Circular RNAs in cancer
Abstract
Circular RNA (circRNA), a subtype of noncoding RNA, has emerged as a significant focus in RNA research due to its distinctive covalently closed loop structure. CircRNAs play pivotal roles in diverse physiological and pathological processes, functioning through mechanisms such as miRNAs or proteins sponging, regulation of splicing and gene expression, and serving as translation templates, particularly in the context of various cancers. The hallmarks of cancer comprise functional capabilities acquired during carcinogenesis and tumor progression, providing a conceptual framework that elucidates the nature of the malignant transformation. Although numerous studies have elucidated the role of circRNAs in the hallmarks of cancers, their functions in the development of chemoradiotherapy resistance remain unexplored and the clinical applications of circRNA-based translational therapeutics are still in their infancy. This review provides a comprehensive overview of circRNAs, covering their biogenesis, unique characteristics, functions, and turnover mechanisms. We also summarize the involvement of circRNAs in cancer hallmarks and their clinical relevance as biomarkers and therapeutic targets, especially in thyroid cancer (TC). Considering the potential of circRNAs as biomarkers and the fascination of circRNA-based therapeutics, the “Ying-Yang” dynamic regulations of circRNAs in TC warrant vastly dedicated investigations.
1 INTRODUCTION
Based on the final draft of the human genome released by the human genome sequencing consortium, researchers found that the vast majority of the human genome could be transcribed into RNA.1 Among this, only about 2% of the entire genome is transcribed into RNA that serves as templates for proteins, while noncoding RNAs comprise the majority of the human transcriptome.2, 3 Since the initial description of circular RNAs (circRNAs) in viroid by Sanger et al.4 several decades ago, circRNAs were long considered to be by-products resulting from splicing errors in eukaryotic cells.5 Advances in high-throughput sequencing technologies and dedicated bioinformatic computational algorithms have elevated circRNAs to the forefront of RNA studies over the past decade.6, 7 Mounting researches have provided persuasive and incontrovertible evidence proving the biological functions of circRNAs in human ontogenesis and various diseases, especially in different types of cancers. For example, CDR1as (antisense to the cerebellar degeneration-related protein 1 transcript) was first reported by Hansen et al.,8 which was subsequently found to be involved in various physiological and pathological conditions, including neuronal connectivity,9 stemness maintenance,10 ischemic brain damage,11, 12 cardio-cerebrovascular diseases,13, 14 and various cancers.15-18
Tumorigenesis and cancer development are influenced by both environmental and genetic factors.19-21 The evolving understanding of cancer genetics and biology has led to a more intricate comprehension of the disease. To encapsulate the complexity of cancer, Hanahan and Weinberg introduced and conceptualized the hallmarks of cancer.22 These hallmarks indicate distinct and supplementary capabilities acquired by human cells as they undergo malignant transformation from normalcy to neoplastic states.23 Various circRNAs participate in these hallmarks across diverse cancers, including thyroid cancer (TC).24-31
TC, the most prevalent endocrine malignancy, arises from the thyroid gland, which is the largest endocrine gland in adults.32 In recent decades, the global incidence of TC has increased dramatically,33, 34 with an age-standardized death rate of 0.53 and an age-standardized disability-adjusted life-years rate of 14.571 in 2021.35 The 5-year relative survival rate of TC was estimated to be 98.5%.36 In China, TC is the most rapidly increasing type among diverse cancers in women and the most frequently diagnosed cancer in women under 30 years.37-39 TC encompasses four histologic subtypes, with papillary thyroid carcinoma (PTC) constituting over 83% of all cases.40 Both follicular thyroid carcinoma and PTC, arising from thyroid follicular cells, are collectively categorized as differentiated thyroid carcinoma (DTC) due to their superior differentiation.32 Anaplastic thyroid carcinoma (ATC), characterized by undifferentiation or poor differentiation, represents the third histopathologic type derived from thyroid follicular cells.32 Malignant tumors originating from parafollicular C cells are termed medullary thyroid carcinoma, comprising less than 2% of thyroid malignancies.41-44 Treatment strategies involving surgery, radioactive iodine, and thyroid-stimulating hormone suppression have proven effective for most patients with DTC.44 However, DTC occasionally recurs after primary treatment,44-46 and recurrent DTC is linked to a poor prognosis. Despite advancements in diagnostic methods and systemic management, the recurrence rate of TC is around 15%,47, 48 and TC mortality has shown a gradual increase.33, 34, 38
Xu et al.49 revealed that, in adults, the number of circRNAs identified in human endocrine tissue, including the thyroid gland, exceeds those found in other tissues. Endocrine malignancies typically result in an imbalance of hormone secretion, influencing organs throughout the body.50 Apart from surgery, endocrine malignancies generally lack customized chemotherapeutic, radiotherapeutic, hormonal, or biologic therapy strategies.51 Given the abundance of circRNAs in the thyroid gland, dysregulated circRNAs may play a more pivotal role in the tumorigenesis, progression, and therapeutic resistance of thyroid tissue compared with other tissues.52 Several studies have explored the expression profiles of dysregulated circRNAs and their roles in the hallmarks of TC over the past few years.53-57 Considering the surge in research achievements and the urgency of creating effective therapeutic strategies, it is meaningful and timely to review the advances in research on circRNAs and their roles in cancer.
In this review, we first provide a concise overview and update on the biogenesis, features, functions, and turnover of circRNAs. Subsequently, we summarize current knowledge regarding their functional mechanisms in each hallmark of cancer and highlight their potential as diagnostic and prognostic biomarkers for cancers, with a particular emphasis on TC. We outline the roles of circRNAs in cancer therapeutic resistance and emphasize their potential as therapeutic targets and agents. Finally, we discuss unresolved questions about circRNAs in cancers that warrant exploration in future research.
2 SUMMARIZATION OF circRNAs AND THEIR FUNCTIONS IN CANCERS
2.1 Classification of circRNAs
CircRNAs exhibit three primary subtypes based on their contained sequences: exonic circRNAs (ecircRNAs), formed from exonic sequences in precursor mRNAs (pre-mRNAs); circular intronic RNAs (ciRNAs), formed from intronic sequences in pre-mRNAs; and exon-intron circRNAs (EIciRNAs), formed from both exonic and intronic sequences in pre-mRNAs.5 EcircRNAs predominantly reside in the cytoplasm, functioning as competing endogenous RNAs (ceRNAs) to sponge microRNAs (miRNAs), thereby protecting mRNAs from miRNA-mediated inhibition.58-60 Conversely, ciRNAs and EIciRNAs are primarily located in the nucleus, where they regulate transcription.61, 62 All three circRNA subtypes consist of sequences derived from a single gene. Recently, two distinct types of circRNAs, fusion circRNAs (f-circRNAs) and read-through circRNAs (rt-circRNAs), have been identified, incorporating sequences from two different genes.63, 64 F-circRNAs originate from fusion genes formed by chromosomal translocations in cancer cells.65-67 Rt-circRNAs result from read-through transcription, producing hybrid transcripts that include coding exons from two adjacent and similarly oriented genes.64, 68 While f-circRNAs are interchromosomal chimeras between distant genes, rt-circRNAs are intrachromosomal chimeras involving adjacent genes on the same strand.63
2.2 Biogenesis of circRNAs
Maintaining cellular physiological balance involves the regulated generation of circRNAs through multiple cis-acting elements and trans-acting factors, mirroring the control of canonical splicing.69, 70 Cis-acting elements govern circRNA biogenesis through lariat-driven and intron pairing-driven circularization.69 In lariat-driven circularization, pre-mRNA partially folds, enabling the downstream donor splicing site to attack the upstream receptor splicing site, forming circRNA with the spliced folded region.71, 72 In intron pairing-driven circularization,73 reverse complementary sequences on the flanks of exons, acting as cis-acting elements, facilitate back-splicing to directly form circRNA.74 CircRNA generation is also subject to regulation by diverse trans-acting factors under specific conditions. During RNA-binding protein (RBP)-driven circularization, RBPs promote circRNA generation by binding to specific sites in the flanking introns, bringing the donor and receptor sites together. Notably, certain RBPs facilitate circRNA generation, while others exert the opposite effect.6
2.3 Features of circRNAs
The foremost distinctive feature of circRNA is its stability, stemming from its closed-loop structure, rendering it resistant to exonucleases.75, 76 Second, circRNAs exhibit abundant expression across various species, with several circRNAs expressed at much higher levels than their cognate linear mRNAs.5, 6, 77 Third, some circRNAs demonstrate conservation among diverse species. Both the circularized exon sequences and the flanking intronic sequences of conserved circRNAs are conserved,78 along with their splice sites and the effects of RBPs on circRNA biogenesis.78-80 Fourth, circRNAs display species-, cell-, tissue-, developmental stage-, and disease-specific expression.49, 55, 81, 82
2.4 Functions of circRNAs
2.4.1 miRNA sponging
Sponging miRNA stands out as a pivotal function of circRNA (Figure 1).5, 70 By binding and sequestering target miRNAs, circRNAs can modulate the expression and function of mRNAs targeted by these miRNAs. For instance, CDR1as harbors over 70 miRNA response elements (MREs) for miR-7.83-87 Similarly, circHIPK3 targets multiple miRNAs, regulating various downstream mRNAs.75, 88 In specific situations, circRNAs act as reservoirs by sponging miRNAs for transportation.89
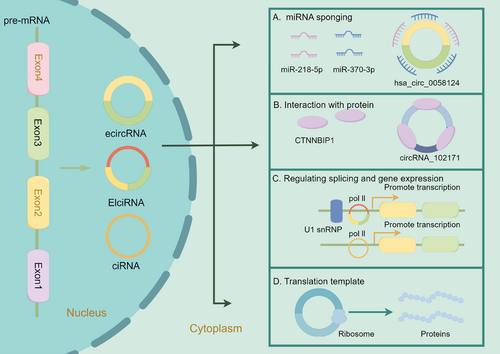
2.4.2 Interaction with proteins
CircRNAs can directly interact with proteins, functioning as protein sponges.90 For instance, the cytoplasm-localized circAnks1a directly interacts with YBX1, enhancing the interaction between YBX1 and transportin-1, thereby promoting the nuclear translocation of YBX1.91 CircAmotl1 can bind to PDK1 and AKT1, enhancing the phosphorylation of AKT1 and facilitating its nuclear translocation, exerting cardioprotective functions.92
Moreover, circRNAs can act as scaffolds, bringing different proteins into proximity to form functional complexes. CircYap binds directly to ACTG and TPM4 and facilitates their interaction by forming a complex that inhibits actin polymerization and fibrosis.93 Similarly, circSKA3 promotes invadopodium formation by forming a complex with Tks5 and integrin β1.94
2.4.3 Regulating splicing and gene expression
EIciRNAs and ciRNAs can modulate the transcription of their parental genes in cis.61, 62 The generation of ecircRNAs from pre-mRNAs influences the splicing process forming mature mRNAs that share splicing sites with the ecircRNAs.72, 95 In hepatocellular carcinoma (HCC), upregulated circRHOT1 recruits TIP60 to the promoter of NR2F6, initiating NR2F6 transcription and promoting HCC development and progression.96 CircSMARCA5 sponges the splicing factor SRSF1 to regulate the splicing of vascular endothelial growth factor A (VEGFA) pre-mRNA.97 Recent studies have confirmed that ecircRNAs can bind to RBPs to modulate both transcription and translation processes. In the nucleus, circAnks1a binds directly to the promoter of the Vegfb gene and promotes its transcription by recruiting YBX1.91 CircFAM120A competes with its cognate mRNA to bind IGF2BP2, promoting the translation of IGF2BP2-unbound FAM120A mRNA.98 CircYap could bind to its cognate linear yes-associated protein (Yap) mRNA and translational initiation factors poly(A)-binding protein and eIF4G. Overexpression of circYap could disrupt the assembly of Yap translation initiation machinery, inhibiting Yap translation.99 Additionally, the antisense circRNA circSCRIB blocks pre-mRNA splicing and post-transcriptional translation of its parental gene.100
2.4.4 Translation template
While previously considered noncoding, a minority of circRNAs has recently been confirmed to have translation potential.101-103 The internal ribosome entry site element and N6-methyladenosine (m6A) modification in circRNAs are assumed to initiate cap-independent translation.101, 104 A 73-amino acid protein termed “circPPP1R12A-73aa” is confirmed to be translated from circPPP1R12A in colon cancer.105 Notably, circPPP1R12A-73aa, encoded by circPPP1R12A and not circPPP1R12A itself, promotes cell proliferation and metastasis via Hippo-YAP signaling.105 CircARHGAP35 is translated into a large protein (1289 amino acids) in an m6A-dependent manner and interacts with TFII-I in the nucleus to promote tumor progression.106 CircE7, generated from oncogenic human papillomaviruses, can also be translated into the E7 oncoprotein.107 SEMA4B-211aa, a novel protein encoded by circSEMA4B, has been shown to inhibit the development of breast cancer (BC) by suppressing the phosphorylation of AKT.108 CircFBXW7 encodes the FBXW7-185aa protein to suppress the progression of triple-negative breast cancer (TNBC) by regulating the expression of FBXW7.109 Wang et al.110 found that ciRNAs containing G-rich repeats in the cytoplasm could serve as templates for repeat-associated non-AUG translation, producing toxic dipeptide repeat proteins.
2.5 Transport and turnover of circRNAs
After circRNA generation, EIciRNAs and ciRNAs often remain in the nucleus, whereas ecircRNAs are typically transported to the cytoplasm.5 Huang et al.111 demonstrated an evolutionarily conserved length-dependent pathway controlling the export of circRNAs. Shorter circRNAs (<400 nucleotides) are preferentially exported by URH49, while the transport of circRNAs >1200 nucleotides is mediated by UAP56.111 The transport regulation of circRNAs between 411 and 1099 nucleotides in length is complicated by the influence of RNA secondary structures.111 Additionally, m6A modification is involved in the export of circRNAs from the nucleus.112 Furthermore, the NXF1–NXT1 pathway plays a crucial role in the nuclear export of repeat-containing ciRNAs,110, 113 and the G-rich sequences and secondary structures of expanded repeats in the ciRNA are important for its stabilization and export mediation from the nucleus to the cytoplasm.110
CircRNAs have a much longer half-life than linear mRNAs, and their degradation mechanisms remain unelucidated.90, 114 Generally, degradation is thought to be initiated by endonucleases followed by a cascade of exonucleases or endonucleases.90 Hansen et al.115 proposed that miR-671 directly cleaves CDR1as in an Ago2-slicer-dependent manner by binding to CDR1as at the near-perfect target site with high conservation for miR-671. Park et al.116 found that m6A modification of circRNAs is recognized by m6A reader protein YTHDF2, which interacts with the adaptor protein HRSP12 to recruit the RNase P/MRP complex that degrades YTHDF2-bound circRNAs. Liu et al.117 reported that circRNAs are degraded by RNase L upon viral infection or poly (I:C) treatment. In addition to RNase L-mediated circRNA degradation under immune conditions, Fischer et al.118 discovered a structure-mediated circRNA decay mode under normal conditions. Besides, as a key component of P-body and RNAi machinery, Drosophila GW182 and its human homologs, TNRC6A/TNRC6B/TNRC6C, have been shown to regulate circRNA degradation by ribonucleases, in a process thought to be independent of the P-body and RNAi machinery.119, 120
Furthermore, circRNAs are enriched in exosomes and released into the extracellular space upon the fusion of multivesicular bodies with cell membranes.121, 122 The discharge of circRNAs from cells into the extracellular space via exosomes is another mechanism for circRNA clearance.123
2.6 Secondary structures of circRNAs
Secondary structures in circRNAs can bind to special proteins117 and modulate circRNA stability, nuclear export, and decay,110, 119 influencing the bond between circRNA and its parental linear mRNA.99
Through bioinformatic analysis, Sun et al.124 found that multiple circRNAs contain internal complementary base-pairing sequences (ICBPS). Complementary paired ICBPSs might enable circRNA to form secondary double-stranded structures. The maximum length of the ICBPS in most circRNAs is under 15 or even 10 nucleotides. Researchers have discovered more than 2000 circRNAs containing over 20 pairs of ICBPS. As the overall length of circRNAs increases, the number and maximum length of ICBPS also tends to increase.124 CircRNAs with a higher probability of internal base pairing are under 200 nucleotides in length.
In circRNAs, ICBPS overlaps with both MREs and open reading frames. Therefore, the double-stranded structure of circRNAs might influence their translation and miRNA sponging. Furthermore, the double-stranded structure of circRNA may also promote its bond with RBPs, facilitating its nuclear export and degradation.124 However, these hypotheses require confirmation in further studies.
3 DYSREGULATED circRNAs INVOLVED IN THE HALLMARKS OF CANCER
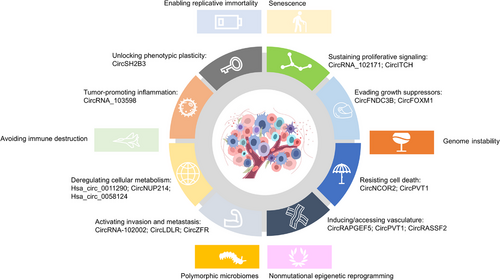
The progression of normal human cells into malignant tumors is a complex, multistep process.125 Hanahan and Weinberg have provided a comprehensive framework for understanding cancer biology, summarizing functional capabilities that define the “Hallmarks of Cancer.”22, 125 These hallmarks consist of eight acquired capabilities: sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing/accessing vasculature, activating invasion and metastasis, deregulating cellular metabolism, and avoiding immune destruction. Additionally, two enabling characteristics are present: genome instability and tumor-promoting inflammation.22 Recent updates include two emerging hallmarks: unlocking phenotypic plasticity and senescent cells, along with two novel enabling characteristics, nonmutational epigenetic reprogramming, and polymorphic microbiomes.23 In the following section, we summarize representative circRNAs participating in these hallmarks in various cancers, especially in TC (Figure 2).
3.1 Sustaining proliferative signaling
The sustained proliferation of cells is a fundamental feature of malignant tumors.22 While normal cells rely on external growth-promoting signals to maintain an active proliferative state, tumor cells can autonomously generate proliferative signaling by disrupting the production of growth factor ligands, expression of receptor molecules, and activation of downstream signaling pathways.22, 125
In HCC, hsa_circRNA_0104348 promotes the proliferation and inhibits apoptosis of HCC cells by regulating the miR-187-3p/RTKN2 axis and modulating Wnt/β-catenin pathways.126 CircSMO promotes the proliferation and migration of glioblastoma (GBM) cells via binding to miR-326 to upregulate CEP85.127 In gastric cancer (GC), circNFATC3 binds to IGF2BP3 to enhance the stability of IGF2BP3 by suppressing TRIM25-mediated ubiquitination, enhancing the IGF2BP3-CCND1 regulatory axis and elevating CCND1 mRNA stability to promote the proliferation of GC cells.128 CircNFATC3 also functions as an oncogene in GC, which promotes cell proliferation via the miR-23b-3p/RAI14 axis.129
Various circRNAs also contribute to the malignant proliferation of TC by activating downstream proliferative signaling cascades responsible for cell proliferation. Overexpression of hsa_circ_0007694 in TC cell lines suppresses cell proliferation, migration, and invasiveness. Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis indicates that dysregulated genes in PTC cell lines overexpressing hsa_circ_0007694 are enriched in mTOR and Wnt signaling, as well as cancer-related pathways. These results suggest a role for hsa_circ_0007694 in TC cell proliferation, marked by the suppression of key proteins involved in these pathways, including p-ATKSer473, p-GSK3BSer9, and Vim.130
The Wnt/β-catenin pathway is a pivotal oncogenic signaling pathway in PTC.131 The downregulation of circRNA_102171 enhances the interaction between CTNNBIP1 and β-catenin, which in turn, inhibits the interaction of β-catenin with TCF3/TCF4/LEF1, resulting in the suppression of target gene expression within the Wnt/β-catenin pathway. Consequently, this downregulation results in the inhibition of PTC cell proliferation both in vitro and in vivo. Notably, circRNA_102171 functions as a molecular sponge for CTNNBIP1 (Figure 1). However, it does not affect the expression of CTNNBIP1 mRNA, instead inhibiting the binding between CTNNBIP1 and β-catenin. Thus, circRNA_102171 facilitates the interaction between β-catenin and TCF/LEF, thereby activating the Wnt/β-catenin pathway and promoting the proliferation of PTC cells.132
Recently, the tumor suppressor circITCH had been identified to be downregulated in PTC tissue.131 Overexpression of circITCH can impair the proliferation and invasiveness of PTC cell lines, promoting apoptosis. This effect is partially reversed by the transfection of miR-22-3p mimics. Additional experiments revealed that circITCH overexpression suppresses the Wnt/β-catenin pathway through the degradation of β-catenin, achieved by upregulating CBL. Therefore, circITCH regulates the Wnt/β-catenin pathway via the miR-22-3p/CBL axis, resulting in the suppression of PTC cell proliferation.131
Yao et al.133 showed that hsa_circ_0058124 exhibits the highest fold-change in PTC tissue compared with normal tissue and is significantly upregulated in invasive tumors compared with that in noninvasive tumors. Knockdown of hsa_circ_0058124 results in significant inhibition of proliferation, migration, and invasiveness, accompanied by increased apoptosis. According to ceRNA theory, hsa_circ_0058124 upregulates the NOTCH pathway suppressor, NUMB, by sponging miR-218-5p. Besides, silencing hsa_circ_0058124 upregulates NOTCH3 and GATAD2A. Further in vitro and in vivo experiments suggested that hsa_circ_0058124 regulates NUMB expression and the downstream NOTCH3/GATAD2A signaling axis by sponging miR-218-5p in PTC.133 Additionally, Liu et al.134 found that hsa_circ_0058124 promotes the proliferation of PTC cells by modulating the miR-370-3p/LMO4 axis (Figure 1).
Consistent with the microarray profiling results of Peng et al.,55 Jin et al.,135 and Zhu et al.,136 circPSD3 (hsa_circ_0004458) has been identified as upregulated in PTC tissue and cell lines.137 Following circPSD3 knockdown in PTC cells, significant suppression of cell proliferation, migration, and invasiveness is observed. Silencing circPSD3 results in the downregulation of PI3K and Akt phosphorylation by increasing miR-637 and decreasing HEMGN levels. Therefore, circPSD3 acts as a sponge for miR-637, modulating HEMGN expression to regulate the PI3K/Akt signaling pathway and promoting PTC progression.137 Moreover, circPSD3 might function as an oncogene, promoting the proliferation of PTC cells by modulating the miR-7-5p/METTL7B/MMP2/MMP9136 and miR-885-5p/RAC1 axis.135
Knockdown of hsa_circ_0067934 induces lower rates of proliferation, migration, and invasiveness while promoting higher apoptosis rates in TC cell lines. These effects were attributed, in part, to the modulation of the epithelial–mesenchymal transition (EMT) and PI3K/Akt signaling pathways.138 Additionally, Zhang et al.139 found that downregulating hsa_circ_0067934 suppresses TC proliferation by regulating the miR-1304/CXCR1 axis. Hsa_circ_0009294, with the highest expression levels among the ecircRNAs generated from SSU72 in thyroid cell lines, was designated as circSSU72.140, 141 Zhang et al.141 confirmed its participation in the proliferation, migration, and invasion of PTC by modulating the miR-451a/S1PR2 axis and downstream Akt pathway. Besides, circNRIP1 could promote the development of PTC via miR-195-5p to regulate the P38 MAPK and JAK/STAT pathways.142 Silencing the upregulated hsa_circ_0005273 in PTC cell lines suppresses proliferation, migration, and invasiveness. Further experiments revealed that hsa_circ_0005273 acts as an oncogene, accelerating PTC proliferation via the miR-1183/SOX2 axis.143 Similarly, CDR1as overexpression promotes proliferation, migration, and invasion while inhibiting apoptosis of PTC cells in vitro by modulating the miR-7/EGFR axis.144 Silencing circRNA_104565 inhibits PTC cell proliferation in vitro and in vivo. Rescue experiments demonstrated that circRNA_104565 promotes cell proliferation by sponging miR-134 to release ELF2.145 Silencing circFAT1(e2) inhibits proliferation, migration, and invasiveness of PTC cells by sponging miR-873 to regulate downstream ZEB1.146
With emphasis on TC, the functional mechanisms of dysregulated circRNAs in hallmarks of TC are summarized in Table 1. To better review the roles of circRNAs in the hallmarks of cancers, representative circRNAs are reviewed based on the roster of hallmark capabilities, providing insights into their involvement in the distinctive features of cancer progression (Figure 2).
| Hallmarks | CircRNAs | Chromosome | Gene symbol | Length (bp) | Expression change | Location | Relationships with the clinical features | Functions | Possible mechanism | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Sustaining proliferative signaling | Hsa_circ_0007694 | – | – | – | Down | – | – | Inhibit proliferation, migration, invasion, promote apoptosis, and arrest cell cycle at S stage | Suppressing p-ATKSer473, p-GSK3BSer9, Vim and regulating mTOR signaling pathways, Wnt signaling pathway, and cancer-related pathways | 130 |
| CircRNA_102171 | SMURF2 | Up | Nucleus | – | Promote proliferation, migration, invasion, inhibit apoptosis | Activate Wnt/β-catenin pathway by interacting with CTNNBIP1 and blocking its interaction with the β-catenin/TCF complex | 132 | |||
| CircITCH | – | ITCH | – | Down | – | Clinical stage, LNM and survival status | Inhibit proliferation, invasion and promote apoptosis | Suppressing activation of Wnt/β-catenin pathway through regulating miR-22-3p/CBL axis | 131 | |
| Hsa_circ_0058124 | chr2 | FN1 | 864 | Up | Nucleus | Tumor size, TNM stage, extrathyroidal extension, LNM, distant metastasis | Promote proliferation, migration, invasion, inhibit apoptosis | Sponging miR-218-5p to upregulate NUMB expression and repress downstream NOTCH3/GATAD2A signaling axis | 133 | |
| Hsa_circ_0058124 | – | – | – | Up | – | – | Promote proliferation, migration and invasion while inhibit apoptosis | Via miR-370-3p/LMO4 axis | 134 | |
| CircPSD3 (hsa_circ_0004458) | – | PSD3 | – | Up | – | – | Promote proliferation, migration, invasion and cell cycle progression while inhibit apoptosis | Via miR-637/HEMGN axis and downstream PI3K/Akt signal pathway | 137 | |
| CircPSD3 (hsa_circ_0004458) | chr8:18656804-18662408 | PSD3 | 448 | Up | – | Tumor size, T stage, LNM, distant metastasis, and TNM stage | Promote proliferation, cell cycle progression, and inhibit apoptosis | Regulating miR-885-5p/RAC1 axis | 135 | |
| CircPSD3 (hsa_circ_0004458) | – | PSD3 | – | Up | – | Advanced TNM stage, tumor size, and LNM | Promote proliferation and invasion | Via miR-7-5p/METTL7B axis | 136 | |
| Hsa_circ_0067934 | – | – | – | Up | – | Tumor sizes, LNM, and AJCC stages | Promote proliferation, migration, invasion, and inhibit apoptosis | Regulating EMT and PI3K/AKT signaling pathways | 138 | |
| Hsa_circ_0067934 | chr3 | PRKCI | – | Up | Cytoplasm | LNM and AJCC stages | Promote proliferation, migration, invasion, and inhibit apoptosis | Regulating miR-1304/CXCR1 axis | 139 | |
| CircSSU72 (hsa_circ_0009294) | chr1: 1477053–1479367 | SSU72 | – | Up | Cytoplasm | Tumor size, capsule invasion and LNM | Promote proliferation, migration, and invasion | Via miR-451a/S1PR2 axis and downstream AKT pathway | 141 | |
| CircNRIP1 | – | NRIP1 | – | Up | – | Advanced TNM stages | Promote proliferation and invasion while inhibit apoptosis | Via modulating miR-195-5p and P38 MAPK and JAK/STAT pathways | 142 | |
| Hsa_circ_0005273 | – | – | – | Up | Cytoplasm | – | Promote proliferation, migration, invasion | Regulating miR-1183/SOX2 axis | 143 | |
| CDR1as | chrX:139865339-139866824 | CDR1 | 1485 | Up | – | Tumor size and LNM | Promote proliferation, migration and invasion while inhibit apoptosis | Via miR-7/EGFR axis | 144 | |
| CircRNA_104565 | – | – | – | Up | – | – | Promote proliferation | Via miR-134/ELF2 axis | 145 | |
| CircFAT1(e2) (has_circ_0001461) | – | FAT1 | – | Up | Cytoplasm | – | Promote proliferation, migration, and invasion | Via miR-873/ZEB1 axis | 146 | |
| Evading growth suppressors | CircTP53 | – | TP53 | – | Up | Cytoplasm | – | Promote proliferation | Via miR-1233-3p/MDM2 axis and downstream p53 pathway | 147 |
| CircWDR27 (hsa_circ_0078738) | chr6:170033042-170058454 | WDR27 | – | Up | Cytoplasm | – | Promote proliferation, migration, invasion, and cell cycle progression while inhibit apoptosis | Via miR-215-5p/TRIM44 axis | 148 | |
| Hsa_circ_0058129 | chr2:216271849-216296687 | FN1 | – | Up | Cytoplasm | – | Promote proliferation, migration, invasion, and cell cycle progression | Via miR-873-5p/FSTL1 axis | 149 | |
| CircFNDC3B (hsa_circ_0006156) | chr3:171965322-171969331 | FNDC3B | 526 | Up | Cytoplasm | Tumor size, LNM, advanced TNM stages and survival status | Promote proliferation, migration, invasion, cell cycle progression, and inhibit apoptosis | Regulating miR-1178/TLR4 axis | 150 | |
| CircFOXM1 (hsa_circ_0025033) | chr12: 2966846–2983691 | FOXM1 | 3410 | Up | Cytoplasm | Tumor size, TNM stage, LNM, nodular Goiter and distant metastasis | Promote proliferation, cell cycle progression | Regulating miR-1179/HMGB1 axis | 151 | |
| CircFOXM1 (hsa_circ_0025033) | chr12:2966846-2983691 | FOXM1 | – | Up | – | – | Promote proliferation, migration, invasion, and inhibit apoptosis | Regulating miR-1231 and miR-1304 | 152 | |
| Resisting cell death | CircNCOR2 (hsa_circ_0000461) | chr12:124911167-124934413 | NCOR2 | 566 | Up | Cytoplasm | – | Promote proliferation, migration and invasion while inhibit apoptosis | Via miR-615a-5p/MTA2 axis | 153 |
| CircPRMT5 | – | PRMT5 | – | Up | – | TNM stage and LNM | Promote proliferation, migration, invasion, and inhibit apoptosis | Regulating miR-30c/E2F3 axis | 154 | |
| Hsa_circ_0000644 | – | KIAA1199 | – | Up | Cytoplasm | Tumor size and LNM | Promote proliferation, migration, and invasion while inhibit apoptosis | Via miR-1205/E2F3 axis | 155 | |
| Hsa_circ_0001666 (hsa-circRNA-000742) | chr6: 70726457–170739638 | FAM120B | – | Up | Cytoplasm | LNM | Promote proliferation and cell cycle progression while inhibit apoptosis | Via miR-330-5p/miR-193a-5p/miR-326/ETV4 axis | 156 | |
| CircRPS28 (hsa_circ_0049055) | – | RPS28 | – | Up | Cytoplasm | – | Promote proliferation, migration, and invasion while inhibit apoptosis | Via miR-345-5p/FZD8 axis | 157 | |
| CircTIAM1 (hsa_circ_0061406) | chr21: 32554737–32567621 | TIAM1 | – | Up | Cytoplasm | Advanced TNM stages, tumor size, LNM | Promote proliferation and migration while inhibit apoptosis | Via miR-646/HNRNPA1 axis | 158 | |
| Hsa_circ_0011385 | – | EIF3I | – | Up | Cytoplasm | – | Promote proliferation, migration, invasion, and inhibit apoptosis and cell cycle arrest | Regulating miR-361-3p, Bax, caspase-3, TIMP, MMP2, and MMP9 | 159 | |
| CircBACH2 (hsa_circ_0001627) | chr6:90959407–90981660 | BACH2 | 2995 | Up | Cytoplasm | Tumor size, TNM stage, LNM and survival status | Promote proliferation, migration, invasion, and inhibit apoptosis | Regulating miR-139-5p/LMO4 axis | 160 | |
| CircPVT1 | – | PVT1 | – | Up | – | T stage, LNM and survival status | Promote proliferation, migration, invasion, and inhibit apoptosis | Regulating miR-126, Bax, Bcl-2 | 161 | |
| Hsa_circ_0102272 | – | RTN1 | 487 | Up | – | TNM stage, histological grade, LNM, and overall survival state and progression-free survival status | Promote proliferation, migration, invasion, and inhibit apoptosis | – | 162 | |
| CircNEURL4 (hsa_circ_0041821) | chr17:7225183-7225329 | NEURL4 | 146 | Down | Cytoplasm | Advanced TNM stage, LNM, and survival status | Inhibit proliferation, migration, and invasion while promote apoptosis | Via miR-1278/LATS1 axis | 163 | |
| CircEIF6 (hsa_circ_0060060) | – | EIF6 | 799 | Up | – | – | Promote proliferation, autophagy, inhibit apoptosis, and promote the cisplatin-resistance | Regulating miR-144-3p/TGF-α axis to promote the cisplatin-resistance of human thyroid carcinoma cells by autophagy regulation | 164 | |
| Hsa_circ_0067934 | chr3 | PRKCI | – | Up | – | – | Promote proliferation, repress ferroptosis and apoptosis | Via miR-545-3p/SLC7A11 axis | 165 | |
| Inducing/accessing vasculature | CircRAPGEF5 (hsa_circ_0001681) | chr7:22330794-22357656 | RAPGEF5 | 516 | Up | Cytoplasm | – | Promote proliferation, migration, invasion | Regulating miR-198/FGFR1 axis | 53 |
| Hsa_circ_0079558 | – | RAPGEF5 | – | Up | – | Advanced TNM stages, tumor size, LNM | Promote proliferation and invasion while inhibit apoptosis | Via miR-26b-5p/MET axis regulating MET/AKT signaling pathway and miR-198/FGFR1 axis | 166 | |
| CircPVT1 | chr8: 128902834–128903244 | PVT1 | – | Up | Cytoplasm | Advanced TNM stages, tumor size, LNM | Promote proliferation, migration, and invasion | Via miR-195/VEGFA axis (and Wnt/β-catenin signaling pathway) | 167 | |
| Hsa_circ_0011058 | – | TMEM222 | – | Up | Cytoplasm | Advanced TNM stage, LNM, nodular goiter and survival status | Promote proliferation, angiogenesis, and inhibit apoptosis and radiosensitivity | Via miR-335-5p/YAP1 axis | 168 | |
| CircRASSF2 (Hsa_circ_0059354) | chr20:4760668-4766974 | RASSF2 | – | Up | Cytoplasm | TNM stages and LNM | Promote proliferation, migration, invasion, angiogenesis, and inhibit apoptosis | Via miR-766-3p/ARFGEF1 axis | 169 | |
| CircRASSF2 (hsa_circ_0059354) | chr20:4760668-4766974 | RASSF2 | – | Up | Cytoplasm | TNM stage, LNM and distant metastasis | Promote proliferation, migration, invasion, cell cycle progression, and inhibit apoptosis | Regulating miR-1178/TLR4 axis | 170 | |
| Activating invasion and metastasis | CircRNA-102002 | – | USP22 | – | Up | Cytoplasm | LNM, higher T stage and survival status | Promote EMT, migration, and invasion | Via miR-488-3p/HAS2 axis | 171 |
| CircLDLR (hsa_circ_0003892) | chr19: 11230767–11238761 | LDLR | 544 | Up | – | – | Promote proliferation, migration, and invasion while inhibit apoptosis | Via miR-195-5p/LIPH axis | 172 | |
| CircLDLR (hsa_circ_0003892) | chr19: 11230767–11238761 | LDLR | 544 | Up | Cytoplasm | Advanced TNM stages, tumor size, LNM and survival status | Promote proliferation, migration, and invasion while inhibit apoptosis | Via miR-637/LMO4 axis | 173 | |
| Hsa_circ_0008274 | – | – | – | Up | – | Advanced TNM stage, LNM, tumor infiltration and survival status | Promote migration and adhesion while inhibit apoptosis | Via miR-154-3p/SLC7A11 axis | 174 | |
| CircRUNX1 (hsa_circ_0002360) | chr21: 36206706–36231875 | RUNX1 | 297 | Up | Cytoplasm | Larger tumor size, advanced TNM stage, extrathyroidal extension and LNM | Promote proliferation, migration, and invasion | Via miR-296-3p/DDHD2 axis | 175 | |
| Hsa_circ_0001018 | – | CCT4 | 348 | Up | Cytoplasm | Advanced TNM stage, LNM and distant metastasis | Promote proliferation, migration, invasion, and cell cycle progression while inhibit apoptosis | Via miR-338-3p/SOX4 axis | 54 | |
| CircVANGL1 | – | VANGL1 | – | Up | Cytoplasm | Advanced TNM stages and LNM | Promote proliferation, migration, and invasion | Via miR-194/ZEB1 axis and downstream EMT pathway | 176 | |
| CircCCDC66 | – | CCDC66 | – | Up | Cytoplasm | Advanced TNM stages, tumor size, LNM and survival status | Promote proliferation, migration, and invasion | Via miR-129-5p/LARP1 axis and downstream EMT pathway | 177 | |
| CircZFR (hsa_circ_0072088) | chr5 | ZFR | – | Up | – | TNM stage, LNM and survival status | Promote proliferation, migration, and invasion | Regulating miR-1261/C8orf4 axis | 57 | |
| CircEIF3I | – | EIF3I | – | Up | – | Tumor size, TNM stage, LNM | Promote proliferation, migration, invasion | Regulating miR-149/KIF2A axis | 178 | |
| Hsa_circ_0062389 | – | PI4KA | – | Up | – | Large tumor size and LNM | Promote proliferation, migration, and EMT | Via miR-1179/HMGB1 axis | 179 | |
| Hsa_circ_0039411 | chr16 | – | – | Up | – | – | Promote proliferation, migration, invasion, and inhibit apoptosis | Regulating miR-1179/ABCA9 axis and miR-1205/MTA1 axis | 180 | |
| Deregulating cellular metabolism | Hsa_circ_0011290 | – | – | – | Up | – | Advanced stages and survival status | Promote proliferation, glycolysis while inhibit apoptosis | Via miR-1252/FSTL1 axis | 181 |
| CircNUP214 | – | NUP214 | – | Up | Cytoplasm | – | Promote proliferation, migration, invasion, glycolysis while inhibit apoptosis | Via miR-15a-5p/HK2 axis | 182 | |
|
CircNUP214 (hsa_circ_0089153) |
chr9 | NUP214 | – | Up | Cytoplasm | – | Promote proliferation, migration, invasion, and inhibit apoptosis | Regulating miR-145/ZEB2 axis | 183 | |
| CircPRKCI (hsa_circ_0122683) | – | PRKCI | – | Up | Cytoplasm | LNM and recurrence | Promote proliferation, migration, invasion, glycolysis while arrest cell cycle | Via miR-335/E2F3 axis | 184 | |
| Hsa_circ_0058124 | chr2 | FN1 | 864 | Up | Cytoplasm | TNM stage | Promote proliferation, migration, invasion, and metabolic abilities | Regulating miR-940/MAPK1 axis | 185 | |
| CircRAD18 | – | – | – | Up | – | – | Promote cell glucose uptake and lactate production as well as proliferation and metastasis | Via miR-516b/PDK1 axis | 186 | |
| CircPUM1 | – | PUM1 | – | Up | – | Advanced TNM stage, LNM and survival status | Promote proliferation, migration, invasion, and glycolysis | Via miR-21-5p/MAPK1 axis | 187 | |
| Tumor-promoting inflammation | CircRNA_103598 | – | – | – | Up | – | Advanced TNM stage, tumor size, metastasis status and survival status | Promote proliferation and OVV mediated antitumor effects | Via miR-23a-3p/IL-6 axis | 188 |
| Unlocking phenotypic plasticity | CircSH2B3 (hsa_circ_0006741) | chr12: 111405107–111451623 | SH2B3 | 759 | Up | – | – | Promote proliferation while inhibit 125I uptake, NIS expression and differentiation of PTC cells | Via miR-4640-5p/IGF2BP2 axis | 189 |
- Abbreviations: circRNA, circular RNA; LNM, lymph node metastasis; TC, thyroid cancer.
3.2 Evading growth suppressors
In addition to achieving self-sufficiency in proliferative signaling, tumor cells possess the ability to circumvent suppressor programs that negatively regulate cell proliferation.22, 125 For instance, canonical suppressor genes TP53 and RB often exhibit functional subversion in various tumors.22 Hanahan and Weinberg125 highlighted that during tumor development, cell cycle arrest induced by antigrowth signals to block proliferation is evaded and short circuited. Several circRNAs have been demonstrated to be involved in the evasion of growth suppressors and cell cycle arrest in different cancers.
Inactivation of p53 is essential for glioma tumorigenesis, in particular GBM. CDR1as can directly bind to the p53 DBD domain, disrupting the formation of the p53/MDM2 complex to protect p53 from ubiquitination and degradation. Thus, CDR1as contributes to the inhibition of gliomagenesis by directly interacting with proteins rather than serving as miRNA sponges.190 In BC, hypoxia-inducible circWSB1 binds to the deubiquitinase USP10, suppressing USP10-mediated p53 stabilization and promoting the progression of BC.191 The upregulated circZFR in cervical cancer promotes p-Rb phosphorylation by binding to SSBP1 and activating CDK2/cyclin E1 complexes, which release activated E2F1 to promote the expression of DNA replication-associated genes and accelerate cell cycle progression.192
In TC, several circRNAs also participate in the mediation of growth suppressor evasion. CircTP53, derived from TP53, exhibits upregulation in TC tissue compared with that in normal tissue.147 CircTP53 levels show a negative correlation with p53 expression in TC tissue. Overexpression of circTP53 promotes the viability and proliferation of PTC cells, reducing p21 at both the mRNA and protein levels, and decreasing p53 expression at the protein level without affecting mRNA levels.147 Cytological experiments confirm that circTP53 might function in TC by sponging miR-1233-3p to release MDM2, acting as an E3 ubiquitin ligase for p53 degradation in the proteasome,193 thereby regulating the p53 signaling pathway.147
CircWDR27 (hsa_circ_0078738) is significantly upregulated in PTC tissue and cell lines compared with that in normal controls,148 consistent with the microarray profiling results of Ye et al.151 Suppression of circWDR27 arrests cells in the G0/G1 phase, promotes apoptosis, and inhibits proliferation, migration, and invasiveness of PTC cells. In vitro and in vivo experiments indicated that circWDR27 serves as a tumor promoter in PTC by modulating the miR-215-5p/TRIM44 axis to accelerate the cell cycle.148
Silencing hsa_circ_0058129 inhibits PTC progression by regulating the miR-873-5p/FSTL1 axis to induce cell cycle arrest.149 Similarly, circFNDC3B inhibition causes G1-phase cell cycle arrest, restrains PTC cell proliferation, migration, and invasion, and promotes apoptosis.150 Rescue experiments confirm that circFNDC3B promotes PTC cell cycle progression via the miR-1178/TLR4 axis.150 Overexpression of circFOXM1 (hsa_circ_0025033) in vitro promotes cell cycle progression and enhances the proliferation of PTC cells, while circFOXM1 knockdown has the contrary effects. Predominantly enriched in the cytoplasmic fractions of PTC cells, circFOXM1 participates in PTC tumorigenesis by regulating the miR-1179/HMGB1 network.151 Moreover, Pan et al.152 found that overexpressing circFOXM1 inhibits apoptosis and enhances the viability, proliferation, migration, and invasiveness of PTC cells by suppressing miR-1231 and miR-1304. The interaction of miR-1231/miR-1304 with circFOXM1 has a synergistic effect.152 This phenomenon confirms that circRNAs could function as ceRNAs by sponging different miRNAs to evade cell cycle arrest modulated by tumor suppressors.
3.3 Resisting cell death
The maintenance and expansion of tumors are influenced by both cell proliferation and death.125 Generally, three major pathways are involved in cell death: apoptosis, autophagy, and necrosis.1, 22
In retinoblastoma, knocking down circFAM158A promotes apoptosis in vitro and in vivo by modulating the miR-138-5p/SLC7A5 axis.194 Downregulation of circCCS in lung cancer cells promotes apoptosis in vitro via regulating the miR-383/E2F7 axis.195 In gastrointestinal stromal tumors (GISTs), circSMA4 inhibits apoptosis via the miR-494-3p/KIT axis and by modulating the downstream JAK/STAT signaling pathway.196 CircPTPN22 stimulates the phosphorylation of Akt and Erk via the miR-6788-5p/PAK1 axis, thus mediating autophagy in GC cells.197 Additionally, circDHX8 can competitively bind to RNF5, inhibiting the interaction between ATG2B and RNF5 to maintain the stability of ATG2B protein, thus promoting autophagy and tumor development in GC.198
In TC, most research has focused on the roles of circRNAs in apoptosis. Silencing or overexpression of circNCOR2 (hsa_circ_0000461) increases or inhibits PTC cell apoptosis, respectively. Primarily distributed in the cytoplasm of PTC cells, circNCOR2 functions via the post-transcriptional regulation of the miR-615a-5p/MTA2 axis.153 Knockdown of hsa_circ_0000644 or circPRMT5 promotes apoptosis and inhibits the proliferation, migration, and invasiveness of PTC cell lines, which could be reversed by overexpressing E2F3 or inhibiting miR-1205 or miR-30c. Considering that E2F3 is a master regulator of DNA damage-induced apoptosis,199 circPRMT5 and hsa_circ_0000644 might serve as carcinogenic circRNAs to suppress PTC apoptosis through modulating the miR-30c/E2F3 and miR-1205/E2F3 axes, respectively.154, 155
Silencing of hsa_circ_0001666 promotes apoptosis and inhibits cell proliferation both in vitro and in vivo, which could be reversed by overexpression of EVT4 or inhibition of miR-330-5p, miR-193a-5p, or miR-326 in vitro. Upregulation of hsa_circ_0001666 in PTC prevents cell death and plays an oncogenic role by regulating EVT4 via acting as a miRNA sponge.156 Silencing circRPS28 (hsa_circ_0049055) induces apoptosis in PTC cells and blocks their proliferation, migration, and invasiveness. CircRPS28, mainly distributed in the cytoplasm, might function as an oncogene in PTC by sponging miR-345-5p to modulate FZD8, thereby preventing cell death.157
Knockdown of upregulated circTIAM1 (hsa_circ_0061406) promotes apoptosis of PTC cells and decreases their migration and proliferation abilities, which can be reversed by inhibiting miR-646. HNRNPA1 is a target of miR-646, and overexpression of HNRNPA1 reverses the antitumor effects of miR-646 overexpression in PTC cells. Therefore, circTIAM1 inhibits PTC apoptosis via the miR-646/HNRNPA1 axis.158 Silencing of the upregulated hsa_circ_0011385 promotes apoptosis and induces cell cycle arrest in PTC cells by modulating miR-361-3p.159 Similarly, silencing circBACH2 enhances apoptosis of PTC cells, and a mechanistic study suggested that circBACH2 might promote PTC by regulating apoptosis via the miR-139-5p/LMO4 axis.160 Tao et al.161 found that circPVT1 plays an essential role in PTC apoptosis and progression by modulating miR-126, a tumor suppressor in PTC.200, 201 Meanwhile, hsa_circ_0102272 might serve as an oncogene in TC by regulating apoptosis; however, the underlying regulatory mechanism requires further investigation.162 These results suggest that circRNAs may be a novel therapeutic target for promoting cell death in TC.
Few studies have been conducted on the role of downregulated circRNAs in PTC apoptosis. CircNEURL4 (hsa_circ_0041821) is downregulated in PTC tissue and cell lines.163 Overexpression of circNEURL4 stimulates PTC cell apoptosis and inhibits PTC cell proliferation in vitro and tumor formation in vivo, which could be reversed by overexpression of miR-1278. CircNEURL4, mainly located in the cytoplasm, might function as a “sponge” to target miR-1278, liberating LATS1 to modulate the apoptosis progress of PTC.
Recent research has focused on the roles of circRNAs in autophagy. The upregulated expression of circEIF6 (hsa_circ_0060060) in TC tissue55 was confirmed in five pairs of ATC tissue and paired normal tissue and in ATC and PTC cell lines compared with that in normal cell lines.164 Cisplatin treatment results in the upregulation of circEIF6 and downregulation of miR-144-3p. TGF-α levels and LC3 II/LC3 I ratios are increased, and cleaved poly (ADP-ribose) polymerase (PARP), cleaved Caspase3, and p62 levels are decreased by overexpression of circEIF6. Furthermore, the effect of circEIF6 overexpression could be reversed by miR-144-3p mimics. Together with additional GFP-LC3 puncta detection used for testing autophagy, these results suggest that circEIF6 induces autophagy and promotes proliferation by upregulating TGF-α during cisplatin treatment, which could be reversed by miR-144-3p.164
Ferroptosis, an iron-dependent form of nonapoptotic regulated cell death, has garnered considerable attention.202 As a reactive oxygen species (ROS)-dependent form of cell death, ferroptosis is characterized by two main biochemical features: lipid peroxidation and iron accumulation. Recently, Li et al.203 found that circSTIL suppresses ferroptosis in colorectal cancer via the miR-431/SLC7A11 signaling axis. CircLRFN5 could modulate ferroptosis in GBM by binding to the PRRX2 protein and promoting its degradation, which downregulates the ferroptosis suppressor GCH1.204 In PTC cells, Wang et al.165 observed that silencing hsa_circ_0067934 increases the levels of ferroptosis-related markers, including Fe2+, iron, and ROS, producing an effect similar to that of erastin stimulation. Further experiments indicated that hsa_circ_0067934 regulates ferroptosis, apoptosis, and proliferation of PTC by modulating the key ferroptosis-negative regulator SLC7A11 through sponging miR-545-3p.165
3.4 Enabling replicative immortality
Generally, cultured cells undergo senescence and subsequently enter a crisis phase after repeated cycles of cell division. This process is regulated by telomeres, which protect the ends of chromosomes and shorten progressively in nonimmortalized cells during each round of DNA replication.5 In tumor cells capable of immortalized division, the specialized DNA polymerase known as telomerase is expressed to circumvent this replicative barrier. By adding telomere repeat segments to telomeric DNA, telomerase extends telomeres.22 As the core catalytic subunit of telomerase, telomerase reverse transcriptase (TERT) plays essential roles in the tumorigenesis and development of various cancers.205
In HCC, Zhang et al.206 investigated the effects of TERT promoter mutations on the expressions of ncRNAs. In the mutant promoter group, 21 circRNAs were significantly upregulated, and 23 circRNAs were significantly downregulated. Among them, bioinformatic analysis indicated that hsa_circ_0003154, hsa_circ_0008952, and hsa_circ_0031584 could play essential roles in the tumorigenesis of HCC.206 In colorectal cancer, hsa_circ_0020397 enhances cell viability and invasion of cancer cells and suppresses their apoptosis by regulating the expression of miR-138 target genes, including PD-L1 and TERT.207 Wang et al.208 found that silencing hsa_circ_0000263 in Hela cells inhibits telomerase activity and promotes apoptosis and radiosensitivity by modulating the miR-338-3p/TERT axis. Additionally, circWHSC1 was shown to promote ovarian cancer progression by upregulating TERT through the sequestration of miR-1182.209
3.5 Inducing or accessing vasculature
Similar to normal tissue, tumor tissue requires vascularization to provide nutrients and oxygen, meeting the increasing metabolic demands of malignant cells while facilitating the removal of metabolic waste and carbon dioxide.22, 210 Tumor vasculature can develop through angiogenesis or by co-opting normal tissue vessels, principally via invasion and metastasis.23 Several circRNAs are known to participate in angiogenesis.
The upregulated circFNDC3B in oral squamous cell carcinoma (OSCC) stimulates angiogenesis by accelerating the ubiquitin degradation of FUS and promoting VEGFA expression and angiogenesis.211 In GC, after being transported from the nucleus to the cytoplasm, m6A-modified circPAK2 interacts with IGF2BPs, forming a circPAK2/IGF2BPs complex to stabilize VEGFA mRNA, thereby promoting angiogenesis and lymph node metastasis (LNM).212 Hsa_circ_0000520 acts as a scaffold, promoting the binding of UBE2V1/UBC13 to Lin28a, which facilitates the ubiquitous degradation of Lin28a in bladder cancer. By increasing PTEN mRNA stability and suppressing the PI3K/AKT pathway, the vasculogenic mimicry (VM) formations of bladder cancer cells are significantly inhibited.213 Similarly, hsa_circ_0000758 accelerates the angiogenesis of bladder cancer by regulating the miR-1236-3p/ZEB2 axis.214 Additionally, hsa_circ_0084043, derived from colorectal cancer-associated fibroblasts (CAFs), had been shown to induce angiogenesis by sponging miR-140-3p, thereby regulating the functions of the VEGF signaling pathway.215
Members of the fibroblast growth factor (FGF) family are endowed with potent proangiogenic activities. Activation of the FGF/FGF receptor (FGFR) system may lead to neovascularization in various human tumors, supporting tumor progression and metastatic dissemination.216 Among them, the role of FGFR1 has been demonstrated previously.217 In PTC, circRAPGEF5 (hsa_circ_0001681) modulates FGFR1 expression by regulating miR-198,53 implying its potential influence on angiogenesis in PTC cells. The tumor-promoting roles of circRAPGEF5 that enhance the proliferation, migration, and invasiveness of PTC cells have been described53; however, further experimental evidence of circRAPGEF5 stimulating angiogenesis is required. Another circRNA derived from RAPGEF5, hsa_circ_0079558, could also regulate FGFR1 expression by modulating the expression of miR-198, which in turn could modulate the angiogenesis of PTC cells.166 Similarly, members of the VEGF family have been identified as inducers of tumor angiogenesis.216 In vivo and in vitro experiments confirmed that circPVT1 regulates the expression of miR-195 to modulate the activities of VEGFA and the Wnt/β-catenin signaling pathway.167 Hence, circPVT1 might play a carcinogenic role in PTC by inducing angiogenesis.
Knockdown of hsa_circ_0011058 inhibits angiogenesis in PTC cells, which manifests as reduced tube formation and downregulation of the angiogenesis activators, VEGFA and FGF-2. The proliferation of PTC cells is also inhibited, whereas apoptosis and radiosensitivity of PTC cells are enhanced. Mainly distributed in the cytoplasm, hsa_circ_0011058 has been shown to regulate YAP1 by sponging miR-335-5p. In vivo experiments suggested that silencing hsa_circ_0011058 inhibits the formation of xenograft tumors and decreases microvessel density in xenograft tumors. In summary, hsa_circ_0011058 is involved in angiogenesis, proliferation, apoptosis, and radioresistance in PTC by modulating the miR-335-5p/YAP1 axis.168
Knockdown of circRASSF2 (hsa_circ_0059354) in PTC cells suppresses angiogenesis, which was assessed using a tube formation assay of human umbilical vein endothelial cells cultured in a PTC cell suspension. Besides, silencing circRASSF2 suppresses cell proliferation, migration, and invasion and promotes apoptosis in PTC cells.169 Rescue experiments demonstrated that circRASSF2 serves as an oncogene in PTC by modulating the miR-766-3p/ARFGEF1 axis.169 Additionally, Wu et al.170 found that circRASSF2 regulates proliferation, migration, invasiveness, cell cycle progression, and apoptosis of PTC cells through the miR-1178/TLR4 axis.
In brief, the above studies suggest that circRNAs play crucial roles in vascular dysregulation, promoting the progression of this hallmark in TC.
3.6 Activating invasion and metastasis
Invasion and metastasis are representative hallmarks of malignant tumors and are usually associated with poor prognosis.22, 24, 125 During the multistep process of invasion and metastasis, cancer cells undergo morphological alterations and changes in cell-cell or cell-matrix interactions, accompanied by dysregulation of E-cadherin, N-cadherin, and extracellular proteases.1, 22, 125 EMT is arguably essential for modulating invasion and metastasis.22
The upregulated circFNDC3B in OSCC promotes EMT and lymphangiogenesis by sequestering miR-181c-5p, leading to the upregulation of SERPINE1 and PROX1.211 Additionally, another circRNA derived from FNDC3B, hsa_circ_0003692, could be translated to a novel protein-FNDC3B-267aa in GC, which inhibits GC migration and metastasis by directly binding to c-Myc and promoting its degradation, thereby suppressing the downstream c-Myc-Snail/Slug axis.218 Similarly, circYAP encodes a novel truncated protein, YAP-220aa, which binds to LATS1 and leads to YAP dephosphorylation and nuclear translocation, thereby activating a host of metastasis-promoting genes in colorectal cancer.219 Furthermore, circYAP transcription is activated by YAP, thus forming a positive feedback loop promoting the liver metastasis of colorectal cancer.219 In addition, hsa_circ_0088036 promotes the invasion and metastasis abilities of bladder cancer cells through the miR-140-3p/FOXQ1 signaling axis.220
LNM is a well-known risk factor for TC recurrence and poor outcomes.46, 221, 222 Mounting evidences suggest that circRNAs can promote EMT in TC to facilitate the invasion-metastasis cascade. Overexpression of circRNA_102002 in PTC causes a noticeable shift in cellular morphology to a spindle shape with increased intercellular mass. This is accompanied by the downregulation of E-cadherin and the upregulation of N-cadherin, Vimentin, Slug, Twist, MMP2, and MMP9, promoting the EMT process and enhancing the migration and invasion of PTC cells. In addition, silencing of circRNA_102002 inhibits lung metastasis of PTC cells in vivo. Further mechanistic investigation suggested that circRNA-102002 promotes EMT, as well as the migration and invasiveness of PTC cells, to facilitate PTC metastasis by modulating the miR-488-3p/HAS2 axis.171
Consistent with the microarray profiling results of Peng et al.,55 circLDLR (hsa_circ_0003892) is upregulated in PTC tissue and cell lines.172, 173 Silencing circLDLR causes decreased migration, invasion, and proliferation and promotes PTC cell apoptosis, resulting in xenograft tumors of smaller size and lighter weight.172, 173 Focusing on the underlying mechanism, Gui et al.172 found that overexpression of circLDLR increases Twist1 levels and decreases E-cadherin expression by modulating LIPH through sponging miR-195-5p to promote the migration and invasion of PTC cells. Jiang et al.173 also observed that circLDLR knockdown decreases the expression of MMP2 and MMP9 via the miR-637/LMO4 pathway, inhibiting migration and invasiveness of PTC cells. Knockdown of hsa_circ_0008274 significantly decreases the expression of ICAM-1, fibronectin, and vitronectin, thereby suppressing cell migration and adhesion, which is abrogated by SLC7A11 overexpression. Mechanistic studies showed that hsa_circ_0008274 modulates SLC7A11 expression by acting as a sponge for miR-154-3p to promote PTC migration and invasion.174
Chu et al.175 discovered that circRUNX1 (hsa_circ_0002360) is upregulated in PTC tissue and cell lines compared with those of normal controls. Higher expression levels of circRUNX1 in PTC tissue are associated with larger tumor size, advanced TNM stage, extrathyroidal extension, and LNM, implying that circRUNX1 is related to stronger migration and invasiveness of PTC. This was confirmed in an in vitro study, as overexpressing circRUNX1 promoted migration, invasion, and proliferation of PTC cells through the miR-296-3p/DDHD2 axis, whereas silencing it exerted opposite functions on PTC cells.
Similarly, hsa_circ_0001018 expression is remarkably increased in PTC tissue and cell lines and is associated with TNM staging, LNM, and distant metastasis in PTC tissue. Overexpression of hsa_circ_0001018 reduces the expression of E-cadherin, enhances the expression of vimentin and fibronectin, promotes the migration and invasion of PTC cells, reduces cell cycle arrest at the G1 phase, and inhibits cell apoptosis by modulating the miR-338-3p/SOX4 axis.54
High circVANGL1 expression is associated with LNM and advanced TNM stages.176 Overexpression of circVANGL1 enhances the migration, proliferation, and invasiveness of PTC cell lines and increases the expression levels of N-cadherin and vimentin, whereas decreasing the expression levels of E-cadherin by modulating the miR-194/ZEB1 axis and the downstream EMT pathway.176 In addition, the knockdown of circCCDC66 suppresses migration, invasiveness, and mouse xenograft tumor generation in PTC cells through the miR-129-5p/LARP1 axis and the downstream EMT pathway in the development of PTC.177 Wei et al.57 found that the migration, invasion, and proliferation of PTC cells are suppressed by circZFR (hsa_circ_0072088) knockdown, which is attenuated by the ectopic expression of TC1. Further results suggest that the circZFR/miR-1261/TC1 cascade might act as a potential target for inactivating invasion and metastasis in PTC therapy.
In vitro and in vivo experiments revealed that overexpressing circEIF3I promotes the migration, invasion, and proliferation of PTC cell lines via the miR-149/KIF2A axis.178 Hsa_circ_0062389 stimulates PTC migration and development partly via the miR-1179/HMGB1 axis,179 whereas hsa_circ_0039411 promotes the migration and invasion of PTC by regulating the expression of ABCA9/MTA1 via miR-1179/miR-1205.180 Therefore, these interactome ceRNA networks are implicated in the invasion and metastasis of PTC and have potential as therapeutic targets in clinical practice.
3.7 Deregulating cellular metabolism
During dysregulated cancer cell proliferation, the energy metabolism of cancer cells is reprogrammed to meet the demands of rapid cell growth and division.22 By consuming more glucose and producing more lactate, cancer cells prefer glycolysis even in the presence of oxygen and functioning mitochondria.223 This phenomenon, termed the Warburg effect, is an inefficient means of generating ATP compared with oxidative phosphorylation.223 Dysregulation of energy metabolism is closely associated with other cancer hallmarks, such as sustained proliferative signaling and evasion of growth suppressors.224
In non-small cell lung cancer (NSCLC), circSLC25A16 stimulates glycolysis and proliferation of NSCLC cells via the miR-488-3p/HIF-1α axis, facilitating the transcription of LDHA.225 Ma et al.226 found that circLIPH promotes glycolysis in pancreatic cancer by sponging miR-769-3p and modulating the downstream GOLM1/PI3K/AKT/mTOR pathways. In pancreatic ductal adenocarcinoma (PDAC), circRREB1 increases PGK1 phosphorylation, enhancing glycolytic flux by disrupting the interaction between PTEN and PGK1. Additionally, circRREB1 directly binds to YBX1, promoting its nuclear translocation and stimulating WNT7B transcription, thereby activating the Wnt/β-catenin pathway to maintain stemness in PDAC.227 Hsa_circ_0004674 promotes the glycolysis and progression of osteosarcoma by regulating the expression of glycolysis-related genes through the miR-140-3p/TCF4 axis.228 Suppression of this axis reduces glucose consumption and lactate accumulation in cancer cells.
Cellular metabolism dysregulation also plays an important role in TC. Glucose metabolic profiling demonstrated that hsa_circ_0011290-depletion suppresses glucose uptake, decreases lactate production, and increases ATP levels. Hsa_circ_0011290 depletion also inhibits proliferation and induces apoptosis in PTC cell lines. FSTL1 transcripts are markedly downregulated in response to hsa_circ_0011290 knockdown, which could be reversed by concurrent miR-1252 inhibition. Similarly, the compromised malignant phenotypes induced by hsa_circ_0011290 silencing could subsequently be stimulated by miR-1252 inhibition. In summary, hsa_circ_0011290 modulates cellular metabolism of PTC via the miR-1252/FSTL1 axis.181
HK2, a critical participant in the Warburg effect, is upregulated in PTC tissue and cell lines and is negatively correlated with miR-15a-5p expression in PTC tissue. HK2 overexpression could reverse the inhibitory effect of miR-15a-5p on glycolysis and the malignant phenotypes of PTC cells.182 Besides, silencing circNUP214 inhibits cell glycolysis, proliferation, migration, and invasion while it induces apoptosis in PTC cell lines, which can be reversed by inhibiting the expression of miR-15a-5p. Therefore, circNUP214 promotes anaerobic glycolysis and PTC progression via the miR-15a-5p/HK2 axis.182 At the same time, Li et al.183 found that circNUP214 promotes PTC development by modulating the miR-145/ZEB2 axis.
Silencing circPRKCI (hsa_circ_0122683) inhibits glucose uptake and lactate production, suppresses the proliferation of PTC cells, and arrests them in the G0/G1 phase, which can be reversed by inhibiting miR-335.184 Further experimental results suggested that circPRKCI acts as an oncogenic participant in PTC carcinogenesis and development by regulating cellular metabolism with precise spatiotemporal control of the miR-335/E2F3 axis.184 Similar to the results of Yao et al.133 and Liu et al.,134 Sun et al.185 found that hsa_circ_0058124 is upregulated in PTC tissue and cell lines compared with those of normal controls. Silencing of hsa_circ_0058124 significantly suppresses the oxygen consumption rate of basal and maximum respiration of TC cells, thereby inhibiting their metabolic activity and suppressing their proliferation, migration, and invasiveness. These effects can be replicated by miR-940 overexpression, and miR-940 inhibition can reverse the suppressive effect of silencing MAPK1 in TC cells.185 Thus, hsa_circ_0058124 may function through the miR-940/MAPK1 axis during PTC metabolism and progression.
Silencing the upregulated circRAD18 inhibits cell glucose uptake, lactate production, and proliferation, as well as metastasis of PTC cells. The underlying downstream molecule was confirmed to be PDK1, a metabolic protein involved in glucose intake, regulated by the circRAD18/miR-516b axis.186 Similarly, circPUM1 accelerates PTC tumorigenesis by dysregulating cellular metabolism via the miR-21-5p/MAPK1 signal axis.187 Thus, circRNAs are essential players in the dysregulated cellar metabolism of various cancers, including TC.
3.8 Avoiding immune destruction
According to the theory of immune surveillance, human cells are dynamically monitored by the immune system, which is capable of discerning and eliminating the newly transformed malignant cells.22 Disruption at any step of the cancer-immunity cycle can impair the immune system's ability to generate effective anticancer immune responses to control tumor progression.229 Moreover, the tumor microenvironment (TME) has been shown to play a critical role in modulating the anticancer immune response.229
Liu et al.230 found that circIGF2BP3 upregulation in NSCLC inhibits CD8+ T-cell responses and causes tumor immune evasion by regulating the miR-328-3p/miR-3173-5p/PKP3 axis and stabilizing the PD-L1 protein in an OTUB1-dependent manner. Serving as a scaffold to enhance the interaction between TRIM25 and IGF2BP, circNDUFB2 inhibits the progression of NSCLC by promoting ubiquitination and degradation of IGF2BPs. In addition, circNDUFB2 overexpression triggers immune responses in NSCLC cells by mediating RIG-I–MAVS signaling cascades, increasing the recruiting of CD8+ T cells and DCs into the TME.231 Furthermore, circFAM53B can be translated into a specific peptide that can be presented by DCs to prime naive T cells, driving antigen-specific adaptive anticancer immunity in BC cells.232 Similarly, circFAT1 can regulate the recruitment of CD8+ cells into the TME and promote immune evasion by activating STAT3.233 In colorectal cancer, circREEP3 promotes tumor progression by recruiting the chromatin remodeling protein CHD7 to the FKBP10 promoter, activating its transcription. Additionally, circREEP3 inhibits anti-tumor immunity by enhancing RNF125-dependent degradation of RIG-1, thereby regulating IFN-β production and CD8+ T cell infiltration into the TME.234
3.9 Genome instability
Genome instability is inherent in most types of cancer cells.22 The irreversible activation of oncogenes and the silencing of tumor suppressor genes are necessary for the initiation of various cancers.235 More than 500 oncogenic driver mutations have been identified in over 28,000 cancer exomes.236 It is important to note that these mutations could also be found in nontumor tissues, which suggests that part of these mutations can drive tumor generation only when they cooperate with other irritants or hallmarks of cancer.235
In a Cadmium (Cd)-induced lung tumor model, DNA damage was identified as a key factor promoting tumorigenesis. The downregulation of circCIMT in this model functioned as a tumor suppressor that inhibited DNA damage by directly binding to APEX1, thereby regulating the DNA base excision repair (BER) pathway, which can remove small and nonhelix-distorting base damages.237 CircSMARCA5 can form an R-loop with its parent gene locus in BC, inhibiting the transcription of its parent gene, SMARCA5. As SMARCA5 is a key player in chromatin remodeling by providing a structural basis for recruiting different DNA damage repair factors in DNA damage regions, circSMARCA5 inhibits DNA damage repair in BC.238 Hsa_circ_0007919 has been shown to increase LIG1 transcription by recruiting FOXA1 and TET1, thereby promoting multiple DNA repair pathways in PDAC.239
3.10 Tumor-promoting inflammation
As an enabling characteristic, tumor-promoting inflammation can provide bioactive molecules to the TME that contribute to multiple hallmark capabilities, including growth, survival, proangiogenic factors, extracellular matrix-modifying enzymes, and inductive signals.22 CircRNAs have been shown to participate in the regulation of immune cells and inflammatory cytokines, modulating the inflammatory TME in various tumors that support tumor progression.224, 240
Chronic inflammation is an essential promoter of all steps in tumor progression and is associated with about 20% of cancer deaths worldwide.241 Sun et al.241 showed that TNFα accelerates the expression of circDMD, which enhances tumorigenesis by activating the canonical NF-κB pathway and promoting VEGFR3 expression through R-loop formation at its promoter, while also regulating the miR-4711-5p/KDM5A axis. In NSCLC, circNOX4 promotes the secretion of interleukin 6 (IL-6) to establish an inflammatory niche via the miR-329-5p/FAP axis, enhancing tumor progression.242 Similarly, IL-6 is also regulated by circFOXK2 in NSCLC, which can sponge miR-149-3p.243 In BC, tumor cell-derived exosomal circSERPINE2 can be absorbed by tumor-associated macrophages (TAMs), enhancing their IL-6 secretion. The increased IL-6, in turn, elevates EIF4A3 and CCL2 levels within tumor cells, which upregulate circSERPINE2 biogenesis in tumor cells and promote the recruitment of TAMs in a positive feedback mechanism.244
In TC, circRNAs also regulate the secretion of inflammatory cytokines and chemokines. IL-6 expression is noticeably upregulated in PTC tissue, positively correlated with circRNA_103598 expression, and negatively correlated with miR-23a-3p expression. Knockdown of circRNA_103598 markedly suppresses the proliferation of PTC cells, which is reversed by the introduction of miR-23a-3p. MiR-23a-3p expression is markedly decreased and negatively correlated with upregulated circRNA_103598 or IL-6 expression in PTC tissue. Suppression of PTC cell proliferation and replication of the oncolytic vaccinia virus (OVV) mediated by miR-23a-3p inhibition is abolished by IL-6 overexpression. Therefore, circRNA_103598 is involved in the progression of PTC and OVV-mediated antitumor effects via modulation of the miR-23a-3p/IL-6 axis.188
However, the role of circRNAs in the regulation of various immune cells and the TME inflammation of TC requires further exploration, considering that inflammation is an essential player in oncogenesis and recurrence.245, 246
3.11 Unlocking phenotypic plasticity
Normal cells are destined to follow a pathway that results in terminal differentiation to maintain the homeostatic functions of the organs. Evasion from end-stage differentiation by unlocking the normally restricted phenotypic plasticity has been recorded as a critical process in tumorigenesis.247 Phenotypic plasticity manifests through three main mechanisms: dedifferentiation, blocked differentiation, and transdifferentiation.23
In GBM, exosomal circCMTM3 derived from GBM stem cells (GSCs) has been shown to promote the phenotypic transition from differentiated glioma cells (DGCs) to VM. Once internalized by DGCs, circCMTM3 binds to CNOT4, suppressing the ubiquitination and degradation of STAT5A and STAT5B. This binding enhances the phosphorylation of STAT5A via the protein scaffold function of circCMTM3, which further activates the transcription of provasculogenic factors.248 In NSCLC, circNOX4 plays an essential role in the phenotypic conversion of normal fibroblasts (NFs) to CAFs.242 Similarly, in PDAC, upregulation of circCUL2 in NFs induces the transition to inflammatory CAF phenotype, which promotes tumor development through IL-6 secretion by regulating the miR-203a-3p/MyD88/NF-κB/IL-6 axis.249 Additionally, the levels of circZEB1 in melanoma cells remain high during phenotypic switching from cancer cells lacking cancer stem cells (CSCs) markers to those expressing CSCs markers, underscoring its regulatory role in the phenotypic plasticity of melanoma.250
Dedifferentiation of PTC cells is related to decreased expression or loss of the sodium iodide symporter (NIS) and deficiencies of NIS in the plasma membrane, which result in the failure of iodine uptake in thyroid cells.251 Recently, Sa et al.189 found that higher expression levels of the aryl hydrocarbon receptor (AhR) are associated with the dedifferentiation of PTC. AhR antagonists inhibit proliferation and increase 125I uptake and the expression of NIS in PTC cells, localized to the membrane of PTC cells, suggesting that AhR antagonists promote the differentiation of PTC cells. CircSH2B3 (hsa_circ_0006741), upregulated in PTC cell lines compared with that in normal cell lines, is downregulated after treatment with AhR antagonists. Furthermore, silencing circSH2B3 upregulates the expression of NIS in PTC cells, increases 125I uptake, and inhibits proliferation. However, overexpression of circSH2B3 leads to contrary effects and reverses the differentiation effects induced by AhR antagonists. MiR-4640-5p suppression might partially reverse the differentiation effect of silencing circSH2B3, whereas IGF2BP2 inhibits the differentiation effect induced by miR-4640-5p overexpression. In addition, as an m6A reader, IGF2BP2 enhances the translocation of AhR from the cytoplasm to the nucleus to promote its function. Therefore, circSH2B3 induces PTC dedifferentiation by modulating the miR-4640-5p/IGF2BP2 axis.189
3.12 Senescence
Senescence is an irreversible process that occurs during aging, wherein dysfunctional or otherwise unnecessary cells are inactivated or deleted, serving as a protective mechanism for maintaining tissue homeostasis. During this process, cell morphology and metabolism undergo changes, and cell division is inhibited. Most importantly, senescence-associated secretory phenotype (SASP) is activated during cellular senescence. While cellular senescence is generally accepted as a tumor-antagonizing player, increasing evidence suggests that senescence can act as a tumor-promoting factor in certain contexts.23 SASP is the principal mechanism through which senescent cells promote tumor development, which could transmit hallmark capabilities to adjacent cells in the TME via paracrine signaling with various molecules.23
Many malignant tumors are associated with aging and senescence, including lung cancer, HCC, colorectal cancer, GC, and BC, among others.252 Various studies have provided evidence that circRNAs play important roles in those tumors.252 One recent review summarized the pathways through which circRNAs regulate cellular senescence.253 On the other hand, Li et al.254 found that patients with nasopharyngeal carcinoma (NPC) who suffered from distant metastasis display senescence-related phenotypes. Silencing circWDR37 enhances cisplatin- or gemcitabine-induced cellular senescence in NPC but suppresses the migration and invasion capabilities of senescent NPC cells in vitro.254 Mechanistically, circWDR37 initiates PKR homodimerization and autophosphorylation. Phosphorylated PKR then induces IKKβ phosphorylation, which binds to and releases p65 from IκBα, triggering NF-κB activation. This activation stimulates the transcription of CCND1 and SASP component genes. Taken together, circWDR37 regulates the senescence-driven metastasis in NPC by modulating PKR activity.254
However, overexpression of circLARP4 induces senescence and inhibits tumor progression in HCC by regulating the miR-761/RUNX3 axis and the downstream p53/p21 pathway.255 CircDnmt1 inhibits cellular senescence and promotes tumor growth by stimulating cellular autophagy through the nuclear translocation of p53 and Auf1.256 In colorectal cancer, circDNA2v directly binds to IGF2BP3, maintaining its stability and sustaining the mRNA levels of c-Myc. Silencing circDNA2v results in the downregulation of c-Myc, which induces tumor cell senescence, release of proinflammatory mediators, and recruitment of cytotoxic T cells.257
Thus, the authentic roles of circRNAs and senescence in various cancers require further exploration. Furthermore, Ding et al.258 demonstrated that human umbilical cord mesenchymal stem cell-derived exosomes (UMSC-Exos) prevent cardiac senescence by delivering circHIPK3, which serves as a scaffold to recruit ubiquitin ligase to degrade HuR. However, whether UMSC-Exos could exhibit similar antisenescence functions in various tumors necessitates further research.
3.13 Nonmutational epigenetic reprogramming
The notion of nonmutational epigenetic reprogramming of gene expression is well acknowledged as the critical mechanism regulating embryonic development and organogenesis.23 Complementary to the theory that tumors result from genomic instability and mutation, named permanent genetic alterations, nonmutational epigenetic reprogramming refers to the gene expression changes modulated by epigenetic manipulations independent of genome reprogramming.23 Surging studies demonstrated that circRNAs promote tumor development and progression by participating in epigenetic reprogramming.
In TNBC, multiple oncogene transcription processes are regulated by YBX1, whose O-GlcNAcylation is modulated by the circZEB1/miR-337-3p/OGT axis.259 Moreover, Lan et al.260 showed that circBRAF can recruit KDM4B to enhance MMP9 and ADAMTS14 expression via H3K9me3 modification in TNBC. Furthermore, circBRAF interacts with IGF2BP3 to regulate mRNA stability through m6A modification, enhancing the expression of VCAN, FN1, CDCA3, and B4GALT3 in TNBC.260 In GC, circRHBDD1 binds to IGF2BP2 to inhibit IGF2BP2 ubiquitination and degradation, thereby IGF2BP2 can enhance PD-L1 mRNA stability through m6A modification.261 Besides, hsa_circ_0000119 promotes ovarian cancer progression by increasing the methylation of CDH13 by regulating the miR-142-5p/DNMT1 axis.262 Furthermore, circGNAO1 can sequester DNMT1 to reduce the methylation of GNAO1 promoter, upregulating the expression of GNAO1 to suppress HCC.263
3.14 Polymorphic microbiomes
Gut microbiota, the bacteria settled in the human gastrointestinal system, is well acknowledged to be an essential player in the onset and progression of major depressive disorders, Alzheimer's disease,264 and various tumors, including gastrointestinal cancers.265-269 The interactions between gut microbiota and circRNAs are gaining momentum in research, which would shed light on the oncogenic mechanisms underlying cancers and provide clues to the development of novel therapeutic interventions.270
Gut microbiota modulated by NLRP3 inflammasome deficiency can ameliorate depressive-like behaviors by affecting astrocyte dysfunction via the regulation of circHIPK2.266 Zhu et al.271 demonstrated that gut microbiota inhibits tumor metastasis in mice models by regulating the IL-11/circRNA/miRNA axis to modulate the expression of genes involved in the stemness of CSCs and EMT. In GC, helicobacter pylori (H. pylori) upregulates the expression of circMAN1A2 in GC cell lines, which accelerates the progression of GC by sequestering miR-1236-3p to modulate MTA2 expression.272 Interestingly, the induced overexpression of circMAN1A2 by H. pylori is not dependent on CagA, one of the most crucial virulence factors of H. pylori.272 Similarly, circPGD can be upregulated by H. pylori infection, which serves as a tumor promoter in GC.273 However, circRNA_15430 is downregulated by H. pylori infection, which functions as a tumor suppressor in GC by regulating the miR-382-5p/ZCCHC14 axis.274
In recent years, the intratumoral microbiome has been discovered in various cancer tissues that were previously considered sterile. Recent studies have revealed the roles of the intratumoral microbiome in tumorigenesis and progression, delineating the underlying carcinogenic mechanisms, including epigenetic modifications, metastasis induction, and immune dysfunctions, among others.275, 276 However, the interplay and crosstalk between circRNAs and the intratumoral microbiome warrant further research.
4 CIRCULAR RINGS IN TC: LANDSCAPES OF DYSREGULATED circRNAs IN TC AND PREDICTED ceRNA NETWORKS
TC is the most prevalent endocrine malignancy, with a notably increasing incidence globally, resulting in a significant financial burden.32 The surge in TC diagnoses may stem from the overdiagnosis associated with the increased clinical utilization of advanced imaging technologies such as ultrasound, computed tomography, and magnetic resonance imaging.37 However, a genuine rise in TC incidence cannot be dismissed, considering the aggravation in tumor size and stage.32, 33, 37-39 Despite a low mortality rate, the high incidence of TC, particularly PTC, imposes a substantial financial burden and negatively impacts the quality of life of patients. Therefore, further exploration of the roles of circRNAs in TC pathogenesis and progression is essential, as it may provide new insights into the development of innovative therapeutic strategies. In this section, we provide a concise overview of the landscapes of dysregulated circRNAs in TC tissues and serum.
4.1 The landscapes of circRNA in TC tissue and predicted ceRNA networks
Although Xu et al.49 identified 3777 circRNAs in normal thyroid tissue, the roles of circRNAs in malignant thyroid tissue were not investigated in this study. In a groundbreaking study, Peng et al.55 distinguished the expression profiles of circRNAs between TC and benign thyroid tissue, marking the beginning of a novel chapter in understanding TC development and progression (Figure 3). They identified 88 significantly upregulated and 10 downregulated circRNAs in PTC tissue compared with matched normal thyroid tissue, and 129 upregulated and 226 downregulated circRNAs in PTC tissue compared with tissue of benign thyroid lesions.55 Among them, 12 upregulated and four downregulated circRNAs were identified in PTC tissue in both comparisons. Notably, the downregulation of hsa_circRNA_100395 appeared to play a critical role in PTC by scavenging miR-141-3p and miR-200a-3p to regulate their downstream cancer-related genes. However, the authentic roles of these circRNAs in the pathogenesis of PTC require further confirmation.55
Microarray data obtained by Peng et al.55 were subsequently analyzed in other studies to profile the ceRNA network in PTC.277 Differential expression analysis of miRNAs and mRNAs between PTC and normal tissue from The Cancer Genome Atlas database led to the construction of a network involving 12 circRNAs, 33 miRNAs, and 356 mRNAs based on ceRNA theory.277 Five hub genes were selected to refine the ceRNA network, establishing a circRNA-miRNA-hub gene subnetwork, which included axes such as hsa_circ_0011385/hsa-miR-204/CDH2, hsa_circ_0011385/hsa-miR-6777/VCAN, and hsa_circ_0067934/hsa-miR-375/IGFBP3/FSTL3.277 KEGG pathway analysis revealed the mRNAs targeted by key circRNAs were enriched in pathways such as the p53 signaling pathway, cell adhesion molecules, cellular senescence, and transcriptional dysregulation in cancer.
Using a different approach, Lou et al.278 identified the key regulation axis in PTC. Analyzing the microarray profiling results of Peng et al.,55 Lou et al.278 recognized circRNAs were significantly differentially expressed when comparing PTC with normal tissues, but not in comparisons between benign lesions and normal tissues. This selection included 13 upregulated and one downregulated circRNA. Among them, the upregulated hsa_circ_0088494 was predicted to target miR-876-3p, which was significantly downregulated in TC tissue and associated with a favorable prognosis. The hsa_circ_0088494/miR-876-3p/CTNNB1/CCND1 axis, deemed important in PTC emergence and progression, was confirmed.
Subsequent studies investigated differentially expressed circRNAs in PTC using microarray analysis.56, 151, 170, 279 A comprehensive analysis identified 678 significantly upregulated and 459 downregulated circRNAs in five PTC tissue samples compared with paired normal tissue samples.151 Similarly, Wu et al.170 identified 478 upregulated and 446 downregulated circRNAs in five PTC tissue samples compared with paired normal tissue samples, while Guo et al.279 found 74 upregulated and 84 downregulated circRNAs in three PTC tissue samples compared with paired normal tissue samples. Ren et al.56 observed that 206 circRNAs were significantly upregulated, and 177 circRNAs were downregulated in PTC tissue compared with that in normal tissue. Bioinformatic analysis indicated that downregulated hsa_circRNA_047771 targets elevated miR-522-3p/miR-153-5p in PTC tissue.56
RNA sequencing (RNA-seq) also had been employed to investigate the differentially expressed circRNAs in PTC compared with that in normal thyroid tissue (Figure 3). Lv et al.280 identified 16,569 circRNAs from four paired PTC tissue and neighboring nontumor tissue samples using RNA-seq. Among them, 301 circRNAs were upregulated and 419 were downregulated in PTC tissue. In another study, Lan et al.281 identified 41 upregulated and 46 downregulated circRNAs in PTC tissue samples from three women with PTC compared with matched normal tissue. KEGG analysis of the parental genes of dysregulated circRNAs showed that the most enriched pathway was autoimmune thyroid disease, a known risk factor for TC.33
Apart from comparing circRNA expression patterns in TC and benign thyroid tissue, studies have delved into comparing circRNA expression patterns in invasive and noninvasive TC tissue. Using a ceRNA microarray, the expression profiles of circRNAs were compared in four invasive PTC tissue samples (with extrathyroidal extension and metastasis), four noninvasive PTC tissue samples (with no extrathyroidal extension or metastasis), and four matched adjacent normal tissue samples.133 A total of 377 circRNAs were upregulated and 230 were downregulated in invasive tumors compared with that in adjacent normal tissue. Compared with that in noninvasive tumors, 42 circRNAs were upregulated and seven were downregulated in invasive tumors. Eleven circRNAs were consistently upregulated, and two were downregulated in both sets of comparisons.133
However, the sample sizes of these studies were relatively small, and the methods and parameters used for identifying dysregulated circRNAs differed, limiting their generalizability. Consensus on the overall landscape of circRNAs in TC is lacking, highlighting the need for further studies to unravel the mystery of these closed rings in TC.
4.2 The landscapes of circRNA serum exosomes
Exosomes, ranging in size from 30 to 150 nm, are actively released by parent cells and taken up by recipient cells. They carry diverse contents, including RNAs, influencing the functions of recipient cells and participating in intricate cell-to-cell communication.282, 283 In PTC, exosomal long noncoding RNAs play pivotal roles in driving the EMT and inducing stemness in PTC cells.284, 285 Concurrently, exosomal miRNAs can orchestrate oncogenic behaviors in PTC cells.286 However, the potential roles of circRNAs in PTC exosomes remain largely unexplored.
Yang et al.287 collected serum samples from three patients with PTC and three patients with benign thyroid goiter. Exosomes were extracted from these samples using exosome isolation kits. The analysis revealed three upregulated circRNAs, including hsa_circ_007293, and 19 downregulated circRNAs in the serum exosomes of patients with PTC. KEGG analysis indicated that differentially expressed circRNAs were enriched in 16 signaling pathways, such as the thyroid hormone, PI3K-Akt, AMPK, ABC transport, pyruvate metabolism, calcium, and phosphatidylinositol signaling pathways. These findings suggest significant roles of exosomal circRNAs in the development and progression of PTC by influencing various pathways, warranting further research.
Lin et al.288 showed that overexpression of hsa_circ_007293 in PTC cell lines led to increased levels of hsa_circ_007293 in the exosomes generated by these cells. These exosomes were absorbed by recipient PTC cells, promoting their proliferation, migration, invasion, and EMT pathways. Hsa_circ_007293 was predominantly enriched in the cytoplasm of PTC cells and targeted miR-653-5p to regulate the expression of PAX6. Additionally, the enhanced proliferation, migration, invasion, and EMT pathways in PTC cells receiving exosomes were effectively suppressed by miR-653-5p overexpression or PAX6 inhibition. Therefore, exosomal hsa_circ_007293 may play a crucial role in promoting the malignant behavior of PTC through the miR-653-5p/PAX6 axis.288
5 DIAGNOSTIC POTENTIAL OF circRNAs
Considering their features of stability, specificity, and abundance, circRNAs are ideal diagnostic and prognostic biomarkers for cancers.6 Furthermore, the relatively stable detection of cicrRNAs in different kinds of body fluids, including in saliva, plasma, urine, and serum, renders them promising candidates as noninvasive liquid biopsy biomarkers for cancers.289 In this section, we briefly introduce the diagnostic value of circRNAs across various cancers and summarize their diagnostic potential for TC.
5.1 CircRNAs as diagnostic biomarkers of circRNAs for cancers
For tumors originating from the digestive system, mounting evidences suggest the potential of circRNAs as diagnostic biomarkers. CircYAP is significantly upregulated in colorectal cancer tissues with liver metastasis. Receiver operating characteristic (ROC) analyses indicated that circYAP could predict liver metastasis in colorectal cancer with an area under the curve (AUC) value of 0.8433.219 Higher expression of circMAN1A2 in GC tissues is associated with advanced stages of GC. Additionally, circMAN1A2 levels are significantly higher in the plasma of patients with GC than that in healthy plasma, indicating its potential as a novel diagnostic biomarker for GC.272 CircMORC3 can serve as a diagnostic biomarker for hypopharyngeal squamous cell carcinoma (SCC) with an AUC of 0.834.290 Higher expression of hsa_circ_0006091 in HCC tissues can distinguish HCC from healthy controls with an AUC of 0.916, which could be improved if combined with levels of AFP or RGS12 as a combined biomarker.291
For tumors located in the respiratory tract, the diagnostic values of circRNA are also investigated. In laryngeal SCC, hsa_circ_0036722 can distinguish laryngeal SCC from adjacent normal tissues with an AUC of 0.838.292 Higher expression of hsa_circRNA_102231 is associated with advanced TNM stages and LNM, which can diagnose lung cancer with an AUC of 0.897.293 Furthermore, Wang et al.294 identified hsa_circ_0001821 and hsa_circ_0077837 as potential diagnostic biomarkers for NSCLC, demonstrating AUC values of approximately 0.90.
The circWSB1 expression level is significantly correlated with the T stage of patients with BC, and the ROC curve demonstrated that the circWSB1 level can identify BC effectively with an ROC of 0.705.191 In cervical cancer, increased expression of circZFR is positively associated with LNM, SCC antigen value, and Ki-67 value. Additionally, the ROC curve proved that the circZFR level can distinguish cervical cancer effectively with an AUC of 0.88.192 The positive relationships between circSMA4 expression and mitotic figures, as well as the malignant degrees of GISTs, were established. Additionally, the ROC curve analysis underlined the nearly perfect diagnostic efficiency of circSMA4 for GISTs with an AUC of 0.9824.196
The diagnostic value of circRNAs in the serum were also investigated. Payervand et al.215 validated the diagnostic potential of a batch of six circulating circRNAs in serum from patients with colorectal cancer, including hsa_circ_0060745, hsa_circ_001569, hsa_circ_007142, hsa_circ_0084043, circBANP, and CDR1as. The results indicated that all AUC values exceeded 0.75, with three values surpassing 0.90, demonstrating the effectiveness of these circRNAs in differentiating patients with colorectal cancer from healthy individuals.215 Sun et al.295 validated the upregulation of hsa_circ_0004001, hsa_circ_0004123, and hsa_circ_0075792 in blood samples from patients with HCC. The levels of these three circRNAs were associated with TNM stages and tumor sizes, which could serve as a noninvasive diagnostic biomarker for HCC. Furthermore, the combined three-circRNA exhibited a higher AUC value for diagnosis, along with enhanced sensitivity and specificity.295 Additionally, circSLC39A5 expression in plasma was significantly associated with satellite nodules, LNM/vascular invasion, total bilirubin levels, and HBsAg levels in patients with HCC, enabling diagnosis of HCC with an AUC of 0.915.296 Additionally, plasma circELMOD3 could identify HCC patients with an AUC of 0.908.297 In addition, the ROC curve suggested that hsa_circ_0023179 could act as a serum marker of NSCLC with an AUC of 0.831.298 CircHERC1 expression in plasma could distinguish patients with lung cancer and cancer-free patients with an AUC of 0.746.299
The circRNAs in saliva and urine also demonstrated their potential as biomarkers. Song et al.300 validated that urinary hsa_circ_0137439 could differentiate patients with bladder cancer from healthy controls with an AUC of 0.890. Moreover, it could distinguish muscle-invasive bladder cancer from nonmuscle-invasive bladder cancer with an AUC of 0.798.300 Yang et al.301 found that urine hsa_circ_0071196 could serve as a potential noninvasive biomarker for detecting bladder urothelial carcinoma with an AUC of 0.935. For the diagnosis of OSCC, the combination of salivary hsa_circ_0001874 and hsa_circ_0001971 showed an AUC of 0.922.302
5.2 CircRNAs as diagnostic biomarkers for TC
5.2.1 Diagnostic biomarkers for TC in tissue samples
In a study investigating the clinical involvement of circRNAs in patients with PTC, Ren et al.56 first explored the diagnostic potential of circRNAs in PTC. Among the dysregulated circRNAs identified by microarray analysis, the most upregulated circRNA in PTC, hsa_circRNA_007148, showed a significant correlation with LNM. Conversely, the most downregulated circRNA, hsa_circRNA_047771, exhibited correlations with BRAFV600 mutation, TNM stage, and LNM. These associations suggested that circRNAs could serve as diagnostic biomarkers in PTC. ROC analyses indicated that hsa_circRNA_007148 and hsa_circRNA_047771 are potential diagnostic biomarkers for PTC, with AUCs of 0.846 and 0.876, respectively.56
Similarly, Guo et al.303 identified 53 dysregulated circRNAs in PTC tissue compared with normal tissue using high-throughput RNA-seq. ROC curve analysis suggested that eight dysregulated circRNAs with AUC > 0.7 could serve as potential diagnostic markers of PTC (Table 2). Among them, chr5:161330882-161336769−, chr9:22046750-22097364+ (hsa_circ_0008796), and chr8:18765448-18804898− (hsa_circ_0002111) were significantly related to the BRAFV600E mutation, and chr12:129699809129700698− was significantly associated with capsular invasion. Additionally, chr5:3852341838530666− (hsa_circ_0072309) was associated with both pT and pN stages.303 Du et al.304 further validated that hsa_circ_0002111 was expressed at significantly higher levels in PTC tissue compared with that in nontumor tissue, closely associated with advanced TNM stage and LNM, making it a potential diagnostic biomarker for PTC, with an AUC of 0.833.304
| CircRNAs | Chromosome | Gene symbol | Expression change | Relationships with the clinical features | Number of patients | Clinical samples | Clinical value | AUC | Sensitivity | Specificity | CI | References | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnostic biomarkers for TC in tissue samples | Hsa_circRNA_047771 | chr18 | NARS | Down | BRAFV600 mutation, TNM stages, and LNM | 40 PTC tissues and matched paratumor tissues | Tumor tissues | Diagnostic biomarker | 0.876 | 0.875 | 0.800 | 95% CI: 0.78–0.94 | 56 |
| Hsa_circRNA_007148 | chr3 | FNDC3B | Up | LNM | 40 PTC tissues and matched paratumor tissues | Tumor tissues | Diagnostic biomarker | 0.846 | 0.825 | 0.775 | 95% CI: 0.75–0.96 | 56 | |
| chr5: 161330882161336769- | chr5 | – | Up | BRAFV600E mutation | 45 PTC tissues and matched paratumor tissues | Tumor tissues | Diagnostic biomarker | 0.878 | – | – | 95% CI: 0.8068-0.9492 | 303 | |
| chr12: 129699809129700698- | chr12 | – | Up | capsular invasion | 45 PTC tissues and matched paratumor tissues | Tumor tissues | Diagnostic biomarker | 0.8099 | – | – | 95% CI: 0.7213-0.8984 | 303 | |
| chr9: 2204675022097364+ (hsa_circ_0008796) | chr9 | – | Up | BRAFV600E mutation | 45 PTC tissues and matched paratumor tissues | Tumor tissues | Diagnostic biomarker | 0.76 | – | – | 95% CI: 0.6610-0.8590 | 303 | |
| chr20: 1745634717465553+ | chr20 | – | Up | – | 45 PTC tissues and matched paratumor tissues | Tumor tissues | Diagnostic biomarker | 0.759 | – | – | 95% CI: 0.6583-0.8597 | 303 | |
| chr7: 116699070116700284+ | chr7 | – | Up | – | 45 PTC tissues and matched paratumor tissues | Tumor tissues | Diagnostic biomarker | 0.7763 | – | – | 95% CI: 0.6794-0.8732 | 303 | |
| chr8: 1876544818804898 (hsa_circ_0002111) | chr8 | – | Up | BRAFV600E mutation | 45 PTC tissues and matched paratumor tissues | Tumor tissues | Diagnostic biomarker | 0.8277 | – | – | 95% CI: 0.7387-0.9166 | 303 | |
| chr7: 2230833822318037- | chr7 | – | Up | – | 45 PTC tissues and matched paratumor tissues | Tumor tissues | Diagnostic biomarker | 0.7017 | – | – | 95% CI: 0.5918-0.8116 | 303 | |
| chr5: 3852341838530666 (hsa_circ_0072309) | chr5 | – | Down | pT and pN stages | 45 PTC tissues and matched paratumor tissues | Tumor tissues | Diagnostic biomarker | 0.7101 | – | – | 95% CI: 0.6029-0.8174 | 303 | |
| Hsa_circ_0002111 | – | PSD3 | Up | Advanced TNM stage and LNM | 82 PTC tissues and matched paratumor tissues | Tumor tissues | Diagnostic biomarker | 0.833 | 0.756 | 0.805 | 95% CI: 0.7713 to 0.8935 | 304 | |
| Hsa_circ_0137287 (chr8:92301363-92307931+) | chr8 | SLC26A7 | Up | Extrathyroidal extension, T stage, LNM, microcarcinoma, and tumor size | 120 PTC and 60 adjacent noncancerous thyroid tissues | Tumor tissues | Diagnostic biomarker | 0.8973 | – | – | 95% CI: 0.8452-0.9494 | 305 | |
| chr5:160757890-160763776- | chr5 | GABRB2 | Up | – | 44 PTC tissues and matched paratumor tissues (training cohort) | Tumor tissues | Diagnostic biomarker | 0.9566 | – | – | 95% CI: 0.9088–1.004 | 281 | |
| chr12:40696591-40697936+ (hsa_circ_0025887) | chr12 | LRRK2 | Up | – | 44 PTC tissues and matched paratumor tissues (training cohort) | Tumor tissues | Diagnostic biomarker | 0.9476 | – | – | 95% CI: 0.9041–0.9911 | 281 | |
| chr7:22330794-22357656- (hsa_circ_0001681, circRAPGEF5) | chr7 | RAPGEF5 | Up | – | 44 PTC tissues and matched paratumor tissues (training cohort) | Tumor tissues | Diagnostic biomarker | 0.9099 | – | – | 95% CI: 0.8433–0.9764 | 281 | |
| chr21:16386665-16415895- | – | – | Up | – | 44 PTC tissues and matched paratumor tissues (training cohort) | Tumor tissues | Diagnostic biomarker | 0.7200 | – | – | 95% CI: 0.6128–0.8272 | 281 | |
| chr22:36006931-36007153- (hsa_circ_0063050) | chr22 | MB | Down | – | 44 PTC tissues and matched paratumor tissues (training cohort) | Tumor tissues | Diagnostic biomarker | 0.9305 | – | – | 95% CI: 0.8796–0.9814 | 281 | |
| chr9:16435553-16437522- (hsa_circ_0086414) | chr9 | BNC2 | Down | – | 44 PTC tissues and matched paratumor tissues (training cohort) | Tumor tissues | Diagnostic biomarker | 0.8192 | – | – | 95% CI: 0.7306–0.9078 | 281 | |
| chr2:179514891-179516047- | chr2 | TTN | Down | – | 44 PTC tissues and matched paratumor tissues (training cohort) | Tumor tissues | Diagnostic biomarker | 0.8270 | – | – | 95% CI: 0.7343–0.9196 | 281 | |
| chr7:91924203-91957214+ | – | – | Down | – | 44 PTC tissues and matched paratumor tissues (training cohort) | Tumor tissues | Diagnostic biomarker | 0.8763 | – | – | 95% CI: 0.8045–0.9481 | 281 | |
| chr5:160757890-160763776- | chr5 | GABRB2 | Up | – | 43 PTC tissues and matched paratumor tissues (test cohort) | Tumor tissues | Diagnostic biomarker | 0.9502 | – | – | 95% CI: 0.8995–1.001 | 281 | |
| chr12:40696591-40697936+ (hsa_circ_0025887) | chr12 | LRRK2 | Up | – | 43 PTC tissues and matched paratumor tissues (test cohort) | Tumor tissues | Diagnostic biomarker | 0.9448 | – | – | 95% CI: 0.8966–0.9931 | 281 | |
| chr7:22330794-22357656- (hsa_circ_0001681, circRAPGEF5) | chr7 | RAPGEF5 | Up | – | 43 PTC tissues and matched paratumor tissues (test cohort) | Tumor tissues | Diagnostic biomarker | 0.9186 | – | – | 95% CI: 0.8607–0.9765 | 281 | |
| chr21:16386665-16415895- | – | – | Up | – | 43 PTC tissues and matched paratumor tissues (test cohort) | Tumor tissues | Diagnostic biomarker | 0.7309 | – | – | 95% CI: 0.6212–0.8407 | 281 | |
| chr22:36006931-36007153- (hsa_circ_0063050) | chr22 | MB | Down | – | 43 PTC tissues and matched paratumor tissues (test cohort) | Tumor tissues | Diagnostic biomarker | 0.7309 | – | – | 95% CI: 0.8794–0.9902 | 281 | |
| chr9:16435553-16437522- (hsa_circ_0086414) | chr9 | BNC2 | Down | – | 43 PTC tissues and matched paratumor tissues (test cohort) | Tumor tissues | Diagnostic biomarker | 0.7309 | – | – | 95% CI: 0.7319–0.9106 | 281 | |
| chr2:179514891-179516047- | chr2 | TTN | Down | – | 43 PTC tissues and matched paratumor tissues (test cohort) | Tumor tissues | Diagnostic biomarker | 0.8253 | – | – | 95% CI: 0.7318–0.9189 | 281 | |
| chr7:91924203-91957214+ | – | – | Down | – | 43 PTC tissues and matched paratumor tissues (test cohort) | Tumor tissues | Diagnostic biomarker | 0.8661 | – | – | 95% CI: 0.7922–0.9401 | 281 | |
| CircRNA_103598 | Advanced TNM stage, tumor size, metastasis status and survival status | 100 PTC tissues and matched paratumor tissues | Tumor tissues | Diagnostic biomarker | 0.9465 | – | – | – | 188 | ||||
| Hsa_circ_0015278 | – | – | Down | Age, extrathyroidal invasion, pathological LNM, pT stage, pTNM stage, relapse rate and survival status | 206 PTC tissues and matched paratumor tissues | Tumor tissues | Diagnostic biomarker | 0.903 | – | – | 95% CI: 0.847–0.932 | 306 | |
| CircBACH2 (hsa_circ_0001627) | chr6:90959407–90981660 | BACH2 | Up | Tumor size, TNM stage, LNM, and survival status | 40 PTC tissues and matched paratumor tissues | Tumor tissues | Diagnostic biomarker | 0.8631 | – | – | 95% CI: 0.7774–0.9489 | 160 | |
| CircFNDC3B (hsa_circ_0006156) | chr3:171965322-171969331 | FNDC3B | Up | Tumor size, LNM, advanced TNM stages, and survival status | 42 PTC tissues and matched paratumor tissues | Tumor tissues | Diagnostic biomarker | 0.891 | – | – | 95% CI: 0.820–0.961 | 150 | |
| Combined prediction index: -0.321× (chr4: 25665378–25667298+) + 0.23×(chr1: 12578718–12579412-) − 2.818× (chr7: 116699071–116700284+) − 1.078× (chr7: 116695750– 116700284+) + 0.689× (chr5: 161330883–161336769−) − 0.723× (chr10: 179994–249088+) +15.627 | – | – | – | – | 95 PTC tissues and matched paratumor tissues | Tumor tissues | Diagnostic biomarker | 0.976 | 0.977 | 0.953 | – | 280 | |
| Diagnostic biomarkers for TC in circulation | CircMAN1A2 | – | MAN1A2 | Up | – | 57 PTC patients and 121 normal controls | Serum | Diagnostic biomarker | 0.734 | 0.509 | 0.884 | – | 307 |
| CircRAPGEF5 (hsa_circ_0001681) | chr7:22330794-22357656 | RAPGEF5 | Up | – | 52 PTC patients and 50 normal controls (Training cohort) | Serum | Diagnostic biomarker | 0.711 | 0.647 | 0.754 | 95% CI: 0.597-0.824 | 308 | |
| Hsa_circ_0058124 | chr2 | FN1 | Up | – | 52 PTC patients and 50 normal controls (Training cohort) | Serum | Diagnostic biomarker | 0.790 | 0.810 | 0.618 | 95% CI: 0.692-0.887 | 308 | |
| CircRAPGEF5 + hsa_circ_0058124 | – | – | – | – | 52 PTC patients and 50 normal controls (Training cohort) | Serum | Diagnostic biomarker | 0.860 | 0.805 | 0.749 | 95% CI: 0.784-0.937 | 308 | |
| CircRAPGEF5 | chr7:22330794-22357656 | RAPGEF5 | Up | – | 61 PTC patients and 61 normal controls (Test cohort) | Serum | Diagnostic biomarker | 0.692 | 0.722 | 0.631 | 95% CI: 0.577-0.807 | 308 | |
| Hsa_circ_0058124 | chr2 | FN1 | Up | – | 61 PTC patients and 61 normal controls (Test cohort) | Serum | Diagnostic biomarker | 0.727 | 0.719 | 0.710 | 95% CI: 0.609-0.844 | 308 | |
| CircRAPGEF5 + hsa_circ_0058124 | – | – | – | – | 61 PTC patients and 61 normal controls (Test cohort) | Serum | Diagnostic biomarker | 0.867 | 0.866 | 0.695 | 95% CI: 0.787-0.947 | 308 | |
| CircRAPGEF5 | chr7:22330794-22357656 | RAPGEF5 | Up | – |
113 PTC patients and 80 patients with thyroid nodules |
Serum | Diagnostic biomarker | 0.684 | – | – | 95% CI: 0.558-0.810 | 308 | |
| Hsa_circ_0058124 | chr2 | FN1 | Up | – |
113 PTC patients and 80 patients with thyroid nodules |
Serum | Diagnostic biomarker | 0.674 | – | – | 95% CI: 0.575-0.774 | 308 | |
| CrcRAPGEF5 + hsa_circ_0058124 | – | – | – | TNM stage, LNM, and distant metastasis |
113 PTC patients and 80 patients with thyroid nodules |
Serum | Diagnostic biomarker | 0.807 | 0.821 | 0.640 | 95% CI: 0.714-0.900 | 308 | |
| Hsa_circ_0124055 | chr3:49514281-49548252 | DAG1 | Up | Tumor size, TNM stage, histological grade, LNM, and survival status | 65 TC patients and 65 normal controls | Serum | Diagnostic biomarker | 0.836 | 0.712 | 0.939 | 95% CI: 0.763-0.908 | 309 | |
| Hsa_circ_0101622 | chr14:31775937-31858211 | HEATR5A | Up | Tumor size, TNM stage, histological grade, LNM, and survival status | 65 TC patients and 65 normal controls | Serum | Diagnostic biomarker | 0.805 | 0.712 | 0.894 | 95% CI: 0.727-0.883 | 309 | |
| Hsa_circ_0124055 + hsa_circ_0101622 | – | – | – | – | 65 TC patients and 65 normal controls | Serum | Diagnostic biomarker | 0.911 | 0.894 | 0.818 | 95% CI: 0.859-0.962 | 309 | |
| Hsa_circ_0021549 | – | – | Down | – | 57 PTC patients and age and sexmatched healthy controls | Whole blood | Diagnostic biomarker | 0.8194 | – | – | 95% CI: 0.7117 to 0.9270 | 279 | |
| CircIPCEF1 | chr: 154520801154544377 | IPCEF1 | Down | LNM | 57 PTC patients and age and sexmatched healthy controls | Whole blood | Diagnostic biomarker | 0.8010 | – | – | 95% CI: 0.7108 to 0.8912 | 279 | |
| Diagnostic biomarkers for LNM in TC | CircUMAD1 (hsa_circ_0001676) + Gal3 | – | – | Up | LNM and lesion side | 23 PTC patients without LNM and 27 PTC patients with LNM | Plasma | Diagnostic biomarker for LNM | 0.87 | 0.741 | 1 | 95% CI: 0.766 to 0.970 | 310 |
| Hsa_circRNA_404686 | – | – | Down | – | 10 PTC patients without LNM and 10 PTC patients with LNM | Plasma | Diagnostic biomarker for LNM | – | – | – | – | 311 |
- Abbreviations: circRNA, circular RNA; LNM, lymph node metastasis; TC, thyroid cancer.
Furthermore, Lan et al.305 demonstrated that the downregulated hsa_circ_0137287 in PTC was significantly lower in patients with more aggressive clinicopathological characteristics, such as extrathyroidal extension, higher T stage, LNM, and larger tumor size. ROC curves suggested that hsa_circ_0137287 is a potential diagnostic biomarker for PTC, with an AUC of 0.8973. Hsa_circ_0137287 also predicts extrathyroidal extension and LNM, with AUCs of 0.6885 and 0.6691, respectively. In their another study exploring circRNA profiles in PTC, several dysregulated circRNAs, including hsa_circ_0001681 (circRAPGEF5), were shown to have diagnostic value.281
Moreover, ROC curves suggested that circRNA_103598, hsa_circ_0015278, circBACH2, and circFNDC3B can distinguish PTC tissue from adjacent normal tissue with AUCs of 0.9645, 0.903, 0.8631, and 0.891, respectively.150, 160, 188, 306 These results demonstrate the potential of circRNAs as clinical diagnostic biomarkers for PTC.
To enhance the sensitivity, specificity, and accuracy of PTC diagnosis, researchers have explored the diagnostic value of combinations of circRNAs. After profiling circRNA expression in PTC using RNA-seq, the diagnostic values of the five most differentially expressed circRNAs in PTC tissue were determined using ROC curves (Table 2).280 Based on a logistic regression model, the authors generated a combined prediction index with an AUC of 0.976, sensitivity of 0.977, and specificity of 0.953 (Table 2).280
Several studies have shown that circRNA expression levels are correlated with the clinical characteristics of patients with PTC. For example, high levels of hsa_circ_0079558, circEIF3I, and circTIAM1 are associated with larger tumor size, advanced TNM stage, and LNM.158, 166, 178 Similarly, higher expression of CDR1as, hsa_circ_0000644, circSSU72, and circPSD3 are associated with LNM and larger tumor size in patients with PTC.135, 136, 141, 144, 155 However, their diagnostic value has not been directly evaluated, warranting further studies.
5.2.2 Diagnostic biomarkers for TC in circulation
The potential value of circulating circRNAs for TC diagnosis is highly promising compared with the invasive nature of detecting circRNAs in tissue. Recently, circMAN1A2 was found to be upregulated in serum samples from 57 patients with TC compared with 121 healthy controls, showing potential as a diagnostic biomarker for TC with an AUC of 0.734.307 However, circMAN1A2 expression levels are also increased in serum samples from patients with other cancers, potentially limiting its specificity for TC diagnosis.
Based on studies about the ceRNA network of circRAPGEF5 and hsa_circ_0058124 in PTC,53, 133, 185 Shi et al.308 observed their elevated expression levels in serum samples from patients with PTC compared with healthy controls and patients with benign thyroid nodules. Following systematic treatment, including surgery, their serum expression levels decreased. ROC analyses demonstrated individual AUCs of 0.711 for circRAPGEF5 and 0.790 for hsa_circ_0058124 in the training cohort. Notably, the combination of these two circRNAs exhibited an enhanced AUC of 0.860. Shi et al.308 found a significant correlation between these circRNAs and TNM staging, LNM, and distant metastasis. For distinguishing patients with PTC from those with benign thyroid nodules, the AUC was 0.684 for circRAPGEF5 and 0.674 for hsa_circ_0058124 in the pooled cohorts (training and test). Importantly, the AUC value for the combination of circRNAs reached 0.807. Logistic regression analysis, even after adjusting for age and sex, indicated that the dysregulated expression of circRAPGEF5, hsa_circ_0058124, and the combination of the two circRNAs could predict the presence of PTC. This held true regardless of whether the reference group consisted of healthy controls or patients with benign thyroid nodules.308 Similarly, Sun et al.309 found higher levels of hsa_circ_0124055 and hsa_circ_0101622 in the plasma of patients with TC than in healthy controls. After surgery, these levels decreased, indicating their potential as diagnostic biomarkers for TC with AUCs of 0.836 and 0.805, respectively. Combining hsa_circ_0124055 with hsa_circ_0101622 increased the AUC to 0.911.
Additionally, two downregulated circRNAs, circIPCEF1 and hsa_circ_0021549, extracted from whole blood samples, show potential as noninvasive diagnostic biomarkers for PTC.279 Furthermore, Lin et al.288 observed higher levels of hsa_circ_007293 in serum exosomes from patients with PTC, correlating with higher TNM stages and an increased risk of LNM. CircFNDC3B and circRASSF2 levels were also higher in serum exosomes of patients with PTC than in healthy controls, suggesting their potential as noninvasive diagnostic biomarkers for PTC.150, 170 However, further research is required to consolidate the potential clinical application of these circRNAs as diagnostic biomarkers for PTC.
5.2.3 Diagnostic biomarkers for LNM in TC
While the majority of studies have concentrated on discerning the presence of PTC in patients, some studies have explored circulating circRNAs as potential biomarkers for predicting LNM in individuals with PTC.310, 311 Yu et al.310 explored Gal3 as a potential marker, given its association with the secretion of metastasis-promoting cytokines.312 They observed higher expression levels of Gal3 in the circulation of patients with PTC with LNM compared with those without LNM, resulting in an AUC of 0.8407 for Gal3 as a predictor of LNM. The circRNA circUMAD1 (hsa_circ_0001676), known to modulate Gal3 by acting as a miR-873 sponge, exhibited significantly higher expression in patients with PTC with LNM. Although circUMAD1 alone had an AUC of 0.7531 for predicting LNM, its combination with Gal3 achieved an enhanced AUC of 0.87, with a sensitivity of 74.1% and specificity of 100%. This suggested that the combination of circUMAD1 and Gal3 held promise as a novel noninvasive biomarker for predicting LNM in patients with PTC.310
In another study by Yang et al.,311 microarray analysis identified hsa_circRNA_404686 and several other circRNAs as potential diagnostic biomarkers for predicting LNM in women with PTC. However, it is important to note that this study focused exclusively on women patients with PTC with thyroid nodules not larger than 1 cm, and further investigations with larger cohorts are warranted for validation.
Several studies have demonstrated the association of dysregulated circRNAs with LNM and metastasis in TC. However, the potential value of circRNA measurement for predicting LNM had not been systematically assessed. For instance, expression levels of circSSU72, circPRMT5, and hsa_circ_0001666 have been significantly linked to LNM in PTC tissue.141, 154, 156 Additionally, the expression levels of circRASSF2, circPSD3, hsa_circ_0004458, hsa_circ_0058124, and circFOXM1 exhibited positive correlations with TNM stage, LNM, and distant metastasis in patients with PTC.133, 135, 136, 151, 170 Moreover, higher expression levels of circSSU72 and hsa_circ_0058124 are associated with capsule invasion (extrathyroidal extension).133, 141 Nevertheless, further studies are essential to evaluate whether these circRNAs can serve as potential diagnostic biomarkers for predicting LNM and distant metastasis in PTC.
6 PROGNOSTIC POTENTIAL OF circRNAs
In addition to their role as diagnostic biomarkers for cancers, researchers have also explored the potential of circRNAs as prognostic biomarkers across various cancer types. CircRNAs, characterized by their closed-loop structures without free tails, demonstrate enhanced stability compared with linear mRNAs and miRNAs in peripheral blood. Studies have suggested that circRNAs are enriched in exosomes, providing an additional layer of protection in the blood.122 CircRNAs can be readily detected in serum samples and other types of body fluids,307 making them ideal noninvasive prognostic biomarkers for various diseases, particularly cancer.24, 290, 313 Furthermore, an increasing body of evidences support the notion that circRNA levels may serve as predictors of responses to specific therapeutic strategies targeting cancers. Continued investigation into the prognostic capabilities of circRNAs and the underlying mechanisms involved could yield valuable insights for their clinical application and contribute to the development of effective therapeutic strategies from the prospect of precision medicine.
6.1 CircRNAs as prognostic biomarkers for cancers
CircIGF2BP3 expression levels were positively correlated with LNM, advanced tumor stages, and shorter overall survival (OS) in NSCLC patients. Multivariate regression analysis results suggest that a higher expression level of circIGF2BP3 is an independent prognostic biomarker for NSCLC.230 Besides, higher expression of hsa_circRNA_102231 is associated with poorer OS of patients with lung cancer.293
Higher expression of circFAM53B and circFAM53B-219 peptides are associated with smaller tumor size and better disease-free survival (DFS) in patients with BC. In multivariate Cox regression analyses, circFAM53B serves as an independent prognostic factor for BC.232 Higher levels of circWSB1 are related to poorer OS of patients with BC and could be a standalone risk biomarker.191 Furthermore, circROBO1 is upregulated in BC-derived liver metastases compared with the BC primary sample and is related to shorter OS.314 In TNBC, lower circFBXW7 expression is negatively associated with tumor size, LNM, poorer OS, and DFS. Additionally, multivariate Cox regression analysis indicated that lower circFBXW7 is an independent prognostic factor for patients with TNBC.109 In patients with HER2+ BC, higher levels of circCDYL2 predicted rapid recurrence and shorter DFS and OS following anti-HER2 therapy.315
The prognostic values of circRNAs were also evaluated in tumors located in the organs of the digestive system. Lower expression of circLARP4 independently predicted poor survival outcomes in patients with HCC.255 Higher circNFATC3 expression levels are associated with poorer OS in GC patients.129 CircROBO1 is significantly upregulated in HCC and higher circROBO1 in HCC is correlated with worse OS and relapse-free survival (RFS).316 Higher expression of hsa_circ_0007919 is significantly associated with vascular invasion, nerve invasion, T stage, LNM, and TNM stage in patients with PDAC.239 Moreover, higher expression of hsa_circ_0007919 can predict poorer OS and DFS of patients with PDAC.239
Higher expression of circCCT3 in patients with multiple myeloma is associated with longer progression-free survival and OS intervals, which could serve as a reliable, independent predictor for prognosis.317 However, Papatsirou et al.318 found that higher levels of circulating CDR1as are correlated with a poorer prognosis in multiple myeloma. Additionally, CDR1as expression levels are negatively associated with glioma grade and serve as an independent molecular biomarker of OS in glioma, particularly in GBM.190
The prognostic value of circRNAs in circulation and other body fluids is also noteworthy. Higher levels of exosomal circAKT3 are associated with poorer OS and RFS in patients with HCC.319 Higher expression of hsa_circ_0004831, circ_PVT1, and hsa_circRNA_001569, in circulation is associated with poorer OS in patients with colorectal cancer.320, 321 Yan et al.322 confirmed that higher levels of hsa_circRNA_100199 in serum are an independent predictor of RFS and OS in patients with acute myeloid leukemia. Moreover, the levels of hsa_circRNA_100199 in serum are significantly lower in patients who achieved complete remission than in those who did not, indicating its value for predicting the therapeutic response.322 Additionally, circVMP1 expression levels in the serum samples of patients with cisplatin-resistant NSCLC are significantly higher than those in cisplatin-sensitive patients, implying its potential as a novel biomarker for cisplatin sensitivity in patients with NSCLC.323
Hsa_circ_0137439 in urine can act as an independent prognostic predictor of RFS and OS for patients with bladder cancer.300 Furthermore, highly expressed circPRMT5 in the serum and urinary exosomes is positively associated with LNM and advanced tumor progression in patients with urothelial carcinoma of the bladder, suggesting its potential as a promising prognostic biomarker.324 Chen et al.325 found that hsa_circ_0000231 expression level was an independent predictor for the poor prognosis of patients with tongue squamous cell carcinoma (TSCC), which was also upregulated in saliva samples of patients with TSCC.
6.2 CircRNAs as prognostic biomarkers for TC
The clinical potential of circRNAs as prognostic biomarkers for TC has been established. Liu et al.162 identified significantly elevated levels of hsa_circ_0102272 in TC tissue and cell lines compared with that in normal controls. High expression of hsa_circ_0102272 is strongly correlated with adverse factors such as LNM, higher TNM stage, and histological grade, leading to poorer OS and progression-free survival. Multivariable regression analysis confirmed hsa_circ_0102272 as an independent predictor of prognosis in patients with TC.162 Similarly, Wang et al.138 observed higher levels of hsa_circ_0067934 in TC tissue associated with larger tumor size, LNM, and higher American Joint Committee on Cancer (AJCC) stage. Kaplan–Meier analysis indicated that elevated hsa_circ_0067934 expression predicted lower survival rates, and Cox proportional hazards regression analysis identified it as an independent risk factor for poor OS.138 Zhang et al.139 further validated the prognostic significance of hsa_circ_0067934.
In patients with TC, higher expression levels of circBACH2, circFNDC3B, circCCDC66, circLDLR, hsa_circ_0124055, and hsa_circ_0101622 are significantly associated with larger tumor size, higher TNM stage, LNM, and poor OS.150, 160, 173, 177, 309 Similarly, increased expressions of circRNA_102002, circPVT1, hsa_circ_0008274, hsa_circ_0011058, circZFR, and circPUM1 are linked to positive LNM, higher TNM stages, and poorer OS.57, 161, 168, 171, 174, 187 Among them, the expressions of hsa_circ_0008274 and hsa_circ_0011058 are closely associated with tumor infiltration and nodular goiter, respectively.168, 174 Additionally, higher expression of circRNA_103598 is associated with a more advanced TNM stage, larger tumor size, metastasis, and poorer OS in patients with PTC.188 Besides, higher expression levels of hsa_circ_0005273 and hsa_circ_0011290 are linked to poor OS in patients with PTC.143, 181
Limited research has explored the prognostic role of downregulated circRNAs in TC. Ding et al.163 found that downregulated circNEURL4 expression in patients with PTC is associated with advanced TNM stages, LNM, and poorer survival.163 The expression level of circITCH is associated with the clinical stage and LNM, with proportional hazards analysis suggesting that circITCH is an independent prognostic marker for PTC.131 Higher expression of hsa_circ_0015278 is significantly correlated with younger age, absence of extrathyroidal invasion, pathological LNM, and lower pT and pTNM stages in patients with PTC.306 Furthermore, higher hsa_circ_0015278 expression is significantly associated with a lower relapse rate and better DFS.
While various studies have reported the differential expression of circRNAs in TC tumor tissue and matched normal tissue and demonstrated their value as prognostic biomarkers in patients with TC (Table 3),57, 163, 168, 174, 187, 306 little attention has been paid to the prognostic role of dysregulated circRNAs in peripheral circulation for TC. This potential, which could serve as candidates for liquid biopsy,326 requires further exploration.
| CircRNAs | Chromosome | Gene symbol | Expression change | Relationships with the clinical features | Number of patients | Clinical samples | Clinical value | Reference |
|---|---|---|---|---|---|---|---|---|
| Hsa_circ_0102272 | – | RTN1 | Up | TNM stage, histological grade, LNM, and overall survival state and progression-free survival status | 58 | Tumor tissues | Poorer overall survival (HR = .24, 95% CI = 1.42 –7.55) and progression-free survival | 162 |
| Hsa_circ_0067934 | – | – | Up | Tumor sizes, LNM, and AJCC stages | 57 | Tumor tissues | Poorer overall survival (RR = 4.385, 95% CI = 1.087–17.544) | 138 |
| Hsa_circ_0067934 | chr3 | PRKCI | Up | LNM and AJCC grades | 50 | Tumor tissues | Poorer overall survival | 139 |
| CircBACH2 (hsa_circ_0001627) | chr6:90959407–90981660 | BACH2 | Up | Tumor size, TNM stage, LNM, and survival status | 40 | Tumor tissues | Poorer overall survival | 160 |
| CircFNDC3B (hsa_circ_0006156) | chr3:171965322-171969331 | FNDC3B | Up | Tumor size, LNM, advanced TNM stages, and survival status | 42 | Tumor tissues | Poorer overall survival | 150 |
| CircCCDC66 | – | CCDC66 | Up | Advanced TNM stages, tumor size, LNM, and survival status | 60 | Tumor tissues | Poorer overall survival | 177 |
| CircLDLR (hsa_circ_0003892) | chr19: 11230767–11238761 | LDLR | Up | Advanced TNM stages, tumor size, LNM, and survival status | 45 | Tumor tissues | Poorer overall survival | 173 |
| Hsa_circ_0124055 | chr3:49514281-49548252 | DAG1 | Up | Tumor size, TNM stage, histological grade, LNM, and survival status | 66 | Tumor tissues | Poorer overall survival | 309 |
| Hsa_circ_0101622 | chr14:31775937-31858211 | HEATR5A | Up | Tumor size, TNM stage, histological grade, LNM, and survival status | 66 | Tumor tissues | Poorer overall survival | 309 |
| CircRNA-102002 | – | USP22 | Up | LNM, higher T stage, and survival status | 50 | Tumor tissues | Poorer overall survival | 171 |
| CircPVT1 | – | PVT1 | Up | T stage, LNM, and survival status | 39 | Tumor tissues | Poorer overall survival | 161 |
| Hsa_circ_0008274 | – | – | Up | Advanced TNM stage, LNM, tumor infiltration, and survival status | 60 | Tumor tissues | Poorer overall survival | 174 |
| Hsa_circ_0011058 | – | TMEM222 | Up | Advanced TNM stage, LNM, nodular goiter, and survival status | 62 | Tumor tissues | Poorer overall survival | 168 |
| CircZFR (hsa_circ_0072088) | chr5 | ZFR | Up | TNM stage, LNM, and survival status | 41 | Tumor tissues | Poorer overall survival | 57 |
| CircPUM1 | – | PUM1 | Up | Advanced TNM stage and LNM and survival status | 54 | Tumor tissues | Poorer overall survival | 187 |
| CircRNA_103598 | – | – | Up | Advanced TNM stage, tumor size, metastasis status, and survival status | 100 | Tumor tissues | Poorer overall survival | 188 |
| Hsa_circ_0005273 | – | – | Up | – | 50 | Tumor tissues | Poorer overall survival | 143 |
| Hsa_circ_0011290 | – | – | Up | Advanced stages and survival status | 50 | Tumor tissues | Poorer overall survival | 181 |
| CircNEURL4 (hsa_circ_0041821) | chr17:7225183-7225329 | NEURL4 | Down | Advanced TNM stage, LNM, and survival status | 68 | Tumor tissues | Poorer overall survival | 163 |
| CircITCH | – | ITCH | Down | Clinical stage, LNM, and survival status | 37 | Tumor tissues | Poorer overall survival | 131 |
| Hsa_circ_0015278 | – | – | Down | Age, extrathyroidal invasion, pathological LNM, pT stage, pTNM stage, relapse rate, and survival status | 206 | Tumor tissues | Poorer disease-free survival | 306 |
- Abbreviations: circRNA, circular RNA; LNM, lymph node metastasis; TC, thyroid cancer.
7 FUNCTIONS OF circRNAs IN TUMOR THERAPEUTIC RESISTANCE
Cancer is one of the most prevalent diseases threatening people's health and causing death every year. In China, it is estimated that nearly 5 million new cancer cases and more than 2.5 million new cancer deaths occur annually.327 The five most common cancers in China are lung cancer, colorectal cancer, TC, liver cancer, and stomach cancer, comprising above 55% of all new cancer cases. The five leading causes of cancer death are lung cancer, liver cancer, stomach cancer, colorectal cancer, and esophageal cancer, accounting for more than 65% of total cancer deaths.327 Generally, after the diagnosis of cancer, patients undergo systemic therapies, including surgery, radiation and/or chemotherapy, targeted therapy, immunotherapy, or hormone therapy.39 The cancer survival rate has increased for most cancer types in the past several years.327, 328 However, one of the major challenging aspects of managing cancers is chemoradiotherapy resistance, which has a significant impact on the efficacy of cancer therapy. The biological mechanisms responsible for chemoradiotherapy resistance in tumor cells and the TME are numerous, and extensive researches have suggested that circRNAs play a contributory role in chemoradiotherapy resistance.
7.1 Functions of circRNAs in chemoradiotherapy resistance
7.1.1 Functions of circRNAs in chemotherapy resistance
Chemotherapy resistance poses formidable obstacles to cancer therapy. By acquiring the ability to resist chemotherapy, tumor cells can evade chemotherapy-induced cell death, leading to tumor recurrence, metastasis, and poorer prognosis. The development of chemotherapy resistance results from complex and interacting mechanisms, including dysregulation of efflux transporters expression, enhanced DNA repair systems, suppression of cell apoptosis/ferroptosis, acceleration of autophagy, reinforcement of EMT and stemness, aberrant expression of targeted genes and related signal pathways, and remodeling of the TME.329-334 Increasing evidences have demonstrated that circRNAs participate in the abovementioned processes.
ABCC1 is one of the most studied ATP-binding cassette (ABC) transporters, which is responsible for multidrug resistance.335 Several studies have validated that various circRNAs affect the chemotherapy resistance of cancers by regulating the expression of ABCC1. For instance, hsa_circ_0076305 enhances ABCC1 expression by sponging miR-186-5p, thus promoting cisplatin resistance of NSCLC.336 Similarly, circPVT1 enhances chemotherapy resistance in lung adenocarcinoma for cisplatin and pemetrexed by regulating the miR-145-5p/ABCC1 axis.337 Moreover, macrophage-derived circTEX2 enhances cisplatin resistance in GC by regulating the miR-145/ABCC1 axis.338 Additionally, circSETDB1 sponges miR-508-3p to modulate the expression of ABCC1, contributing to paclitaxel resistance of ovarian cancer cells.339
Different circRNAs also contribute to chemotherapy resistance by influencing the activities of DNA repair systems, evasion of programmed cell death, and reinforcement of EMT and stemness. In bladder cancer, circSTX6 increases the expression of SUZ12 by sponging miR-515-3p and interacting with the mRNA stabilizer PABPC1, enhancing the chemoresistance of bladder cancer cells to cisplatin by facilitating DNA damage repair and inhibiting apoptosis.340 CircVDAC3 mediates trastuzumab deruxtecan resistance in HER2-low BC by regulating ferroptosis through its binding to HSPB1 protein and inhibiting its ubiquitination and degradation.341 In NSCLC, circVMP1 inhibits apoptosis and upregulates EMT by regulating the miR-524-5p-METTL3/SOX2 axis, which results in cisplatin resistance.323 Moreover, circSYT15 acts as a sponge for miR-503-5p to regulate the expression of RSF1 in cervical cancer, suppressing apoptosis, and enhancing drug resistance.342 In bladder cancer, circPTK2 binds to PABPC1 and stabilizes SETDB1 mRNA to promote its expression, facilitating SETDB1-mediated EMT and gemcitabine resistance.343 CircBACH1 promotes the stemness and chemotherapy resistance of BC cells via the miR-217/G3BP2 signaling pathway.344
The dysregulation of p53 and other targeted genes, as well as associated signaling pathways, also play important roles in circRNA-related chemotherapy resistance. CircGLIS3 promotes the progression of prostate cancer by regulating the p53 signaling pathway through the miR-661/MDM2 axis; knocking down this pathway may improve the response of prostate cancer cells to enzalutamide.345 In NPC, hsa_circ_0067717 serves as a scaffold for TRIM41 and p53, stimulating TRIM41-induced p53 degradation through ubiquitination and enhancing paclitaxel chemoresistance.346 Zhu et al.347 found that upregulated circNUP50 promotes platinum resistance in ovarian cancer by accelerating p53 ubiquitination through binding to both p53 and UBE2T, as well as by modulating the miR-197-3p/G3BP1 axis. The authors designed a co-delivery nanosystem comprising both platinum and si-circNUP50, which overcame platinum resistance in an in vivo tumor model effectively.347 Hsa_circ_0097922 silencing increased the chemotherapy sensitivity of BC to tamoxifen in vitro and in vivo partly through regulating the miR-876-3p/ACTN4 axis, which might serve as a potential therapeutic target for BC treatment.348 CircCDYL2 stabilizes GRB7 by preventing its proteolytic ubiquitination and promoting its interaction with FAK in BC, which activates downstream PI3K/AKT and RAS/ERK signaling pathways to enhance trastuzumab resistance.315
CircRNAs also regulate the remodeling of TME to affect chemotherapy sensitivity. In ovarian cancer, circITGB6 promotes an M2 macrophage-dependent cisplatin resistance by forming a circITGB6/IGF2BP2/FGF9 RNA-protein ternary complex by directly interacting with IGF2BP2 and FGF9 mRNA, which stabilize FGF9 mRNA and induce polarization of TAMs toward M2 phenotype.349 In PDAC, silencing circFARP1 in CAFs inhibits the ability of CAFs to induce tumor cell stemness and gemcitabine resistance by regulating the secretion of leukemia inhibitory factor (LIF) and downstream STAT3 signaling pathway, which is modulated by the circFARP1/miR-660-3p/LIF axis and direct interaction between circFARP1 and CAV1.350 In NSCLC, circCPA4 upregulates intracellular and extracellular exosomal PD-L1 levels by sponging miRNA let-7, inactivating CD8+ T cells, promoting cell stemness, enhancing EMT, and increasing resistance to cisplatin.351
7.1.2 Functions of circRNAs in radiotherapy resistance
Whether cancer cells are sensitive to radiotherapy might be influenced by various internal and external factors, including cell cycle regulation and the promotion and inhibition of apoptosis.352 In lung cancer, hsa_circ_0006420 induces G2/M arrest by regulating DNA damage repair pathway-related proteins and promotes cell proliferation and EMT in a p53-dependent manner, increasing radiation resistance by interacting with HUR and PTBP1 in the nucleus.353 Exosomal circPRRX1 was proved to suppress the radiation sensitivity of GC cells in vitro, which functions as ceRNA by regulating miR-596/NKAP crosstalk.354 Hsa_circRNA_101491 can suppress the radiosensitivity of esophageal SCCs by regulating miR-125a-5p to modulate EMT and apoptosis.355 In BC, circABCC1 enhances radiotherapy resistance by regulating miR-627-5p to upregulate the expression of ABCC1.356 CircFIP1L1 regulates apoptosis and radiosensitivity in NPC by modulating the miR-1253/EIF4A3 axis and stabilizing PTEN mRNA.357
The dysregulated DNA damage repair system is another mechanism leading to radiotherapy resistance.352 In NPC, circCDYL2 recruites EIF3D to the 5′-UTR of RAD51 mRNA, promoting the translation of RAD51 and enhancing homologous recombination repair capability as well as radiotherapy resistance.358 In HCC, circEYA3 enhances the radiotherapy resistance by binding to IGF2BP2 and increasing its ability to stabilize DTX3L mRNA, attenuating radiation-induced DNA damage in HCC cells.359 Silencing hsa_circ_0005615 increases the radiosensitivity of colorectal cancer in vivo by regulating the miR-665/NOTCH1 axis.360 Similarly, the knockdown of hsa_circ_0067835 enhances the radiosensitivity of colorectal cancer by modulating the miR-296-5p/IGF1R axis.361
7.2 CircRNAs and their role in TC resistance: potential therapeutic targets
131I therapy is a common treatment for recurrent and metastatic DTC based on cancer cell iodide uptake.362 However, resistance to 131I treatment poses a significant challenge, with patients exhibiting refractory DTC facing a mean survival of less than five years and a 10-year survival rate of less than 10%.189, 363
Addressing 131I resistance in DTC, Chen et al.364 identified circNEK6 (hsa_circ_0088483) as one of the most upregulated circRNAs in TC, particularly in 131I-resistant DTC tissue and cells. CircNEK6 suppression enhances the 131I radiosensitivity of DTC cells by upregulating the inhibitory effect of miR-370-3p on MYH9 expression, resulting in the inhibition of cell proliferation, migration, and invasiveness, induction of cell apoptosis, and DNA damage in 131I-resistant DTC cells.364 Additionally, circNEK6 regulates the miR-370-3p/FZD8 axis and downstream c-myc and cyclin D1, activating the Wnt signaling pathway and influencing TC progression.365
The involvement of circRNAs in the chemosensitivity and radiosensitivity of TC has also been explored. For instance, circEIF6 upregulation in ATC and PTC cells after cisplatin treatment led to suppressed cisplatin sensitivity. CircEIF6 knockdown regulates miR-144-3p/TGF-α, enhancing the chemosensitivity and suppressing proliferation and autophagy in TC cells.164 Besides, hsa_circ_0011058 knockdown enhances the radiosensitivity of PTC cells through the miR-335-5p/YAP1 axis, modulating angiogenesis, proliferation, and apoptosis.168
Several other circRNAs have been implicated in TC treatment resistance. For example, circSH2B3 induces PTC dedifferentiation by modulating the miR-4640-5p/IGF2BP2 axis, suggesting its potential as a target for redifferentiation treatment in radioiodine-refractory PTC.189 Hsa_circ_0079558, promoting the proliferation and migration of PTC cells, was identified as a potential therapeutic target by regulating the miR-26b-5p/MET axis and the downstream MET/Akt signaling pathway, as well as the miR-198/FGFR1 axis. These effects were reversible with PHA665752, a MET-specific small-molecule inhibitor, highlighting its therapeutic potential.166
Concludingly, circRNAs and their associated pathways play pivotal roles in the progression and resistance of TC, presenting promising opportunities as therapeutic targets.
8 CONCLUSION AND PROSPECTS
Despite the conceptual distinctions among these hallmarks, their regulation is interconnected in cancer,23 and this holds true for TC as well. CircRNAs engage in different hallmark capabilities, contributing to overlapping and complementary functions. For instance, circTIAM1 can modulate apoptosis, migration, and proliferation of PTC cells, influencing hallmark capabilities like resisting cell death and sustaining proliferation signaling.158 While mounting evidences underscore the pivotal roles of circRNAs in modulating hallmark capabilities, more comprehensive profiling of the regulatory network of TC is imperative.
The escalating prevalence of TC, particularly PTC, has emerged as a significant global medical challenge.33 Ongoing advancements in research methods are poised to elucidate the roles of various circRNAs in diverse hallmark capabilities and their interplay across these capabilities. As prominent contributors to cancer, including TC, circRNAs have garnered significant attention. However, further investigations are warranted to translate these findings into clinical applications. While many studies have explored the “miRNA sponge” functions of circRNAs in TC, a notable lack of information prevails regarding other functional roles. For instance, circFNDC3B has been identified as an oncogenic player in TC through miR-1178 sponging.150 Still, additional research is needed to determine whether it also functions as a template for encoding novel oncogenic proteins in TC, as observed in colon cancer.366 Additionally, the regulatory mechanisms governing the upregulation or downregulation of circRNAs in TC and their secretion into exosomes for transmitting functions to receptor cells remain unclear.
The diagnostic and prognostic value of circRNAs in cancer research is a key area of focus. Two prospective observational studies demonstrated the potential of circRNAs as clinical biomarkers. Chen et al.367 found that hsa_circ_0089762, hsa_circ_0064644, and hsa_circ_0089763 in plasma are of importance for the early diagnosis of post-stroke cognitive impairment (ChiCTR2000035074). Yuan et al.368 demonstrated that the expression levels of CDR1as were an independent predictive factor for pathological complete response in patients with locally advanced BCs receiving neoadjuvant therapy. Moreover, lower CDR1as expressions were associated with poorer DFS, RFS, and distant DFS, and could serve as independent prognostic factors (NCT02199418 and NCT02221999).368 However, the clinical samples were relatively small, and the studies were conducted in a single center. More clinical studies enrolling larger cohorts and multi-center trials are needed to accumulate evidence supporting the general application of circRNAs as tangible and prevalent diagnostic and prognostic biomarkers in the clinic. For TC, previous studies have primarily detected circRNAs in tissues using invasive methods, limiting their utility for preoperative diagnosis. Further researches are required to explore the role of circRNAs in the blood for noninvasive diagnosis of TC. Moreover, while most studies focused on biomarkers distinguishing patients with TC from those without tumors, biomarkers capable of differentiating malignant nodules from benign ones are more valuable to clinicians, especially those measurable in peripheral blood. It is crucial to consider that a specific circRNA synthesized in diverse kinds of tumors can be transferred to the peripheral blood.369 Therefore, before clinical application, dysregulated circRNAs must be confirmed to originate from TC, and not from other tumors. Thus, the genuine diagnostic and prognostic roles of circRNAs for TC in peripheral blood should be further investigated.
With the increasing evidences of artificial circRNAs in various diseases,370-375 developing synthetic circRNAs as precise therapeutic strategies for TC is of great potential, such as artificial miRNA “sponges” or translation templates, especially considering the cell- and tissue-specific expression patterns of circRNAs. Synthetic circRNA sponging oncogenic miR-21 was engineered based on the ceRNA theory, which was effective in suppressing gastric carcinoma cell and lung cancer cell proliferation in vitro.371, 376 Recently, Kasamatsu et al.377 developed a synthetic circRNA containing binding sequences for miR-1269a and validated its function in OSCC cell lines, where it inhibited tumorigenesis by regulating the miR-1269a/PLCG2 axis. Researches further studied the functions of artificial circRNAs in vivo. Wang et al.378 constructed a circRNA containing multi-miR binding sites, which induced loss-of-function of both miR-21 and miR-93 in vitro and in vivo. The results suggested the potential of artificial circRNA in molecular therapeutics for esophageal carcinoma.378 Bayat et al.379 designed and synthesized a circular decoy, named CM21D, with three binding sites for miR-21 using the tRNA-splicing mechanism in GBM cell models. In vitro and in vivo experiments demonstrated the greater efficiency of CM21D at suppressing tumor growth by targeting miR-21 to rescue the expression of miR-21 target genes, than its linear form, LM21D.379 Later, Adibzadeh et al.380 used the CRISPR/RMCE hybrid system to generate recombinant CHO cells capable of synthesizing CM21D successfully, which further highlighted the potential for developing synthetic circRNAs as precise therapeutic strategies. Moreover, synthetic circRNAs capable of producing functional proteins have also been designed and fabricated.381, 382
Apart from synthesizing the forged circRNA decoys to exhibit as tumor suppressors, researchers have also designed siRNAs targeting oncogenic circRNAs to inhibit tumor development and metastasis. Wang et al.383 found that exosome-delivered siRNA of ciRS-122 suppressed glycolysis and reversed chemoresistance in colorectal cancer by modulating the miR-122–PKM2 pathway in vitro and in vivo. Meng et al.316 developed nanoparticles (NPs) loaded with siRNA of circROBO1, which inhibited HCC progression without apparent toxicity in vivo. In a patient-derived tumor xenograft model, Yang et al.384 validated that the tail vein injection of shRNA targeting circPTK2 significantly inhibited tumor metastasis. Furthermore, the development of physicochemical technologies also demonstrated the potential of synthetic circRNAs as siRNAs in vitro and in vivo, shedding light on their application as therapeutic platforms for gene-silencing in various cancers.385, 386
Although the studies about therapy methods using circRNA had been successfully conducted in vivo, preclinical application and clinical trials are still in the primary stages. Before the wide clinical application of circRNA as a cancer therapy, several limitations and challenges should be resolved.
First, the accurate delivery of circRNAs or siRNAs to target tissues or cells is fundamental for circRNA-based cancer therapy.387 Fortunately, the development of exosome-based, NPs-based, adeno-associated virus (AAV) vector-based, and other delivery strategies have laid a solid foundation for the future of circRNA-based precision medicine.387-389 Exosomes are widely recognized as promising drug carriers due to their low immunogenic potential, ability to cross biological barriers, unique stability, and biocompatibility.39, 390, 391 Moreover, their surface could be monitored to further improve its inherent capacity to deliver RNAs to target tumor sites.391, 392 Zhou et al.393 re-engineered the exosomes to select and encapsulate specific artificial circRNAs, which successfully transferred antitumor artificial circRNAs to bladder cancer cells. Furthermore, growing researches suggest that exosomes derived from mesenchymal stem cells (MSCs) possess remarkable therapeutic potential.391, 394 By transferring the naturally retained regenerative capabilities and biological cargos, including miRNAs, lncRNAs, and circRNAs, of their parental stem cells, these EVs demonstrated their therapeutic abilities in various pathological situations, especially in various cancers.391 For instance, exosomal hsa_circ_0030167 derived from bone marrow MSCs suppressed the progression and stemness of pancreatic cancer cells by sponging miR-338-5p to regulate the downstream wif1/Wnt8/β-catenin axis.395 Hsa_circ_0037104 in human umbilical cord-derived MSCs-derived exosomes could inhibit the proliferation and metastasis of cholangiocarcinoma cells by regulating the miR-620/APAF1 axis.396 In addition, exosomes derived from MSCs can be modified to carry specific therapeutic circRNAs through exogenous or endogenous loading.391, 392
NP-mediated delivery systems are also widely used in anticancer therapy due to their targeted delivery, extended cargo release, and higher stability.397, 398 Furthermore, the ligands on the NP surface could be manufactured to be actively recognized by the targeting receptors overexpressed on tumor cells, enhancing its efficacy and exemption of side effects.399 Moreover, NP-based stimuli-responsive release systems, which enable the appointed discharge of cargo upon internal and external stimuli, would further heighten its clinical prospect.400, 401 For example, has_circ_0001073 delivered by NPs could suppress tumor growth in a xenograft BC model.402 Polyethylenimine-based NPs delivered synthetic circRNAs decoys targeting miR-21 exhibited remarkable suppression of tumor progression in a lung adenocarcinoma xenograft mouse model.403 Additionally, AAV vectors, incorporating synthetic circRNAs with binding sites for miR-132 and miR-212, were administered and selectively expressed in cardiomyocytes within an in vivo model of cardiovascular disease.404 However, the potential long-term immunological and off-target effects cannot be entirely ruled out.
Second, off-target effects must be overcome before the adoption of circRNA-based therapies as a viable therapeutic option in the clinic, considering that one specific circRNA could be involved in various diseases, even types of cancers, and that one specific circRNA could function by regulating diverse miRNAs and downstream pathways.5, 88 The meticulous design of sequence compositions, chemical modifications, and cell-specific promoters of artificial circRNAs might decrease the underlying off-target effects.387, 388, 404 Furthermore, the CRISPR/Cas system, along with elaborately considered delivery methods, can contribute to the reduction of off-target effects.98, 389 Third, the expression of artificial circRNAs in vivo needs to be appropriately modulated.405 The customization of engineered circRNA regulators406 and CRISPR/Cas gene editing strategy407, 408 could provide promising solutions to these issues.
Due to their unique closed-loop structure, low immunogenicity, and extremely high stability, circRNA-based therapeutic platforms have evoked a surge of research interest.409, 410 In the future, an enhanced understanding of the biological functions of circRNAs and the implications of their dynamic networks in TC will open novel avenues for developing circRNA-based therapeutics for TC, playing a vital role in the precision treatment of TC.
AUTHOR CONTRIBUTIONS
Yang Guo, C. W., and L. Z. conceived this manuscript. Yang Guo drafted the manuscript and prepared the figures. Qiang Huang and Yu Heng collected the related references and prepared tables. They contribute equally to this manuscript. Yujuan Zhou, Hui Chen, and Chengzhi Xu participated in the discussion and offered valuable recommendations for the manuscript. Chunping Wu, Lei Tao, and Liang Zhou supervised and revised the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
Graphic Abstract and Figure 1 was created using Figdraw (www.figdraw.com). Besides, we would like to thank Editage (www.editage.cn) for English language editing. The present study was supported by grants from the Shanghai Sailing Program (21YF1405600 and 23YF1404700), National Natural Science Foundation of China (82403387) and Science and Technology Development Fund of Shanghai Pudong New Area (PKJ2024-Y02), Clinical Research Plan of SHDC (SHDC2020CR6011), Shanghai Municipal Key Clinical Specialty (SHSLCZDZK00801), and Science and Technology Commission of Shanghai Municipality (19411961300).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




