Role of N6-methyladenosine RNA modification in cancer
Abstract
N6-methyladenosine (m6A) is the most abundant modification of RNA in eukaryotic cells. Previous studies have shown that m6A is pivotal in diverse diseases especially cancer. m6A corelates with the initiation, progression, resistance, invasion, and metastasis of cancer. However, despite these insights, a comprehensive understanding of its specific roles and mechanisms within the complex landscape of cancer is still elusive. This review begins by outlining the key regulatory proteins of m6A modification and their posttranslational modifications (PTMs), as well as the role in chromatin accessibility and transcriptional activity within cancer cells. Additionally, it highlights that m6A modifications impact cancer progression by modulating programmed cell death mechanisms and affecting the tumor microenvironment through various cancer-associated immune cells. Furthermore, the review discusses how microorganisms can induce enduring epigenetic changes and oncogenic effect in microorganism-associated cancers by altering m6A modifications. Last, it delves into the role of m6A modification in cancer immunotherapy, encompassing RNA therapy, immune checkpoint blockade, cytokine therapy, adoptive cell transfer therapy, and direct targeting of m6A regulators. Overall, this review clarifies the multifaceted role of m6A modification in cancer and explores targeted therapies aimed at manipulating m6A modification, aiming to advance cancer research and improve patient outcomes.
1 INTRODUCTION
Cancer is the second leading cause of death and imposes tremendous personal and societal burdens.1 Development, invasion, and metastasis of cancer involve an imbalance between cancer and immune cells. This imbalance is related to multiple factors, including exogenous (environmental pollution, chronic irritation, poisons or drugs, microbial action, and personal habits) and endogenous (genetic, epigenetic, and endocrine factors). Epigenetic processes include DNA methylation, histone modification, chromatin remodeling, noncoding RNA (ncRNA) and RNA modification.2, 3 Among the various RNA modification mechanisms, N6-methyladenosine (m6A), first discovered in mammalian cells in 1974, is the most abundant.4 m6A, a dynamic and reversible RNA modification, is regulated by various proteins, including writers (methyltransferases), erasers (demethylases), and readers (m6A-binding proteins). Previous studies have extensively investigated these proteins. However, recent research has increasingly focused on the posttranslational modifications (PTMs) of these regulatory proteins, which can significantly impact their activity and function. Exploring PTMs of m6A regulators represents a promising avenue for future research. m6A modification plays critical roles in RNA splicing, translation, stability, degradation, and translocation5 and can also reverse regulate chromatin status,6 ultimately influencing a range of human diseases, including cancer7 and other diseases such as cardiovascular diseases,8 autoimmune diseases,9 central nervous system diseases,10 reproductive system diseases,11 and metabolic diseases.12 Therefore, elucidating the role of m6A modifications in cancer may provide better targets for treatment.
Programmed cell death (PCD) represents a highly orchestrated form of cellular demise and is among the processes regulated by m6A modifications, impacting the interplay between cancer and immune cells. Recent studies indicate that m6A RNA modification can govern tumor-associated immune cells, encompassing macrophages, neutrophils, dendritic cells (DCs), T cells, B cells, and natural killer (NK) cells. m6A modification crucially influences the growth, polarization, activation, and differentiation of these immune cells within the tumor microenvironment (TME). Moreover, m6A may be pivotal in anticancer immunity, particularly in cancers associated with specific microorganisms. The modification regulates the life cycle of several pathogens, and certain pathogens can produce oncogenic proteins that promote carcinogenesis by altering m6A methylation levels in the human body. Thus, targeting m6A modification in microorganisms presents a promising avenue for future cancer therapies.13
Various cancer treatments have been rapidly developed and widely used, including surgery,14 chemotherapy,15 radiotherapy,16 targeted therapy,17 endocrine therapy,18 and immunotherapy.18 In contrast to other therapies, immunotherapy focuses on the interactions between immune and cancer cells.19 Immunotherapy consists of gene therapy, immune checkpoint blockade (ICB), cytokine therapy, adoptive cell transfer (ACT) treatment,20 and direct targeting of m6A regulators. Although this strategy has achieved a degree of return, some patients still do not benefit because of multiple factors, such as immune evasion21 and drug resistance.22 However, the underlying mechanisms have not been fully elucidated. Therefore, we reviewed recent studies on m6A-based immunotherapies. Targeting m6A modifications may shed new light on improving immunotherapy.
In this review, we first provide a comprehensive summary of the regulatory proteins involved in m6A modification, encompassing the writers (methyltransferases), erasers (demethylases), and readers (m6A-binding proteins). Additionally, we elucidate their PTMs, including methylation, acetylation, lactylation, ubiquitination, SUMOylation, phosphorylation, and O-GlcNAcylation. PTMs play a pivotal role in regulating the activity or stability of m6A regulatory proteins and impact their functions in cancer cells. Subsequently, we delve into the interplay between m6A modification and chromatin accessibility within cancer cells. Furthermore, our focus lies on recent advancements in understanding the implications of m6A modification in cancer from three distinct perspectives. First, m6A modifications are intricately involved in various PCD mechanisms such as autophagy, ferroptosis, pyroptosis, cuproptosis, and disulfidoptosis, which exhibit dual roles in cancer processes. Second, m6A modification exerts influence on the TME by modulating proliferation, polarization, recruitment, and activity of diverse immune cells including macrophages, neutrophils, DCs, T cells, B cells, and NK cells. Last, in certain microorganism-associated cancers, m6A could potentially affect pathogen life cycles while specific oncogenic proteins derived from cancer-related microorganisms can alter human m6Amethylation patterns as well. We summarize the roles played by Helicobacter pylori (Hp), Fusobacterium nucleatum (Fn), hepatitis B virus, Epstein-Barr virus (EBV), and Human papillomavirus (HPV). At the end, we highlight how m6A modification plays an important role in cancer immunotherapy including RNA therapy, ICB, cytokine therapy, ACT, and direct targeting treatment of m6A regulatory proteins. A deeper investigation into the mechanisms underlying m6A may shed new light on future cancer research and treatment.
2 REGULATORY PROTEINS OF M6A MODIFICATION
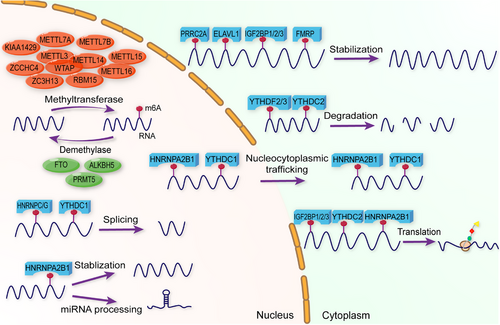
m6A modification is regulated by diverse proteins including writers, erasers, and readers. Writers, specifically methyltransferases, methylate mRNA bases. Erasers, known as demethylases, remove these modifications. Readers are proteins that modulate mRNA metabolism by selectively binding to m6A modifications. Each regulator plays a crucial role in m6A processes, influencing both physiological functions and pathological conditions (Figure 1 and Table 1).
| Type | Regulator | Function | References |
|---|---|---|---|
| Writer | METTL3 | Catalyze m6A RNA modification | 23, 24 |
| METTL14 | Catalyze m6A RNA modification | 23-25 | |
| WTAP | Assist the localization of METTL3/METTL14 complex into nuclear speckles | 26 | |
| METTL5 | Promote 18S rRNA m6A modification | 27 | |
| METTL16 | Promote U6 small nuclear RNA m6A modification and S-adenosylmethionine (SAM) synthetase pre-mRNA | 32, 33 | |
| KIAA1429 | Methylate several target RNA in m6A manner | 35 | |
| ZCCHC4 | Promote 28S rRNA m6A modification | 27, 37 | |
| METTL7A | The sequence between 76 and 172 aa of METTL7A contributes to the methylation of adenosine at 481 of LOC606724. | 28 | |
| METTL7B | Induce m6A modification of GPX4, HMOX1, and SOD1 mRNA. | 30 | |
| ZC3H13 | Anchor WTAP in nuclear and enhance its activity | 38 | |
| RBM15 | Catalyze m6A RNA modification | 36 | |
| Eraser | FTO | Remove m6A modification | 44 |
| ALKBH5 | Remove m6A modification | 47 | |
| PRMT5 | Inhibit RNA m6A methylation by enhancing the nuclear translocation of ALKBH5 | 50 | |
| Reader | YTHDF1 | Promote m6A-modified mRNA translation | 51, 52, 54 |
| YTHDF2 | Recognize m6A modification and degrade mRNA | 51 | |
| YTHDF3 | Enhance the translation of m6A-enriched transcripts and mRNA degradation | 53 | |
| YTHDC1 | Promote targeted mRNA splicing and nuclear export of mRNA | 55, 70 | |
| YTHDC2 | Enhance targeted mRNA translation efficiency and decrease mRNA abundance | 56 | |
| IGF2BP1 | Promote m6A-modified mRNA stability and translation in cytoplasm | 59 | |
| IGF2BP2 | Promote m6A-modified RNA translation in cytoplasm | 57 | |
| IGF2BP3 | Promote m6A-modified RNA translation in cytoplasm | 58 | |
| HNRNPC | Promote m6A-modified RNA splicing in nuclear | 74 | |
| HNRNPA2B1 |
Interact with and promote primary miRNA processing in nuclear Promote target mRNA nucleocytoplasmic trafficking Promote mRNA stabilization |
60, 61, 72, 73 | |
| HNRNPG | Promote m6A-modified RNA splicing | 75 | |
| PRRC2A | Stabilize m6A-modified mRNA | 62 | |
| ELAVL1 | Interact with other m6A regulators and stabilize target RNA | 40, 63, 76 | |
| FMRP | Stabilize m6A-modified mRNA | 64 |
- Abbreviations: METTL3, methyltransferase-like 3; METTL14, methyltransferase-like 14; WTAP, Wilms tumor 1-associating protein; METTL5, methyltransferase-like 5; METTL16, methyltransferase-like 16; ZCCHC4, zinc finger CCHC domain-containing protein 4; METTL7A, methyltransferase-like 7A; METTL7B, methyltransferase-like 7B; ZC3H13, zinc finger CCCH domain containing protein 13; RBM15, RNA binding motif protein 15; FTO, Fat mass and obesity-associated protein; ALKBH5, human AlkB homolog 5; PRMT5, Protein arginine methyltransferase 5; YTHDF1, YT521-B homology (YTH) domain-containing family protein 1; YTHDF2, YT521-B homology (YTH) domain-containing family protein 2; YTHDF3, YT521-B homology (YTH) domain-containing family protein 3; YTHDC1, YT512-B homology domain-containing protein 1; YTHDC2, YT512-B homology domain-containing protein 2; IGF2BP1, insulin-like growth factor 2 mRNA-binding protein 1; IGF2BP2, insulin-like growth factor 2 mRNA-binding protein 2; IFG2BP3, Insulin-like growth factor 2 mRNA-binding protein 3; HNRNPC, heterogeneous nuclear ribonucleoprotein C; HNRNPA2B1, heterogeneous nuclear ribonucleoprotein A2B1; HNRNPG, heterogeneous nuclear ribonucleoprotein G; PRRC2A, proline-rich coiled-coil 2A; ELAVL1, ELAV-like RNA-binding protein 1; FMRP, fragile X mental retardation protein
2.1 m6A writers
m6A writers primarily comprise the m6A methyltransferase complex, which includes methyltransferase-like 3 (METTL3), methyltransferase-like 14 (METTL14), and Wilms tumor 1-associating protein (WTAP).23 METTL3, the first discovered writer and a key member of this complex, is a 70-kDa protein that contains a methyltransferase domain that methylates RNA. Similarly, METTL14 can methylate RNA. In addition, when METTL3 and METTL14 form a complex with a 1:1 stoichiometry,24 METTL3 and METTL14 have much better m6A methyltransferase activity than either alone.25 Although WTAP does not directly methylate RNA, it significantly influences the structure and substrate specificity of the METTL3/METTL14 complex. In nuclear speckles, WTAP is associated with the accumulation of METTL3 and METTL14.26 Moreover, other important methyltransferases have been reported, including methyltransferase-like 5 (METTL5),27 methyltransferase-like 7A (METTL7A),28, 29 methyltransferase-like 7B (METTL7B),30, 31 methyltransferase-like 16 (METTL16),32, 33 KIAA1429,34, 35 RNA binding motif protein 15 (RBM15),36 zinc finger CCHC domain-containing protein 4 (ZCCHC4),37 and zinc finger CCCH domain containing protein 13 (ZC3H13).38 Emerging evidence has revealed the important role of writers in tumorigenesis,39 tumor metastasis,40 and immunotherapy.41 m6A writers can upregulate the m6A methylation of cancer-related genes. For example, Wei et al.42 demonstrated that METTL3 accelerates gastric cancer (GC) progression through the ADAMTS9-mediated phosphatidylinositol-3 kinase (PI3K)/V-akt murine thymoma viral oncogene homolog (AKT) pathway. Zhou et al.43 found that the METTL3/YTHDF2 m6A axis accelerated colorectal carcinogenesis. Collectively, m6A writers promote RNA methylation and induce multiple biological functions.
2.2 m6A erasers
m6A erasers, also known as m6A demethylases, remove m6A from RNA and decrease m6A levels. Fat mass and obesity-associated protein (FTO) has been considered as the first m6A demethylase since He et al.44 discovered that m6A was the main substrate of FTO in nuclear RNA in 2011. FTO not only plays an essential role in obesity by regulating adipogenic pathways and inducing preadipocyte differentiation to facilitate adipogenesis but also participates in tumor processes.45, 46 Another key demethylase, human AlkB homolog 5 (ALKBH5), also plays a role in m6A modifications associated with various diseases, especially tumors. ALKBH5 belongs to the alkB family of dioxygenases, which regulate oxidative demethylation to modulate the repair of N-alkylated nucleobases.47, 48 ALKBH5 participates in multiple cancer or noncancer processes.49 Protein arginine methyltransferase 5 (PRMT5) is a new demethylase that inhibits RNA m6A modification by enhancing the nuclear translocation of ALKBH5.50 In summary, m6A erasers play important roles in demethylating RNA and inducing subsequent functions.
2.3 m6A readers
m6A readers, also called m6A-binding proteins, can recognize and bind to m6A-modified transcripts to regulate the expression and function of several genes during various processes. Several m6A readers have been identified, including the YTHDF family,51-54 YTHDC family,55, 56 IGF2BP family,57-59 RNA-binding protein heterogeneous nuclear ribonucleoprotein (HNRNP) family,60, 61 proline-rich coiled-coil 2A (PRRC2A),62 ELAV-like RNA-binding protein 1 (ELAVL1),63 and fragile X mental retardation protein (FMRP).64 The YTHDF family includes three proteins: YTHDF1, YTHDF2, and YTHDF3. YTHDFs have different functions. YTHDF1 recognizes m6A-modified RNA and promotes its translation into the cytoplasm. YTHDF2 participates in the degradation of m6A-modified RNA. YTHDF3 enhances mRNA translation and degradation. YTHDFs regulate cancer progression in an m6A-dependent manner. For instance, Chen et al.65 found that YTHDF1 could facilitate the translation of FOXM1 via m6A modification, which subsequently promotes breast cancer (BC) progression. However, a new model for YTHDFs provided by Zaccara and Jaffrey66 illustrates that YTHDFs function together to mediate the degradation of m6A-modified RNA. Liquid‒liquid phase separation (LLPS) is the formation of several membraneless condensates. Interestingly, YTHDFs have the potential to form condensates. Specifically, the N-terminal of YTHDFs is mainly an intrinsically disordered region (IDR), whereas the C-terminal consists of the m6A-binding YTH domain. Both play vital roles in the formation of the condensates.67 Zou et al.68 found that YTHDF1 and YTHDF2 can form different granules because of their diverse low-complexity regions. In addition, Fu et al.67 found that under oxidative stress, YTHDF1 and YTHDF3 are abundant in stress granules rather than in processing bodies (P-bodies). However, YTHDF2 was abundant in both stress granules and P-bodies.67 Stress granules and P-bodies have several functions, including metabolic reprogramming, targeting and silencing of specific mRNAs via the RNA-induced silencing complex, and restoring and permitting the translation of key specific mRNAs.69 YTHDCs are YTH domain-containing proteins, including YTHDC1 and YTHDC2. YTHDC1 mediates nuclear export of m6A-modified mRNA and facilitates mRNA splicing.55, 70 Recent studies have also demonstrated its ability to form membraneless organisms such as nuclear condensates via LLPS, which may be associated with gene expression, transcript splicing, and nucleocytoplasmic export.71 YTHDC2 enhances translation efficacy and destabilizes cytoplasm.56 IGF2BP family refers to Insulin-like growth factor 2 mRNA-binding proteins, which mainly consist of IGF2BP1, IGF2BP2, and IGF2BP3. IGF2BPs are key readers of m6A modifications that stabilize mRNA and promote its translation in the cytoplasm.59 HNRNPA2B1 has multiple functions in m6A modification, including processing of miRNA,72 promotion of nucleocytoplasmic trafficking,73 and stabilization of m6A-modified mRNA.60, 61 HNRNPC is an abundant nuclear RNA-binding protein that recognizes and splices m6A-modified RNA.74 HNRNPG recognizes RNA via RNA recognition motif (RRM) and Arg-Gly-Gly (RGG) motifs and splices m6A-modified RNA.75 PRRC2A plays a role in target RNA stabilization. For example, PRRC2A stabilizes Olig2 mRNA via the GGACU motif.62 ELAVL1 (also known as HuR) interacts with other m6A regulators and promotes m6A-modified RNA stabilization.40, 76 FMRP sustains m6A-modified stability and maintains its expression via m6A modification.77 Collectively, m6A-binding proteins recognize m6A-modified mRNA and influence their stability, localization, and translation. Notably, condensate formation of reader proteins induced by LLPS is an important direction for future research.
3 PTMs OF M6A REGULATORS
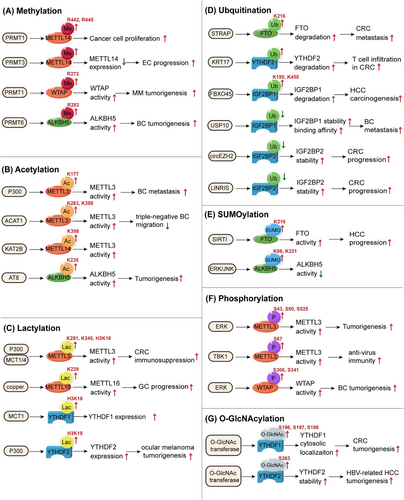
As a posttranscriptional RNA modification, m6A is regulated by writers, erasers, and readers. Moreover, these regulatory proteins undergo PTMs. We investigated various forms of modifications, such as methylation, acetylation, lactylation, ubiquitination, SUMOylation, phosphorylation, and O-GlcNAcylation (Figure 2 and Table 2).
| Modification | Regulator | Site | Factors | Function | References |
|---|---|---|---|---|---|
| Methylation | METTL14 |
R442 R445 |
PRMT1 | Methylate METTL14 and promote cancer cell proliferation | 78 |
| METTL14 | PRMT3 | Methylate METTL14 and upregulate its expression to promote EC progression | 79 | ||
| WTAP | R272 | PRMT1 | Methylate WTAP and promote the oxidative phosphorylation of MM | 80 | |
| ALKBH5 | R283 | PRMT6 | Methylate ALKBH5, which promotes aerobic glycolysis and tumorigenesis in BCs | 81 | |
| Acetylation | METTL3 | K177 | P300 | Catalyze METTL3 acetylation and affect subcellular localization of METTL3 to impede cancer metastasis in BC | 83 |
| METTL3 |
K263 K388 |
ACAT1 | Catalyze METTL3 acetylation, inhibit migration of cancer cells via NR2F6/ACAT/METTL3 axis | 84 | |
| METTL3-METTL14-WTAP complex | Sulfatide | Acetylation of the writer complex down-regulates MTF1 expression and the growth of HCC cells | 85 | ||
| METTL14 | K398 | KAT2B | Promote METTL14 acetylation and protein stability | 86 | |
| ALKBH5 | K235 | AT8 | Regulate demethylase activity and promote tumorigenesis | 87 | |
| IGF2BP2 | K530 | SIRT1 | Loss of SIRTI acetylates IGF2BP2 to recruit the nuclease XRN2 and degrade the ATP6V1A transcript. | 88 | |
| Lactylation | METTL3 |
H3K18 K281 K345 |
P300 | H3K18la facilitate the transcription of METTL3 to promote its expression; lactylation of METTL3 at K281 and K345 enhance its capture of m6A-modified RNA to promote immunosuppression in CRC | 95 |
| METTL16 | K229 | Copper | Lactylation of METTL16 at K229 promotes its methyltransferase activity and promotes FDX1 expression to induce cuproptosis | 96 | |
| YTHDF2 | H3K18 | P300 | H3K18la facilitates the transcription of YTHDF2 and then promote the degradation of m6A-modified PER1 and TP53 mRNAs to promote tumorigenesis in ocular melanoma | 93 | |
| YTHDF1 | H3K18 | MCT1 | H3K18la facilitates the transcription of YTHDF1 and its protein expression by binding to the promoter | 94 | |
| Ubiquitination | YTHDF2 | KRT17 | Promote YTHDF2 degradation and therefore stabilize CXCL10 mRNA to induce T cell infiltration in CRC | 102 | |
| IGF2BPs | TRIM25, circNDUFB2 | Degrade IGF2BPs proteins and activate antitumor immunity during NSCLC progression | 103 | ||
| IGF2BP2 | circEZH2 | Block IGF2BP2 degradation and promote its function of stabilizing CREB1 mRNA to promote CRC progression | 105 | ||
| IGF2BP2 | K139 | LINRIS | Block IGF2BP2 ubiquitination and facilitate its glycolysis in CRC | 106 | |
| IGF2BP1 | USP10 | Block IGF2BP1 ubiquitination and stabilize IGF2BP1, enhance its binding to m6A-modified CPT1A mRNA, leading to breast cancer metastasis | 107 | ||
| IGF2BP1 |
K190 K450 |
FBXO45 | Increase IF2BP1 ubiquitination and subsequent PLK1 upregulation to promote HCC carcinogenesis | 104 | |
| FTO | K216 | STRAP | Increase FTO ubiquitination and promote its degradation to stabilize MTA1 mRNA and promote CRC metastasis | 101 | |
| METTL14 | HRD1 | Promote METTL14 ubiquitination and degradation to suppress endoplasmic reticulum-related liver disease | 100 | ||
| SUMOylation | YTHDF2 | K281, K571, K572 | PIAS1 | Promote viral RNA decay to restrict EBV replication | 110 |
| YTHDF2 | K571 | SUMO1 | Increase its binding affinity of m6A-modified mRNAs and decrease-related gene expression | 111 | |
| METTL3 | K177, K211, K212, K215 | SUMO1 | Represses METTL3 m6A methytransferase activity | 113 | |
| ALKBH5 | K86, K321 | ERK/JNK | Inhibit ALKBH5 demethylation activity and increase m6A modification to protect genomic integrity of cells in response to ROS | 109 | |
| FTO | K216 | SIRT1 | Promote FTO SUMOylation and its degradation to decrease GNAO1 expression and promote HCC | 112 | |
Phosphorylation |
METTL3 | S43, S50, S525 | ERK | Stabilize m6A methyltransferase complex to upregulate m6A levels and promote tumorigenesis | 117, 118 |
| METTL3 | S67 | TBK1 | Phosphorylate METTL3 and promote its activation, enhancing antivirus immunity | 120 | |
| WTAP | S306, S341 | ERK | Stabilize m6A methyltransferase complex to upregulate m6A levels and promote tumorigenesis | 117 | |
| WTAP | S341 | ERK | Promote WTAP phosphorylation and stabilize WTAP protein, further promoting RNA m6A methylation of ENO1, impacting the glycolytic activity of BC cells. | 119 | |
| YTHDF2 |
S39, T381 |
EGFR, SRC, ERK | Promote YTHDF2 phosphorylation and stabilization, subsequently facilitate m6A-modified LXRA and HIVEP2 mRNA decay to promote glioblastoma tumorigenesis | 121 | |
| O-GlcNAcylation | YTHDF2 | S263 | OGT | Enhance YTHDF2 stability and oncogenic activity in HBV-related HCC | 123 |
| YTHDF1 |
S196, S197, S198 |
OGT | Promote YTHDF1 cytosolic localization and upregulate downstream target gene expression to promote CRC tumorigenesis | 124 |
3.1 Methylation
Arginine methylation of m6A regulatory proteins, induced by protein arginine methyltransferases (PRMTs), plays a vital role in cancer initiation and progression. Methylated METTL14 participates in cancer development. For instance, PRMT1-mediated methylation of METTL14 at the C-terminus is important for its function in catalyzing m6A modification, which promotes cancer cell proliferation.78 PRMT3 methylates METTL14, which downregulates its expression and promotes EC progression by influencing glutathione peroxidase 4 (GPX4) expression.79 WTAP is methylated by PRMT1, which targets the oxidative phosphorylation of multiple myeloma (MM) cells and promotes carcinogenesis via m6A modification of NDUFS6.80 PRMT6 can methylate the m6A eraser ALKBH5 at R283, which upregulates LDHA expression and promotes aerobic glycolysis and tumorigenesis in BC.81 In conclusion, arginine methylation of the m6A regulator is a potent target for cancer modulation.
3.2 Acetylation
Acetylation is a PTM.82 METTL3 is acetylated by P300 at K177. Acetylated METTL3 dampens its subcellular localization and function in BC cells, impeding cancer metastasis.83 Similarly, METTL3 is acetylated by acetyl-CoA acetyltransferase 1 (ACAT1) at residues K263 and K388, which stabilizes METTL3 and suppresses triple-negative BC migration and invasion via the NR2F6/ACAT/METTL3 axis.84 Acetylation of the m6A writer complex METTL3–METTL14–WTAP can be induced by sulfatide, thus regulating MTF1 expression to promote the growth of hepatocellular carcinoma (HCC) cells.85 KAT2B, a lysine acetyltransferase, catalyzes METTL14 acetylation at K398 and increases METTL14 protein stability to upregulate m6A methylation of Spi2a mRNA, which inactivates the NF-κB pathway.86 ALKBH5 is acetylated by lysine acetyltransferase 8 (AT8) at K235, which enhances its demethylase activity.87 IGF2BP2 is acetylated at K530. Loss of its deacetylase SIRT1 recruits nuclease XRN2 to degrade the ATP6V1A transcript.88 All the abovementioned studies show that acetylation can regulate the function of m6A regulatory proteins to influence disease processes.
3.3 Lactylation
The Warburg effect involves an increase in glycolytic metabolism even in the presence of O2, which is crucial for carcinogenesis, metastasis and drug resistance.89, 90 Lactate, a product of glycolytic metabolism, has recently gained increasing attention owing to its potential biological functions in cancer. Lactylation is a novel epigenetic modification.91 In 2019, Zhang et al.92 reported the metabolic regulation of histone lactylation gene expression. Current evidence indicates that lactylation is involved in m6A modifications. Histone lactylation is abundant in the promoters of m6A regulators and significantly influences their expression to a great extent. For instance, YTHDF1 and YTHDF2 expression are facilitated by H3K18la.93, 94 In contrast, lactylation directly modifies m6A regulators. Xiong et al.95 identified two lactylation modification sites, K281 and K345, in the zinc-finger domain of METTL3. The lactylation of METTL3 enhances its binding to Jak1 mRNA and therefore promotes the immunosuppression of colorectal cancer (CRC). Sun et al.96 found that high copper stress could induce METTL16 lactylation at K229, which upregulates the activity of METTL16 and, therefore, promotes FDX1 expression to facilitate carcinogenesis in GC. In summary, lactylation is important and may provide a new strategy for m6A modification in cancer cells.
3.4 Ubiquitination
Ubiquitin is a small protein consisting of 76 amino acids that assists in protein degradation by the S26 proteasome.97 Protein ubiquitination is a ubiquitin-dependent PTM that affects all cellular processes.98 Ubiquitination plays a key role in translation by modifying ribosomal and regulatory proteins.99 In this study, we focused on its role as an m6A regulator. METTL14 is ubiquitinated and degraded by HRD1 to suppress endoplasmic reticulum-related liver diseases.100 Serine/threonine kinase receptor-associated protein (STRAP) ubiquitinates FTO at K216, promotes its degradation, stabilizes MTA1 mRNA, and promotes CRC metastasis.101 KRT17 promotes YTHDF2 degradation via ubiquitination, which stabilizes CXCL10 mRNA and induces T-cell infiltration in CRC.102 IGF2BP protein ubiquitination can be upregulated by circNDUFB2 and TRIM25 (a kind of E3 ubiquitin ligase), leading to antitumor immunity in non-small cell lung cancer (NSCLC).103 IGF2BP1 is ubiquitinated by the E3 ubiquitin ligase FBXO45 at K190 and K450, which subsequently induces PLK1 upregulation and HCC carcinogenesis.104 Additionally, blocking the ubiquitination of IGF2BP proteins can suppress their degradation and promote their binding to RNA.105-107 These studies demonstrate that ubiquitination mainly affects m6A regulator proteins by promoting degradation.
3.5 SUMOylation
SUMOylation is a novel PTM associated with small ubiquitin-like modifiers that participate in cancer development of cancer.108 SUMOylation of m6A regulators influences their functions. For example, extracellular signal-regulated kinase (ERK)/c-Jun N-terminal kinases (JNK) signaling-induced SUMOylation of ALKBH5 represses its demethylase activity.109 YTHDF2 can be SUMOylated by PIAS1 at K281, K571, and K572, thereby facilitating the degradation of viral RNA and suppresses the replication of EBV.110 Additionally, YTHDF2 SUMOylation at K571 promotes mRNA decay and tumorigenesis.111 FTO is modified by SIRT1 at K216, which promotes FTO SUMOylation and degradation, decreases GNAO1 expression, and promotes HCC.112 METTL3 is reported to be SUMOylated at K177, K211, K212, and K215, which represses METTL3 m6A methyltransferase activity.113
3.6 Phosphorylation
Phosphorylation is an extensively studied PTM of proteins.114 Over the past 25 years, protein phosphorylation has been observed at the serine, threonine, and tyrosine residues.115 Protein phosphorylation regulates important cellular functions.116 ERK phosphorylates and stabilizes the METTL3/14-WTAP methyltransferase complex, which increases m6A methylation and promotes tumorigenesis.117 Specifically, METTL3 is typically modified at S43, S50, and S525,117, 118 whereas WTAP is modified at S306 and S341.117, 119 METTL3 is also phosphorylated at residue S67, which promotes its activation and helps m6A modification to stabilize IRF3 mRNA, leading to antiviral immunity.120 YTHDF2 can be phosphorylated and stabilized by EGFR/SRC/ERK signaling at S39 and T381, which facilitates the degradation of LXRA and HIVEP2 mRNA, leading to glioblastoma (GBM) tumorigenesis.121 Therefore, phosphorylation mainly stabilizes m6A regulators and activates their function in target mRNAs to modulate downstream gene expression and cellular processes. Current studies on SUMOylation of m6A regulators are limited and require further research.
3.7 O-GlcNAcylation
O-linked N-acetylglucosaminylation (O-GlcNAcylation) is a type of glycosylation related to O-GlcNAc at serine or threonine residues.122 Yang et al.123 found that YTHDF2 is modified by O-GlcNAc transferase 8(OGT8) at S263, which stabilizes YTHDF2 and enhances its activity by inhibiting its ubiquitination. Subsequently, O-GlcNAc-modified YTHDF2 stabilizes the minichromosome maintenance protein 2/5 transcripts to facilitate the onset of HBV-related HCC.123 YTHDF1 O-GlcNAcylation at S196, S197, and S198 promotes YTHDF1 cytosolic localization and upregulates the expression of downstream target genes to promote CRC tumorigenesis.124 The O-GlcNAcylation of m6A regulators has a significant impact on diseases, and related research has great potential.
These studies show that diverse PTMs in m6A regulators significantly influence their functions in cancer.
4 M6A MODIFICATIONS WITH CHROMATIN REGULATION IN CANCER
Chromatin and transcriptional states, which are dynamically regulated by epigenetic modification networks, are critical for establishing and maintaining cellular identity. Studies have shown that the m6A modification can regulate the chromatin state, known as chromatin accessibility, to affect transcriptional activity (Figure 3).
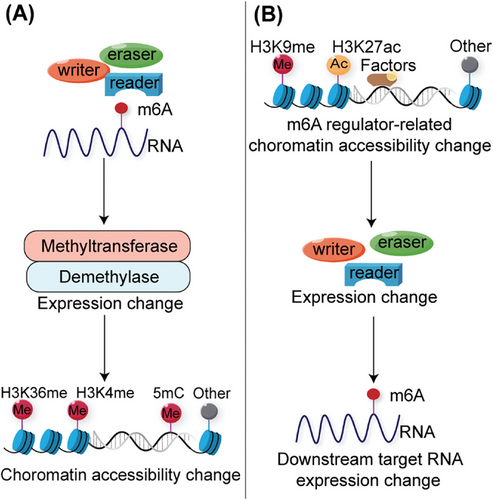
Changes in chromatin accessibility due to m6A-modified target genes significantly impact downstream transcription levels. Liu et al.6 discovered that METTL3 mediates m6A modification of chromosome-associated regulatory RNAs, including promoter-associated RNAs, enhancer RNAs, and repeat RNAs. YTHDC1 facilitates the nuclear degradation of a subset of these m6A-modified RNAs, ultimately leading to reduced chromatin accessibility and downstream transcription inhibition.6 The interactions between RNA m6A and DNA/histone modifications are important for physiological and pathogenic processes. In esophageal squamous cell carcinoma (ESCC) cells, METTL3 mediates m6A modification of the DNA demethylase TET1. The m6A reader FXR1 recognizes m6A RNA and recruits TET1 to genomic sites to demethylate DNA, leading to chromatin accessibility and reprogramming of gene transcription.125 In PDAC cells, m6A super-enhancer RNAs modified by the METTL3-CFL1 complex are recognized by YTHDC2, which recruits H3K4 methyltransferase MLL1 to catalyze H3K4me3 deposition, thus enhancing local chromatin accessibility and oncogene transcription.126 ALKBH5-demethylated lncRNA SNHG15 promotes myeloma tumorigenicity by increasing chromatin accessibility and recruiting the H3K36me3 modifier SETD2.127
Changes in the chromatin state of m6A regulators alter the levels of m6A modifications in downstream target genes, influencing their transcriptional activity. PARP1 regulates the transcription factor NFIC, and the activation of METTL3 transcription relies on PARP1 in conjunction with the METTL3 promoter. Upon irradiation or treatment with a PARP1 inhibitor, PARP1 dissociates from the METTL3 promoter chromatin. This leads to reduced accessibility of nuclear factor I-C (NFIC) and TATA-box binding protein (TBP), resulting in the repression of METTL3 expression and RNA m6A methylation.128 KDM4C regulates ALKBH5 expression to maintain leukemia stem cell (LSC) function in acute myeloid leukemia (AML) by increasing chromatin accessibility at ALKBH5 locus, reducing H3K9me3 levels, and promoting v-myb avian myeloblastosis viral oncogene homolog (MYB) and RNA Polymerase II (Pol II) recruitment. ALKBH5 affects the mRNA stability of the receptor tyrosine kinase AXL, which is recognized by YTHDF2 in an m6A-dependent manner.129 Temozolomide (TMZ) induces a SOX4-mediated increase in chromatin accessibility at METTL3 by promoting H3K27ac levels and recruiting RNA polymerase II to upregulate METTL3 expression, which further promotes m6A deposition on the histone methyltransferase EZH2, inhibits nonsense-mediated mRNA decay, and maintains GBM stem cell properties. This eventually led to increased TMZ resistance in GBM.130
The above studies, including the direct or indirect regulation between RNA m6A and DNA/histone modifications, demonstrate extensive interactions between these epigenetic regulatory events and provide new targets for tumor therapy.
5 M6A MODIFICATIONS WITH PCD IN CANCER
PCD is a crucial and well-organized mechanism that maintains cellular homeostasis in response to internal and external stimuli. Within TMEs, the interplay between pro-death and pro-survival pathways shapes the complexity and variability of tumor immunity. Previous research underscores PCD's pivotal role in cancer processes and in anticancer immunity.131-134 Various factors regulate PCD, with recent attention focused on m6A modifications. Here, we explore the interrelationship between m6A and diverse forms of PCD—including autophagy, ferroptosis, pyroptosis, cuproptosis, and disulfidaptosis—in both cancer progression and antitumor immunity. Our findings aim to inform novel strategies for future cancer treatments (Table 3).
| PCD | Cancer | Regulator | Function | References |
|---|---|---|---|---|
| Autophagy | HCC | YTHDF1 | HIF-1α-induced YTHDF1 upregulates ATG2A and ATG14 translation to promote HCC autophagy and malignancy. | 140, 141 |
| HCC | IGF2BP1 | circMDK modified by IGF2BP1 promotes HCC proliferation and metastasis via the miR-346/874-3p-ATG16L1 axis. | 142 | |
| HCC | WTAP | WTAP-mediated m6A modification regulates LKB1 and decreases phosphorylation of AMPK to restrain cell autophagy and promote HCC proliferation. | 144 | |
| HCC | METTL3 | M6A-modified FOXO3 by METTL3 activates autophagy-associated pathways and promotes sorafenib resistance. | 152 | |
| EOC | FTO | circRAB11FIP1 promotes FTO-associated proteins expression and mediates mRNA expression levels of ATG5 and ATG7 in an m6A dependent manner to facilitate autophagy flux. | 143 | |
| ccRCC | FTO | FTO/autophagy/SIK2 axis promotes the progression of ccRCC. | 145 | |
| GC |
FTO, YTHDF2 |
FTO and YTHDF2 regulate ULK1 expression to modulate autophagy activation and drug resistance. | 149 | |
| LSCC | IGF2BP3 | IGF2BP3 regulates TMA7-mediated autophagy and Cisplatin resistance in LSCC. | 151 | |
| SCLC | METTL3 | METTL3 targets DCP2 to induce Pink1–Parkin pathway-mediated mitophagy and mitochondrial damage to promote chemoresistance in SCLC. | 150 | |
| Ferroptosis | GC | YTHDF2 | Hypoxia-induced lncRNA CBSLR interacts with YTHDF2 to decrease CBS mRNA and therefore reduce methylation of the ACSL4 leading to ferroptosis resistance in GC. | 187 |
| PTC | FTO | FTO downregulates SLC7A11 in an m6A dependent manner through ferroptosis to Inhibit PTC proliferation, migration, and invasion. | 158 | |
| BC | METTL14 | M6A-modified FGFR4 reduces ferroptosis in recalcitrant HER2-positive BC via the β-catenin/TCF4-SLC7A11/FPN1 axis. | 165 | |
| HB | METTL3 | METTL3-mediated SLC7A11 m6A modification enhances HB ferroptosis resistance. | 156 | |
| Pyroptosis | LC |
METTL3, YTHDF2 |
M6A modification of lncRNA LINC00969 at posttranscriptional levels inhibits pyroptosis and promotes acquired gefitinib resistance in lung cancer. | 172 |
| Cuproptosis | GC | METTL16 | Lactylation of METTL16 promotes cuproptosis in GC by upregulating m6A modification of FDX1 mRNA and expression of FDX1 protein. | 96 |
- Abbreviations: HCC, hepatocellular carcinoma; EOC, epithelial ovarian cancer; ccRCC, clear cell renal cell carcinoma; GC, gastric cancer; LSCC, laryngeal squamous cell carcinoma; SCLC, small cell lung cancer; PTC, papillary thyroid cancer; BC, breast cancer; HB, hepatoblastoma; LC, lung cancer.
5.1 Autophagy
Autophagy is highly conserved in eukaryotes. It maintains cellular homeostasis and metabolism.135 When stressed by internal or external stimuli, cells combine autophagosomes with lysosomes to form autolysosomes, membrane structures that engulf and degrade aged or injured organelles, misfolded proteins, and pathogens to regulate cell homeostasis of cells.136 Additionally, this process plays a vital role in tumors by promoting and inhibiting tumorigenesis and progression.137
m6A modifications are closely associated with autophagy. The upregulation of m6A RNA modification is helpful for autophagosome formation when nutrients are deficient.138 The m6A modification can regulate autophagy-related genes (ATGs), ultimately influencing their function and promoting the onset of various diseases, including tumors.139 For instance, Hao et al.138 reported that YTHDF3 responds to METTL3-associated m6A hypermethylation and recruits eIFs to promote FOXO3 translation, consequently activating a subset of ATGs and leading to autophagy. In liver tissues, m6A RNA modification plays a complex role in tumor progression. If not addressed, liver fibrosis can progress to cirrhosis and cancer. Hepatic stellate cells (HSCs) play an important role in myofibroblast matrix production during this physiological and pathological process. Shen et al.140 showed that YTHDF1 stabilizes BECN1 mRNA and promotes autophagy activation via m6A modification in HSCs. The hypoxia-inducible factor-1α (HIF-1α) drove YTHDF1 to bind to the m6A-modified mRNA of ATG2A and ATG14, which can contribute to the translation of ATG2A and ATG14, thereby promoting the survival of HCC under hypoxic conditions and its progression.141 One study identified an oncogenic circRNA, circMDK, as a potential biomarker for HCC, because its upregulation with m6A modification upregulates ATG16L1, resulting in the activation of the PI3K/AKT/mTOR signaling pathway to promote cell proliferation, migration, and invasion.142 Interestingly, circRNAs have gained considerable attention in recent years. CircRAB11FIP1 mediates the expression of ATG5 and ATG7 via m6A, thus promoting epithelial ovarian cancer.143 LKB1 is regulated by WTAP via m6A modification and then phosphorylated AMPK. At the same time, researchers found that knockdown of WTAP could upregulate the level of autophagy and inhibit hepatoblastoma (HB) cell proliferation.144 Similarly, the progression of clear cell renal cell carcinoma (ccRCC) can be regulated by FTO because it inhibits autophagy and promotes tumorigenesis through an m6A-IGF2BP2-dependent mechanism, indicating that FTO can be a prognostic biomarker and a promising target in ccRCC.145
In parallel, m6A-dependent autophagy plays an important role in antitumor drug resistance. From a pharmacokinetic perspective, m6A modifications influence drug transport and metabolism. This may be related to several membrane transporter proteins such as ATP-binding cassette proteins. In addition, m6A can alter drug targets to regulate drug response and resistance.146 Autophagy promotes anticancer drug resistance to protect tumor cells from survival.147 Furthermore, m6A modification can modulate ATGs (ATG5 and ATG7) to regulate the formation and progression of autophagosomes, thus influencing autophagy and promoting the survival and anticancer resistance of tumor cells.148 However, the underlying mechanisms remain unclear. FTO-mediated cisplatin resistance in GC is attributed to the inhibition of Unc-51-like kinase (ULK1)-mediated autophagy.149 Sun et al.150 found that METTL3 is a marker for poor prognosis of small-cell lung cancer (SCLC) and is highly expressed in chemoresistant SCLC cells through Pink1–Parkin pathway-mediated mitophagy. Translation machinery-associated 7 homolog (TMA7) plays a key role in the carcinogenesis of laryngeal squamous cell carcinoma (LSCC) and cisplatin resistance via the IGF2BP3/TMA7/UBA2 axis.151 In liver cancer, sorafenib resistance is induced by m6A-dependent, FOXO3-mediated autophagy.152 m6A plays a crucial role in the onset, progression, and drug resistance of multiple tumors by regulating autophagy, suggesting a promising breakthrough in future antitumor treatments.
5.2 Ferroptosis
Ferroptosis, first described by Dixon et al. in 2012,153 is a new nonapoptotic form of PCD. Biochemically, ferroptosis is a ROS-dependent PCD characterized by iron accumulation and lipid peroxidation.154 Emerging evidence has demonstrated its mechanisms, including the suppression of system Xc−, GPX4, mitochondrial voltage-dependent anion channels, and P53.155 System Xc is an amino acid antitransporter composed of two subunits, SLC7A11 and SLC3A2.153 GPX4 plays a pivotal role in the induction and regulation of ferroptosis by inhibiting lipid peroxide formation.
Studies have indicated that m6A modification can regulate cancer cell ferroptosis via m6A-modifying ferroptosis-associated mRNA to modulate these mechanisms. For instance, SLC7A11 has been widely demonstrated to be m6A-modified by several m6A regulators. SLC7A11 mRNA stability can be promoted, and its expression can be upregulated in a METTL3 manner, resulting in tumor growth and ferroptosis resistance in HB156 and lung adenocarcinoma.157 SLC7A11 can be downregulated by FTO to inhibit thyroid cancer progression via ferroptosis.158 ALKBH5 can decrease the expression of SLC7A11 by repressing the m6A modification, which promotes ferroptosis in CRC159 and thyroid cancer.160 NF-κB activating protein (NKAP) serves as a novel suppressor of ferroptosis. NKAP binds to m6A and promotes SLC7A11 mRNA splicing to protect GBM cells from ferroptosis.161 METTL16 increases GPX4 expression by modifying m6A to inhibit ferroptosis and promote BC.162 The mature GPX4 mRNA contains three m6A binding motifs. RUNX1 intronic transcript 1 (RUNX1-IT1) directly binds to IGF2BP1 and promotes LLPS to increase GPX4 mRNA stability, thereby blocking ferroptosis and promoting BC carcinogenesis.163 Erianin, a low-molecular-weight bibenzyl natural product extracted from Dendrobium chrysotoxum, induces ferroptosis in renal cancer stem cells by promoting the m6A methylation of ALOX12 and P53 mRNA.164 m6A-modified FGFR4 reduces ferroptosis in recalcitrant HER2-positive BC via the β-catenin/TCF4-SLC7A11/FPN1 axis.165
Furthermore, m6A-associated ferroptosis plays an important role in anticancer immunity and immunotherapy. Immunotherapy-activated CD8+ T cells can regulate tumor ferroptosis to enhance the antitumor effects.166 Several studies have aimed to bioinformatically analyze the relationship between m6A modification, ferroptosis, and immunity in cancers. SLC17A9 is associated with tumor immune infiltration, m6A modification, and ferroptosis in HCC.167 Li et al.168 found that the expression of BTBD10 (an activator of the Akt family) was correlated with some m6A-associatedgenes, ferroptosis-related genes, and immune checkpoints in HCC. Wang et al.169 showed that YTHDF1 suppresses CD8+ T cell-related anticancer effects and ferroptosis by stabilizing programmed cell death ligand 1 (PD-L1) transcripts, which are important for prostate cancer cells to evade effector T cell cytotoxicity and CD8+ T cell-mediated ferroptosis. In summary, the m6A modification regulates ferroptosis in cancer cells by modulating the expression of ferroptosis-associated mRNAs. m6A-modulated ferroptosis also participates in cancer immunity and immunotherapy. However, the underlying mechanisms remain largely unknown. Therefore, further research is required to develop novel cancer treatment strategies.
5.3 Pyroptosis
Pyroptosis is a gasdermin-mediated PCD process mainly activated by caspases. Pyroptotic cells are swollen and their plasma membrane ruptures. METTL3 suppresses pyroptosis in retinal pigment epithelium cells by targeting the miR-25-3p/PTEN/Akt signaling cascade.170 YTHDF1 inhibits caspase-1-dependent pyroptosis by upregulating the WW domain-containing E3 ubiquitin protein ligase 1.171 LINC00969 promotes acquired gefitinib resistance by decreasing NLRP3 levels via m6A modification to inhibit pyroptosis in lung cancer.172 Additionally, bladder cancer, GC, BC, and melanoma are associated with pyroptosis-related lncRNAs and m6A modification.173-176 However, the underlying mechanisms remain to be elucidated.
5.4 Cuproptosis
Cuproptosis is a newly identified form of PCD that is dependent on copper (Cu). This process is characterized by Cu-targeting and binding to lipoylated components within the tricarboxylic acid cycle, which ultimately induces proteotoxic stress and leads to cell death. Current research indicates that cuproptosis may be correlated with various cancer signaling pathways, including EGFR, PDK1, PI3K, MAPK, MYC, and Notch.177 Sun et al.96 found that tumor tissues had higher Cu and lactate contents than normal tissues in GC. In addition, they demonstrated that under high Cu stress, lactylation of METTL16 at K229 upregulated the m6A methylation levels of FDX1 mRNA and FDX1 protein expression, which triggered cuproptosis.96 Nucleophosmin 1 (NPM1) is a biomarker for gastrointestinal cancer. Researchers found that NPM1 is associated with anticancer immunity, m6A modification, and cuproptosis. Cuproptosis-related genes can be used to predict the prognosis of various cancers, including lung adenocarcinoma,178, 179 BC,180 and HCC181, 182 in an m6A-associated manner. However, little is known about the specific role of m6A and further studies are needed to uncover this mystery.
5.5 Disulfidptosis
In contrast to other types of PCDs, cells with high SLC7A11 expression can accumulate cellular disulfides such as cystine. This accumulation induces disulfide stress, which leads to an increasing number of disulfide bonds within the actin cytoskeleton, damaging the cytoskeletal structure, and consequently resulting in cell death.183-185 Disulfidopathy is a novel approach for metabolic cancer therapy. Recent studies have suggested that metabolic therapy using glucose transporter inhibitors can facilitate disulfidptosis and dampen cancer development.186 Disulfidopathy is a potential target for cancer treatment. However, current studies require bioinformatics analyses. Further research is required to explore this mechanism, and m6A modifications may provide an important perspective.
m6A modification plays a pivotal role in regulating the survival and death of cancer cells. PCD serves as a critical modulator of cancer immunity, influencing the function of immune cells and leading to diverse outcomes. Consequently, targeting m6A could be considered for cancer immunotherapy.
6 M6A MODIFICATIONS IN CANCER-ASSOCIATED IMMUNE CELLS
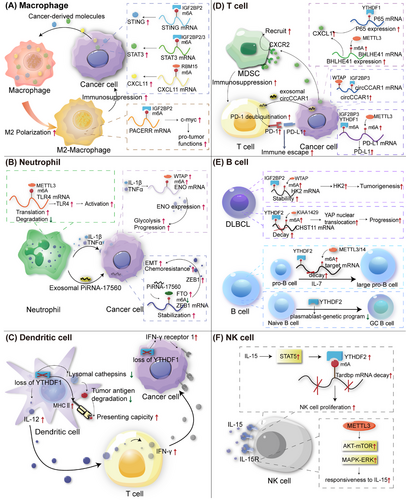
Previously, we analyzed multiple PTMs of m6A regulators and m6A modifications in cancer cells. However, the TME is dynamic and complex, including not only cancer cells but also noncancer cells, including various immune cells that play an essential and frontline role in fighting against viruses, bacteria, and cancer cells by triggering innate and adoptive immune responses.188 Dysregulation and dysfunction of immune cells participate in cancer initiation, progression, invasion, and metastasis, as well as immunotherapy resistance.189-191 Emerging evidence has shown that m6A plays an important role in the growth, differentiation, polarization, migration, and activation of immune cells. Here, we summarized the role of m6A in several cancer-related immune cells, including macrophages, neutrophils, DCs, T cells, B cells, and NK cells (Figure 4 and Table 4).
| Cell | Cancer | Function | References |
|---|---|---|---|
| Mø | Glioma | ALKBH5 expression recruits M2 macrophages in glioma to promote cancer proliferation, migration, and invasion. | 198 |
| PC | LncRNA–PACERR interacts with miR-671-3p and IGF2BP2 to promote M2 polarization to promote cancer proliferation, migration, and invasion. | 199 | |
| HCC | ALKBH5 promotes JNK and ERK pathways via upregulating MAP3K8 to recruit PD-1+ macrophages by expressing IL-8 to promote cancer growth and metastasis. | 202 | |
| CRC | CircASPH interacts with IGF2BP2 and promotes its stability to activates STING signaling pathway, leading to M2-TAMs infiltration in CRC. | 201 | |
| ccRCC | RBM15 enhances the stability of CXCL11 mRNA and upregulates its expression in an m6A-dependent manner to promote M2 polarization and infiltration, leading to cancer growth and metastasis. | 203 | |
| BC | LINC00657 activates TGF-β signaling pathway and induces M2-TAMs infiltration by m6A modification in BC. | 204 | |
| NE | PTC | METTL3 alters IL-8 expression to recruit TANs via c-Rel and RelA inactivation of NF-κB to suppress cancer progression. | 207 |
| BC | IL-1β and TNFα secreted from C5aR1+ neutrophils upregulate WTAP expression and m6A levels via ERK1/2 to increase ENO1, leading to promote cancer glycolysis. | 119 | |
| BC | Senescent neutrophils-derived exosomal piRNA-17560 enhances FTO and decrease m6A levels to strengthen ZEB1 transcripts stability and expression leading to chemoresistance and EMT in cancer cells. | 208 | |
| DC | cancers | YTHDF1 promotes lysosomal proteases and suppress cross-presentation of engulfed tumor neoantigens, inducing immune escape. | 224 |
| GC | YTHDF1 loss in GC promotes recruitment of mature DCs with increased MHCII expression and IL-12 secretion and upregulates expression of IFN-γ receptor 1 and JAK/STAT1 signaling pathway to restore sensitivity to antitumor immunity. | 225 | |
| T | NSCLC | METTL3-modified circIGF2BP3 upregulates PKP3 expression and induce PD-L1 expression by deubiquitination to inhibit CD8+ T-cell anticancer efficacy. | 234 |
| BC | METTL3/IGF2BP3 axis upregulates m6A levels of PD-L1 mRNA to increase its expression, dampening CD8+ T cell response. | 235 | |
| CRC | METTL14 loss of TAMs promotes CD8+ T cell dysfunction and promote cancer progression. | 236 | |
| CRC | METTL3 activates the m6A–BHLHE41–CXCL1 axis to increase MDSC accumulation and decrease CD8+ T cells. | 237 | |
| CRC | YTHDF1 suppress CD8+ T cell infiltration and dampens anticancer immunity via an m6A-p65–CXCL1/CXCR2 axis to promote CRC. | 238 | |
| CRC | KRT17 promotes T cell infiltration via YTHDF2–CXCL10 axis in CRC to control cancer growth. | 102 | |
| HCC | WTAP-mediated circCCAR1 promotes CD8 + T-cell dysfunction and anti-PD1 resistance to promote cancer growth and metastasis. | 239 | |
| CRC | m6A modification levels increase translation of immune checkpoints and restrain CD8+ T cell function and infiltration to promote cancer growth and immune escape. | 240 | |
| BLCA | YTHDF2 regulates RIG-I-mediated innate immune and decrease CD8+ T cell recruitment to promote cancer progression and immune evasion. | 241 | |
| B | DLBCL | METTL3 upregulates pigment epithelium-derived factor (PEDF) expression to promote proliferation of DLBCL. | 253 |
| DLBCL | NCBP1-enhanced METTL3 regulates c-MYC expression via NCBP1/METTL3/m6A/c-MYC axis to promote DLBCL progression. | 254 | |
| DLBCL | KIAA1429 regulates the m6A modification of its downstream CHST11 and Hippo-YAP pathway to promote DLBCL progression. | 255 | |
| NK | Cancers | YTHDF2 regulates NK cell function and proliferation by forming a STAT5–YTHDF2 positive feedback loop. | 266 |
| Cancers | METTL3 modifies m6A methylation of SHP-2 and regulates NK cell response to IL-15 associated with AKT and MAPK signaling pathway in order to affect anticancer immunity. | 267, 268 |
- Abbreviatios: Mø, macrophage; NE, neutrophil; DC, dendritic cell; NK, natural killer cell; PC, pancreatic cancer; BC, breast cancer; GC, gastric cancer; NSCLC, non-small cell lung cancer; CRC, colorectal cancer; HCC, hepatocellular carcinoma; BLCA, bladder carcinoma; DLBCL, diffuse large B-cell lymphoma.
6.1 Macrophages
Macrophages, derived from the mononuclear phagocyte system, play essential roles in various biological and pathological processes.192 Macrophages are commonly divided into M1 and M2 types. M1 macrophages are usually considered to have antitumor functions, whereas M2 macrophages are thought to have protumor functions. Additionally, tumor-associated macrophages (TAMs) significantly influence various stages of cancer progression, including angiogenesis, tumorigenesis, metastasis, invasion, and hypoxia induction, by modulating the tumor tissues.193-195 m6A RNA modification is a regulator of macrophage activation.196 METTL3-deficient TAMs eventually lead to tumorigenesis by increasing the infiltration of regulatory T cells (Tregs) and reducing the number of Th1 and CD8+ cells through the NF-κB and STAT3 signaling pathways, resulting in the reduced therapeutic efficacy of anti-PD-1 therapy.197 A previous study showed that silencing ALKBH5 significantly reduces the infiltration of M2 macrophages in gliomas, suggesting that ALKBH5 may be a potent predictor of sensitivity to immunotherapy in some cancers, especially gliomas.198 Liu et al.199 found that lncRNA–PACERR increases the number of M2 TAMs in pancreatic cancer (PC) cells in an IGF2BP2/m6A manner. IGF2BP3 upregulates and activates the STAT3 pathway to promote M2-TAM polarization and immunosuppression in gallbladder cancer.200 CircASPH interacts with IGF2BP2, stabilizes it to activate STING signaling, and promotes M2-TAM infiltration in CRC.201 In hepatoma tissues, M2 macrophages exhibit high PD-L1 expression. ALKBH5 recruits PD-L1+ TAMs by regulating MAP3K8 expression and activating the ERK/JNK and IL-8 pathways to promote HCC progression.202 RBM15, an oncogene in a number of tumors, enhances the stability of CXCL11 mRNA via m6A RNA modification to facilitate M2 macrophage polarization and infiltration, boosting the progression of ccRCC.203 LINC00657 activates the transforming growth factor-β (TGF-β) signaling pathway and induces M2-TAM infiltration by m6A modification in BC.204 In conclusion, m6A plays a crucial role in the activation, polarization, migration, and infiltration. Targeting m6A in TAMs may alter the suppressive immune microenvironment to promote anticancer immunity.
6.2 Neutrophils
Neutrophils, which account for the majority of granular leukocytes, play essential roles in tumorigenesis, progression, and invasion. Tumor-associated neutrophils (TANs) influence the activation and function of other immune cells.205 The m6A modification plays a crucial role in neutrophil activation. METTL3 enhances m6A methylation, thereby increasing the translation of toll-like receptor 4 (TLR4) mRNA, a key factor in neutrophil activation.206 The m6A modification also influences neutrophil infiltration and migration. In papillary thyroid cancer (PTC), METTL3 deficiency leads to increased IL-8 expression, which recruits TANs and promotes PTC progression through c-Rel and Rel A inactivation of the NF-κB pathway, highlighting the anticancer function of METTL3 in PTC.207 piRNA-17560 increases FTO levels in BC cells by enhancing its stability. Senescent neutrophils are abundant in therapy-treated tissues and can produce exosomal piRNA-17560, contributing to chemoresistance and epithelial–mesenchymal transition (EMT) in BC cells in an m6A-dependent manner.208 In addition, Ou et al.119 revealed a correlation between m6A and TANs in BC, identifying a novel subset of C5aR1-positive neutrophils implicated in promoting BC progression. Mechanistically, C5aR1-positive neutrophil-derived IL1β and TNFα can activate ERK1/2 signaling, phosphorylating and stabilizing WTAP to promote BC cells by facilitating glycolysis in BC cells.119 Solute carrier family 2 member 1 (SLC2A1) plays an essential role in cellular glycometabolism. A pancancer analysis identified SLC2A1 as a m6A-related potential biomarker for prognosis and immunotherapy. SLC2A1 positively correlates with neutrophils, providing a new strategy for cancer immunotherapy.209 Neutrophil extracellular traps (NETs) are extracellular fibrous structures produced by neutrophils that regulate NETosis.210-213 NETs can impair autophagic flux, resulting in abnormal autophagy, which induces sepsis-associated acute lung injury via METTL3.214 Researchers have found that METTL5 is strongly and positively associated with immune cell infiltration, including neutrophil infiltration, in HCC.215 Recent studies have demonstrated that NETs are associated with m6A modifications in sepsis-associated acute lung injury. NETs mediate m6A modification through METTL3, subsequently inducing ferroptosis inacute lung injury.216 NETs also participate in cancer invasion, evasion, angiogenesis, and metastasis by regulating the TME.217-220 However, the association between m6A and NETs remains unclear. These studies indicate that m6A participates in TAN activation and infiltration of TANs. Understanding the link between m6A and NETs is a potential direction for future research.
6.3 Dendritic cells
DCs, known for dendritic pseudopodia, were first discovered by Steinman in 1973.221 DCs are regarded as the most professional antigen-presenting cells (APCs); they play a key role in anticancer immunity and therapy.222 For instance, DCs are necessary for T cell-mediated antitumor immunity by activating T cells and presenting tumor antigens.223 Recently, m6A modification-mediated DCs and cancer cells have been subjected to new trials. YTHDF1 can increase the expression of lysosomal cathepsins in DCs. Downregulation of cathepsins enhances the cross-presenting ability of wild-type DCs. Moreover, the efficacy of PD-L1 therapy is promoted in Ythdf1−/− mice, indicating that m6A modification and YTHDF1 can modulate anticancer immunity in DCs.224 Similarly, in gastric tumors, the loss of YTHDF1 can recruit mature DCs, which subsequently promote sensitivity to antitumor effect.225 In HCC, Wang et al.215 showed that METTL5 expression was positively correlated with the infiltration of immune cells, including DCs. Gong et al.226 demonstrated that the expression levels of METTL14, ZC3H13, and APC (an antagonist of the Wnt signaling pathway) positively correlated with DCs in BC. Downregulation of METTL14 and ZC3H13 correlated with a poor prognosis. Endothelin-converting enzyme 2 is a prognostic biomarker associated with m6A modifications in lung adenocarcinoma. Endothelial-converting enzyme 2 expression was significantly negatively correlated with DC infiltration.227 Glycolipid transfer protein expression is associated with m6A-related genes and DCs in cervical cancer (CC).228 YTHDC2 is correlated with immune infiltration levels of DCs in head and neck squamous cell carcinoma (HNSCC).229 Briefly, m6A modification influences DC activation and infiltration. More importantly, as the strongest APC, targeting the m6A modification of DCs may shed new light on DC-based immunotherapy.
6.4 T cells
T lymphocytes (T cells) play a major role in adaptive cellular immunity and participate in humoral immunity induced by thymus-dependent antigens.230 Functionally, T cells are divided into helper T cells (Ths), cytotoxic T lymphocytes (CTLs), Tregs, and exhausted T cells (Texs).230, 231 T cells are important for anticancer immunity. Recent studies have shown that the growth and activation of T cells are modulated by m6A RNA modification.232, 233 For instance, m6A-modified circIGF2BP3 can inhibit the CD8+ T cell response and induce cancer escape via PD-L1 deubiquitination in NSCLC.234 In BC, the upregulation of the m6A modification of PD-L1 mRNA via the METTL3/IGF2BP3 axis can downregulate T cell antitumor immune activation to inhibit tumor immune surveillance.235 Dong et al.236 demonstrated a negative relationship between m6A levels and dysfunctional CD8+ T cells in CRC. METTL3 methylates BHLHE41 mRNA and upregulates its expression, leading to increased CXCL1 expression, which, in turn, recruits immunosuppressive myeloid-derived suppressor cells (MDSCs) to dampen T cells. Additionally, silencing METTL3 in CRC sustains the activation and proliferation of both CD4+ and CD8+ T cells, thereby suppressing tumor progression.237 YTHDF1 in CRC also recruits MDSCs by activating the m6A–p65–CXCL1 axis to inhibit T-cells, subsequently promoting CRC. This implies that targeting FTHDF1 is a good strategy for boosting anti-PD1 therapy.238 Keratin 17 (KRT17) plays a protective role in CRC by promoting T cell infiltration in a YTHDF2 dependent manner.102 In HCC, CD8+ T cell dysfunction can lead to immune evasion. HCC cell-derived exosomal circCCAR1 is stabilized by WTAP and IGF2BP3, which can be taken up by CD8+ T-cells. It stabilizes PD-1 by promoting its deubiquitination. At the same time, it facilitates the dysfunction of CD8+ T cells, resulting in immunosuppression.239 Intriguingly, Li et al. found that methionine restriction reduces tumor growth and enhances antitumor immunity by increasing the number and cytotoxicity of tumor-infiltrating CD8+ T cells in a YTHDF1-dependent manner, suggesting that targeting methionine metabolism or YTHDF1 is a potential target for tumor immunotherapy.240 Zhang et al.241 showed that YTHDF2 inhibits its downstream target RIG-I, thereby facilitating immune evasion in bladder carcinoma (BLCA). YTHDF2-deficient BLCA cells implanted in recipient mice activated innate immune responses and recruited CD8+ T cells.241 HNRNPC interacts with m6A modifications during the immune processes. Cheng et al.242 suggested that this gene could enhance the activation of Tregs, leading to immune escape and poor prognosis in prostate cancer. Additionally, some studies have revealed a crosstalk between m6A modification and T-cell exhaustion in anticancer immunity and immunotherapy. The inhibition of METTL3 or IGF2BP3 can enhance antitumor immunity through PD-L1-mediated T-cell exhaustion in BC as well.235 A previous study reported that lncRNA–AC026356.1, a downstream target of METTL14/IGF2BP2, is positively correlated with T cell exhaustion in lung adenocarcinoma.243 CCL8 andIL-1b can make hypoxia zones to recruit TAMs and cytotoxic T cells. The recruited immune cells can then be reprogrammed for immunosuppression in GBM.244 This study illustrates a new mechanism of hypoxia in tumors. Previous studies have also reported a correlation between hypoxia and m6A modification.187, 245 In conclusion, m6A participates in T cell-mediated antitumor immunity mostly by regulating T cell activation and PD-1/PD-L1. Therefore, Tregs and Texs are potential targets for future immunotherapy.
6.5 B cells
B lymphocytes (B cells), a type of lymphocyte differentiated from mammalian bone marrow lymphoid stem cells, participate in B-cell-mediated humoral immunity.246, 247 m6A is a key factor in the development, differentiation, and function of B cells. METTL3–METTL14 complex-mediated m6A modification is crucial for IL-7-induced pro-B cell proliferation via YTHDF2.248 Loss of METTL14 eventually represses the transition of pre-B cells from large to small.248, 249 Interestingly, another study showed that the loss of METTL3 in the pro-B stage slightly influences B cells in liver fibrosis.250 These results indicate that the link between m6A modification and B cell development may be associated with different stages. B-cell differentiation is important for antibody-mediated immunity and is determined by transcription factors. YTHDF2 promotes the formation of germinal centers by suppressing the plasmablast genetic program.251 Several B cell lymphomas are associated with m6A modifications. piRNA-30473 upregulates WTAP expression. Increased WTAP stabilizes its target mRNA, hexokinase 2 (HK2), which promotes tumorigenesis in diffuse large B-cell lymphoma (DLBCL).252 METTL3 is also functionally upregulated in DLBCL tissues.253 Furthermore, Meng et al.254 verified that NCBP1 enhances METTL3 by maintaining METTL3 mRNA stabilization and mediating c-MYC to promote DLBCL proliferation. Chen et al.255 found that KIAA1429/YTHDF2 suppression of carbohydrate sulfotransferase 11 (CHST11) inactivates the Hippo– yes associated protein (YAP) pathway via m6A RNA modification in DLBCL. Overall, m6A plays key roles in the development, differentiation, and function of B cells. In addition, m6A modification occurs in B cell lymphomas, especially DLBCL, thus providing an avenue to better understand and treat these malignancies.
6.6 NK cells
NK cells are cytotoxic lymphocytes derived from bone marrow lymphoid progenitor cells that play a vital role in nonspecific and specific immunity.256, 257 Unlike T and B cells, NK cells can kill pathogens or cancer cells without prior sensitization.258 NK cells have gained attention owing to their heterogeneous characteristics and functions in antitumor immunity and immunotherapy.259 Studies have reported alterations in NK cell levels in various tumors, including lung adenocarcinoma,260 HCC,261, 262 bladder cancer,263 GC,264 and HNSCC.265 IL-15 is an important factor in the proliferation, development, and function of NK cells. Interactions between IL-15 and m6A regulate the anticancer immunity of NK cells. A previous study reported that the expression level of YTHDF2 is regulated in NK cells. Upregulated YTHDF2 forms a STAT5–YTHDF2 loop that promotes the proliferation and antitumor immunity of NK cells.266 METTL3 enhances the NK cell response to IL-15, which is dependent on the activation of AKT–mTOR and MAPK–ERK. Src homology 2-containing protein tyrosine phosphatase 2 (SHP-2), encoded by Ptpn11, is an essential factor in IL-15-induced ERK activation. METTL3 deficiency reduces SHP-2 expression.267, 268 The relationship between m6A and NK cells is unclear, and further studies are needed to reveal the role of m6A in tumor-infiltrating NK cells.
TAM heterogeneity is closely associated with tumorigenesis and immune evasion. m6A modification plays an important role in the regulation of immune cell infiltration in the TMEs. Therefore, targeting m6A-regulated immune cells may be an attractive therapeutic strategy for restoring antitumor immunity.
7 M6A MODIFICATIONS IN MICROORGANISM-ASSOCIATED CANCERS
Recently, microorganisms have been studied for their roles in carcinogenesis and therapeutic responses. Previous studies have shown that microorganisms increase mutagenesis, regulate oncogenic genes, and modulate immunity.269, 270 Microorganisms may influence tumor onset and progression by modulating m6A modification.271 A previous study reported that microbial pathogens can disrupt pulmonary immune homeostasis by altering host m6A modifications, thereby influencing NSCLC development and outcomes.271 Therefore, we reviewed the interplay between m6A modifications and specific cancer-related microorganisms, such as Hp, Fn, hepatitis B virus, Epstein–Barr virus, and human papilloma virus, to provide insights into the epigenetic mechanisms and treatment strategies of microorganism-associated cancers (Table 5).
| Pathogen | Cancer | Regulator | Function | References |
|---|---|---|---|---|
| Hp | GC | FTO | CagA+ Hp increases the expression of FTO, which downregulates the m6A level of CD44 mRNA to promote tumorigenesis. | 275 |
| Fn | CRC | METTL3 | Fn decreases METTL3 expression and m6A levels of KIF26B to promote CRC progression. | 281 |
| ESCC | METTL3 | Fn upregulates the expression of METTL3 to promote ESCC proliferation and metastasis by promoting c-Myc mRNA methylation. | 283 | |
| HBV | HCC | YTHDF2 | YTHDF2 can promote the expression of MMP2 and MMP5 to promote tumorigenesis and progression of HBV-associated HCC. | 123, 187 |
| HCC | IGF2BP3 | HBV-pgRNA can upregulate IGF2BP3 expression to promote HCC. | 289 | |
| HCC | YTHDC1 | YTHDC1 and FMRP can promote the nuclear export of HBV-related transcripts to influence the life cycle of HBV. | 291 | |
| HCC |
ALKBH5 WTAP |
ALKBH5 interacting with macrophages and WTAP interacting with natural killer T cells may influence the progression of liver fibrosis in HBV infection. | 293 | |
| EBV | cancers | METTL14 | Viral-encoded latent oncoprotein EBNA3C can bind to METTL14 and promote tumorigenesis. | 299 |
| GC | WTAP | EBER1 regulates can downregulates WTPA expression to promote the migration of EBV-associated GC. | 300 | |
| GC | METTL3 | EBV-circRPMS1 upregulates METTL3 expression to promote the progression of EBV-associated GC. | 302 | |
| HPV | CC | IGF2BP2 | E6/E7 proteins regulate the expression of MYC via IGF2BP2 to promote CC. | 307 |
| cancers | IGF2BP1 | IGF2BP1 can stabilize E7 transcripts to influence HPV-associated cancers. | 308 | |
| CC | ALKBH5 | E7 can increase ALKBH5 expression and enhance PAK5 expression to promote tumorigenesis and metastasis of CC. | 309 | |
| CC | METTL3 | METTL3 inhibitors combined with anti-PD1 therapy enhance the efficacy of immunotherapy in CC. | 311 |
- Abbreviations: GC, gastric cancer; HCC, hepatocellular carcinoma; CRC, colorectal cancer; ESCC, esophageal squamous cell carcinoma; CC, cervical cancer.
7.1 Helicobacter pylori
Hp infection is one of the main causes of GC. Typically, Hp infection first induces nonatrophic gastritis, which can develop into atrophic gastritis, gastric polyps, and ultimately, GC.272 Mechanistically, a recent review summarized that Hp may impair gastric epithelial cells through oxidative stress, DNA damage, impairment of DNA repair pathways, and endoplasmic reticulum stress.273 Recently, Li et al.274 performed a comprehensive analysis of differences in m6A modifications during Hp infection. They found an increasing level of m6A in Hp infection and a significantly different expression of m6A regulators, indicating that m6A modification might correlate with Hp infection.274 FTO promotes tumorigenesis in chronic CagA + Hp infection by regulating CD44 mRNA m6A methylations.275 Additionally, Hp infection has an undesirable effect on cancer immunotherapy by decreasing the efficacy of anti-PD1 immunotherapy.276 Some researchers have suggested that increasing the expression of PD-L1 could be an early response to Hp infection.277 The relationship between Hp infection and host cell DNA impairment has been studied to some extent. However, the relationship between Hp and m6A modifications in gastric carcinogenesis and disease progression remains largely unknown. Further studies are required to provide additional insights into Hp-related GC treatment.
7.2 Fusobacterium nucleatum
Fn is an opportunistic pathogen in human body.278 Emerging research has reported that Fn is associated with CRC.279, 280 Herein, from the perspective of m6A modification, we present illustrative research on Fn and CRC. Fn decreases METTL3 expression and m6A levels in KIF26B by activating YAP signaling and inhibiting FOXD3 (forkhead Box D3, a transcription factor for METTL3) expression to promote CRC progression.281 Xu et al.282 indicated that Fn infection-induced microRNA-4717-3p excessive maturation via METTL3-dependent m6A modification suppressed the expression of mitogen-activated protein kinase 4 and its anticancer function to promote CRC proliferation. Fn not only facilitates CRC, but also correlates with ESCC. Researchers have found that the upregulated expression of METTL3, which is induced by Fn, could promote ESCC proliferation and metastasis by promoting c-Myc mRNA methylation in a YTHDF1-dependent manner.283 Additionally, Fn-related m6A modifications are involved in CRC immunotherapy. Fn promote the expression of PD-L1 and mediate immune escape in CRC via m6A-modified IFIT1.284 In conclusion, Fn is involved in cancer progression and immunotherapy. Further studies are needed to explore strategies for treating cancers, particularly CRC.
7.3 Hepatitis B virus
HBV is a member of the Hepadnaviridae family of enveloped viruses, with a double-stranded DNA genome of 3200 bp in length.285 HBV infection is also associated with HCC. In the present study, we clarified the function of m6A modification during this process. First, HBV RNA is predominantly modified by m6A in the coding region of HBx.286, 287 Kim discovered an m6A site at nt 1616 of the HBV genome, indicating that m6A modification may regulate HBx protein expression to modulate the HBV life cycle.288 Yang et al. found that YTHDF2 stabilizes the transcripts of minichromosome maintenance protein 2 (PPM2) and minichromosome maintenance protein 5 (PPM5) via m6A modification, which promotes the tumorigenesis and progression of HBV-associated HCC.123 HBV-pgRNA (pregenomic RNA) upregulated IGF2BP3 expression to promote HCC. Furthermore, interferon (IFN)-α−2a can increase pgRNA m6A modification and degrade its stability.289 Kim et al. demonstrated that HBV could increase the m6A modification of phosphatase and tensin homolog (PTEN) RNA, leading to decreased RNA stability and PTEN protein expression. PTEN downregulation can affect nonspecific immunity by inhibiting interferon regulatory factor 3 (IRF-3) nuclear import. Simultaneously, it activates the PI3K/AKT pathway, which influence HCC development.290 The m6A modification can also affect HBV RNA localization. YTHDC1 and FMRP promote nuclear export of HBV transcripts. The loss of YTHDC1 and FMRP can inhibit reverse transcription in HBV, affecting the HBV life cycle.291 Additionally, the m6A modification modulates immune cell infiltration in HBV-HCC. In a recent study, patients were divided into two clusters: cluster A and B. Relatively, the overall survival rate of cluster A was higher than that of addition, and immune cell infiltration of the two clusters was significantly different.292 Another study pointed out that ALKBH5 interacting with macrophages and WTAP interacting with NK T cells may be key factors in the progression of liver fibrosis during HBV infection.293 In summary, the m6A modification regulates HBx RNA and protein levels to modulate the HBV life cycle. HBV infection influences both oncogene expression and immune cell infiltration in an m6A-dependent manner to regulate HCC progression.
7.4 Epstein‒Barr virus
EBV is an oncogenic herpes virus linked to various cancers, including Burkitt's lymphoma, nasopharyngeal carcinoma (NPC), and EBV-associated GC.294 Recent studies have indicated that the m6A modification may be involved in this process. First, m6A modification mediates mRNA decay during EBV lytic reactivation to regulate its life cycle.295 The m6A modification can promote EBV lytic reactivation by attenuating IFN signaling.296 Additionally, WTAP deposits m6A marks on EBV transcripts and recruits YTHDF reader proteins to activate the CNOT1 RNA decay pathway.297 The tumorigenic function of EBV may be related to virus-encoded latent oncoproteins such as EBNA2.298 One study examined the role of EBNA3C, which binds METTL14 to promote tumorigenesis.299 In addition, EBER1 can activate the NF-κB signaling pathway, which downregulates WTPA expression to promote the migration of EBV-associated GC.300 Liu et al.301 divided patients with NPC into two subgroups: an m6A high-score group and an m6A low-score group. They found that the m6A high-score group was related to immune suppression and poorer survival, while the m6A low-score group was related to a better response to immunotherapy; therefore, m6A modification is likely related to NPC progression and immunotherapy effects.301 Zhang et al.302 demonstrated that EBV-circRPMS1 promotes EBV-associated GC progression by recruiting Sam68 to METTL3 and upregulating METTL3 expression. Overall, m6A participates in the lytic reactivation of EBV and its life cycle. The m6A modification may regulate oncoproteins to influence EBV-associated cancers; however, it remains unclear whether this modification can be applied in EBV-associated cancer treatment.
7.5 Human papillomavirus
HPV is a small double-stranded DNA virus that infects the squamous epithelia and promotes tumorigenesis.303 Globally, 4.5% of cancers are caused by HPV, of which squamous epithelium-associated cancers account for a large proportion, including CC.303, 304 HPV is associated with several cancer processes, including initiation, progression, invasion, and metastasis, with unclear mechanisms.305 In the present study, we focused on m6A modifications. Sustained expression of HPV E6/E7 oncogenes alters cancer cell growth.306 The m6A modification can modulate E6/E7 expression to influence cancer cells. E6/E7 proteins regulate the m6A methylation levels of MYC mRNA in an IGF2BP2-dependent manner, which promotes several biological and pathological processes in CC, including aerobic glycolysis, proliferation, and metastasis.307 Wang et al.308 showed that E7 transcripts are stabilized by the m6A reader IGF2BP1. Interestingly, upon heat stress, the m6A-modified E7 reversed the fate of IGF2BP1. Based on this, they provided a treatment strategy for HPV-associated cancers that depend on heat.308 Huo et al.309 found that E7 increases ALKBH5 expression and enhances PAK5 expression by downregulating m6A modification levels, which promotes the tumorigenesis and metastasis of CC. Additionally, the m6A modification influences the antiviral treatment of HPV-associated cancers. IFN-ε is a key cytokine that helps the human body defend against viral infection, especially in epithelial cells. HPV can influence the m6A RNA modification of IFNE via WTAP to regulate IFN-ε, subsequently influencing the nonspecific immune responses to HPV in condyloma acuminata.310 Importantly, METTL3 has been identified as a mediator of the immunosuppressive TME in HPV-associated cancers. In vivo cell-derived xenograft models showed that METTL3 inhibitors combined with anti-PD1 therapy enhanced the efficacy of immunotherapy in CC.311 Collectively, m6A modifications interact with HPV infections and influence immunotherapy.
Microorganisms regulate the expression of host tumor-related genes through m6A modifications. However, even after the microorganisms are eradicated, these epigenetic changes can persist and continue to drive tumorigenesis, which is called “the hit-and-run model.” Understanding this interaction provides crucial insights into the mechanisms underlying microorganism-related cancers and highlights potential targets for therapeutic intervention in m6A modification pathways.
8 M6A MODIFICATIONS IN CANCER IMMUNOTHERAPY
Cancer immunotherapy has attracted increasing attention in recent years. Distinct from therapies that directly influence cancer cells or tissues, such as surgery and chemotherapy, immunotherapy aims to enhance the interaction between cancer and immune cells, boosting the immune response and suppress cancer progression.20 Additionally, clinical data have demonstrated that patients with high sensitivity to immunotherapy have longer survival rates and decreased recurrence rates, highlighting the significance of immunotherapy.312 However, numerous factors, including the limited efficacy of immunotherapy, complicated TME, immune escape, and tumor heterogeneity, have made it difficult for every cancer patient to benefit. Interestingly, the m6A modification may play a role in improving these effects. For instance, the m6A-associated TME can be analyzed to aid immunotherapy.313 Therapeutic resistance is also associated with m6A modification.314 Herein, we expound on several advances in cancer immunotherapy from an m6A modification perspective with the hope of providing novel insights into immunotherapy enhancement. RNA therapy, immune checkpoint inhibitors (ICIs), cytokine therapy, ACT therapy, and direct targeting of m6A regulator therapy are mainly included (Figure 5).
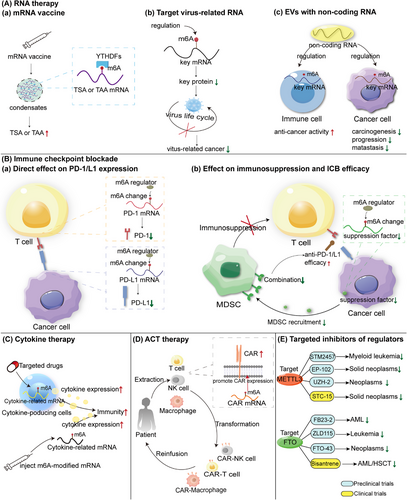
8.1 RNA therapy
As a carrier of genetic information, RNA plays an essential role in diverse biological processes, as well as in disease processes such as cancer. Specific chemical modifications of RNA directly influence its molecular function.315 m6A, the most abundant RNA modification, regulates both mRNA and ncRNA stability, translocation, and translation efficacy to modulate anticancer immunity.316, 317 Moreover, targeting m6A modifications of both mRNAs and ncRNAs may have potential value in cancer immunotherapy. Thus, we have clarified several recent advances and prospects.
8.1.1 Prospect of cancer mRNA vaccines with m6A modification
With the recent application of COVID-19 mRNA vaccines, such as the BNT162b2 vaccine, mRNA vaccines have been extensively studied over the last 5 years.318 In addition to infectious diseases, the potent preventive and therapeutic value of mRNA vaccines in oncology is unclear and attractive. Several clinical trials are ongoing to test its efficacy and safety, including NCT04534205 and NCT03313778.319 In contrast to other types of cancer vaccines, including peptide, protein, and cellular vaccines, mRNA vaccines have a more persistent expression of tumor-associated antigens or tumor-specific antigens (TAAs or TSAs) and a lower risk of human genome alteration caused by injected nucleotide sequences than DNA vaccines.320, 321 However, a few challenges restrict the development of mRNA vaccines, including the balance of antigen expression and adjuvant effects, stability and safety, appropriate delivery methods for vaccines, and targeted tumor antigen mutation.322 Here, we provide insights into the m6A modifications.
The m6A modification may enhance mRNA vaccine expression, which is a crucial aspect of mRNA vaccine function. Katalin Karikó and Drew Weissman, who were awarded the 2023 Nobel Prize in Medicine or Physiology, discovered in 2005 that modified nucleosides such as m5C, m6A, m5U, s2U, and pseudouridine can modulate mRNA activity. In particular, pseudouridine has been shown to reduce mRNA degradation and increase protein expression, forming the basis for COVID-19 mRNA vaccines.323 These results highlighted the crucial role of RNA modification in the development of mRNA vaccines. Modification of the 3ʹ-UTR improves mRNA vaccine stability and translation efficiency.324 Recent findings by He et al.325 suggested that m6A modification was predominantly found in the 3′-UTR and coding sequences (CDS). Future research should explore how altering m6A modifications affects mRNA vaccine stability and translation efficacy. In addition, m6A modification may facilitate mRNA vaccine activation via LLPS, a process that forms cellular membraneless components (e.g., biomolecular condensates, including the P-body, ribosomes, stress granules, and autophagosomes).326 LLPS is involved in the processes of cancer.327 Emerging evidence has shown that m6A promotes the formation of transcriptional condensates, thereby enhancing gene expression.328 This relationship may offer a novel approach for mRNA vaccine development. Although the mechanisms of LLPS remain largely unknown, IDRs are widely regarded to correlate with condensate formation.329 Lee et al.328 found that arginine residues in YTHDC1 IDR2 are important for condensate formation, which promotes gene activation. Additionally, Chen et al.330 reported that YTHDC1 undergoes LLPS and forms nuclear YTHDC1–m6A condensates, which maintain mRNA stability and control myeloid leukemic differentiation. YTHDF1, another YTH domain-containing protein, targets mRNAs for degradation by promoting P-body formation via LLPS.331 These studies indicate that m6A modifications may affect mRNA translation, stability, and activation via LLPS. The potential of modulating condensate formation to enhance mRNA vaccine efficacy, particularly in stabilizing and activating tumor antigen-related mRNA, remains an intriguing area for future research.
8.1.2 Targeting viral RNA m6A modification to treat virus-associated cancer
Viral infection is the first step in virus-induced cancer. Persistent and chronic infections cause inflammation, eventually leading to cancer initiation. Therefore, the inhibition of viral action and stimuli is important. Recent studies have shown that m6A modifications may shed new light on antiviral treatments at the epigenetic level. For example, the HBV vaccine has been widely used to prevent HBV infection, whereas therapeutic vaccines exhibit limited efficacy and weaken immune responses, especially in chronic HBV infection, which may progress to HBV-associated HCC.332 Moreover, HBV is often reactivated after discontinuation of nucleoside analogs that target HBV DNA replication.333 Future studies should focus on interrupting HBV RNA expression. Kim et al. identified m6A at nt 1616 in the coding region of HBx, which regulates HBx protein expression. After silencing YTHDF2 and methyltransferases, both HBx RNA and HBx protein expression levels were notably increased.288 This study suggests that m6A modification could be a novel target for treating HBV-associated HCC. During HPV infection, the HPV E7 oncotranscripts confer thermal vulnerability through IGF2BP1-dependent m6A modifications. Heat stress induces the formation of distinct m6A-modified E7 mRNA–IGF2BP1 granules, which can be resolved by the ubiquitin–proteasome system. This provides a potential heat treatment strategy for HPV-associated cancers.308 In summary, targeting viral RNA and inhibiting viral morbigenous protein expression by m6A modification provides some clues. However, several issues remain unresolved. First, its application in treatment remains elusive, and clinical trials should be performed to determine whether altering m6A modifications can efficiently alter viral RNA expression in patients. Second, appropriate drugs targeting viral RNA modifications are expensive. Third, the mechanisms of targeting RNA m6A modifications in other viruses, including bacteria, fungi, and other pathogenic microorganisms, which may lead to cancer, need to be explored.
8.1.3 m6A and extracellular vesicles loaded with ncRNA
Thus, m6A has the potential to be a target for tumor immunotherapy. Extracellular vesicles (EVs) have been engineered as carriers of ncRNA, which play a vital role in both immune cells and cancer cells.334 These ncRNAs target m6A regulators and regulate the m6A modification of specific mRNAs to modulate several physiological or pathological processes. Cigarette smoking induces M2-TAMs to secrete EVs carrying circEML4. These EVs are transported into NSCLC cells, where circEML4 reduces ALKBH5 levels and increases the suppression of cytokine signaling 2 (SOCS2) mRNA m6A modification in the nucleus, resulting in decreased SOCS2 expression and an activated JAK–STAT signaling pathway. Consequently, EVs promote the progression, migration, invasion and metastasis.335 This study explains the mechanism by which cigarette smoking induces NSCLC from epigenetic and immunological perspectives. Importantly, we have developed a new hypothesis regarding NSCLC treatment by cutting off the transportation of circEML4-loaded vesicles from M2-TAMs to NSCLC cells. You et al.336 constructed EVs with high CD47 expression, which were derived from macrophages and loaded with short interfering RNA against YTHDF1 to treat GC by self-presentation of immunogenic tumors and blockade of CD47. Notably, these particles can preferably interact with regulatory protein α, which can help TAMs to kill cancer cells with more effective delivery and lower toxicity.336 Recently, exosomes with a diameter of approximately 100 nm have attracted increasing amount of attention.337 The lncRNA MiR4458HG derived from HCC can be packaged within exosomes to promote M2-TAM polarization by increasing ARG1 expression. Additionally, these EVs increase IGF2BP2 expression in HCC cells, which stabilizes SLC2A1 and HK2 mRNA to promote HCC progression.338 Exosomal circVMP1 upregulates METTL3 expression and m6A modification of SOX2 to facilitate NSCLC progression.339 Conversely, m6A modifications mediate the formation of ncRNA-loaded EVs. In HCC cells, WTAP and IGF2BP3 stabilized circCCAR1 and circCCAR1-loaded exosome formation. These exosomes can be taken up by CD8+ T cells, where circCCAR1 promotes PD-1 deubiquitination and stability, ultimately promoting CD8+ T cell dysfunction and HCC immune escape.239 Similar results have been previously reported. For instance, the formation of adipocyte exosomes carrying lncRNAs LOC606724 and SNHG1 is promoted by METTL7A in an m6A-dependent manner.28 In addition, ncRNA vaccines have been studied in recent years. Li et al.340 developed a circRNA vaccine that drives immunity in hard-to-treat malignancies. Despite the promise of circRNA vaccines, their low immunogenicity and insufficient pro-inflammatory microenvironment remain significant challenges. From an m6A perspective, we propose two solutions: first, m6A modifications may stabilize circRNAs and enhance their functionality; second, EVs with m6A modifications could serve as effective delivery carriers, facilitating the entry of circRNA vaccines into cells. These hypotheses warrant further investigation. In conclusion, EVs loaded with ncRNAs play crucial roles in cancer progression, anticancer immunity, and immunotherapy. Future research may focus on the application of m6A modifications and EVs. Specifically engineered EVs can be constructed to modulate the m6A modification of key target mRNAs, thereby regulating cancer cell processes and immune cell functions. Additionally, m6A modification of ncRNAs can enhance the formation and function of critical EVs in target cells. The potential use of EVs with m6A modifications as carriers for ncRNA vaccines is also worth exploring.
8.2 Immune checkpoint blockade
ICB has been widely applied in diverse diseases, especially cancer, wherein ICIs block the interaction between receptors on the surfaces of immune and tumor cells, thereby inhibiting the dysfunction of immune cells and enhancing their anticancer immunity.341 The use of ICIs has significantly prolonged the survival of many cancer patients. Current evidence suggests that m6A modifications closely influence immune checkpoint-blocking therapy by directly regulating immune checkpoint expression and modulating immune cell suppression. This reduces drug resistance and enhances treatment efficacy.
8.2.1 The role of m6A in anti-PD-1/PD-L1 therapy
Anti-PD-1/PD-L1 therapy is the most prevalent immune checkpoint-blocking therapy. PD-1 (programmed cell death protein-1, also called CD279) is an important immunosuppressive molecule that is mainly expressed on the surface of immune cells such as macrophages. PD-1 can downregulate functions of the human immune system.342, 343 PD-L1, also regarded as a ligand of PD-1, also called CD274. It is mainly expressed in tumors and tumor-related cells.344 The PD-1/PD-L1 axis inhibits cytotoxic T cell-mediated tumor responses to induce immune escape.345 Recent studies have reported the function of m6A in PD-1/PD-L1 immunotherapy.
m6A modification regulates PD-1/PD-L1 expression, thereby regulating PD-1/PD-L1-related immune evasion by directly affecting related mRNA stability or influencing its modification. Researchers have found that circIGF2BP3 can upregulate the expression of PKP3, which stabilizes OTUB1 mRNA and thus promotes PD-L1 deubiquitination, ultimately increasing the expression of PD-L1 and simultaneously promoting immune evasion in NSCLC.234 METTL14 upregulates lncRNA MIR155HG m6A modification and its stability, which promotes the expression of PD-L1, leading to immune escape in HCC.346 ALKBH5 stabilizes its target ZDHHC3 mRNA, which in turn stabilizes PD-L1 to promote immune escape from glioma.347 METTL3 upregulates PD-L1 expression by modifying lncRNA MALAT1, which promotes the progression and immune evasion of PC.348 METTL16 can increase the expression of PD-L1 in CRC.349 ALKBH5 orchestrates PD-1 expression in intrahepatic cholangiocarcinoma (ICC) and colon adenocarcinoma, with strong expression of ALKBH5 leading to high sensitivity to anti-PD1 immunity in tumors.350, 351 These studies demonstrated that m6A modification promotes PD-1/PD-L1-related cancer immune evasion by regulating its expression. Targeting the functions of m6A regulators or m6A sites is a potential therapeutic strategy for cancer immunotherapy. Regulation of m6A modification may alter cancer immune evasion and improve the treatment efficacy of the traditional anti-PD-1/PD-L1 strategy by regulating PD-1/PD-L1 expression. For instance, IOX1, a specific ALKBH5 inhibitor, significantly decreases PD-L1 expression and prolongs survival in mice.347 In summary, targeting m6A modifications to regulate PD-1/PD-L1 expression to reduce immune evasion and improve immunotherapy is valuable.
Similar to chemotherapy, drug resistance is a significant challenge in immunotherapy, driven by multiple underlying mechanisms.352 One such mechanism is the link between epigenetics and immune cell dysfunction, which is the focus of our discussion. Targeting m6A modifications can reduce the immunosuppressive state of immune cells and promote their infiltration, thereby alleviating drug resistance and enhancing anti-PD-1/PD-L1 therapy. Wang et al.353 found that the depletion of METTL3 and METTL14 enhances responses to anti-PD-1 therapy in colorectal cancer by stabilizing Stat1 and Irf1 mRNA and promoting IFN-γ–Stat1–Irf1 signaling via YTHDF2. Metabolic stress and starvation can increase FTO expression in melanoma cells, which eventually promotes proliferation, migration, and evasion of melanoma cells, as well as increases anti-PD-1 resistance.354 YTHDF1, which is of great significance, promotes colorectal cancer via an m6A–p65–CXCL1/CXCR2 axis.238 Interestingly, researchers have also reported that high YTHDF1 expression is related to a better prognostic outcome in patients with NSCLC because of better infiltration of lymphocytes and downregulation of PD-L1.51 This study provides researchers with a new approach to enhance anti-PD-1 efficacy in NSCLC. The METTL3 inhibitor STM2743 downregulates the m6A methylation of BHLHE41 and the expression of CXCL1, thereby decreasing MDSC migration. Downregulated MDSC migration dampens their suppressive function in CD8+ T cells; as a result, it improves anti-PD-1 therapy in CRC.237 Similarly, Bao et al.238 found that targeting YTHDF1 with siYTHDF1 suppresses its binding to m6A-modified p65 mRNA and decreased CXCL1 expression, leading to reduced MDSC migration and better anti-PD-1 efficacy. KRT17 promotes YTHDF2 degradation. Degradation of YTHDF2 decreases the decay of m6A-modified CXCL10 mRNA and increases its expression, promoting cytotoxic T lymphocyte (CTL) infiltration into the tumor tissue. KRT17 synergized with anti-PD-1 and showed satisfactory efficacy in CRC.102 Inhibition of ALKBH5 downregulates Dickkopf-related protein 1 (DKK1) expression via the Wnt/β-catenin pathway, subsequently leading to less MDSC recruitment and better anti-PD-1 outcomes in CRC.355 In nonalcoholic fatty liver disease-related HCC (NAFLD-HCC), METTL3 increases SCAP mRNA translation in an m6A-dependent manner, which induces cholesterol production and inactivates CD8+ T cells. Inhibition of METTL3, including the small-molecule inhibitor STM2457 and the nanoparticle siMETTL3, can effectively restore CD8+ T-cell function and boost anti-PD-1 treatment.356 In conclusion, the m6A modification is a potent target for the reversal of immunosuppression and restoration of immune cell function, leading to a decrease in resistance and improvement in the efficacy of anti-PD-1/PD-L1 therapy.
8.2.2 Other immune checkpoints
In addition to PD-1/PD-L1, other immune checkpoints, such as CTL-associated antigen-4 (CTLA-4), mitogen-activated protein kinase, TIM family, and T-cell Ig and ITIM domains, are of great significance and have potential therapeutic effects.357-360 Therefore, the role of m6A modifications in these immune checkpoints is worth exploring. For example, m6A-modified circQSOX1 promotes the expression of PGAM1, which induces immune evasion by activating glycolysis and inactivating anti-CTLA-4 therapy in CRC.361 Thus, Sh-circQSOX1 synergized with anti-CTLA-4 therapy may overcome resistance to anti-CTLA-4 treatment. Additional immune checkpoints may play a role in immunotherapy via m6A modification.
8.3 m6A modification and cytokine therapy
Cytokines are secreted by both immune and nonimmune cells. Cytokines play a key role in anticancer immunity.362, 363 Over the past 30 years, cytokines and cytokine receptors have gained increasing attention because of their vital roles in several physiological and pathological processes. Cytokine therapy is a novel cancer immunotherapy. Diverse cytokines have been considered immunotherapeutic targets, such as IL-1,364 IL-2,365 IL-6,366 IL-8,367 and IL-15.368 Here, we reviewed the role of m6A in cancer cytokine immunotherapy. The m6A modification modulates cytokine expression to recruit immune cells. For instance, METTL3 deficiency improves IL-8 production by PTC cells, which recruit TANs and promotes PTC progression.207 Targeting the upregulation of METTL3 and mRNA m6A modifications may reduce IL-8 secretion and decrease TAN infiltration, thereby enhancing the efficacy of immunotherapy in PTC. YTHDF1 promotes HCC progression by increasing IL-6 secretion and recruiting MDSCs. Reducing YTHDF1, for instance with lipid nanoparticle-encapsulated siRNA against YTHDF1 (LNP-siYTHDF1), can alleviate MDSC-induced CD8+ T cell exhaustion and improve anticancer immunity.369 YTHDF1 loss in GC increases IL-12 expression and DC recruitment, which restores sensitivity to anticancer immunity.225 Cytokines are multifunctional and important biomolecules that participate in anticancer immunity. Cytokine therapy alters the TME and improves interactions between immune and cancer cells. Based on previous studies, the m6A modification may provide a new strategy for cytokine therapy. m6A plays a vital role in cytokine secretion by recruiting tumor-associated immune cells to influence cancer progression. Future research should target cytokine-related mRNA m6A modifications in cancer and immune cells to change specific cytokine expression, thus enhancing the efficacy of immunotherapy. In addition, we suggest that constructing and delivering m6A-modified cytokine-related mRNAs into the TME to persistently increase or decrease cytokine expression may be helpful in cancer immunotherapy. However, further studies are needed to confirm this hypothesis.
8.4 ACT therapy
ACT therapy refers to the modification of immune cells to express a chimeric antigen receptor (CAR) for the treatment of different types of cancers, especially leukemia and lymphoma.370-372 The CAR consists of an antigen-binding region, a transmembrane region, and a signal transmembrane region. Typically, CAR immune cells are derived from a patient's peripheral blood, engineered to express CARs in vitro, and reinjected into the patient after expansion. Therefore, CAR immune cells can specifically recognize and interact with cancer cells without antigen processing or presentation.372 CAR-T, CAR-NK, and CAR-macrophage therapies have been studied, with CAR-T therapy being the most abundant.373 Recently, scientists reported that super CAR-T cells target multiple tumor-associated antigens and exhibit improved antitumor ability.374 However, challenges such as primary resistance, relapse, and adverse effects are unsolved.375 Although CAR-T therapy has been successful in treating leukemia and lymphoma, a number of solid tumors cannot obtain satisfactory results, which is probably related to a lack of tumor-specific targets, immunosuppressive TME, problems with homing and access to the tumor site, and lack of CAR-T cell expansion.376 Interestingly, non-m6A-related neoantigen-coding lncRNAs are regarded as vital factors in glioma progression and may provide an important direction for CAR-T therapy.377 The m6A modification is still worth considering for applications in CAR-T therapy. First, m6A modification regulates immunosuppressive TME.378 Targeting m6A may relieve the immunosuppressive TME and enhance the efficacy of CAR immune cell therapy. METTL3 plays a role in T cell dysfunction via MDSC recruitment.237 METTL14 is also associated with T-cell dysfunction.236 The expansion of CAR immune cells is essential. Targeting m6A modifications promotes immune cell proliferation. For example, YTHDF2 regulates NK cell function and proliferation.266 These results may aid future CAR-NK cell therapy. In addition, it is worth exploring whether targeting the m6A modification of immune cells derived from patients can promote CAR expression, which may promote CAR immune cell therapy efficacy.
8.5 Direct-targeted treatment of m6A regulators
m6A modifications play various roles in the occurrence and development of tumors, and therapies targeting m6A-related molecules are also diverse.379, 380 Multiple studies have shown that m6A modifications affect the sensitivity of chemotherapy drugs as a direct target and thus affect the treatment of cancer.381-384 Therefore, an increasing number of therapies that directly target m6A regulators have been reported. The two most widely regulated proteins are METTL3 and FTO, which coordinate during the dynamic reversible m6A modification process. At present, a small-molecule inhibitor targeting METTL3 has entered clinical research, and the other four are in preclinical research, all of which are used to treat cancer or leukemia. The same is true for small-molecule inhibitors that target FTO.
8.5.1 METTL3-targeted drugs
In 2017, METTL3 was shown to promote the development of AML.385 In 2021, STM2457, a small-molecule inhibitor of METTL3 with in vivo activity, was first identified and further demonstrated to be effective against AML progression.386 STM2457 is a potent and highly specific inhibitor of the METTL3–METTL14 catalytic activity, without affecting other RNA methyltransferases. By binding to METTL3, STM2457 inhibits the translation of m6A-positive genes, thereby impeding the progression of AML while sparing normal hematopoietic stem cells and other normal cells. In vivo assays have confirmed that STM2457 inhibits the proliferation and expansion of AML cells and significantly prolongs the lifespan of mice, without causing significant toxic side effects or affecting body weight. In April 2021, STM2457 entered preclinical research trials for the treatment of myeloid leukemia, conducted by The Wellcome Trust Sanger Institute, the University of Cambridge, and Storm Therapeutics Ltd. In addition to AML, STM2457 alone or in combination with other drugs has shown therapeutic effects in a variety of tumors, including HCC,356, 387 ICC,388 neuroblastoma,389, 390 colorectal cancer,391 renal cell carcinoma,392 and NSCLC.393, 394
With the continuous progress of research, a new generation of METTL3 inhibitors on STC-15 was discovered, which is a derivative of STM2457, the first molecule specifically targeting an RNA methyltransferase enzyme to enter clinical development. In November 2022, STORM Therapeutics Ltd. began a phase I clinical trial for advanced solid tumors. In June 2024, the research team presented interim phase 1 clinical data for STC-15 at the American Society of Clinical Oncology 2024, STC-15 was well tolerated, and clinical activity was observed across the pharmacologically active dose range in patients with advanced cancer. They will also study STC-15 in combination with checkpoint inhibitors.
Two other drugs targeting METTL3 in preclinical studies are EP-102 and UZH2. EP-102 inhibits AML cell proliferation and acts synergistically with venetoclax, a selective BCL-2 inhibitor.395 Preclinical research trials on EP-102 indications for solid tumors have been conducted by EPICS Therapeutics, Ltd. in December 2023. The other UZH2 showed target engagement in cells and reduced the m6A/A levels of polyadenylated RNA in AML and prostate cancer cell lines.396 It is undergoing preclinical studies for cancer by the University of Zürich.
8.5.2 FTO-targeted drugs
FTO promoted cancer cell growth, self-renewal, metastasis, and immune escape. FB23-2 is an analog of FTO that selectively inhibits meclofenamic acid (MA); however, its activity is significantly higher than that of MA and FB23.397 It promoted AML cell differentiation/apoptosis and inhibited the progression of primary cells in xenotransplanted mice.398 In April 2019, a preclinical study of FB23-2 for the treatment of AML was conducted by Shanghai Institute of Materia Medica, Chinese Academy of Sciences. ZLD115 is a flexible alkaline side-chain-substituted benzoic acid FTO inhibitor derived from FB23. It showed a better drug similarity than FB23. ZLD115 exhibited significant antiproliferative activity in leukemic NB4 and MOLM13 cell lines and antileukemic activity in xenograft mice without substantial side effects.399 In July 2023, the Shanghai Institute of Materia Medica, Chinese Academy of Sciences, and Hangzhou Institute of Advanced Research, University of Chinese Academy of Sciences, began a preclinical trial of ZLD115 for the treatment of leukemia.
FTO-04, a derivative of FB23 created through rational design, effectively hinders the formation of neurospheres by CSCs in GBM patients, while leaving nonmalignant neural stem cells unaffected.400 Through further rational structure-based enhancements, FTO-04 was refined to FTO-43, an exceptionally selective oxetanyl-class inhibitor of FTO. FTO-43 elevates the m6A levels in GC cells to a degree comparable to that of FTO knockdown, impeding the in vitro growth of GC, GBM, and AML cells.401 In August 2022, FTO-43 was used to treat tumors in preclinical studies conducted at the University of California in San Diego.
CS1 (bisantrene) and CS2 (brequinar) have been identified as specific inhibitors of FTO, which can inhibit the self-renewal and immune evasion of cancer stem cells and exhibit potent antitumor effects in many types of cancer. They can significantly attenuate the self-renewal and reprogramming of leukemia stem/initiating cells by inhibiting the expression of immune checkpoint genes, especially LILRB4.402 CS1 and CS2 showed higher efficacy than FB23-2 at inhibiting AML cell viability. Among the FTO-targeted drugs, bisanthrene was the first inhibitor to enter clinical trials. In this phase II study, the clinical safety and efficacy of bisantrene were evaluated in an initial cohort of patients with relapsed/refractory AML.403 Of the 10 patients enrolled in the study, four showed a clinical response to bisantrene with acceptable cardiac toxicity. Given the observed low toxicity, a follow-up study is planned to combine bisantrene with a complementary antileukemia therapy. These findings suggest that bisantrene has a promising antileukemic activity and an acceptable safety profile. Small-molecule drugs targeting m6A regulators are gradually being developed and optimized. In the future, more of these drugs are expected to enter clinical research, either alone or in combination with chemotherapeutic agents or checkpoint inhibitors, to treat cancer. Thus, m6A can be leveraged in cancer immunotherapy through indirect RNA therapy, ICIs, cytokine therapy, and direct targeting of m6A regulators.
9 CONCLUSION AND PROSPECTS
m6A, the most prevalent form of RNA modification, has garnered significant attention for its pivotal role in human diseases, particularly cancer. Writers, erasers, and readers collectively regulate the dynamic m6A processes. Importantly, the expression and activity of these regulatory proteins can be modulated by various chemical modifications such as lactylation, acetylation, ubiquitination, phosphorylation, SUMOylation, and O-GlcNAcylation. Furthermore, m6A modification closely intertwines with chromatin accessibility, thereby influencing transcriptional activity. Drugs targeting PTMs of m6A regulators offer promising avenues for novel cancer treatment strategies.
PCD exhibits dual roles in tumorigenesis, influenced in part by the substances released into the intracellular environment during the process. PCD plays a crucial role in modulating tumor immunity. Targeting m6A modifications directly affects cancer cell death by influencing PCD-related pathways. Conversely, m6A modifications impact immune cell function through their effects on PCD. Moreover, m6A is involved in various processes within tumor-associated immune cells, including proliferation, differentiation, polarization, recruitment, and activation. Therefore, further investigation into the role of m6A in cancer cell-immune cell interactions holds promise for developing novel strategies to enhance cancer immunotherapy.
m6A modifications play a pivotal role in microorganism-associated cancers. On one hand, m6A influences the RNA expression and life cycle of specific pathogens, affecting their infection and oncogenic potential. Conversely, m6A modifications can impact the efficacy of anti-infection and anticancer treatments. Additionally, microorganisms utilize a “hit-and-run” mechanism to induce lasting epigenetic changes in host cells through modulation of m6A modifications. Together, the interplay between m6A modifications and microorganisms in microorganism-associated cancers represents a promising avenue for future prevention and treatment strategies.
Our focus centers on the role of m6A modifications in immunotherapy, with potential future benefits in enhancing the expression of TAAs or TSAs to improve mRNA vaccine efficacy. The process may involve m6A-related LLPS. Notably, targeting m6A modifications in virus-related cancers holds therapeutic promise. Engineered EVs can specifically modulate m6A modifications on key target mRNAs, influencing cancer cell–immune cell interactions. m6A modifications may enhance ICB by regulating the expression of immunosuppressive molecules and immune cell infiltration, thereby alleviating immunosuppression and drug resistance.Moreover, constructing and delivering m6A-modified cytokine-related mRNAs into the TME could persistently alter cytokine expression, potentially aiding cancer immunotherapy. Despite successful ACT outcomes in specific leukemias and lymphomas, challenges such as primary resistance, relapse, and adverse events persist, particularly in solid tumors. The development of drugs targeting m6A regulators shows promise, with several in clinical trials and numerous others in preclinical stages, highlighting significant potential for future clinical applications. In the future, there will be more drugs, alone or in combination, for the treatment of advanced cancers.
AUTHOR CONTRIBUTIONS
Q. Y., G. N. N., Z. S. W., and G. L. M. wrote the manuscript and generated figures and tables. H. B., W. C., and G. C. L. revised the manuscript. S. Q. Y. and L. Z. B. projected and edited the manuscript. Y. S. M. and X. Y. F. reviewed the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No.82103591), Natural Science Foundation of Chongqing, China (CSTB2023NSCQ-MSX0408), the Key Program of Natural Science Foundation of Chongqing (cstc2020jcyj-zdxmX0020), and Young Ph.D. Incubation Program of Xinqiao Hospital (2022YQB095). We thank all of the authors who contributed to the knowledge reviewed in this article.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




