Subsequent Survival and Loss of Lifetime for Patients With Progression-Free 24 Months After Treatment in Nasopharyngeal Carcinoma: A Comprehensive Nationwide Population-Based Analysis
Yang Liu, Yaqian Han, and Mei Feng contributed equally to this work.
Funding: This work was supported by the National Natural Science Foundation of China (81172125), National Key Research and Development Program of China (2023YFC2411602), National High Level Hospital Clinical Research Funding (2022-CICAMS-80102022203), Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (2021-I2M-C&T-A-018), Beijing Xisike Clinical Oncology Research Foundation (Y-HR2020QN-0984) and the Beijing Hope Run Fund (LC2021L06 and LC2022A26).
ABSTRACT
Currently, there is little evidence supporting the use of early endpoints to assess primary treatment outcomes in nasopharyngeal carcinoma (NPC). We aim to explore the relationship between 24-month progression-free survival (PFS24) and subsequent overall survival (sOS) as well as loss of lifetime (LoL) in NPC patients. sOS is defined as survival from the 24-month point or progression within 24 months leading to mortality. LoL represents the reduction in life expectancy due to NPC, compared to the general population matched by age, sex, and calendar year. The standardized mortality ratio (SMR) is defined as the ratio of observed mortality to expected mortality. The study included 6315 patients from nonendemic and endemic regions of China. Among them, 5301 patients (83.9%) achieved PFS24, with a 5-year sOS of 90.2% and an SMR of 1.0. Over a 10-year period following treatment, the mean LoL was only 0.01 months/year. For most subgroups, patients achieving PFS24 exhibited comparable sOS and LoL with the general population. However, patients failing to achieve PFS24 showed significantly worse outcomes, with 5-year sOS of 21.9%, SMR of 23.7, and LoL of 6.48 months/year. These notable outcome disparities highlight the importance of PFS24 in NPC risk stratification, patient monitoring, and study design.
1 Introduction
Nasopharyngeal carcinoma (NPC) is the most predominant head and neck cancer in China. A majority of NPC patients present with locally advanced disease, with distant metastases primarily responsible for treatment failure [1, 2]. The overall survival (OS) rates at 5 years for non-metastatic NPC have reached 80%–90% in the era of comprehensive treatment [1]. Despite implementation of modern treatments, a notable subset of patients still encounter disease progression or recurrence [1, 2]. Consequently, there is a need to improve the overall outcomes in this setting, including early detection of recurrence through close surveillance and the development of more effective treatment modalities through prospective clinical trials.
Progression-free survival at 24 months (PFS24) is recognized as a key milestone for patient stratification in a variety of malignant hematologic tumors, because subsequent overall survival (sOS) rates after PFS24 are comparable with that of the general (background) population [3-6]. These novel survival assessment metrics represent meaningful clinical benchmarks to predict the outcomes of cancer patients. However, the implications of PFS24 on NPC remain unclear. The influence of achieving PFS24 on sOS and loss of lifetime (LoL) requires further study. If confirmed, however, PFS24 may become a valid endpoint to assist in the optimization of posttreatment surveillance strategies as well as the acceleration of the clinical trial process by establishing PFS24 as a surrogate endpoint.
To determine the reliability of PFS24 in a real-world setting, we assessed sOS and LoL stratified by PFS24 using large-scale individual data of patients with NPC from North, Central, Southwest, and South China.
2 Results
2.1 Patient Demographics and Survival Outcomes
This comprehensive analysis included 3052 patients from the National Cancer Center cohort (nonendemic region, Beijing) and 3263 patients from the endemic regions, with 1996 patients from Guangzhou, 717 from Sichuan, and 550 from Hunan. Figure 1 provides the comprehensive overview of the process for selecting patients. The ratio of patients from endemic regions to those from nonendemic regions was 1.1:1. Cohort-specific and aggregated patient features are presented in Table S1. With a median age of 47 years, the cohort had a male-to-female ratio of 2.9:1. The majority of patients presented with locally advanced disease (48.2% stage III and 37.4% stage IV). The primary treatment modalities included intensity-modulated radiation therapy (IMRT) alone in 21.1% of cases, concurrent chemoradiotherapy (CCRT) in 44.9%, and induction chemotherapy (IC) followed by CCRT (IC+CCRT) in 25.2%. A detailed summary is presented in Table 1.
| Characteristics | No. of patients (%) | 5-Year OS (95% CI) | p value | SMR (95% CI) | p value |
|---|---|---|---|---|---|
| All patients | 6315 (100) | 83.1 (82.0, 84.2) | 3.0 (2.9, 3.1) | <0.001*** | |
| Sex | <0.001*** | ||||
| Male | 4685 (74.2) | 81.4 (80.1, 82.8) | 3.0 (2.9, 3.1) | <0.001*** | |
| Female | 1630 (25.8) | 86.3 (84.3, 88.3) | 2.8 (2.6, 3.0) | <0.001*** | |
| Age (years)a | <0.001*** | ||||
| <48 | 3181 (50.4) | 86.5 (85.1, 87.9) | 8.0 (7.8, 8.3) | <0.001*** | |
| ≥48 | 3134 (49.6) | 78.8 (77.1, 80.5) | 2.1 (2.0, 2.2) | <0.001*** | |
| KPS score | 0.200 | ||||
| ≥80 | 6076 (96.2) | 83.2 (82.1, 84.3) | 3.0 (2.9, 3.1) | <0.001*** | |
| <80 | 239 (3.8) | 79.2 (73.1, 85.8) | 3.2 (2.7, 3.7) | <0.001*** | |
| Pathology | <0.001*** | ||||
| WHO II | 969 (15.3) | 78.3 (75.5, 81.1) | 3.4 (3.2, 3.6) | <0.001*** | |
| WHO III | 5158 (81.7) | 83.8 (82.6, 85.0) | 2.9 (2.8, 3.0) | <0.001*** | |
| Other | 188 (3.0) | 79.7 (73.2, 86.8) | 2.8 (2.3, 3.3) | <0.001*** | |
| AJCC 8th T stage | <0.001*** | ||||
| T1 | 816 (12.9) | 89.7 (87.3, 92.1) | 1.6 (1.4, 1.9) | <0.001*** | |
| T2 | 1017 (16.1) | 88.4 (86.1, 90.7) | 2.1 (1.9, 2.4) | <0.001*** | |
| T3 | 2885 (45.7) | 86.3 (84.9, 87.8) | 2.4 (2.3, 2.6) | <0.001*** | |
| T4 | 1597 (25.3) | 69.6 (67.0, 72.3) | 5.5 (5.4, 5.7) | <0.001*** | |
| AJCC 8th N stage | <0.001*** | ||||
| N0 | 744 (11.8) | 91.0 (88.5, 93.6) | 1.2 (1.0, 1.4) | 0.300 | |
| N1 | 2189 (34.7) | 87.8 (86.1, 89.4) | 2.2 (2.0, 2.3) | <0.001*** | |
| N2 | 2404 (38.1) | 81.1 (79.3, 83.0) | 3.5 (3.4, 3.7) | <0.001*** | |
| N3 | 978 (15.5) | 69.6 (66.3, 73.1) | 6.9 (6.6, 7.2) | <0.001*** | |
| AJCC 8th clinical stage | <0.001*** | ||||
| I | 188 (3.0) | 96.9 (93.9, 100.0) | 0.6 (0.3, 1.3) | 0.098 | |
| II | 723 (11.4) | 91.7 (89.3, 94.2) | 1.5 (1.3, 1.7) | 0.006** | |
| III | 3045 (48.2) | 89.6 (88.4, 90.9) | 1.8 (1.7, 2.0) | <0.001*** | |
| IVA | 2359 (37.4) | 70.3 (68.1, 72.5) | 5.7 (5.6, 5.9) | <0.001*** | |
| EBV DNA (copies/mL)b | <0.001*** | ||||
| <2000 | 4824 (76.4) | 83.7 (82.5, 84.9) | 2.3 (2.2, 2.5) | <0.001*** | |
| 2000–20,000 | 942 (14.9) | 79.8 (76.4, 83.4) | 3.9 (3.6, 4.2) | <0.001*** | |
| >20000 | 549 (8.7) | 76.2 (71.2, 81.5) | 6.5 (6.0, 6.9) | <0.001*** | |
| LDH (U/L)c | <0.001*** | ||||
| <240 | 5604 (88.8) | 83.9 (82.7, 85.0) | 2.9 (2.8, 3.0) | <0.001*** | |
| ≥240 | 711 (11.2) | 72.8 (68.7, 77.1) | 6.9 (6.5, 7.4) | <0.001*** | |
| Treatment modality | <0.001*** | ||||
| CCRT | 2835 (44.9) | 83.7 (82.1, 85.3) | 3.4 (3.2, 3.5) | <0.001*** | |
| IMRT | 1333 (21.1) | 83.8 (81.6, 86.0) | 1.7 (1.6, 1.8) | <0.001*** | |
| IC+CCRT | 1594 (25.2) | 81.2 (78.6, 84.0) | 5.0 (4.7, 5.3) | <0.001*** | |
| IC+IMRT | 234 (3.7) | 71.1 (63.9, 79.1) | 5.7 (5.1, 6.3) | <0.001*** | |
| CCRT+AC | 135 (2.1) | 78.2 (71.0, 86.1) | 7.6 (6.6, 8.6) | <0.001*** | |
| IC+CCRT+AC | 145 (2.3) | 85.8 (77.7, 94.6) | 4.7 (3.7, 6.0) | 0.004** | |
| IMRT+AC | 13 (0.2) | 65.8 (43.3, 100.0) | 13.7 (10.9, 17.2) | 0.026* | |
| IC+IMRT+AC | 26 (0.4) | 96.0 (88.6, 100.0) | 1.8 (0.4, 7.5) | 0.659 |
- Abbreviations: AC, adjuvant chemotherapy; AJCC, American Joint Committee on Cancer; CCRT, concurrent chemoradiotherapy; CI, confidence interval; EBV DNA, Epstein–Barr virus DNA; IC, induction chemotherapy; IMRT, intensity-modulated radiation therapy; KPS, Karnofsky Performance Status; LDH, lactate dehydrogenase; OS, overall survival; SMR, standardized mortality ratio.
- a Age was dichotomized into two subgroups based on the median value.
- b The cutoff of pretreatment cfEBV-DNA was set at 2000 copies/mL based on previous well-recognized studies.
- c The cutoff values of LDH (240 U/L) have demonstrated powerful prognostic value in previous research.
- * p < 0.05.
- ** p < 0.01.
- *** p < 0.001.
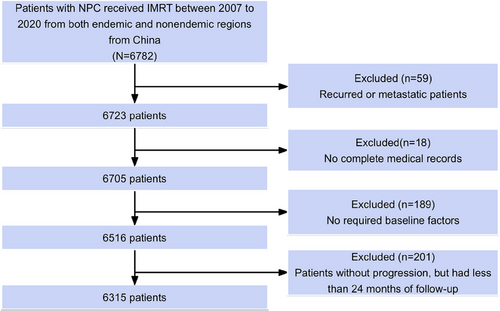
The median follow-up period for survivors was 76 months, of which 1548 patients (24.5%) experienced progression and 1006 patients (15.9%) died. The 5-year OS and PFS rates across all cohorts were 83.1% and 74.9%, respectively. The OS and PFS curves are presented in Figure S1. Univariate analysis revealed that sex, age, pathology, TNM staging, Epstein–Barr virus (EBV) DNA copies, lactate dehydrogenase (LDH), and treatment modality were significantly correlated with 5-year OS (Table 1). Figure 2A shows the annual hazard rate trajectory. Following initial treatment, 1014 (65.5%) cases of progression and 440 (43.7%) deaths occurred within 24 months. In concordance, smoothed hazard plots revealed that the peak annual hazard of progression was 10.3% within the initial 24 months and then decreased continuously. Furthermore, after 24 months, the annual hazard of death remained essentially constant at 4.0%.
The 5-year OS was considerably lower in the entire study cohort (83.1%) compared with that in the matched background population (94.7%). Additionally, the SMR from the initial treatment was 3.0 (Figure 2B). Of note, Kaplan–Meier analysis had an estimated 2-year PFS rate of 83.8%, which was most similar to the 5-year OS rate (83.1%) compared with PFS at 12 months (90.3%) and 36 months (79.9%). This suggests that the PFS24 milestone is an important metric for subsequent evaluations.
2.2 PFS24 and Subsequent OS
All 6315 patients completed adequate follow-up for the PFS24 assessment. Of these, 5301 (83.9%) achieved progression-free status after 24 months. In cases who achieved PFS24, the median OS was not reached (5-year sOS, 90.2%; Figure 2C). Minimal disparity was observed in the PFS24-associated actual death rate compared with the expected death rate (SMR 1.0, p = 0.478). Table 1 lists the OS and SMR at 5 years for the subgroups. Conversely, for patients experiencing progression within 24 months, the median sOS was limited to 25.0 months. The 5-year sOS rate post-progression was 21.9%, with an SMR of 23.8 (p < 0.001; Figure 2D).
2.3 Exploratory Subgroup Analysis Between PFS24 and Subsequent OS
Substantial variability of the clinical outcomes in the NPC subgroups prompted us to examine subgroup-specific outcomes contingent upon PFS24 (Table 2). Patients in most subgroups exhibited actual death rates similar to the expected death rates after reaching PFS24.
| Outcomes from time of achieving PFS24 (n = 5301) | Outcomes from time of failing PFS24 (n = 1014) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | No. of patients (%) | 5-Year sOS (95% CI) | p value | SMR (95% CI) | p value | No. (%) | 5-Year sOS (95% CI) | p value | SMR (95% CI) | p value |
| All patients | 5301 (83.9) | 90.2 (89.0, 91.4) | 1.0 (1.0, 1.1) | 0.478 | 1014 (16.1) | 21.9 (18.9, 25.4) | 23.8 (23.5, 24.1) | <0.001*** | ||
| Sex | 0.090 | 0.020* | ||||||||
| Male | 3890 (83.0) | 89.8 (88.4, 91.2) | 1.0 (0.9, 1.1) | 0.482 | 795 (17.0) | 20.1 (16.8, 24.0) | 22.8 (22.4, 23.1) | <0.001*** | ||
| Female | 1411 (86.6) | 91.2 (88.9, 93.5) | 1.1 (0.9, 1.3) | 0.471 | 219 (13.4) | 29.2 (22.6, 37.6) | 28.2 (27.4, 29.1) | <0.001*** | ||
| Age (years) | <0.001*** | <0.001*** | ||||||||
| <48 | 2705 (85.0) | 93.6 (92.2, 95.0) | 2.4 (2.1, 2.7) | <0.001 | 476 (15.0) | 25.3 (20.6, 31.1) | 72.4 (71.5, 73.4) | <0.001*** | ||
| ≥48 | 2596 (82.8) | 86.5 (84.5, 88.5) | 0.8 (0.7, 0.9) | 0.005 | 538 (17.2) | 19.1 (15.4, 23.6) | 15.9 (15.5, 16.2) | <0.001*** | ||
| KPS score | 0.900 | 0.040* | ||||||||
| ≥80 | 5108 (84.1) | 90.2 (89.0, 91.4) | 1.1 (1.0, 1.2) | 0.403 | 968 (15.9) | 22.6 (19.5, 26.2) | 24.1 (23.8, 24.1) | <0.001*** | ||
| <80 | 193 (80.8) | 92.1 (86.0, 98.6) | 0.8 (0.4, 1.5) | 0.524 | 46 (19.2) | 7.3 (2.0, 26.8) | 18.4 (17.1, 19.9) | <0.001*** | ||
| Pathology | 0.300 | 0.100 | ||||||||
| WHO II | 767 (79.2) | 87.2 (84.3, 90.3) | 1.2 (1.0, 1.5) | 0.136 | 202 (20.8) | 16.4 (11.3, 23.6) | 22.3 (21.6, 23.0) | <0.001*** | ||
| WHO III | 4388 (85.1) | 90.9 (89.6, 92.2) | 1.1 (1.0, 1.2) | 0.553 | 770 (14.9) | 23.7 (20.1, 28.0) | 24.0 (23.6, 24.4) | <0.001*** | ||
| Other | 146 (77.7) | 93.5 (86.9, 100.0) | 0.6 (0.2, 1.3) | 0.111 | 42 (22.3) | 26.1 (14.2, 47.9) | 22.1 (20.5, 23.9) | <0.001*** | ||
| AJCC 8th T stage | <0.001*** | <0.001*** | ||||||||
| T1 | 732 (89.7) | 92.3 (89.5, 95.1) | 0.8 (0.6, 1.0) | 0.082 | 84 (10.3) | 35.2 (25.3, 49.0) | 18.0 (16.9, 19.1) | <0.001*** | ||
| T2 | 885 (87.0) | 93.6 (91.2, 96.0) | 0.8 (0.6, 1.1) | 0.187 | 132 (13.0) | 32.6 (24.0, 44.1) | 24.2 (23.2, 25.3) | <0.001*** | ||
| T3 | 2477 (85.9) | 93.3 (91.7, 94.8) | 0.7 (0.6, 0.9) | 0.001 | 408 (14.1) | 23.1 (18.3, 29.1) | 20.6 (20.1, 21.1) | <0.001*** | ||
| T4 | 1207 (75.6) | 80.8 (77.5, 84.1) | 2.1 (1.9, 2.3) | <0.001 | 390 (24.4) | 14.2 (10.3, 19.5) | 29.0 (28.4, 29.6) | <0.001*** | ||
| AJCC 8th N stage | 0.090 | 0.030* | ||||||||
| N0 | 700 (94.1) | 91.7 (88.5, 95.1) | 0.6 (0.4, 0.9) | 0.001 | 44 (5.9) | 14.1 (4.7, 42.2) | 16.1 (15.0, 17.3) | <0.001*** | ||
| N1 | 1952 (89.2) | 91.0 (89.1, 93.0) | 1.0 (0.8, 1.1) | 0.730 | 237 (10.8) | 22.9 (16.8, 31.3) | 16.0 (15.5, 16.6) | <0.001*** | ||
| N2 | 1966 (81.8) | 90.3 (88.4, 92.2) | 1.2 (1.1, 1.4) | 0.064 | 438 (18.2) | 22.6 (18.2, 28.2) | 27.1 (26.5, 27.7) | <0.001*** | ||
| N3 | 683 (69.8) | 85.9 (82.0, 90.0) | 1.7 (1.5, 2.1) | 0.002 | 295 (30.2) | 22.2 (17.1, 28.7) | 32.4 (31.7, 33.2) | <0.001*** | ||
| AJCC 8th clinical stage | <0.001*** | <0.001*** | ||||||||
| I | 186 (98.9) | 93.0 (86.5, 99.9) | 0.5 (0.2, 1.2) | 0.043 | 2 (1.1) | 50.0 (12.5, 100.0) | 12.5 (7.2, 21.5) | 0.527 | ||
| II | 668 (92.4) | 92.3 (89.2, 95.4) | 0.9 (0.7, 1.2) | 0.480 | 55 (7.6) | 31.6 (18.2, 55.0) | 15.3 (14.0, 16.6) | <0.001*** | ||
| III | 2697 (88.6) | 94.3 (93.0, 95.6) | 0.6 (0.5, 0.8) | <0.001 | 348 (11.4) | 29.7 (24.2, 36.5) | 18.0 (17.5, 18.6) | <0.001*** | ||
| IVA | 1750 (74.2) | 83.3 (80.8, 86.0) | 1.9 (1.8, 2.1) | <0.001 | 609 (25.8) | 17.1 (13.7, 21.3) | 29.1 (28.6, 29.6) | <0.001*** | ||
| EBV DNA (copies/mL) | 0.600 | 0.200 | ||||||||
| <2000 | 3216 (66.7) | 90.5 (89.2, 91.8) | 1.0 (0.9, 1.2) | 0.505 | 715 (14.8) | 22.0 (18.6, 26.1) | 22.2 (21.7, 22.7) | <0.001*** | ||
| 2000–20,000 | 769 (81.6) | 88.4 (83.8, 93.2) | 1.2 (0.9, 1.5) | 0.424 | 173 (18.4) | 25.6 (18.4, 35.7) | 17.9 (17.2, 18.7) | <0.001*** | ||
| >20,000 | 423 (77.0) | 90.4 (84.6, 96.5) | 1.1 (0.7, 1.8) | 0.654 | 126 (23.0) | 12.5 (4.5, 34.5) | 36.5 (35.2, 37.8) | <0.001*** | ||
| LDH (U/L) | 0.600 | 0.030* | ||||||||
| <240 | 4778 (85.3) | 90.4 (89.1, 91.6) | 1.0 (1.0, 1.1) | 0.600 | 826 (14.7) | 22.9 (19.6, 26.8) | 21.7 (21.4, 22.1) | <0.001*** | ||
| ≥240 | 523 (73.6) | 88.2 (83.0, 93.8) | 1.2 (0.9, 1.6) | 0.418 | 188 (26.4) | 19.8 (13.4, 29.2) | 36.3 (35.1, 37.6) | <0.001*** | ||
| Treatment modality | <0.001*** | 0.200 | ||||||||
| CCRT | 2422 (85.4) | 89.6 (87.8, 91.4) | 1.1 (1.0, 1.2) | 0.012 | 413 (14.6) | 19.6 (15.4, 25.0) | 32.8 (32.2, 33.4) | <0.001*** | ||
| IMRT | 1139 (85.4) | 91.1 (89.1, 93.2) | 1.3 (1.1, 1.4) | 0.002 | 194 (14.6) | 22.4 (16.7, 30.0) | 12.4 (11.9, 12.9) | <0.001*** | ||
| IC+CCRT | 1301 (81.6) | 92.5 (90.0, 95.1) | 0.7 (0.6, 0.9) | 0.237 | 293 (18.4) | 25.9 (19.6, 34.3) | 29.9 (29.1, 30.7) | <0.001*** | ||
| IC+IMRT | 186 (79.5) | 81.6 (73.2, 91.0) | 1.2 (0.9, 1.6) | 0.009 | 48 (20.5) | 19.7 (10.4, 37.3) | 24.0 (22.6, 25.6) | <0.001*** | ||
| CCRT+AC | 102 (75.6) | 94.6 (88.8, 100.0) | 2.4 (1.8, 3.2) | 0.948 | 33 (24.4) | 21.3 (10.5, 42.9) | 42.7 (40.2, 45.3) | <0.001*** | ||
| IC+CCRT+AC | 118 (81.4) | 96.7 (94.8, 98.6) | 1.0 (0.3, 2.9) | 0.733 | 27 (18.6) | 27.4 (19.9, 34.9) | 27.2 (24.2, 30.6) | <0.001*** | ||
| IMRT+AC | 10 (76.9) | – | 1.2 (0.5, 3.4) | 0.110 | 3 (23.1) | – | 27.3 (21.0, 35.4) | 0.194 | ||
| IC+IMRT+AC | 23 (88.5) | – | 10.3 (7.3, 14.5) | <0.001 | 3 (11.5) | – | 83.8 (67.7, 103.7) | 0.427 | ||
- Abbreviations: AC, adjuvant chemotherapy; AJCC, American Joint Committee on Cancer; CCRT, concurrent chemoradiotherapy; CI, confidence interval; EBV DNA, Epstein–Barr virus DNA; IC, induction chemotherapy; IMRT, intensity-modulated radiation therapy; KPS, Karnofsky Performance Status; LDH, lactate dehydrogenase; PFS, progression-free survival; SMR, standardized mortality ratio.; sOS, subsequent overall survival.
- * p < 0.05.
- *** p < 0.001.
Of note, despite significant the divergence of OS among the subgroups with distinct baseline EBV DNA abundance (Table 1), these differences disappeared after achieving PFS24, with a 5-year sOS of 90.5% for the <2000 subgroup, 88.4% for the 2000–20,000 subgroup, and 90.4% for >20,000 subgroup (p = 0.600; Table 2). Similarly, comparable sOS values were observed between LDH-stratified groups after achieving PFS24 (90.4% for <240 U/L subgroup, and 88.2% for ≥240 U/L subgroup, p = 0.600).
Subsequent analyses examined outcomes related to clinical staging (Table 3). In early- to mid-stage disease, PFS24 was achieved in 98.9% of stage I, 92.4% of stage II, and 88.6% of stage III patients. For patients achieving PFS24, sOS at 5 years was 93.0%, 92.3%, and 94.3% for stage I, II, and III patients, respectively, which is similar to the general population matched by age and sex (92.2%, 94.3%, and 94.6%, Figure 2E; Table 3). The SMRs for stage I, II, and III patients achieving PFS24 were 0.5, 0.9, and 0.6, respectively. Conversely, for patients failing PFS24, the 5-year sOS was markedly lower at 50.0%, 31.6%, and 29.7% for stages I, II, and III, respectively (Figure 2F), with corresponding SMR values of 12.5, 15.3, and 18.0.
| 3 Years from time point | 5 Years from time point | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PFS time point | No. of patients | SMR (95% CI) | p value | No. at risk | Actual 3-year OS (%) | Expected 3-year OS (%) | No. at risk | Actual 5-year OS (%) | Expected 5-year OS (%) |
| Patients achieving PFS24 | |||||||||
| All stages (I—IVA) | |||||||||
| 12 months | 5710 | 1.7 (1.6, 1.8) | <0.001*** | 2868 | 92.7 (92.0, 93.5) | 97.0 | 1693 | 87.8 (86.7, 88.9) | 94.5 |
| 24 months | 5301 | 1.0 (1.0, 1.1) | 0.478 | 2169 | 94.1 (93.2, 94.9) | 97.1 | 1111 | 90.2 (89.0, 91.4) | 94.6 |
| 36 months | 3818 | 0.8 (0.7, 0.9) | <0.001*** | 1626 | 94.9 (94.0, 95.8) | 97.0 | 795 | 91.7 (90.4, 93.0) | 94.6 |
| Stage I | |||||||||
| 12 months | 187 | 0.5 (0.2, 1.2) | 0.042* | 95 | 98.6 (96.7, 100.0) | 96.7 | 57 | 97.5 (94.7, 100.0) | 92.6 |
| 24 months | 186 | 0.5 (0.2, 1.2) | 0.043* | 75 | 97.5 (94.6, 100.0) | 96.8 | 30 | 93.0 (86.5, 99.9) | 92.2 |
| 36 months | 138 | 0.4 (0.1, 1.3) | 0.006** | 56 | 98.9 (96.7, 100.0) | 95.6 | 26 | 90.9 (82.3, 100.0) | 92.9 |
| Stage II | |||||||||
| 12 months | 699 | 1.2 (1.0, 1.4) | 0.275 | 325 | 95.0 (93.2, 96.9) | 96.9 | 236 | 92.6 (90.1, 95.2) | 94.3 |
| 24 months | 668 | 0.9 (0.7, 1.2) | 0.480 | 267 | 96.0 (94.0, 97.9) | 96.9 | 193 | 92.3 (89.2, 95.4) | 94.3 |
| 36 months | 482 | 0.7 (0.5, 1.0) | 0.035* | 227 | 97.7 (96.1, 99.3) | 97.0 | 158 | 93.5 (90.4, 96.7) | 93.9 |
| Stage III | |||||||||
| 12 months | 2843 | 1.0 (0.9, 1.2) | 0.736 | 1446 | 96.0 (95.2, 96.8) | 97.1 | 858 | 92.1 (90.8, 93.5) | 94.5 |
| 24 months | 2697 | 0.6 (0.5, 0.8) | <0.001*** | 1129 | 96.9 (96.0, 97.7) | 97.1 | 558 | 94.3 (93.0, 95.6) | 94.6 |
| 36 months | 1956 | 0.5 (0.4, 0.7) | <0.001*** | 832 | 96.2 (95.1, 97.3) | 96.9 | 381 | 94.7 (93.2, 96.2) | 94.6 |
| Stage IVA | |||||||||
| 12 months | 1981 | 3.2 (3.1, 3.4) | <0.001*** | 1033 | 86.9 (85.2, 88.6) | 97.1 | 559 | 79.5 (77.2, 81.8) | 94.8 |
| 24 months | 1750 | 1.9 (1.8, 2.1) | <0.001*** | 698 | 89.0 (87.1, 90.9) | 97.2 | 330 | 83.3 (80.7, 85.9) | 95.1 |
| 36 months | 1242 | 1.3 (1.2, 1.5) | 0.012* | 511 | 91.5 (89.6, 93.4) | 95.3 | 230 | 86.5 (83.8, 89.4) | 95.3 |
| Patients failing PFS24 | |||||||||
| All stages (I—IV) | |||||||||
| 12 months | 605 | 32.6 (32.2, 33.1) | <0.001*** | 91 | 24.3 (20.8, 28.4) | 97.0 | 37 | 15.7 (12.5, 19.7) | 95.1 |
| 24 months | 1014 | 23.8 (23.5, 24.1) | <0.001*** | 179 | 30.5 (27.5, 33.9) | 97.2 | 68 | 21.9 (18.9, 25.4) | 95.2 |
| 36 months | 1220 | 19.9 (19.6, 20.1) | <0.001*** | 210 | 31.4 (28.5, 34.5) | 97.1 | 81 | 23.3 (20.4, 26.6) | 95.0 |
| Stage I | |||||||||
| 12 months | 1 | 21.7 (14.4, 32.7) | – | – | – | – | – | – | |
| 24 months | 2 | 12.5 (7.2, 21.5) | 0.527 | 1 | 50.0 (12.5, 100.0) | 98.6 | 1 | 50.0 (12.5, 100.0) | 98.6 |
| 36 months | 4 | 11.4 (8.2, 15.7) | 0.071 | 1 | 25.0 (4.6, 100.0) | 98.6 | 1 | 25.0 (4.6, 100.0) | 98.6 |
| Stage II | |||||||||
| 12 months | 24 | 21.3 (19.3, 23.6) | <0.001*** | 3 | 41.7 (26.0, 66.9) | 97.0 | 2 | 13.9 (2.6, 73.7) | 93.8 |
| 24 months | 55 | 15.3 (14.0, 16.6) | <0.001*** | 10 | 47.4 (35.6, 63.1) | 97.0 | 5 | 31.6 (18.2, 55.0) | 94.7 |
| 36 months | 74 | 11.4 (10.5, 12.4) | <0.001*** | 15 | 43.6 (32.5, 58.6) | 96.2 | 8 | 32.8 (21.1, 51.0) | 94.3 |
| Stage III | |||||||||
| 12 months | 202 | 28.1 (27.2, 28.9) | <0.001*** | 41 | 30.7 (24.4, 38.3) | 97.3 | 22 | 24.6 (18.6, 32.5) | 95.6 |
| 24 months | 348 | 18.0 (17.5, 18.6) | <0.001*** | 77 | 38.2 (32.9, 44.4) | 97.1 | 33 | 29.7 (24.2, 36.5) | 94.8 |
| 36 months | 416 | 15.6 (15.2, 16.1) | <0.001*** | 93 | 41.1 (36.1, 46.9) | 97.1 | 39 | 32.0 (26.7, 38.4) | 94.8 |
| Stage IVA | |||||||||
| 12 months | 378 | 36.5 (35.9, 37.2) | <0.001*** | 47 | 20.1 (16.0, 25.1) | 96.7 | 13 | 10.6 (7.2, 15.5) | 94.7 |
| 24 months | 609 | 29.1 (28.6, 29.6) | <0.001*** | 90 | 24.9 (21.3, 29.1) | 97.2 | 30 | 17.1 (13.7, 21.3) | 95.4 |
| 36 months | 726 | 24.4 (24.0, 24.8) | <0.001*** | 101 | 24.8 (21.4, 28.7) | 97.1 | 33 | 17.6 (14.3, 21.5) | 95.3 |
- Abbreviations: CI, confidence interval; OS, overall survival; PFS, progression-free survival; SMR, standardized mortality ratio.
- * p < 0.05.
- ** p < 0.01.
- *** p < 0.001.
For advanced stage IVA disease, however, PFS24 was only achieved in 74.2% of the patients. Patients achieving PFS24 had a 5-year sOS rate of 83.3% with an SMR of 1.9, which indicates a significantly worse survival compared with the general population (95.1%; Figure 2G; Table 3). For patients failing to achieve PFS24, the 5-year sOS rate was considerably lower at 17.1% (general population, 95.5%; Figure 2H), with a high SMR of 29.1.
2.4 Loss of Residual Lifetime Analysis and Cumulative Mortality
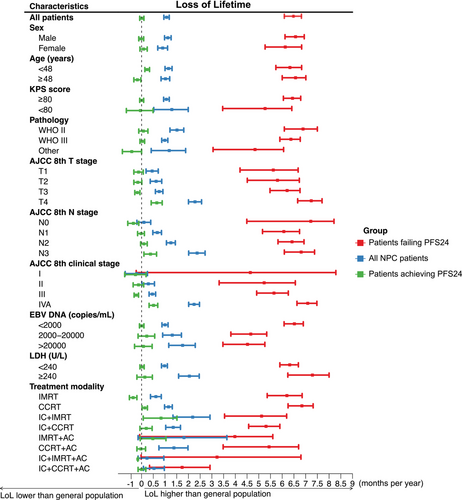
Throughout the posttreatment phase, NPC patients experienced a reduction in residual lifetime by 1.09 months per year within the initial 10 years, compared to the expected survival for the background population. The detailed LoL outcomes by NPC subgroup are presented in Figure 3 and Table S2. For patients achieving PFS24, the residual lifetime was reduced by 0.01 months/year. In most patients achieving PFS24, the reduction in lifetime was less pronounced and statistically insignificant compared with the background population, even among those with an age ≥48 years, KPS score <80, EBV DNA ≥2000 copies/mL, and an LDH ≥240 U/L (Figure 3). For T4, N3, and stage IVA disease, however, LoL remained significantly high at 0.65, 0.38, and 0.51 months/year, respectively. For patients failing to achieve PFS24, the corresponding LoL estimates were 6.48 months/year (Figure 3).
The 5-year cumulative incidence of mortality in all patients was 17.3%, whereas a reduction to 6.0% was observed in patients who successfully achieved PFS24 (Figure S2A). Similarly, the cumulative incidence of progression at 5 years was 25.1% in all patients with NPC, which decreased to 10.8% in patients achieving PFS24 (Figure S2B). Multivariate analysis for individuals achieving PFS24 revealed that age >48 years, advanced T and N stage, high EBV DNA copies, and treatment without concurrent chemotherapy were related factors for late relapse (Table S3). Additionally, for individuals achieving PFS24, age >48 years, advanced T stage, and treatment without concurrent chemotherapy were strongly correlated with late mortality (Table S4).
2.5 Sensitivity Analysis Comparing PFS24 With Alternative Time Points
In the context of sensitivity analysis, we examined outcomes at the additional time points of PFS12 and PFS36. The squared linear correlation after adjustment for measurement error was strong, with an R2 coefficient of 0.94 between the treatment effect of PFS24 and 5-year OS (Figure 4). The squared linear correlations of PFS12 and PFS36 with 5-year OS were suboptimal, yielding coefficients R2 of 0.92 and 0.88, respectively.
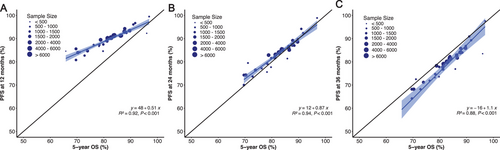
For patients achieving PFS24, we observed no significant difference in the actual death rate at PFS24 compared with the expected death rate (SMR 1.0, p = 0.478); however, the actual death rates for patients achieving PFS12 and PFS36 were significantly different compared with those of the background population (SMR: 1.7 at 12 months and 0.7 at 36 months; Table 3). Furthermore, for patients failing to achieve the selected time point, there was minimal difference in 5-year sOS rates at 24 (22.0%) and 36 (23.3%) months, suggesting that there was minimal advantage in extending the timeframe beyond 24 months. Consistent outcomes were noted in patients with early-to-mid-stage disease (Table 3); however, for stage IVA patients, despite achieving PFS12, PFS24, or PFS36, the three- and 5-year sOS remained significantly lower than the expected survival rate in the background population, and the SMR was significantly higher (Table 3).
3 Discussion
Using extensive multicenter data from both endemic and nonendemic regions, the prognostic value of PFS24 for subsequent survival and LoL was assessed in the context of a real-world contemporary treatment paradigm for NPC. Irrespective of stage, PFS24 proved to be an important milestone for subsequent outcomes in NPC. Patients exhibiting no progression within the initial 24 months following treatment experienced significantly superior long-term outcomes compared with those failing to achieve PFS24. Specifically, for stage I–III, patients who achieved PFS24 showed a comparable sOS and LoL compared with a general population matched by age and sex (i.e., these patients were “truly curable”). In this early-to-intermediate setting, PFS24 may be considered a reliable marker for disease cure in daily practice and a surrogate endpoint for clinical trials involving NPC patients. In contrast, for stage IVA patients, the overall outcome remained unsatisfactory, despite achieving PFS24, with sOS and LoL significantly inferior to the background population. These observations suggest that PFS24 is a useful milestone for optimizing posttreatment surveillance and incorporating it into the clinical trial process as a surrogate endpoint.
Multiple pretreatment characteristics have been demonstrated as important prognostic factors and have been integrated into risk stratification and treatment modification models [7-12]. In the present study, most NPC patients stratified by pretreatment factors were initially at high risk or both progression and mortality; however, a markedly attenuated significance of these risk features was observed after achieving PFS24, even in the high-risks with high EBV DNA copies and LDH. These results suggest that pretreatment risk factors gradually lose prognostic significance during dynamic surveillance.
In the present study, PFS24 status was significantly associated with sOS and LoL in the entire population, beyond prognostic categorization at diagnosis, and may be considered a prognostic indicator. Most individuals who achieved PFS24 had life expectancies comparable with the general population, demonstrating an SMR of 1.0 and a LoL of merely 0.01 months/year. Therefore, for patients who have reached PFS24, caregivers may recommend a lower intensity of surveillance to minimize mental stress as well as medical costs for surveillance workups. However, a subgroup analysis revealed that stage IV was an exception. Even with the most advanced treatment modality, the prognosis for stage IVA patients remained poor [13, 14]. This suggests that despite improved sOS and lower LoL in stage IVA patients who reached PFS24, they remained essentially “incurable,” thus justifying more intensive treatment for this substage of patients.
Shorter intervals for tumor progression are typically concomitant with diminished OS rates in various solid malignancies [15-17], including NPC [18, 19]. Consistent with previous studies, for patients unable to attain progression-free status at 24 months, the median OS following relapse was merely 25 months. This highlights the limited success of treatment in patients with early disease deterioration and necessitates more accurate prediction for patients with a potential for early progression, and consequently, more intensive therapy.
Besides the prognostic value of PFS24 on sOS, we also found a robust linear correlation between PFS24 and 5-year OS, with an R2 coefficient of 0.94. For stage I to III patients without disease progression at 24 months, the sOS and LoL were similar to the age- and gender-adjusted normal background population. These results indicate the potential value of PFS24 as an early endpoint in clinical trial design. Knowledge of early efficacy endpoints is imperative to facilitate the rapid incorporation of optimal therapeutic strategies into clinical practice.
Two previous meta-analyses based on data from randomized controlled trials (RCTs) examined the correlation between surrogate endpoints, such as EFS and PFS, with OS [20-22]; however, these two studies provided limited insights into NPC survival, primarily attributable to the frequently restricted follow-up intervals and the high selectivity observed in the enrolled patient cohorts. Compared with these studies, our study has several notable advantages. First, individual data were derived from patients who received therapeutic interventions outside the framework of clinical trials, providing prognosis estimates in real-world settings that can be extrapolated to the general population. Second, the datasets were from various multicenter cohorts, including both endemic and nonendemic patients, with reliable data quality and long follow-up periods. This illustrates the applicability of our findings to both endemic and nonendemic practices. Third, this study used SMR and LoL data matched to the general population, which is suitable for quantifying long-term survival and does not rely on accurate cause-of-death reporting. Collectively, PFS24 is a convincing and effective surrogate for OS, and warrants further assessment of its validity as a study endpoint for prospective settings involving NPC.
There are several limitations to this study. First, its retrospective design introduces potential biases, which is an inherent limitation. Second, the method employed for detecting EBV DNA are prone to considerable variation among laboratories, which could compromise their broader applicability. Third, differences in electronic health record systems, clinician documentation practices, and patient reporting can introduce biases. These considerations necessitate a cautious approach when interpreting our findings and their applicability to other contexts. Consequently, additional validation in varied clinical environments is needed to support the conclusions drawn from this study.
In conclusion, the evaluation of PFS24 enables the stratification of subsequent prognosis in non-metastatic NPC patients. Most patients achieving PFS24 exhibit favorable subsequent survival and minimal LoL, which are comparable to the general population and represent a curable entity. Nevertheless, stage IVA patients still succumb to significant and persistent disease failure despite achieving PFS24. This represents an incurable setting and warrants indefinite surveillance to timely detect recurrence. Not surprisingly, patients with early disease progression have a markedly unfavorable prognosis and require more effective subsequent line therapy. Our findings suggest that PFS24 is a robust metric for recommending a surveillance strategy and a promising surrogate endpoint for clinical trial design in NPC.
4 Materials and Methods
4.1 Study Design
In the present study, 6782 patients with non-metastatic NPC were retrospectively reviewed from the period spanning January 2007 to December 2020. The inclusion criteria encompassed patients treated with IMRT and those with complete baseline characteristics. To ensure analysis reliability, patients without progression but with follow-up periods of less than 24 months were excluded. Ultimately, the final cohort consisted of 6315 patients with non-metastatic NPC from four distinct regions of China. Clinical and demographic information were systematically collected. The study protocol was reviewed and approved by the Medical Research Ethics Committee of the National Cancer Center, Chinese Academy of Medical Sciences (Approval No. 23/353-4095). Given the retrospective nature of the study, which involved the use of anonymized data, the Committee waived the requirement for informed consent from individual participants. All procedures adhered to ethical standards as outlined in the Declaration of Helsinki, and the confidentiality and privacy of all patients were strictly maintained throughout the study.
4.2 Treatment and Follow-up
Each eligible patient received curative IMRT, with doses of 2.0–2.27 Gy per fraction, administered 5 days per week over a treatment duration of 6–7 weeks. The treatment targeted primary tumors and metastatic lymph nodes with doses exceeding 66 Gy, while regions prone to infiltration were given a minimum of 50 Gy. The chemotherapy regimen—including induction, concurrent, and adjuvant phases—was selected at the discretion of the treating clinician, with platinum-based agents being the most commonly used.
Systematic evaluations were conducted every 3 months during the initial 2 years, transitioning to semi-annual evaluations for the following 3–5 years, and subsequently transitioning to annual examinations.
4.3 Statistical Analysis
Categorical variables were described using frequencies and percentages, and continuous variables were summarized as medians with ranges. Continuous variables were classified using widely accepted cutoff points. Prior research has established that EBV DNA load cutoffs of 2000 and 20,000 copies/mL possess significant prognostic implications [8, 23]. Kaplan–Meier analysis was used to generate survival estimates. The annual hazard rates delineated the temporal trajectories of progression and morality, whereas the Epanechnikov kernel was used for smoothing. Statistical significance was defined as a two-sided p value of 0.05. Data analyses were conducted utilizing SPSS (version 26.0, IBM) and R software (version 4.2.0; http://www.r-project.org/).
OS represented the time interval from the initiation of treatment to death or last follow-up. PFS was defined as the time interval from the initiation of treatment to distant metastasis, local-regional recurrence, or death from any cause. PFS24 was defined as the status of survival without progression 24 months after initial treatment. The sOS is defined as survival from the 24-month point or progression within 24 months leading to mortality. A general population was derived from Chinese life tables and matched with the study cohort by age, gender, and calendar year using a conditional approach. LoL represented the reduction in life expectancy due to NPC, compared to the general population, such as cancer. The 10-year LoL was determined by calculating the area under the survival curves between NPC patients and the general population from treatment initiation to a 10-year follow-up period. The SMR was defined as the ratio of observed mortality to expected mortality. Expected survival was estimated using a conditional method, with the “survexp” function in R software.
For the sensitivity analysis, we investigated the relationship between the distributions of multipoint PFS rates and 5-year OS rates through linear regression analysis conducted in R software. This analysis was performed on subgroups defined by predefined, widely recognized prognostic factors. We assessed the correlation between PFS rates at additional landmark time points—namely at 12, 24, and 36 months—and the 5-year OS rates. The squared linear correlation coefficient (R2) was calculated to evaluate the association between PFS rates at various time points and 5-year OS, producing corresponding values for each subgroup. An R2 value approaching 1 indicated a stronger correlation. Based on prior studies, R2 values were categorized as excellent (R2 > 0.9), very good (R2 > 0.75), good (R2 > 0.5), moderate (R2 > 0.25), and poor otherwise.
Author Contributions
Yang Liu: conceptualization, methodology, formal analysis, investigation, writing –original draft, visualization. Yaqian Han: data curation, investigation. Mei Feng: data curation, investigation. Ye Zhang: data curation, investigation. Kai Wang: data curation, investigation. Yuan Qu: data curation, investigation. Xuesong Chen: data curation, investigation. Jianghu Zhang: data curation, investigation. Jingwei Luo: data curation, investigation. Runye Wu: data curation, investigation. Ye-Xiong Li: data curation, investigation. Xiaodong Huang: data curation, investigation. Qiuyan Chen: data curation, investigation. Jingbo Wang: funding acquisition, conceptualization, methodology, formal analysis, investigation, writing – original draft, visualization. Junlin Yi: funding acquisition, project administration, resources, writing – review and editing, conceptualization. All authors have read and approved the final manuscript.
Acknowledgments
We thank all the patients and their families for their support. This work was presented in part as a poster at the 2024 American Society for Radiation Oncology (ASTRO) Annual Meeting, American, Washington, October 2, 2024. This work was supported by the National Natural Science Foundation of China (81172125), National Key Research and Development Program of China (2023YFC2411602), National High Level Hospital Clinical Research Funding (2022-CICAMS-80102022203), Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (2021-I2M-C&T-A-018), Beijing Xisike Clinical Oncology Research Foundation (Y-HR2020QN-0984) and the Beijing Hope Run Fund (LC2021L06 and LC2022A26).
Ethics Statement
The study protocol was reviewed and approved by the Medical Research Ethics Committee of the National Cancer Center, Chinese Academy of Medical Sciences (Approval No. 23/353-4095). Given the retrospective nature of the study, which involved the use of anonymized data, the Committee waived the requirement for informed consent from individual participants. All procedures adhered to ethical standards as outlined in the Declaration of Helsinki, and the confidentiality and privacy of all patients were strictly maintained throughout the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The datasets used or analyzed in this study are available upon request from the corresponding author.




