Epithelial Atg5 Deficiency Intensifies Caspase-11 Activation, Fueling Extracellular mtDNA Release to Activate cGAS–STING–NLRP3 Axis in Macrophages During Pseudomonas Infection
Junyi Wang, Lei Zhang, and Yingying Liu have contributed equally to this work.
Funding: This work was supported by the National Institutes of Health Grant (R01 AI138203), the National Natural Science Foundation of China (82370022, 82200079, and 82300088), the Natural Science Foundation of Sichuan (2024NSFSC1524), the Chengdu High-Level Key Clinical Specialty Construction Project (ZX20201202020), and the Third People's Hospital of Chengdu Scientific Research Project (CSY-YN-03-2024-008, 2023PI01, 2023PI12, and 2023PI15). The UND Imaging Core, and Histology Core were supported by NIH P20 GM113123, U54GM128729, and UND SMHS funds. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abstract
Pseudomonas aeruginosa (P. aeruginosa) infections pose a significant threat to public health, underscoring the need for deeper insights into host cellular defenses. This study explores the critical role of autophagy-related protein 5 (ATG5) in lung epithelial cells during P. aeruginosa infection. Single-cell RNA transcriptomics revealed a pronounced enrichment of autophagy pathways in type II alveolar epithelial cells (AEC2). Using a conditional Atg5 knockout murine model, we demonstrated that ATG5 deficiency in AEC2 compromises survival, hampers bacterial clearance, and increases pathogen dissemination. Additionally, the loss of ATG5 exacerbated inflammatory responses, notably through the activation of the AKT/PI3K/NF-κB axis and pyroptosis, which culminated in severe lung injury and epithelial barrier disruption. Mechanistically, the absence of ATG5 disrupted mitophagy, leading to intensified mitochondrial damage. This exacerbated condition coupled with the activation of gasdermin D (GSDMD) by the noncanonical caspase-11, enhancing the release of mitochondrial DNA (mtDNA), which in turn activated cGAS–STING–NLRP3 signaling in macrophages. These findings highlight the essential role of ATG5 in modulating immune responses and suggest potential therapeutic targets for managing P. aeruginosa-induced pulmonary infections.
1 Introduction
Pseudomonas aeruginosa, an opportunistic Gram-negative bacterium, is notably prevalent in infections afflicting immunocompromised individuals, those with severe burns, or patients suffering from chronic pulmonary conditions such as cystic fibrosis and chronic obstructive pulmonary disease [1]. This pathogen is part of the ESKAPE group, which includes Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, P. aeruginosa, and Enterobacter. These pathogens are known for their multidrug resistance, positioning them as critical targets for novel therapeutic strategies [2]. The World Health Organization has prioritized carbapenem-resistant strains of P. aeruginosa for new drug development, underlining the urgent need for innovative interventions in clinical settings [3]. Therefore, a deeper understanding of host–pathogen interactions is crucial for developing strategies that leverage host defensive processes to prevent and treat bacterial infections effectively.
Autophagy, a fundamental cellular process, plays a crucial role in maintaining cellular integrity and energy homeostasis during stress conditions by degrading and recycling cytoplasmic components. This process involves the formation of autophagosomes, double-membraned vesicles that encapsulate and transport cellular debris to lysosomes. The initiation of canonical autophagy involves the fusion of FIP200- and autophagy-related protein (ATG) 16L1-positive vesicles, leading to the formation of a prophagophore. Two interconnected ubiquitin-like enzymatic cascades, involving ATG7, ATG3, and a complex comprising ATG12, ATG5, and ATG16L1, lipidate the mammalian paralogue of the ATG8 family, LC3-I, transforming it into LC3-II. The production of LC3-II is essential for the assembly, elongation, and closure of autophagosomes, illustrating the intricate molecular choreography of this process [4].
Pyroptosis, a form of programmed cell death, plays pivotal roles in cellular immune defense through its classical and nonclassical pathways. The classical pathway, triggered by caspase-1 activation via inflammasome complexes like NLRP3, detects microbial products or cellular stress, leading to the cleavage of gasdermin D (GSDMD). This cleavage results in pore formation in the cell membrane, cell lysis, and the release of proinflammatory cytokines, such as IL-1β and IL-18. In contrast, the nonclassical pathway involves caspase-11 (caspase-4/5 in humans), which also can cleave GSDMD to induce pyroptosis [5]. Concurrently, the cyclic GMP–AMP synthase (cGAS)–stimulator of interferon genes (STING pathway, activated by cytosolic DNA from pathogens or damaged mitochondria, triggers the production of type I interferons, enhancing antiviral and antibacterial defenses.
Recent studies have highlighted the pivotal role of autophagy in defending mammals against microbial threats. Notably, the depletion of key autophagic proteins, like ATG5, ATG16L1, or ATG7 in macrophages, not only enhances the growth of Mycobacterium tuberculosis but also increases host susceptibility [6]. The dynamic interplay between autophagy, inflammasomes, pyroptosis, and the cGAS-STING pathway is essential for orchestrating cellular responses to infection. This is particularly crucial in the lungs, a primary site of attack by pathogens like P. aeruginosa, which can trigger severe immune reactions. Previous research from our group demonstrated increased inflammasome activation and pyroptosis in P. aeruginosa-induced sepsis following Atg7 depletion in mice [7], raising significant questions about how autophagy modulates the frontline defenses in lung epithelial cells. This study delves into the critical role of ATG5 within lung epithelial cells, orchestrating a multifaceted defense against P. aeruginosa infection. By regulating inflammatory responses, maintaining mitochondrial homeostasis, controlling caspase-11 activation, and limiting the release of extracellular mitochondrial DNA (mtDNA), ATG5 enhances cellular resilience. These findings have deepened our understanding of the nuanced interplay between epithelial autophagy and host defense mechanisms, underscoring potential therapeutic strategies to modulate inflammation and enhance pathogen clearance in respiratory infections.
2 Results
2.1 Alveolar Epithelial Deficiency in Atg5 Increased Susceptibility to P. aeruginosa Infection
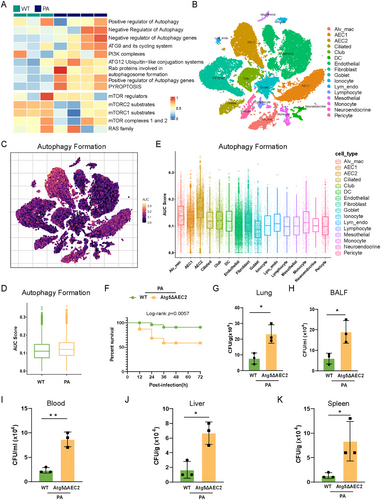
By analyzing our lung bulk RNA-seq dataset, comprising three wild-type (WT) mice and four P. aeruginosa-infected mice at 24 h postinfection, we uncovered a pronounced enrichment of autophagy-related pathways upon P. aeruginosa infection, as determined by single-sample gene set enrichment analysis (ssGSEA; Figure 1A). To delve into the enrichment of autophagy formation signature across distinct cell types, we employed our single nucleus RNA sequencing (SnRNA-seq) dataset derived from two WT mice and two P. aeruginosa-infected mice at 24 h postinfection (Figures 1B and S1). AUCell analysis revealed an escalated autophagy formation signature enrichment in P. aeruginosa-infected lungs compared to control lungs, with type II alveolar epithelial cells (AEC2) exhibiting the highest enrichment of this signature (Figure 1C–E). Through protein–protein interaction (PPI) analysis with the bulk RNA-seq dataset (Figure S2), we found that ATG5, a pivotal protein involved in the autophagy formation, plays a key role in the interaction network within P. aeruginosa-infected lungs. Therefore, we examined the role of ATG5 in AEC2 during P. aeruginosa infection, utilizing a mouse pneumonia model. PAO1 (1 × 107 CFU/mouse) was intranasally delivered into each of AEC2-specific Atg5 conditional knockout (Atg5ΔAEC2) mice and their littermate WT counterparts. Upon PAO1 infection, deficiency of Atg5 in AEC2 reduced the survival rate, resulting in a mortality rate of 45% for Atg5ΔAEC2 mice, while WT mice exhibited a mortality rate of 13% after 72 h (Figure 1F). Moreover, Atg5ΔAEC2 mice exhibited impaired bacterial clearance in both lungs and bronchoalveolar lavage fluid (BALF) compared to WT mice (Figure 1G,H). Notably, there was a marked increase in bacterial burdens in the blood, spleen, and liver of Atg5ΔAEC2 mice, indicating enhanced dissemination of P. aeruginosa (Figure 1I–K). Collectively, these findings underscore the indispensable role of ATG5 in epithelial cells for resistance to P. aeruginosa infection.
2.2 Alveolar Epithelial Deficiency in Atg5 Exacerbated the Inflammatory Response and Activation of the AKT/PI3K/NF-κB Axis in Lungs During P. aeruginosa Infection
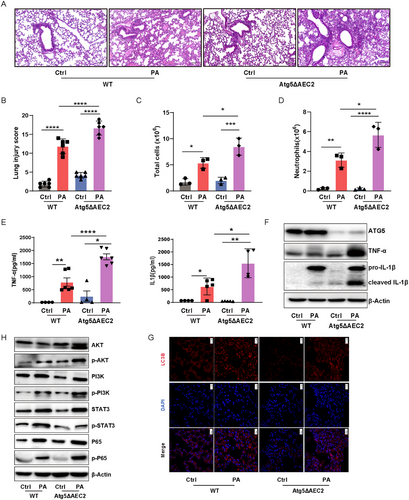
To gain deeper insight into the lethality associated with P. aeruginosa infection in Atg5ΔAEC2 mice, lung samples were stained with hematoxylin and eosin staining (H&E) and scored in a blinded manner. The assessment revealed more severe lung injuries and heightened infiltration of inflammatory cells in Atg5ΔAEC2 mice in comparison to their WT counterparts post P. aeruginosa infection (Figure 2A,B). Confirmation of increased total cells in BALF, notably neutrophils, further emphasized the heightened inflammatory state in Atg5ΔAEC2 mice following exposure to PAO1 (Figure 2C,D). Given the pivotal role of cytokines in acute lung injury, we measured the levels of proinflammatory factors TNF-α and IL-1β, which were found to be significantly elevated in BALF from Atg5ΔAEC2 mice compared to WT mice postinfection (Figure 2E). Western blotting validation underscored that the absence of Atg5 and autophagy in AEC2 aggravated the production of TNF-α and IL-1β in the lungs during P. aeruginosa infection (Figures 2F,G and S3). Furthermore, immunoblotting revealed heightened activation of AKT, PI3K, and NF-κB in the lungs of Atg5ΔAEC2 mice compared to WT mice after PAO1 infection (Figures 2H and S3). Unexpectedly, while overall STAT3, another key proinflammatory pathway, increased, there was no evident elevation in phosphorylated-STAT3 during P. aeruginosa infection in Atg5ΔAEC2 mice (Figures 2H and S3). Similarly, our airway epithelial cell delivery experiments revealed that lungs treated with Atg5-knockdown epithelial cells exhibited more severe lung injury and heightened infiltration of inflammatory cells following P. aeruginosa infection compared to those treated with control siRNA-transfected epithelial cells. Notably, administration of the AKT inhibitor AKTi ½ or the NF-κB inhibitor JSH-23 significantly alleviated the exacerbated lung injury and inflammatory cell infiltration induced by epithelial Atg5 knockdown (Figure S3). These data suggest that epithelial ATG5 protects against lung injury upon infection, potentially by controlling the inflammatory response and regulating the AKT/PI3K/NF-κB axis.
2.3 Loss of Atg5 Intensified the Dysregulation of Tight Junctions in Alveolar Epithelial Cells During P. aeruginosa Infection
The lung epithelial barrier serves as a crucial defense against bacterial infiltration, preventing bacterial entry into the circulatory system and thereby mitigating bacterial dissemination [8]. To further understand bacterial dissemination in Atg5ΔAEC2 mice, we conducted an assessment of the tight junction protein ZO-1 through immunofluorescent staining (Figure S4A). Remarkably, ZO-1 expression exhibited a marked decrease in lung samples of Atg5ΔAEC2 mice compared to their WT counterparts in response to P. aeruginosa infection, a trend that was consistently confirmed by western blotting (Figure S4B,C). Correspondingly, the targeted knockdown of Atg5 using siRNA resulted in a more pronounced reduction in ZO-1 levels in the murine AEC2 (MLE-12 cell line) following stimulation with P. aeruginosa (Figure S4D–F). These data underscore the critical role of ATG5 in the maintenance of epithelial tight junctions during P. aeruginosa infection
2.4 Alveolar Epithelial Atg5 Deficiency Intensified Inflammasome Activation and Pyroptosis in Lungs Upon P. aeruginosa Infection
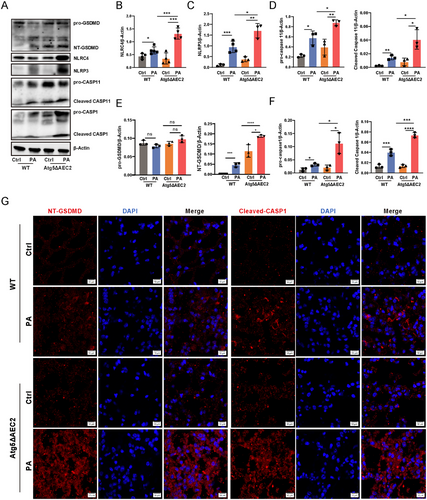
Excessive inflammasome activation is known to contribute to exacerbated pathological damage [9]. We probed inflammasome expression levels through western blotting, revealing a substantial increase in NLRP3 and NLRC4 expression in Atg5ΔAEC2 mice compared to WT mice upon P. aeruginosa infection (Figure 3A–F). Additionally, the levels of procaspase-11 and cleaved caspase-11 were elevated in mice lacking alveolar epithelial Atg5 compared to control mice following infection (Figure 3A–F). These results underscore that Atg5 deficiency intensifies the activation of pulmonary NLRP3, NLRC4, and caspase-11 inflammasomes induced by P. aeruginosa. Given the established link between inflammasome activation and a form of inflammatory cell death known as pyroptosis [10], we investigated the expression of pyroptosis-related proteins. Our analysis revealed an increase in procaspase-1 and cleaved caspase-1 in lung tissues of Atg5ΔAEC2 mice after P. aeruginosa infection (Figure 3A–F). The activation of caspase-1 results in the cleavage of gasdermin D (GSDMD), releasing the N-terminal GSDMD (NT-GSDMD) and inducing pyroptotic cell death [10]. Notably, the concurrent enhancement of NT-GSDMD levels was observed alongside increased caspase-1 cleavage in Atg5 conditional knockout mice during infection (Figure 3A–F). Immunofluorescence further illustrated a substantial increase in cleaved caspase-1 and NT-GSDMD specks in Atg5ΔAEC2 mice compared to WT mice (Figure 3G). Moreover, mice with AEC2 Atg5 deletion exhibited an augmentation in cleaved IL-1β compared to WT mice in response to P. aeruginosa challenge (Figure 2F). In summary, these findings establish the protective role of epithelial ATG5 against P. aeruginosa-induced injury by finely modulating inflammasome activation and pyroptosis in the lungs.
2.5 Loss of Atg5 Intensified Activation of Noncanonical Caspase-11 Inflammasome and GSDMD in Alveolar Epithelial Cells Upon P. aeruginosa Infection
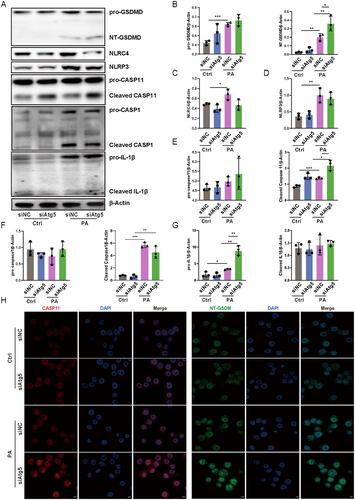
To further explore the mechanisms of Atg5 in regulating inflammasomes and pyroptosis in alveolar epithelial cells during P. aeruginosa infection, we employed siRNA to interfere with Atg5 expression in MLE-12 cells. Unexpectedly, Atg5 loss did not augment the activation of NLRP3 and NLRC4 in AEC2 following P. aeruginosa exposure (Figure 4A–G). Moreover, there was no increase observed in the levels of cleaved caspase-1 and cleaved IL-1β in Atg5-silenced epithelial cells compared to control cells in response to P. aeruginosa infection (Figures 4A–G and S5). In contrast, an increase was noted in cleaved caspase-11 in Atg5 siRNA-transfected cells compared to control siRNA-transfected cells following exposure to P. aeruginosa (Figure 4A–G). While western blot showed unchanged procaspase-11 levels, immunofluorescence demonstrated an increase in total caspase-11 expression, possibly due to its ability to encompass both procaspase-11 and its cleaved form (Figure 4H). Caspase-11 orchestrates GSDMD activation through noncanonical inflammasome signaling. Consistently, this intensified activation of caspase-11 coincided with an increase in NT-GSDMD levels (Figure 4A–G). Immunofluorescence further illustrated augmented expression of NT-GSDMD in cells transfected with Atg5 siRNA compared to those transfected with control siRNA upon infection (Figure 4H). Taken together, these data indicate that ATG5 primarily implicates in the regulation of noncanonical caspase-11 inflammasome-mediated GSDMD activation in alveolar epithelial cells during P. aeruginosa infection.
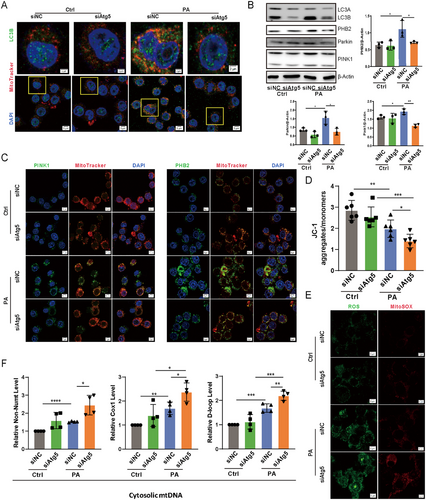
2.6 Loss of Atg5 Impedes Mitophagy and Aggravated Mitochondrial Damage in Epithelial Cells During P. aeruginosa Infection
Mitochondria represent a major source of reactive oxygen species (ROS), which can cause cellular damage if not effectively managed [11]. To counteract this, mitophagy—a specialized form of autophagy—plays a crucial role in mitigating mitochondrial ROS (mtROS) by facilitating the removal of damaged mitochondria [12, 13]. To investigate the role of mitophagy in modulating inflammatory responses during P. aeruginosa infection, we employed two complementary approaches: pretreatment with the mitochondrial division/mitophagy inhibitor Mdivi-1 and knockdown of Atg5 in mouse lung tissues via siRNA transfection. Both interventions significantly reduced lung injury and inflammatory cell infiltration compared to controls following P. aeruginosa infection (Figure S6), highlighting the critical role of mitophagy in maintaining tissue homeostasis under infection-induced stress. Moreover, in Atg5-silenced MLE-12 cells, LC3B puncta, a marker associated with autophagosome formation, displayed decreased accumulation around mitochondria in response to P. aeruginosa, indicating a compromised mitophagic process compared to control cells (Figure 5A). Surprisingly, the knockdown of Atg5 in epithelial cells led to a decrease in the expression of PINK1, Parkin, and PHB2 upon P. aeruginosa treatment, implying that the loss of Atg5 not only disrupts mitophagy by impeding autophagosomal formation but also inhibits both ubiquitin-dependent and receptor-mediated mitophagy pathways during infection (Figure 5B,C). Consistent with this, silencing Atg5 in MLE-12 cells resulted in aggregated mitochondrial damage, as evidenced by a substantial decrease in mitochondrial membrane potential (Figure 5D) and a significant increase in the production of both total ROS and mtROS during PAO1 infection (Figure 5E). Additionally, considering that mtDNA is released under various pathological conditions, such as oxidative stress and mitochondrial dysfunction [14], we measured cytosolic mtDNA, which was significantly elevated in Atg5-knockdown cells compared to control cells postinfection (Figure 5F). These findings collectively underscore the essential role of ATG5 in mitophagy and the maintenance of mitochondrial homeostasis in alveolar epithelial cells during P. aeruginosa infection.
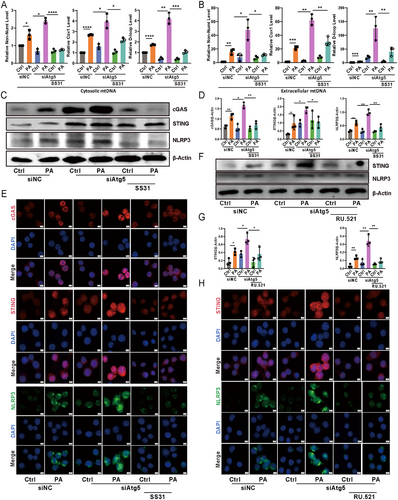
2.7 Loss of Atg5 in Epithelial Cells Facilitated Extracellular mtDNA Release During P. aeruginosa Infection, Subsequently Triggering cGAS–STING–NLRP3 Axis Activation in Macrophages
Recent work highlighted the role of GSDMD in promoting the release of mtDNA from the cytosol into the extracellular space [15]. We speculated that silencing Atg5 would lead to the accumulation of mtDNA in the extracellular space. Quantitative PCR analysis revealed a significant increase in mtDNA levels in the culture medium supernatant of MLE-12 cells with Atg5 knockdown following P. aeruginosa infection, compared to control cells (Figure 6B). The escape of mtDNA into the cytosol triggers the activation of the cGAS–STING–NLRP3 axis [16]. The extracellular release of mtDNA may subsequently activate immune cells, offering a plausible explanation for the observed discrepancy where alveolar epithelial Atg5 deficiency intensified NLRP3 inflammasome activation in lungs, but loss of Atg5 in alveolar epithelial cells did not. To further investigate this phenomenon, we exposed RAW264.7 murine macrophages to the 0.22 um filtered medium supernatant of P. aeruginosa-infected MLE-12 cells. Western blotting and immunofluorescence results demonstrated a significant increase in the expression of cGAS, STING, and NLRP3 in macrophages treated with supernatant from Atg5-silenced MLE-12 cells compared to control cells (Figure 6C–E). Consistent with these findings, our airway epithelial cell delivery experiments showed that lungs receiving Atg5-knockdown epithelial cells exhibited increased expression of cGAS, STING, and NLRP3 in macrophages following P. aeruginosa infection, compared to lungs treated with control siRNA-transfected epithelial cells (Figure S7). To validate these findings, we employed the mitochondria-targeted peptide SS-31, known for neutralizing ROS and inhibiting mtDNA release [17]. Indeed, SS-31 markedly decreased mtDNA levels in both the cytoplasm and culture medium supernatant of Atg5-knockdown epithelial cells during P. aeruginosa infection (Figure 6A,B). Furthermore, the heightened expression of cGAS, STING, and NLRP3 in macrophages treated with supernatant from P. aeruginosa-infected Atg5-silenced MLE-12 cells was significantly mitigated by SS-31 treatment of the epithelial cells (Figure 6C–E). In addition, we used RU.521, a selective inhibitor targeting the catalytic site of cGAS [18]. This inhibitor significantly suppressed downstream STING and NLRP3 expression in macrophages treated with supernatant from Atg5-silenced MLE-12 cells infected with P. aeruginosa (Figure 6F–H). This observation suggests that inhibiting cGAS activity, which impairs cGAMP production, indirectly affects STING expression. This suggests a possible feedback mechanism wherein the downstream signaling components of the cGAS-STING pathway are modulated by upstream inhibition. Altogether, these results underscore the pivotal role of ATG5 in controlling extracellular mtDNA accumulation from alveolar epithelial cells and subsequent cGAS–STING–NLRP3 signaling activation in macrophages during P. aeruginosa infection.
3 Discussion
The investigation into the essential role of autophagy in P. aeruginosa infection has yielded insights into the intricate landscape of host–pathogen interactions and immune defense mechanisms [1, 19]. Our bulk RNA-seq results are consistent with previous studies that autophagy is an important characteristic of lungs upon P. aeruginosa invasion [20, 21]. Of note, employing single-cell transcriptomics, our investigation may advance current understanding by revealing that AEC2 manifests the strongest activity in autophagy formation during P. aeruginosa infection, spotlighting a cell-type-specific engagement of autophagy. The cell-type specificity observed underscores the intricate nature of autophagy engagement in the pulmonary infection, accentuating the imperative to dissect these responses within the epithelial cell milieu. Our study involving conditional Atg5 knockout mice emphasizes the crucial role of epithelial autophagy in the face of P. aeruginosa infection. The observed reduction in survival and increased bacterial dissemination echoes earlier study where autophagy induced by type III secretion system (T3SS) toxins was recognized for enhancing the clearance of this pathogen from human corneal epithelial cells [22]. Similarly, autophagy has been identified as a protective mechanism, safeguarding AEC2 against Mycobacterium tuberculosis infection [23]. In the context of fungal infection, ATG5 and ATG16L1 contribute to plasma membrane repair through lysosomal exocytosis, safeguarding epithelial cells against Candida albicans (C. albicans) [24]. Vaginal epithelial cells with active autophagy exhibited resilience against C. albicans infection, while those with defective autophagy succumbed to the onslaught [25]. Our study extends these observations, revealing worsened lung injury, hyperactivated inflammasomes, heightened pyroptosis and inflammatory responses, and aggravated epithelial barrier dysregulation in epithelial Atg5-deficient mice following P. aeruginosa infection, aligning with our earlier reports on the severity of lung injury and enhanced inflammasome activation in Atg7−/− mice [7]. This collective evidence supports the pivotal role of epithelial ATG5-mediated autophagy in shielding against microbial-induced pulmonary injury through mechanisms, such as bacterial clearance, modulation of proinflammatory signaling, and maintenance of barrier integrity. However, this protective role contrasts with the reported response to viral infections. Contrary to bacterial and fungal infections, respiratory syncytial virus replication is facilitated by autophagy in epithelial cells [26]. Another study points toward autophagy promoting H9N2 virus replication in alveolar epithelial cells by modulating oxidative stress via the Akt/TSC2/mTOR signaling pathway [27]. These distinctions underscore the pathogen-dependent role of epithelial autophagy in infections, urging further exploration to unravel the intricacies of these divergent responses and inform potential therapeutic interventions.
In a recent study, P. aeruginosa biofilm was implicated in the activation of NLRP3 inflammasomes in macrophages [28]. Notably, NLRP3 was found to selectively drive IL-1β secretion in P. aeruginosa-infected neutrophils, thereby regulating the severity of infection [29]. The T3SS and the Type I CRISPR-Cas system were found to play a pivotal role in promoting the assembly and activation of the NLRC4 inflammasome [1]. This process occurs in macrophages that are subjected to the challenge of P. aeruginosa and is finely orchestrated by the autophagy. Our prior investigations revealed that the silencing of Atg7 in alveolar macrophages amplifies NLRC4 inflammasome activation and pyroptosis subsequent to P. aeruginosa infection [30]. However, it is worth noting that these insights primarily stem from experiments involving immune cells. Herein, our study unveils an unexpected facet of ATG5 involvement, highlighting its role in activating the noncanonical caspase-11 inflammasome in lung epithelial cells during P. aeruginosa stimulation, while showing no influence on NLRP3 and NLRC4 activation. This discovery adds complexity to our comprehension of autophagy regulation in inflammasome activation during bacterial infections. Caspase-11, directly recognizing bacterial lipopolysaccharide, cleaves GSDMD, initiating both pyroptosis and NLRP3-dependent caspase-1 activation and IL-1β maturation in bone-marrow-derived macrophages [31]. Intriguingly, our results demonstrate that while GSDMD cleavage increased alongside caspase-11 activation in alveolar epithelial cells with Atg5 loss upon P. aeruginosa infection, no concurrent activation of caspase-1 or maturation of IL-1β was observed. These findings align with recent work by Wang et al., who elucidated the roles of caspase-1 and caspase-11 in Burkholderia pseudomallei infection [32]. They found that caspase-1 predominantly governs the production of IL-1β and IL-18, as well as pyroptosis in infected macrophages, whereas caspase-11 orchestrates pyroptotic cell death specifically in infected lung epithelial cells. Similarly, the observed differences between siRNA-mediated Atg5 knockdown in epithelial cells and genetic Atg5 deficiency in Atg5ΔAEC2 mice likely stem from inherent differences in these experimental models. The in vivo model incorporates systemic and microenvironmental factors, including interactions with immune cells and other stromal cells, which may amplify the effects of ATG5 deficiency and contribute to the observed discrepancies. Nevertheless, further studies are imperative to elucidate the regulatory role and underlying mechanisms of ATG5-mediated autophagy in governing noncanonical caspase-11 inflammasome-mediated GSDMD activation in alveolar epithelial cells under bacterial challenges.
Upon cleavage, the N-terminal domain of gasdermins infiltrates cellular membranes, forming oligomeric membrane pores that lead to membrane rupture and ensuing pyroptotic cell death. These membrane pores enable the discharge of activated cytokines or other damage-associated molecular patterns, intensifying the subsequent inflammatory responses [33]. H7N9 virus-induced pyroptosis in alveolar epithelial cells involves gasdermin E, resulting in the release of cytosolic contents that precipitate a cytokine storm [34]. In our study, the loss of Atg5 in epithelial cells facilitated the extracellular release of mtDNA during P. aeruginosa challenge. Our observation aligns with recent studies demonstrating that the N-terminal domain of GSDMD, in permeabilizing the plasma membrane, plays a crucial role in releasing mtDNA. This process occurs in two steps: an initial relocation to the cytosol and a subsequent release into the cellular milieu following severe plasma membrane destabilization during pyroptosis [15]. Furthermore, our observation that the extracellular release of mtDNA from epithelial cells subsequently activates the cGAS–STING–NLRP3 axis in macrophages during P. aeruginosa infection. Similar mechanistic insights have been uncovered in the context of XBP1 deficiency, where impaired mitophagy facilitates the extracellular release of mtDNA. This occurs through hepatocyte pyroptosis, subsequently activating the macrophage STING pathway during acute liver injury [35]. This provides a possible explanation for our observed paradox: while alveolar epithelial Atg5 deficiency heightened NLRP3 inflammasome activation in the infected lungs, the loss of Atg5 did not elicit the same response in alveolar epithelial cells. Collectively, these findings reveal a pathway of mtDNA release, offering insight into the intercellular regulatory processes dependent on epithelial ATG5 and their implications for immune responses during microbial challenges. However, another consideration is the potential involvement of additional danger-associated molecular patterns (DAMPs) in the activation of the cGAS/STING/NLRP3 signaling pathway observed in this study. It is important to acknowledge that other DAMPs released by dying epithelial cells could also contribute to this activation. Future studies are warranted to explore the relative contributions of other DAMPs alongside mtDNA in this context, offering a quite comprehensive understanding of the molecular signals driving the cGAS/STING/NLRP3 pathway during P. aeruginosa infection.
Mitochondrial damage serves as a critical trigger for P. aeruginosa-induced activation of inflammasomes, a process meticulously regulated by autophagy [36]. The failure of autophagosome formation, a key player in cellular quality control, results in the aggregation of mitochondrial damage [37]. Our investigations into the impact of Atg5 deficiency in epithelial cells upon stimulation by P. aeruginosa have unveiled an impairment in mitophagy, culminating in the aggregation of mitochondrial damage. This compromised condition is marked by a diminished mitochondrial membrane potential, heightened mtROS production, and the accumulation of cytoplasmic mtDNA. These findings resonate with prior research underlining the pivotal role of ATG5 in preserving mitochondrial homeostasis during microbial challenges [38]. However, an unforeseen revelation unfolded as a reduction in the expression of pivotal mitophagy regulators, specifically PINK1, Parkin, and PHB2, was noted in Atg5-silenced epithelial cells exposed to P. aeruginosa. This unexpected decrease implies that the loss of Atg5 not only hampers autophagosomal formation but disrupts both ubiquitin-dependent and receptor-mediated mitophagy pathways. While JC-1 staining provided valuable insights into mitochondrial membrane potential, it offers a limited perspective on overall mitochondrial function. Future endeavors aimed at unraveling the molecular interactions involving ATG5, PINK1, Parkin, and PHB2, combined with more comprehensive functional assays, hold the potential to provide insights into the intricate role of ATG5 in mitophagy, particularly in the context of bacterial infections.
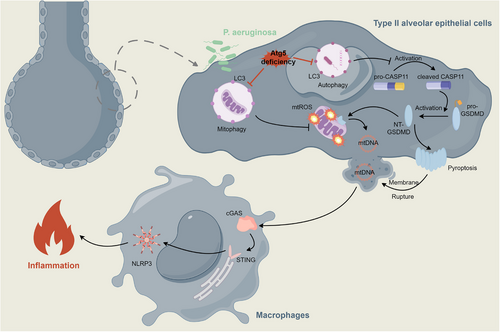
In conclusion, our work illuminates the essential role of epithelial ATG5 in the defense against P. aeruginosa. Atg5 deficiency heightens vulnerability, compromising the clearance of bacteria and exacerbating systemic dissemination. The aggravated tissue inflammatory response, involving the activation of the AKT/PI3K/NF-κB axis, inflammasomes, and pyroptosis, culminates in severe lung injury. Mechanistically, the interference with mitophagy due to Atg5 loss in epithelial cells intensifies mitochondrial damage. This, combined with the enhanced activation of GSDMD mediated by the noncanonical caspase-11 inflammasome, amplifies the extracellular release of mtDNA, triggering cGAS—STING–NLRP3 signaling in macrophages (Figure 7). Our findings broaden the understanding of microbial infections, particularly those involving bacteria that have evolved mechanisms to hijack autophagosome formation, evading host elimination. The compromised autophagy observed in Atg5-deficient conditions may contribute to a protracted inflammatory state and increased tissue injury. Targeting autophagy pathways might potentially disrupt bacterial survival mechanisms and alleviate the excessive inflammation and injury associated with certain infections, paving the way for innovative therapeutic interventions.
In acknowledging the limitations of our study, it is essential to note that we primarily focused on the role of ATG5-dependent autophagy in the host defense against P. aeruginosa. Future investigations should investigate the complexities of ATG5-independent autophagy to provide a more comprehensive understanding of the host's multifaceted responses to microbial challenges. Moreover, this study did not address whether P. aeruginosa naturally evades host defenses by targeting or blocking the autophagic pathway in epithelial cells. In addition, the findings of this study are primarily based on murine models and in vitro systems, which, while highly informative, may not fully capture the complexity of human pulmonary infections. Future research incorporating human tissue models or clinical samples will be essential to validate and extend the translational relevance of our findings.
4 Materials and Methods
4.1 Mice
Six to eight weeks old C57BL/6 mice were purchased from the Jackson Laboratory. The Atg5ΔAEC2 mice were generated on a C57BL/6J background by crossing Atg5f/f mice with SftpcCreER mice to produce SftpcCreER;Atg5f/f mice [39]. To induce Atg5 knockout specifically in AT2 cells, tamoxifen was administered intraperitoneally at a dose of 50 mg/kg once daily for 5 consecutive days. The mice were bred and housed at the University of North Dakota, with all animal procedures approved by the University of North Dakota Institutional Animal Care and Use Committee (2210-0045) and conducted in compliance with animal care and institutional protocols.
4.2 Cell Culture, Transfection, and Treatment
Murine epithelial cells (MLE-12) and macrophage cells (RAW264.7) were acquired from the American Type Culture Collection. RAW264.7 cells were cultured in high glucose Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum and 100 U/mL penicillin–streptomycin antibiotics, maintained in a 37°C incubator with 5% CO2. MLE-12 cells were cultured in Dulbecco's modified Eagle medium/nutrient mixture F-12 medium. Transfection of MLE-12 cells with small interfering RNAs was carried out using RNAiMAX (Invitrogen), following the manufacturer's instructions. The Atg5 siRNA was procured from Santa Cruz Biotechnology.
4.3 Bacteria Preparation and Infection Experiments
P. aeruginosa strain PAO1 was graciously provided by Dr. S. Lory (Harvard Medical School, Boston, MA, USA). The bacteria were cultivated in lysogeny broth (LB) medium at a constant temperature of 37°C with shaking at 220 rpm until reaching an OD600 of 0.6–0.8. For in vivo experiments, mice were anesthetized with ketamine (80 mg/kg) and xylazine (10 mg/kg), then intranasally instilled with 1 × 107 clonal-forming units (CFUs) of PAO1 in 30 µL phosphate-buffered saline (PBS). The health status of mice was closely monitored, and euthanasia was performed upon observing moribund conditions to generate survival curves using Kaplan–Meier methods and to facilitate additional assessments.
For airway epithelial cell delivery experiments, we utilized a previously established intratracheal delivery model in mice, which achieves a cell-retention efficiency exceeding 10% [40]. C57BL/6J mice were administered 20 µL of 2% polidocanol (PDOC; Sigma-Aldrich, St. Louis, MO, USA) in PBS intratracheally to prepare the lungs. After 24 h, MLE-12 cells transfected with Atg5 siRNA or siNC (1 × 107 cells per mouse) were delivered via intratracheal injection. The next day, mice were infected with P. aeruginosa (PAO1) and lung tissues were harvested 24 h postinfection for analysis. For inhibitor experiments, mice were pretreated with the AKT inhibitor AKTi ½ (20 mg/kg) or the NF-κB inhibitor JSH-23 (40 mg/kg) via intraperitoneal injection for 2 consecutive days prior to PAO1 infection. Lung tissues were collected 24 h after infection for further analysis.
For Atg5 knockdown in vivo experiments, 20 µg of Atg5 siRNA or siNC was mixed with 7.5 µL of Entranster-in vivo transfection reagent (Engreen Biosystem) and 7.5 µL of saline to a total volume of 30 µL. The mixture was administered intranasally to C57BL/6J mice. After 24 h, mice were infected with PAO1 (1 × 10⁷ CFU). Lung tissues were harvested 24 h postinfection for analysis. For mitophagy inhibition experiments, mice were pretreated with the mitochondrial division/mitophagy inhibitor Mdivi-1 (50 mg/kg) via gavage for 5 consecutive days. Following pretreatment, mice were infected with PAO1 (1 × 10⁷ CFU). Lung tissues were collected 24 h postinfection for further analysis.
In the epithelial infection experiments, MLE-12 cells were infected with PAO1 at an MOI of 20 for 2 h after cell fusion reached 60%–80%. To assess the impact of SS-31, MLE-12 cells were treated with 200 nM SS-31 along with PAO1. Following the infection, supernatant was collected, subjected to centrifugation, and bacteria were removed using a 0.22 µm filter. The resulting supernatant was then utilized to culture RAW264.7 cells for 24 h. For RU.521, RAW264.7 cells were pretreated with 20 uM RU.521 for 2 h, followed by culturing with the supernatant of PAO1-treated MLE-12 cell medium for 24 h.
4.4 Bacterial Burden Assay
Lung, spleen, and liver homogenates, BALF, and blood collected from mice were appropriately diluted in PBS to various concentrations. Subsequently, the diluted samples were evenly spread on LB agar dishes. These dishes were incubated overnight in a 37°C environment, after which bacterial colonies were counted for analysis.
4.5 Histological Analysis
Lung tissues were fixed in 4% paraformaldehyde (PFA), followed by a 48-h dehydration in 75% alcohol at 4°C. Subsequently, the tissues were embedded in paraffin using a routine histological procedure. H&E staining, following the standard staining protocol, was then performed for histological examination. The lung injury was scored in a blinded manner according to previous method [41].
4.6 Mitochondrial Membrane Potential Assay
The assessment of mitochondrial membrane potential was conducted employing the JC-1 Mitochondrial Membrane Potential Assay Kit (Cayman Chemical), following the manufacturer's guidelines. In brief, MLE-12 cells, infected with PAO1 for 2 h as described earlier, were treated with 1 µg/mL of JC-1 and incubated for 30 min. Fluorescence intensity was quantified using a fluorescence plate reader at 560-nm excitation with a 595-nm emission filter and at 485-nm excitation with a 535-nm emission filter.
4.7 Measurement of Reactive Oxygen Species
Intracellular ROS were assessed using H2DCF-DA staining solution (Invitrogen), following the manufacturer's stipulated guidelines. mtROS levels were quantified employing MitoSOX Red Mitochondrial Superoxide Indicators (Invitrogen). MLE-12 cells, subjected to the specified treatments, were incubated with 0.5 µM MitoSOX reagent for 30 min. Following two washes, fluorescence emissions were recorded using confocal laser scanning microscopy at 595 nm.
4.8 Immunoblotting
Lung tissues or whole-cell lysates were prepared in RIPA buffer (Thermo Fisher), enriched with a protease inhibitor cocktail and a phosphatase inhibitor cocktail (Invitrogen). Protein concentrations were quantified using the BCA Protein Assay Kit (Thermo Fisher). Subsequently, 20 µg of protein were electrophoretically separated on SDS–PAGE gels and transferred onto PVDF membranes. The membranes were then blocked in 5% skim milk in 1 × TBST for 1 h, followed by an incubation with primary antibodies (1:1000 dilution; ATG5, TNF-α, IL-1β, PI3K, p-PI3K, AKT, p-AKT, p-P65, CASP1, CASP11, NLRP3, GSDMD, PINK1, Parkin, and PHB2 from Cell Signaling Technology; NLRC4 from ABclonal; P65 from Invitrogen; STAT3 and p-STAT3 from Santa Cruz Biotechnology; ZO-1 from Abcam; cGAS and STING from Proteintech) at 4°C overnight. Subsequent incubation involved secondary antibodies conjugated to HRP (ABclonal, 1:10,000 dilution). Signal detection was achieved using an enhanced chemiluminescence detection kit (Cytiva).
4.9 Immunofluorescence and Confocal Microscopy
Lung sections (5 µm thickness) and cells cultured on coverslips underwent fixation in 4% PFA, permeabilization in 0.1% Triton X-100, and blocking in PBS supplemented with 10% goat serum. Primary antibodies (1:200 dilution; ZO-1 from Abcam; LC3B, NLRP3, PINK1, PHB2, cleaved GSDMD, and cleaved CASP1 from Cell Signaling Technology; cGAS and STING from Proteintech) were incubated in the blocking buffer at 4°C overnight. Subsequently, Goat anti-Mouse Alexa Fluor 488 Antibody, Goat anti-Mouse Alexa Fluor 594 Antibody, Goat anti-Rabbit Alexa Fluor 488 Antibody, and Goat anti-Rabbit Alexa Fluor 555 Antibody (Invitrogen; 1:1000 dilution) were employed to bind primary antibodies at room temperature for 1 h. Nuclei were counterstained with DAPI, and images were acquired using an Olympus FV3000 confocal microscope. For mitochondrial labeling, cells were incubated with MitoTracker Red (Invitrogen, M7512).
4.10 Enzyme-Linked Immunosorbent Assay
Paired antibodies (capture and detection) and standard recombinant mouse IL-1β and TNF-α were used to determine mouse cytokine concentrations in BALF according to manufacturer's instructions.
4.11 Measurement of mtDNA
MLE-12 cells underwent PBS washing and harvesting, with 10% reserved for whole-cell DNA extraction. The remaining cells were resuspended in prechilled mitochondrial extraction buffer. Subsequently, these cells were subjected to 20 passes through a 25-G syringe on ice and two-step centrifugation to facilitate separation of mitochondria and cytosolic fractions [42]. The purification of DNA from whole-cell extracts, cytosolic fractions, or culture media was carried out using the DNA Extraction Kit (TIANGEN) per manufacturer's instructions. mtDNA was quantified by qPCR using primers specific for the mitochondrial D-loop region (Forward: 5′-AATCTACCATCCTCCGTGAAACC-3′, Reverse: 5′-TCAGTTTAGCTACCCCCAAGTTTAA-3′), cytochrome c oxidase (Forward: 5′-GCCCCAGATATAGCATTCCC-3′, Reverse: 5′-GTTCATCCTGTTCCTGCTCC-3′) or a specific region of mtDNA that is not inserted into nuclear DNA (Forward: 5′-CTAGAAACCCCGAAACCAAA-3′, Reverse: 5′-CCAGCTATCACCAAGCTCGT-3′). Nuclear DNA encoding telomerase reverse transcriptase (Forward: 5′-CTAGCTCATGTGTCAAGACCCTCTT-3′, Reverse: 5′-CTAGCTCATGTGTCAAGACCCTCTT-3′), 18S ribosomal RNA (Forward: 5′-TAGAGGGACAAGTGGCGTTC-3′, Reverse: 5′-CGCTGAGCCAGTCAGTGT-3′), and β2 microglobulin (Forward: 5′-ATGGGAAGCCGAACATACTG-3′, Reverse: 5′-CAGTCTCAGTGGGGGTGAAT-3′) was used for normalization.
4.12 Transcriptome Analysis
The transcriptome library was prepared using the TruSeq RNA Sample Preparation Kit (Illumina, San Diego, CA, USA) with 5 µg of total RNA as input. PolyA selection was performed using oligo(dT) beads to isolate mRNA, which was subsequently fragmented. Complementary DNA (cDNA) synthesis was conducted using the SuperScript Double-Stranded cDNA Synthesis Kit with random hexamer primers (Invitrogen, CA). The resulting cDNA underwent end-repair, phosphorylation, and addition of an “A” base in accordance with Illumina's library preparation protocol. Library size selection was performed on a 2% low range ultra agarose gel, followed by amplification using Phusion DNA Polymerase with 15 PCR cycles. Paired-end sequencing was carried out on the Illumina HiSeq X Ten platform by Majorbio (Shanghai, China). Raw paired-end reads were trimmed and quality-checked using SeqPrep and Sickle with default parameters. Clean reads were then aligned to the reference genome in orientation mode using TopHat (version 2.0.0). ssGSEA was performed via GSVA package (1.42.0; method = “ssgsea,” kcdf = “Poisson”) [43]. PPI analysis was performed utilizing the STRING database. Autophagy-related signatures are available in Table S1.
SnRNA-seq was performed by SingulOmics Corporation (https://singulomics.com/). After quality control (nFeature_RNA > 400 and nFeature_RNA < 4000 and percent.mt < 5) and normalization with SCTransform(), integration was performed by FindIntegrationAnchors() and IntegrateData() function in Seurat (v4.1.1) [44, 45]. Principal components analysis and clustering were conducted with 30 PCs and a resolution of 0.4 in FindClusters(). Single-cell differential expression gene (DEGs) analyses were performed via MAST R package (1.20.0) [46]. Likelihood ratio tests between the full and reduced model formulas were used to identify DEGs (Benjamini and Hochberg method was performed for multiple testing corrections, FDR < 0.05). Autophagy formation gene signature is available in Table S1. Calculation and visualization of AUC scores were conducted via AUCell package (1.16.0) in R (4.1.0) [47, 48].
4.13 Statistical Analysis
All data were presented as mean ± SD from at least three independent experiments. Statistical analyses were done using Graphpad Prism (8.0.2). Multiple group comparisons were analyzed using one-way ANOVA or two-way ANOVA. Two group comparisons were analyzed by nonpaired t-test. Statistical significance was defined as a p value < 0.05.
Author Contributions
Junyi Wang and Min Wu designed research; Junyi Wang, Lei Zhang, Yingying Liu, Yao Liu, Anying Xiong, and Qin Ran performed experiments; Junyi Wang, Guoping Li, Colin Combs, and Min Wu wrote the manuscript; Xiang He, Vincent Kam Wai Wong, Colin Combs, and Guoping Li provided regents and critical suggestions. All the authors contributed to the article and approved the submitted version.
Acknowledgments
We thank the University of North Dakota Histology Core and Imaging Core for tissue processing and microscopy support. We express our gratitude to Angela Floden at the University of North Dakota for her support in breeding mice. The schematic is produced via Figdraw.
Ethics Statement
All animal procedures approved by the University of North Dakota Institutional Animal Care and Use Committee (2210-0045).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All data supporting the findings of this study are available from the authors upon reasonable request. The RNA-seq and SnRNA-seq data were deposited into the National Genomics Data Center of China National Center for Bioinformation under accession number CRA024835.




