Macrophage Signaling Pathways in Health and Disease: From Bench to Bedside Applications
Yongquan Chi and Haipeng Jiang authors contributed equally to this work.
ABSTRACT
Macrophages exhibit remarkable functional plasticity by dynamically polarizing into proinflammatory or antiinflammatory subsets in response to microenvironmental cues. This duality underpins their pivotal roles in immune defense, tissue homeostasis, and disease progression; however, the molecular mechanisms governing their polarization and crosstalk across various pathologies remain incompletely defined. This review systematically delineates macrophage biology, emphasizing the interplay between subset-specific signaling networks and their context-dependent activation in both health and disease. The heterogeneity of macrophages is characterized by detailing the distinctions between tissue-resident and monocyte-derived origins, as well as their polarization states. Core pathways regulating phagocytosis, tissue repair, immune modulation, and neuroprotection are dissected, along with their dysregulation in autoimmune disorders, neurodegeneration, cancers, and cardiovascular diseases. Notably, microenvironmental factors such as damage-associated molecular patterns, pathogen-associated molecular patterns, and metabolic intermediates dynamically reshape macrophage phenotypes through NLR Family Pyrin Domain Containing 3 (NLRP3) inflammasome activation or signal transducer and activator of transcription (STAT)-mediated transcriptional control. Preclinical and clinical evidence underscores potential therapeutic targets and emerging strategies. The significance of this review lies in its integrative analysis of signaling crosstalk, paradoxical pathway roles, and translational implications for precision therapies. These insights into macrophage functions and signaling pathways provide a robust foundation for future disease intervention and personalized medicine.
1 Introduction
Since Élie Metchnikoff's discovery of macrophages at the end of the 19th century [1], these cells have been recognized as pivotal in immune regulation, tissue homeostasis, and disease genesis [2, 3]. Initially identified as innate immunophagocytic sentinel cells, macrophages are now known for their remarkable plasticity, capable of dynamically polarizing into proinflammatory (M1) or antiinflammatory (M2) states in response to environmental cues. Over the past two decades, advancements in molecular biology and single-cell histology have unveiled the complexity of macrophage signaling networks. Through the activation of various receptors, such as Toll-like receptor (TLR), NLR, and interleukin 1 receptor (IL-1R), macrophages detect pathogen- or injury-associated molecules and modulate immune responses via signal transduction pathways, including mitogen-activated protein kinase (MAPK), nuclear factor kappa B (NF-κB), and JAK–STAT. Macrophages play a crucial role in coordinating immune responses, maintaining homeostasis, participating in angiogenesis and neuronal networks, and driving cancer progression. They are involved in immune diseases and contribute to cardiovascular and other pathological processes. These studies highlight the essential functions of macrophages in health and offer new insights into their roles in chronic inflammation, cancer, autoimmunity, and other diseases.
Macrophages express multiple pattern-recognition receptors (PRRs), regulatory receptors, chemotaxis/activation-associated cytokine receptors, antigen-processing and presentation molecules, and the characteristic CD14 surface marker. These receptors have multiple functions, including phagocytosis, involvement in inflammatory responses, antigen processing and presentation, and immunomodulation. However, despite significant advancements in mechanistic studies, the translation of these findings to clinical practice remains fragmented, underscoring the necessity for a systematic integration of macrophage signaling pathways and their complex roles. The development of therapeutic strategies targeting macrophages is motivated by their dual roles in both protection and destruction within various disease contexts. For example, the dysregulation of signaling pathways, including TLR, JAK–STAT, PI3K–AKT, and NF-κB, is closely associated with chronic inflammatory diseases such as rheumatoid arthritis (RA) and atherosclerosis, as well as with the immunosuppressive effects mediated by tumor-associated macrophages (TAMs) [4, 5]. A thorough examination of the association between these pathways and the macrophages and the complex roles of these pathways and macrophages, this review provides insights into macrophage signaling pathways and their roles in health and disease, particularly the key molecular mechanisms involved in the regulation of their polarization and function. The focal point of this study will be the examination of the impact that disparate signaling pathways exert on the polarized state of macrophages. Additionally, this review will discuss the functional effects of these pathways in various diseases, including inflammatory responses, tumor immunity, and autoimmunity. Furthermore, it will introduce the potential clinical applications of macrophage signaling pathways, with a particular emphasis on developing novel therapeutic strategies that target these pathways.
The review systematically integrates the environment-dependent activation features of macrophage signaling pathways in physiology and pathology, providing a comprehensive overview. By combining preclinical and clinical evidence, it identifies interferable targets (e.g., colony stimulating factor 1 receptor, STAT3, NLRP3 inflammatory vesicle inhibitors) and explores their potential application in clinical therapy. The study aims to provide a theoretical basis and experimental support for future disease intervention and individualized therapy.
2 Overview of Macrophage Biology
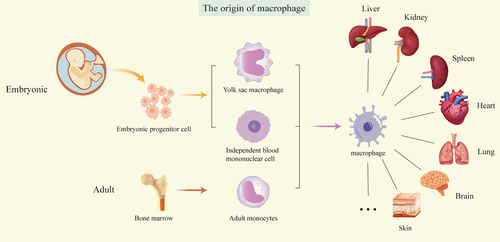
Macrophages can be classified into two distinct types: tissue-resident macrophages (TRMs) and monocyte-derived macrophages (MoMFs) [6-8]. The prevailing perspective suggests that macrophages originate from the mononuclear phagocyte system [9] and that tissue macrophages are continuously replaced by monocytes in the circulation [10]. However, recent advancements in fate-tracing animal models and single-cell sequencing technologies have facilitated a more nuanced understanding of the origins of TRMs [11, 12]. These studies have revealed that TRMs predominantly originate from yolk sacs, fetal liver, and bone marrow hematopoietic stem cells [13]. The origins of TRMs vary slightly across different tissues and organs; some are exclusively derived from the yolk sac (e.g., brain microglia), while others arise from both the yolk sac and the fetal liver (e.g., dermal Langerhans cells, alveolar macrophages, hepatic Kupffer cells (KCs), and cardiac macrophages) [14]. However, intestinal and cutaneous dermal macrophages are derived primarily from bone marrow-derived monocytes and are continuously replenished after birth by circulating monocytes [15-17].
The role of TRMs in antitumor effects is multifaceted, exhibiting both pro- and antitumorigenic functions. As demonstrated by María Casanova-Acebes et al., TRMs enhance tumor immunity and response to immunotherapy, promote epithelial–mesenchymal transition (EMT) and invasiveness of tumor cells, induce potent regulatory T-cell (Treg) responses, and shield tumor cells from adaptive immune attack [18]. Additionally, TRMs gather near tumor cells during the early stages of tumor formation [3] (Figure 1).
2.1 Macrophage Plasticity and Polarization
Macrophages are a key type of immune cells that fulfill a dual role in the inflammatory response by transitioning between proinflammatory and antiinflammatory subtypes, depending on the prevailing context.
2.1.1 M1 Macrophages
When exposed to Th1 cytokines such as interferon - γ (IFN-γ), tumor necrosis factor - α (TNF-α), and IL-2 or lipopolysaccharide (LPS), macrophages undergo a shift toward the M1 phenotype. Metabolically, glycolysis is a major source of energy. Stimulation of LPS leads to proinflammatory polarization of macrophages and a pro-Th1 cellular response accompanied by metabolic reprogramming [19], characterized by increased aerobic glycolysis and disruption of the tricarboxylic acid (TCA) cycle. In contrast, CD40 signaling has been shown to promote proinflammatory and antitumor polarization through an as yet poorly understood metabolic programming involving fatty acid oxidation without inhibiting TCA cycle, suggesting therapeutic potential [20]. A number of factors contribute to M1 polarization, including LPS, IFN-γ, and TNF-α. M1 macrophages are polarized through a variety of signaling pathways, such as NF-κB, interferon regulatory factors (IRFs), PI3K/Akt, and STAT1 [21, 22]. Based on our previous studies, macrophages can be converted to an M1 phenotype under the influence of the action of FSTL1 and binding to PKM2, thereby attenuating liver inflammation and fibrosis [23].
2.1.2 M2 Macrophages
In addition, as a reply to Th2-type cytokines, macrophages are known to polarize toward the M2 phenotype, such as IL-4, IL-10, and IL-13, as well as certain TLR ligands. Metabolically, oxidative phosphorylation (OXPHOS) dominates in these cells. M1 macrophages are known to inhibit tumor growth, whereas specific subpopulations of M2 macrophages have been observed to promote tumor progression, metastasis, angiogenesis, and tissue repair. The M2 macrophage population has been further classified into M2a, M2b, M2c, and M2d subtypes.
2.1.2.1 M2a
M2a macrophages represent a prominent line of research within the broader category of M2 macrophages [24]. The initial identification of these cells was made in a 1992 study that demonstrated increased activity and CD206 expression in surface mouse peritoneal macrophages stimulated by IL-4 [25]. The macrophage-polarized M2a phenotype is characterized by elevated expression levels of cell-surface markers, notably CD206, in response to cytokines such as IL-13. In contrast, the expression levels of CD163 and CD86 are comparatively low to moderate [26]. Additionally, M2a cells have been shown to produce various cytokines, including IL-10, TGF-β, chemokine (C-C Motif) Ligand (CCL17, CCL18, CCL22, and CCL24) [24, 25, 27].
Additionally, M2a macrophages have been demonstrated to exhibit profibrotic functions [28]. The polarization of M2a macrophages is facilitated by signaling pathways mediated by PI3K/Akt and STAT6. Macrophages express both type I and type II IL-4 receptors. The activation of these receptors leads to the phosphorylation and subsequent dimerization of STAT6. Once activated, the STAT6 dimer translocates to the nucleus, where it initiates the expression of target genes. In contrast, type I IL-4 receptors activate only insulin receptor substrates (IRSs), which do not translocate to the nucleus [24]. However, activated IRSs have been observed to initiate signaling pathways, including PI3K/Akt-mediated signaling pathways. The STAT6 protein has been demonstrated to regulate the expression of genes related to M2 polarization, a downstream target of peroxisome proliferators-activated receptor (PPAR-γ), and to have a positive regulatory role [29].
2.1.2.2 M2b
The binding of LPS to antiovalbumin IgG/OVA or antisheep erythrocyte IgG/erythrocyte immune complexes (ICs) has been shown to promote a shift from M1 to M2 in the macrophage phenotype, characterized by a decrease in IL-12 production and an increase in IL-10 levels. The distinctive M2 subtype, initially described in 2002, has been designated as M2b, and LPS + IC has been identified as a conventional M2b inducer [30]. M2b macrophages are distinguished by their unique features that set them apart from other M2 subpopulations. They are capable of transferring the Th1-cell response to that of Th2-cells, primarily through the secretion of IL-4 and IL-13. A subsequent study revealed that whole-body irradiation-induced miR-122 decreased GAS5 expression, leading to increased CCL1 levels and promoting macrophage conversion to the M2b phenotype, although the mechanism of radiation-induced miR-122 remains to be elucidated [31].
M2b cells are distinguished by their elevated CCL1 production, a characteristic that distinguishes them from other M2 cell types. CCL1 interacts with the cell surface receptor CCR8, attracting monocytes, dendritic cells, and natural killer (NK) cells. The release of CCL1 is crucial for the maintenance of M2b identity, as its inhibitory effect can lead to the transformation of M2b cells into M0 or M1 macrophages [32]. Other markers associated with M2b macrophages include CD86, IL-10, and TNF-α. CD86, also known as B7-2, serves as a marker for both M1 and M2b macrophages, allowing M2b to differentiate from other M2 macrophages. Conversely, IL-10's efficacy in differentiating M2b from other subtypes is limited due to its coproduction by M2c and M2d cells [33]. Finally, although TNF-α is recognized for its paradoxical antiinflammatory effects, it has been identified as a marker for M2b in several studies [30].
2.1.2.3 M2c
IL-10, TGF-β, and glucocorticoids stimulate M2c macrophages, leading to increased expression of CD163, TLR1/8, and the Tyro3–Axl–MerTK pathway, while decreasing CD86 levels. These macrophages secrete IL-10, TGF-β, CCL16, CCL18, and CXCL13 [34, 36]. M2c macrophages are polarized through STAT3, MAPK/ERK, and PI3K/Akt mediated signaling pathways. GAS6, produced by M2c macrophages, interacts with MerTK to induce IL-10 production, which further activates M2c macrophages, creating a positive feedback loop that enhances M2c polarization. This polarization is critical for regulating tissue repair, as signaling through these pathways leads to the activation of multiple genes associated with anti-inflammation, matrix remodeling, angiogenesis, and phagocytosis [30, 37]. Furthermore, M2c macrophages possess the capacity to stimulate and induce Treg in both in vitro and in vivo assays, indicating that the effects of M2c macrophages are mediated by Treg [37].
2.1.2.4 M2d
The initial identification of M2d macrophages was made in the ascites fluid of ovarian cancer patients in 2007. These cells are distinguished by their low expression of CD86 and high expression of CD163, and upon stimulation with LPS, IL-6, and A2R ligands, they produce M-CSF, IL-10, and CCL18. The binding of IL-6 to the IL-6 receptor/gp130 receptor complex initiates the recruitment of JAK1/2, leading to the phosphorylation of STAT3. This process results in the dimerization of STAT3, which subsequently translocates to the nucleus to activate gene transcription. Leukemia inhibitory factor, a member of the IL-6 family, induces the production of IL-6 by binding to its receptor. This mechanism allows M2d macrophages to utilize IL-6 in an autocrine manner [38]. Additionally, M2d macrophages are involved in tumor progression, angiogenesis, and extracellular matrix (ECM) remodeling through the secretion of IL-10, TGF-β, vascular endothelial growth factor (VEGF), and matrix metalloproteinase 9 (MMP9), while exhibiting low levels of IL-12, TNF, and IL-1β expression [39]. Moreover, other macrophage subpopulations, including Mhem, Mox, M4, and MHb, have been postulated (Table 1).
| Macrophage subset | Stimuli | Markers | Secreted molecules | Functions | References |
|---|---|---|---|---|---|
| M1 | IFN-γ, LPS, CD40 | MHCII, CD40, CD68, CD80, CD86, TLR2/4, IL-1R | TNF-α, IL-1α, IL-1β, IL-6, IL-12, IL-23, COX-2, iNOS | Proinflammation, pro-Th1 cellular response, inhibit cancer growth | [19-23] |
| M2a | IL-4, IL-13 | CCL17, CD206, CD209, HLA-DR, Dectin-1 | IL-10, TGF-β, CCL17, CCL18, CCL22, CCL24 | Anti-inflammation, tissue repair, metastasis | [24-28] |
| M2b | LPS + ICs | CD86, CCL1, SPHK1, TNF-α | IL-10, SPHK1, CCL1, TNF-α, | Immunoregulation, tumor progression | [30-33] |
| M2c | IL-10, TGF-β, glucocorticoids | CD163, TLR1/8, Tyro3–Axl–MerTK | IL-10, TGF-β, CCL16, CCL18, CXCL13 | Immunosuppression, phagocytosis, angiogenesis | [30, 34–37] |
| M2d | LPS, IL-6/A2R ligands | CD163 | IL-10, IL-6, CCL18, M-CSF | Tumor progression, angiogenesis | [38, 39] |
In summary, macrophages, as a critical component of the immune system, can exhibit a dual role in inflammatory regulation by transitioning between proinflammatory M1 and antiinflammatory M2 subtypes. M1 macrophages are formed under the stimulation of Th1 cytokines or LPS and are powered by glycolysis and polarized through various signaling pathways. In contrast, M2 macrophages respond to Th2 cytokines and are iso-polarized, predominantly oxidative phosphorylated, and subdivided into subtypes such as M2a, M2b, M2c, and M2d, each with unique markers and polarization signaling pathways. M2 macrophages respond to Th2-type cytokine iso-polarization by oxidative phosphorylation, which is subdivided into four subtypes, each with unique markers, functions, and polarization signaling pathways. The polarization process of these subtypes involves specific signaling pathways.
3 Macrophage Signaling Pathways
Macrophage polarization phenotype and functional regulation are contingent on the integrated action of complex signaling networks. Among them, core pathways such as NF-κB, JAK/STAT, PI3K/Akt, and MAPK regulate key transcription factors through cascade reactions, forming molecular switches for different polarization phenotypes. The molecular mechanisms of the signaling networks and their interactions require systematically analyzed (Figure 2).
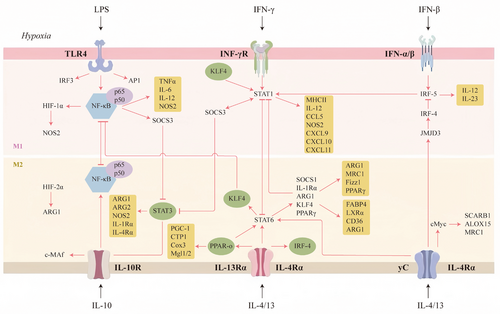
3.1 Classical Polarization Regulatory Pathways
3.1.1 JAK–STAT Pathway
The JAK–STAT signaling pathway represents a pivotal signal transduction pathway for the activation of cytokines (e.g., IL-4, IL-6, IFN-γ, etc.), and its core mechanism involves the triggering of the phosphorylation of the JAK kinase upon the binding of transmembrane receptors to ligands. This, in turn, activates the STAT proteins to form a dimer and enter the nucleus to regulate the expression of target genes [40]. In the context of macrophage polarization, this pathway exerts a regulatory role in M1 and M2 phenotypic transitions through the activation of distinct STAT isoforms. For instance, IFN-γ drives M1 polarization and induces the secretion of proinflammatory factors such as TNF-α and IL-6 through the activation of STAT1. In contrast, IL-4 and IL-13 upregulate anti-inflammatory markers, including arginase 1 (Arg1) and YM1, via STAT6. Additionally, IL-10 promotes M2 polarization and inhibits inflammatory responses through the action of STAT3 [41]. Differential activation of STAT is the molecular basis of macrophage functional plasticity, which directly affects the dynamic balance of inflammatory diseases and tumor microenvironment (TME).
It has been established that STAT1 and STAT5 are core drivers of M1 polarization. JAK1 and JAK2 are activated by the binding of IFN-γ to its receptor, which then leads to the phosphorylation of STAT1 and the formation of a homodimer that directly binds to the promoter regions of proinflammatory genes (e.g., NOS2), while repressing M2-associated gene expression [42]. In contrast, STAT5 enhances the antigen-presenting ability of macrophages by regulating the secretion of cytokines such as IL-12. Finally, STAT3 and STAT6 dominate M2 polarization. Notably, IL-4/IL-13 upregulates markers such as CD206 and Arg1 through the activation of STAT6, thereby promoting tissue repair. In addition, IL-10 induces STAT3 suppressor of cytokine signaling 3 (SOCS3) expression, creating a negative feedback loop to inhibit JAK1 activity, thus suppressing M1 polarization and enhancing immune tolerance. The sustained activation of STAT3 is also associated with a protumorigenic phenotype in TAMs promoting angiogenesis and immune escape through the secretion of factors such as VEGF, TGF-β, and so on [43, 44].
3.1.2 NF-κB Pathway
NF-κB is a family of pleiotropic transcription factors involved in numerous physiological and pathological processes by regulating inflammation, immune responses, cell proliferation, and apoptosis. The family members include RelA (p65), RelB, c-Rel, p50 (derived from p105), and p52 (derived from p100) [45], all of which have Rel homology domains that function through the formation of heterodimers or homodimers, and whose classical and nonclassical activation pathways play different roles in macrophage polarization. The classical pathway is triggered by TLR4/LPS, TNF-α, and so on, which degrades IκB protein through phosphorylation by IKK complex [46-48], releasing the p50–RelA dimer into the nucleus, driving the expression of proinflammatory genes (e.g., inducible nitric oxide synthase [iNOS], IL-6), and directly promoting the polarization of M1-type macrophages. In contrast, the nonclassical pathway is activated by stimuli such as CD40 and BAFF-R, and it relies on NIK kinase to process p100 into p52. This processed p52 then binds to RelB, thereby regulating genes associated with lymphoid organ development. While it can be hypothesized that the nonclassical pathway may play a role in the regulation of M2-type polarization, its precise function remains to be elucidated.
In M1-type polarization, NF-κB activation is closely associated with glycolytic metabolic reprogramming. For instance, enhanced NF-κB signaling in BCL3 knockout macrophages resulted in the upregulation of glycolysis rate-limiting enzymes (e.g., HK2, LDHA), along with a significant increase in M1 markers (IL-6, iNOS) [49], suggesting that NF-κB reinforces the proinflammatory phenotype by activating glycolysis. In addition, the TLR/NF-κB signaling pathway plays a crucial importance in the polarization of macrophages. TLR, a member of the PRR family, recognizes pathogen-associated molecular patterns (PAMPs) and is integral to the immune response to pathogens. Phosphorylation of MyD88 is the first step in TLR signaling, but TLR3 operates through a TRIF-dependent pathway [50, 51]. Subsequent to this, TLR activation triggers the expression of various downstream factors, including NF-κB, AP1, CREB, and IRF3/7, which subsequently facilitate the transcription of genes associated with inflammatory factors, adhesion molecules, and other substances [51, 52]. M1 polarization can be induced by bacterial LPS or viral RNA, both of which activate the TLR/NF-κB pathway and lead to the expression of proinflammatory cytokines.
The effects of NF-κB signaling pathway on macrophage M2 polarization are multifaceted and environment-dependent. During the process of M2 polarization, NF-κB activation by IL-1β induces the expression of the STAT6-dependent factor VEGF-A, which promotes tissue repair. Conversely, the antiinflammatory properties of macrophages can be enhanced by inhibiting NF-κB phosphorylation, for instance, with a JSH-23 inhibitor [53]. The term “LPS-tolerant” refers to a specific type of macrophages characterized by diminished restimulation potential, which are thought to exhibit M2-like properties. These macrophages are distinguished by the accumulation of (inhibitory) p50 NF-κB homodimers. Research has demonstrated that the inhibition of the p50 NF-κB subunit can hinder the progression of tolerance and the expression of cytokines associated with the M2 phenotype [54]. The NF-κB pathway is a central hub for macrophage polarization through a dual mechanism of inflammatory signaling dominance and metabolic regulation. Its targeted intervention (e.g., inhibition of the classical pathway or modulation of metabolic enzymes) may develop a novel strategy for the management of inflammatory diseases.
3.1.3 PI3K–Akt–mTOR Pathway
The PI3K/Akt signaling pathway is critically important, as its activation leads to the phosphorylation of tyrosine residues, facilitates various signaling processes for extracellular cytokines and other signals in the ECM, and ultimately enhances cell viability while inhibiting cellular senescence and death [55, 56]. Moreover, this pathway plays a pivotal role in regulating various aspects of macrophages, including survival, migration, proliferation, and the coordination of their responses to diverse metabolic and inflammatory signals [55, 57]. Multiple studies have consistently emphasized the pivotal function of PI3K/Akt in macrophage polarization and exosome promotion of the transition of M1 macrophages to M2 through the PI3K/Akt pathway [58, 59]. Researchers developed a novel therapeutic nanofiber based on rhubarbic acid, a unique drug that activates the PI3K/AKT/mTOR pathway, inhibits the NF-κB and STAT3 pathways, and progresses the conversion of M1 macrophages to M2, leading to effective treatment of retinal ischemia/reperfusion injury [60]. Another significant finding is the role of Saikosaponin D, which was shown to downregulate STAT6 and PI3K/AKT/mTOR pathways in IL-4-stimulated RAW 264.7 cells, thereby inhibiting cellular M2 polarization [61].
3.1.4 IRFs Family
An important family of transcription factors is known as the IRFs, comprising 9 members (IRF1-9) in humans, with IRF3, IRF5, and IRF7 exhibiting particularly notable functions in innate immunity and macrophage polarization [62-64], characterized by a conserved N-terminal DNA-binding domain featuring a unique “tryptophan pentad repeat” structure that recognizes interferon-stimulated response elements in target gene promoters. Functionally, IRFs are pivotal regulators of innate and adaptive immunity, particularly in mediating IFN-α/β responses, antiviral defense, and inflammation.
In macrophages, IRFs play a crucial role in maintaining immune homeostasis by regulating the polarization phenotypes and functional plasticity of these cells. For instance, IRF5 has been shown to drive the expression of proinflammatory factors such as IL-12 and TNF-α in M1-type macrophages, while IRF4, in conjunction with STAT6, has been observed to synergistically induce an antiinflammatory/restorative phenotype (e.g., upregulation of IL-10, Arg1) in M2-type macrophages [65]. Additionally, IRF3 and IRF7 are significantly important in regulating immune responses. IRF1 and IRF8 are known to have complex functions that reinforce NF-κB-mediated inflammatory signaling while also mediating the virally triggered type I interferon response [66]. A functional imbalance of IRFs (e.g., IRF5 overexpressing systemic lupus erythematosus (SLE), IRF4 defects and immune dysregulation) has been shown to lead to aberrant macrophage activation and promote autoimmune diseases, chronic inflammation, and tumor progression. It has become a core target of the macrophage immunoregulatory network by integrating signaling pathways such as JAK–STAT and TLR [67].
IRF5 has been shown to possess a multitude of functions, such as activating genes that encode inflammatory cytokines, type I interferons, and tumor suppressors. Recently, IRF5 has been recognized as a crucial transcription factor in M1 macrophage the differentiation of M1 macrophages because M1 macrophages express IRF5 in large numbers, and the expression of IRF5 in M2 macrophages is associated with the overall expression of genes characteristic of M1 macrophages [68]. Furthermore, research indicates that the knockdown of IRF5 in iNOS−/− M1 macrophages results in a decreased expression of M1-characteristic cytokines. These findings identify tyrosine residues 74th and 104th of IRF5 as being critical for inducing IL-12/p40 promoter activation. This research unveils a novel mechanism that regulates M1 macrophage differentiation through nitration of IRF5 tyrosine residues [69].
Additionally, evidence suggests that IRF4 functions as a negative regulator of inflammation in adipose tissue macrophages during diet-induced obesity, partially by modulating M2-like macrophage polarization [70]. Consequently, distinct isoforms of IRF evidently exhibit varied roles in regulating macrophage phenotypes.
3.1.5 Notch Signaling Pathway
The Notch signaling pathway, which is highly conserved in both vertebrates and invertebrates, significantly influences various aspects of postnatal development and tissue renewal. It plays a crucial role in regulating cell fates during proliferation, differentiation, and apoptosis [71-73] . Mammals express four transmembrane Notch receptors (Notch1 to Notch4) and five classical transmembrane ligands, divided into two families: Delta-like 1, 3, and 4, and the JAG-type ligands Jagged-1 and Jagged-2. These homologous genes exhibit highly conserved structural domain architectures with high structural fidelity. Notch proteins represent a family of single-pass transmembrane receptors, distinguished by three primary modular components: the Notch extracellular domain, the Notch intracellular domain, which contains essential regulatory motifs such as ankyrin repeats and a transactivation domain [74, 75]. The cellular specificity and spatial distribution patterns of both Notch receptors and their corresponding ligands fundamentally regulate the dynamics of signal transduction, acting as critical determinants of signaling amplitude and temporal duration. Additionally, RBP-J plays a pivotal role in orchestrating context-dependent transcriptional regulation.
Macrophages have been shown to stably express Notch ligands and receptors Notch1, 2, and 4, suggesting a potential role for Notch signaling in the activation of these cells and the regulation of their multifaceted biological properties [76]. Subsequent studies have confirmed that LPS can specifically enhance the expression of Notch1 through the activation of macrophage pathways that are either MyD88-dependent or independent. This activation leads to the expression of downstream genes. When Notch signaling is activated, there is an increase in the secretion of inflammatory factors such as IL-6 and iNOS, a reduction in the release of IL-10, and a polarization of macrophages toward the M1 phenotype [77]. In a subsequent study, Keewan and Naser [78] investigated the roles of RBP-J-mediated Notch signaling and TLR signaling as essential regulators of macrophage function, demonstrating a synergistic regulatory effect on these cells. Feedback mechanisms, which follow the expression of downstream genes Hes1 and Hey1, modulate the regulatory influence of Notch signaling on macrophages.
Xu et al. [79] clearly demonstrated that the RBP-J-dependent classical Notch pathway activates the TLR4 molecule, which is activated via the MAP kinase-interacting kinase–eIF4E–interleukin-1 receptor. The activation of the TLR4 molecule by the RBP-J-dependent classical Notch pathway is associated with the MAP kinase-interacting kinase–eIF4E–IRAK2 pathway. This pathway has been shown to induce high expression of IRF8, which activates the expression of molecular markers related to M1 polarization. Notably, this pathway has been shown to progress M1 inflammatory responses in macrophages.
In the study by Wang et al. [80], it was observed that SOCS3 functions as a downstream molecule of the Notch signaling pathway, regulating the polarization of macrophages. The RBP-J-mediated Notch signaling pathway has been found to regulate M1 and M2 macrophages through SOCS3. Emerging evidence from recent investigations highlights the pivotal regulatory role of the Notch–RBP-J signaling axis in orchestrating lineage commitment, spatial distribution, and effector functions of distinct hepatic macrophage subsets during metabolic dysfunction-associated steatohepatitis (MASH). This offers novel mechanistic insights into myeloid cell dynamics under inflammatory conditions. These findings propose that pharmacological modulation of RBP-J-mediated transcriptional programs could serve as a potential strategy for mitigating disease progression in the pathogenesis of MASH [81]. Complementary research further elucidates the capacity of Notch-mediated signaling to influence macrophage polarization states in hepatic disorders, particularly concerning the remodeling of the inflammatory microenvironment [82]. Xu et al. [83] utilized a combined obesity-ethanol steatohepatitis murine model to demonstrate a concurrent elevation of Notch1 receptor activation and proinflammatory M1-polarization markers within liver-resident macrophages. In contrast, genetic ablation of Notch1 resulted in an inability to upregulate M1-associated transcriptional signatures in hepatic myeloid populations.
Collectively, the functional polarization and effector activities of macrophages are coordinated through the interplay of multiple signaling cascades. These cascades include key regulators such as the JAK–STAT axis, the NF-κB network, the PI3K–Akt–mTOR pathway, the IRF transcription factor family, and Notch-mediated signaling. Notably, the JAK–STAT cascade plays a pivotal role in determining M1/M2 phenotypic commitment through the differential phosphorylation of STAT1 and STAT6 isoforms. In contrast, the NF-κB system exerts a dual regulatory influence over inflammatory responses and tissue repair mechanisms, which is mediated through its canonical and alternative activation routes, respectively. The PI3K–Akt–mTOR pathway exerts a crucial function in metabolic reprogramming, while the IRF5/8 and IRF4/3 pathways exhibit an antagonistic relationship in regulating inflammatory and antiinflammatory genes. The Notch signaling pathway, through its interaction with the RBP-J axis, contributes to M2-like polarization within the microenvironment, thereby facilitating tumor development. The intricate interplay among these pathways is crucial for maintaining physiological homeostasis, with their imbalance potentially leading to various pathologies, including tumors, neurodegenerative diseases, and autoimmune diseases.
4 Divergent Functions of Macrophages
4.1 Phagocytosis
As myeloid-derived immune sentinels, macrophages perform dual immunological roles as professional phagocytes and antigen-presenting cells. These versatile cells orchestrate the elimination of pathogens, initiate inflammatory cascades, and clear cellular debris through their engulfment processes. Recent research underscores their essential contributions to tissue development and metabolic homeostasis, mediated by finely tuned phagocytic activity and cytokine-mediated intercellular communication. Phagocytosis, a specialized mechanism of engulfment, enables both professional phagocytes (e.g., macrophages) and nonspecialized cell types (e.g., epithelial cells) to detect, internalize, and degrade particulate matter larger than 0.5 µm in diameter, serving as a critical defense strategy for maintaining homeostatic equilibrium during pathogenic incursions [84]. The ability of macrophages to recognize foreign pathogens or foreign objects is based on multiple receptors (e.g., FcγR) on their surface. Among them, PRRs play a critical role. These immune surveillance receptors specifically detect PAMPs—evolutionarily conserved structural motifs exclusive to microbial pathogens., such as LPS and peptidoglycan in bacteria, and nucleic acids in viruses. In addition to these mechanisms, macrophages can recognize pathogens labeled by complement proteins via complement receptors (CR). When the complement system is activated, complement components, such as C3b, are deposited on the surface of pathogens, and macrophage CRs (e.g., CR3, CR4) recognize these markers, thereby initiating phagocytosis. A 2007 investigation by Green and colleagues [85] demonstrated the recruitment of autophagy-related (Atg) proteins, including Beclin 1, LC3, Atg5, and Atg7, to maturing phagosomes in macrophages following TLR-mediated signaling. This phenomenon was subsequently characterized as the noncanonical LC3-associated phagocytosis pathway, which is distinct from classical autophagy processes. In addition, recent findings have demonstrated that rhamnose, produced by prokaryotic metabolism, promotes SLC12A4 activation through binding to SLC12A4. This, in turn, induces modulation of Rac1 and CDC42 activity and promotes phagocytosis by macrophages, thereby contributing to the alleviation of sepsis [86] (Figure 3).
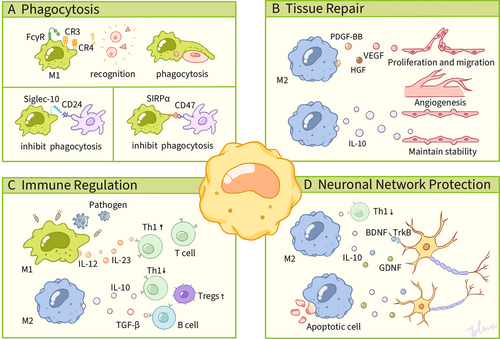
The macrophage-expressed inhibitory receptor Siglec-10 engages with tumor-associated CD24 through interactions at its extracellular domain, which triggers intracellular signaling via immunoreceptor tyrosine-based inhibitory motifs (ITIMs) located within its cytoplasmic tail [87]. This molecular recognition event facilitates the recruitment of Src-family tyrosine kinases (SFKs) that phosphorylate the ITIM tyrosine residues, thereby initiating a phosphorylation cascade that mobilizes Src homology 2 (SH2) domain-containing phosphatases, including SHP-1 and SHP-2. The subsequent binding of SHP-1 to the phosphorylated ITIM domains mediates substrate dephosphorylation through its catalytic activity, ultimately leading to actin cytoskeletal reorganization that enables phagocytic clearance. Additionally, SHP-1 negatively regulates intracellular signaling involving cell adhesion molecules, ECM factors, hormones, cytokines, and growth factors [88]. Consequently, the interaction of CD24 with Siglec-10 impedes phagocytosis by macrophages and fosters evasion from tumors. Blocking CD24 or Siglec-10 expression by gene or antibody facilitates macrophage phagocytosis and inhibits tumor growth [89, 90].
SIRPα serves as the endogenous ligand for CD47 [91], a surface-expressed transmembrane glycoprotein predominantly localized on myeloid lineage cells, including macrophages, monocytes, and dendritic cells. The architecture of the SIRPα receptor consists of an extracellular region with three immunoglobulin-like domains, a single transmembrane module, and cytoplasmic tyrosine kinase phosphorylation motifs. Essential to its inhibitory function, the intracellular segment contains ITIMs that mediate the suppression of signal transduction [92, 93]. This counter-regulatory mechanism operates through the competitive antagonism of ITAM-coupled receptor activation. Effective signal inhibition requires the phosphorylation of ITIM tyrosine residues within cytoplasmic sequences, which facilitates the recruitment and enzymatic activation of SH2 domain-containing phosphatases SHP-1 and SHP-2. These effector molecules, equipped with SH2 phosphotyrosine-binding modules, execute downstream dephosphorylation cascades [92, 93]. The enzymatic activation of SHP-1 and SHP-2 phosphatases triggers the phosphorylation of myosin IIA, acting as a molecular switch that antagonizes nonmyosin IIA activity. These nonmuscle myosin isoforms play a critical role in regulating phagocytic machinery by coordinating phagolysosome maturation and orchestrating target engulfment in macrophages. When myosin IIA is dephosphorylated in macrophages, actin depolymerization occurs, resulting in reduced phagocytosis [94, 95]. The CD47–SIRPα interaction between malignant cells and phagocytic effector cells initiates tyrosine phosphorylation of ITIMs within SIRPα’s cytoplasmic domain through SFK-mediated activation. This signaling cascade facilitates recruitment of protein tyrosine phosphatases SHP-1/SHP-2, which contributes to reduced phagocytosis [96].
4.2 Tissue Repair
Tissue regeneration and repair are fundamental biological mechanisms crucial for maintaining organismal homeostasis and ensuring survival [97]. Following tissue damage caused by pathogen invasion or physical trauma, necrotic cells release endogenous damage-associated molecular patterns (DAMPs), while invading microbes secrete PAMPs. Together, these signals activate innate immune signaling cascades that drive inflammatory responses [98]. This molecular alert system mobilizes a complex inflammatory cascade characterized by the sequential recruitment, clonal expansion, and functional maturation of diverse effector populations. These include immune effectors such as neutrophils, macrophages, innate lymphoid cells, NK cells, and adaptive lymphocytes, as well as stromal components like fibroblasts, epithelial and endothelial lineages, and progenitor cells. Collectively, these elements establish an integrated cellular network that promotes regenerative processes [99]. Under physiological conditions, a self-limited inflammatory phase facilitates the precise architectural restoration of native tissue matrices. In contrast, persistent dysregulation of healing mechanisms can lead to maladaptive fibroproliferative responses, which are characterized by excessive ECM deposition that compromises parenchymal functionality and may culminate in terminal organ insufficiency [100]. Therefore, the spatiotemporal regulation of repair pathways is essential. Among the various contributors to tissue restoration, macrophages are identified as pivotal regulators due to their plastic functional adaptability and context-dependent polarization states [101]. They have been shown to play crucial regulatory roles throughout all phases of repair and fibrosis [102]. Recent strides in regenerative medicine and molecular biology have underscored the pivotal role of macrophages in promoting regeneration across diverse tissues, including the heart, liver, kidney, muscle, and nervous system. The capacity of macrophages to transition between distinct phenotypes in response to microenvironmental signals renders them promising therapeutic targets for enhancing tissue repair and regeneration [103].
The modulation of chemokine receptor signaling in mononuclear phagocytes alters their migratory patterns and functional roles in regenerative processes. Experimental evidence demonstrates that M2-polarized macrophages act as crucial regulators of wound resolution and neovascularization [104], thereby highlighting their therapeutic potential in regenerative medicine. In contrast, clinical analyses indicate that the dynamics of TAM infiltration correlate with negative clinical outcomes in cohorts treated with antiangiogenesis therapies [105]. Yang et al. [106] established the proangiogenic functions of M2-like macrophages through a multiplatform analysis that incorporated human pancreatic ductal adenocarcinoma specimens, murine xenograft models, and The Cancer Genome Atlas genomic datasets. In contrast to M0 macrophage-derived exosomes (MDEs), M2 MDEs promote ex vivo and in vivo angiogenesis and tumor progression. Given that conventional anti-VEGF drugs are often ineffective in pancreatic cancer, proangiogenic M2 MDEs associated with TAMs represent emerging therapeutic targets in pancreatic carcinogenesis. Guo et al. [107] demonstrated that Runt-related transcription factor 1 (RUNX1) drives CCL2 expression in colorectal adenocarcinoma through transcriptional upregulation of hematological cell lineage 2 (HCL2), establishing RUNX1 as a critical transcriptional mediator of HCL2-dependent chemokine signaling. In their study of colorectal cancer (CRC), Guo et al. [107] found that RUNX1 recruits macrophages and induces M2-polarized TAM in CRC by promoting the production of CCL2 and the activation of the hedgehog pathway. Emerging evidence reveals macrophages induce endothelial activation through paracrine secretion of angiogenic mediators such as VEGF, driving pathological neointimal hyperplasia. Furthermore, these immune sentinels preserve vascular homeostasis through dual mechanisms: production of immunomodulatory cytokines (e.g., IL-10) and regulation of ECM composition and dynamics, effectively attenuating inflammatory cascades. A recent research by Zenget al. found that macrophages in skin wounds in a diabetic state polarize to M1 type, targeting vascular endothelial cell HELZ2 protein by secreting extracellular vesicles carrying miR-ERIA, thereby inhibiting vascular endothelial cell migration and tube-forming ability and ultimately leading to delayed wound healing [108]. Furthermore, macrophages have been shown to maintain vascular endothelial cell stability by secreting antiinflammatory cytokines (e.g., IL-10) and regulating ECM components to reduce inflammatory responses.
4.3 Immune Regulation
Immunoregulation is defined as the complicated interactions between immune molecules, immune cells, and the immune system with other bodily systems during immune responses. These interactions establish a regulatory network that coordinates and constrains various processes, thereby ensuring that the immune response is appropriately calibrated in terms of strength and quality to maintain the stability of the body's internal environment. Typically, systematic analysis of immunometabolic pathways focuses on macrophages, which are central to both pro- and anti-inflammatory immune responses [109]. It is now widely acknowledged that these cells play a pivotal role in immune regulation, a process that is critical for maintaining health. A mounting body of evidence suggests a critical functional linkage between the bioenergetic reprogramming of polarized macrophages (M1/M2 phenotypes) and their immunomodulatory capacities. Systematic elucidation of the dynamic interactions governing metabolic reprogramming dynamics and PRR cascades within these immune cells may establish a conceptual foundation for designing targeted intervention strategies against chronic inflammatory pathologies [110].
IFN-γ and LPS have been shown to trigger macrophages, leading to the TCA cycle displayed through integrated transcriptional and metabolic pathways. Inhibition of succinate dehydrogenase by the citric acid metabolite succinate in the TCA cycle is a key feature of IFN-γ/LPS-polarized macrophages. Emerging pharmacological studies have demonstrated that succinate, acting as an immune-regulating metabolite, possesses dual therapeutic properties, which include both significant attenuation of inflammatory cascades and effective suppression of pathogenic microorganisms. It is noteworthy that alterations in metabolite concentrations have the capacity to directly modify the function of signaling pathways. HIF-1α stabilization, induced by LPS-induced succinate accumulation in macrophages, has been shown to promote proinflammatory cytokine IL-1β expression [111]. The proteostatic maintenance of HIF-1α drives glycolytic reprogramming in proinflammatory macrophages. This oxygen-sensitive transcription factor orchestrates the transcriptional activation of essential enzymatic components involved in carbohydrate metabolism, such as the lactate export machinery (MCT4/SLC16A3) and hexose uptake systems (GLUT1/SLC2A1). M1 macrophages have also been shown to exhibit an enhanced pentose phosphate pathway, which produces NADPH [112]. It has been established that NADPH plays a crucial role as a cofactor for LPS-induced iNOS, facilitating the catabolism of arginine into NO and l-citrulline. It is noteworthy that NADPH not only generates NO and ROS, but also contributes to the production of the antioxidant glutathione, which is vital for maintaining redox homeostasis and averting cellular damage from ROS [113]. Additionally, NO serves as a central regulatory mediator that governs metabolic reprogramming in classically activated macrophages. Through dual molecular mechanisms involving the nitrosylation of the [4Fe–4S] enzymatic complex and the consequent irreversible disruption of mitochondrial electron transfer, this gaseous signaling molecule fundamentally compromises mitochondrial bioenergetic efficiency. It effectively uncouples the progression of the TCA cycle from ATP synthase activity, while imposing energetic constraints on oxidative metabolism [114].
Distinct bioenergetic configurations characterize macrophage polarization states, with M2 variants exhibiting significant divergence from their proinflammatory M1 counterparts. This dichotomy reflects their specialized roles in immunoregulation and stromal maintenance. Central to this metabolic compartmentalization is the differential engagement of ATP synthesis pathways: M1 polarization preferentially drives flux through the glycolytic pathway, often accompanied by the accumulation of itaconate, whereas M2 activation coordinates mitochondrial oxidative metabolism through the TCA cycle, coupled with mitochondrial OXPHOS, thus optimizing the electron transport chain for energy production. This process is facilitated by the glutamate metabolic pathway, which involves β-fatty acid oxidation and α-ketoglutarate. The IL-4/IL-13 signaling axis orchestrates metabolic reprogramming in macrophages by transcriptionally enhancing fatty acid β-oxidation and mitochondrial biogenesis. This process is mechanistically dependent on the integrated activation of STAT6, members of the PPAR nuclear receptor family, and PGC-1β. M1 and M2 macrophages exhibit opposing arginine metabolism; M1 macrophages upregulate iNOS to metabolize l-arginine into the antimicrobial substances NO and l-citrulline, while M2 macrophages catalyze l-arginine to urea and l-ornithine through the induction of Arg1 [117, 118].
The M1/M2 polarization state of macrophages has been demonstrated to exhibit bidirectional roles in immunomodulation. It has been shown that M1-type macrophages activate antitumor immunity by secreting proinflammatory factors such as TNF-α and IL-12, whereas M2-type macrophages promote immunosuppression and tissue repair through mediators such as IL-10 and TGF-β [119]. Notably, 3,3′,5-triiodothyronine (T3) has been found to have a dual regulatory role, promoting M1 polarization to enhance inflammatory response and inhibiting M2 activity to maintain immune homeostasis, illustrating a dynamic regulatory mechanism with implications for autoimmune disease and tumor therapy [120]. M2-type polarization of TAMs is a fundamental factor in tumor immune escape, which promotes angiogenesis and suppresses T cell function by secreting molecules such as VEGF and PD-L1. Recent studies have developed pH-responsive nanoparticles, which can reprogram TAM to the M1 phenotype by precisely modulating lysosomal function, significantly enhancing antigen presentation efficiency and activating CD8+ T cells [121]. In addition, a combination of GM-CSF secretion mediated by engineered bacteria and SIRPα–siRNA delivery has been shown to synergistically block the CD47–SIRPα immune checkpoint pathway, thereby significantly enhancing the antitumor efficacy [122].
4.4 Neuronal Network Protection
The neuronal network is comprised of highly specialized neurons and their synaptic connections, which achieve information integration and regulation through electrochemical signaling. It is a central carrier of nervous system function. Macrophages (e.g., microglia, which are resident macrophages in the central nervous system [CNS]) can regulate the microenvironment of neural stem cells. These cells play a critical role in safeguarding neuronal networks across multiple levels, including the regulation of the inflammatory microenvironment, the maintenance of neurohomeostasis, the execution of phagocytic clearance and damage repair, and the regulation of metabolism and energy homeostasis. M2-type macrophages, in particular, have been shown to inhibit excessive inflammatory responses and reduce neuronal apoptosis by secreting antiinflammatory factors such as IL-10 and TGF-β.
In a spinal cord injury model, TREM2 knockout macrophages significantly improved neuronal survival by decreasing levels of proinflammatory factors TNF-α and IL-1β [123]. Macrophages also promote neuronal survival and axonal regeneration by secreting BDNF) and NT-3 [124]. Following spinal cord injury, an increase in the number of dorsal root ganglion macrophages (DRGMacs) is observed, primarily through self-renewal. A subset of DRGMacs undergoes a transformation into a microglial-like state following nerve injury, with these cells potentially migrating from the spinal cord to DRG and contributing to neuroprotection and repair. A further subset of DRGMacs displays characteristics analogous to satellite glial cells, which have been observed to express macrophage-associated genes post-nerve injury and potentially contribute to immune responses and neuroprotection [125]. Research has demonstrated that fumarate hydratase (FH) modulates the proinflammatory and reparative functions of macrophages by regulating mitochondrial RNA release and interferon-β signaling pathways. Inhibition of FH enhances the antiinflammatory phenotype of macrophages and attenuates neuronal damage in neurodegenerative lesions [126, 127]. Furthermore, the study identified that MDE carry noncoding RNA species, such as miR-155 and miR-21, which traverse the neurovascular unit, achieving cerebral parenchymal infiltration through selective modulation of tight junction complexes and endothelial transcytosis pathways, thereby modulating synaptic plasticity and neuronal electrical activity [128].
Accumulating experimental evidence delineates the pathophysiological contributions of myeloid phagocytes, particularly circulating monocytes, TRMs, and microglial cells, in mediating neuroimmune interactions that drive inflammation-associated axonal degeneration, CNS structural compromise, and resultant neurobehavioral alterations in mammalian models [129, 130]. Notably, cerebral phagocytic populations constitutively express PRRs, including TLR4 and NLRP3 inflammasomes, which mechanistically enable their capacity to mediate structural plasticity through dendritic spine pruning and synaptic remodeling [131, 132]. The functional polarization of these immunoregulatory cells is not merely determined by passive cytokine exposure; rather, it is shaped by the dynamic equilibrium between neurodestructive mediators (e.g., IL-1β/TNF-α) and neurotrophic factors (e.g., glial cell-derived neurotrophic factor [GDNF]/insulin-like growth factor 1), which collectively dictate their dichotomous roles in neural circuit disruption versus restoration. Furthermore, the adrenergic signaling–Arg1–polyamine metabolic axis in muscular macrophages mediates neuroprotective adaptations during infectious challenges, where activation of the β2-adrenergic receptor triggers pharmacological induction of Arg1 enzymatic activity, thereby elevating polyamine biosynthesis to prevent infection-associated neurodegeneration [133].
During the process of tissue healing following acute injury, macrophages are responsible for the removal of cellular debris from the affected area through a process known as thrombospondin-1 (TSP-1)-dependent phagocytosis. In addition to this primary function, these cells also release metabolites, such as adenosine, which play a crucial role in promoting angiogenesis and axon regeneration. The activation of calcitonin gene-related peptide (CGRP) neurons has been observed to enhance the phagocytic activity of macrophages. It has been demonstrated that CGRP neurons are responsible for accelerating the repair of skin and muscle injuries. The neuropeptide CGRP exhibits dual immunomodulatory functions through coordinated molecular mechanisms. It mediates neutrophil efferocytosis via Rho GTPase-dependent cytoskeletal reorganization and drives macrophage phenotypic switching toward an immunoregulatory (M2-like) state through TSP-1-mediated autocrine/paracrine signaling loops [124].
Macrophages, a fundamental component of organismal homeostasis, function as a regulatory axis that orchestrates a multifaceted physiological barrier through phagocytosis. This process encompasses the elimination of metabolic byproducts, the orchestration of tissue repair microenvironments, the maintenance of immune dynamic homeostasis, and the engagement in neural synaptic pruning. However, their high degree of plasticity endows macrophages with the capacity to dynamically transform in pathological microenvironments. In such microenvironments, macrophages can either initiate a protective program by sensing injury signals through PRRs or be driven by abnormal microenvironments to develop proinflammatory or profibrotic phenotypes. These phenotypes ultimately influence the direction of disease progression.
5 Macrophages in Diseases
5.1 Autoimmune Diseases
Macrophages play a crucial role in the progression of various autoimmune disorders due to their diverse functional repertoire, which includes immunoregulation, proinflammatory activation, and stromal remodeling. These myeloid sentinels orchestrate complex chemokine networks through the dynamic secretion of soluble mediators that recruit and prime adaptive immune cell populations, thereby establishing self-perpetuating inflammatory circuits via paracrine signaling mechanisms.
SLE is an autoimmune disease characterized by an overactive immune system, the presence of autoantibodies, and multisystem damage, resulting in a wide heterogeneity of clinical manifestations .The etiology of SLE is complicated and involves both genetic susceptibility and environmental factors [134, 135]. One hypothesis is that residual cellular debris generated by increased cell death initiates immune overactivity and induces immunologic loss [136]. Research efforts have increasingly concentrated on the immunomodulatory effects of regulated cell death modalities, particularly their ability to undermine immunological tolerance and dynamically reconfigure adaptive immune circuits. The study of the role of innate immune cells in the pathogenesis of SLE has also attracted considerable interest, as there is a disruption in the homeostasis between macrophage subtypes in SLE, which is more pronounced in the affected organs. LPS leakage from damaged intestinal barriers was found to induce GSDMD-mediated cellular pyroptosis through the TLR4/caspase 11 pathway in MRL/lpr mice. Moreover, pyroptotic macrophages promoted the differentiation of naive B cells into plasmoblasts and plasma cells, which may exacerbate the pathogenesis of lupus. Additionally, inhibition of caspase 11 and intestinal barrier repair with antibiotics effectively inhibited the caspase 11/GSDMD pathway and attenuated the manifestation of lupus in mice [137] (Figure 4) .
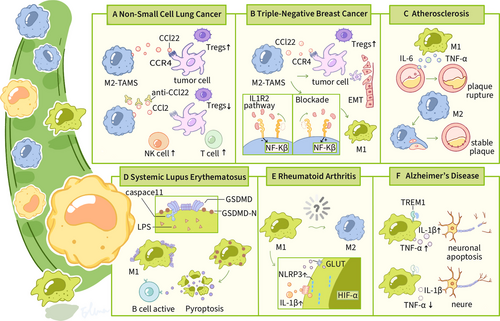
RA, a prevalent immune-mediated disorder, is characterized by a complex disease progression involving inflammatory cascades that target the synovium and lead to destructive polyarticular involvement. This often results in irreversible joint degradation and significant functional impairment [138]. Pathological analyses indicate a marked dysregulation in macrophage polarization dynamics, with a shift in the balance from proinflammatory (M1) to antiinflammatory (M2) phenotypes, favoring dominant M1 activation throughout the pathogenesis of RA [139, 140]. Macrophages are critical in the initiation and maintenance of synovitis in RA, where they can act as antigen-presenting cells leading to T-cell-dependent B-cell activation and production of damaging cytokines, but macrophages are also involved in maintaining tissue homeostasis/repair [141]. Pathological and physiological macrophages differ in phenotype and function (e.g., cytokine secretion) and exhibit polymorphism. Reprogramming M1 macrophages to M2 using targeted IL-10 gene therapy prevents joint inflammation and damage associated with arthritis [142]. Rosmarinic acid, a polyphenolic derivative derived from Prunus serotina, orchestrates immunometabolic reprogramming through the dual-axis suppression of the ERK/hypoxia-inducible factor 1 alpha (HIF-1α)/GLUT1 signaling cascade. This targeted kinase modulation attenuates Warburg-like glycolysis, facilitating a phenotypic transition from classically activated macrophages to alternatively polarized phenotypes. This metabolic switch not only ameliorates synovitis-mediated joint destruction but also enhances chondroprotective repair mechanisms in RA models [143]. Thus, modulation of joint-associated macrophage subtypes has significant therapeutic potential.
Synovial tissue is the primary site of joint inflammation in RA patients. Chronic synovitis results in irreversible damage to cartilage and bone. TAMs are evolutionarily conserved innate immune effectors that originate from fetal liver-derived precursors or bone marrow myeloid progenitors. These sentinel cells perform organ-specific homeostatic functions, ranging from the efferocytic clearance of apoptotic debris to the sequestration of pathogens, as exemplified by the alveolar surveillance mechanisms that maintain sterility during respiratory gas exchange. Crucially, macrophages act as immunoregulatory rheostats, coordinating a biphasic inflammatory cascade that initiates neutrophil recruitment and subsequently resolves sterile injury. During parenchymal damage, local macrophage pools are augmented by circulating monocyte-derived counterparts, establishing heterotypic cellular crosstalk networks that calibrate the intensity of inflammation [144]. The dynamic equilibrium between CD169+ synovial-resident macrophages and CCR2+ monocyte-derived infiltrating macrophages dictates the temporal progression of synovitis and the fidelity of articular regeneration processes in rheumatoid joints [145]. Landmark clinical observations, particularly the pathognomonic association of STM (CD68+CD163−) density gradients with radiographic joint space narrowing [146], coupled with spatial transcriptomic evidence demonstrating the colocalization of macrophage activation markers (CD86/MHC-II) with subchondral bone erosion foci in high-DAS28 joints [147], have provided mechanistic insights into macrophage-mediated synoviocyte hyperplasia and osteoclast activation during the progression of RA.
5.2 Neurodegenerative Diseases
As the predominant neurodegenerative dementia subtype, Alzheimer's disease (AD) clinically manifests as relentless mnestic dysfunction and multidomain cognitive deterioration. Advancing chronological age emerges as the primary nonmodifiable determinant in AD pathogenesis, and both are associated with chronic inflammation (sometimes referred to as “inflammatory aging”) and an impaired immune response. Microglia are the major resident macrophages in the brain, accounting for 10% of all glial cells. In the early stages of AD, they play a protective role by phagocytosing β-amyloid (Aβ) and tau proteins, but as the disease progresses, prolonged activation releases proinflammatory factors (e.g., IL-1β, TNF-α) that exacerbate neuroinflammation [148-150]. In recent years, it has been found that macrophages of peripheral origin can enter the AD brain through the blood–brain barrier and synergize with central microglia to regulate pathological processes. For example, CX3CR1+ BAMs are enriched around Aβ plaques, but their clearance is affected by lipid metabolic status [150-152]. Microglia rely on the TREM2 receptor to recognize and phagocytose Aβ, while secreting insulin-degrading enzyme to directly degrade Aβ. Knockdown of the myeloid triggering receptor TREM1 protects glucose metabolism in peripheral macrophages and oxidative phosphorylation in brain neurons. Restoration of immune responses that support healthy brain function [153]. In addition, a study shows that MDM activation is independent of TREM2 and that blocking monocyte migration with anti-Ccr2 antibody completely abolishes the cognitive-improving effects of anti-PD-L1 treatment in Trem2−/−5XFAD mice [154]. On the other hand, high-fat diet and APOE4 genotype exacerbate lipid droplet deposition in microglia, leading to decreased phagocytosis through inhibition of the PI3K/AKT pathway. Knockdown of Fit2, a key gene for lipid droplet formation, was shown to increase Aβ clearance by 40% [150]. Activated microglia are converted to a glycolytic phenotype, leading to lactate accumulation and disruption of neuronal mitochondrial function [155, 156].
Macrophages play a dynamic, double-edged role in AD and their function is coregulated by genetic, metabolic and microenvironmental factors. Future studies need to combine single-cell multiomics, organoid models, and clinical cohorts to analyze the spatio-temporal specific functions of different subpopulations of macrophages and to develop stage-appropriate therapeutic strategies.
5.3 Cancers
TAMs constitute critical stromal constituents of the neoplastic niche, exerting pleiotropic regulatory effects on malignant proliferation, VEGF-mediated neovascularization, and PD-L1-dependent immune checkpoint activation [157-159]. Ontogenetically stratified, these myeloid populations segregate into embryonically derived tissue-resident and hematopoietic progenitor-derived monocytic lineages. Functionally, these myeloid infiltrates pervasively colonize solid neoplasms, driving oncogenic processes, angiogenic switching, metastatic dissemination, and desmoplastic stromal barrier formation, ultimately facilitating immune-privileged tumor evolution.
Triple-negative breast carcinoma (TNBC), defined by the immunohistochemical absence of estrogen receptor, progesterone receptor, and HER2/neu expression, represents the most therapeutically challenging breast cancer subtype with aggressive metastatic potential. While bidirectional tumor–stroma signaling has been extensively characterized in mammary malignancies [160], the precise molecular circuitry through which TNBC cells reprogram TAMs remains enigmatic. Emerging data delineate a feedforward TNBC–TAM communication IL-6/TGF-β1-mediated paracrine signaling induces constitutive HLF activation in neoplastic cells. Mechanistically, HLF orchestrates ferroptosis resistance via transcriptional upregulation of GGT1, thereby enabling glutathione-mediated redox homeostasis and fueling TNBC malignant progression. These findings unveil the HLF/GGT1 regulatory node as a promising therapeutic vulnerability in TNBC pathobiology [161]. A monocyte-derived subpopulation of STAB1+TREM2high lipid-associated macrophages (LAM) expanded in patients resistant to immune checkpoint blockade (ICB) therapy; this LAM subpopulation is immunosuppressive and acquires tumor-promoting capacity upon recruitment to the tumor site via the cancer-associated fibroblast (CAF)-driven CXCL12–CXCR4 axis, which supports an immunosuppressive microenvironment [162]. IL-1 receptor type 2 (IL1R2) orchestrates breast tumor-initiating cell (BTIC) stemness maintenance and neoplastic expansion in TNBC. Mechanistically, preclinical TNBC models revealed that pharmacological IL1R2 antagonism potently suppressed CCL2-mediated myeloid cell infiltration and M2-like TAM polarization, concomitantly impairing BTIC clonogenicity while mitigating CD8+ T-lymphocyte dysfunction. This multimodal immunomodulation consequently attenuated oncogenic progression and conferred significant overall survival extension in murine TNBC systems through STAT3 signaling axis inactivation and glutathione peroxidase 4-mediated ferroptosis sensitization. This suggests that targeting this molecule may improve patient treatment [163]. In addition, TAM secretes IL-1β and TNF-α, which activate the IKK/NF-κB pathway in TNBC cells via IL1R/TNFR, inducing increased autophagy and promoting cell migration and invasion. Animal models showed that targeted inhibition of this pathway significantly reduced lung metastasis [164]. In conclusion, TAM plays a central role in TNBC progression through polarization regulation, metabolic interaction and immune escape mechanisms.
Non-small cell lung cancer (NSCLC) is the most prevalent form of lung cancer, constituting 80–85% of all lung cancers. It encompasses primarily squamous cell carcinoma, adenocarcinoma, and large cell carcinoma. TAMs in the interstitium of NSCLC are predominantly of the M2 phenotype (CD68+TGF-β1+), which is significantly higher than that in paracancerous tissues, and the M2 phenotype inhibits antitumor immunity by secreting factors such as IL-10 and TGF-β, promoting angiogenesis and stromal remodeling [165]. The infiltration level and spatial distribution of TAMs were found to have a significant impact on the outcome of NSCLC patients treated with ICB [166]. It is also significant that the degree of TAM infiltration in NSCLC was associated with upregulation of CD27, ITGAM, and CCL5 gene expression. The protein products of these genes play a key role in controlling tumor macrophage polarization and may be novel immunotherapeutic targets [165]. An in vitro coculture model study showed that NSCLC cells are capable of inducing macrophages to express an immunosuppressive M2-type phenotype, particularly high expression of Arg1. This model provides an effective tool to study the biological functions of macrophages in NSCLC and to develop therapeutic strategies against macrophages.
Esophageal squamous cell carcinoma (ESCC) is a malignant tumor derived from esophageal squamous epithelial cells and is the major pathological type of esophageal cancer. TAMs potentiate neoplastic invasion through paracrine secretion of protumorigenic factors while simultaneously driving VEGF-A-driven neovascularization. TAM abundance exhibits a linear correlation with CD31+ microvessel density indices, suggesting their dual role in stromal remodeling and angiogenic switch regulation during ESCC progression [167]. The M2-polarized phenotype of TAMs is an important feature of the ESCC microenvironment, and M2-type macrophages promote tumor immune escape and angiogenesis by secreting factors such as VEGFA and IL-10 [168]. And, CAFs recruit and induce tumor cell invasion and angiogenesis by secreting PAI-1, CCL2, and other factors to recruit and induce macrophage M2 polarization while enhancing migration and invasion of ESCC cells [169]. Therapeutic strategies that target the Hippo/YAP–CD24/Siglec-15 signaling nexus exhibit bimodal therapeutic efficacy in ESCC by suppressing Hippo/YAP-driven oncogenic signaling cascades and enhancing macrophage-mediated efferocytosis through the blockade of the STAT6-mediated “don't eat me” signal (CD47/SIRPα axis). This mechanism holds significant translational potential in the context of precision oncology for ESCC [170].
5.4 Liver Diseases
5.4.1 Viral Hepatitis
Viral hepatitis is a liver disease caused by hepatitis viruses and is classified as an infectious disease. The primary clinical manifestations include decreased appetite, nausea, upper abdominal discomfort, liver pain, and fatigue. Some patients may also experience jaundice, fever, and liver enlargement, often accompanied by impaired liver function. A subset of patients may develop chronic hepatitis, which can lead to cirrhosis, and in a few cases, progress to liver cancer. Diagnosis of viral hepatitis primarily relies on identifying the etiology, which encompasses five types: A, B, C, D, and E. We will focus specifically on hepatitis B and C viruses [171].
Despite their distinct virological profiles, hepatitis B virus (HBV) and hepatitis C virus (HCV) share immunopathogenic mechanisms, where the balance between hepatic viral eradication and persistence is governed by the dynamic equilibrium between innate PRRs and adaptive lymphocyte-mediated immune surveillance [172]. KCs, the liver's specialized macrophages strategically located in sinusoidal endothelial fenestrae, function as immunological gatekeepers. They paradoxically maintain defense against hepatotropic pathogens while also mediating inflammatory cascades that promote fibrosis [172].
Both HBV and HCV are primarily transmitted through percutaneous and sexual routes, with perinatal transmission occurring predominantly in the case of HBV [173-175]. Infections caused by these viruses may either resolve spontaneously or progress to chronic liver disease characterized by ongoing viral replication within hepatocytes [176]. Persistent hepatic inflammation serves as a critical pathophysiological driver of hepatic fibrogenesis, the progression of cirrhosis, and ultimately hepatocarcinogenesis [177]. Immunocompetent hosts who achieve spontaneous viral resolution exhibit a broad-spectrum adaptive immunity characterized by polyfunctional CD4+ T helper (Th1/Tfh) cells, cytotoxic CD8+ T effector memory re-expressing CD45RA (TEMRA) cells, and neutralizing antibody-producing B cell clones that target conserved viral epitopes. In contrast, chronic HBV/HCV carriers demonstrate attenuated adaptive immunity [178]. This observation underscores the importance of strong, multiepitope-specific T and B cell responses in the clearance of infection, which can only be achieved following effective innate immune responses [179].
HBV-infected hepatocytes release viral progeny particles, HBsAg, and the nonstructural secretory variant HBeAg into systemic circulation, with these viral components being routinely quantified in patient serum [174]. Nevertheless, conclusive evidence of productive HBV replication in nonhepatocytic cell populations has yet to be definitively established. Crucially, the potential intracellular retention of HBV antigens within KCs under physiological conditions, along with the capacity of human KCs to internalize intact virions or viral subcomponents ex vivo, remain uncharacterized. In contrast, in vitro models employing THP-1 monocytes, PBMCs, and dendritic cells have demonstrated HBV ligand–receptor interactions that trigger downstream immunostimulatory cascades. Mechanistic studies reveal TLR2-mediated recognition of HBcAg–hsp70 complexes and heparan sulfate proteoglycan (HSPG)-dependent viral attachment in THP-1 cells, culminating in NF-κB-dependent cytokine production (IL-6, IL-12, TNF-α) and inflammasome priming through ASC speck formation [180]. However, since HBcAg is exclusively found within infected hepatocytes or viral particles, the potential interaction of HBcAg with KCs via HSPG or other extracellular receptors such as TLR2 remains uncertain. Multiple receptor–ligand interactions between KC surface molecules and HBV components across experimental systems. Notably, HBsAg demonstrates CD14-dependent binding to peripheral monocytes and mannose receptor-mediated engagement with dendritic cells [174]. Furthermore, HBsAg–albumin complexes may enhance scavenger receptor-mediated endocytosis of viral antigens by sinusoidal endothelial cells and KCs through opsonization mechanisms [173, 174]. Within the hepatic immunological niche, dendritic cells and MoMFs coordinate adaptive immunity via antigen cross-presentation while secreting cytokines that modulate HBV cccDNA transcriptional activity [181]. Crucially, M1-polarized macrophages produce IL-1β and IL-6 that suppress HBV replication through JAK/STAT1 pathway activation and proteasomal degradation of viral core particles.
The HCV contains a 9.6 kilobase positive-sense RNA genome that translates into a polyprotein precursor subsequently cleaved into structural components (core, E1/E2 glycoproteins) and nonstructural regulators (NS1–NS5). Postreplicative assembly yields 55–65 nm enveloped virions packaging genomic RNA within nucleocapsid complexes [171]. In contrast to HBV, HCV employs a multistep entry pathway involving hepatocyte surface molecules beyond claudin-1/occludin tight junctions—including EGFR, EphA2, HSPG, LDL-R, SR-B1, and tetraspanin CD81. Notably, only a subset of these receptors (e.g., CD81, SR-B1) are functionally expressed on KCs. Experimental evidence indicates HCV–E2 glycoprotein engages KCs through CD81-mediated interactions [171], while DC-SIGN—a hepatocyte-absent C-type lectin receptor—mediates viral attachment to KC surface glycans through high-mannose N-linked glycosylation sites on HCV envelope proteins. [176, 177]. Although it is unlikely that HCV can replicate within KC, activation of these cells by HCV and its proteins has been documented. Specifically, HCV core and NS3 proteins stimulate CD14+ KCs and MoMFs, derived from human liver perfusate, via TLR2, resulting in the production of proinflammatory cytokines such as IL-1β, IL-6, and TNF, as well as the immunosuppressive cytokine IL-10 [182, 183]. Contemporary studies have elucidated that TLR4 mediates NS3 detection in purified liver-derived KCs through differential centrifugation and adherence-based isolation protocols, triggering MyD88-dependent TNF-α secretion [182]. Nevertheless, the limited secretory capacity of HCV core and NS3 proteins from infected hepatocytes curtails their extracellular availability for KC pattern recognition via canonical TLR pathways. A plausible alternative hypothesis posits that phagocytic clearance of HCV-infected hepatocytes by KCs could facilitate intracellular exposure to viral RNA-derived PAMPs within endosomal compartments.
5.4.2 Alcohol-Associated Liver Disease
Alcohol-associated liver disease (ALD) represents a preeminent global health burden, accounting for the majority of chronic hepatopathies worldwide [184-187] and constituting the principal indication for hepatic transplantation in Western healthcare systems [188]. Within its clinical spectrum, alcoholic hepatitis emerges as a critical syndrome of acute-on-chronic hepatic decompensation, exhibiting a 28-day mortality risk exceeding 30% and 90-day mortality rates reaching 50% in severe presentations. Orthotopic liver transplantation persists as the sole disease-modifying intervention for eligible candidates meeting stringent abstinence criteria, though its application remains constrained by donor organ scarcity and complex ethical considerations [189-191]. The pathophysiological continuum of ALD is inextricably linked to ethanol-induced enteric dysbiosis, characterized by Bacteroidetes depletion and Proteobacteria expansion [192-194]. These microbial community perturbations potentiate gut barrier dysfunction, driving endotoxin translocation and hepatic inflammasome activation [195-197]. The dysbiosis of intestinal flora, in turn, induces intestinal barrier dysfunction and permits the transfer of live bacteria from the gut to the liver [198, 199]. Alcohol-associated liver injury has been shown to induce changes in the composition and phenotype of hepatic macrophages and a decrease in the number of CRIg+ KC. These changes have been demonstrated to impair the clearance of pathogenic bacteria. The detrimental effects of ethanol-induced CRIg suppression in KCs were effectively neutralized through soluble CRIg-Ig supplementation, demonstrating hepatoprotective benefits in murine models of alcohol-related hepatic injury. Alcohol has been shown to reduce CRIg expression in the liver by altering the composition and phenotype of hepatic macrophages, thereby compromising the hepatic firewall [200]. This compromised CRIg expression directly impairs hepatic clearance mechanisms for gut-derived pathogens, establishing a pathophysiological link to aggravated hepatic pathologies ranging from terminal hepatic conditions (such as cirrhotic degeneration) to elevated risks of disseminated infections in alcohol-associated hepatitis. These cascading effects position CRIg modulation as a novel therapeutic frontier in managing ethanol-related hepatic disorders [201, 202] (Figure 5).
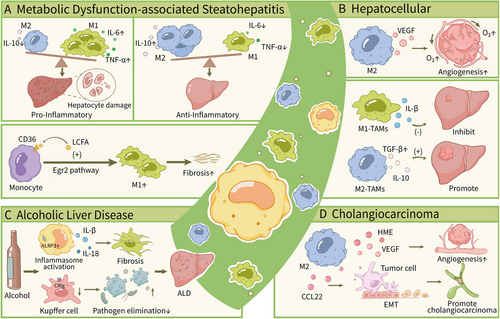
5.4.3 Metabolic Dysfunction-Associated Steatohepatitis
Formerly classified as NAFLD, the newly defined MASLD diagnostic entity describes hepatic disorders characterized by aberrant intracellular lipid accumulation [203]. The disease continuum progresses from benign steatotic manifestations through inflammatory MASH phases toward irreversible fibrotic damage [204]. Despite established correlations between fibrotic burden and clinical outcomes in MASLD [205], the fundamental mechanisms mediating disease advancement persist as unresolved scientific questions, creating critical barriers to developing effective antifibrotic MASH interventions [206]. In light of these observations, macrophages have been shown to exhibit both proinflammatory and antiinflammatory functions in the context of MASH [207, 208]. Specifically, m1-type macrophages have been observed to secrete cytokines such as TNF-α and IL-6, which have been implicated in the induction of hepatocellular injury and fibrosis. Conversely, M2-polarized macrophages demonstrate antiinflammatory potential via IL-10 secretion, concurrently facilitating tissue regeneration through cytokine-mediated pathways. Within hepatic fibrogenesis, hepatic stellate cells (HSC) and their transdifferentiated myofibroblast derivatives serve as central mediators. Contemporary research reveals that macrophage-mediated regulation of HSC activation states constitutes a critical immunofibrogenic axis driving ECM deposition [209].
A defining pathological hallmark of MASH involves extensive infiltration of circulatory monocytes that undergo terminal differentiation into macrophage populations, ultimately displacing embryonically-derived KCs [210]. These infiltrating myeloid cells, classified as scar-associated macrophages (SAMs) [211, 212], exhibit phenotypic parallels with lipid-encapsulating macrophages (LAMs) observed encircling necrotic adipocytes in obese adipose depots, warranting their designation as hepatic LAMs [212]. Mechanistic studies confirm SAMs mediate cellular crosstalk with stromal constituents, directly propelling MASH-to-fibrosis transition across species [212, 213]. Crucially, the zinc-finger transcription factor Egr2 governs monocyte-to-hLAM differentiation in MASH microenvironments. Pathological accumulation of saturated long-chain fatty acids (LCFAs) activates macrophage Egr2 signaling, a metabolic reprogramming process suppressed by unsaturated LCFA counterparts. Pharmacological modulation of this monocyte-specific saturated LCFA–Egr2 pathway consequently represents a rational therapeutic strategy for MASH intervention [214].
XBP1 expression was found to be significantly increased in liver samples from MASH patients. In a mouse model, the deletion of hepatocyte-specific Xbp1 in subjects fed a high-fat or methionine/choline-deficient diet resulted in a substantial inhibition of steatohepatitis development [215]. Furthermore, the study observed that macrophage-specific Xbp1 knockout mice exhibited reduced sensitivity to high-fat and methionine/choline-deficient diets in comparison with wild-type Xbp1FL/FL mice. Notably, the study observed an anti-inflammatory M2 polarization in the macrophages of Xbp1-deficient mice. The severity of steatohepatitis was found to be reduced in Xbp1-deficient macrophages through decreased NLRP3 expression and proinflammatory cytokine secretion [215]. Furthermore, the severity of steatohepatitis was also reduced in Xbp1-deficient Nlrp3 knockout mice compared with wild-type Nlrp3FL/FL mice. Furthermore, the study observed that xbp1-deficient macrophages inhibited hepatic stellate cell activation by decreasing TGF-β1 expression, leading to a reduced incidence of fibrotic changes in macrophage-specific Xbp1 knockout mice compared with wild-type Xbp1FL/FL mice.
5.4.4 Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC) represents the most prevalent form of primary liver cancer, constituting between 75 and 85% of cases. It is marked by a high morbidity and mortality rate, with the majority of patients encountering a lack of surgical candidates due to delayed diagnosis and treatment facing challenges such as an immunosuppressive microenvironment and drug resistance [216]. Within the TME of HCC, TAMs function as pivotal immunomodulators, orchestrating disease progression through polarization (M1/M2 phenotypic switch) and intricate intercellular interactions [217].
In the context of HCC, M1 has been shown to impede tumor progression through a variety of mechanisms [218-220]. Current research endeavors have centered on the regulation of genes or proteins capable of inducing macrophage polarization or infiltration. For instance, stromal cell protein spondin2 (SPON2) has been shown to promote M1-like macrophage infiltration by activating RhoA and Rac1 and increasing F-actin reorganization through SPON2-α4β1 integrin signaling [221]. Conversely, high expression of sirtuin1 (SIRT1) in HCC cells has been observed to regulate M1 polarization through the NF-κB pathway [222]. Furthermore, elevated expression of retinoic acid-inducible gene I (RIG-I) has been shown to induce apoptosis in HCC by promoting M1 polarization in mouse peritoneal macrophages via the RIG-I/MAVS/TRAF2/NF-κB pathway [223]. IL-12-hyperexpressing monocytes exhibit suppressed phosphorylation of STAT3 (p-STAT3) and diminished c-Myc signaling activity, a molecular reprogramming that drives macrophage polarization toward proinflammatory M1 states while exerting tumor-suppressive effects on HCC progression [224]. Furthermore, activated (M2) macrophages have been observed to secrete the cytokine CCL22, which has been implicated in enhancing tumor invasion and inducing EMT through Smad2/3 and Smad1/5/8 activation and Snail upregulation [225]. Furthermore, CCL17, another cytokine secreted by M2 macrophages, has been shown to be closely associated with tumor stemness and EMT in the TGFβ1 and Wnt/β-catenin signaling pathways [226].
5.4.5 Cholangiocarcinoma
Cholangiocarcinoma (CCA) is a highly aggressive malignant neoplasm that originates from the epithelium of the bile ducts. CCA is characterized by difficulties in early diagnosis, poor prognosis, and limited therapeutic options. The M2-polarized TAM population in TME demonstrates bifunctional capacity: First, through immunosuppressive cytokine secretion (notably IL-10/TGF-β), they establish an immune-evasive milieu; second, via sustained production of proangiogenic mediators (VEGF) and MMPs, they actively potentiate CCA growth, local infiltration, and distant metastasis. Furthermore, the interaction between TAM and CCA cells can form an immunosuppressive microenvironment, thereby weakening the efficacy of chemotherapy and immunotherapy. Consequently, targeting the polarization state or signaling pathway of TAM has emerged as a novel strategy for CCA treatment.
Experimental evidence reveals M2-polarized TAMs remodel the immune landscape of intrahepatic CCA (iCCA) through multifactorial mechanisms: cytokine release (including TNF-α, ICAM-1, IL-6) and cancer cell EMT induction [227]. Complementary in vivo studies demonstrate iCCA-derived factors accelerate M2 differentiation of human monocytic leukemia-derived THP-1 cells, with resultant IL-10-secreting macrophages potentiating HCC proliferation, metastatic propensity, and mesenchymal transition cascades [228]. Macrophage population and phenotype are positively correlated with angiogenesis and clinical prognosis in HCC. CD14 + CD16 + monocytes from CCA patients express high levels of angiogenic factor-related genes (epithelial regulatory proteins, VEGF-A and CXCL3) and predict tissue invasiveness in iCCA [229]. The angiogenic cascade in neoplasms is mechanistically linked to macrophage-derived metalloelastase (HME) and VEGF signaling pathways. Intriguingly, M2-polarized TAMs generated through ICC-secreted cytokine stimulation exhibit dual proangiogenic effects: enhanced microvessel density and transcriptional upregulation of VEGF biosynthesis [230].
5.5 Cardiovascular Disease
Cardiovascular disease (CVD) is a complex group of disorders characterized by atherosclerosis, myocardial infarction (MI), and stroke, with core pathological mechanisms involving vascular endothelial damage, lipid deposition, chronic inflammation, and immune cell infiltration. Macrophages are the major immune cell type within atherosclerotic plaques. They play a dual role in CVD through phenotypic polarization. In particular, macrophages with a TAM-like phenotype (similar to M2 type) may promote tissue repair in atherosclerotic plaques by secreting antiinflammatory factors and stabilize plaques by inhibiting inflammation through phagocytosis of apoptotic cells. In contrast, M1-type macrophages release proinflammatory factors and MMPs, accelerating the risk of plaque rupture. Recent studies have focused on modulating macrophage polarization homeostasis or targeting their metabolic reprogramming (e.g., cholesterol metabolism, glycolysis) to inhibit plaque progression and stabilize the vascular microenvironment, providing new strategies for the treatment of CVD.
Angiopoietin-like proteins 3 (ANGPTL3) demonstrates potent atherogenesis-promoting activity through multitiered mechanisms. Akt pathway activation via phosphorylation mediates ANGPTL3-driven upregulation of TLR4, potentiating robust NF-κB nuclear translocation upon LPS challenge. This signaling cascade culminates in proinflammatory cytokine biosynthesis and M1-skewed polarization in human THP-1 macrophages. In vivo validation using ANGPTL3-overexpressing murine models reveals amplified TLR4-mediated plaque inflammation driving atherosclerotic lesion progression [231].
The mTOR signaling network serves as a multifunctional regulator of cellular homeostasis, coordinating processes including growth regulation, metabolic adaptation, and survival pathways [232]. While mTOR hyperactivation demonstrates pathological correlations with atherogenesis, its cell-specific contributions to plaque evolution and macrophage functionality remain mechanistically elusive. Paradoxically, whereas pharmacological mTORC1 inhibition confers vascular protection [233], genetic ablation of mTORC2 in macrophages precipitates exacerbated atherosclerotic manifestations characterized by complex lesion morphology and elevated apoptotic indices [234]. In vitro mechanistic studies reveal mTORC2-mediated suppression of FoxO1 transcriptional activity, effectively constraining inflammasome assembly and IL-1β production—central mediators of vascular inflammation. Importantly, pharmacological FoxO1 antagonism demonstrates therapeutic efficacy in mitigating inflammation driven by mTORC2 deficiency across experimental models. Notably, the collective deletion of macrophage mTOR disrupts mTORC1- and mTORC2-dependent pathways, resulting in minimal changes in plaque size or complexity, suggesting a balanced yet opposing role of these signaling arms [231].
25-Hydroxycholesterol (25-HC) is an oxidized product of cholesterol, produced by the enzyme cholesterol 25-hydroxylase. Classified within a family of bioactive oxysterols, 25-HC is endogenously synthesized by cells during cholesterol homeostasis dysregulation and immunostimulation [235]. Macrophage-derived 25-HC exacerbates atherosclerotic progression through dual mechanisms: autocrine/paracrine-mediated plaque destabilization and intraplaque smooth muscle cell migration impairment. Mechanistically, 25-HC potentiates proinflammatory activation in lipid-engorged macrophages while restricting vascular smooth muscle cell motility. Experimental investigations further reveal 5-HC modulates membrane cholesterol dynamics, disrupting TLR4 signaling architecture to amplify NF-κB-driven inflammatory transcriptomes and augment apoptotic susceptibility in lesional macrophages [236].
6 Potential Therapeutic Targets to Cure Liver Injury by Modulating Macrophages
An additional crucial function of macrophage polarization in liver injury, specific pathways, and chemokines within macrophages significantly impacts the progression of NAFLD, MASH, ALF, and acute-on-chronic liver failure (ACLF). The following therapeutic targets may be explored for the modulation of macrophages to facilitate the treatment of liver injury (Table 2).
| Targets | Names | Effects related to MASH or distinguishing features | Clinical trial stage | References |
|---|---|---|---|---|
| CCR2/CCR5 | Cenicriviroc | Antifibrotic effects in animal models, potential fibrosis regression | III | [307] |
| Gemcabene | Anti-inflammation, decrease LDL-C, CRP, IL-6, IL-1 levels, significant reduction in fibrosis progression | III | [308, 309] | |
| PPAR | Lanifibranor | Improve liver enzymes, with more frequent diarrhea, nausea, anemia, etc. than placebo group | III | [310-312] |
| Saroglitazar | Improve ALT and LFC, improvement in insulin resistance and atherogenic dyslipidemia | Launched | [313, 314] | |
| MBX 8025 | Adverse effects: more transaminase elevation at higher doses | II | [315] | |
| PXL065 | Improve adiponectin level, with no peripheral oedema or anemia | IV | [316] | |
| Pioglitazone | Improve insulin resistance and lipid metabolism | II | [317] | |
| GLP-1R | Semaglutide | No significant difference in patients with improvement in fibrosis stage, great improvements in liver biochemistry and steatosis combined with semaglutide and firsocostat | III | [318, 319] |
| Liraglutide | Enhance insulin secretion, inhibit glucagon secretion anti-inflammatory and antioxidant | III | [320] | |
| FXR | Obeticholic acid | Fibrosis improvement, with pruritus more frequently | III | [321] |
| Cilofexor | Great improvements in liver biochemistry and steatosis combined with semaglutide and firsocostat | II | [318] | |
| Tropifexor | Sustained decreases in ALT and HFF, with pruritus more frequently | II | [322] | |
| Galectin-3 | Belapectin | Safe but no significant reduction in fibrosis | II | [323] |
| THR-β | Resmetirom | Significant reduction in hepatic fat, expedited approval from the US FDA | III | [324] |
| FGF21 | Pegozafermin | Clinically meaningful reductions in liver fat, measures of liver function, and circulating lipids | II | [325, 326] |
| Efruxifermin | Reduce HFF and liver injury markers, with longer-duration studies warranted | II | [327] | |
| SCD | Aramchol | The primary endpoint of a reduction in liver fat did not meet the prespecified significance level with Aramchol 600 mg | III | [328] |
| ASK1 | Selonsertib | No significant effect of liver biomarkers | III | [215] |
| ACC | Firsocostat | Great improvements in liver biochemistry and steatosis combined with semaglutide and firsocostat | II | [318] |
| Clesacostat | The first clinical research to assess histological endpoints using diacylglycerol acyltransferase 2 inhibitor (DGAT2i) and DGAT2i + acetyl-coenzyme A carboxylase (ACC) inhibitor | II | [238, 329] | |
| DGAT-2 | Ervogasta | [238] |
6.1 CCL2, CCR2/5 Antagonist
In MASLD, macrophages are recognized as playing a critical role, with elevated periportal macrophages serving as an early histological indicator. Specifically, periportal CCR2+ inflammatory macrophages accumulate in the periportal areas of patients with MASH, which correlates with the severity of the disorder and the presence of fibrosis [237]. Upon activation, macrophages develop secretory competence, enabling the production of a diverse array of immunomodulatory molecules. Notably, these include chemokines such as CCL2 (MCP-1), CCL3 (MIP-1α), and CXCL8 (IL-8), as well as proinflammatory cytokines like IL-1β, IL-6, and TNF-α. This multifaceted repertoire of mediators plays a crucial role in orchestrating the pathological inflammatory cascades that underlie disease pathogenesis.
A notable trend of upregulation in chemokines and their receptors has been identified in patients diagnosed with MASH. This trend encompasses the upregulation of CCL3-5/CCR-5, as well as the chemokine CCL2. Furthermore, hepatic expression of additional cytokines, such as CD44 and CD62E (E-Selectin), has been significantly elevated in MASH patients, indicating their involvement in leukocyte recruitment to inflammatory sites. CD44 interacts with components of the ECM, including osteopontin, to regulate macrophage recruitment to the liver [238]. Additionally, it plays a role in macrophage activation triggered by DAMPs, PAMPs, and saturated fatty acids. Given that the CCL2/CCR2 axis is crucial for recruitment of inflammatory monocytes to the injured liver and for driving the progression of hepatic fibrosis, various studies have explored strategies to mitigate MASH-induced liver injury by blocking this axis. The administration of CCL2 inhibitors in mouse models of steatohepatitis induced by a methionine–choline-deficient diet and chronic liver injury (CCl4-induced) has demonstrated a reduction in monocyte/macrophage infiltration and an improvement in MASH progression [239].
Cenicriviroc (CVC), an orally bioavailable dual chemokine receptor antagonist targeting CCR2/CCR5 with optimal pharmacokinetic properties, was originally developed for HIV management [240] through suppression of Ly6C+ monocyte infiltration, CVC depletes hepatic macrophage reservoirs, consequently attenuating fibrotic progression in chronic liver diseases [241]. Clinical validation across randomized placebo-controlled studies and phase IIb trials confirms its therapeutic efficacy in fibrosis, demonstrating acceptable tolerability and sustained hepatic benefits.
Previous studies have investigated the function of CCR2 in concanavalin A-induced immune liver damage with a particular focus on the dual CCR2/CCR5 antagonist, CVC. Demonstrated to be safe in clinical trials and effective in various animal disease models, CVC was evaluated for its effects on liver macrophage populations. Flow cytometry analysis revealed that CVC treatment significantly reduced the overall proportion of liver macrophages in Con A-administered mice, specifically decreasing the levels of MoMFs while leaving KCs unaffected. CVC intervention in Con A-challenged murine models elicited marked depletion of hepatic proinflammatory CX3CR1+CCR2+Ly6C+ macrophage subpopulations. Complementary immunohistochemical quantification revealed attenuated CCR2+ monocyte accumulation in therapeutic cohorts. These congruent findings establish CVC's dual mechanism, suppression of CCR2+ monocyte activation, and blockade of hepatic leukocyte trafficking. The therapeutic efficacy of CVC was also assessed through the measurement of serum transaminase levels and the evaluation of liver tissue necrosis, both of which demonstrated a positive response, indicating that CVC significantly mitigates liver injury induced by Con A. Complementary experimental evidence from TUNEL assays and ROS histochemical detection revealed CVC-mediated attenuation of hepatocellular apoptosis and oxidative damage in Con A-challenged murine livers. Extended pharmacological investigations validate CVC's multifunctional hepatoprotective profile, demonstrating antifibrotic efficacy and steatosis suppression across diverse hepatic pathologies [242, 243]. Overall, these findings validate CVC's pharmacological promise in mitigating Con A-triggered immune-mediated hepatic injury.
In murine studies, pharmacological targeting of the CCL2–CCR2 pathway has been shown to reduce monocyte infiltration and the accumulation of MoMF in the injured liver. Consequently, the use of CCL2 inhibitors, in conjunction with CCR2/5 antagonists, has the potential to enhance fibrosis outcomes by diminishing monocyte infiltration and modifying hepatic macrophage subsets. The CCR2/5 pathway has emerged as a prominent goal in the treatment of MASH [244].
6.2 PPAR Agonist
PPARs are ligand-activated core transcription factors that adjust adipocyte disintegration, lipid storage, and insulin sensitivity. This transcription factor facilitates hepatic lipogenesis and steatosis, promotes fatty acid absorption and triglyceride synthesis in hepatocytes, and enhances the secretion of adipokines via SREBP-1c [245]. Conversely, PPAR-γ also protects the liver by inhibiting the proinflammatory polarization of macrophages, thereby reducing oxidative stress and reversing the activation of HSCs [246, 247].
The inactivation of PPAR expression results in the activation of HSCs, an inflammatory response, and increased lipogenesis; conversely, the activation of PPARs can prevent and suppress liver fibrosis. Notably, targeted therapies aimed at activating PPAR expression have demonstrated potential efficacy in the treatment of hepatic diseases associated with liver fibrosis [248].
Multiple synthetic PPAR-α agonists have undergone rigorous clinical assessment. Notably, fenofibrate exhibited therapeutic efficacy in ameliorating histopathological features within experimental murine steatohepatitis models [249]. Concurrently, bezafibrate administration demonstrated dual pharmacodynamic effects—modulating lipometabolic pathways and restoring hepatic architecture in monosodium glutamate-induced rodent models substantiating its therapeutic potential for MASLD management [250].
Certain synthetic PPAR-γ agonists have been shown to mitigate liver inflammation and activate HSCs. Pioglitazone, in particular, has the potential to improve hepatic steatosis, inflammation, and fibrosis in MASLD by downregulating platelet-derived growth factor and TIMP-2 [251]. Pioglitazone demonstrates geroprotective potential with therapeutic implications for senescent hepatic fibrogenesis [251]. Mechanistic parallels emerge for rosiglitazone, which ameliorates acetaminophen-induced acute hepatotoxicity while attenuating diet/age-driven hepatic lipid accumulation and MASH progression [252, 253]. Notably, the PPARβ/δ agonist KD3010 exerts ECM remodeling inhibition in experimental fibrosis models (CCL4 cholestatic), achieved through dual suppression of HSC activation and inflammatory cascade potentiation [254]. L-165041, a synthetic PPAR-β/δ ligand, has also been shown to prevent hepatic steatosis by ameliorating liver inflammation and lipid accumulation in mice subjected to a Western diet [255]. Consequently, there is a pressing need to explore additional PPAR-β/δ agonists for the treatment of liver diseases. Experimental evidence of Wy-14,643-mediated oxidative damage attenuation implicates PPARα-regulated genomic targets that modulate hepatic redox homeostasis and/or sustain mitochondrial β-oxidative flux as critical mediators of APAP hepatoprotection [255]. Further support for the hypothesis that Wy-14,643 mediates protection against ischemia–reperfusion injury (IRI) in fatty livers through the attenuation of the inflammatory response has been provided by studies examining adhesion molecule expression.
Research has revealed a previously unrecognized hepatoprotective mechanism mediated by PPAR-α during acetaminophen-induced liver injury. In this experimental paradigm, prophylactic treatment with the synthetic PPAR-α activator Wy-14,643 effectively prevented all signs of hepatic injury in genetically unmodified mice following administration of a hepatotoxic APAP dosage. The safeguarding effect was confirmed through multiple parameters: preserved macroscopic hepatic appearance, microscopic evaluation of hematoxylin–eosin-stained tissue samples showing absence of notable pathological alterations, and substantially reduced plasma concentrations of hepatocellular enzymes (AST/ALT). Furthermore, in the context of IRI, pretreatment with Wy-14,643 demonstrated protective effects in both MASH and steatosis models, leading to a significant reduction in ALT levels and areas of hepatocellular necrosis [256, 257].
6.3 Glucagon-Like Peptide-1 Receptor Agonists
Gut-derived hormones are currently utilized in the clinical management of metabolic diseases, exemplified by glucagon-like peptide-1 receptor (GLP-1R) agonists such as semaglutide and liraglutide, as well as GLP-1/GIP (glucose-dependent insulinotropic polypeptide) dual agonists like tirzepatide. Additionally, GLP-1/glucagon dual agonists, including cotadutide, are under advanced clinical development [258, 259]. Their influence on liver inflammation and fibrosis is primarily considered to be indirect, potentially through mechanisms such as reduced energy supply and improved hepatocyte metabolism. Sort of evidence proposes that hepatic KCs may possess functional GLP-1 receptors, implying possible susceptibility to pharmacological activation through GLP-1R agonists [260]. Of particular clinical relevance, therapeutic targeting of this pathway has demonstrated efficacy in achieving histological improvement of MASH in phase II clinical trials [261], findings that have stimulated expanded clinical evaluation of this molecular mechanism in subsequent large-scale investigations. Furthermore, tirzepatide exhibits a pronounced dose-dependent effect on body weight reduction, particularly when compared with semaglutide and dulaglutide [262]. Given this body of evidence, certain GLP-1R agonists and tirzepatide are emerging as promising therapeutic options for MASLD and MASH, especially in individuals with concurrent type 2 diabetes mellitus or obesity [263, 264].
The GLP-1R demonstrates extensive expression throughout multiple anatomical systems, spanning pancreatic tissue, central nervous networks, gastrointestinal components, cardiovascular structures, hepatic parenchyma, adipose deposits, and musculoskeletal elements [265]. Binding of GLP-1 to its receptor stimulates insulin secretion while inhibiting glucagon secretion, leading to improved glucose metabolism and lower blood glucose levels. Furthermore, GLP-1R plays a role in regulating gastric emptying, gastric acid secretion, and gastric motility; it also suppresses food and water intake, thereby contributing to weight loss. Recently, GLP-1R agonists and GLP-1R/glucagon receptor (GCGR) dual-target agonists have demonstrated efficacy in the treatment of MASH [266].
Studies have evaluated the potential beneficial effects of GLP-1R and GCGR dual agonists on liver fibrosis in rodent models. These dual-target agonists have been shown to improve liver conditions in rats and mice with CCl4-induced hepatic fibrosis. The underlying mechanism involves the inhibition of TGF-β and the downstream Smad signaling pathways, which ultimately contribute to the reversal of hepatic fibrosis [266]. Furthermore, the histological features of MASH, such as steatosis and ballooning, have also been found to improve with the administration of GLP-1R agonists, including liraglutide and semaglutide [262]. Experimental evidence illustrates liraglutide's capacity to regulate immune cell differentiation patterns. Demonstrated through preclinical models, the GLP-1R agonist lixisenatide ameliorates atherosclerotic pathology in insulin-resistant murine subjects through phenotypic alteration of macrophages [267]. Research by Bruen et al. has documented liraglutide's dual capacity to direct monocyte-derived cell phenotypes while influencing cellular differentiation toward M2-resolution specialized macrophages [268. Complementing these findings, in vitro investigations have revealed liraglutide administration induces antiinflammatory polarization of KCs [269].
There is emerging evidence suggesting that GLP-1RAs may confer benefits in liver fibrosis associated with MASLD. While the precise mechanisms by which GLP-1RAs may reduce liver fibrosis remain unclear, several experimental studies indicate that these agents could modulate ECM homeostasis. This regulatory mechanism appears to be mediated via dual suppression of oxidative stress mediators and downregulation of MAPK cascade activity, thereby impeding the assembly of BATF/JUN transcriptional complexes involving basic leucine zipper ATF-like transcription factors [270]. However, it remains uncertain whether this mechanism is applicable to human livers [271]. A recent systematic review and network meta-analysis highlighted that semaglutide, liraglutide, and the combination of vitamin E with pioglitazone exhibited the highest probability of ranking as the most effective interventions for achieving resolution of MASH [272].
6.4 Thyroid Hormone Receptor-Beta Agonist
As previously mentioned, PPARs, liver X receptors, FXRs, and thyroid hormone receptors (THRs) are recognized as prominent therapeutic target receptors. While FXRs have been extensively studied, this review will primarily focus on THRs, with a particular emphasis on THR-β. THR-β is predominantly expressed in the liver, and THR-β agonists have shown efficacy in reducing LDL levels, TG, and hepatic steatosis in humans. Additionally, THR-β facilitates fatty acid catabolism and stimulates mitochondrial biogenesis, resulting in decreased lipotoxicity and enhanced liver function, which ultimately leads to a reduction in liver fat [273].
Resmetirom (MGL-3196) represents a novel investigational orally bioavailable selective THR-β agonist with pioneering therapeutic potential [274]. Data from mechanistic studies implicate macrophage-mediated processes in its anti-MASH activity [275]. Practically positioned downstream of PI3K/AKT signaling, the NF-κB cascade constitutes a principal regulatory axis for inflammatory mediators, whose aberrant activation is fundamentally linked to MASH pathogenesis. Concurrently, STAT3 stimulation exerts context-dependent immunomodulatory effects, demonstrating dual functionality in both fibrogenic progression and inflammatory modulation during MASH evolution [274]. Through Gene Ontology and Kyoto Encyclopedia of Genes and Genomes enrichment analyses conducted in both the resmetirom and control groups, Wang et al. [276] suggest that resmetirom has the potential to ameliorate MASH by restoring the expression of RGS5, which subsequently deactivates the STAT3 and NF-κB signaling pathways.
6.5 Fibroblast Growth Factor 21
Fibroblast growth factor 21 (FGF21) is a member of the FGF superfamily and serves as an endocrine factor closely related to FGF19 and FGF23, which are secreted by the liver to regulate the intake of simple sugars and the preference for sweet foods [277]. This regulatory function is mediated through signaling via FGF21 receptors located in the paraventricular nucleus of the hypothalamus. FGF21 promotes thermogenesis through endocrine signaling to adipose tissue, which helps prevent adipose tissue dysfunction and reduces lipid spillage into the liver [278]. Additionally, it enhances fatty acid oxidation and cholesterol clearance through autocrine signaling to the liver, thereby mitigating hepatic lipotoxicity [279]. Furthermore, FGF21 inhibits the activation of KCs, monocyte recruitment, and the formation of LAMs and SAMs, which may impede collagen accumulation and reduce hepatic fibrosis. Recent studies have demonstrated that FGF21 can diminish alcohol preference and cravings, leading to a reduction in alcoholic liver injury [280]. Consequently, FGF21 represents a promising target for mitigating alcohol-induced hepatic damage.
During the development of MASH, evidence suggests that FGF21 inhibits the activation of KCs and MoMFs. This inhibition prevents the accumulation of CD36high KCs and CD36highCD9high MoMFs under dietary conditions that promote MASH. These findings indicate that FGF21 reduces LAMs and SAMs, thereby inhibiting the synthesis of collagen type I alpha 1 and preventing fibrogenesis [281].
Distinct from canonical fibroblast growth factors, FGF21 exhibits absent heparin affinity, enabling systemic circulation as an endocrine mediator. This pleiotropic hormone exerts profound regulatory effects on whole-body metabolic homeostasis while demonstrating hepatoprotective capacities [282]. Preclinical and clinical investigations collectively validate FGF21's cytoprotective function in hepatic systems [283]. Experimental models employing FGF21-deficient rodents fed lipotoxic diets display aggravated hepatocellular injury, a phenotype rescued through exogenous FGF21 administration [284, 285]. Mechanistic studies further reveal FGF21's capacity to attenuate carbon tetrachloride-induced acute hepatic damage via SIRT1-dependent autophagic induction [285]. Clinical observations demonstrate significantly elevated circulating FGF21 concentrations in ACLF patients, showing positive correlations with hepatic injury biomarkers including CRP and transaminases. Particularly, FGF21 transcription is regulated by CHOP—a terminal effector of endoplasmic reticulum (ER) stress cascades [286]. Paradoxically, increased FGF21 expression reciprocally inhibits the eIF2α–ATF4–CHOP signaling axis, creating negative feedback to mitigate ER stress-mediated hepatocyte injury [281]. Beyond ER stress modulation, oxidative challenges induce dramatic upregulation of both hepatic FGF21 production and systemic secretion. This adaptive response potentially represents the liver's endogenous mechanism to bolster antioxidant defenses and counteract toxic insults [287]. Moreover, clinical evidence indicates diverse changes in serum FGF21 levels among HBV-infected patients under varying clinical conditions. Serum levels of FGF21 were positively correlated with serum aminotransferases, suggesting that FGF21 may serve as an indicator of liver damage associated with HBV infection. CHB patients demonstrate diminished circulating FGF21 concentrations, particularly pronounced in cases of HBV-induced cirrhotic complications, a phenomenon potentially reflecting impaired hepatic biosynthetic capabilities. Conversely, the paradoxical FGF21 surge detected in CHB subjects progressing to HCC suggests diagnostic value for this hepatokine in tracking malignant transformation within this clinical cohort [288].
The intrinsic elevation of FGF21 across multiple pathological states positions this hepatokine as a compelling candidate for emerging as a multipurpose diagnostic and prognostic biomarker. Clinical investigations revealed a 25-fold surge in circulating FGF21 concentrations within 2 h posttransplantation, whereas maximal ALT/AST concentrations emerged at 24-h postprocedural intervals [289]. Temporal trajectory analysis identified concordance between 2-h FGF21 peaks and subsequent 24-h transaminase elevations, establishing its potential as an early-warning indicator with high predictive accuracy for IRI in transplant recipients [290]. While further mechanistic investigations and population-specific validation remain prerequisites for clinical implementation, this emerging pleiotropic hormone exhibits substantial therapeutic potential for both managing and monitoring diverse metabolic pathologies.
The therapeutic potential of natural FGF21 is constrained by its short half-life of approximately 2 h. Consequently, researchers are actively developing a long-acting glycosylated and polyglycolated FGF21 analogue known as pegozafermin, which exhibits an extended in vivo half-life. This analogue demonstrates promise for the treatment of MASH and severe hypertriglyceridemia. In clinical trials, pegozafermin successfully achieved both primary endpoints, indicating potential efficacy in treating MASH. Notably, all three dosage groups (15 mg or 30 mg weekly, or 44 mg biweekly) exhibited one phase of improvement in defibrase, without exacerbation of MASH or worsening of fibrosis, and did so without significant side effects [279, 291].
6.6 Galectin-3 Antagonist
Galectin-3 (Gal - 3), a protein of significant importance, is instrumental in the pathogenesis of MASH and fibrosis, as well as in various fibrotic conditions affecting the lungs, heart, kidneys, liver, and vascular system. It interacts with receptors such as TLR4, TGF - β, dectin-1, and TREM2, leading to a range of intracellular effects. Within cells, Galectin-3 is associated with AKT phosphorylation, transcriptional regulation, Wnt/β-catenin signaling, and the regulation of apoptosis. Extracellularly, it plays a role in inflammation, cell proliferation, differentiation, and migration. Additionally, Galectin-3 promotes morphogenesis in endothelial cells and facilitates tumor angiogenesis [292].
As an upstream factor of TREM2, Gal-3 act a pivotal part in modulating macrophage polarization, specifically reducing M1 polarization while promoting M2 polarization [293]. Additionally, Gal-3 activates NLRP3 inflammatory vesicles by interacting with TLR4 in the liver through its carbohydrate-recognition-binding domains, thereby facilitating the development and expression of inflammatory cells. This research finds a significant association between Gal-3 and progression of chronic MASH in mice subjected to a high-fat diet [294].
Accumulating research identifies Gal-3 as a pathogenic factor in hepatic fibrogenesis, where pharmacological blockade demonstrates therapeutic efficacy against fibrotic resolution [295, 296]. The multifactorial pathogenesis of tissue fibrogenesis involves intricate crosstalk between immunocytes and activated myofibroblasts, with Gal-3 emerging as a pivotal molecular bridge [294]. Functioning as a pleiotropic modulator, Gal-3 orchestrates cellular responses in both stromal (myofibroblasts) and innate immune compartments (macrophages), critically influencing fibrotic progression. Models of carbon tetrachloride-induced hepatic fibrosis reveal that genetic ablation of Gal-3 suppresses stellate cell transdifferentiation and collagen biosynthesis, demonstrating impaired TGF-β responsiveness in Gal-3-deficient cells compared with wild-type counterparts. Mechanistically, Gal-3 deficiency disrupts TGF-β receptor membrane localization while attenuating β-catenin phosphorylation and nuclear trafficking, though Smad2/3 activation remains unaffected. These findings establish Gal-3's indispensable role in TGF-β-driven ECM remodeling through noncanonical signaling pathways. Specifically, Gal-3 mediates IL-4-dependent macrophage polarization toward profibrotic phenotypes [297], with IL-4/IL-13-stimulated macrophages amplifying fibrogenic gene networks that perpetuate collagen deposition and disease advancement. This molecular triad positions Gal-3 as a critical nexus integrating macrophage–fibroblast interactions within the fibrotic microenvironment. A deeper understanding of the mechanisms regulating tissue fibrosis, along with targeted strategies to inhibit Gal-3 in the liver, provides a compelling rationale for developing novel therapeutic approaches for patients suffering from liver fibrosis.
Galectin therapeutics has engineered GR-MD-02, a modified pectic polysaccharide derived from Malus domestica, through synthetic modification processes. This galactoarabino–rhamnogalacturonan polymer contains a heterogeneous sugar composition dominated by galacturonic acid, galactose, arabinose, and rhamnose residues. Preclinical analyses demonstrate its superior binding specificity for Gal-3 over related galectin isoforms. Initial clinical evaluation in a 2015 Phase 1 trial (NCT01899859) involving 31 MASH patients with progressive fibrosis demonstrated acceptable safety profiles and biological activity. Subsequent Phase 2 investigations comprised two parallel cohorts: the MASH FX study (NCT02421094, n = 30) enrolling subjects with significant fibrosis (F3) and the larger MASH CX trial (NCT02462967, n = 162) focusing on cirrhotic patients. Despite these extensive evaluations, GR-MD-02 failed to achieve statistically significant improvements in the primary histological endpoint of fibrosis regression through quantitative MRI-based assessment (LiverMultiSca). Secondary biomarkers including magnetic resonance elastography and vibration-controlled transient elastography (FibroScan) measurements similarly showed no clinically meaningful alterations. The pleiotropic involvement of Gal-3 in chronic inflammatory pathologies has solidified its status as a viable therapeutic target across multiple disease states. This molecular rationale has stimulated substantial commercial investment, with numerous biopharmaceutical entities pursuing diverse targeting approaches including small molecule inhibitors, monoclonal antibodies, and carbohydrate-based antagonists. These strategies include the design and synthesis of powerful small molecule antagonists (such as glycomimetics) and larger biologics derived from natural sources, all aimed at therapeutic intervention for various cancers, fibrosis, and other liver diseases [298]. Gal3-targeting therapies may be utilized either as standalone treatments or in combination with existing medications.
The pivotal involvement of Gal-3 in orchestrating inflammatory cascades and fibrotic remodeling across multiple organ systems has positioned its pharmacological targeting as a priority in novel therapeutic development. Preclinical investigations and emerging clinical evidence collectively substantiate the antifibrotic potential of Gal-3 blockade. In concanavalin A-challenged murine hepatitis models, prophylactic administration of the Gal-3 antagonist TD139 attenuated hepatic inflammatory responses through immunomodulatory mechanisms [299]. DAVANAT—a carbohydrate-based Gal-3 inhibitor demonstrated therapeutic efficacy in experimental cholangitis induced by Novosphingobium aromaticivorans [300] and dextran sulfate sodium-mediated colitis [301] via suppression of NLRP3 inflammasome activation and IL-1β downregulation in macrophage populations. Further supporting this therapeutic paradigm, the orally bioavailable Gal-3 inhibitor GB1211 exhibited dual antifibrotic activity in murine models of hepatotoxic (CCl4-induced) and pulmonary (bleomycin-induced) fibrosis through distinct pathway modulation [302]. Clinically, elevated Gal-3 expression correlates with hepatic decompensation risks, particularly in cirrhotic progression and chronic HBV infections. Patients with HBV-associated ACLF displaying Gal-3 promoter methylation exhibited accelerated disease trajectories characterized by reduced survival durations, elevated 3-month mortality indices, and higher MELD prognostic scores [303]. Moreover, given the involvement of Gal-3 in nearly all inflammatory processes activated during acute intravascular hemolysis, this molecule may represent a viable therapeutic target. Future research is essential to elucidate the precise role of Gal-3 in the development of acute and chronic tissue injuries caused by acute intravascular hemolysis and to further explore its previously demonstrated therapeutic effects in fibrotic diseases, thereby positioning Gal-3 as a potential therapeutic target in hemolysis-induced organ damage.
Furthermore, investigations have revealed temporal elevation of Gal-3 expression during initial hepatic insult phases following α-galactosylceramide administration, detected systemically in circulation and hepatic compartments. Immunohistochemical analyses identified Gal-3-expressing macrophages migrating to inflammatory foci with distinct aggregation patterns. These observations propose macrophage-derived Gal-3 as a promising diagnostic indicator for acute hepatocellular damage in histopathological evaluations [304].
Numerous medications are available for the management of liver inflammation. Inhibitors of apoptosis signal-regulating kinase 1 (ASK1) () have shown efficacy in halting disease progression, which encompasses inflammation, liver fibrosis, insulin resistance, and hepatic lipid accumulation [305, 306]. Additionally, agonists of the A3 adenosine receptor can induce apoptosis in inflammatory cells and inhibit the NF-κB signaling pathway. Below outlines the various drugs, their corresponding targets, and their clinical trial statuses (Table 2).
7 Conclusion and Prospectives
As indicated above, macrophages, a pivotal component of the immune system, fulfill a variety of functions. These include maintaining body homeostasis, regulating immune responses, and participating in numerous disease processes. The complexity and diversity of their signaling pathways determine the plasticity and functional diversity of macrophages in different microenvironments. A comprehensive examination of these pathways and their roles in health and disease will facilitate a more profound comprehension of their functions in physiological and pathological processes. This, in turn, will provide new targets and strategies for the treatment of various diseases.
Signaling pathways such as JAK–STAT, NF-κB, and PI3K–Akt–mTOR play a pivotal role in the regulation of macrophage polarization and function, influencing the proinflammatory (M1) and antiinflammatory (M2) phenotypic transitions of macrophages by regulating the activities of key transcription factors. For instance, the JAK–STAT pathway drives M1- and M2-type polarization through the phosphorylation of STAT1 and STAT6, respectively. The NF-κB pathway promotes M1-type proinflammatory responses and M2-type tissue repair functions through classical and nonclassical pathways, respectively. The PI3K–Akt–mTOR pathway affects macrophage energy acquisition and functional status through metabolic reprogramming.
Maladaptive polarization patterns and functional impairment of macrophages represent key pathophysiological hallmarks across various pathological conditions. In autoimmune disorders such as SLE and RA, disturbances in the homeostatic equilibrium of macrophages, specifically the M1/M2 ratio, drive pathogenic cascades by triggering excessive immune responses and resulting in parenchymal injury. In neurodegenerative contexts like AD, prolonged activation of microglial populations—macrophages residing in the CNS—perpetuates neuroinflammatory cascades through the continuous release of proinflammatory mediators. In TMEs, TAMs promote neoplastic progression and immune evasion via paracrine signaling of proinflammatory cytokines and angiogenic factors. Cardiovascular pathologies illustrate the role of macrophages in regulating atherosclerotic plaque dynamics, where the states of polarization critically influence lesion stability and pathological evolution.
Despite the relatively deep understanding of macrophage signaling pathways and their roles in disease, many questions remain to be further investigated, and future research needs to be focused on. With the development of new technologies, future studies can utilize these technologies to deeply resolve macrophage signaling pathways and their roles in disease. For example, single-cell sequencing technology can help us more precisely identify the heterogeneity and functional status of macrophages in different disease states. While advances in basic research have identified numerous therapeutic candidates through the elucidation of mechanisms, significant translational barriers remain in clinical implementation. Enhancing the continuity of translation between experimental models and human trials is vital for the advancement of precision treatment paradigms. Pharmacological agents that modulate key signaling nodes, such as the JAK–STAT and NF-κB pathways, have demonstrated preclinical efficacy across various disease models. However, these agents necessitate rigorous molecular optimization and multicenter validation to establish their clinical relevance.
Author Contributions
Yongquan Chi and Haipeng Jiang: writing—original draft; Yongquan Chi, Haipeng Jiang, Jianhua Rao, and Yongsheng Li: writing—review and editing; Yiyuan Yin, Xinyu Zhou, and Yiyouyou Shao: formal analysis and resources; Jianhua Rao and Yongsheng Li: conceptualization; Jianhua Rao: funding acquisition. All authors have read and approved the final manuscript.
Acknowledgments
Figure 2 was created with MedPeer.cn. The project was supported by “Bottleneck” Technical Breakthrough Talent Support Special Project of Jiangsu Province's “333 High-Level Talents,” Jiangsu Province Hospital (the First Affiliated Hospital with Nanjing Medical University), Clinical Capacity Enhancement Project (JSPH-MB-2023-9, JBGS202501), and the Innovation and Entrepreneurship Training Program for College Students of Nanjing Medical University (202410312048Z).
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors disclose no conflicts of interest.
Open Research
Data Availability Statement
No data were used for the research described in the article.




