Structural basis of neuropeptide Y signaling through Y1 and Y2 receptors
Abstract
Neuropeptide Y (NPY), a 36-amino-acid peptide, functions as a neurotransmitter in both the central and peripheral nervous systems by activating the NPY receptor subfamily. Notably, NPY analogs display varying selectivity and exert diverse physiological effects through their interactions with this receptor family. [Pro34]–NPY and [Leu31, Pro34]–NPY, mainly acting on Y1R, reportedly increases blood pressure and postsynaptically potentiates the effect of other vasoactive substances above all, while N-terminal cleaved NPY variants in human body primary mediates angiogenesis and neurotransmitter release inhibition through Y2R. However, the recognition mechanisms of Y1R and Y2R with specific agonists remain elusive, thereby hindering subtype receptor-selective drug development. In this study, we report three cryo-electron microscopy (cryo-EM) structures of Gi2-coupled Y1R and Y2R in complexes with NPY, as well as Y1R bound to a selective agonist [Leu31, Pro34]–NPY. Combined with cell-based assays, our study not only reveals the conserved peptide-binding mode of NPY receptors but also identifies an additional sub-pocket that confers ligand selectivity. Moreover, our analysis of Y1R evolutionary dynamics suggests that this sub-pocket has undergone functional adaptive evolution across different species. Collectively, our findings shed light on the molecular underpinnings of neuropeptide recognition and receptor activation, and they present a promising avenue for the design of selective drugs targeting the NPY receptor family.
1 INTRODUCTION
The neuropeptide Y (NPY) receptor subfamily belongs to class A G-protein coupled receptor (GPCR) β branch and comprises four receptor subtypes (Y1R, Y2R, Y4R, and Y5R), mediating different types of effector proteins signaling pathways, including Gi or Go proteins.1-3 They can be activated by three structurally related, but functionally diverse endogenous peptides: NPY, peptide YY, and pancreatic polypeptide (PP).4, 5 All three peptides comprise 36 amino acids each with amidated C-terminal ends and share highly conserved amino acid sequences.6, 7 Among them, NPY could specifically activate Y1R, Y2R, and Y5R, and it is broadly distributed in the central and peripheral nervous system, regulating various physiological processes such as food intake, stress response, anxiety, and memory retention.8, 9
NPY is cleaved into two N-terminal truncated NPY variants during cellular metabolism: NPY3−36 and NPY2−36, processed by dipeptidyl peptidase IV and aminopeptidase P (AmP), respectively.10-13 The truncated NPY peptides lose their binding affinity for Y1R, but they retain a similar binding affinity with Y2R.14, 15 In addition, the synthesized NPY analog [Leu31, Pro34]–NPY is reported to be specific agonist at Y1R, sharing similar activation potency with NPY for Y1R.16-19 Y1R and Y2R are known to be primarily expressed at the post- and presynaptic membranes, respectively.20-22 [Pro34]–NPY and [Leu31, Pro34]–NPY, when acting on Y1R, primarily raise blood pressure and postsynaptically potentiate the effects of other vasoactive substances. In contrast, N-terminally truncated NPY mostly mediates angiogenesis and inhibits neurotransmitter release through Y2R-mediated processes.23-27 These results suggest that Y1R and Y2R exhibit different ligand recognition mechanisms and could potentially allow for selective drug development for corresponding condition. However, selective agonist development would require the identification of the underlying mechanisms of receptor–peptide ligand interactions. Although structural information of Y1R- and Y2R has been reported,28-30 the underlying ligand selectivity and receptor activation mechanisms remain unclear. Recently, structural studies on the NPY1R have elucidated the molecular mechanisms underlying Y1R recognition of NPY. However, the molecular basis for why Y1R requires the complete N-terminus of NPY for optimal activation and why [Leu31, Pro34]–NPY selectively activate Y1R rather than Y2R remains unclear. Our study has provided new insights to address these mentioned issues.
In this study, we present three single-particle cryo-electron microscopy (cryo-EM) structures of the Y1R/Y2R–Gi2 signaling complexes bound to endogenous peptide NPY, as well as Y1R–Gi2 in complex with a selective ligand [Leu31, Pro34]–NPY. Moreover, we performed structural comparison and identified a unique sub-pocket in Y1R that accommodates the N-terminal NPY residues. Further, the results of our adaptive evolution analysis demonstrated that positive Darwinian selection occurred specifically in Y1R from Osteichthyes. In addition, our structural analysis reveals that the residue Pro34 substitution in NPY specifically interrupted the extensive NPY–Y2R interaction but is more compatible with Y1R. Our study elucidates key residues required for NPY peptide recognition and deciphers the plasticity of orthosteric site of NPY receptor subtypes in response to the same ligand stimuli.
2 RESULTS
2.1 Cryo-EM structures of Y1R/Y2R–Gi2 signaling complexes
In order to investigate Y1R and Y2R activation potency in response to NPY variants, including N-terminally truncated NPY and NPY mutants (Figure 1A), we measured the cAMP inhibition ability of wildtype Y1R and Y2R elicited by different agonists in HEK293 cells (Figures 1A–C). Our results indicated that deleting the N-terminal residues (Y1 and P2) of NPY (NPY3−36) and NPY13−36 remarkably reduced Y1R activation compared to NPY (>50 folds), whereas [Leu31, Pro34]–NPY analog retained a potency nearly identical to NPY (Figure 1B). In contrast, the NPY3−36 variant exhibited slightly reduced Y2R activation and [Leu31, Pro34]–NPY was ∼100-fold less potent than NPY on Y2R activation (Figure 1C). These observations suggest that Y1R and Y2R display different ligand recognition mechanisms.
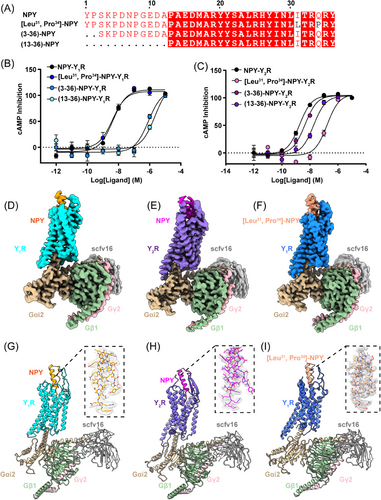
To gain a more thorough understanding of how Y1R and Y2R recognize NPY or NPY mutants, we determined the structures of NPY-bound Y1R, NPY-bound Y2R, and [Leu31, Pro34]–NPY-bound Y1R in complex with the Gi2 protein using cryo-EM single-particle technique (Figures 1D–I and S1 and 2, and Table S1). The final cryo-EM maps of NPY–Y1R–Gi2 (PDB ID:8K6M), NPY–Y2R–Gi2 (PDB ID:8K6N), and [Leu31, Pro34]–NPY–Y1R–Gi2 (PDB ID: 8K6O) displayed global nominal resolutions of 3.5, 3.2, and 3.3 Å after refinement, respectively (Figures S1 and 2 and Table S1). The Y1R and Y2R structure shared a canonical seven-transmembrane (TM) helical architecture and an intracellular amphipathic helix 8. The seven TM helix domains of both receptors could be visibly distinguished in the cryo-EM maps (Figure S3), and the high-resolution density maps allow us to unambiguously build the NPY, [Leu31, Pro34]–NPY, NPYRs, Gαi2, Gβ, Gγ, and scFv16 (Figures 1D–I and S3). However, probably due to the NPY flexibility in the extracellular region, we failed to model the residues 10−15 from NPY and [Leu31, Pro34]–NPY and the residues 10−14 from NPY in Y1R and Y2R complex structures, respectively (Figures 1G–I).
By comparing NPY–Y1R or NPY–Y2R with their inactive states (PDB ID: 5ZBQ and 7VGX, respectively),31, 32 we noticed that the intracellular end of TM6 in two receptors exhibited an obvious outward shift to accommodate the Gαi2 α5 helix and TM7 also adopted an inward displacement similar to other active class A GPCR structures33-35 (Figures S4A and B). As the NPY–Y1R and [Leu31, Pro34]–NPY–Y1R structures share highly similar conformation with a Cα root mean square deviation (RMSD) of 0.569 Å (279–279 atoms), we use the NPY–Y1R–Gi2 and NPY–Y2R–Gi2 structures to analyze the overall Y1R and Y2R structures in this section (Figure S4C).
Although Y1R and Y2R shared only 30% sequence identity, these two receptors activation complex structures resembled a similar conformation with a Cα RMSD of 0.905 Å (213–213 atoms) (Figure S4D). It is noteworthy that the extracellular end of TM1, TM4, and TM5 in Y1R exhibited a slight outward movement relative to that in Y2R (Figure S4D). The ligand NPY displayed similar binding pattern both in the Y1R and Y2R structures and folded into a canonical PP-fold,36 the C-terminal regions (residues 32−36) were inserted into an orthosteric pocket of Y1R and Y2R, while the N-terminals (residues 1−10) folded back to form extensive interactions with the NPY α-helix (residues 15−31) (Figures 1G–H and S5A–C).
2.2 A sub-pocket in Y1R determines NPY selectivity
The NPY–Y1R with NPY–Y2R structural comparison revealed remarkable displacements (∼9 Å) in the NPY N-terminal part (Figure S5D). In Y1R, the NPY N-terminal end could be found in a cavity composed of the extracellular ends of TM5, TM6, and ECL2. However, the NPY N-terminal part in Y2R extended toward the ECL2 region, forming relatively few contacts with the receptor. In accordance with the NPY pharmacological assay, the structure revealed different NPY recognition mechanisms in Y1R and Y2R.
In detail, the NPY N-terminal Y1 and P2 residues fitted into the amphiphilic cavity formed by hydrophobic (P183ECL2, F199ECL2, and F2866.58) and polar (E182ECL2 and D200ECL2) residues, and the corresponding [Leu31, Pro34]–NPY residue adopted a conformation similar to that of NPY (Figure S6A). Therefore, we defined the cavity in Y1R as a unique sub-pocket (Figures 2A and B), where the clusters of hydrophobic residues (P183ECL2, F199ECL2, and F2866.58) formed a hydrophobic contact network with Y1 and P2 residues and D200ECL2 and E182ECL2 established a polar network with the NPY peptide Y1 and K4 side chains (Figure 2B). In addition, two critical residues R2086.35 and F2866.58 bifurcated the NPY binding pocket in Y1R into two cavities, a sub-pocket, and a major orthosteric pocket (Figure 2B). In contrast to the Y1R NPY recognition mode, Y2R did not contain the equivalent sub-pocket for NPY binding (Figures 2C and D).
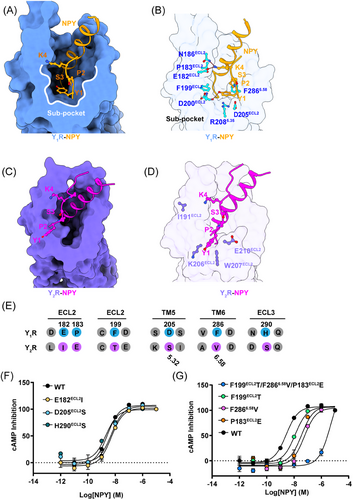
To further identify the key residues of the Y1R sub-pocket for the NPY N-terminus recognition, we conducted a series of mutagenesis analyses. The Y1R and Y2R sequence alignment results confirmed that the Y1R sub-pocket residues (E182ECL2, D200ECL2, P183ECL2, F199ECL2, and F2866.58) were not conserved (Figure 2E). We replaced these key residues with the corresponding residues in Y2R and our mutagenesis test demonstrated that mutations E182I, D205S, and H290S in Y1R did not affect the receptor activation potency (Figure 2F and Table S2). However, the hydrophobic residues of sub-pocket mutations (P183ECL2E, F199ECL2T, F2866.58 V, and P183ECL2E/F199 ECL2T/F2866.58 V) all severely reduced Y1R potency to NPY (Figure 2G and Table S2). Our results indicate that the sub-pocket-forming hydrophobic residues contribute to the Y1R-mediated NPY recognition.
Compared with other β-branch structures of class A GPCRs, for instance, the endogenous endothelin ET-1 peptide-bound ETB receptor structure revealed a similar peptide–receptor binding mechanism as that in Y1R, in which the extracellular part of the receptor (ECL2, TM6, and ECL3) is widely involved in the N-terminal ET-1 binding33 (Figure S6B). However, the N-terminal ET-1 region mainly forms hydrogen bonds and electrostatic interactions with ET-1 and no such Y1R sub-pocket has been observed in the ETB–ET1 structure (Figures S6B–C).
Our structures revealed a sub-pocket, which plays important roles in the N-terminal NPY recognition and receptor activation of Y1R. Our observation was consistent with previous Y1R-related NPY NMR modeling and docking studies.37 Taken together, our study provides insights into the detailed NPY N-terminus-Y1R interactions and helps in fully understanding the different selectivity mechanisms of various N-terminally truncated NPY peptides upon Y1R and Y2R activation.
2.3 Adaptive evolution of the Y1R sub-pocket
NPY and Y1R evolutionary dynamics in early vertebrates have already been thoroughly studied.2, 38 However, the evolutionary fate of Y1R sub-pocket is elusive. We collected 22 genomes of vertebrates and invertebrates to reveal the related evolutionary processes. Our results revealed that NPY and Y1R were present in vertebrates such as Cyclostomata, Chondrichthyes, Osteichthyes, and Tetrapoda, but not in invertebrates (Figure 3A). We selected six classical vertebrate Y1Rs for signal pathway detection and the results demonstrated that Cyclostomata (pmY1R/Petromyzon marinus), Osteichthyes (drY1R/Danio rerio), and Tetrapoda (hY1R/Homo Sapiens, and cpbY1R/Chrysemys picta bellii) Y1Rs could be activated by NPY, while such activation is significantly weakened (scY1R EC50 = 175.1 nM and ccY1R could not be activated by NPY) in Chondrichthyes (scY1R/Scyliorhinus canicula and ccY1R/Carcharodon carcharias) (Figures 3A and B). The branch-site model has proved to be a useful tool for detecting biological hypotheses of positive selection and generative mutation research and functional analysis.39 So, we used a branch-site model to assess whether the Y1Rs underwent positive selection.40, 41 As shown in Figure 3C, the Y1R sequences exhibited a relevant nonsynonymous (dN)/synonymous (dS) substitution rate ratio (branch-site dN/dS of ω > 1; Figures 3A and C) that was highly significant (likelihood ratio tests [LRTs], p < 0.05; Figure 3C) only in the Osteichthyes lineage. In contrast, Chondrichthyes did not exhibit this ratio, suggesting that positive Darwinian selection occurred specifically in Osteichthyes, but not in Chondrichthyes Y1Rs (Figure 3C). And we found that the sub-pocket residues Q182 and R203 in Osteichthyes Y1R were under positive selection (Figure 3C). Furthermore, according to sequence logos between Osteichthyes and Chondrichthyes Y1Rs, the six sub-pocket residues were non-conserved (Figure 3D).
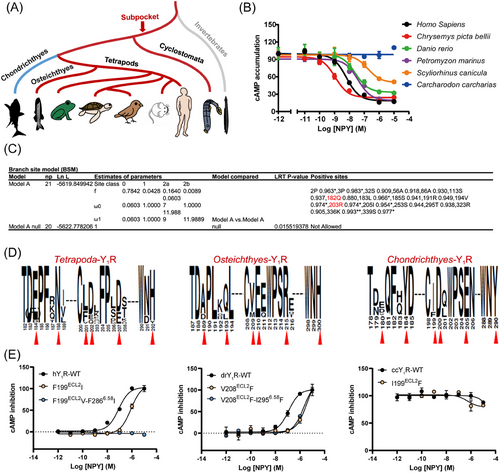
To verify the Y1R-related functional differences between Chondrichthyes and other vertebrates, three vertebrate species (human/Homo Sapiens, zebrafish/Danio rerio, and Carcharodon/Carcharodon carcharias representing Tetrapoda, Osteichthyes, and Chondrichthyes, respectively) were selected to test the biological experimental research (Figure 3E). We used the NPY peptide to activate the different Y1Rs. NPY activated humans and zebrafish, but not Carcharodon, Y1Rs. To further investigate the function of the six residues (E182ECL2, Q186 ECL2, F199 ECL2, D200ECL2, D2055.32, F2866.58, and H290ECL3) in the sub-pocket, the human Y1R residues (hY1R) were mutated to the corresponding residues in the Chondrichthyes orthologs. Similar to the effect of mutating the corresponding key residues in Y2R, residue F199ECL2 and F2866.58 substitutions markedly affected the hY1R activation efficacy in response to the NPY peptide (Figure 3E). In addition, residues N186 and D205 in human Y1R sub-pocket under positive selection were also engaged in NPY recognition (Figure S7A). Next, Carcharodon Y1R (ccY1R) (Q181ECL2, Y184 ECL2, I199 ECL2, E2055.32, and Y290ECL3) and zebrafish Y1R (drY1R) (A188 ECL2, Q192 ECL2, V208 ECL2, R2145.32, and I2956.58) residues were reversely mutated to the corresponding hY1R. We observed that mutated residues in Carcharodon Y1R to the corresponding hY1R does not rescue their ability to respond to the NPY. From an evolutionary perspective, the reason is that the protein conformation of Carcharodon Y1R has undergone significant changes, and rescuing their ability to respond to NPY may require more than a few point mutations (Figures 3E and S7B-C). Taken together, our results indicated that the positive selection pressure in Osteichthyes Y1R maintains the sub-pocket and contributes to retaining the Gi signaling. In contrast, Chondrichthyes were not constrained by the selection pressure, and the mutations in the sub-pocket amino acids led to changes in their Gi signaling.
2.4 Conserved common site for NPY recognition by NPYRs
Overall, the NPY-bound Y1R and Y2R structures shared a similar conformation and central residues, E15–L31 (α-helix region) of NPY formed approximately five α-helical turns and the base of the NPY α-helix overlay in the two structures, while the amino terminus of the Y1R helix was rotated approximately 10˚ inwards compared with that in Y2R (Figure S8A). The residue R25 of NPY in the Y1R structure formed hydrogen bonds with D1042.68, which could not be observed in the NPY-Y2R structure, potentially contributing to the inwards shift of the NPY-bound Y1R α-helix (Figure S8B). In addition, the hydrogen bonds between the residue R25 of NPY and D1042.68 further stabilized the NPY–Y1R interactions. Consistent with these structural observations, a D104A2.68 substitution reduced the potency of the Y1R response for NPY (∼73 folds) (Figure S8C).
NPY in the Y1R and Y2R complex structures adopted similar conformations in the recognition by the NPYR orthosteric pockets (Figures 4A–D). The C-terminus of NPY (residues 33−36), which was confirmed to display major importance for all NPYR bindings,3, 42 adopted an extended conformation and reaches far into the cores of Y1R and Y2R, contacting all TM helices except for TM1, as well as residues in ECL2 and ECL3 through an extensive interface of hydrophobic and polar interactions (Figures 4A–D). The NPY C-terminal-bound Y1R structure exhibited both important common characteristics and notably distinct features compared to the NPY-bound Y2R structure (Figures 4A–D). The structural comparison revealed that the Y1R-bound NPY side chains R35 and Y36 overlaid well with those of Y2R-bound NPY, while the NPY residues R33 and Q34 occupied different binding sites to those of Y2R-bound NPY (Figure 4E).
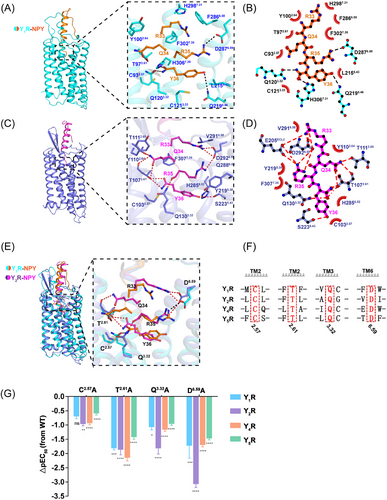
At the bottom region of the orthosteric peptide-binding pocket, polar receptor residues formed an extensive polar interaction network with amidated Y36 both in the Y1R-and Y2R-bound NPY structures, structurally supporting the fact that this amidation modification was necessary for the NPYR activity.43, 44 The Y36 amidation group and hydrogen bond established polar contacts with residues T1072.61 and S2235.46 in the NPY-Y2R structure (Figures 4C and D), whereas the amidation and carbonyl groups of Y36in the Y1R-bound NPY formed hydrogen bonds with residues Q1203.32 and H3067.39, respectively (Figures 4A and B). In addition, residue Y36 was further coordinated by Q2195.46 through polar interactions and C932.57 through van der Waals contacts in the NPY-Y1R structure (Figures 4A and B). On the same Y36 orientation, residue R35 was fastened mainly through polar interactions by D6.59 in the NPY–Y1R and NPY–Y2R structures (Figures 4A–D). Notably, previous studies reported that the ionic interaction of residue D6.59 is key for positively charged ligand recognition.45
To further identify the common binding sites NPYRs, we conducted a series of mutagenesis analyses. Our NPYR sequence alignments indicated that residues (C2.57, T2.61, Q3.32, and D6.59) in TM2, TM3, and TM6, which form polar interactions and van der Waals contacts with the C-terminus residues Y36 and R35, were conserved among the four receptor subtypes (Figures 4E and F). Mutation of these conserved residues (C2.57A, T2.61A, Q3.32A, and D6.59A) in Y1R, Y2R, Y4R, and Y5R significantly impaired receptor activity (Figures 4G, S9–12 and Table S2), supporting the essential role of these residues in NPY recognition. Taken together, the structural observations and mutagenesis analyses revealed that NPYRs share common binding sites to bind NPY residues Y36 and R35 and adopt different molecular patterns in the interaction with NPY.
2.5 The different recognition patterns in Y1R and Y2R
Our cAMP assay data demonstrated [Leu31, Pro34]–NPY retained similar activation potency as NPY on Y1R, but displayed higher selectivity for Y1R over Y2R, indicating that both Y1R and Y2R has different mechanisms for ligand recognition. Our structures of Y1R and Y2R in complex with NPY or [Leu31, Pro34]–NPY offer templates to understand the ligand recognition or selectivity. In the case of the NPY-Y1R structure, the residue Q34 is observed to interact with only T972.61 in Y1R through hydrogen bonding (Figure 5A). Whereas the side chain of Q34 in Y2R displayed a different conformation and was projected into a cavity shaped by TM2 and TM7, interacting with T1072.61, Y1102.64, T1112.65, and F3077.35 in Y2R (Figures 5B and S13A).
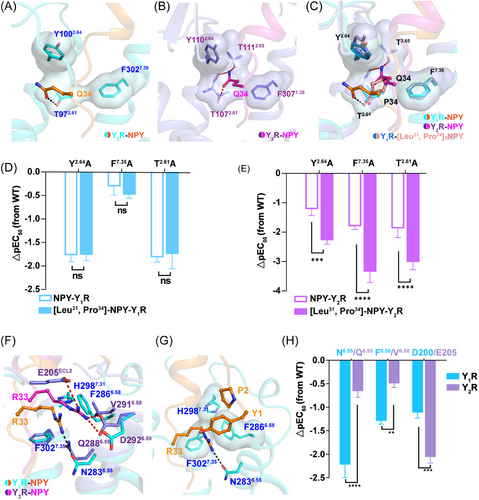
To further analyze the selectivity of [Leu31, Pro34]–NPY, we determined the structure of the [Leu31, Pro34]–NPY-bound Y1R in complex with the Gi2 protein. The C-terminal parts of NPY and [Leu31, Pro34]–NPY adopted similar binding pose in the orthosteric pocket of Y1R (Figure S13B). Residue P34 of [Leu31, Pro34]–NPY share a similar position with residue Q34 of NPY (Figures 5C and S13B and C). The substitution of Q with P at position 34 of NPY disrupted the interactions between Q34 and T972.61 in Y1R. The residue P34 in [Leu31, Pro34]–NPY peptide was not observed to make polar interaction with Y1R, and this kind of peptide did not influence Y1R signaling activation. Comparing to structures of Y1R bound NPY or [Leu31, Pro34]–NPY, the residue Q34 of NPY makes hydrogen bonds with T1072.61 and T1112.65 in Y2R (Figure 5C). These residues T1072.61, Y1102.64, T1112.65, and F3077.35 that involved in ligand binding pocket are conserved in both Y1R and Y2R. We next investigated whether these conserved residues play different roles in Y1R and Y2R, the residues T2.61, Y2.64, T2.65, and F7.35 were replaced with Ala both in Y1R or Y2R. The results of our functional assays showed that all mutants of Y2R significant lost NPY-induced signal transduction, which further confirm the important roles of these residues in Y2R (Figure S13D). By contrast, T2.65A and F7.35A mutations in Y1R displayed similar activation potency as wild-type receptor in response to NPY (Figure S13D).
To further compare the pharmacological featured of NPY and [Leu31, Pro34]–NPY, we measured the NPY or [Leu31, Pro34]–NPY-induced Y1R and Y2R activation. For Y1R, NPY and [Leu31, Pro34]–NPY displayed similar potencies on Y1R or mutants (Figure 5D). However, both NPY analogs exhibited different activation potencies for Y2R and its mutants (Figure 5E).
In addition, structural comparisons of Y1R and Y2R revealed a notable displacement of R33 from NPY peptides in two receptors (Figure 5F). In Y2R structure, the residue R33 is observed to interact with D2926.59 and forms polar interactions with R35 and E205ECL2 via hydrogen bonding (Figure 5F). In Y1R structure, the side chain of R33 in NPY is found to insert a cavity shaped by TM6 and TM7, establishing hydrophobic contacts with residues F3027.35, H2987.31, and F2866.58 in Y1R (Figure 5F). Consistent with the structural observations, the results of mutagenesis studies and functional assays showed that the mutation E205ECL2A influenced NPY induced Y2R activation potency, whereas the mutation N2836.55A reduced the Y1R activation induced by NPY (Figures 5H, S9–12 and Table S2). Notably, the residues F2866.58 in Y1R appears to be a key facet to shape the sub-pocket (Figure 5G).
2.6 Structural basis of NPY mediated Y1R and Y2R activation
The structural comparison of our two Gi2-coupled NPY receptors with other class A GPCRs revealed similar conformations.46 TM6 and TM7 of Y1R and Y2R adopted nearly identical conformations to the active structures of the neurotensin receptor NTS1,47 orexin receptors,35 and the endothelin ETB receptor33 (Figure S14). Furthermore, the structural comparison of two Y1R and Y2R receptors with the antagonist-bound NPYRs (PDB ID: 5ZBH, 5ZBQ, and 7DDZ),31, 32 supports the contention that these two complexes were in the active state (Figures 6A and D). Compared with structure of antagonist BMS-193885 bound Y1R (PDB ID: 5ZBH), the activated Y1R complex displayed pronounced outward displacements (∼8 Å, measured at Cα of T2586.30) of the TM6 at cytoplasmic region, and ∼5 Å inward shift of TM7 (measured at Cα of Y3207.53) (Figure 6A).
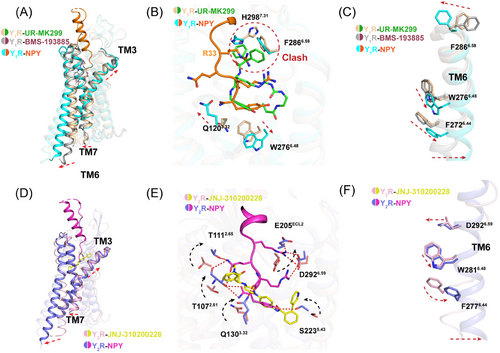
The residue F2866.58 in Y1R was demonstrated to play important role in receptor activation as well as ligand selectivity. The detailed comparison of both active and inactive structures reveals a significant movement of F2866.58 toward receptor helical core, which may initiate the cascade of conformational changes upon agonist binding. Meanwhile, the microswitch residue W6.48 is observed to display rotameric change (Figures 6C and F), synergistically, F6.44 in P-I-F motif underwent conformational changes to facilitate G-protein coupling (Figure S15). In addition, residue Q3.32 conserved in Y1R and Y2R formed hydrogen bonds with NPY, leading to a slight upward movement of TM3 (Figures 6A, B, D, and E).
3 DISCUSSION
NPY serves a critical role in modulating a variety of physiological processes in both the central nervous system and peripheral tissues. It exerts its effects through binding to G protein-coupled NPY receptors, with the Y1 and Y2 receptor subtypes being particularly noteworthy. Gaining a comprehensive understanding of the structural mechanisms through which Y1R and Y2R interact with NPY is indispensable for the rational design of selective drugs.
Although it reported some structures of the NPY-bound receptor complex48, 49 during the preparation of our manuscript, some new insights about ligand selectivity mechanisms of NPYRs were still obtained in our research. Here, we present three cryo-EM structures of Gi2-coupled Y1R and Y2R bound to either NPY or [Leu31, Pro34]–NPY. These structures reveal a conserved orthosteric peptide-binding pocket in both Y1R and Y2R that interacts with the C-terminal region of NPY. The extreme C-terminal dipeptide with amidated modification (R35-Y36-NH2) is buried in the bottom of orthosteric pocket, which is highly conserved between the two NPYR subtypes. Through a combination of structural observations and alanine mutagenesis analysis, we demonstrate that the binding of the NPY C-terminus is critical for the activation potency or efficacy of NPY.
In contrast, distinct physiochemical environments surrounding a tetrapeptide (Y1-P2-R33-Q34) between Y1R and Y2R serve as determinants for two NPYR subtype preference. Intriguingly, we identified a distinct sub-pocket in Y1R that contributes to ligand selectivity and the sub-pocket of Y1R plays an essential role in the recognition of the N-terminus of NPY and receptor activation. Notably, Y1-P2-R33 forms a specific interaction network with hydrophobic residues F3027.35, H2987.31, F2866.58, enhancing the Y1R selectivity.
Moreover, comparisons of the NPY- Y1R, NPY–Y2R, and [Leu31, Pro34]–NPY–Y1R complex structures highlight the plasticity of orthosteric pockets across receptor subtypes. Substituting Q34 with P34 disrupts the original polar and hydrophobic interactions with Y2R, which plays a more indispensable role of Y2R to interact with NPY than Y1R, thus leading to the Y2R selectivity.
In summary, we offer detailed molecular maps depicting the binding of NPY peptides to various NPY receptor subtypes, thereby shedding light on subtype-specific interaction patterns. This critical insight substantially broadens our knowledge of ligand recognition and signal transduction within the NPY–GPCR system. Consequently, our findings establish a robust foundation for the future development of selective drugs aimed at specific NPY receptor targets, thereby paving the way for more precise and efficacious therapeutic strategies.
4 MATERIALS AND METHODS
The details of the protein expression and purification, cryo-EM structure determination, and pharmacological experiments are provided in Supplementary Information Methods.
AUTHOR CONTRIBUTIONS
W. Y. and Z. S. initiated structural studies of NPYRs and their ligands. S. S. and C. S. designed the expression constructs, purification, and preparation of the NPY–Y1R–Gi2, NPY–Y2R–Gi2 and [Leu31, Pro34] NPY–Y1R–Gi2 complexes. S. S., L. C., and C. Z. carried out cryo-EM screening, data collection, and model building and refinement in the study. Y. D., C. W., and K. W. performed functional assays. Z. Y. and H. H. contributed in purification of scFv16. H. C. performs bioinformatics analysis under the direction of C. D. S. S. and Y. D. prepared figures. S. S., C. S., and Y. D. planned and coordinated the entire project under the supervision of W. Y. and Z. S. W. Y., F. Y., C. D., and Z. S. supervised the overall project and wrote the manuscript. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
We appreciate the staff of Cryo-EM Center in Southern University of Science, Technology for cryo-EM data collection and Sichuan University West China Cryo-EM Center. Additionally, we acknowledge the resources provided by the Duyu High Performance Computing Center at Sichuan University and the Big Data Platform at West China Hospital of Sichuan University (WCH-BDP). This work was supported by the National Natural Science Foundation of China (31972916 to Z. S., 32100988 to W. Y., 82271190 to L. C., 32270438, to C. D. 32170498), the China Postdoctoral Science Foundation (2023M732474, GZB20230480 to S. S.), Science and Technology department of Sichuan Province (2022ZYD0085 to Z. S., 2022YFH0116 to C. D.), 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYYC20023 to Z. S., ZYJC21050 to C. D.). National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (Z2023JC003 to C. D.) the National Key Research and Development Program of China.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Structural data from this study have been deposited in the Protein Data Bank (PDB) under coordinate accession numbers 8K6M (cryo-EM structure of the NPY–Y1R–Gi2 complex), 8K6O (cryo-EM structure of the [Leu31, Pro34]–Y1R–Gi2 complex) and 8K6N (cryo-EM structure of the NPY–Y2R–Gi2 complex). Additionally, the Electron Microscopy Data Bank (EMDB) accession numbers EMD-36923, EMD-36925, and EMD-36924 correspond to the cryo-EM structures mentioned above. All remaining data generated or analyzed during this research are available within the published article and its Supplementary Information files.




