Epigenetic modifications in obesity-associated diseases
Abstract
The global prevalence of obesity has reached epidemic levels, significantly elevating the susceptibility to various cardiometabolic conditions and certain types of cancer. In addition to causing metabolic abnormalities such as insulin resistance (IR), elevated blood glucose and lipids, and ectopic fat deposition, obesity can also damage pancreatic islet cells, endothelial cells, and cardiomyocytes through chronic inflammation, and even promote the development of a microenvironment conducive to cancer initiation. Improper dietary habits and lack of physical exercise are important behavioral factors that increase the risk of obesity, which can affect gene expression through epigenetic modifications. Epigenetic alterations can occur in early stage of obesity, some of which are reversible, while others persist over time and lead to obesity-related complications. Therefore, the dynamic adjustability of epigenetic modifications can be leveraged to reverse the development of obesity-associated diseases through behavioral interventions, drugs, and bariatric surgery. This review provides a comprehensive summary of the impact of epigenetic regulation on the initiation and development of obesity-associated cancers, type 2 diabetes, and cardiovascular diseases, establishing a theoretical basis for prevention, diagnosis, and treatment of these conditions.
1 INTRODUCTION
Over the years, obesity has been regarded as an appearance problem, but evidence shows that fat accumulation and ectopic deposition are the pathogenic factors for various diseases, and there is a growing awareness that obesity is a disease in itself. The global number of obese people tripled from 1975 to 2016, and the rate continues to rise.1 The increase in the number of obese individuals can be attributed to a host of problems, including alterations in dietary habits, a sedentary lifestyle, and the consumption of high-energy foods. Generally speaking, an overweight person with a body mass index (BMI) of 25 kg/m2 is considered overweight, and a person with a BMI of 30 kg/m2 is classified as obese. However, the solely use of height and weight for assessing obesity is less reliable. The inclusion of additional measures such as waist circumference, waist-to-hip ratio, body fat rate (BFR), visceral fat content, subcutaneous fat levels, and other indicators can enhance the precision of obesity classification.2-4
A large proportion of cancer cases in many regions and countries can be attributed to obesity and overweight.5 Compared with metabolically healthy individuals with normal weight, metabolically healthy obese individuals have an approximately 30% increased risk of obesity-associated cancers, while the risk is even more significant for metabolically unhealthy obese individuals.6 In America, obesity has gradually surpassed tobacco as the leading preventable cancer cause.7 Prostate cancer (among males) and breast cancer (BC) (among females) have emerged as the leading types of cancer, surpassing lung cancer, as per the latest research data from the American Cancer Society. The increased incidence of BC in women is largely associated with declining fertility rates and weight gain, which also related to the rising incidence of uterine corpus cancer.8 In addition, obesity represents a significant risk factor for type 2 diabetes (T2D). Modest weight-loss can mitigate T2D-associated complications and delay the progression of the disease.9 Obese patients with high BFR are prone to insulin resistance (IR) and hyperinsulinemia. As the condition progresses, individuals with impaired ability to tolerate glucose can eventually develop into T2D.10 Cardiovascular diseases (CVDs) remain the leading contributor to the global health burden, encompassing conditions such as hypertension, atherosclerosis, coronary heart disease (CHD), heart failure, and other related ailments. Obesity and metabolic disorders not only elevate the susceptibility to CVDs but also intensify the physiological impairment caused by preexisting CVDs.11, 12
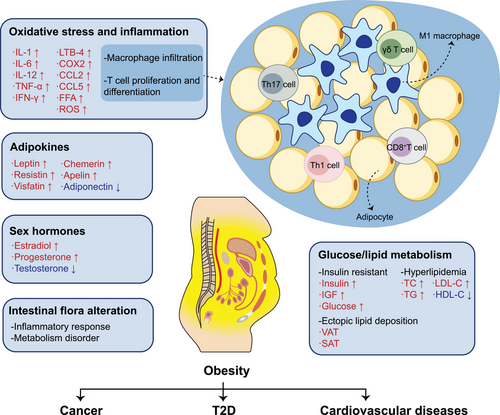
Obesity can cause systemic metabolic abnormalities, and the accompanying chronic inflammatory response, endocrine changes, and the release of various cytokines are the main pathogenic factors of obesity-associated diseases (Figure 1). In obese patients, the adipocytes hypertrophy and the abnormal lipids accumulation damage angiogenesis, causing local tissue hypoxia and necrosis, and ultimately remodeling cardiovascular system.13 The adipokines, inflammatory factors, and hormones released by adipose tissue (AT) can affect tumor metabolism reshaping. AT also regulate atherosclerosis and hypertension process by releasing adipokines and inflammatory factors.14, 15 Additionally, adipokines released from AT can regulate the sensitivity to insulin, while inflammatory factors and the release of free fatty acids (FFAs) can also affect glucose transport, thus promoting IR.16, 17 In addition to progressing to T2D, IR can not only increase the risk of CVDs by increasing vascular stiffness but also promote tumor cell proliferation.17, 18
The rapid increasing incidence of obesity-associated diseases cannot be solely explained by genetics. Changes in diet and lifestyle habits can drive reversible and heritable genetic changes between cellular and individual generations through epigenetic modifications. Inconsistent with the traditional Mendelian inheritance laws, epigenetics modifies gene function without changing DNA sequences, it does fill the gap between unexplained alterations of gene expression patterns and the deletion of gene changes in pathogenesis. Various epigenetic regulatory mechanisms collaborate to uphold genetic stability, and the alterations of epigenetic regulation in individuals with obesity can potentially lead to the development of subsequent diseases.19-22 Hence, conducting comprehensive research on the involvement of epigenetic modifications in the progression of obesity-associated diseases will contribute to the prevention and therapy.
Here, we review the involvement of DNA methylation (DNAm), histone modification, noncoding RNAs (ncRNAs), and chromatin remodeling in the etiology of obesity-associated cancers, T2D, and CVD. We also outline the pivotal role of macrophages in obesity-triggered inflammation and the accompanying epigenetic regulation. Furthermore, dietary regulation, physical exercise, and bariatric surgery can induce epigenetic alterations to prevent and treat obesity-associated diseases. These specific epigenetic variations could serve as biomarkers for diseases diagnosis, and the advent of epigenetic drugs also provides new therapeutic strategies for obesity-associated diseases.
2 DNAm IN OBESITY-ASSOCIATED DISEASES
DNAm, the common epigenetic modifications, methylates the 5th carbon atom of cytosine using the methyl provided by S-adenosyl methionine (SAM) under the catalysis of DNA methyltransferases (DNMTs) and converts the cytosine into 5-methylcytosine (5mC).23 In mammals, CpG dinucleotides are sites where DNAm usually takes place, and the areas that contain high CpG dinucleotides are called the CpG islands (CGIs).24 Mechanistically, obesity-induced activities of DNMTs and demethylases can result in diverse CpG site methylation levels across regions, thereby influencing the suppression or activation of relevant gene expression25-27 (Figure 2).
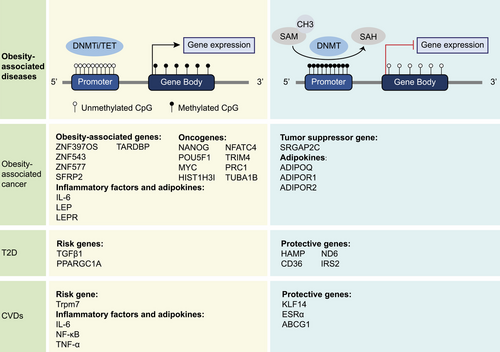
2.1 Obesity-associated DNAm and cancer development
Cancer initiation and development are mainly characterized by hypermethylation on CGIs of tumor suppressor gene promoters and global DNA hypomethylation.28 The expression levels of obesity-related genes due to DNAm changes might affect cancer progression. Some overexpressed obesity-related genes were associated with tumor-promoting factors, while other underexpressed counterparts could inhibit cancer development.29 Specific CpG methylation patterns are associated with obesity.30 Thus, DNAm in diverse specific genes in cancers is conducive to differential diagnosis and targeted therapy. For instance, the combined methylation levels of zinc finger protein genes ZNF397OS and ZNF543 could distinguish obesity-associated colorectal tumors from other colorectal tumors, contributing to precise medical management.31 Additionally, ZNF577 hypermethylation might be the epigenetic biomarker of obesity-associated BC and is also relevant to diet patterns.32, 33 Secreted frizzled-related protein type 2 (SFRP2) is a potential epigenetic biomarker of colorectal cancer, which could inhibit Wnt signaling. BMI could influence the DNAm level of SFRP2 in patients with colorectal cancer.34 Long interspersed nucleotide element 1 (LINE-1)is often used to represent the global DNAm level, showing hypomethylation in tumors, and was considered to be statistically significant with BMI in colon cancer and BC.35, 36 Ten-eleven translocation methylcytosine dioxygenases (TETs) are the main DNA demethylases that catalyze 5-methylcytosine (5mC) into 5-hydroxymethylcytosine (5hmC) and play an important role in maintaining pluripotency of stem cells.26 Obesity promoted the self-renewal of triple-negative breast cancer (TNBC) stem cells by inducing oxidative stress, which was triggered by the increased expression of TAR DNA binding protein (TARDBP) mediated by ten-eleven translocation methylcytosine dioxygenase 1 (TET1).37
Obesity can increase oncogenes expression or inhibit expression of tumor suppressor genes through DNAm regulation.38, 39 For instance, obesity-induced inflammation led to hypomethylation of the promoter regions of oncogenes such as Nanog homeobox, POU class 5 homeobox 1 (POU5F1), and MYC proto-oncogene (MYC) in colon cells, which may eventually cause colon cancer.40 In tumors of obese patients with colon cancer, the promoter of oncogene histone linker 1 with histone H3.1 (HIST1H3I) is hypomethylated, while the promoter of tumor suppressor gene Slit-Robo Rho GTPase-activating protein 2C (SRGAP2C) is hypermethylated.41 Additionally, the intron of oncogene nuclear factor of activated T-cells cytoplasmic 4 (NFATC4) demonstrates hypermethylation.41 The alterations in gene expression resulting from differential methylation regions will contribute to the diagnosis of obesity-associated cancers. Nonalcoholic steatohepatitis (NASH) is highly correlated with overnutrition and IR. In some cases, patients with NASH might give rise to hepatocellular carcinoma (HCC). A genome-wide DNAm analysis in liver tissue samples exhibited a decrease in DNAm of tumor-related genes in NASH-associated precancerous tissue, and this phenomenon continued and intensified in NASH-related HCC.42 These overexpressed tumor-related genes such as tripartite motif-containing 4 (TRIM4), protein regulator of cytokinesis 1 (PRC1), tubulin alpha 1b (TUBA1B), are closely related to inflammation and malignant differentiation.
Epigenetic modifications may influence both lipid metabolism and immune response. Epigenetic research showed significantly different epigenome methylation profiles of periprostatic AT between obese/overweight and normal-weight patients with prostate cancer. Thirty-eight genes were found to be related to fatty acid accumulation in AT and tumor immune evasion.43 AT, as the primary endocrine and metabolic organ, playing a significant role in obesity-associated cancers by secreting inflammatory factors, adipocytokines and hormones that can stimulate an adverse TME. Colorectal cancer patients with elevated DNAm levels of inflammatory factors, specifically interleukin-6 (IL-6), in their visceral AT (VAT) are at an increased risk of developing colorectal cancer.44 It is suggested that 25-hydroxyvitamin D partly mediates this process. Various adipokines have been proven to stimulate tumor cell expansion through intracellular signal pathways.45 For instance, leptin exerts its effects on antiapoptotic, inflammation promotion, and angiogenesis through signaling pathways such as JAK/STAT3, MAPK, and PI3K.46 On the other hand, resistin promotes tumor cells proliferation, migration, and adhesion by affecting MAPK, PI3K, and NF-κB signaling pathways.47, 48 Recent research suggested that adipokines also impact cellular communication in the cancerous process. For instance, adipose stromal cells (ASCs) could secrete adipokines to affect the gap junction loci, which affected the intercellular communication in endometrial tumors of obese patients.49 Mechanistically, adipokines caused promoter hypermethylation and long-term silence of gap junction protein alpha 1 (GJA1) that encoded gap junction protein connexin-43. In addition, organic pollutants accumulating in AT might cause an oncogenic impact through the endocrine pathway.50 Exposure to endocrine-disrupting compounds might lead to intergenerational epigenetic alterations.51 Bisphenol A (BPA) is an endocrine disruptor with estrogenic activity and has been confirmed as a common carcinogenic chemical substance in environment that promote the progression of both hormone-sensitive and nonhormone-sensitive cancers.52, 53 A high-fat diet (HFD) can increase the incidence of offspring BC induced by BPA, which is associated with DNAm-mediated changes in gene expression.54 BPA can accumulate in AT, and the accumulated BPA can cause changes in lipid metabolism, further aggravating the obese state, and this vicious cycle may be one of the causes of cancer.55
Leptin and adiponectin secreted by adipocytes are crucial in development of cancer. In comparison with normal-weight individuals, obese/overweight individuals get higher levels of leptin and lower levels of adiponectin in their serum.56 When the normal organism is in a state of hunger or reduction of body fat, the serum leptin content significantly decreases to store energy.57 On the contrary, the rising leptin content in response to increased body fat resists the obese state by inhibiting appetite. This negative feedback between adipocytes and the central nervous system helps maintain the lipid metabolism of the organism to keep weight. Previous research has indicated that overexpression of leptin and leptin receptors significantly affect tumor proliferation, invasion, and metastasis, accelerating the process of tumor deterioration and decrease the survival rates.58-61 Intriguingly, leptin not only promotes tumor progression by regulating angiogenesis, inhibiting apoptosis, and remodeling the TME but also plays a critical role in antitumor immunotherapy through innate and adaptive immune response.62, 63 Some obese/overweight cancer patients experience better prognoses and responses to immunotherapy due to the “leptin paradox” than their normal-weight counterparts.62 In untreated and newly diagnosed patients with renal cell carcinoma, promoter methylation in the leptin gene (LEP), as well as leptin receptor gene (LEPR), is remarkably higher in tumor tissues compared with normal adjacent tissues.64 Additionally, hypermethylation of leptin receptor gene correlated with recurrence and shorter recurrence-free survival. In contrast, increased expression of LEP in ovarian cancer patients was related to longer lifetimes and lower recurrence rates.65 In addition to solid tumors, the degree of LEP methylation could also be used to predict the prognosis of acute myeloid leukemia (AML).66 Leptin has a dual effect on cancer progression, its antitumor immunomodulatory effects and mechanisms remain unclear. Correspondingly, due to the pleiotropic effects of leptin, its complex regulation has become an obstacle to its widespread clinical application.
Adiponectin is mainly involved in glucose and lipid metabolism, and it is known to bind to three receptors, namely adiponectin receptor 1 (ADIPOR1), adiponectin receptor 2 (ADIPOR2), and T-cadherin. Adiponectin exerts an inhibitory effect on tumors, and promoter methylation of the adiponectin gene (ADIPOQ) is associated with cancer risk.67 Adiponectin can counteract the effects of leptin, exerting an antitumor effect by inhibiting angiogenesis, cell proliferation, tumor invasion, and metastasis.68 For patients with pancreatic cancer, treatment with the methylation-blocking drug 5-Azacytidine has been shown to improve the transcription level of ADIPOR1 and ADIPOR2 to enhance adiponectin signaling.69 This inhibitory effect on tumors by adiponectin is mainly reflected in antiproliferation. Furthermore, studies have demonstrated that chronic energy restriction can impede mammary tumor growth by altering the DNAm of LEP and ADIPOR1.70 Obesity was found to promote tumor progression by increasing leptin expression synergized with decreasing adiponectin expression. Meanwhile, the increased leptin levels can also directly reduce adiponectin transcription. Consequently, the alteration in leptin-to-adiponectin ratio plays a crucial role in linking obesity to cancer progression.71
2.2 DNAm profiles in T2D and complications
By examining the DNAm profiles of individuals with T2D, researchers have identified novel candidate genes associated with T2D and enhanced comprehension of the disease's pathogenesis.72 Obesity primarily leads to the development of T2D by inducing IR and disrupting lipid metabolism. Researchers have reported hypermethylation of the hepcidin antimicrobial peptide (HAMP) gene promoter in the livers of mice predisposed to T2D and obese women exhibiting IR.73 HAMP can serve as a candidate gene for assessing susceptibility to T2D and potentially mitigating excessive insulin secretion. CD36 plays a role in the uptake of fatty acids and the regulation of lipid metabolism. Hypermethylation and polymorphism of the CD36 promoter may be linked to the progression of obesity and T2D.74 In obese and T2D patients, there is an increase in CD36 promoter methylation compared with thin individuals. Additionally, reduced plasma sCD36 levels lead to an increase in low-density lipoprotein cholesterol (LDL-C) levels.74 In obese individuals, those with T2D have hypermethylation of the insulin receptor substrate 2 (IRS2) promoter CpG sites in the liver, and the reduced expression of IRS2 is closely related to IR.75 Among patients with T2D, the elevated expression of DNA damage-inducible β (Gadd45β), in conjunction with TET1, stimulates the DNA demethylation of the PPARG coactivator 1 alpha (PPARGC1A) promoter, resulting in heightened gluconeogenesis and reduced glucose tolerance among patients.76 DNAm alterations are evident across all three stages of adipogenesis in obese individuals, and these differentially methylated genes may play a role in the development of T2D primarily by impairing the function of mature adipocytes.77
DNAm profiles varies among different T2D subgroups, and the initiation of diabetic complications can be predicted according to the methylation risk scores of subgroups.78 Advanced diabetes patients commonly experience a range of complications, increasing the complexity of treatment. Compared with normal mice, obese/diabetic mice show different expression patterns of DNMTs and TET proteins in their kidney tissues, which may promote the progression of diabetic nephropathy by regulating DNAm levels.79 Ten-eleven translocation methylcytosine dioxygenase 2 (TET2) can demethylate the CGI of transforming growth factor β1 (TGFB1) to promote glomerulosclerosis and accelerating the progression of diabetic nephropathy.80 In addition, DNAm markers can also be used to evaluate the risk stratification of T2D nephropathy and predict the occurrence of diabetic retinopathy.81, 82 Due to technological limitations, mitochondrial epigenetic regulation has often been ignored. However, recent studies have also demonstrated the utility of mitochondrial DNA (mtDNA) methylation in the pathogenesis of IR in skeletal muscle and diabetic retinopathy.83 Elevated levels of FFAs in the liver can enhance the mitochondrial translocation of DNA methyltransferase 1 (DNMT1), resulting in the hypermethylation of NADH-dehydrogenase 6 (ND6) on mtDNA.84 The inhibition of ND6 leads to systemic IR due to mitochondrial dysfunction.
2.3 DNAm and metabolic risk factors in CVDs
DNAm markers can be utilized to assess the individual's cardiac metabolic risk and offer assistance in the context of obesity-induced CVDs.85-87 A meta-analysis of genome-wide DNAm in obese adults shows that BMI-related CpG sites methylation with are associated with metabolic parameters of atherosclerosis and hyperlipidemia.88 In the whole-genome association study, one-third of the differentially methylated genes related to CHD are associated with obesity, among which estrogen receptor 1 ATP binding cassette subfamily G member 1 (ABCG1), and IL6 have been identified as candidate genes for CHD in multiple studies.89 Krüpple-like factor 14 (KLF14) is a key metabolic-related transcription factor, and its deficiency can accelerate the formation of atherosclerosis.90 The high methylation status of KLF14 is closely related to obesity indices, lipid profiles, blood pressure status, and IR status, which can provide reference for the precise treatment of cardiometabolic diseases.91
Abnormal secretion of adipokines is an important pathogenesis of CVDs caused by obesity. Leptin can stimulate carotid sinus nerve to increase blood pressure. The high expression of leptin and leptin receptor in carotid corpuscles is positively correlated with the promoter demethylation of transient receptor potential melastatin 7 (Trpm7).92 Leptin-activated pSTAT3 can regulate the expression of Trmp7 through epigenetic modification and increase the risk of hypertension in obese patients.92 Inflammation is the basic feature of atherosclerosis, and the release of inflammatory factors and the recruitment of immune cells are accompanied by the whole course of the disease. The promoters of the IL-6 and nuclear factor kappa B (NF-κ B) genes were found to be hypomethylated in the peripheral leukocytes of obese patients.93, 94 Upregulation of IL-6 and NF- κ B in leukocytes is closely associated with a decreased level of adiponectin.93 In obese patients, anti-inflammatory effect of adiponectin is inhibited, leading to an acceleration of the atherosclerosis process. In addition, another study shows that the hypomethylation of IL-6 and tumor necrosis factor-alpha (TNF-α) genes in obese patients can lead to endothelial dysfunction by causing inflammation and oxidative stress, thus promoting the occurrence of atherosclerosis and coronary artery disease.94
DNAm changes typically precede changes in gene expression, and detecting DNAm can be beneficial for early disease diagnosis.95 Furthermore, in obese/overweight state, DNAm regulates and reprograms gene expression patterns, which means that DNAm will become a pivotal target for treatment and drug development.
3 HISTONE MODIFICATION IN OBESITY-ASSOCIATED DISEASES
Histones are involved in the formation of nucleosomes alongside DNA, and the N-terminal end of histones can be easily modified by histone epigenetic modifying enzymes. Such modifications are associated with obesity development through the regulation of related gene expression, including methylation, acetylation, ubiquitination, phosphorylation, and others.96 Histone modifications occur on specific tails enriched with lysine and arginine residues, influencing gene expression through posttranslational modification. The dysregulation of these modifications can affect DNA repair, cell cycle regulation, and apoptosis, leading to the accumulation of genetic and epigenetic alterations that drive obesity-associated complications. Here, we mainly elaborate on the role of histone methylation and acetylation in obesity-associated diseases.
3.1 The impact of histone modifications on cancer-related gene expression
Adding a different number of methyl groups to various residue sites of histone has an effect on gene expression, and different methylation degrees further complicate histone modification and regulation of gene expression.97 The consumption of a HFD was shown to upregulate the transcription of MYC and led to hypomethylation of histone H4K20 by Jumonji domain-containing Histone Demethylases (JHDM) in the promoter region of the MYC regulatory genes.98 This leads to proliferation of prostate cancer cells and increased tumor burden. Histone methyltransferase SET domain-containing 2 (SETD2) could catalyze H3K36me3, which is a well-known conserved epigenetic modification.99 Deficiency in SETD2 was known to regulate cholesterol homeostasis and activate c-Jun/activator protein 1 (AP-1) via triggering lipid accumulation to promote HCC progression.100
Histone acetylation mainly occurs on the lysine residues in histone tails, which is a reversible dynamic equilibrium process, and the disruption of this balance has been linked to tumorigenesis. Chromatin becomes slack due to histone acetylation, thereby increasing DNA accessibility and promoting transcriptional activity. In steatotic hepatocytes, hyperacetylation of genome-wide histones could initiate carcinogenesis by damaging DNA.101 Recent studies have found that the concurrent activation of acetylated and trimethylated H3K9 in mice can lead to NASH-related HCC, and deacetylation of H4K16 promotes cancer progress by silencing genes associated with cell death.102, 103 In addition to these findings, histone modifications caused by the interactions between a HFD and intestinal microbiome are significant during gastrointestinal tumor development. Enhancer histone methylation and acetylation induced by intestinal microflora have been associated with the signal pathway of the occurrence and development of colon cancer under obesity exposure.104
3.2 Histone acetylation and methylation in initiation and progression of T2D
PPAR γ in AT can promote the growth and differentiation of adipocytes, and can reduce insulin secretion by pancreatic β cells and increase insulin sensitivity.105 The acetylation of PPAR γ is essential for its activation. Targeting Sirtuin 1 (SIRT1) can increase the expression levels of genes related to insulin sensitivity and ameliorate obesity-induced T2D.106 Furthermore, inhibitors of histone deacetylase 3 (HDAC3) can also activate PPAR γ to reverse IR caused by HFD.107 Inhibition of histone deacetylases (HDACs) can also be used to prevent cardiomyopathy caused by diabetes. HDAC3 increases the H3 histone deacetylation of dual specificity phosphatase 5 (DUSP5) gene promoter in the heart of diabetic mice, which leads to cardiac dysfunction by inhibiting the expression of DUSP5. Blocking HDACs can serve as a preventive measure against diabetic cardiomyopathy. Specifically, HDAC3 stimulates H3 histone deacetylation of the DUSP5 gene promoter in the hearts of diabetic mice, resulting in cardiac dysfunction through suppressing the expression of DUSP5.108 HDAC3 inhibitors can also mitigate diabetic liver injury by upregulating nuclear factor erythroid 2-related factor 2 (Nrf2), and alleviate T2D-induced aortic fibrosis and inflammation by enhancing fibroblast growth factor 21 (FGF21) synthesis.109 The role of HDAC3 inhibitors in the treatment of diabetes has been widely recognized, and selective HDAC3 inhibitors can become potential antidiabetic drugs. The therapeutic potential of HDAC3 inhibitors in managing diabetes is widely acknowledged, and high selective HDAC3 inhibitors have the ability to develop into promising antidiabetic medications.110
In addition to histone acetylation, the modification of histones through methylation is critical in pathogenesis and progression of T2D. Premature senescence of adipocyte precursor cells and disruption in adipogenesis can lead to early IR. In adipocytes of individuals with T2D, reduced enrichment of lysine 4 tri-methylated H3-histone (H3K4me3) and subsequent increasing expression of mitochondrial transcription factor A (TFAM) promotes premature senescence of adipocytes, inhibits adipocyte differentiation, and accelerates the progression of T2D.111 Pancreatic β cells are crucial cells in the regulation of glucose homeostasis, and the defective adaptive response of β cells is one of the pathogenic mechanisms of T2D. PPAR γ agonists can upregulate the histone lysine methyltransferase SET domain containing 7 (SETD7) in β-cells, which increases pancreatic and duodenal homeobox 1 (PDX1).112 PDX1 drives β-cell adaptation to protect β cell function, thus regulating glucose sensitivity and insulin secretion.112
3.3 The dual role of histone acetylation in vascular diseases
Lysine acetylation is implicated in numerous cardiac disease processes, including cell metabolism, gene transcription, and enzyme activity, and is associated with metabolic disorders and heart failure.113 Histone acetylation is involved in the regulation of smooth muscle cells (SMCs) differentiation and remodels vessels, which leads to the initiation of hypertension in individuals with metabolic syndrome.114 Obesity-induced hyperleptinemia may lead to histone acetylation and methylation of Trpm7, thereby promoting hypertension through upregulating Trpm7 expression.92 Reduced expression of the histone deacetylase Sirtuin 6 (SIRT6) in SMCs within atherosclerotic plaques may compromise telomere integrity, leading to senescence of SMCs and inflammation.115 Conversely, the lack of histone deacetylase 9 (HDAC9) can mitigate endothelial–mesenchymal transition (EMT) that preserve plaques stability and decelerate the progression of atherosclerosis.116 It is suggested that diverse HDACs may play different roles in vascular diseases.
Abnormal lipid metabolism may prompt modifying enzymes and binding proteins to recognize, produce and eliminate posttranslational modification, thereby regulating the expression of related genes and promoting the advancement of obesity-associated diseases. With the development of mass spectrometry, additional histone acylation modifications have been discovered, which enriches the level of protein posttranslational modification regulations. Currently, various HDAC inhibitors have been approved for clinical application.
4 ncRNAs IN OBESITY-ASSOCIATED DISEASES
ncRNAs refer to a class of RNAs that cannot encode proteins and are categorize into two groups: housekeeping ncRNAs and regulatory ncRNAs.117 Housekeeping ncRNAs are essential in fundamental cellular processes, while regulatory ncRNAs are mainly involved in modulating gene expression. Here, we will introduce epigenetic regulations of long ncRNAs (lncRNAs), micro RNAs (miRNAs), and circular RNAs (circRNAs) in obesity-associated diseases.
4.1 ncRNAs in obesity-associated diseases
LncRNAs are a type of ncRNAs lacking open reading frames and having a length greater than 200 nucleotides. They can be classified into five types: sense, antisense, bidirectional, intronic, and intergenic ncRNAs.117 Although lncRNAs have been regarded as “transcriptional noise” for a long time, studies have shown that lncRNAs affect cell proliferation, differentiation, apoptosis, and carcinogenic pathways.
4.1.1 The impact of lncRNAs in the link between obesity and cancer
Recent research has substantiated that lncRNAs in AT act as a bridging molecule linking obesity with cancer.118-120 Communication between AT-derived stem cells (ADSCs) and HeLa cells could alter the lncRNA expression profile in ADSCs, consequently enhancing the migratory potential of cervical cancer cells.121 In patients with obesity and diabetes, an increase in carcinogenic lncRNAs was observed in subcutaneous AT (SAT). These lncRNAs are involved in carcinogenic networks and may interact with multiple oncogenes, thereby elevating the risk of cancer.118 In a simulated state of excessive obesity using mature adipogenic medium, lncRNA plasmacytoma variant translocation 1 (PVT1) promoted cell epithelial-mesenchymal transition (EMT) and enhanced cell viability and migration ability of TNBC cells by inhibiting the expression of cancer suppressor gene p21.122
A substantial number of lncRNAs have been demonstrated to be linked to cancer. Alterations in their expression levels and mutations have the potential to facilitate the initiation and progression of cancer. In the context of BC, lncRNA AP001429.1 can target multiple miRNAs, potentially exerting anticancer effects. Recent study has indicated that obesity can reduce the expression of lncRNA AP001429.1 in patients with BC, thus promoting cancer progression.123 Downregulation of lncRNA five prime to Xist (FTX) and overexpression of lncRNA small nucleolar RNA host gene 20 (SNHG20) promoted the development of nonalcoholic fatty liver disease (NAFLD) into HCC by modulating the polarization of Kupffer cells.124, 125 The leptin and estradiol levels in postmenopausal obese women are high, and the two hormones contribute to angiogenesis, survival, cell proliferation, and migration. A study of obese ovariectomized mice with a HFD suggested that the cotreatment of leptin and estradiol could increase the expression of lncRNA nuclear-enriched abundant transcript 1 (NEAT1).126 This increase could induce carcinogenesis of endometrial tissue through NEAT1/mmu-miR-204-5p/IGF1 axis. LncRNAs play a crucial role in predicting the prognosis of obesity-associated esophageal squamous cell carcinoma (ESCC).127 Specifically, the lncRNA SLC25A21-AS1, associated with lipid metabolism, is recognized as promoting ESCC progression via the nucleophosmin-1 (NPM1)/c-Myc signaling pathway.128 With the in-depth investigation of genome-wide association studies in tumor samples, the potential for lncRNAs to function as diagnostic markers and therapeutic targets for cancer has emerged.
4.1.2 The role of lncRNAs in the pathogenesis of T2D
Diabetes can be categorized based on clinical characteristics, and the expression levels of lncRNAs in different clusters of diabetes vary significantly. This varied lncRNAs expression may be associated with the expression of genes involved in lipid metabolism and white blood cell fractions.129 The lncRNA noncoding repressor of NFAT (NRON) plays a crucial role in regulating glucose and lipid metabolism. Its deficiency has been associated with enhanced insulin sensitivity and improved serum lipid status, making it a potential target for obesity treatment.130 Mechanistically, deletion of NRON leads to the maintenance of lipid homeostasis through upregulating expression level of FGF21 and activating AMPK.130 Dysfunction of white AT (WAT) has been associated with the onset of IR, and there is an upregulation of lncRNA ADIPINT in WAT of obese individuals.131 ADIPINT functions by binding to pyruvate carboxylase which can modulate energy metabolism, thereby regulating lipid content and IR.131 lncRNA RAP2 is a white adipocyte-selective RNA and has the ability to interact with the RNA binding protein Igf2bp2, forming a nucleoprotein complex.132 The LncRAP2–Igf2bp2 complex plays a crucial role in maintaining lipid metabolism homeostasis, and its decreased expression is associated with obesity-related susceptibility to T2D.132 Except for the interaction with proteins, the crosstalk between lncRNA and miRNA is also one of the pathogenesis of diabetes. In the islets of obese and diabetic mice, as well as in the serum of patients with T2D, reduced expression of LncRNA Kcnq1ot1 leads to decreased levels of cyclin D1 (Ccnd1) and cyclin D2 (Ccnd2) through the targeting of miR-15b-5p.133 The absence of Kcnq1ot1 inhibits β-cell proliferation, impairs insulin synthesis and secretion, and decreases glucose tolerance.133 lncRNA βFaar is markedly downregulated in the islets of obese mice, and its deletion can result in β-cell damage and apoptosis induced by obesity.134 Mechanistically, βFaar upregulates islet-specific genes by sponging miR-138-5p and inhibits the degradation of TRAF3 interacting protein 2 (TRAF3IP2) by binding to the SMAD specific E3 ubiquitin protein ligase 1 (SMURF1), thereby suppressing β-cell apoptosis.134 In conclusion, the intricate network of lncRNAs and their interactions with various molecules and pathways have highlighted their significant role in the pathogenesis of T2D.
4.1.3 Obesity-induced lncRNA alterations on CVDs
In the obese state, lncRNAs may induce vascular endothelial dysfunction, consequently elevating the risk of CVDs. Maternal weight gain during pregnancy has been associated with reduced expression of the lncRNA KLRK1-AS1 in offspring, leading to impaired function of endothelial colony forming cells in repairing the vascular barrier.135 Furthermore, exercise has been shown to downregulate the expression of lncRNA MALAT1 while increasing the levels of miR-320a in serum of obese children/adolescents.136 This in turn reduces the levels of endothelial dysfunction markers such as vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), and E-selectin.136 Notably, polymorphisms in the lncRNA PVT1 gene have been implicated as a potential cause of dyslipidemia.137 PVT1 has also been found to increase the risk of essential hypertension by modulating blood lipid levels.137 Moreover, a HFD has been shown to cause myocardial injury by provoking an inflammatory response and promoting cell apoptosis. Ghrelin, a polypeptide with cardioprotective properties, has been observed to target the lncRNA H19/miR-29a/insulin-like growth factor 1 (IGF-1) signaling pathway and the lncRNA HOTAIR/miR-196b/IGF-1 signaling pathway, thereby mitigating cardiomyocyte injury associated with obesity.138, 139 These studies not only enhance our comprehension of the mechanisms by which lncRNAs regulate obesity-associated CVDs, but also offer novel avenues and insights for future research and clinical interventions.
4.2 MiRNAs in obesity-associated diseases
MiRNAs are endogenous small ncRNAs whose length is about 22 nucleotides. iRNAs repress translation through complete and incomplete base complementary pairing with mRNA, regulating the function of the target genes.
4.2.1 The role of miRNAs in obesity-associated cancers and potential therapeutic applications
The modifications of miRNAs have vital roles in diverse cancerous progress, and differential expression of miRNAs can serve as biomarkers and therapeutic targets.140 In HCC, miR-148a and miR-22-3p could participate in tumor progression by regulating lipid metabolism.141, 142 Peroxisome proliferator-activated receptor γ (PPARγ) is abundant in AT and known for its role in modulating energy metabolism, inflammation, adipogenesis, cell differentiation, and antitumor effect.143, 144 Obesity might decrease PPARγ by inducing overexpression of miR-27b, miR-130b, and miRNA-138, which could increase the susceptibility of colorectal cancer.145 Obesity increases the risk of BC through various molecular mechanisms, which are partly regulated by miRNA. Upregulated miRNA-3184-5p and downregulated miRNA-181c-3p that targeted forkhead box protein P4 (FOXP4) and PPARα, respectively, could promote proliferation and invasive ability of adipocyte-induced BC cells.146 Many miRNAs are now recognized as valuable diagnostic and prognostic markers for cancer, these miRNAs that play an oncogenic role are defined as oncomiRs. Chronic inflammation induced by obesity can also modulate the expression of cancer-related miRNAs and inflammatory miRNAs (inflamma-miRs). For instance, obese patients with BC have an increased expression of miRNA-21 (oncomiR and inflamma-miR) and a decreased expression of tumor suppressor miRNA-34a compared with normal-weight patients.147 In addition, miRNA-21 and miRNA-146a (inflamma-miR) were significantly increased in obese patients with lymph node metastasis. MiRNA-10b could mediate the interaction between obesity and BC in postmenopausal women by regulating cancer-related genes.148 MiRNA is a promising novel noninvasive biomarker, and there are methods for detecting miRNAs in feces, blood, and urine of patients.149, 150 Endogenous mature miRNA is highly stable in serum and is not easily degraded by RNA enzymes in the blood. Research showed that miRNA-223 in the urine of patients with endometrial cancer (EC) is evidently increasing, and there were differential expressions of miRNAs between normal-weight and obese patients.151 Detecting miRNAs in body fluid will be a powerful tool for screening, diagnosis, and prognosis of cancer. However, the modification of miRNA is complex and variable. Combined detection is necessary to enhance the diagnostic value, and the differences in expression among diverse individuals may limit detection application. Thus, expanding the sample size for further study can help use miRNAs as diagnostic markers of tumors in clinical applications.
The interactions between miRNAs and adipokines play an important role in biological functions of obesity-associated cancers. Adipokines could upregulate oncogenic miRNAs and downregulate antitumoral miRNAs, ultimately promoting tumor development.152 Additionally, adipokines can also affect antitumor therapy via miRNAs. Leptin could upregulate coactivator Mediator complex subunit 1 (Med1), an anchor subunit of the human mediator complex, by decreasing miRNA-205 expression to diminish the hormonal therapy effect of tamoxifen in BC.153 Overexpressed miRNA-628 could dampen the proliferation of prostate cancer cells and reduce EMT, invasive ability, and the characteristics of stem cells, while leptin can promote the development of prostate cancer by downregulating the expression of miRNA-628.154 Meanwhile, miRNA-628 could increase the sensitivity of prostate cancer cells to chemotherapeutic drugs by promoting cell apoptosis. In the case of pancreatic ductal adenocarcinoma, leptin can upregulate miRNA-342-3p to inhibit tumor suppressor (KLF6), which was associated with increased gemcitabine resistance.155 Obesity could increase leptin expression by downregulating tumor suppressor p16INK4A expression, which promoted the precancerous development of BC.156 Interestingly, p16INK4A in breast adipocytes could also mediate miRNA-141 and miRNA-146b-5p to combine with leptin mRNA, negatively regulating leptin expression. Collectively, the complex interaction between miRNAs and adipokines is believed to function in the progression of obesity-associated cancers.
Extracellular vehicles (EVs), including but not limited to microvesicles, exosomes, apoptotic bodies, and oncosomes, are membranous vesicles that could shuttle amongst cells in the extracellular matrix, which participate in cell communication, migration, angiogenesis, and cancer cell proliferation. AT-derived EVs involve the occurrence and metastasis of tumors and can transport miRNAs to mediate cell-to-cell communications157 (Figure 3). For example, miRNA-23a/b was transported into HCC cells by AT-derived exosomes and played a pivotal role in tumor proliferation, metastasis, and 5-fluorouracil (5-FU) resistance by targeting von Hippel-Lindau (VHL)/hypoxia-inducible factor 1α (HIF-1α) axis.158 Instead, tumor cells could also secrete exosomes containing miRNAs that act on adipocytes. For instance, BC cells secreted exosomes containing miRNA-155, which could induce beige/brown adipocyte differentiation and alter the surrounding adipocyte metabolism to promote cancer progression.159 Adipocytes differentiate into white, beige, and brown adipocytes. White adipocytes are primarily used for energy storage, while brown adipocytes can generate energy by burning fat, and beige adipocytes are an intermediate state between them. MiRNA-155 could downregulate PPARγ expression, making adipocytes use energy more efficiently, and this high metabolic state contributed to the growth of breast tumors. In addition, tumor cell-derived EVs could also cause abnormal glucose metabolism leading to disorders of systemic glycemic control. For instance, miRNA-122 carried by BC cells-derived EVs acts on pyruvate kinase M (PKM) in β-cells, thus inhibiting insulin secretion, and contributing to the development of a hyperglycemic state that favors tumor growth.160 Recent studies have also suggested that the specific miRNome profile of platelets in patients with visceral obesity was involved in the initiation of colon cancer, and the regulation of platelet activation may become another breakthrough in the therapy of obesity-associated cancers.161

MiRNAs are direct or indirect factors in cellular communication between adipocytes and tumor cells162, 163; thus, utilizing the transport function of exosomes may provide a new direction for cancer treatment. Scientists have leveraged AT-derived exosomes to transport miRNAs to tumors which triggers apoptosis of cancer cells and enhances the sensitivity to chemotherapy (Figure 3). Here, miRNAs downregulate the expression of target genes by binding to the mRNA of carcinogenic factors. For example, AT-derived mesenchymal stem cells (AMSCs) secrete exosomes with miR-122 to suppress the expression of a disintegrin and metalloprotease 10 (ADAM10), insulin-like growth factor receptor 1(IGF1R), and cyclin G1 (CCNG1) in HCC cells, resulting in arrest of cell cycle and apoptosis, and improve chemotherapy efficacy.164 AMSC-derived exosomes can delivery miR-145 to BC cells to prevent cancer progression by suppressing the expression of Rho-associated coiled-coil containing protein kinase 1 (ROCK1), matrix metalloproteinase 9 (MMP9), erb-b2 receptor tyrosine kinase 2 (ERBB2) and upregulation of tumor protein p53 (TP53).165 Similarly, miR-199a enhances liver cancer sensitivity to doxorubicin by inhibiting the mTOR pathway.166 MiR-424-5p restrained the expression of programmed death-ligand 1 (PD-L1) on TNBC cells, which create an inflammatory milieu.167 AMSC-derived exosomes, serving as endogenous carriers, exhibit stability and low immunogenicity, rendering them an innate vehicle for miRNAs transportation. They can effectively counteract the drawbacks of instability and susceptibility to degradation of exogenous miRNAs. Despite the promising prospects, exosomes still face technical impediments in terms of separation, identification, and commercialization, and their clinical applications remain in the nascent stage.
4.2.2 MiRNAs as potential biomarkers and therapeutic targets for T2D
The fact that miRNAs can be used as markers to predict the risk of T2D is significant in early disease detection and intervention. The study of plasma miRNA in preadolescent children provides valuable insights into the potential biomarkers associated with obesity and IR.168 In this research, miR-18a-5p, miR-146a-5p, miR-152-3p, and miR-423-3p could potentially serve as important indicators for identifying individuals at risk for developing T2D.168 Upregulation of miR-155, miR-29, and miR-548ag is observed in T2D patients, and targeting these miRNAs may decrease fasting plasma glucose levels and improve glucose tolerance.169-171 AT serves as the primary origin of serum exosomes that can serve as indicators of AT function and potential prognostic markers for T2D. In obese patients with T2D, there is an elevation in hsa-miR-551b-3p levels in both VAT and serum exosomes compared with obese patients without T2D, suggesting a potential association between miRNAs secretion and obesity-induced lipid remodeling.172 Furthermore, miR-27-3p overexpression in exosomes originating from M1 macrophages results in impaired mitochondrial autophagy through the suppression of ras homolog family member T1 (Rhot1) gene, which encodes mitochondrial rho GTPase 1.173 Mitochondria dynamics are believed to contribute to the advancement of diabetes, as autophagic impairments within the mitochondria can exacerbate oxidative stress in β cells and foster IR.174 Consequently, the induction of mitochondrial autophagy holds promise for potential therapeutic benefits in the treatment of diabetes.
MiRNA plays a key role in diabetic nephropathy, diabetic retinopathy, and diabetic heart disease, and can be used as a new marker and therapeutic target for the prevention and treatment of diabetic complications in the future. Rab3A/27A system is an important protein family involved in extracellular vesicles secretion, which affects intercellular communication by regulating the transport of miRNAs in vesicles and participates in podocyte injury in diabetic nephropathy. Under obese status, miR-223-3p leads to high blood glucose, abnormal insulin release, abnormal development of the ocular vascular system, and diabetes with retinopathy. The expression of miR-340-5p is significantly increased in heart tissue of diabetic patients, targeting myeloid cell leukemia 1 to induce mitochondrial dysfunction, worsen oxidative stress, and play a crucial role in the development of diabetic cardiomyopathy.
4.2.3 MiRNAs as predictive biomarkers for obese patients with CVDs
MiRNAs can be utilized to predict the early onset of CVDs in obese patients. In the baboon model of early atherosclerosis, changes in the miRNA expression profile were observed, and a significant correlation was found between miR-5001 and miR-7975 and lesion load.175 However, further exploration is required to elucidate the specific mechanism. Furthermore, in comparison with healthy individuals, patients at high risk of CVDs exhibited varying degrees of increased plasma miR-182-5p, miR-199a-5p, miR-193a-5p, and miR-155-5p.176 Due to chronic inflammation resulting in myocardial damage and reduced cardiac contractility, obese patients are susceptible to left ventricular dysfunction. Consequently, the expression levels of miR-101-3p, miR-140-3p, and miR-99a-5p in the plasma of obese patients can serve as predictive markers for early myocardial injury.177 Patients with coronary artery disease exhibit distinct miRNA expression profiles in both epicardial and VATs compared with healthy individuals.178 The differential expression of certain miRNAs in these ATs is associated with varying clinical outcomes. Exosomes derived from visceral fat carry miR-27b-3p into endothelial cells, thereby targeting the PPAR α/NF- κ B pathway.179 This mechanism can exacerbate atherosclerosis by promoting inflammation.
4.3 CircRNAs in obesity-associated diseases
CircRNAs are characterized by their closed loop shape that lacks 3′ polyadenylation (poly(A)) tails and 5′ cap, making them stable and resistant to degradation. circRNA, as a competing endogenous RNA (ceRNA), could competitively combinate with miRNA to attenuate negative regulation of downstream target genes and increase target gene expression.180 Although the primary function of circRNAs is to act as miRNA “sponges” in regulating target gene expression, some circRNAs could also exert their functions by interacting with proteins.181 CircRNAs have been confirmed to be involved in lipid synthesis and decomposition, and its imbalance can lead to a series of metabolic diseases.182, 183
CircRNAs play a dual role in regulating inflammation in AT. For instance, circRNAs participated in regulating inflammation response through an obesity-associated ceRNA network, which was related to lipid metabolic disorders.184 On the contrary, circARF3 (ADP-ribosylation factor 3) could also strengthen mitophagy to subdue adipose inflammation by combining miRNA-103.,185 but whether changes in this inflammatory state affect tumor development have not been confirmed. A recent study identified circRNAs profiles in white and brown AT, which provided circRNA candidates for regulating adipose formation.186, 187 Studies suggested that circSAMD4A increased preadipocyte differentiation,188 and circArhgap5-2 played an essential role in adipogenesis and lipid metabolism.189
Evidence suggests that downregulated hsa_circ_000839 in patients with BC exhibits a negative correlation with BMI, but the underlying mechanism is still unclear.190 A transcriptome study of circRNAs was conducted, focusing on obese and postmenopausal patients with EC and revealed the presence of 174 differentially expressed circRNA in EC tissues compared with adjacent normal tissues.191 Nonetheless, additional functional research is required to determine the circRNAs that can offer diagnostic and prognostic assistance in relation to obesity-related EC. The plasma of TNBC patients with high BFR showed increased expression of exo-cirCRIM1 derived from adipocytes.192 Exo-cirCRIM1 can increase protein glycosylation of fructose-bisphosphatase 1 (FBP1) by suppressing miR-503-5p and thus weakening its glycolytic inhibition, which promotes TNBC progression. While circRNAs were confirmed to contribute to obesity and cancer development, whether circRNAs play a part in the interaction between obesity and cancer has been rarely explored.
SIRT1 is involved in regulating insulin sensitivity and oxidative stress, and it plays a protective role in T2D.193 In T2D patients, the expression of hsa_circ_0115355 was abnormal, and the reduced hsa_circ_0115355 hindered its competitive binding with miR-145, resulting in decreased expression of SIRT1.193 The upregulation of circ LRP6 in damaged β cells leads to increased apoptosis, decreased insulin release, and induction of oxidative stress, exacerbating T2D by targeting the miR-9-5p/protein N-arginine methyltransferase-1 (PRMT1) signaling pathway.194 Forkhead box O1 (FOXO1) is a negative regulator of insulin signal and plays an important role in the regulation of blood glucose.195 Oleic acid can promote the activation of circ HIPK3, leading to increased fat deposition and IR by sponging miR-192-5p and upregulating FOXO1.196
Due to their tissue specificity and conservation, circRNAs can serve as potential biomarkers for diagnosing coronary artery disease.197 For instance, in comparison with healthy individuals, the expression of hsa_circ_0124644 was significantly upregulated in the peripheral blood of patients with coronary artery disease and validated in larger sample sizes.198 Mechanistically, circRNAs can competitively bind to miRNAs to regulate the expression of genes associated with coronary artery disease.87 CircRNAs derived from extracellular vesicles can also elevate the risk of hypertension and atherosclerosis by modulating blood lipids, lipid deposition, and endothelial cell function.199
Evidence shows that ncRNAs have significant biological functions and participate in forming complex gene regulatory networks. Exosomes assist in transporting ncRNAs to distant organs, amplifying the negative effects of AT. These molecules interact with each other, facilitate intercellular communication and collectively contributing to the progression of obesity-associated diseases.
5 CHROMATIN REMODELING IN OBESITY-ASSOCIATED DISEASES
Changes in the position and structure of nucleosomes give rise to alterations in gene transcriptional activity controlling gene expression, which is called chromatin remodeling. Histone covalent modification and ATP-dependent chromatin remodeling are two types of chromatin remodeling. According to the unique domains found in catalytic ATPases and associated subunits, chromatin remodeling complexes are categorized into four subfamilies, namely: switching defective/sucrose nonfermenting (SWI/SNF), imitation switch (ISWI), chromodomain helicase DNA-binding, and inositol requiring 80 (INO80) family remodelers.200 These ATP-dependent remodelers exert a DNA translocation mechanism to achieve nucleosome assembly, chromatin access, and editing of nucleosome.201 The energy produced by ATP hydrolysis powers the “sliding” of nucleosomes on DNA. Remodelers could expose or cover binding sites at promoters and enhancers of the gene via a loose and tight nucleosome arrangement, which led to gene silencing or expression.201 Adipocytes can provide energy to ATP-dependent chromatin remodeling complexes, the accessibility of chromatin with transcription factors increases, and gene transcription starts.
Compared with healthy-weight patients, there is an enrichment of chromatin modification and remodeling genes in tumor tissues of obese patients with prostate cancer, which provides a potential connection between obesity and cancer progression.202 Chromatin remodeling protein scaffold/matrix attachment region-binding protein 1 (SMAR1) was known for its role in recruiting HDAC1/mSin3a complex to PPARγ promoter to attenuate PPARγ expression level, which had a negative effect on adipocytes differentiation and adipogenesis.203 Apart from this, SMAR1 was also proved to be a suppressor in tumor progression.204 The inhibitory effect of SMAR1 on adipogenesis and tumorigenesis might provide a novel thread to obesity and obesity-associated cancer. AT-rich interactive domain-containing protein 1A (ARID1A), a DNA-binding component of the SWI/SNF chromatin-remodeling complex, was corroborated to play a crucial role in the pathogenesis of NASH.205 In addition to contributing to steatohepatitis, loss of ARID1A has epigenetic effects on development of established HCC. While overexpression of ARID1A promoted tumor initiation, loss of ARID1A could be discovered in established tumor that degraded chromatin accessibility and downregulated expression of genes relative to tumor progression.206 Transcriptional corepressors SWI/SNF complex could be recruited by nuclear receptor Nur77 dampening expression of CD36 and fatty acid-binding protein 4 (FABP4) and subsequently inhibited uptake and transport of fatty acid, reducing the energy source necessary for BC development.207 The chronic stimulation of obesity can result in cellular adaptation, leading to the differentiation of cancer cells into stem cell-like characteristics. Mechanistically, prolonged exposure to obesity enhances the chromatin accessibility of the transcription factor CCAAT/enhancer-binding protein beta (C/EBPB), thereby promoting the increased expression of downstream genes associated with cancer dedifferentiation.208
Inflammatory stress can lead to β cells damage, which constitutes one of the pathogenic processes in diabetes. The Vitamin D receptor shuttles between chromatin remodeling complexes BRG1-associated factors (BAF) and polybromo-associated BRG1-associated factors (PBAF), regulating nuclear chromatin accessibility to protect β cells through the modulation of anti-inflammatory protein expression.209 In obese/diabetic mice and diabetic patients, the downregulated expression of DNA binding protein CCCTC-binding factor (CTCF) in β cells leads to dysregulation of chromatin accessibility.210 Dietary intervention can restore CTCF expression and protect β cell function and maintain glucose homeostasis through chromatin remodeling of genes related to glucose metabolism and stress response.210 Poly (ADP-ribose) polymerase 1 (PARP-1), a DNA binding protein, plays a role in DNA repair and chromatin remodeling, and is activated in diabetic cardiomyopathy. Mechanistically, PARP-1 targets the SIRT1/peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC-1α) axis, leading to increased oxidative stress, inflammation, and fibrosis in myocardium. BAF60a is a subunit of SWI/SNF chromatin remodeling complex which is an important regulator of adipose inflammation induced by obesity.211 In obese and T2D mice, macrophage infiltration in AT was associated with decreased expression of BAF60a in stromal vascular fractions.211 The abnormal expression of BAF60a and its downstream effector activating transcription factor 3 (ATF3) promotes the release of inflammatory cytokines and aggravates the T2D process.211
Although chromatin remodeling in obesity-associated diseases has recently attracted research interest, this field is relatively young.
6 EPIGENETIC MODIFICATIONS OF MACROPHAGES IN OBESITY-INDUCED INFLAMMATION
It is widely recognized that immune regulation plays a critical role in obesity-induced inflammation in AT. Hyperplastic AT, often accompanied by abnormal immune cell infiltration and continuous chronic low-level inflammation, can not only cause obesity-related metabolic complications such as IR and CVDs but also promote the development of cancers.212, 213 Infiltration of macrophages in AT of obese patients increased from under 10% in the normal state to nearly 40%, and the phenomena of macrophage infiltration were more prominent in VAT.214, 215 With an increase in lipid accumulation, hypertrophic adipocytes are encircled by macrophages, resulting in the formation of crown-like structures (CLS), which are associated with a negative impact on cancer prognosis.216, 217 Macrophages can be classified into two phenotypes: M1 (proinflammatory state) and M2 (anti-inflammatory state).218 Diet-induced obesity was confirmed to convert macrophages to M1 polarized chronic inflammatory condition in AT.219 Here, M1 macrophages can produce cytokines such as TNF-α and IL-6 to promote progression of obesity-associated diseases.220 Considering that macrophage polarization is largely influenced by environment, the role of epigenetic modifications in this area has attracted scholars’ attention.
Obesity mainly causes an increase in the infiltration of M1 macrophages. The cytokines secreted by M1 macrophages can accelerate lipid metabolism disorder, chronic inflammation, cell apoptosis, and other biological processes in AT, which is particularly common in metabolic diseases. For instance, in animal models with a HFD, lipotoxic hepatocytes-derived extracellular vesicles released miR-192-5p and miR-9-5p to facilitate M1 macrophage activation, promoting the development of NAFLD.221, 222 Conversely, M2 macrophages can suppress obesity-induced inflammation and hinder the advancement of cardiovascular metabolic disorders. Research has demonstrated that exosomes containing IL-4 can stimulate macrophages to differentiate into anti-inflammatory phenotypes and enhance glucose uptake by upregulating PPAR γ.223 Administering exosomes with IL-4 via intraperitoneal injection to obese mice can reprogram circulating inflammatory signals and mitigate the inflammatory response of cardiovascular system.223
Central obesity is a leading risk factor for IR, and IR is also the most crucial link in the carcinogenesis mechanism that is associated with the decline of glucose uptake and utilization rates. HFD could lead to M1 polarization of bone marrow macrophages (BMMs), and the elevated miR-143-5P derived from BMMs could induce IR in hepatocytes.224 In addition, high expression of miR-27-3p in M1 macrophages induced by a HFD leads to the disruption of mitochondrial autophagy, which exacerbates IR.173 Berberine is known to alleviate obesity, IR and liver injury by inhibiting macrophages from releasing miR-155-5p, which protects the liver from lipotoxicity.225 Another study suggested that berberine could also partly inhibit lncRNA Gomafu expression from suppressing obesity-induced inflammation through M2 polarization of macrophages.226 Downregulation of lysine (K)-specific demethylase 2A (Kdm2a) increased the H3K36me2 level and enhanced chromatin accessibility, which promotes the conversion of macrophages to an anti-inflammatory state.227 Silencing of Kdm2a effectively mitigated the progression of both IR and obesity. Additionally, employing miRNA to regulate the differentiation and proliferation of protective subsets of lipid-associated macrophages has the potential to prevent obesity-induced metabolic disorders.228 M2-like macrophages are capable of maintaining metabolic homeostasis, and overexpressed miR-690 in M2 bone marrow-derived macrophages enhanced insulin sensitivity both in vivo and in vitro, which might also be available for metabolic therapy of metabolic diseases.229
The relationship between chronic inflammation and cancer has been confirmed. Inflammation in the AT microenvironment can lead to changes in metabolic syndrome, which is believed to be associated with cancer risk and progression.230 Macrophages comprise the primary constituent of abnormally infiltrated immune cells in the vicinity of AT and are major participants to the chronic inflammation induced by obesity. In addition to macrophages, obesity has an impact on numerous other immune cells. As an example, prolonged chronic inflammation overstimulates T cells, leading to T cell exhaustion and increased PD-1 expression, which ultimately promoted tumor growth.231 This suggests that obese patients may be more responsive to immune therapy. In general, the changes in immune cells and inflammation caused by obesity play an important role in the process of tumor development. Intervention of AT inflammation may become a new strategy for the treatment of obesity-associated diseases and epigenetic modifications will contribute to this strategy.
7 PREVENTION AND TREATMENT OF OBESITY-ASSOCIATED DISEASES
Obesity is a significant contributing factor to various metabolic disorders and certain types of cancer. Reversing obesity state represents a potential breakthrough in the prevention and treatment of these conditions. Managing weight presents a significant challenge for obese individuals, and achieving successful weight control necessitates a combination of diverse approaches and long-term persistence. Dietary regulation, physical exercise, and bariatric surgery can reduce the risk of obesity-associated diseases by addressing weight control at the root, while epigenetic drugs and immunotherapy can enhance the effectiveness of conventional treatments for obesity-associated diseases (Figure 4). According to classical genetic theory, phenotypic changes are based on changes in DNA sequences. However, with the emergence of epigenetics, mechanisms that induce phenotypic alterations are becoming varied and complex. Exploring the epigenetic modifications of diseases is helpful to the prevention and treatment. In particular, specific epigenetic changes could be considered valuable biological markers in the predisease state and early stage of diseases development, which can provide new methods to detect and treat diseases early and prolong the lifespan of patients. Hence, exploring the field of epigenetic modification is vital as it provides prospects for clinical application.
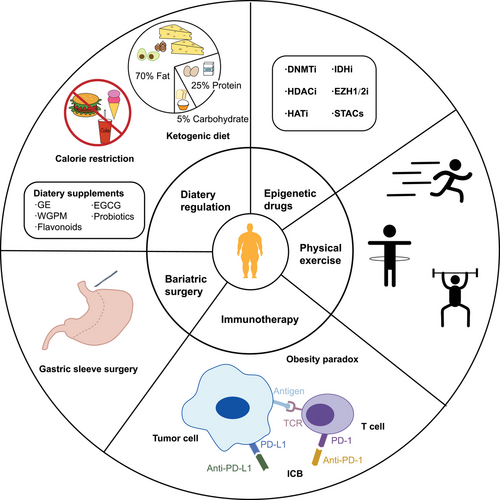
7.1 Dietary regulation
With the change in dietary habits, high-fat/fast food has become a common dish on the table. Consumption of a HFD can alter the global DNAm patterns, thereby elevating the susceptibility to obesity-associated diseases.232, 233 Rats fed a high-sugar and high-fat cafeteria diet exhibited adipocyte hypertrophy and IR in the VAT, which was associated with DNA hypomethylation of the solute carrier family 27 member 3 (Slc27a3).234 An obese diet could also affect the gut microbiome to stimulate histone modification of enhancers in the colon, which might be relative to the promotion of colon cancer.104 Moreover, HFD, which is rich in dietary vegetable fat n-6 linoleic acid, was known to regulate DNAm alterations of farnesoid-X-receptor (Fxr) and prostaglandin-endoperoxide synthase-2 (Ptsg-2) genes, which involve in the development of colonic inflammation and cancer.235 Over-consumption of fructose for an extended period might promote lipid accumulation and downregulated expression of LEP and miR-192, which was also related to prostate cancer pathogenesis.236 Excessive fructose consumption elevates the risk of developing T2D by impacting the regulatory network of miRNAs, thereby diminishing insulin sensitivity and promoting the accumulation of TG.237 Consequently, it is crucial to restrict fructose consumption in our regular diet. Proper dietary interventions can mitigate the adverse effects of obesity and decrease the initiation and progress of associated complications.232 Research demonstrated that following a two-year weight loss dietary intervention, TXNIP DNA hypermethylation, regulating glucose homeostasis, enhanced pancreatic islet function and lowered the risk of T2D.238 In addition, dietary intervention for individuals in early T2D has the potential to mitigate the impairment on β cells due to lipotoxicity and inflammation by reinstating the chromatin reprogramming.210 Improved diet quality can alter the levels of DNAm in cardiometabolism-related genes, which is linked with increased risk of hypertension and CHDs.239 Energy restriction has always been the main intervention for weight loss, but it poses significant challenges to long-term dietary intervention due to its impact on health and low compliance.
Certain nutrients are thought to mitigate the impact of obesity, and their proper intake may help prevent obesity-associated diseases. Animal research suggested that adding grape powder to HFD could reduce oncomiR-34a and oncomiR-21 expression to prevent obesity-induced prostate cancer.240 Genistein (GE) is a bioactive nutritional compound that is abundant in soybean products and is considered a potential antitumor agent. Nowadays, GE consumption could prevent advanced BC through epigenetic modification and even inhibit HFD-induced BC promotion and metabolism disorders in offspring.241 Maternal consumption of GE can impact the initial colonization of intestinal microbiota, global DNAm levels and the expression of key cancer-associated genes in offspring.242 Whole grain proso millet (WGPM) has been shown to substantially reduce blood glucose and lipid levels in mice with T2D by altering the expression profile of miRNA and inhibiting gluconeogenesis-related pathways.243 Flavonoids are prevalent in plants and have demonstrated the ability to enhance endothelial function and decrease the CVDs risk through epigenetic regulation.244 Epigallocatechin-3-gallate (EGCG), which is rich in green tea, can act as an epigenetic regulator to inhibit ROS production and exert an antioxidant effect, contributing to prevent CVDs and diabetes.245 The gut microbiota in obese patients diminishes ethanolamine metabolism, elevates intestinal permeability, and contributes to chronic inflammation. Innovative probiotics have the potential to reinstate microbial ethanolamine metabolism and alleviate intestinal permeability and inflammation in obese and diabetic patients by targeting the AT-rich interaction domain 3a (ARID3a)/miR-101a/zona occludens-1 (Zo1) axis.246 Furthermore, supplementing the diet with new probiotics can decrease the levels of miR-155-5p and miR-125b-5p in the plasma of obese patients, thereby regulating inflammation and lipogenesis.247 While dietary supplements are generally a safe and convenient means of prevention, additional clinical experimental evidence is needed to determine appropriate optimal dosages and supplementation method.
When glucose levels are insufficient under the state of starvation, the lipids are decomposed into glycerol and fatty acids by fat mobilization, producing acetyl-CoA for energy and ketone bodies to supply the brain and heart. The ketogenic diet is characterized by high-fat and low-carbohydrate, similar to the state of hunger, producing a large number of ketone bodies. At present, studies have shown that a ketogenic diet helps to inhibit the progression of metabolic syndrome, diabetes, CVDs, and cancer.248-250 Nevertheless, some studies indicate that a long-term ketogenic diet may lead to IR and elevated LDL levels.251, 252 Consequently, numerous uncertainties persist regarding the effectiveness of ketogenic diet treatment for metabolic diseases. We all know that cancer cells generate energy mainly through glycolysis, and damaged mitochondrial function prevents them from utilizing ketone bodies for energy production like normal cells. Thus, tumor cells are more sensitive to glucose deficiency. A ketogenic diet inhibits the initiation of the primary tumor and systemic metastasis by lower blood glucose levels and increasing ketone bodies. However, the biological mechanisms of the antitumor effect of ketone bodies remain unclear. The latest study showed that β-hydroxybutyric acid, a ketone body, could bind to the cell surface receptor Hcar2, increasing the level of transcription factor Hopx to suppress the proliferation of colon cancer cells.253 β-hydroxybutyric acid can mediate histone lysine 3-hydroxybutyrylation modification which has been identified as a noval epigenetic modification that links fatty acid metabolism to cancer progession.254 β-Hydroxybutyrate dehydrogenase 1 (BDH1) is the primary rate-limiting enzyme in ketone body metabolism and is closely related to the overall survival rate of patients with HCC. Metastasis-associated protein 2 (MTA2) could inhibit the level of BDH1 and increase histone β-hydroxybutyrylation, thus promoting the proliferation of HCC stem cells.255 β-Hydroxybutyrylation of tumor suppressor factor p53 could inhibit cancer cell apoptosis and dampen the function that maintains cancer cell growth arrest.256 However, β-hydroxybutyrylation might also play an antitumor role in inhibiting the critical enzyme of methionine metabolism, as tumors are highly dependent on methionine metabolism.257 There are diverse results treated with ketone bodies in different tumors under different physiological conditions. While the ketogenic diet may impede the tumor's growth rate, persistent weight loss may engender cachexia. Recent studies indicate that the utilization of glucocorticoids alongside a ketogenic diet may avert the onset of cachexia and correspondingly elongate survival rates.258 To date, most of the experiments conducted have only been based on animal models, and further studies are required to determine how the ketogenic diet can be safely applied in clinical anticancer therapy.
7.2 Physical exercise
In addition to healthy dietary intervention, regular physical exercise is also an effective preventive measure for obesity-associated diseases.259-262 In a study focusing on postmenopausal overweight/obese women, miR-122 levels in the plasma of the participants decreased significantly following a 12-month weight-loss regimen of diet and exercise that reduced BC risk.263 Physical exercise can stimulate the release of ncRNAs and alleviate metabolic abnormalities by impacting intertissue communication.264 The myokine irisin, primarily derived from contracted skeletal muscles, plays a key role in improving glucose tolerance and suppressing IR.265 From a mechanistic standpoint, miRNAs released from skeletal muscle may be involved in the regulation of irisin expression.265 Exercise can influence the miRNA expression profile in exosomes derived from myotubes and upregulate the expression of genes associated with anti-inflammatory responses and appetite regulation.266 The effects of exercise are comprehensive, and designing a tailored exercise plan based on individual conditions can enhance insulin sensitivity and cardiopulmonary health. Integrating it with dietary regulation can produce multiplied benefits.
7.3 Bariatric surgery
A sedentary state is ubiquitous during daily study and work, and physical exercise and calorie restriction are two major measures to manage weight. Although caloric restriction can limit the glucose supply to tumors, it can perturb water and electrolyte metabolism, causing physical damage to patients. Therefore, it is not advisable for patients to attempt this method without professional guidance. Furthermore, recent research suggested that long-term weight loss interventions had a better effect on cancer prevention and mitigation than short-term approaches.20, 267, 268 Epigenetic modification is a dynamic process that can be reversed by long-term weight loss interventions, thereby reducing the risk of obesity-associated diseases. The potential harmful effects of obesity are well-documented. In young individuals, obesity can lead to changes in DNAm, and over time, tumor-prone gene signature appears in aging individuals.269 Obesity-associated DNAm changes and the upregulation of oncogenes remain unchanged after short-term weight loss, but long-term weight loss reverses the tumor-prone gene signature.
Except for diet control and physical exercise, long-term weight loss induced by bariatric surgery could change the metabolic status through epigenetic regulation, thus reducing the risk of obesity-related complications.270, 271 According to a cohort study of 30318 obese patients (BMI ≥ 35) that showed bariatric surgery significantly reduced the incidence and mortality of obesity-associated cancers.272 The plasma miRNA expression profile in T2D patients undergoes changes following bariatric surgery. This analysis of changes can help identify potential therapeutic targets for T2D. Differentially expressed miRNAs in obese patients tended to be normalized after bariatric surgery, which was thought to reduce the risk of colorectal cancer.273 Gastric sleeve surgery can prompt the upregulation of tumor suppressor miR-122 in serum, thus reducing the risk of cancer.274 In addition, the marked upregulation of lncRNA Gm19619 triggered by a HFD can enhance hepatic gluconeogenesis and lipid accumulation.275 Following vertical sleeve gastrectomy, the expression of lncRNA Gm19619 is reduced, leading to improved glucose and lipid metabolism.275 Sleeve gastrectomy can reverse the upregulation of lncRNA taurine-upregulated gene 1 (TUG1) induced by high sugar and high fat intake, leading to decreased TUG1 levels which in turn help in regulating blood sugar levels in patients with T2D.276 Hence, bariatric surgery presents a promising approach for preventing complications in obese patients.
7.4 Epigenetic drugs
The diversity and uncertainty of epigenetic modification pose significant challenges to drug therapy related to epigenetics. Resveratrol or 5-aza could reduce triglyceride accumulation in hepatoma cells, and the resveratrol could reverse promoter DNAm of Nrf2-Keap1 (aberrant DNAm in human cancers) to attenuate NAFLD.277 HCC patients induced by NAFLD exhibit overexpression of Squalene Epoxidase (SQLE), which triggers a series of oxidative stress events. These events include the expression of DNMT3A and the silencing of DNMT3A-induced Phosphatase and Tensin Homolog (PTEN), ultimately activating AKT–mTOR.278 Inhibitors of SQLE, such as the antifungal drug terbinafine, could suppress this series of chain reactions and offer a new approach for HCC target therapy. As a target point, the fatty acid-binding (FABP4) could indirectly lead to overexpression of DNMT1 and silence tumor suppressor gene p15INK4B in AML cells.279 Bioactive compound honokiol and adiponectin could inhibit leptin-induced tamoxifen resistance in BC.153 Maternal generation with HFD during pregnancy can induce epigenetic alteration in offspring. DNMT inhibitor hydralazine and HDAC inhibitor valproic acid suppresses BC progress in mice offspring of the high-fat group but increases the incidence and burden of BC in the control group.280 Currently, approved epigenetic drugs are mainly prescribed for treating hematological malignant tumors (Table 1), while a large number of epigenetic drugs for solid tumors are under clinical trials. This will provide a new direction for cancer treatment.
| Type of drug | Mechanism of drug | Drug name | Cancer type |
|---|---|---|---|
| Inhibitor of DNA methyltransferases (DNMTi) | Demethylation under low concentrations and cytotoxic effects under high concentrations | Azacitidine291 | Myelodysplastic syndromes, MDS |
| Decitabine292 | |||
| Inhibitor of enhancer of zeste homolog 1/2 (EZH1/2i) | Inhibiting histone methyltransferase enhancer of zeste homolog 1/2 (EZH1/2i) activity to regulate the expression level of cancer-related genes, and impede abnormal cell proliferation |
Tazemetostat293 Valemetostat294 |
Epithelioid Sarcoma, ES Relapsed or refractory adult T-cell leukemia/lymphoma, R/R ATL |
| Inhibitor of histone deacetylases (HDACi) | Increasing the transcription of genes associated with tumor suppression, cell cycle stagnation, cell differentiation, and apoptosis under the slack chromatin state | Vorinostat295 | Cutaneous T cell lymphoma, CTCL |
| Belinostat296 | Peripheral T cell lymphoma, PTCL | ||
| Romidepsin297 | CTCL and PTCL | ||
| Chidamide298 | PTCL | ||
| Panobinostat299 | Multiple Myeloma, MM | ||
| Inhibitor of the mutant isocitrate dehydrogenase enzymes (IDHi) | Combined with mutant IDH1/IDH2 can reduce the level of tumor metabolite 2-hydroxyglutarate (2-HG) and promote normal cell differentiation | Ivosidenib300 | IDH1-mutated AML |
| Enasidenib301 | IDH2-mutated AML |
Though there are no epigenetic drugs approved for the treatment of T2D and CVDs in clinical application, some drugs are undergoing clinical trials (Table 2). Investigating the epigenetic genome of obese patients and advancing the development of targeted chromatin-modifying drugs have the potential to minimize side effects and facilitate precision treatment.86 Thiazolidinediones are antidiabetic drugs that target PPARγ, but their usage is restricted due to their potential impact on heart and kidney function. HDAC3 inhibitors have the ability to induce posttranslational modifications of PPARγ and could potentially offer a safer therapeutic approach for T2D by enhancing insulin sensitivity.107 Bromodomain and extraterminal (BET) proteins act as histone acetylation readers in chromatin remodeling. The BET inhibitor apabetalone has the potential to reduce the risk of CVDs and T2D by suppressing monocyte inflammatory activation.281
| Type of drug | Drug name | Diseases type | Phase | NCT number |
|---|---|---|---|---|
| DNMTi | Hydralazine | T2D/atherosclerosis/CVDs/ hypercholesterolemia/ hypertension | Phase III | NCT00000620 |
| Diabetic nephropathy | Phase IV | NCT02046395 | ||
| Hypertension | Phase II | NCT00000499 | ||
| Hypertensive renal disease | Phase II/III | NCT00582777 | ||
| Heart Failure | Phase II/III | NCT01255475 | ||
| Phase II | NCT01516346 | |||
| HDACi | Sodium phenylbutyrate | Diabetes/IR | Phase IV | NCT00533559 |
| Not applicable | NCT00771901 | |||
| Prediabetics | Not applicable | NCT05028803 | ||
| Ricolinostat | Painful diabetic peripheral neuropathy | Phase II | NCT03176472 | |
| Inhibitor of histone acetyltransferases (HATi) | Curcumin | T2D | Phase IV | NCT04528212 |
| T2D (IR and oxidative stress) | Not applicable | NCT02529982 | ||
| Metabolic syndrome (gut barrier function) | Not Applicable | NCT03542240 | ||
| NAFLD with T2D | Phase II/III | NCT02908152 | ||
| Hypercholesterolemia/ hypertension | Not applicable | NCT03542240 | ||
| Atherosclerosis | Not applicable | NCT02998918 | ||
| Sirtuin-activating compounds (STACs) | Resveratrol | T2D (endothelial function) | Not applicable | NCT01038089 |
| Not applicable | NCT01881347 | |||
| T2D | Phase I | NCT01677611 | ||
| T2D (inflammation) | PhaseIII | NCT02244879 | ||
| Diabetes with neuropathic complication | Not applicable | NCT05172947 | ||
| Prediabetics | Not applicable | NCT02565979 | ||
| Metabolic syndrome | Not applicable | NCT01714102 | ||
| Diabetic nephropathy | Early Phase I | NCT02704494 | ||
| Heart failure (metabolic and skeletal muscle function) | Phase II | NCT03525379 | ||
| Diastolic heart failure/ hypertension | Phase I | NCT01185067 | ||
| Coronary artery disease | Not applicable | NCT02137421 | ||
| Not applicable | NCT06095635 | |||
| CVDs (postmenopausal women) | Phase I/II | NCT01564381 | ||
| CVDs (primary and secondary Prevention) | Phase II | NCT01449110 | ||
| Atherosclerosis | Not applicable | NCT02998918 | ||
| Peripheral artery disease | Phase III | NCT03743636 | ||
| Not applicable | NCT02246660 | |||
| Vascular system injuries/endothelial disfunction | Not applicable | NCT01668836 | ||
| Hypertension | Phase I | NCT02616822 | ||
| Hypercholesterolemia | Phase II | NCT02409537 |
- Data resource: https://www.clinicaltrials.gov.
In comparison with traditional drugs, epigenetic drugs show certain advantages, the development of epigenetic drugs faces significant challenges due to high costs and individual variability. Although epigenetic therapy has brought great convenience to diseases treatment, it still has a long way to go before its wide application in clinics.
7.5 Immunotherapy in obesity-associated cancers
Tumor cells evade immune surveillance and malignantly proliferate in the organism due to their low immunogenicity and the release of immune suppressing molecules. Recent years, immunotherapy targeting tumor immune evasion has brought hope to cancer patients. However, the response to immunotherapy varies among patients with obesity-associated cancers, and the specific mechanisms by which obesity affects the tumor immune microenvironment are not clear. In general, with the progression of obesity, the infiltration of myeloid-derived suppressor cells increases in tumors, while CD8+ T cells decrease, leading to inhibition of antitumor immunity.282, 283 However, some obese patients show better response to immunotherapy compared with normal-weight patients. For instance, researchers have constructed a DNAm-based biomarker for BMI (DM-BMI) model to investigate the relationship between obesity and BC. They found a positive correlation between DM-BMI and immunotherapy checkpoint inhibitor response markers, indicating that obese cancer patients may be more sensitive to immune checkpoint blockade therapy than normal-weight patients.284 In obese patients, increased interferon-gamma and leptin can upregulate the expression of PD-1/PD-L1, and anti-CTLA-4 therapy has also been demonstrated to be more effective.285 In addition, the expression of Nod-like receptor family pyrin domain containing 3 (NLRP3) inflammasome increases in AT of obese patients.286 The NLRP3 inflammasome can produce inflammatory factors, including IL-1β and IL-18, while promoting the upregulation of PD-1/PD-L1 expression, resulting in an immune-suppressive microenvironment.286 Current research indicates that miR-223-3p can downregulate the NLRP3 inflammasome, thereby mitigating tumor progression and offering a novel approach to overcoming immunotherapy resistance.287 Variances in obese cancer patients’ responsiveness to immunotherapy may be associated with obesity-induced epigenetic modifications. Subsequent research may unveil novel therapeutic targets for cancer. Presently, the confluence of immunotherapy and chemotherapy with epigenetic drug therapy has the potential to augment the efficacy of singular treatments while mitigating adverse drug effects, thus emerging as promising anticancer strategies.
8 CONCLUSION
The increased prevalence of obesity can be attributed to the daily consumption of high-energy diets and a reduction in physical activity levels. Except for stirring up physical anxiety, obesity is considered to weaken immunity system, lead to metabolic and endocrine dysfunction, disrupt fertility, and its long-term chronic inflammation exacerbates obesity complications incidence and progression. Obesity alters the overall metabolic environment, and adverse signals from AT can be relayed to adjacent or distant organs through intercellular communication. AT is one of the earliest sites of IR to appear. In obesity, AT can lead to aberrant expression of adiponectin, leptin, and other adipokines through epigenetic modifications. This can impact β cell insulin secretion, induce inflammatory responses, trigger endothelial dysfunction, myocardial damage, and contribute to cardiometabolic diseases. Additionally, adipokines can regulate angiogenesis, cell proliferation and reshape TME to promote cancer development. Excessive fat accumulation can induce macrophage infiltration in AT, and increase the release of inflammation factors through epigenetic regulation, aggravating the level of inflammation.
Currently, obesity is a significant risk factor for cancer, T2D and CVDs. Interventions for AT-induced inflammatory state, and lifestyle changes such as diet, exercise, and even surgery can reverse the obesity-induced abnormal signaling pathways. The temporary and reversible nature of epigenetics provide a noval treatment option for obesity-associated diseases. The emergence of epigenetic editing has offered technical support for investigating disease pathogenesis and clinical translation.288 However, epigenetic editing technology is associated with drawbacks such as off-target effects and limited short-term efficacy, while delivery of CRISPR/Cas in vivo encounters substantial challenges.289 In addition, with the appearance of genetic and epigenetic landscape and entropy, we have a more comprehensive understanding of diseases pathogenesis, and more efficient targeted drugs were invented with new experimental approaches.290 To date, a variety of epigenetic drugs are in the onset stage. Epigenetic drugs have the capability to precisely target specific genes and signaling pathways, enabling tailored treatment. Moreover, their reversibility allows for dynamic treatment strategies. Epigenetic drugs hold promise for targeting diverse diseases and have broad applicability. Nevertheless, the off-target effects of epigenetic drugs may lead to unforeseen side effects by targeting unintended genes or pathways, and the potential long-term impact on the body remains unconfirmed. Consequently, achieving high selectivity and ensuring safety are critical challenges in the development of epigenetic drugs.
Meanwhile, detection of DNAm and ncRNAs offer a noninvasive means of diagnosing obesity-associated diseases, providing convenience to patients. The emergence of exosomes also facilitates the application of exogenous small ncRNAs for therapy. Currently, exogenous ncRNAs are mainly transported by adipocytes-derived exosomes, which makes target positioning difficult and may cause negative effects on nontargeted cells or organs. The high cost of large-scale production and unknown safety have hindered the clinical application of ncRNAs. In the future, people should overcome the difficulty of delivering different ncRNAs at the same time and focus on developing safer and more specific vehicles. In summary, epigenetics holds significant therapeutic potential for obesity-associated diseases, yet it is confronted with ethical, safety, and feasibility challenges, necessitating continual improvement and validation in clinical practice.
AUTHOR CONTRIBUTIONS
Writing—original draft; conceptualization; visualization: Yiqian Long. Supervision; funding acquisition: Chao Mao. Project administration; funding acquisition: Shuang Liu. Writing—review and editing; project administration; supervision; funding acquisition: Yongguang Tao. Writing—review and editing; project administration; supervision; funding acquisition: Desheng Xiao.
All authors have read and approved the article.
ACKNOWLEDGMENTS
Not applicable.
CONFLICT OF INTEREST STATEMENT
All authors disclosed no relevant relationships.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




