Stable Expressed DNMT3A Mutants Predict a Poor Prognosis in Acute Myeloid Leukemia Patients Without Receiving Hematopoietic Stem Cell Transplantation
Xiang Zhang, Lixia Liu contributed equally to this work.
Funding: This study was funded by the Natural Science Foundation of Zhejiang Province (LY21H080003), Zhejiang Provincial Key Research and Development Program (2021C03123), and National Natural Science Foundation of China (82370163, 82170144, 81771779).
Dear Editor,
DNA methyltransferase 3A (DNMT3A) is wildly recognized as a tumor suppressor gene. Its deficiency leads to expanded hematopoietic stem cells (HSCs) pool, blocked HSCs differentiation, genomic instability, and a risk of malignant transformation in clonal hematopoiesis [1]. DNMT3A mutation (DNMT3AMut) is prevalent in adults, particularly in monocytic, and cytogenetically normal cases, affecting 25% of acute myeloid leukemia (AML) patients [1]. Although the distribution pattern of DNMT3AMuts has been well characterized in AML, its prognostic significance remains controversial.
To better understand the reasons behind DNMT3AMut prognostic heterogeneity, we conducted a retrospective study, as detailed in two previous studies [2, 3]. Our findings show that DNMT3AMuts with stable expressed mutants are associated with poor prognosis in AML patients without receiving hematopoietic stem cell transplantation (HSCT). In this study, we enrolled 485 adult de novo AML patients, of whom 98 (20.2%) were found to have DNMT3AMuts. Our results indicate the distribution pattern of DNMT3AMuts and clinical characteristics of AML patients with these mutations were similar to previous reports. In our cohort, patients with DNMT3AMuts showed a relatively shorter overall survival (OS), relapse-free survival (RFS) and disease-free survival (DFS) (Figure 1A).
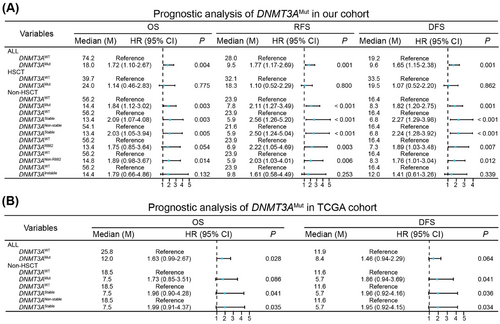
We analyzed how different therapeutic strategies, with or without HSCT, affected the prognosis of various subgroups of DNMT3AMut patients. In the HSCT group, DNMT3AMut patients exhibited comparable OS, RFS, and DFS with DNMT3A wild-type (DNMT3AWT) patients (Figure 1A). Conversely, DNMT3AMut patients showed poorer OS, RFS, and DFS in the non-HSCT group, and the disparity between DNMT3AMut and DNMT3AWT patients was more pronounced in the non-HSCT group compared to the overall cohort (Figure 1A). Therefore, HSCT overcame the poor prognosis of DNMT3AMut, indicating DNMT3AMut patients without receiving HSCT were primarily responsible for the unfavorable outcomes.
To further explore key factors contributing to poor prognosis of DNMT3AMut patients, we focused on DNMT3AMut types in the non-HSCT group. Near 50% of DNMT3AMut variants in AML are heterozygous DNMT3AR882, the hotspot mutation [1]. As reported, DNMT3AR882 played a dominant-negative role against DNMT3AWT via formatting dimers, leading to genome-wide hypomethylation [1]. However, DNMT3Anon-R882 has been infrequently studied. Recently, Yung-Hsin Huang et al. systematically studied protein stability of 253 disease-associated DNMT3AMut variants and their methyltransferase activity, and provided a comprehensive view on the implications of various DNMT3AMut variants [4]. In our cohort, we identified 112 DNMT3AMut variants. We first categorized DNMT3AMut patients into two groups: DNMT3AR882 and DNMT3Anon-R882. Except for OS, both of DNMT3AR882 and DNMT3Anon-R882 patients a had worse RFS and DFS compared to DNMT3AWT patients, indicating that DNMT3AR882 and DNMT3Anon-R882 did not effectively distinguish between prognostic outcomes (Figure 1A). Accurately, nearly half of DNMT3Anon-R882 variants were missense mutations, displaying similar mutant stability and diminished methyltransferase activity with DNMT3AR882, so we further divided DNMT3AMut patients into stable and instable DNMT3AMut groups (Supplementary Materials and Methods). Notably, stable but not instable DNMT3AMut patients exhibited worse OS, RFS and DFS compared to DNMT3AWT patients (Figure 1A). Additionally, we observed similar results when comparing stable DNMT3AMut to nonstable DNMT3AMut patients (nonstable DNMT3AMut included both instable DNMT3AMut and DNMT3AWT) (Figure 1A). Thus, stable DNMT3AMut was responsible for poor prognosis of DNMT3AMut patients in the non-HSCT group.
As stable DNMT3AMut serves as a strong prognostic factor for AML patients, we were interested in exploring whether differences existed in distribution patterns and clinical characteristics between stable and instable DNMT3AMut. In our cohort, we identified stable DNMT3AMut in 51 patients and instable DNMT3AMut in 25 patients, while 22 DNMT3AMut cases were undefined. Apart from a relatively higher frequency of NPM1 mutation in stable DNMT3AMut patients, the distribution pattern of stable DNMT3AMut was similar to that of instable DNMT3AMut. Furthermore, compared to 412 nonstable DNMT3AMut patients, 51 stable DNMT3AMut patients presented a distribution pattern similar with DNMT3AMut compared to DNMT3AWT patients. Definitely, stable DNMT3AMut did not exhibit a specific distribution pattern. We also compared the baseline clinical characteristics of stable DNMT3AMut with those of instable DNMT3AMut or nonstable DNMT3AMut patients. However, stable DNMT3AMut patients did not obviously exhibit distinct clinical features. Although there were no significant statistical differences, the stable DNMT3AMut group showed a relatively lower complete remission rate and a higher relapse rate compared to both instable DNMT3AMut and nonstable DNMT3AMut groups, regardless of whether all patients or non-HSCT patients were considered, which possibly contributed to poor prognosis of stable DNMT3AMut patients.
To determine whether stable DNMT3AMut was an independent risk factor for poor prognosis in non-HSCT patients, we divided patients into stable DNMT3AMut group and nonstable DNMT3AMut group, and displayed univariate analysis for OS, RFS, or DFS, respectively. Notably, stable DNMT3AMut exhibited an adverse effect on OS, RFS and DFS. Following this, we conducted multivariate analyses, confirming that stable DNMT3AMut was an independent adverse risk factor for OS, RFS or DFS in the non-HSCT AML patients (Table S1).
Given its strong prognostic role, we sought to validate the clinical significances of stable DNMT3AMut in external cohorts. Herein, we analyzed data from The Cancer Genome Atlas TCGA (TCGA) database, and found that stable DNMT3AMut consistently indicates poor prognosis in the non-HSCT patients (Figure 1B).
Although most evidences supported that DNMT3AMut was a biomarker for poor prognosis in AML, the role of DNMT3AMut in AML has not been widely accepted. In our cohort, DNMT3AMut patients exhibited a shorter OS, RFS, and DFS in the entire cohort, consistent with results from most other reported cohorts. Most stable DNMT3AMut variants are distributed across the methyltransferase domain, which is known to act as a dominant negative, resulting in decreased DNA methylation activity and a poor prognosis. HSCT eliminates leukemia cells, improves the hematopoietic function of the bone marrow, and enhances patient prognosis. A study has shown that HSCT improves outcomes of DNMT3AMut AML patients [5], and our findings also indicate that HSCT reduces the prognostic gap between DNMT3AMut and DNMT3AWT patients. In contrast, the prognostic disparity existed between DNMT3AMut and DNMT3AWT patients in the non-HSCT group. DNMT3AR882, the hotpot mutation in DNMT3AMut variants, was recognized as the primary factor contributing to the poor prognosis associated with DNMT3AMut [1]. Nearly 50% of DNMT3AMut variants are attributed to DNMT3AR882, but significant heterogeneity also exits in DNMT3Anon-R882 [1]. A recent study indicated that certain missense DNMT3Anon-R882 variants also shared similar protein stability and methyltransferase activity features with DNMT3AR882, thus, classifying DNMT3AMut into DNMT3AR882 and DNMT3Anon-R882 was not accurate [4]. Herein, we classified DNMT3AMut as stable or instable DNMT3AMut according to mutant stability, and found that stable DNMT3AMut, rather than instable DNMT3AMut, was an independent predictor for relatively shorter OS, RFS and DFS. Furthermore, stable DNMT3AMut primarily worsened the prognosis for AML genetic subtypes, indicating stable DNMT3AMut was a dominant contributor for poor prognosis in the non-HSCT patients. In the future, we aim to demonstrate the prognostic value of stable DNMT3AMut through prospective multicenter clinical studies.
Collectively, stable DNMT3AMut, but not instable DNMT3AMut, should be regarded as a strong predictor of poor prognosis in AML patients without receiving HSCT, thereby providing insights into the mechanism underlying AML pathogenesis.
Author Contributions
Xiang Zhang designed this study. Xiang Zhang and Xiong Ni collected clinical data and updated follow-up. Xiong Ni and Jin Jie guided clinical managements for patients. Xiang Zhang, Lixia Liu, and Jiayue Qin displayed data analysis. Xiang Zhang and Jiayue Qin wrote the manuscript. Jie Jin provided critical comments on this study. Xiong Ni and Jie Jin revised the manuscript. All authors have read and approved the final manuscript.
Acknowledgments
We would like to thank Hongyan Tong, Wenjuan Yu, and Yanan Zhu from the Department of Hematology, the First Affiliated Hospital, Zhejiang University College of Medicine, as well as Jianmin Wang, Jianmin Yang and Lei Gao from the Department of Hematology, Institute of Hematology, Changhai Hospital, for managing patients and supporting our work. We also thanked Jiewen Sun from Women's Hospital and Institute of Genetics, Zhejiang University School of Medicine for providing advices on English language writing. This study was funded by the Natural Science Foundation of Zhejiang Province (LY21H080003), Zhejiang Provincial Key Research and Development Program (2021C03123), and National Natural Science Foundation of China (82370163, 82170144, 81771779).
Ethics Statements
This study was approved by the ethical review committees of the First Affiliated Hospital of Zhejiang University School of Medicine (IIT20220659A) and Changhai Hospital (B2022-035). All procedures in studies involving human participants were performed in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments. Written informed consent was obtained from these patients.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.




