Transforming the untransformable with knockout minicircles: High-efficiency transformation and vector-free allelic exchange knockout in the fish pathogen Photobacterium damselae
Graphical Abstract
We describe efficient vector-free allelic exchange mutagenesis in Photobacterium damselae subsp. piscicida, potentially adaptable to any other bacterial species. It involves electroporation of minimalistic constructs containing only homology arms and selection markers, knockout minicircles, into cells rendered competent using concentrated sucrose solution. This approach is appropriate for genetic targets located on small plasmids and does not induce off-target mutations, as demonstrated by knockout inactivation of aip56 gene and sequencing of multiple mutagenesis clones, respectively.
Abstract
Gene inactivation studies are critical in pathogenic bacteria, where insights into species biology can guide the development of vaccines and treatments. Allelic exchange via homologous recombination is a generic method of targeted gene editing in bacteria. However, generally applicable protocols are lacking, and suboptimal approaches are often used for nonstandard but epidemiologically important species. Photobacterium damselae subsp. piscicida (Pdp) is a primary pathogen of fish in aquaculture and has been considered hard to transform since the mid-1990s. Consequently, conjugative transfer of RK2/RP4 suicide vectors from Escherichia coli S17-1/SM10 donor strains, a system prone to off-target mutagenesis, was used to deliver the allelic exchange DNA in previous studies. Here we have achieved efficient electrotransformation in Pdp using a salt-free highly concentrated sucrose solution, which performs as a hypertonic wash buffer, cryoprotectant, and electroporation buffer. High-efficiency transformation has enabled vector-free mutagenesis for which we have employed circular minimalistic constructs (knockout minicircles) containing only allelic exchange essentials that were generated by Gibson assembly. Preparation of competent cells using sucrose and electroporation/integration of minicircles had virtually no detectable off-target promutagenic effect. In contrast, a downstream sacB selection apparently induced several large deletions via mobilization of transposable elements. Electroporation of minicircles into sucrose-treated cells is a versatile broadly applicable approach that may facilitate allelic exchange in a wide range of microbial species. The method permitted inactivation of a primary virulence factor unique to Pdp, apoptogenic toxin AIP56, demonstrating the efficacy of minicircles for difficult KO targets located on the high copy number of small plasmids.
1 INTRODUCTION
Photobacterium damselae subsp. piscicida (Pdp) is a fish host-adapted facultatively intracellular pathogen of the Vibrionaceae and a challenge to aquaculture throughout temperate marine waters (Andreoni & Magnani, 2014; Romalde, 2002). To date, there is no reliable control measure for photobacteriosis as both antibiotics and vaccines are ineffective or only partially effective against Pdp (Andreoni & Magnani, 2014; Håstein et al., 2005; Munang'andu, 2018). The pathogen possesses multiple virulence factors including a secreted toxin AIP56 that cleaves nuclear factor-κB transcription factor, stopping the upregulation of the inflammatory genes and the downregulation of antiapoptotic genes in macrophages and neutrophils (Nunez-Diaz et al., 2018; Pereira et al., 2014; Valderrama et al., 2019; do Vale et al., 2005). This critical virulence factor lacks homologs among known proteins, and the Pdp genome encodes a plethora of other not-yet-characterized in silico predicted proteins whose function is completely unknown (Baseggio et al., 2021). Gene inactivation is a robust way to determine or confirm a protein function (Nakashima & Miyazaki, 2014; Xu & Zhang, 2016), which, in pathogenic bacteria, can promote development of disease control measures such as protein subunit vaccines or nutritional therapy (Lu et al., 2021). Consequently, established or putative virulence factors are common gene knockout (KO) targets. The accuracy of the protein characterization hinges on the isogenicity of the KO mutant, because the phenotype may be affected by undesired (secondary, off-target) mutations elsewhere in the genome (Johnson et al., 2003). Yet, reliable mutagenesis protocols are scarce for nonmodel organisms, and approaches demonstrated to have high rate of off-target mutations are widely used (Babic et al., 2008; Ferrières et al., 2010; Strand et al., 2014).
Targeted gene KO in a wide range of bacteria can be achieved via allelic exchange by homologous recombination (Nakashima & Miyazaki, 2014; Xu & Zhang, 2016). In contrast to Red/ET recombination and CRISPR-Cas9 methods that require introduction of plasmids for expression of gene-editing enzymes (Nakashima & Miyazaki, 2014; Xu & Zhang, 2016), this approach relies on endogenous enzymatic machinery conserved across bacterial taxa (Michel & Leach, 2012). To generate the allelic exchange construct (AEC), the desired mutation (deletion, insertion, point mutation) is spliced between 0.5 and 1.5 kb regions homologous to sequences flanking the targeted locus (Nakashima & Miyazaki, 2014; Xu & Zhang, 2016). Next, the AEC is conventionally cloned into a plasmid vector containing marker genes for mutant selection, which is nonreplicating (suicide vector) or conditionally replicating in the recipient bacterial strain. Gibson assembly (GA) appears to be the most rapid and efficient approach to date, allowing simultaneous generation of the AEC and cloning into a plasmid vector in a single reaction (Huang & Wilks, 2017; Rudenko & Barnes, 2018). The next step is delivery of the allelic exchange plasmid, which ideally should be conducted via transformation of chemically competent or electrocompetent cells (Drury, 1994; Wirth et al., 1989). However, universal techniques to render cells competent are lacking and require optimization for many bacterial species and strains (Monk, 2012; Wang et al., 2020; Yildirim et al., 2016). Among epidemiologically important taxa, Pdp has been evidently poorly transformable since multiple conventional protocols were tested and shown inefficient (Cutrín et al., 1995, 2000). When a high-efficiency transformation method is not available, an allelic exchange vector may be delivered by conjugation (Ferrières et al., 2010; Strand et al., 2014). To date, conjugative transfer of pir/RP4 suicide vectors from SM10/S17-1 λ pir donor strains of Escherichia coli was the only method used to deliver the allelic exchange DNA in Pdp KO mutagenesis (Abushattal et al., 2022; Naka et al. 2005, 2007; Osorio et al., 2015). These donors contain the chromosomally integrated RP4::Mu conjugative transfer cluster (Simon et al., 1983), which was repeatedly shown to introduce off-target mutations via Mu phage integration into the allelic-exchange vector and/or the recipient's DNA, as well as Mu phage-mediated and RP4-mediated transfer and illegitimate integration of E. coli genes into the target host (Ferrières et al., 2010; Strand et al., 2014). Although alternative vectors and donors were designed (Babic et al., 2008; Ferrières et al., 2010; Strand et al., 2014), conjugation as a biological process has a mutagenic effect as the entrance of single-strand DNA (ssDNA) via conjugation activates the stress response SOS that involves error-prone replication and increased translocation/recombination of mobile elements (Baharoglu et al., 2010; Virolle et al., 2020). Further, whether DNA is delivered via conjugation or transformation, suicide vectors often integrate via illegitimate recombination (Johnson et al., 2003). In some cases, frequency of the off-target mutations generated by suicide vectors can be sufficient for the latter to be used in random mutagenesis (Desomer et al., 1991).
In the present study, we have achieved high-efficiency transformation in Pdp using a very simple protocol that utilizes only nonionic osmotic pressure (sucrose) to render the cells competent. Originally developed for streptococci (Framson et al., 1997), this salt-free approach also performs well in Vibrio (Wang & Griffiths, 2009), and may potentially be a method of choice for a wide range of bacteria. High-efficiency transformation has allowed us to mutagenize Pdp with nonreplicating DNA without conjugation, or even propagation of mutagenic DNA in E. coli. To further decrease the probability of secondary mutations, instead of conventional cloning of an AEC into a suicide vector, we have employed a vector-free “minicircle” approach where homology arms were spliced with marker genes and circularized by GA. Minicircles were constructed and successfully integrated (single-crossover mutants obtained) for two KO targets, a ubiquitous ssrA/smpB ribosome rescue system (Karzai et al., 2000) and AIP56 toxin, which is unique to Pdp (do Vale et al., 2005). In the latter case, double-crossover mutants were also obtained with ease despite the expectation that aip56 may be very hard to knock out due to its location on high copy number of small plasmids (Freitas et al., 2022). Our donor- and vector-free allelic exchange based on transformation of sucrose-treated competent cells with KO minicircles is a major improvement over previously used mutagenesis techniques in Pdp. The approach offers lower probability of secondary mutations and is potentially applicable to a broad range of bacterial species, including allegedly hard-to-transform species.
2 MATERIALS AND METHODS
2.1 Bacterial strains and routine culture
Strains were isolated from yellowtail kingfish (Seriola lalandi) during 2015–2016 disease outbreaks in South Australia and Western Australia, identified and deposited at the Department of Primary Industries and Regional Development (DPIRD), Western Australia as Pdp AS-16-0540-1 and Pdp AS-16-0555-7, and subsequently deposited as QMA0505 and QMA0506 at The University of Queensland (UQ), where isolates were sequenced (Baseggio et al., 2021). Bacteria were cultured at 25°C on Tryptic soy agar (TSA) or in Tryptic soy broth (TSB), unless otherwise specified.
2.2 Preparation of electrocompetent cells
Initially, we tested best-performing conventional protocol in Pdp according to benchmarking performed by Cutrin et al. (1995) as described, except using TSB to grow cells instead of Brain heart infusion broth. Subsequently, cells were rendered competent using a salt-free protocol developed for preparation of electrocompetent streptococci (Framson et al., 1997) with minor modifications. The overnight TSB cultures were diluted to 103 colony-forming unit (CFU)/mL (OD600 = 0.025 in Eppendorf BioPhotometer 6131) in 150–300 mL TSB, and grown to early exponential phase (104–105 CFU/mL; OD600 = 0.25–0.4). Harvested cultures were transferred to 50 mL tubes and chilled on ice; pelleted for 5 min at 2000g, 4°C; resuspended in 50–100 mL of ice-cold 0.625 M sucrose solution in water (pellets combined) and pelleted for 10 min at 18 500g, 4°C; resuspended in 10 mL of ice-cold 0.625 M sucrose (pellets combined), transferred into 15 mL tube, pelleted for 15 min at 18 500g, 4°C, and resuspended in 0.5–1 mL of the same buffer (or remaining supernatant if pellet was loose). This preparation was also attempted at room temperature using TSB supplemented with 1% salt to grow the culture. Competent cells were aliquoted by 50–90 μL into chilled 1.5 mL tubes and stored at – 80°C.
2.3 Transformation efficiency estimation
Competent cells were defrosted on ice, mixed with 100 ng of a plasmid carrying antibiotic resistance marker, pET-28a(+), pLZ12spec, or pUC19, in 10 μL H2O, transferred to chilled 0.1 cm gap electro cuvettes (Sigma), and electroporated at the default P1 in Eppendorf Eporator (1250 V, 5 ms, 600 Ω). Cells were recovered in 0.5–1 mL of 0.25 M sucrose TSB for 2.5 h, 50 μL aliquots were spread on 75 μg/mL kanamycin (Km), 200 μg/mL spectinomycin, or 1 μg/mL ampicillin plates, and transformants were counted after 48 h. Viable cell counts were performed by Miles and Misra method (Miles et al., 1938) immediately after preparation, after defrosting, after electroporation, and after the recovery period.
2.4 Allelic exchange KO mutagenesis
2.4.1 PCR used in mutagenesis
Primers used in allelic exchange mutagenesis of aip56 and ssrA-smpB are listed in Tables 1 and 2, respectively. They were designed using Geneious Prime 2020.2.2 and Primer-BLAST (NCBI). Annealing temperatures were determined by NEB Tm Calculator v1.13.0 (for this GA overhang parts were excluded from chimeric primers used to amplify the homology arms). Colony PCR screenings were performed using OneTaq® Quick-Load® 2X Master Mix with Standard Buffer (NEB). Amplifications of GA fragments were performed using Q5 Hot Start High-Fidelity 2X Master Mix (NEB). Products were visualized on 1% TAE gel stained with SYBR™ Safe DNA Gel Stain (Invitrogen) and size was determined using Fast DNA Ladder (NEB) marker.
| Purpose | Primer name | Sequence | Product (bp) |
|---|---|---|---|
| To amplify kanR2 gene (selectable marker; kanamycin resistance) | Km_F | ATGAGCCATATTCAACGGGAA | 816 |
| Km_R | TTAGAAAAACTCATCGAGCATC | ||
| To amplify sacB transcript (counter-selectable marker; sucrose sensitivity) | sacB_F | ACAAAGTCATCGGGCATTATC | 2085 |
| sacB_R | CGGCTGACATGGGAATTCTG | ||
| To amplify aip56 upstream homology arm and splice it with sacB (F primer overhang) and kanR2 (R primer overhang) | aip56_up_F | gttaaaaaggatcagaattcccatgtcagccg | 887 |
| TTTGTTGTCCGCTGTTACTTG | |||
| aip56_up_R | cctagagcaagacgtttcccgttgaatatggctcat | ||
| GATTATTGAGTATTTTTTCACTGTGA | |||
| To amplify aip56 downstream homology arm and splice it with kanR2 (F primer overhang) and sacB (R primer overhang) | aip56_down_F | attgcagtttcatttgatgctcgatgagtttttctaa | 924 |
| TTCGATGTAATTCTGCTCTG | |||
| aip56_down_R | tttatgttcagataatgcccgatgactttgt | ||
| GCATCGTTAAGGTCATGTGT | |||
| To verify crossover in upstream homology region | out_ aip56_F | ACGTAATGTGTCGCCCAACT | 2755 |
| Km_R | TTAGAAAAACTCATCGAGCATC | ||
| To verify crossover in downstream homology region | Km_F | ATGAGCCATATTCAACGGGAA | 2673 |
| out_aip56_R | AGCGTTTTCTATACTGTTTTTGGTA | ||
| To screen for aip56 deletion | aip56_F | TCACGTTACAGGCTCTAGTG | 388 |
| aip56_R | GCATTCAACTGAACTGTCGG | ||
| To amplify across the aip56 allelic exchange region | out_aip56_F | ACGTAATGTGTCGCCCAACT | 4656 in KO |
| out_aip56_R | AGCGTTTTCTATACTGTTTTTGGTA | 5335 in WT |
- Note: Lowercase letters indicate Gibson assembly overhangs.
- Abbreviations: KO, knockout; WT, wild type.
| Purpose | Primer name | Sequence | Product (bp) |
|---|---|---|---|
| To amplify kanR2 gene (selectable marker; kanamycin resistance) | Km_F | ATGAGCCATATTCAACGGGAA | 816 |
| Km_R | TTAGAAAAACTCATCGAGCATC | ||
| To amplify sacB transcript (counter-selectable marker; sucrose sensitivity) | sacB_F | ACAAAGTCATCGGGCATTATC | 2085 |
| sacB_R | CGGCTGACATGGGAATTCTG | ||
| To amplify ssrA upstream homology arm and splice it with sacB (F primer overhang) and kanR2 (R primer overhang) | ssrA_up_F | gttaaaaaggatcagaattcccatgtcagccg | 1137 |
| GGGCCACGAAAAACCTGAAC | |||
| ssrA_up_R | cctagagcaagacgtttcccgttgaatatggctcat | ||
| GTTGACCTCTTAAGTCTCTGTTGC | |||
| To amplify ssrA downstream homology arm and splice it with kanR2 (F primer overhang) and sacB (R primer overhang) | ssrA_down_F | attgcagtttcatttgatgctcgatgagtttttctaa | 1407 |
| AATTCGTATACGAAGACGTCC | |||
| ssrA_down_R | tttatgttcagataatgcccgatgactttgt | ||
| TGAGTTTCGCAATGACAGCA | |||
| To verify crossover in upstream homology region | out_ssrA_F | TGGAACCCCAATGCTAGTTGA | 2019 |
| Km_R | TTAGAAAAACTCATCGAGCATC | ||
| To verify crossover in downstream homology region | Km_F | ATGAGCCATATTCAACGGGAA | 2657 |
| out_ssrA_R | CACGGTGGTGAGGTGACTC | ||
| To verify ssrA-smpB deletion | ssrA_F | CTGCTCAGAGCCTGCTATCC | 145 |
| ssrA_R | CAGACACGCCATGCAAAGAC | ||
| smpB_F | TAACAGGCGCAGTAAACCGT | 156 | |
| smpB_R | CCTTATCACGCTGCCAGTCA | ||
| To amplify across the allelic exchange region (not used) | out_ssrA_F | TGGAACCCCAATGCTAGTTGA | 3860 in KO |
| out_ssrA_R | CACGGTGGTGAGGTGACTC | 4019 in WT |
- Note: Lowercase letters indicate Gibson assembly overhangs.
- Abbreviations: KO, knockout; WT, wild type.
2.4.2 Construction of the allelic exchange “KO minicircles”
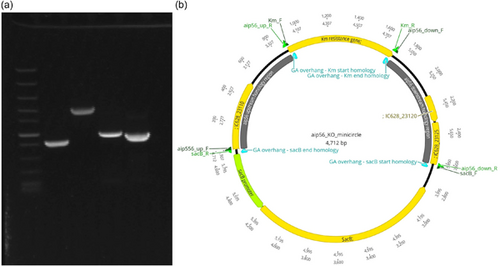
Circular minimalistic AECs (“KO” minicircles) for inactivation of aip56 and ssrA/smpB genes were designed to contain homology arms spliced between selectable and counterselectable marker genes (Figures 1b and 2b). kanR2 gene (selectable marker conferring Km resistance; expressed under target gene transcription elements) and sacB gene with promoter and terminator (counterselection marker conferring sucrose sensitivity) were amplified using Km_F/R and sacB_F/R primer sets (Tables 1 and 2), from 0.5 ng of pET-28a(+) (Addgene; #2526) and pRE107 (Addgene; #43829) plasmids, respectively. The pET-28a reaction was treated postamplification with DpnI (NEB) to eliminate transformation background produced by pET-28a(+) plasmid used as a PCR template. The latter was extracted immediately before amplification to ensure that methylation required for DpnI restriction was retained. Homology regions (0.9–1.4 kb sequences upstream and downstream from aip56 and ssrA-smpB targets) were amplified from Pdp strain QMA0505 gDNA (CP061854-60) using chimeric primers containing GA overhangs homologous to the ends of kanR2 and sacB (aip56_up_F/R, aip56_down_F/R, ssrA_up_F/R, and ssrA_down_F/R reactions; Tables 1 and 2). Fragments (Figures 1a and 2a) were spliced into KO minicircles (Figures 1b and 2b) using NEBuilder HiFi DNA Assembly Cloning Kit (NEB) in 1:1:1:1 molar ratio in 30 μL reactions for 2 h (total of 0.75 pmol DNA, maximum recommended input). Schematic representations of minicircles in Figures 1b and 2b were created in Geneious Prime 2020.2.2.
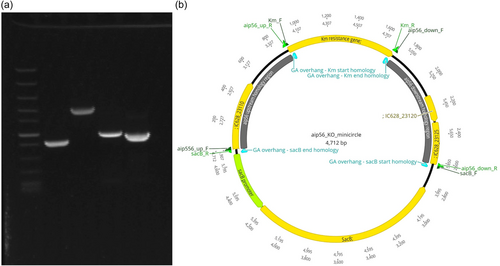
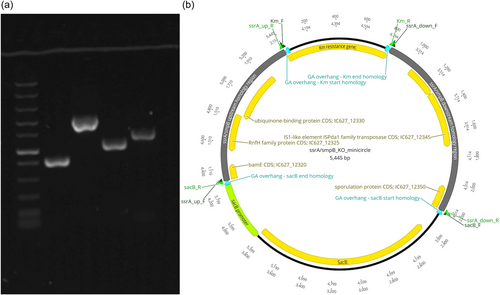
2.4.3 Minicircle transformation and selection of aip56 and ssrA-smpB meroploids
GA reactions of aip56- and ssra/smpB-KO minicircles were purified using AMPure XP magnetic beads (Beckman Coulter), eluted in 20 μL dH2O, and quantified by Qubit. Approximately 0.7 μg of purified minicircles in 10 μL was used to transform QMA0505 (UQ)/AS-16-0540-1 (DPIRD) strain of Pdp (Baseggio et al., 2021) as described (subsection 2.3), except for the extension of the recovery period to 4 h to allow for chromosomal integration of kanR2 for expression, and the exclusion of 0.25 M sucrose from the recovery medium, as it is lethal after integration of sacB-containing minicircles. Transformation with ssDNA was also attempted. For this, half of the purified assembly reactions (10 μL) were denatured at 95°C for 2 min and snap cooled. Single-crossover mutants (meroploids containing integrated minicircles) were selected on 75 μg/mL Km and confirmed by colony PCR screening for the presence of recombination (crossover) in upstream or/and downstream homology regions—amplification across the homology regions where one primer binds to kanR2 sequence and the second primer binds to a sequence outside the homology (out_aip56_F/Km_R, Km_F/out_aip56_R, out_ssrA_F/Km_R, and Km_F/out_ssrA_R reactions; Tables 1 and 2).
2.4.4 Selection of aip56- and ssrA-smpB KO mutants
To select double-crossover clones (KO strains) of aip56, single-crossover mutants were grown to saturation in nonsupplemented TSB, subcultured once, and plated onto agar containing 75 μg/mL Km lethal to wild type (WT) and 15% w/v sucrose (lethal to meroploids). Emergent clones were picked, streaked on the fresh plates, subcultured one more time, and screened for the loss of target genes by colony PCR using an aip56-specific primer set (aip56_F/R; Table 1) (Abushattal et al., 2020). aip56 deletion was further confirmed by amplification across the allelic exchange region yielding a shorter product compared to the WT strain (out_aip56_F/R reaction; Table 1). Single-crossover mutants of ssrA-smpB were grown in multiple liquid culture conditions: TSB, TSB supplemented with 7.5% or 15% sucrose, TSB supplemented with 7.5% or 15% sucrose and 75 μg/mL Km, with decreased agitation or stationary. Most attempts included multiple cultures ranging from 10 to 96. These were plated on 75 μg/mL Km/15% sucrose plates or 50 μg/mL Km/7.5% sucrose plates, and emergent colonies were screened using ssrA- and smpB-specific primer sets (ssrA_F/R, smpB_F/R; Table 2).
2.4.5 Simultaneous selection of aip56-KO clones and “revertant” clones from multiple independent cultures
Multiple aip56 KO clones and clones that reverted to WT genotype (“revertants”) were selected in parallel from independent broth cultures of the single meroploid clone as follows: QMA0786 meroploid used to generate the initial aip56 KO clone QMA0648 was recovered from the frozen stock on 75 μg/mL Km agar and individual colonies were picked to initiate four independent cultures in nonsupplemented TSB, subcultured once, and spread on 15% sucrose plates. Individual colonies were picked and patched onto both 15% sucrose and 75 μg/mL Km/15% sucrose plates. Suspected KO clones (growth on both plates) and clones that reverted to the WT (no growth on Km plates) were PCR-screened with aip56_F/R and KmF/R primer sets (Table 1).
2.5 Sequencing of mutagenesis clones and detection secondary of mutations
To detect secondary mutations, DNA was extracted from selected clones generated during aip56 mutagenesis (QMA0648, QMA0780-89) using Quick-DNADNA Miniprep Plus Kit (Zymo Research). Short-read (Illumina) library preparation and sequencing were performed at the University of Queensland Sequencing Facility (UQ, Brisbane, Australia). Sequencing libraries were prepared using the Nextera XT DNA Library Prep Kit (Illumina), purified with 30 µL of AxyPrep Mag PCR Clean-up beads (Axygen), quantified on the PerkinElmer LabChip GX with the DNA High Sensitivity Reagent Kit (PerkinElmer; CLS760672), and pooled in an equimolar ratio. Sequencing was performed using the Illumina NextSeq500 (NextSeq control software v4.0.0/Real-Time Analysis v2.11.3). The library pool was diluted and denatured according to the standard NextSeq protocol and sequenced to generate paired-end 151 bp reads using a 300-cycle NextSeq500/550 Mid Output Reagent Kit v2.5 (Illumina). After sequencing, fastq files were generated using bcl2fastq2 (v2.20.0.422). For better comparability/assessment of the initial frequency of mutation, WT strain QMA0505 was also resequenced along with mutagenesis clones as Illumina reads for it's published genome C06185-460 were obtained using different sequencing kit and instrument (Baseggio et al., 2021). Reads were trimmed using fastp 0.23.2 and paired during export into Geneious Prime 2023.0.4. Using the latter software, reads were mapped to the CP061854-60 genome (Baseggio et al., 2021) using Geneious assembler under default settings. Variants were identified using “Find variations/SNPs” command with minimum coverage set to 30 and minimum variant frequency set to 0.9. Where annotations differed between the polymorphism tracks, alignment of the reads was examined for strains/contigs lacking the identified variants, and the latter were assigned if lower than set coverage was the reason for the variant not being annotated. Where lower variant frequency was the reason, reads with and without the variant were counted and polymorphism was assigned for over 0.6% frequency cases. QMA0505 read alignment at each variant position was examined to identify whether mutation is de novo. Regions of no read coverage indicating putative large deletions were identified using “Find High/Low coverage” command with a number of sequences under the low coverage flag set to “0.” Where large regions of no coverage were found long-read (Nanopore) sequencing was carried out to confirm the putative large deletions. For the latter, libraries were prepared using Rapid Barcoding Kit 24 V14 (SQK-RBK114.24) and sequenced on a MinION Mk1C device equipped with FLO-MIN114 (R10) flow cell. Nanopore reads were assembled using Flye v2.9, annotated with Prokka v1.12, and exported to Geneious. Regions containing putative deletions were extracted from lon-gread assemblies and the CP061854-60 genome sequence, and aligned using Clustal Omega.
3 RESULTS AND DISCUSSION
3.1 Salt-free preparation of electrocompetent Pdp
We aimed to inactivate two targets via gene KO mutagenesis: AIP56 toxin and SsrA-SmpB ribosomal rescue system, an established and recently proposed virulence factors in Pdp, respectively (Baseggio et al., 2021). For the purpose of obtaining isogenic KO mutants, we wanted to avoid mating with SM10/S17-1 λ pir donor strains of E. coli due to high off-target mutation rate produced by RP4::Mu conjugative transfer cluster (Ferrières et al., 2010; Strand et al., 2014). Transformation is a more straightforward and rapid approach compared to conjugation since allelic exchange DNA is delivered directly to the target organism. Furthermore, ssDNA entering the recipient's cell via conjugation activates bacterial stress response SOS which induces error-prone recombination (Baharoglu et al., 2010; Virolle et al., 2020). Consequently, transformation with double-stranded DNA (dsDNA) is likely to offer lower probability of secondary mutations. For transformation via electroporation, cells are typically washed in hypertonic solutions, as high osmotic pressure stabilizes the cell membrane and decreases cell lysis during electroporation. Most protocols rely on salt ions to create high osmotic pressure; however, salts cause arcing during electroporation and must be washed off in advance. Washed cells are typically resuspended in an electroporation solution containing glycerol that prevents freezing damage and allows storage (Wirth et al., 1989). However, many bacterial species and strains are not readily transformable by standard methods (Aune & Aachmann, 2010), and high transformation efficiency is required for the delivery of nonreplicating DNA in allelic exchange mutagenesis. Case in point, previous attempts to render Pdp cells electrocompetent using the outlined basic approach were not particularly successful. Multiple conditions were evaluated by Cutrin et al. in 1995, and according to the authors, excessive cell lysis occurred when cells were grown in a salty medium (standard for marine species) and prepared using low ionic strength buffers, and arcing occurred when cells were prepared using “isotonic for the bacteria” (i.e., high ionic strength) buffers (Cutrín et al., 2000). Of all the evaluated conventional methods, the highest efficiency of 9.8 × 102 CFU/μg DNA was achieved with a laborious multiple-buffer multiple-reagent protocol immediately after preparation without defrosting/prior storage (Cutrín, 1995). We attempted the same procedure in our laboratory and obtained around 102 CFU/μg transformation efficiencies with pET-28a(+) and pUC19 plasmids.
Since higher efficiencies are required for transformation with nonreplicating DNA, we attempted a rapid and straightforward single-buffer protocol originally developed by Dunny et al. (1991) for gram-positive bacteria. The method was subsequently applied by Framson et al. (1997) for group B streptococci and optimized to contain no salt in the buffer, which rendered this single-buffer protocol to be also a single reagent. The sole ingredient in the wash/electroporation buffer is 0.625 M sucrose, which works as a hypertonic stress agent (nonionic osmotic pressure), electroporation medium, and cryoprotectant. Following the above protocol, we have repeatedly achieved transformation efficiencies of 106–107 CFU/μg DNA in Pdp using pET-28a(+) and pUC19 vectors, and somewhat lower efficiencies of 105–106 CFU/μg DNA were obtained with streptococcal pLZ12spec vector. Notably, these efficiencies were estimated with defrosted cells previously stored at −80°C. We performed the protocol with only minor modifications: TSB was used instead of Todd–Hewitt broth (used to grow streptococci) with no supplementation with glycine, which is used to weaken cell walls in gram-positive bacteria but unnecessary for gram-negative preparations. Notably, supplementation of the growth medium with salt used to culture marine bacteria including Pdp was also routinely omitted for competent cell preparation to reduce the chances of arcing. Yet, there were no arcing events or reduction in transformation efficiency in several preparations from cultures grown in TSB supplemented with 1% salt (1.5% final salt concentration). This suggests that the method can potentially be used for strictly halophilic bacteria if salts from the growth medium are efficiently washed off by the sucrose solution. There was only minor reduction in the number of viable cells after defrosting and a 10-fold reduction after the electroporation. We have also tried room-temperature preparation and transformation, which may be used as a more convenient alternative to traditional ice-cold technique (Tu et al., 2016). This approach was functional but 10–100 times less efficient.
We have efficiently transformed sucrose-treated electrocompetent Pdp with minimalistic AEC generated by GA (KO minicircles) targeting aip56 and ssrA-smpB loci (see subsections 3.2 and 3.3), which demonstrates that achieved transformation efficiency is sufficient for allelic exchange mutagenesis with nonreplicating DNA. The most prominent difference between this protocol and the conditions evaluated by Cutrín et al. (1995) is the composition of the hypertonic (osmotic stress) buffer. The former has increased sucrose concentration and a complete absence of salt; thus, the osmotic pressure created is completely nonionic. This is highly advantageous because any unwashed salts can cause arcing, while washing/resuspension steps require extra time and handling, and may lower the stabilizing effect on the membranes created by a hypertonic buffer. Further, divalent cations from salts may be used as cofactors by nucleases targeting the transforming DNA (Wang & Griffiths, 2009). Indeed, salt-free preparations dramatically improve transformation efficiency in Vibrio parahaemolyticus, which was explained by deactivation of extracellular DNase abundantly secreted by Vibrio (Wang & Griffiths, 2009). In contrast, highly concentrated sucrose solution may be conveniently used as both hypertonic buffer and electroporation buffer as it creates nonionic osmotic pressure, which efficiently stabilizes cell membranes without adversely affecting the electroporation or augmenting DNase activity. Moreover, it has cryoprotective properties, which allow freezing of competent cells without the addition of glycerol.
3.2 “KO minicircles”: circular AECs generated by GA
Nonreplicating plasmids (suicide vectors) are most often used to deliver mutagenic DNA constructs in allelic exchange mutagenesis. Yet, suicide vectors are notorious for illegitimate integration into host chromosomes and plasmids and other kinds of off-target mutagenesis (Johnson et al., 2003). Illegitimate recombination is very common in bacteria, and it is facilitated in plasmid sequences by the abundance of repeats and common/universal motifs (Desomer et al., 1991; Li et al., 2018; Oliveira et al., 2010; de Vries & Wackernagel, 2002). Remarkably, some suicide plasmids illegitimately integrate at frequencies sufficient for random mutagenesis experiments (Desomer et al., 1991). In some circumstances, plasmid backbones may be necessary, such as when transformation is not available, so DNA is delivered by conjugation, or when transformation is low in efficiency, so very large quantities of DNA are required the plasmid is replicated in and extracted from E. coli for electroporation. However, whenever high-efficiency transformation and other factors permit, vector-free KO approach should be considered as an alternative offering lower off-target mutagenesis probability. Vector-free AECs in previous studies were Fusion PCR products circularized by ligation (Gomaa et al., 2017) and linear GA products (Wu et al., 2019). Here we combined these two approaches: we used GA as a more convenient alternative to Fusion PCR technique (Huang & Wilks, 2017; Rudenko & Barnes, 2018), but employed it to generate circular constructs/products, as they are more stable compared to linear DNA. The constructs, here dubbed “KO minicircles,” were generated to knock out aip56 and ssrA-smpB ribosomal rescue loci (Figures 1 and 2). Minicircles were designed for marked nonpolar deletions and contained four fragments assembled in the following order: -> upstream homology arm -> selectable marker gene (kanR2, Km resistance) -> downstream homology arm -> counter-selectable marker transcript (sacB, sucrose sensitivity) ->. In-frame insertion of the selectable marker gene (without transcription elements) in the place of the KO target does not cause polar effects, facilitates mutant selection, and helps to maintain purity of the culture. Alternatively, if unmarked deletion is desired, a selectable marker gene (with transcription elements) can be placed outside the homology regions along with a counter-selectable marker. Overall, the minicircle approach is exceptionally versatile and may be used with any selectable and counter-selectable marker/s, and for other genetic modifications including site-directed mutagenesis and gene knock-in, e.g., insertion of the WT gene for the KO rescue.
3.3 aip56 and ssrA-smpB mutagenesis: Selection of single-crossover mutants
High frequency of single-cross mutants (meroploids with integrated minicircles) in four transformations (aip56 and ssrA-smpB minicircles, dsDNA, and ssDNA each) provided evidence that: (1) transformation efficiency is sufficient for delivery of nonreplicating allelic exchange vectors or vector-free constructs; (2) KO minicircles generated by GA readily integrate into chromosomes and plasmids; and (3) electroporation with KO minicircles is a straightforward and efficient way to deliver allelic exchange DNA. Only ~0.7 μg of assembly products (0.65 μg of aip56 and 0.78 μg of ssrA-smpB construct DNA) were used in mutagenesis, which were delivered either double-stranded or denatured. Many Km-resistant clones were obtained in all transformations, although around two times more in transformations with ssDNA (Table 3). Randomly chosen selected colonies were PCR-screened for the presence of crossover using primer sets where one primer binds to the Km sequence and the second one binds to the genomic sequence outside the homology region. Over 70% of PCR-screened colonies yielded products of expected size (Figure 3 and Table 3), which unambiguously showed successful integration of minicircles. In the case of aip56, both homology arms recombined equally well (Figure 3a,b), while, in the case of ssrA, the upstream region was apparently less recombinable (Figure 3d,c).
| Number of clones | ds-aip56 | ss-aip56 | ds-ssrA | ss-ssrA |
|---|---|---|---|---|
| Selected on kanamycin plates | 32 | 54 | 60 | 112 |
| PCR-screened for minicircle integration (via single crossover) | 14 | 14 | 14 | 14 |
| PCR-positive for minicircle integration (confirmed single-crossover mutants) | 10 | 11 | 13 | 11 |
| Independent nonselective broth cultures | 8 | Not used | 8 | Not used |
| Selected on kanamycin/sucrose plates | 30–200 | - | None | - |
| PCR-screened for deletion of the target gene from each selection | 6 per culture (×8) | - | - | - |
| PCR-negative for the target gene (confirmed KO mutants) | 3/3/0/3/5/1/2/0 (out of six screened) | - | - | - |
- Abbreviations: ds, double-stranded; KO, knockout; ss, single-stranded.
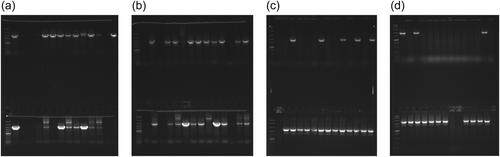
3.4 aip56 and ssrA-smpB mutagenesis: Selection of double-crossover mutants
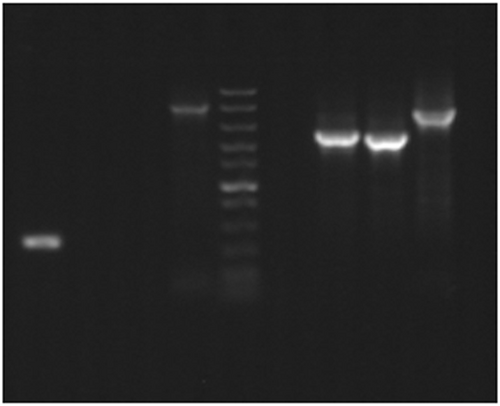
To select double-crossover mutants, meroploids obtained from dsDNA-minicircle transformations were used, as more likely to be isogenic (Baharoglu et al., 2010; Virolle et al., 2020). Multiple aip56 KO clones were obtained with ease after two subcultures of single-crossover mutants in nonselective broth (TSB) and plating on agar supplemented with sucrose (lethal to single crossovers) and Km (lethal to WT). Randomly chosen clones were PCR-screened for the loss of aip56 gene (Table 3). Several aip56-negative clones were further confirmed to be KO mutants by amplification across the allelic exchange region yielding a shorter product compared to WT strain (Figure 4). PCR screening for aip56 deletion showed that many meroploids were escaping sucrose counterselection, which is often weak and inefficient (Cianfanelli et al., 2020; Lazarus et al., 2019), despite omission of salt supplementation in selective plates. Since salt inhibits sacB expression (Blomfield et al., 1991), the latter marker may be suboptimal for slight halophiles such as Pdp and completely inappropriate for obligate halophiles. Nonetheless, double-crossover mutants were selected from 75% of the broth cultures and accounted for 16.65%–83% of the screened colonies (Table 3). Such a success rate was unexpected since aip56 is encoded on a highly abundant, small (<10 kB) plasmid that was anticipated to be hard to eliminate (Freitas et al., 2022). Potentially, aip56 KO would have been harder, perhaps impossible, to achieve using a suicide vector, since integration of a long vector backbone sequence with plasmid replication elements is likely to compromise the aip56 plasmid stability and/or replication . AIP56 is a known toxin characterized at the protein level, which is a primary virulence factor of Pdp causing apoptosis in professional phagocytic cells (Pereira et al., 2014; do Vale et al., 2005). However, isogenic KO mutants lacking the gene have not been characterized yet, which hampers the research aiming at a complete understanding of Pdp pathogenicity (Freitas et al., 2022).
Although both ssrA-smpB homology arms were efficiently recombining (Figure 3 and Table 3), double-crossover mutants were not obtained when followed as per aip56 KO selection despite multiple subsequent attempts involving up to 24 independent single-crossover cultures. Also, based on the hypothesis that ssrA KO mutant (if viable) would be generally impeded in growth and more sensitive to oxidative stress, antibiotics, and high sugar concentrations, we tried the following modifications to the selective conditions: stationary broth cultures, broth cultures with sucrose and sucrose/Km, and lower Km and sucrose concentrations on selective plates, but still we were unable to select ssrA-negative clones. Notably, supplementation of broth cultures with sucrose and lower sucrose concentration on plates dramatically increased the number of single-crossover mutants escaping sacB counterselection, and thus, decreased chances to detect double-crossover mutants, if any were present at minor frequency. Thus, either some alternative counter-selectable markers are required to obtain ssrA KO mutant, or ssrA may be essential in Pdp. Indeed, ssrA-smpB is essential in obligatory intracellular pathogens (Karzai et al., 2000), and Pdp causes primarily intracellular infections and shows relatively slow growth on laboratory culture media.
3.5 Further aip56 mutagenesis and detection of secondary mutations via whole-genome sequencing
3.5.1 Mutagenesis clones used for sequencing
We aimed to confirm that electroporation of KO minicircles into sucrose-treated competent cells can indeed generate highly isogenic KO mutants. However, sequencing of a single KO clone is not sufficient for this purpose as secondary mutations can occur during culturing either completely at random or can be associated with a particular selective pressures or/and inactivation of a particular gene (a target mutation). Thus, we obtained more aip56 KO mutants from several independent broth cultures of a single meroploid clone (clone used to select the initial KO strain). Also, from the same individual broth cultures we have obtained clones that regained the WT genotype (“revertants”), which originate in single-crossover cultures in the same manner as KO clones except for the second crossover happening on the same rather than the opposite homology arm. Since our meroploid contained sacB counterselectable marker conferring sucrose sensitivity, it was possible to select both kinds of clones in parallel on the sucrose agar, which was followed by plating on Km agar to differentiate between revertant/WT and KO genotypes (kanamycin-sensitive and kanamycin-resistant phenotype respectively). Plenty of clones were no longer sensitive to sucrose in three out of four nonselective meroploid broth cultures, and 30%–53% of the clones selected on sucrose were resistant to Km (Table 4). In most cases, PCR screening of selected clones for the presence of aip56 and kanR2 genes has confirmed either revertant to WT genotype (positive for aip56/negative for kanR2) or a KO genotype (negative for aip56/positive for kanR2) (Table 4, Figure 5). To detect and track secondary mutations, we sequenced 11 clones generated during mutagenesis: three pairs of KO and revertant clones selected on sucrose from three independent cultures, the initial aip56 KO clone selected on sucrose/kanamycin agar, and four meroploid clones selected on Km: two from ds aip56-minicircle transformation and two from ss aip56-minicircle transformation (Table 5).
| Number of clones | Culture 1 | Culture 2 | Culture 3 | Cultures 4–6 |
|---|---|---|---|---|
| Selected on sucrose plates | ~50 | ~100 | ~50 | None |
| Picked and patched on sucrose plates and kanamycin plates | 14 | 13 | 15 | - |
| Resistant to kanamycin | 5 | 4 | 8 | - |
| PCR-screened for aip56 gene and kanR2 gene | 5 | 4 | 10 | - |
| PCR-positive for aip56/PCR-negative for kanR2 (revertants to WT) | 2 | 1 | 4 | - |
| PCR-negative for aip56/PCR-positive for kanR2 (KOs) | 2 | 2 | 3 | - |
- Abbreviations: KO, knockout; WT, wild type.
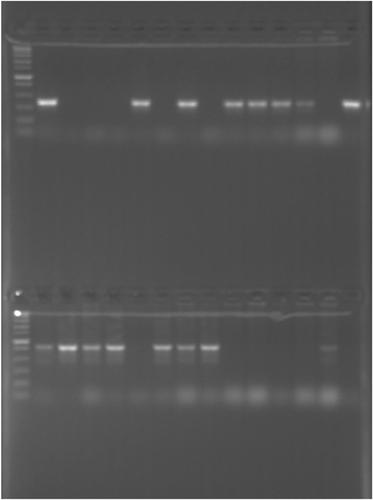
| Strain | Genotype | Parent strain | Selection | Accession numbers |
|---|---|---|---|---|
| QMA0505 | WT | - | N/A | SRR25336394 |
| QMA0648 | KO | QMA0786 | Sucrose/kanamycin | SRR25322645 |
| QMA0780 | Revertant (WT) | QMA0786 | Culture 1 | SRR25337471 |
| Sucrose | SRR25316070 | |||
| JAUYUZ000000000 | ||||
| QMA0781 | Revertant (WT) | QMA0786 | Culture 2 | SRR25337470 |
| Sucrose | SRR25316069 | |||
| JAUYVA000000000 | ||||
| QMA0782 | Revertant (WT) | QMA0786 | Culture 3 | SRR25322644 |
| Sucrose | ||||
| QMA0783 | KO | QMA0786 | Culture 1 | SRR25337469 |
| Sucrose | SRR25316068 | |||
| JAUYVB000000000 | ||||
| QMA0784 | KO | QMA0786 | Culture 2 | SRR25337468 |
| Sucrose | SRR25316067 | |||
| JAUYVC000000000 | ||||
| QMA0785 | KO | QMA0786 | Culture 3 | SRR25337467 |
| Sucrose | SRR25316066 | |||
| JAUYVD000000000 | ||||
| QMA0786 | Meroploid | QMA0505 | ds-MC transformation kanamycin | SRR25322643 |
| QMA0787 | Meroploid | QMA0505 | ds-MC transformation kanamycin | SRR25322642 |
| QMA0788 | Meroploid | QMA0505 | ss-MC transformation kanamycin | SRR25322641 |
| QMA0789 | Meroploid | QMA0505 | ss-MC transformation kanamycin | SRR25322640 |
- Abbreviations: ds, double-stranded; KO, knockout; N/A, not available; ss, single-stranded; WT, wild type.
3.5.2 Secondary mutations
Short reads obtained from Illumina sequencing (SRR25322640–45, SRR25336394, SRR25337467–70) were mapped onto WT reference genome CP061854–60 (Baseggio et al., 2021) to detect small variants and regions of no read coverage indicating putative large deletions. Except for two de novo substitutions in one of the ss-minicircle transformation meroploid clones, all small mutations were variations already existing within the original cell population that increased in frequency (Tables 5 and 6). Small mutations appeared to be due to random replication errors, unlikely to have functional significance, and were mostly copy-number variations within repetitive sequence regions. In contrast, several de novo large deletions were found in KO and revertant clones selected on sucrose plates (Tables 5 and 7). They were evident as areas of no read coverage such as in the case of intended aip56 deletion (Figure 6), and were subsequently confirmed via long-read Nanopore sequencing (SRR253160-66; JAUY(VA-D, UZ)000000000). Five out of six sucrose-selected clones contained one large deletion, which was unique and not linked to broth culture history, that is, different variants present in KO and revertant clones selected in parallel from the same culture (Tables 5 and 7). These five deletions were flanked by transposable elements (TE), which are recognized as primary mediators of evolution in Pdp. Indeed, TE movement is promoted by stress (Twiss et al., 2005) and 15% sucrose in the medium creates high-osmolarity environment stressful to bacteria (Cesar et al., 2020). On the other hand, the KO clone originally selected on Km/sucrose agar did not have any large deletion, which may be incidental or somehow linked to the presence of Km in the medium. Unlike the small variants, the identified large deletions ranging from 8.5 to 25.2 kb were likely to be phenotypically significant. In particular, two sucrose-selected aip56 KO clones, QMA0784 and QMA0785, contained deletions of the same genomic region except for a 3 kb difference. These were acquired independently as they were absent in both QMA0786 meroploid parent strain and two “sister” revertant clones QMA0781 and QMA0782 (Tables 5 and 7). Such independent deletions of the 22/25 kb sequence, which includes several known important genes happening twice, suggest that this change may be favored in aip56-deficient mutants cultured on agar with high sucrose concentrations.
| Mutation | Reference nucleotides | CDS/location | Protein effect | Strains | De novo |
|---|---|---|---|---|---|
| (Tandem repeat) deletion | C1 | 16S rRNA | - | QMA0787 | No |
| (GG)3 > (GG)2 | 25,133–25,134 | ||||
| (Tandem repeat) deletion | C1 | Group II intron reverse transcriptase/maturase | Frameshift | QMA0780 | No |
| (AA)3 > (AA)2 | 96,594–96,595 | ||||
| Substitution | C1 | IS91 family transposase | Substitution | QMA0788 | Yes |
| A > T | 149,093 | D > E | |||
| Substitution | C1 | IS91 family transposase | Substitution | QMA0788 | Yes |
| G > T | 149,097 | P > H | |||
| (Tandem repeat) deletion | C1 | Outside CDS | - | QMA0648 | No |
| (ACTCGCTT)12 > (ACTCGCTT)10 | 760,176–760,191 | QMA0780 | |||
| QMA0785 | |||||
| QMA0789 | |||||
| (Tandem repeat) insertion | C1 | Outside CDS | QMA0781 | No | |
| (TCGCCAC)9 > (TCGCCAC)10 | 846,596 | QMA0784 | |||
| Deletion | C1 | Hypothetical protein | Frameshift | QMA0781 | No |
| −TC | 858,221–858,222 | ||||
| (Tandem repeat) insertion | C1 | Bifunctional methylenetetrahydrofolate dehydrogenase/methenyltetrahydrofolate cyclohydrolase FolD | Truncation | QMA0784 | No |
| (TCGCTAC)12 > (TCGCTAC)14 | 864,195 | QMA0787 | |||
| QMA0789 | |||||
| (Tandem repeat) insertion | C1 | Outside CDS | - | QMA0782 | No |
| (GAGGTTTC)9 > (GAGGTTTC)10 | 2,529,776 | QMA0784 | |||
| QMA0788–89 | |||||
| Substitution/deletion | C2 | Outside CDS | - | QMA0648 | No |
| CT > G− | 744,231–744,232 | QMA0782–88 |
- Abbreviations: CDS, protein coding sequence; rRNA, ribosomal RNA.
| Mutation | Reference nucleotides | CDS within product/locus tag | Strains | |
|---|---|---|---|---|
| IC627_21610 | Hypothetical protein | |||
| Deletion 1 | C2 | IC627_21615 | IS1-like element ISPda1 family transposase | QMA0780 |
| IC627_21620 | IS3 family transposase | |||
| 11, 287 bp | 1,109,902–1,121,188 | IC627_21625 | IS3 family transposase | (revertant, culture1) |
| IC627_21630 | Site-specific integrase | |||
| IC627_21635 | Phosphoribosyltransferase | |||
| IC627_21640 | DNA-protecting protein DprA | |||
| IC627_21645 | Recombinase family protein | |||
| IC627_21650 | DUF3404 domain-containing protein | |||
| IC627_17620 | IS1-like element ISPda1 family transposase | |||
| Deletion 2 | C2 | IC627_17625 | Type VI secretion system tip protein VgrG | QMA0781 |
| IC627_17630 | IS91-like element ISPda2 family transposase | |||
| 26, 434 bp | 368,062–394,495 | IC627_17635 | Hypothetical protein | (revertant, culture2) |
| IC627_17640 | Hcp family type VI secretion system effector | |||
| IC627_17645 | Penicillin-binding protein 1B | |||
| IC627_17650 | Exoribonuclease R | |||
| IC627_17655 | Molybdopterin adenylyltransferase | |||
| IC627_17660 | Isoprenylcysteine carboxylmethyltransferase family protein | |||
| IC627_17665 | M48 family metallopeptidase | |||
| IC627_17670 | Hypothetical protein | |||
| IC627_17675 | IS1-like element ISPda1 family transposase | |||
| IC627_17680 | Fic family protein | |||
| IC627_17685 | IS1-like element ISPda1 family transposase | |||
| IC627_17690 | Hypothetical protein | |||
| IC627_17695 | DUF3010 family protein | |||
| IC627_17700 | DEAD/DEAH box helicase | |||
| IC627_17705 | Hypothetical protein | |||
| IC627_17710 | Leucine-rich repeat domain-containing protein | |||
| IC627_17715 | DUF3820 family protein | |||
| IC627_17720 | NUDIX domain-containing protein | |||
| IC627_17725 | Galactosyl transferase | |||
| IC627_17730 | Cation:proton antiporter | |||
| IC627_17735 | NAD(P)H-dependent oxidoreductase | |||
| IC627_17740 | Iron-containing alcohol dehydrogenase | |||
| IC627_17745 | Gfo/Idh/MocA family oxidoreductase | |||
| IC627_17750 | Hypothetical protein | |||
| IC627_17755 | tRNA (pseudouridine(54)-N(1))-methyltransferase TrmY | |||
| IC627_17760 | IS1-like element ISPda1 family transposase | |||
| IC627_17765 | DUF2955 domain-containing protein | |||
| IC627_17770 | HlyD family secretion protein | |||
| IC627_20615 | NAD(P)H-hydrate epimerase | |||
| Deletion 3 | C2 | IC627_20620 | Hypothetical protein | QMA0783 |
| IC627_20625 | hypothetical protein | |||
| 8, 547 bp | 927,902–936,451 | IC627_20630 | Hypothetical protein | (KO, culture 1) |
| IC627_20635 | IS1 family transposase | |||
| IC627_20640 | IS1-like element ISPda1 family transposase | |||
| IC627_20645 | SCO family protein | |||
| IC627_20650 | DUF368 domain-containing protein | |||
| IC627_20655 | IS1 family transposase | |||
| IC627_20660 | IS1 family transposase | |||
| IC627_20665 | IS1-like element ISPda1 family transposase | |||
| IC627_20670 | Hypothetical protein | |||
| IC627_20675 | Glyoxalase | |||
| IC627_20680 | IS1 family transposase | |||
| IC627_05605 | IS1 family transposase | |||
| Deletion 4 | C1 | IC627_05610 | Hypothetical protein | QMA0784 |
| IC627_05615 | DUF2947 domain-containing protein | |||
| 25, 214 bp | 1,148,799–1,174,012 | IC627_05620 | NAD-dependent succinate-semialdehyde dehydrogenase | (KO, culture 2) |
| IC627_05625 | NUDIX domain-containing protein | |||
| IC627_05630 | IS1-like element ISPda1 family transposase | |||
| IC627_05635 | Phosphatase PAP2 family protein | |||
| IC627_05640 | FAD-binding oxidoreductase | |||
| IC627_05645 | Aquaporin Z | |||
| IC627_05650 | LysR family transcriptional regulator | |||
| IC627_05655 | Cation transporter | |||
| IC627_05660 | NYN domain-containing protein | |||
| IC627_05665 | CG2 omega domain protein | |||
| IC627_05670 | YggN family protein | |||
| IC627_05675 | Hypothetical protein | |||
| IC627_05680 | Phospholipase A | |||
| IC627_05685 | YgiQ family radical SAM protein | |||
| IC627_05690 | IS1-like element ISPda1 family transposase | |||
| IC627_05695 | YgiQ family radical SAM protein | |||
| IC627_05700 | AAA family ATPase | |||
| IC627_05705 | IS1-like element ISPda1 family transposase | |||
| IC627_05710 | IS1-like element ISPda1 family transposase | |||
| IC627_05715 | IS1-like element ISPda1 family transposase | |||
| IC627_05720 | IS1-like element ISPda1 family transposase | |||
| IC627_05725 | Hypothetical protein | |||
| IC627_05730 | IS1-like element ISPda1 family transposase | |||
| IC627_05735 | Hypothetical protein | |||
| IC627_05740 | Hypothetical protein | |||
| IC627_05745 | IS1-like element ISPda1 family transposase | |||
| IC627_05615 | DUF2947 domain-containing protein | |||
| Deletion 5 | C1 | IC627_05620 | NAD-dependent succinate-semialdehyde dehydrogenase | QMA0785 |
| 22, 213 bp | 1,149,105–1,171,327 | IC627_05625 | NUDIX domain-containing protein | (KO, culture 3) |
| IC627_05630 | IS1-like element ISPda1 family transposase | |||
| IC627_05635 | Phosphatase PAP2 family protein | |||
| IC627_05640 | FAD-binding oxidoreductase | |||
| IC627_05645 | Aquaporin Z | |||
| IC627_05650 | LysR family transcriptional regulator | |||
| IC627_05655 | Cation transporter | |||
| IC627_05660 | NYN domain-containing protein | |||
| IC627_05665 | CG2 omega domain protein | |||
| IC627_05670 | YggN family protein | |||
| IC627_05675 | Hypothetical protein | |||
| IC627_05680 | phospholipase A | |||
| IC627_05685 | YgiQ family radical SAM protein | |||
| IC627_05690 | IS1-like element ISPda1 family transposase | |||
| IC627_05695 | YgiQ family radical SAM protein | |||
| IC627_05700 | AAA family ATPase | |||
| IC627_05705 | IS1-like element ISPda1 family transposase | |||
| IC627_05710 | IS1-like element ISPda1 family transposase | |||
| IC627_05715 | IS1-like element ISPda1 family transposase | |||
| IC627_05720 | IS1-like element ISPda1 family transposase | |||
- Abbreviations: CDS, protein coding sequence; KO, knockout.
Thus, it appears that prolonged exposure to high sucrose concentrations used for sacB counterselection might have created stressful conditions, which activated TE movement in Pdp. This indicates that alternative negative selection markers should be considered for TE-rich bacteria, and may potentially allow to obtain a viable ssrA-smpB mutant in Pdp. In contrast, preparation of the competent cells using high sucrose buffer and electroporation and integration of the allelic exchange DNA in the form of the minicircles had virtually no mutagenic effect (even when minicircles were delivered as ssDNA).
4 CONCLUSIONS
We describe donor- and vector-free allelic exchange knockout employing “minicircles” in nonstandard epidemiologically relevant bacterial pathogen Photobacterium damselae subsp. piscicida (Pdp). Pdp is not readily transformable by standard techniques that involve competent cell preparations using hypertonic saline buffers (Cutrín et al., 1995). As a result, delivery of the mutagenic DNA via conjugation of pir/RP4 suicide vectors from SM10/S17-1 λ pir donor strains of E. coli has been used in previous genetic modifications of Pdp, a method notorious for the high rate of the off-target mutation (Babic et al., 2008; Ferrières et al., 2010; Strand et al., 2014). Here we have achieved high transformation efficiency comparable to commercial E. coli preparations via a simple single-buffer single-ingredient protocol. This method avoids salt and uses concentrated sucrose solution as an all-in-one hypertonic wash buffer, cryoprotecting medium, and electroporation buffer. Nonionic osmotic pressure stabilizes membranes for electroporation and, unlike ions from salts, does not interfere with electric discharge and does not contribute to DNase activity (Wang & Griffiths, 2009). Potentially, this approach may be generally efficient in bacteria as it was successfully used in other Vibrionaceae (Wang & Griffiths, 2009) and phylogenetically distant Streptococcaceae (Framson et al., 1997).
High-efficiency transformation is required for allelic exchange mutagenesis using nonreplicating DNA. The latter is conventionally an allelic exchange construct (AEC) cloned in a suicide vector. However, plasmid backbones increase the chances of secondary mutations (Desomer et al., 1991; Li et al., 2018; Oliveira et al., 2010; de Vries & Wackernagel, 2002). Here we have mutated Pdp via vector-free allelic exchange employing KO minicircles, nonreplicating circular minimalistic AEC containing only mutagenesis essentials that were generated by GA (Gibson et al., 2009). Electroporated KO minicircles targeting aip56 and ssrA-smpB loci readily integrated into plasmid and chromosomal DNA, respectively, evident by highly efficient selection of single-crossover mutants. Furthermore, KO mutants of the aip56 gene encoded on highly abundant small (<10 kb) plasmid were selected with ease. This was potentially facilitated by the minicircle approach, as integration of a longer sequence containing a suicide vector backbone is likely to affect host plasmid stability and/or replication. We summarize the employed method using aip56 KO as an example below, which is schematically represented in Figure 7: aip56-KO minicircle of 4.7 kb (0.9 kb sequence upstream from aip56 -> selectable marker gene (kanR2, Km resistance, 0.8 kb) -> 0.9 kb sequence downstream from aip56 -> counterselectable marker transcript (sacB, sucrose sensitivity, 2.1 kb) was generated by GA (Figure 1) and electroporated into Pdp cells rendered competent using highly concentrated sucrose solution (as described in subsection 3.1). Single-crossover mutants were selected by the gain of Km resistance, and confirmed by PCR from Km gene to genomic sequence outside the homology arms (Figure 3a,b), along with the gain of sucrose sensitivity. Double-crossover mutants were selected by loss of sucrose sensitivity, then confirmed by the lack of aip56-specific PCR amplification and by PCR across allelic exchange locus yielding shorter product than in the WT parent, accounting for the size difference of aip56 and kanR2 (Figure 4).
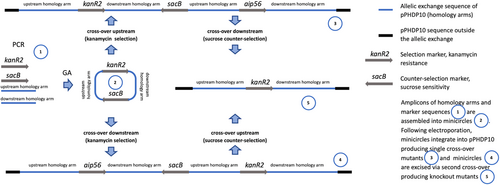
Whole-genome sequencing of multiple clones generated during aip56 mutagenesis did not reveal any secondary mutations that could be attributed to sucrose treatment used to render the cells competent or electroporation/integration of minicircles (Tables 5–7). However, it identified several large deletions associated with the mobilization of TEs by prolonged exposure to high sucrose concentrations used for sacB counterselection (Tables 6 and 7). In this respect, our vector-free method is highly flexible as minicircles can be designed/assembled to contain alternative selection markers or/and to generate other kinds of mutations. To conclude, electroporation of minicircles into sucrose-treated cells is an efficient and versatile approach for allelic exchange allowing to generate highly isogenic mutants in Pdp and potentially most of the bacterial species.
AUTHOR CONTRIBUTIONS
Oleksandra Rudenko: Conceptualization (lead); investigation (lead); methodology (lead); validation (lead); visualization (lead); writing—original draft (lead); writing—review and editing (equal). Laura Baseggio: Conceptualization (supporting); investigation (supporting); methodology (supporting); validation (supporting); writing—review and editing (equal). Fynn McGuigan: Investigation (supporting); methodology (supporting); validation (supporting); writing—review and editing (equal). Andrew C. Barnes: Conceptualization (supporting); funding acquisition (lead); resources (lead); writing—review and editing (equal).
ACKNOWLEDGMENTS
We thank Fisheries Research and Development Corporation (FRDC) for funding the research, and the Department of Primary Industries and Regional Development (DPIRD) in Western Australia for supplying Photobacterium damselae subsp. piscicida strains. Open access publishing facilitated by The University of Queensland, as part of the Wiley - The University of Queensland agreement via the Council of Australian University Librarians.
CONFLICT OF INTEREST STATEMENT
None declared.
ETHICS STATEMENT
None required.
Open Research
DATA AVAILABILITY STATEMENT
Sequencing data were made available under BioProject PRJNA994685: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA994685, which includes deposition of Illumina reads (SRR25322640-45, SRR25336394, SRR25337467-71), and MinION reads (SRR25316066-70) and assemblies (JAUYUZ000000000, JAUYVA000000000, JAUYVB000000000, JAUYVC000000000, JAUYVD000000000).