: 0.62–1.12, p = 0.229), and arterial embolism and thrombosis of lower extremity artery (OR = 0.69, 95% CI: 0.21–2.29, p = 0.5413).
Conclusion
Based on the results of our two-sample MR analysis, we found no significant association between cheese intake and the risk of PVDs. We firmly believed that these findings had the potential to enhance the efficacy of prevention strategies for PVDs, at both the national and clinical level, by improving our understanding of the risk factors involved in their development. Low-sugar, low-salt and low-fat cheese may be a good choice for people at high risk of PVDs.
Abbreviations
-
- GWAS
-
- genome-wide associations studies
-
- MR
-
- Mendelian randomization
-
- PVDs
-
- peripheral vascular diseases
-
- SNPs
-
- single nucleotide polymorphisms
1 INTRODUCTION
Peripheral vascular diseases (PVDs) are defined to often include any or all atherosclerotic diseases beyond cardiac disease, such as leg artery, carotid artery, and aortic diseases and may also be associated with peripheral venous disease and lymphatic disease [1]. These conditions impact more than 230 million adults globally and are linked to a heightened risk of multiple unfavorable clinical outcomes, such as coronary artery disease, stroke, and leg complications [1-3]. While medical therapies for patients with PVDs continue to evolve, nonpharmacologic interventions such as smoking cessation, diet and exercise have shown beneficial effects in minimizing disease progression and development [4, 5]. These recommendations are predominantly derived from the literature characterizing risk factors of atherosclerosis. Unfortunately, the literature regarding the role of dietary intake and nutritional status in patients with PVDs remains limited. Cheese is an important dietary source of calcium, vitamins, unsaturated and branched-chain fats, which can have positive effects on health, while the LDL-cholesterol derived from saturated fats in cheese may have adverse effects on PVDs [6]. Moreover, the current literature on the potential adverse or beneficial effects of cheese intake on PVDs remains controversial, with varying conclusions reported in published studies.
Several observational studies have suggested that cheese intake may have a protective effect against cardiovascular diseases [7, 8]. A systematic review demonstrated that moderate cheese intake (50 g/day) was associated with a lower cardiovascular disease risk, while a multinational prospective cohort study found that cheese intake was linked to a lower risk of mortality and major cardiovascular events [9]. Additionally, a Mendelian randomization (MR) analysis found that cheese intake was inversely associated with peripheral artery disease (PAD), with no observed associations for pulmonary embolism (PE) [10]. Nevertheless, given that PAD is an atherosclerotic occlusive disease, some studies suggested that cheese intake may have a positive association with atherosclerosis. Recio et al. found that cheese intake was positively correlated with carotid intima-media thickness and subclinical atherosclerosis among middle-aged Mexican women in a cross-sectional study [11]. Observational studies, however, were susceptible to bias due to reverse causality and confounding factors. To address these issues, we conducted a two-sample MR study to illustrate the causal link between cheese intake and PVDs.
MR has emerged as a widely utilized epidemiological approach to explore the potential causal link between genetically predicted exposure factors and clinical outcomes through the use of single nucleotide polymorphisms (SNPs) as instrumental variables (IVs) [12]. The MR study leveraged the principle of Mendelian inheritance, where alleles were randomly assigned during conception, thereby minimizing potential confounding factors and reverse causality inherent in observational studies [13]. In this study, we employed a two-sample MR study to evaluate the causal link between cheese intake and PVDs in the European population using data obtained from genome-wide association studies (GWAS).
2 MATERIALS AND METHODS
2.1 Study design
In accordance with the fundamental assumptions of MR analysis, a rigorous two-sample MR was performed to scrutinize the potential causal link between cheese intake and PVDs [14]. Given that all data utilized in this study were obtained from previously published studies, the original authors provided ethical approval and informed consent.
2.2 Study exposures and outcomes
The GWAS data for cheese intake were derived from a large cohort study involving approximately 500,000 individuals conducted by the UK Biobank (http://www.nealelab.is/uk-biobank/) [15]. The study collected genotypic and various phenotypic data and was approved by the research ethics committee. Participants in the cohort were invited to the local evaluation center for data collection using a touch-screen questionnaire or standardized anthropometry. Participants' intake of cheese as an exposure factor was extracted by a questionnaire asking about the frequency of cheese intake. The participants were asked, “How often do you eat cheese (Include cheese in pizzas, quiches, cheese sauce etc)? Please provide an average considering your intake over the last year.” The optional answers for participants included never, less than once a week, once a week, 2–4 times a week, 5–6 times a week, once or more daily, do not know, and prefer not to answer. In total, 451,486 European participants' cheese intake data were obtained. The GWAS summary statistics have been included in the IEU OpenGWAS database and are easily available for researchers to download (GWAS ID: ukb-b-1489) [16, 17].
We obtained GWAS summary statistics of PVDs (all subtypes) from two publicly available cohorts, namely, the UK Biobank and the FinnGen cohorts. GWAS summary statistics for PAD (N = 1230 cases, 359,964 controls), PVD (N = 1456 cases, 461,554 controls), PE (N = 1846 cases, 461,164 controls), and deep vein thrombosis (DVT) (N = 6795, no available cases/controls) were derived from the UK Biobank of European ancestry. GWAS summary statistics for peripheral angiopathy (N = 193 cases, 162,201 controls), aortic dissection (N = 470 cases, 218,322 controls), aortic aneurysm (AA) (N = 2825 cases, 215,967 controls), other PVDs (N = 1037 cases, 217,755 controls), and arterial embolism and thrombosis of lower extremity artery (N = 471 cases, 218,321 controls) were extracted from the FinnGen release 6 (R6) with imputed genotype data, including up to 260,405 individuals of Finnish descent [18]. The detailed descriptions of included traits are summarized in Table 1.
| Exposure | Trait | ID | Number of SNPs | Population | Sample size | Case/control | Database | Consortium | Year |
|---|---|---|---|---|---|---|---|---|---|
| Cheese intake | Cheese intake | ukb-b-1489 | 9,851,867 | European | 451,486 | NA | UK Biobank | MRC-IEU | 2018 |
| Outcomes | |||||||||
| Peripheral artery disease | Peripheral artery disease | ukb-d-I9_PAD | 9,637,467 | European | 361,194 | 1230/359,964 | UK Biobank | NA | 2018 |
| Peripheral vascular disease | Diagnoses - secondary ICD10: I73.9 peripheral vascular disease, unspecified | ukb-b-4929 | 9,851,867 | European | 463010 | 1456/461,554 | UK Biobank | MRC-IEU | 2018 |
| Peripheral angiopathy | Peripheral angiopathy | finn-b-DM_PERIPH_ANGIOPATHY | 16,380,190 | European | 162,394 | 193/162,201 | FinnGen Biobank | NA | 2021 |
| Aortic dissection | Dissection of aorta (no controls excluded) | finn-b-I9_AORTDIS_EXNONE | 16,380,466 | European | 218,792 | 470/218,322 | FinnGen Biobank | NA | 2021 |
| Aortic aneurysm | Aortic aneurysm (no controls excluded) | finn-b-I9_AORTANEUR_EXNONE | 16,380,466 | European | 218,792 | 2825/215,967 | FinnGen Biobank | NA | 2021 |
| Other peripheral vascular diseases | Other peripheral vascular diseases (no controls excluded) | finn-b-I9_OTHPER_EXNONE | 16,380,466 | European | 218,792 | 1037/217,755 | FinnGen Biobank | NA | 2021 |
| Pulmonary embolism | Diagnoses - main ICD10: I26.9 pulmonary embolism without mention of acute cor pulmonale | ukb-b-18366 | 9,851,867 | European | 463,010 | 1846/461,164 | UK Biobank | MRC-IEU | 2018 |
| Deep vein thrombosis | Age deep-vein thrombosis (DVT, blood clot in leg) diagnosed | ukb-d-4012_irnt | 13,449,539 | European | 6795 | NA | UK Biobank | MRC-IEU | 2018 |
| Arterial embolism and thrombosis of lower extremity artery | Arterial embolism and thrombosis of lower extremity artery (no controls excluded) | finn-b-I9_ARTEMBTHRLOW_EXNONE | 16,380,466 | European | 218,792 | 471/218,321 | FinnGen Biobank | NA | 2021 |
- Abbreviations: NA, not available; SNP, single nucleotide polymorphism.
As shown in Figure 1, a genetic variant can be used to estimate a causal effect, and the following three core IV assumptions were met: (a) relevance assumption, which posits that the genetic variants exhibited a robust association with cheese intake; (b) independence assumption, which stipulates that the genetic variation is not associated with any underlying confounders; (c) exclusion assumption, which postulates that the genetic variants were not related to PVDs, except through cheese intake. In accordance with stringent criteria for strong correlation and independence, we selected instrumental SNPs linked to exposure factors with genome-wide level of statistical significance (p < 5 × 10−8) and not in linkage disequilibrium (R2 < 0.01 and clumping window >10,000 kb) [19]. In addition, the strength of individual SNPs was verified by computing the F-statistic. SNPs with F-statistics greater than 10 were deemed powerful enough to counteract the effects of potential biases. Finally, SNPs with inconsistent allele and frequency information in both the exposure and outcome groups were excluded.
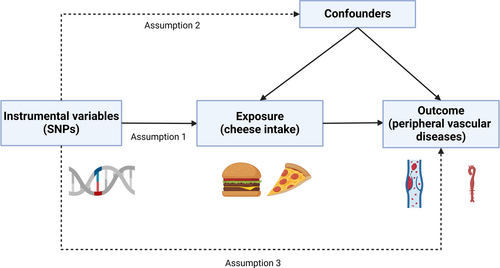
Three key assumptions of the Mendelian randomization study. (1) The selected genetic variant was strongly associated with cheese intake (relevance assumption); (2) the genetic variation was not related to any potential confounding factors (independence assumption); (3) the genetic variants were not related to peripheral vascular diseases (PVDs) except through cheese intake (exclusion assumption).
2.3 Statistical analysis
This study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology Using MR Statement (https://pubmed.ncbi.nlm.nih.gov/34698778/). Inverse-variance weighted analysis (IVW) was employed as the primary analytical approach, which provided a reliable and efficient causal estimate in the absence of horizontal pleiotropy or heterogeneity. Moreover, to ensure the robustness and consistency of the results, various MR methods were employed, including (a) the weighted median method, which calculated the median of the IV estimates and produced unbiased results, even if up to 50% of the IVs were invalid [20]. (b) MR-Egger regression method, which provided a consistent causal estimate even if the IVs exhibited pleiotropic effects [21]. (c) Weighted mode method, which was less biased than other methods, as it reduced bias effectively, yet it may be less precise. However, the outcome may be vulnerable to outlier SNPs. In the sensitivity analyses, both the MR Egger and IVW method were employed to evaluate heterogeneity among the SNPs by computing the Cochran Q statistics, which demonstrated that heterogeneity was statistically significant (p < 0.05) [22]. Horizontal pleiotropy was assessed using the MR Egger intercept test, which referred to a genetic variant with more than one independent phenotypic effect that may influence causal pathways [23]. Furthermore, leave-one-out analyses were conducted to identify whether any individual instrumental SNP biased the MR estimates. The odds ratio (OR) and its 95% confidence interval (CI) were used to display the causal association between cheese intake and PVDs. Forest plots, scatter plots, and funnel plots were used to visually display the outcomes of this MR study. The primary analysis employed the “TwoSampleMR” package of the R program (version 4.1.0) to investigate the impact of cheese intake on PVDs.
3 RESULTS
3.1 Details of SNPs
The numbers of SNPs related to cheese intake (GWAS ID: ukb-b-1489) were 9,851,867, and those for PAD (GWAS ID: ukb-d-I9_PAD), PVD (GWAS ID: ukb-b-4929), peripheral angiopathy (GWAS ID: finn-b-DM_PERIPH_ANGIOPATHY), aortic dissection (GWAS ID: finn-b-I9_AORTDIS_EXNONE), AA(GWAS ID: finn-b-I9_AORTANEUR_EXNONE), other PVDs (GWAS ID: finn-b-I9_OTHPER_EXNONE), PE (GWAS ID: ukb-b-18366), DVT (GWAS ID: ukb-d-4012_irnt), and arterial embolism and thrombosis of lower extremity artery (GWAS ID: finn-b-I9_ARTEMBTHRLOW_EXNONE) were 9,637,467, 9,851,867, 16,380,190, 16,380,466, 16,380,466, 16,380,466, 9,851,867, 13,449,539, and 16,380,466 respectively. The detailed descriptions of the included traits are summarized in Table 1.
3.2 Causal effects of cheese intake on PVDs
As presented in Table 2, IVW results revealed no causal association between genetically predicted cheese intake and PAD (OR = 1.00, 95% CI: 1.00–1.00, p = 0.953), PVD (OR = 1.00, 95% CI: 0.99–1.00, p = 0.265), peripheral angiopathy (OR = 0.56, 95% CI: 0.09–3.66, p = 0.566), aortic dissection (OR = 0.69, 95% CI: 0.19–2.55, p = 0.583), AA (OR = 0.92, 95% CI: 0.46–1.82, p = 0.809), other PVDs (OR = 0.99, 95% CI: 0.44–2.21, p = 0.979), PE (OR = 1.00, 95% CI: 1.00–1.00, p = 0.635), DVT (OR = 0.83, 95% CI: 0.62–1.12, p = 0.229), and arterial embolism and thrombosis of lower extremity artery (OR = 0.69, 95% CI: 0.21–2.29, p = 0.5413) by using 63, 36, 62, 61, 61, 61, 43, 63, and 61 SNPs as the instruments, respectively. Similar results were obtained using the weighted median, MR-Egger, and weighted mode methods, but with poor precision (Table 2). The association between cheese intake and PVDs was further elucidated in the forest plot (Figure 2) and the scatter plot (Figure 3). Furthermore, the leave-one-out sensitivity analysis illustrated in Figure 4 demonstrated that the observed association was not driven by a single SNP.
| Outcomes | Number of instruments | Method | Heterogeneity | Pleiotropy | |||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | Q statistic | p | Intercept | p | |||
| Peripheral artery disease | 63 | MR egger | 1.00 | 0.99, 1.01 | 0.668 | 76.26 | 0.090 | 4.40 × 10−5 | 0.648 |
| 63 | Weighted median | 1.00 | 1.00, 1.00 | 0.879 | |||||
| 63 | Inverse variance weighted | 1.00 | 1.00, 1.00 | 0.953 | 76.53 | 0.101 | |||
| 63 | Weighted mode | 1.00 | 0.99, 1.01 | 0.797 | |||||
| Peripheral vascular disease | 36 | MR egger | 1.00 | 0.99, 1.02 | 0.750 | 49.59 | 0.041 | −7.10 × 10−5 | 0.595 |
| 36 | Weighted median | 1.00 | 0.99, 1.00 | 0.060 | |||||
| 36 | Inverse variance weighted | 1.00 | 0.99, 1.00 | 0.265 | 50.01 | 0.048 | |||
| 36 | Weighted mode | 1.00 | 0.98, 1.01 | 0.366 | |||||
| Peripheral angiopathy | 62 | MR egger | 0.28 | 0.00, 694.89 | 0.753 | 46.42 | 0.901 | 0.012 | 0.853 |
| 62 | Weighted median | 0.68 | 0.05, 9.29 | 0.771 | |||||
| 62 | Inverse variance weighted | 0.58 | 0.09, 3.66 | 0.566 | 46.46 | 0.916 | |||
| 62 | Weighted mode | 7.88 | 0.02, 2621.44 | 0.488 | |||||
| Aortic dissection | 61 | MR egger | 0.00 | 0.00, 0.57 | 0.035 | 66.64 | 0.231 | 0.096 | 0.041 |
| 61 | Weighted median | 0.75 | 0.13, 4.41 | 0.746 | |||||
| 61 | Inverse variance weighted | 0.69 | 0.19, 2.55 | 0.583 | 71.57 | 0.146 | |||
| 61 | Weighted mode | 0.23 | 0.01, 8.61 | 0.428 | |||||
| Aortic aneurysm | 61 | MR egger | 5.45 | 0.31, 96.67 | 0.252 | 109.3 | 1.055 × 10−4 | −0.031 | 0.217 |
| 61 | Weighted median | 1.00 | 0.46, 2.18 | 0.995 | |||||
| 61 | Inverse variance weighted | 0.92 | 0.46, 1.82 | 0.809 | 106.5 | 1.512 × 10−4 | |||
| 61 | Weighted mode | 1.03 | 0.26, 4.04 | 0.962 | |||||
| Other peripheral vascular diseases | 61 | MR egger | 22.87 | 0.76, 689.85 | 0.077 | 38.59 | 0.982 | −0.054 | 0.068 |
| 61 | Weighted median | 1.44 | 0.44, 4.66 | 0.546 | |||||
| 61 | Inverse variance weighted | 0.99 | 0.44, 2.21 | 0.979 | 42.05 | 0.962 | |||
| 61 | Weighted mode | 2.18 | 0.25, 18.98 | 0.481 | |||||
| Pulmonary embolism | 43 | MR egger | 1.01 | 0.99, 1.02 | 0.486 | 40.88 | 0.476 | −7.40 × 10−5 | 0.534 |
| 43 | Weighted median | 1.00 | 1.00, 1.00 | 0.714 | |||||
| 43 | Inverse variance weighted | 1.00 | 1.00, 1.00 | 0.635 | 41.27 | 0.503 | |||
| 43 | Weighted mode | 1.00 | 0.99, 1.01 | 0.696 | |||||
| Deep vein thrombosis | 63 | MR egger | 0.86 | 0.25, 2.96 | 0.815 | 75.35 | 0.102 | −5.70 × 10−4 | 0.957 |
| 63 | Weighted median | 0.79 | 0.53, 1.16 | 0.225 | |||||
| 63 | Inverse variance weighted | 0.83 | 0.62, 1.12 | 0.229 | 75.36 | 0.119 | |||
| 63 | Weighted mode | 0.79 | 0.34, 1.82 | 0.585 | |||||
| Arterial embolism and thrombosis of lower extremity artery | 61 | MR egger | 3.70 | 0.02, 616.22 | 0.618 | 61.11 | 0.400 | −0.029 | 0.510 |
| 61 | Weighted median | 1.37 | 0.22, 8.38 | 0.733 | |||||
| 61 | Inverse variance weighted | 0.69 | 0.21, 2.29 | 0.5413 | 61.57 | 0.419 | |||
| 61 | Weighted mode | 1.58 | 0.05, 50.05 | 0.796 | |||||
- Abbreviations: CI, confidence interval; OR, odds ratio.
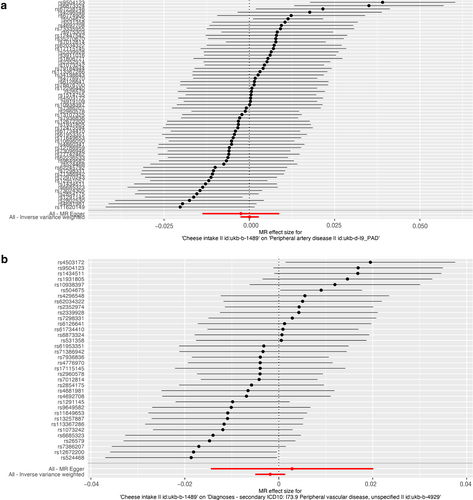
Forest plot of single nucleotide polymorphisms associated with cheese intake and the risk of peripheral artery disease (a), PVD (b), peripheral angiopathy (c), aortic dissection (d), aortic aneurysm (e), other PVDs (f), pulmonary embolism (g), deep vein thrombosis (h), and arterial embolism and thrombosis of lower extremity artery (i). PVD, peripheral vascular disease.

Forest plot of single nucleotide polymorphisms associated with cheese intake and the risk of peripheral artery disease (a), PVD (b), peripheral angiopathy (c), aortic dissection (d), aortic aneurysm (e), other PVDs (f), pulmonary embolism (g), deep vein thrombosis (h), and arterial embolism and thrombosis of lower extremity artery (i). PVD, peripheral vascular disease.
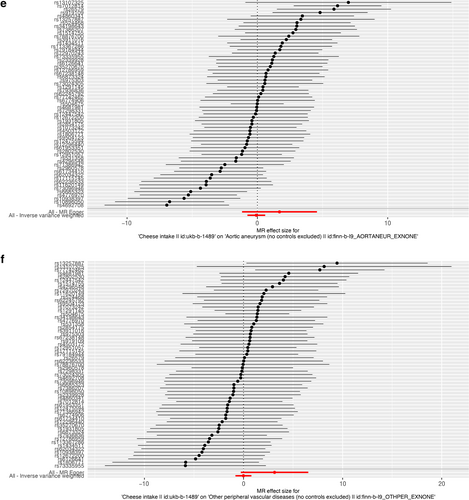
Forest plot of single nucleotide polymorphisms associated with cheese intake and the risk of peripheral artery disease (a), PVD (b), peripheral angiopathy (c), aortic dissection (d), aortic aneurysm (e), other PVDs (f), pulmonary embolism (g), deep vein thrombosis (h), and arterial embolism and thrombosis of lower extremity artery (i). PVD, peripheral vascular disease.
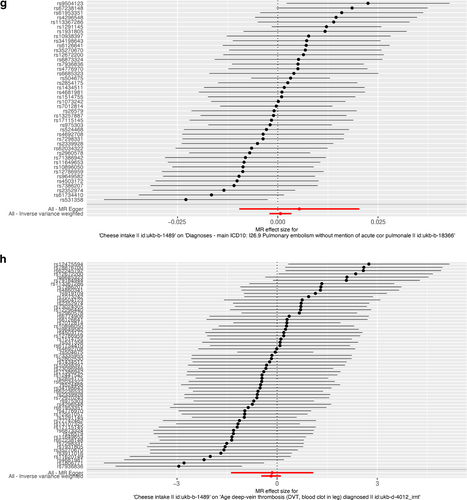
Forest plot of single nucleotide polymorphisms associated with cheese intake and the risk of peripheral artery disease (a), PVD (b), peripheral angiopathy (c), aortic dissection (d), aortic aneurysm (e), other PVDs (f), pulmonary embolism (g), deep vein thrombosis (h), and arterial embolism and thrombosis of lower extremity artery (i). PVD, peripheral vascular disease.
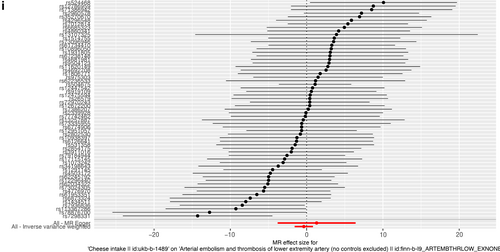
Forest plot of single nucleotide polymorphisms associated with cheese intake and the risk of peripheral artery disease (a), PVD (b), peripheral angiopathy (c), aortic dissection (d), aortic aneurysm (e), other PVDs (f), pulmonary embolism (g), deep vein thrombosis (h), and arterial embolism and thrombosis of lower extremity artery (i). PVD, peripheral vascular disease.
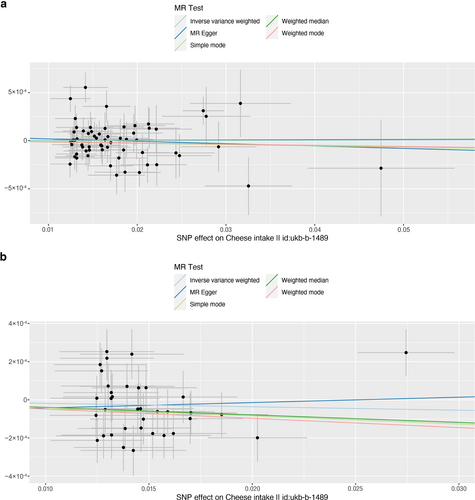
Scatter plot of single nucleotide polymorphisms associated with cheese intake and the risk of peripheral artery disease (a), PVD (b), peripheral angiopathy (c), aortic dissection (d), aortic aneurysm (e), other PVDs (f), pulmonary embolism (g), deep vein thrombosis (h), and arterial embolism and thrombosis of lower extremity artery (i). PVD, peripheral vascular disease.
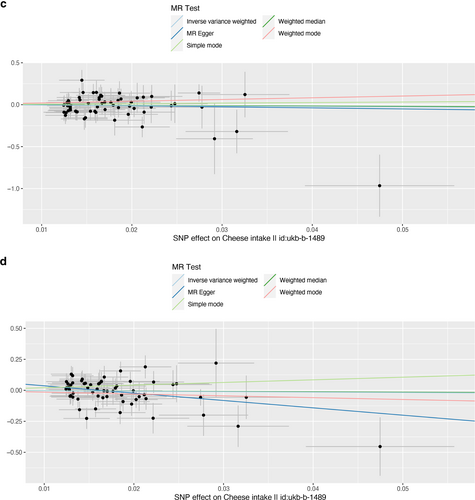
Scatter plot of single nucleotide polymorphisms associated with cheese intake and the risk of peripheral artery disease (a), PVD (b), peripheral angiopathy (c), aortic dissection (d), aortic aneurysm (e), other PVDs (f), pulmonary embolism (g), deep vein thrombosis (h), and arterial embolism and thrombosis of lower extremity artery (i). PVD, peripheral vascular disease.
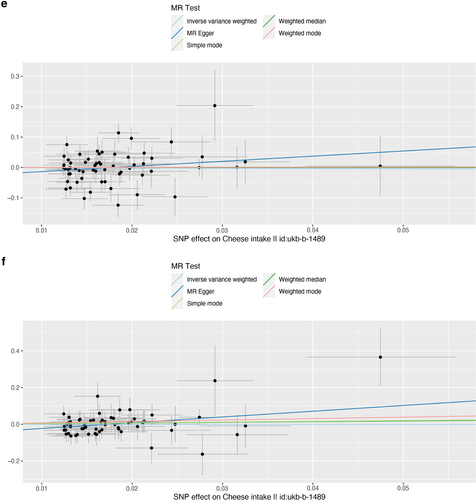
Scatter plot of single nucleotide polymorphisms associated with cheese intake and the risk of peripheral artery disease (a), PVD (b), peripheral angiopathy (c), aortic dissection (d), aortic aneurysm (e), other PVDs (f), pulmonary embolism (g), deep vein thrombosis (h), and arterial embolism and thrombosis of lower extremity artery (i). PVD, peripheral vascular disease.
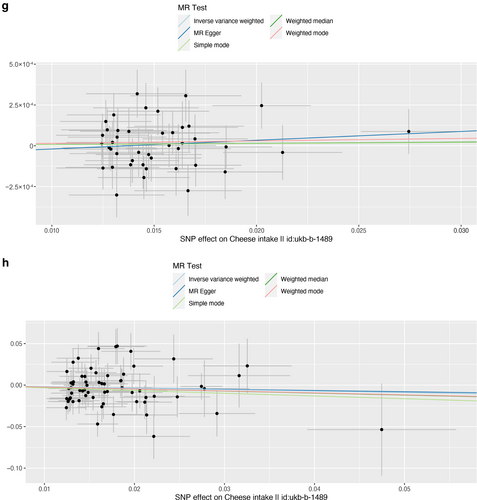
Scatter plot of single nucleotide polymorphisms associated with cheese intake and the risk of peripheral artery disease (a), PVD (b), peripheral angiopathy (c), aortic dissection (d), aortic aneurysm (e), other PVDs (f), pulmonary embolism (g), deep vein thrombosis (h), and arterial embolism and thrombosis of lower extremity artery (i). PVD, peripheral vascular disease.
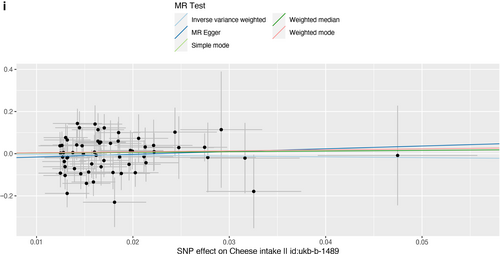
Scatter plot of single nucleotide polymorphisms associated with cheese intake and the risk of peripheral artery disease (a), PVD (b), peripheral angiopathy (c), aortic dissection (d), aortic aneurysm (e), other PVDs (f), pulmonary embolism (g), deep vein thrombosis (h), and arterial embolism and thrombosis of lower extremity artery (i). PVD, peripheral vascular disease.
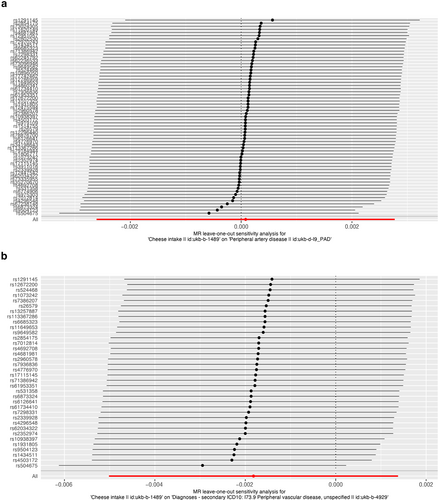
Leave-one-out of single nucleotide polymorphisms associated with cheese intake and of the risk of peripheral artery disease (a), PVD (b), peripheral angiopathy (c), aortic dissection (d), aortic aneurysm (e), other PVDs (f), pulmonary embolism (g), deep vein thrombosis (h), and arterial embolism and thrombosis of lower extremity artery (i). PVD, peripheral vascular disease.
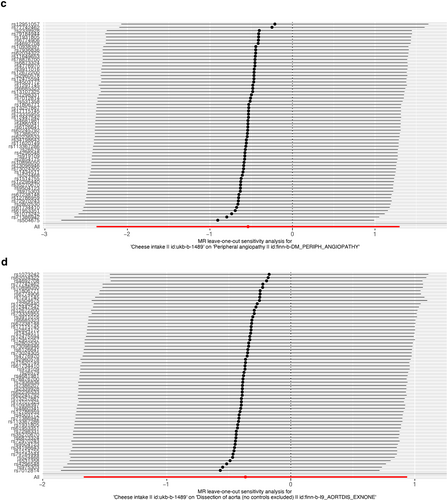
Leave-one-out of single nucleotide polymorphisms associated with cheese intake and of the risk of peripheral artery disease (a), PVD (b), peripheral angiopathy (c), aortic dissection (d), aortic aneurysm (e), other PVDs (f), pulmonary embolism (g), deep vein thrombosis (h), and arterial embolism and thrombosis of lower extremity artery (i). PVD, peripheral vascular disease.
Leave-one-out of single nucleotide polymorphisms associated with cheese intake and of the risk of peripheral artery disease (a), PVD (b), peripheral angiopathy (c), aortic dissection (d), aortic aneurysm (e), other PVDs (f), pulmonary embolism (g), deep vein thrombosis (h), and arterial embolism and thrombosis of lower extremity artery (i). PVD, peripheral vascular disease.
Leave-one-out of single nucleotide polymorphisms associated with cheese intake and of the risk of peripheral artery disease (a), PVD (b), peripheral angiopathy (c), aortic dissection (d), aortic aneurysm (e), other PVDs (f), pulmonary embolism (g), deep vein thrombosis (h), and arterial embolism and thrombosis of lower extremity artery (i). PVD, peripheral vascular disease.
Leave-one-out of single nucleotide polymorphisms associated with cheese intake and of the risk of peripheral artery disease (a), PVD (b), peripheral angiopathy (c), aortic dissection (d), aortic aneurysm (e), other PVDs (f), pulmonary embolism (g), deep vein thrombosis (h), and arterial embolism and thrombosis of lower extremity artery (i). PVD, peripheral vascular disease.
The MR-Egger intercepts of PAD (MR-Egger regression intercept = 4.40 × 10−5, p = 0.648), PVD (MR-Egger regression intercept = 7.10 × 10−5, p = 0.595), peripheral angiopathy (MR-Egger regression intercept = 0.012, p = 0.853), aortic dissection (MR-Egger regression intercept = 0.096, p = 0.041), AA (MR-Egger regression intercept = 0.031, p = 0.217), other PVDs (MR-Egger regression intercept = 0.054, p = 0.068), PE (MR-Egger regression intercept = 7.40 × 10−5, p = 0.534), DVT (MR-Egger regression intercept = 5.70 × 10−4, p = 0.957), and arterial embolism and thrombosis of lower extremity artery (MR-Egger regression intercept = 0.029, p = 0.510) suggested that the results were not biased by genetic pleiotropy. Heterogeneity tests demonstrated that there was no heterogeneity between cheese intake and PAD, peripheral angiopathy, aortic dissection, other PVD, PE, DVT, and arterial embolism and thrombosis of lower extremity artery (all p > 0.05), but heterogeneity was observed in the Q test analysis on the association between cheese intake and PVD and AA (all p < 0.05) (Table 2). Additionally, the funnel plot, presented in Figure 5, indicated that the results were not affected by horizontal pleiotropy.
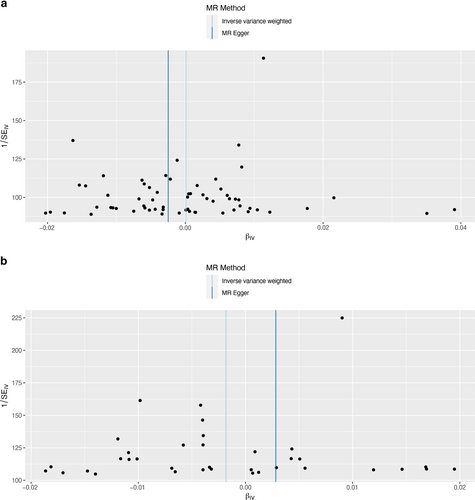
Funnel plots to visualize overall heterogeneity of Mendelian randomization estimates for the effect of cheese intake on the risk of peripheral artery disease (a), PVD (b), peripheral angiopathy (c), aortic dissection (d), aortic aneurysm (e), other PVDs (f), pulmonary embolism (g), deep vein thrombosis (h), and arterial embolism and thrombosis of lower extremity artery (i). PVD, peripheral vascular disease.
Funnel plots to visualize overall heterogeneity of Mendelian randomization estimates for the effect of cheese intake on the risk of peripheral artery disease (a), PVD (b), peripheral angiopathy (c), aortic dissection (d), aortic aneurysm (e), other PVDs (f), pulmonary embolism (g), deep vein thrombosis (h), and arterial embolism and thrombosis of lower extremity artery (i). PVD, peripheral vascular disease.
Funnel plots to visualize overall heterogeneity of Mendelian randomization estimates for the effect of cheese intake on the risk of peripheral artery disease (a), PVD (b), peripheral angiopathy (c), aortic dissection (d), aortic aneurysm (e), other PVDs (f), pulmonary embolism (g), deep vein thrombosis (h), and arterial embolism and thrombosis of lower extremity artery (i). PVD, peripheral vascular disease.
Funnel plots to visualize overall heterogeneity of Mendelian randomization estimates for the effect of cheese intake on the risk of peripheral artery disease (a), PVD (b), peripheral angiopathy (c), aortic dissection (d), aortic aneurysm (e), other PVDs (f), pulmonary embolism (g), deep vein thrombosis (h), and arterial embolism and thrombosis of lower extremity artery (i). PVD, peripheral vascular disease.
Funnel plots to visualize overall heterogeneity of Mendelian randomization estimates for the effect of cheese intake on the risk of peripheral artery disease (a), PVD (b), peripheral angiopathy (c), aortic dissection (d), aortic aneurysm (e), other PVDs (f), pulmonary embolism (g), deep vein thrombosis (h), and arterial embolism and thrombosis of lower extremity artery (i). PVD, peripheral vascular disease.
4 DISCUSSION
In the present investigation, we leveraged summary-level data from the extensive GWAS to scrutinize the potential causal link between cheese intake and PVDs among the European population. Our rigorous two-sample MR analyses yielded compelling evidence in support of null associations between genetically determined cheese intake and risk of PAD, PVD, peripheral angiopathy, aortic dissection, AA, other PVDs, PE, DVT, and arterial embolism and thrombosis of the lower extremity artery. Furthermore, robust sensitivity analyses using several reliable methodologies provided strong assurance that the observed MR analysis results were not influenced by pleiotropic effects.
Given the widespread consumption of cheese and the high morbidity of PVDs worldwide, elucidating the impact of cheese intake on PVDs is of great importance. Although cheese is a full-fat dairy product that may be intuitively linked to increased risk of PAD and abdominal AA due to its high saturated fatty acid content and effect on blood cholesterol levels [24-26], a study by Ageno et al. failed to demonstrate LDL-cholesterol as a risk factor for DVT and PE [27]. Moreover, a MR analysis indicated that cheese intake was inversely associated with PAD, with no observed associations for PE [10]. It is worth noting that evaluating the effect of cheese on PVDs based solely on saturated fatty acids may be inadequate, as the impact of food on a single biomarker such as blood cholesterol may not fully capture the risk of PVDs. Consequently, the current literature regarding the potential benefits or drawbacks of cheese intake on PVDs remains controversial and limited. To address this gap, we adopted a food matrix approach and applied a MR study to explore the link between cheese intake and PVDs more specifically.
In this MR study, we conducted the first comprehensive and systematic assessment of the relationship between cheese intake and PVDs. Unlike most previous studies, our study showed no association between cheese and PVDs. There are several plausible reasons for this discrepancy. Firstly, reverse causality and residual confounding may have affected previous observational studies. For instance, prior studies have not adjusted for the effect of salt in cheese, which has been associated with an increased risk of hypertension, a key risk factor for PAD, aortic dissection, and AA [28-32]. Hence, the role of salt in cheese cannot be overlooked when exploring the relationship between cheese intake and PVDs. In addition, individuals with higher levels of education may exhibit a greater tendency toward cheese intake, in contrast to those with lower levels of education. This observation implied that education level is an important upstream factor in determining health outcomes. It is plausible that a higher education level promoted the adoption of healthier lifestyle choices, consequently reducing the likelihood of being exposed to various health risks [33, 34]. Secondly, the data from previous studies were obtained on the basis of self-reported cheese intake. The potential misclassification of habitual cheese intake, which may result in inaccurate measurement of long-term cheese intake, cannot be overlooked [9]. Thirdly, the lack of association between cheese intake and PVDs in this MR study may be attributed to the counterbalancing effects of both cardio protective and cardio-detrimental components of cheese and dietary components. As a result, the overall effect of cheese intake on PVDs may be neutral. Studies have shown that the saturated fatty acids and sugars contained in cheese can lead to endothelial dysfunction, oxidative stress, inflammation and metabolic disorders [35, 36]. However, a mechanism for beneficial effect of cheese consumption could be the effect of the high content of calcium in cheese. Previous studies showed that dietary calcium with fat generates calcium soup and increases the excretion of lipid in feces and significantly reduces serum cholesterol and triglyceride levels [37]. Moreover, cheese is classified as a probiotic food, containing a significant amount of live microorganisms, mainly Lactobacillus and Bifidobacterium genera [38]. Clinical trials have demonstrated that these probiotic bacteria have beneficial effects on immunity, inflammation, and cardiovascular risk factors [39]. Therefore, low-sugar, low-salt and low-fat cheese may be a good choice for people at high risk of PVDs. Lastly, the distribution of habitual cheese intake populations in the real world was not random, which may be influenced by factors such as the popularity of cheese culture in different areas as well as age and gender. These factors may play a significant role in the results of previous observational studies. Our study underscored the importance of considering various confounding factors in exploring the relationship between cheese intake and PVDs. Our findings may have important implications for public health policies and dietary guidelines.
This study included several advantages. Firstly, it was the first analysis to employ the MR methodology to evaluate the causal link between cheese intake and various PVDs, hence mitigating potential confounding and reverse causality, which is inherent in conventional epidemiologic investigations. Secondly, the results from diverse complementary analyses were largely consistent with each other, further corroborating the causal inference. Thirdly, the large number of cases in summary-level data which were used by us substantially enhanced the statistical power to detect causal associations. Notably, our results were less susceptible to population stratification bias, given that the analyzed data were exclusively from individuals of European descent.
This study, however, has limitations that should be acknowledged. Firstly, the participants were of European descent in the study, and therefore, the generalizability of our findings to other populations, such as Asians, should be interpreted with caution. Secondly, due to the lack of information on the types and amounts of cheese consumed, we were unable to assess the potential effects of cheese diversity and quantity on PVDs risk. Additionally, the original GWAS did not provide information on whether participants consumed cheese with other dietary components, which could have affected our results. Thirdly, the use of summary-level data in this study prevented us from examining the possibility of a non-linear causal relationship between cheese intake and PVDs risk. Therefore, further MR studies with individual-level data and longitudinal designs are needed to explore the potential dose-response associations between cheese intake and PVDs risk. Fourthly, the dietary questionnaire used to collect information on cheese intake may be susceptible to measurement errors. Fifthly, there was a possibility of sample overlap between the United Kingdom Biobank and FinnGen Biobank populations, which may increase the likelihood of false positives. However, as our results are negative, the population overlap is unlikely to affect our conclusion. Sixthly, although the definition and classification of PVDs used in our study was based on standard clinical criteria, variations in them across studies could lead to differences in results. Finally, despite using several sensitivity analyses, the potential bias introduced by horizontal pleiotropy and SNP heterogeneity cannot be completely excluded.
5 CONCLUSIONS
In conclusion, this two-sample MR analysis provided genetic evidence that cheese intake was not associated with PVDs, including PAD, PVD, peripheral angiopathy, aortic dissection, AA, other PVDs, PE, DVT, and arterial embolism and thrombosis of the lower extremity artery. In the future, more randomized trials or cohort studies are necessary to validate the causal relationship between cheese intake and PVDs. We firmly believe that these findings have the potential to enhance the efficacy of prevention strategies for PVDs, at both the national and clinical level, by improving our understanding of the risk factors involved in their development. Low-sugar, low-salt and low-fat cheese may be a good choice for people at high risk of PVDs.
AUTHOR CONTRIBUTION
Yuqing Huang, Bin Zhang and Song Wen designed the study. Song Wen drafted the article. Yuqing Huang, Guodong He and Zehan Huang conducted data acquisition. Yuqing Huang, Guodong He and Zehan Huang performed data analysis. Yuqing Huang, Guodong He, Zehan Huang, and Bin Zhang performed manuscript revision. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.




