Low versus high peripheral oxygen saturation directed oxygen therapy in critically ill patients: a multicenter randomized controlled trial
Xiaobo Yang, Xuehui Gao, Xiang Zheng, Xu Zhao, and Yanli Liu contributed equally to this work.
Abstract
Whether low peripheral oxygen saturation (SpO2) directed oxygen therapy is associated with lower mortality in critically ill patients needs further exploration. Adult critically ill patients from 11 intensive care units in China were screened. Participants were randomly assigned to the low SpO2 (90%–95%) group or the high SpO2 (≥96%) -group. The primary outcome was 28-day all-cause mortality. The secondary outcomes were hours free from ventilators and from renal replacement therapy (RRT) within 14 days. Note that 857 patients in the low SpO2 group and 849 in the high SpO2 group were included. In the low SpO2 group versus the high SpO2 group, the time-weighted average of the fraction of inspired oxygen (FiO2) was significantly lower (33.5 ± 9.7% vs. 39.6 ± 9.3%, p < 0.001), and so was the time-weighted average of SpO2 (95.9 ± 1.8% vs. 98.0 ± 1.9%, p < 0.001). Within 28 days after randomization, 172 (20.1%) in the low SpO2 group and 193 (22.7%) in the high SpO2 group died (p = 0.180). Ventilator-free time and RRT-free time were not significantly different within 14 days. In critically ill patients, low SpO2directed oxygen therapy did not decrease 28-day mortality, 14-day ventilator-free time, or 14-day RRT-free time.
1 INTRODUCTION
Oxygen therapy has been employed in clinical settings for over a century.1 It has become a standard intervention to administer supplemental oxygen to a wide array of patients experiencing acute and critical illnesses, irrespective of their baseline oxygen saturation levels. The administration of oxygen is often considered a routine part of patient care, with the primary aim of ensuring adequate oxygen delivery to tissues and vital organs. However, despite its widespread use, oxygen therapy may not always confer benefits and can, in fact, be harmful in certain patient populations. Without vigilant monitoring and appropriate titration, the delivery of oxygen can lead to either hypoxia or hyperoxia, both of which pose significant risks to patient health and recovery.2
Experimental studies show that the sword of oxygen is double-edged and either edge cuts. Hypoxia can lead to cell injury and organ failure, as the lack of sufficient oxygen impairs cellular respiration and energy production, ultimately resulting in cellular dysfunction and, if not promptly corrected, organ system collapse.3 On the other hand, hyperoxia may also cause cell, tissue, or organ injury due to enhanced oxidative stress and inflammation.4, 5 The excess of oxygen can lead to the formation of reactive oxygen species, which are highly reactive molecules capable of damaging cellular components, including lipids, proteins, and DNA.6 This oxidative damage can trigger apoptosis, or programmed cell death, and can also activate inflammatory pathways, leading to a pro-inflammatory state that can exacerbate tissue injury and impede the healing process. The promotion of oxygen free radical generation overwhelms the body's antioxidant defenses, leading to the activation of both anti-inflammatory and pro-inflammatory factors.7, 8 This dual activation can create a detrimental environment for cells, tipping the balance toward cell death through apoptosis or necrosis; moreover, the change in the internal immune state induced by hyperoxia can lead to an increased risk of infection.6-8 The implications of these findings are significant for the clinical management of patients requiring oxygen therapy.
To optimize oxygen therapy, clinical studies have been conducted. Some studies have demonstrated that excessive oxygen therapy may worsen outcomes in patients with conditions such as myocardial infarction9 and resuscitation from cardiac arrest.10 For general patients in the intensive care unit (ICU), retrospective studies showed that hypoxemia was associated with an increased risk of ventilator -associated pneumonia11 and mortality.12-14 Through retrospective analyses of data from two electronic medical record databases, van den Boom et al. found that the optimal saturation of pulse oximetry (SpO2) for critically ill patients was between 94% and 98%.15 In the 2016 Effect of Conservative versus Conventional Oxygen Therapy on Mortality Among Patients in an Intensive Care Unit (Oxygen-ICU) study, Girardis et al. showed that ICU mortality was lower in the conservative oxygen therapy group (target arterial oxyhemoglobin saturation between 94% and 98% or partial pressure of arterial oxygen (PaO2) between 70 and 100 mmHg) than in the conventional oxygen group (target arterial oxyhemoglobin saturation ≥ 97%).16 However, the sample size of this study was relatively small. In 2017, the British Thoracic Society Emergency Oxygen Guideline recommended that the SpO2 of most acute patients should be set to 94%–98%.17 However, it has been reported that the target value of the British Thoracic Society guidelines will still lead to a large number of hyperxemia.18 The 2018 clinical practice guidelines for acute patients suggest that the SpO2 of patients receiving oxygen therapy should not exceed 96%, which applies to almost all hospitalized patients.19 Large-scale randomized studies are needed to guide oxygen therapy in general ICU patients.
In recent years, several such studies have been reported: the Intensive Care Unit Randomized Trial Comparing Two Approaches to Oxygen Therapy (ICU-ROX) study,20 the Pragmatic Investigation of Optimal Oxygen Targets Trial (PILOT) study,21 the Liberal Oxygenation versus Conservative Oxygenation in ARDS (LOCO2) study,22 the Handling Oxygenation Targets in the ICU (HOT-ICU) study,23 the Optimal Oxygenation in the Intensive Care Unit (O2-ICU) study,24 and the Conservative versus Liberal Oxygenation Targets in Intensive Care Unit Patients (ICONIC) study.25 Almost concurrently with these studies, we were conducting the Peripheral Oxygen Saturation Directed Oxygen Therapy (POSDOT) study to determine whether lower SpO2 targets could provide better outcomes for critically ill patients than higher SpO2 targets.
2 RESULTS
2.1 Characteristics of the patients
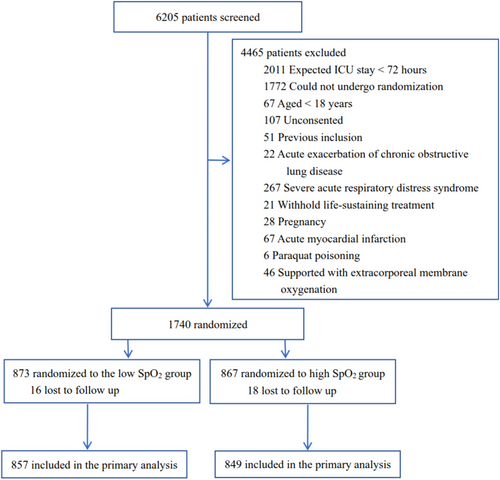
From May 2019 to May 2022, we enrolled 1740 patients in 11 general ICUs from 10 tertiary hospitals in China. However, the enrollment was heavily influenced by the COVID-19 pandemic, we decided to stop the study, and our decision was approved by the ethics committee of our institution. Loss to follow-up occurred in 34 patients, which left an intention-to-treat (ITT) population of 1706, with 857 assigned to the low SpO2 group and 849 to the high SpO2 group (Figure 1). Their mean age was 59.7 ± 15.6 years, 1087 (63.7%) were male, and 1138 (66.7%) were medical patients (Table 1). At admission, 479 (28.1%) patients and 1067 (62.5%) were experiencing shock and respiratory failure, respectively. Their Acute Physiology and Chronic Health Evaluation II (APACHE II) score was 15.9 ± 7.3, and Charlson Comorbidity Index was 1 [0, 3]. The interval between ICU admission and randomization was 17.2 ± 13.6 h. A total of 1445 (84.7%) patients had an ICU stay of no less than 72 hours.
| Demographic | All patients (n = 1706) | Low SpO2 group (n = 857) | High SpO2 group (n = 849) |
|---|---|---|---|
| Age (years) | 59.7 ± 15.6 | 60.2 ± 15.1 | 59.2 ± 16.0 |
| Sex: male (%) | 1087 (63.7%) | 548 (63.9%) | 539 (63.9%) |
| Admission | |||
| Medical | 1138 (66.7%) | 566 (66.0%) | 572 (67.4%) |
| Planned surgery | 165 (9.7%) | 82 (9.6%) | 83 (9.8%) |
| Emergency surgery | 403 (23.6%) | 209 (24.4%) | 194 (22.9%) |
| Organ failure | |||
| Shock | 479 (28.1%) | 237 (27.7%) | 242 (28.5%) |
| Septic | 299 (17.5%) | 145 (16.9%) | 154 (18.1%) |
| Hypovolemia | 137 (8.0%) | 72 (8.4%) | 65 (7.7%) |
| Cardiac | 35 (2.1%) | 16 (1.9%) | 19 (2.2%) |
| Obstructive | 8 (0.5%) | 4 (0.5%) | 4 (0.5%) |
| APACHE II score | 15.9 ± 7.3 | 15.9 ± 7.4 | 16.0 ± 7.2 |
| Charlson Comorbidity Index | 1 [0, 3] | 1 [0, 2] | 1 [0, 3] |
- Note: Data were presented as mean ± standard deviation, count (percentage) or median [interquartile range].
- Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; ICU, intensive care unit; SpO2, peripheral oxygen saturation.
2.2 Mechanical ventilation and oxygen support
There were no significant differences between the low SpO2 group and the high SpO2 group in the percentages of patients receiving mechanical ventilation at randomization (respectively, 62.5% vs. 63.3%, p = 0.762) and ever during the study period (respectively, 68.1% vs. 69.4%, p = 0.583). Compared to the low SpO2 group, a higher time-weighted average FiO2 (39.6 ± 9.3% vs. 33.5 ± 9.7%, p < 0.001) led to a higher time-weighted average SpO2 (98.0 ± 3.6% vs. 95.9 ± 1.8%, p < 0.001) in the high SpO2 group (Table 2). The distribution of time-weighted average FiO2 and time-weighted average SpO2 are presented in Table 3.
| Treatment | Low SpO2 group (n = 857) | High SpO2 group (n = 849) | p-value |
|---|---|---|---|
| Mechanical ventilation at randomization | 536 (62.5%) | 537 (63.3%) | 0.762 |
| Ever receiving mechanical ventilation during the study | 584 (68.1%) | 589 (69.4%) | 0.583 |
| Oxygen support | |||
| Time-weighted FiO2 (%) | 33.5 ± 9.7 | 39.6 ± 9.3 | <0.001 |
| Time-weighted SpO2 (%) | 95.9 ± 1.8 | 98.0 ± 1.9 | <0.001 |
| ABG data | |||
| Before inclusion | |||
| FiO2 from ABGa (%) | 43.0 ± 14.1 | 43.4 ± 13.8 | 0.721 |
| PaO2 from ABGa (mmHg) | 102.4 ± 36.6 | 105.9 ± 38.1 | 0.135 |
| After inclusion | |||
| Time-weighted FiO2 from ABGb (%) | 33.9 ± 12.8 | 41.3 ± 12.4 | <0.001 |
| Time-weighted PaO2 from ABGb (mmHg) | 87.6 ± 14.4 | 100.6 ± 19.3 | <0.001 |
- Note: Data were presented as mean ± standard deviation or count (percentage).
- Abbreviations: ABG, arterial blood gas analysis; FiO2, fraction of oxygen; PaO2, partial pressure of oxygen; PEEP, positive end-expiratory pressure; SpO2, peripheral oxygen saturation.
- a Data from 506 patients in the low SpO2 group and 500 patients in the high SpO2 group.
- b Data from 598 patients in the low SpO2 group and 597 patients in the high SpO2 group.
| Outcomes | Low SpO2 group (n = 857) | High SpO2 group (n = 849) | p-value |
|---|---|---|---|
| Primary outcome | |||
| 28-Day mortality | 172 (20.1%) | 193 (22.7%) | 0.180 |
| Secondary outcomes | |||
| Ventilator free time in 14 days (h) | 59 [24, 105] | 63 [24, 114] | 0.737 |
| Renal replacement free time in 14 days (h) | 120 [74, 195.5] | 120 [75, 200] | 0.670 |
- Note: Data were presented as count (percentage) or median [interquartile range].
- Abbreviation: SpO2, peripheral oxygen saturation.
In patients with data of FiO2 and PaO2 from arterial blood gas analysis, before inclusion, FiO2 (43.0 ± 14.1% in the low SpO2 group vs. 43.4 ± 13.8% in the high SpO2 group, p = 0.721) and PaO2 (102.4 ± 36.6 mmHg in the low SpO2 group vs. 105.9 ± 38.1 mmHg in the high SpO2 group, p = 0.135) were not different between the two groups (Table 2). After inclusion, time-weighted average FiO2 and time-weighted average PaO2 were significantly lower in tlow SpO2 group than in the high SpO2 group (33.9 ± 12.8% vs. 43.4 ± 13.8%, p < 0.001 and 87.6 ± 14.4 mmHg vs. 100.6 ± 19.3 mmHg, p < 0.001, respectively).
2.3 Outcomes
Within 28 days after randomization, 172 (20.1%) patients in the low SpO2 group and 193 (22.7%) in the high SpO2 group died (risk ratio [RR] 0.88, 95% confidence interval [CI] [0.74–1.06], p = 0.180) (Table 3). The log-rank test of the probability of survival between the two groups since randomization was not different either (p = 0.181, Figure 2). As for the secondary outcomes, ventilator-free time (59 [24, 105] h in the low SpO2 group vs. 63 [24, 114] h in the high SpO2 group, p = 0.737) and renal replacement therapy (RRT)-free time (120 [74, 195.5] h in the low SpO2 group vs. 120 [75, 200] h in the high SpO2 group, p = 0.670) in 14 days were not different (Table 3). Subgroup analyses based on baseline characteristics showed a borderline non-significant decrease in 28-day mortality in female patients, and a borderline significant decrease in 28-day mortality in patients with a Charlson Comorbidity Index greater than 0. Univariate and multivariate Cox regression analyses showed age, shock at mission, and respiratory failure at admission were risk factors for death (Table S1). In either analysis, high SpO2 was not a risk factor for death.

3 DISCUSSION
Our multicenter, randomized clinical trial showed that low SpO2-directed oxygen therapy did not decrease 28-day mortality, 14-day ventilator-free time, or 14-day RRT-free time in critically ill patients.
SpO2 is employed routinely in clinical practice and commonly in trials exploring the correlation between oxygen therapy and clinical outcomes in critically ill patients. Based on data from more than 30,000 patients from two electronic medical record databases, van den Boom and colleagues revealed that compared patients with 40% of the time SpO2 between 94% and 98%, patients with 80% of the time SpO2 within the same range had a nearly halved risk of hospital death in a retrospective study.15 Although, in our study, the time-weighted average SpO2 was between 94% and 98% in the low SpO2 group, we did not find a difference in mortality in 28 days, ventilator-free time in 14 days, or RRT-free time in 14 days compared with the high SpO2 group. Our findings are consistent with findings from the ICU-ROX study20 and the PILOT study.21 The ICU-ROX investigators randomized 1000 adult mechanically ventilated patients into the conservative-oxygen group (97% as upper limit of SpO2 and FiO2 as low as possible to 21%) and the usual oxygen group (no upper limit of SpO2 and FiO2 above 30%), and they found no difference in ventilator-free days or 180-day mortality.20 In the PILOT study, Semler and colleagues assigned mechanically ventilated patients into three groups based on SpO2 target, the low SpO2 group (88%–92%), the intermediate SpO2 group (92%–96%), and the high SpO2 group (96%–100%); they found no difference in ventilator-free days or 28-day mortality among these groups.21
Other two commonly used targets in oxygenation studies in ICUs are PaO2 and arterial oxyhemoglobin saturation (SaO2). Compared with patients with SaO2 ≥ 97%, Girardis and colleagues found that patients with SaO2 between 94% and 98% or PaO2 of 70–100 mmHg had a significantly reduced chance of ICU death from 20.2% to 11.6%, of shock from 10.6% to 3.7%, and of bacteremia from 10.1% to 5.1%.16 However, Oxygen-ICU was an early-terminated single-center randomized controlled study with only 480 patients included. These findings were not replicated by the LOCO2 study22 and the HOT-ICU study.23 In the LOCO2 study, Barrot and colleagues included 205 patients with acute respiratory distress syndrome and found no difference in 28-day mortality between patients with PaO2 from 55 to 70 mmHg and patients with PaO2 from 90 to 105 mmHg.22 In the HOT-ICU study on adult patients with acute respiratory failure, 1441 were randomized into the lower oxygenation group (target PaO2 60 mmHg) and 1447 into the higher oxygenation group (target PaO2 90 mmHg), and no difference in 90-day mortality was found.23
The difference between mean/median FiO2 in the high target group and mean/median FiO2 in the low target group varies. It increases from 3% in the Oxygen-ICU study,16 approximately 5% in the ICU-ROX study,20 approximately 6% in our study, approximately 10% in the O2-ICU study24 and the LOCO2 study,22 approximately 12.6% in the ICONIC study,25 and approximate 15% in the PILOT study21 and the HOT-ICU study.23 The different differences in FiO2 between the high target group and the low target group in these studies make it difficult to conduct a meta-analysis.
In the low SpO2 group/conservative group, the actual SpO2 levels were commonly higher than the predefined upper limit of the SpO2 target. In the low SpO2 group/conservative group, the time-weighted average of SpO2 was 93.4% in the study of Panwar and colleagues,26 95.9% in our study, and approximately 94% in the PILOT study21; the predefined upper limit of the SpO2 target was 92%, 95%, and 92%, respectively.
Even in pO2-targeted study, the actual SpO2 levels were commonly higher than the anticipated SpO2 levels in the lower target group. The actual SpO2 was approximately 93% in the LOCO2 study22 and in the HOT-ICU study,23 and the anticipated SpO2 levels were 88%–92% and approximately 90%, respectively. Whether compliance with the study protocol will make a difference is unknown. The bottom line is an extremely large number of participants should be included to make a firm conclusion on whether there will be a significant difference in mortality between conservative oxygen therapy and liberal oxygen therapy in critically ill patients, and we are hoping that the Mega-ROX trial (ACTRN12620000391976) will shed some light on the question.
The strengths of our study lie in the pragmatic protocol allowing us to maintain routine practice except for oxygenation targets and differences in SpO2 and FiO2 between the two groups. However, our study had some limitations. First, our study stopped prematurely because of the COVID-19 pandemic. However, it was unlikely the planned sample size would make a difference in the primary outcome. Second, a large proportion of patients screened were not randomized. For one thing, one liaison at each study center was asked to screen as many patients as possible, and patients admitted into the ICU during weekends or holidays were easily categorized as being unable to be randomized. For another, the plan to include patients with an ICU stay of at least 72 hours aimed at a longer duration of oxygen treatment in the ICU, but it also led to the unnecessary exclusion of potentially eligible patients with an uncertain length of ICU stay at the time of screening. The screening process is difficult to regulate and monitor.27 Third, the SpO2 target was not achieved in the low SpO2 group, which may attenuate the treatment effect. Fourth, data on arterial blood analysis were not mandatorily collected. The relationship between arterial SpO2 and PaO2 is not linear, and compared with the PaO2 target, SpO2 targe is a relatively less precise titration of oxygenation. But SpO2 has the advantage of real-time continuous monitoring. Since no compensation was given to the patients, our ethics committee deemed it would be immoral to conduct arterial blood gas analyses only for the study. In the present trial, PaO2 and FiO2 were obtained from arterial blood gas analyses at the discretion of study physicians. Because pO2 was measured at different times and could not be measured continuously, SpO2 was used to titrate oxygen.23, 25 In the ICONIC study, only 68.3% and 52.9% of patients in the low oxygenation group and the high oxygenation group, respectively, had 50% or more of pO2 measurements within the predetermined ranges.25 This information was not available in the HOT-ICU study.26 The SpO2 values concomitant to pO2 measurements of only three to four times per day were sufficient to differentiate between the low oxygenation group and the high oxygenation group.25 Fifth, all participating study centers were tertiary hospitals from China, which would restrict the generalizability of this study. Sixth, data on ischemic events, including myocardial infarction, ischemic stroke, and intestinal ischemia, were unavailable. Seventh, although the free time in hours of respiratory and of renal support with machines wwas planned and explored as possible outcome measures for future trials, we failed to find differences in these two aspects. For convenience, a fixed time frame of 14 days for the secondary outcomes was used, which was the same as the duration of the oxygen fraction manipulation in the present study. Secondary outcomes within 14 days of enrollment, such as ventilator-free days, were also used by Gelissen and colleagues in the O2-ICU study.24
4 CONCLUSION
In critically ill patients, the low SpO2-directed oxygen therapy did not lead to a lower 28-day mortality, a longer duration free from ventilator, or a longer duration free from RRT in 14 days.
5 MATERIALS AND METHODS
5.1 Study design
POSDOT study was a multicenter randomized controlled trial, conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice. The protocol (Supporting Information 1) and oral or written informed consent were approved by the ethics committee of each institution, and informed consent was obtained from the patients or legal representatives.
5.2 Patients
All patients aged greater than or equal to 18 years were eligible if they were admitted to the ICU with an expected length of stay no less than 72 hours. Exclusion criteria were as follows: pregnancy, acute exacerbation of chronic obstructive pulmonary disease, severe acute respiratory distress syndrome (defined as PaO2/the fraction of oxygen (FiO2) ≤ 100 mmHg and PEEP ≥ 10 cmH2O while on a ventilator), acute myocardial infarction, paraquat poisoning, receiving extracorporeal membrane oxygenation, withholding or withdrawal of life-sustaining treatment, failure to screen within 48 h since admission to ICU, refusal to be included, prior participation in this study or other interventional studies within 3 months, and any other reasons deemed inappropriate by the study doctors.
5.3 Randomization and intervention
Enrolled patients were randomized in a 1:1 ratio to the low SpO2 group (SpO2 target set at 90%–95%) or the high SpO2 group (SpO2 target set at 96%–100%) according to a computer-generated sequence concealed in closed opaque envelopes based on random block sizes of 2, 4, 6, 8, or 10 with stratification on each participating site.
This was an open-label trial to account for the impossibility of concealing the treatment assignments. For patients mechanically ventilated, if SpO2 was not within the predefined range, FiO2 was adjusted by 0.05 (absolute value) every 30 min. In the low SpO2 group, when SpO2 was between 90% and 95%, FiO2 was administered as low as possible to 21%. In the high SpO2 group, FiO2 was adjusted to reach SpO2 higher than 96% as long as FiO2 ≥ 30%. For patients not mechanically ventilated, the flow of oxygen was titrated every 30–60 min. For patients with severely poor peripheral perfusion when SpO2 was unattainable, saturation of oximetry from arterial blood analysis was used. The SpO2 alarms on all monitors were set accordingly. Clear signs were written or hung at the bedside of enrolled patients. Nurses and physicians caring for these patients adjusted FiO2 to achieve the target SpO2. FiO2 and SpO2 were recorded every 6 h. The intervention was stopped at 14 days, death, or ICU discharge, whichever came first.
In case of a procedure, such as endotracheal intubation, fibroscopy or tracheotomy, FiO2 adjustment was determined by the treating clinicians. We recommended following the protocol as much as possible and returning to the protocol as soon as possible. If a patient's condition deteriorates, the treating clinicians could change the SpO2 target if they feel it was in the best interest of the patient. All other treatments, including tracheal intubation, ventilator setting except FiO2, vasoactive drugs, imaging examination, and microbiological specimen collection, referred to the routine of each institution.
5.4 Data collection
A standardized case report form was used to collect data. At enrollment, we collected demographic data, type of admission (medical, elective surgical or emergency surgical), APACHE II score, shock,26 respiratory failure (PaO2/ FiO2 < 300 mmHg), and Charlson Comorbidity Index. Time-weighted averages of FiO2 and SpO2 were calculated.16, 27 Arterial blood gas analysis was performed at the discretion of the treating physicians according to the routine of each institution. Time-weighted averages of FiO2 and pO2 from arterial blood gas analysis were calculated in the same way.
5.5 Outcomes
The primary outcome was 28-day all-cause mortality. Follow-up phone calls were made after discharge to confirm whether patients were alive 28 days after inclusion. The secondary outcomes were hours of free time from ventilators and from RRT within 14 days after inclusion when patients were still in the ICU included in the present study.
5.6 Statistical analysis
On the basis of previous data from our pilot study with a 28-day mortality of 30%, the originally planned sample size was 2148 patients to detect an absolute difference in mortality of 6% between the low SpO2 group and the high SpO2 group (ɑ = 0.05, β = 80%).
All analyses were conducted following the ITT principle. Continuous data were presented as the mean ± standard deviation (SD) or median interquartile range. Unpaired Student's t-test was used to evaluate differences between the two groups. Otherwise, the Mann–Whitney U test was used. Categorical data were expressed as counts (percentages) and analyzed using the chi-square test. Mortality over time was assessed with Kaplan–Meier analysis and log-rank test. The exploratory post hoc subgroup analyses were conducted to assess whether there was heterogeneity in treatment effects on the primary outcome. The RR with 95% CI was used for the presentation of the mortality rate ratio between the two groups when appropriate. Univariate and multivariate Cox regression analyses were used to explore the risk factors for 28-day mortality. A p-value < 0.05 was considered statistically significant. We used Stata/IC 15.1 software (StataCorp) for statistical analysis.
AUTHOR CONTRIBUTIONS
Drafting of the manuscript: Xiaobo Yang, Xuehui Gao, Xiang Zheng, Xu Zhao, Yanli Liu, Lu Zhang, Junli Sun, Peng Wang, Zhengqin Xu, Ronghua Hu, and Hongbin Li. Critical revision of the manuscript: Hong Qi, Yin Yuan, Wei Chen, Jie Liu, Guangqing Huang, Fengsheng Cao, and Keke Xin. Administrative and technical support: Li Yu, Min Yu, Xiaoyun Liu, Li Zhang, Siyuan Chang, Xiaojing Zou, Hong Liu, Zhaohui Fu, Huaqing Shu, Yuan Yu, Jiqian Xu, Shiying Yuan, and You Shang. Acquisition, analysis, and interpretation of data: All Authors. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
We thank all the doctors and nurses for their help in this study. This study was supported by National Key Research and Development Program from Ministry of Science and Technology of the People's Republic of China, Grant Nos.: 2021YFC2500805 and 2021YFC2500801; Wu Jieping Medical Foundation, Grant No.: 320.6750.18011.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
This study was approved by the ethics committee of Tongji Medical College (No. 2017-S024) and ethics committees of other study sites. Informed consent was obtained from all participants. This trial was registered on Clinicaltrials.gov (NCT02999932).
Open Research
DATA AVAILABILITY STATEMENT
Data will be made available 2 years after publication upon reasonable request by researchers who provide a methodologically sound proposal and whose use of the data has been approved by the study authors and the ethics committee of the corresponding authors.




