Effect of different calcium sulfate aspect ratios and chitosan addition methods on the interface properties of calcium sulfate/chitosan composite bone cement
Abstract
It is a great challenge to improve the properties of pure calcium sulfate bone cement. Here, two calcium sulfate hemihydrate powders with different aspect ratios were prepared, and three chitosan addition methods were adopted to investigate the effect on the surface and interface properties of calcium sulfate/chitosan composite bone cement. The results showed that it lacked chemical bond between chitosan and calcium sulfate, and the short rod calcium sulfate displayed better interface adhesion with chitosan owing to its smaller size, so it possessed shorter setting time, better compressive strength and slower degradation than the long rod calcium sulfate, while the biocompatibility had no remarkable difference. Moreover, chitosan solution as liquid phase was a better solidification mode than citric acid, and calcium sulfate/chitosan composite as solid phase was the worst mode because of poor interface compatibility. Conclusively, it was a simple, low-cost, and effective preparation process to choose short rod calcium sulfate powder as solid phase and 1 wt% chitosan solution as liquid phase, which could achieve calcium sulfate/chitosan composite bone cement with the best properties, including setting time, compressive strength, degradation rate, and biocompatibility, displaying a promising application in bone defect repair.
1 INTRODUCTION
Recently, more and more patients are suffering from bone defects. For the larger bone defect area, bone fillers materials were needed to be implanted to guide new bone growth. Both autologous and allogeneic bone grafts have their inherent drawbacks, so biomaterials are gradually used for bone defects repair.1-3 Accordingly, bone cement, a plastic dough composed of solid powder and solidification liquid, can be easily processed into various shapes to provide personalized treatment for the defects, which has been widely adopted in clinical practice for bone defects repair.4-6
Presently, polymethylmethacrylate (PMMA), calcium phosphate, and calcium sulfate bone cements are mainly popular in the market.7-9 However, they have respective demerits, for example, PMMA bone cement would release toxic monomer, produce high-setting temperature, and lack osteoconductivity.10, 11 Calcium phosphate cement exists difficulty of slow solidification and incomplete degradation.12, 13 Comparatively speaking, calcium sulfate bone cement (CSC) is a degradable biological bone cement with good biocompatibility and osteoconductivity.14-16 However, their rapid degradation and brittleness are expected to be further improved. Many literature proved that the types of calcium sulfate (α-calcium sulfate and β-calcium sulfate) and the aspect ratios of calcium sulfate had significant effect on the properties of CSC.17, 18 Especially, the introduction of some biomass polymers including gelatin, hyaluronic acid, chitosan, and silk fibroin to CSC could greatly improve various aspects properties.19-22
Chitosan is a product of chitin deacetylation group, which is the only natural alkaline polysaccharide and possesses excellent biological functions.23-25 Many literature showed that chitosan was used to improve the properties of CSC.26-28 However, chitosan was introduced only acting as an auxiliary component with other polymers or inorganic particles, or in the form of micro-sphere, which would brought about complicated preparation process and high costs. Therefore, it is necessary to explore an effective and simple preparation process to achieve calcium sulfate/chitosan composite bone cement with high quality and low-cost.
Based on this, here, we try to study the preparation of calcium sulfate with different aspect ratios, and design three different preparation methods to obtain the injectable calcium sulfate/chitosan composite bone cements, which was noted as follows, І: chitosan solution as liquid phase was mixed with calcium sulfate powder. П: chitosan powder and calcium sulfate powder as solid phase was mixed with citric acid as liquid phase. Ш: calcium sulfate/chitosan composite powder as solid phase was mixed with citric acid as liquid phase. Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), scanning electron microscopy (SEM), setting time, electromechanical universal tester, in vitro soaking in simulated body fluid (SBF) and in vitro cell culture experiment were carried out. The main purpose of study was to provide a simple, effective, and low-cost preparation process to acquire a more desirable calcium sulfate/chitosan composite bone cement than CSC, including setting time, compressive strength, degradation, and biocompatibility, by investigating the effect of aspect ratios of calcium sulfate and three solidification methods of the addition of chitosan on the properties of calcium sulfate/chitosan composite bone cement, so as to display a promising in bone defect repair application.
2 RESULTS
2.1 Calcium sulfate powder morphology
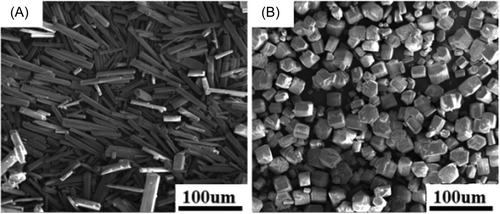
Figure 1 shows the calcium sulfate morphology prepared under different conditions. It can be concluded that the pH of the solution had a great influence on the morphology of calcium sulfate. When the pH value of the system was 3.5, calcium sulfate mainly existed in the shape of short rod and hexagonal prism, whose aspect ratio was about 1~2. While the pH value was 2.0, calcium sulfate displayed long rod with its aspect ratio of 5~10 or so.
2.2 Characterization of calcium sulfate-based bone cement
2.2.1 FT-IR analysis
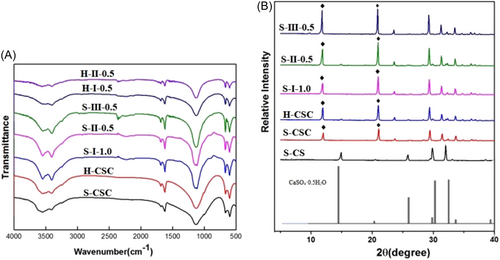
IR spectrum of different samples is given in Figure 2A. For the pure CSC, it could be seen that the spectrum was accord with the relative literature.29 After the chitosan was introduced, the infrared spectra of the composites formed by different aspect ratios of calcium sulfate hemihydrate and different solidification methods were similar to those of pure calcium sulfate, expect for the newly added chitosan peaks. However, there was a subtle difference among different composites, comparing with the s-І−1.0 and H-І−0.5, it could be found that the characteristic peak of 1620 cm−1 for H-I–0.5 was weakened. Likely, H-II-0.5 also had weaker characteristic peak of 1620 cm−1. In addition, S-III–0.5 displayed the strongest characteristic peak of 1620 cm−1 than S-II–0.5 and S-I–1.0.
2.2.2 XRD analysis
Figure 2B shows XRD spectra of different samples. It could be found that the characteristic peaks of short rod calcium sulfate powder was consistent with the Standard XRD card of hemihydrate calcium sulfate (No: 05-0681), indicating that the powder was hemihydrate calcium sulfate. Moreover, the characteristic peaks of both short and long-rod calcium sulfate bone cement were attributable to dihydrate calcium sulfate. For the calcium sulfate/chitosan composites bone cement obtained by different solidification methods with short rod calcium sulfate as raw material, XRD patterns contained the characteristic peaks of hemihydrate and dihydrate calcium sulfate. Comparing with the characteristic peak intensity of different samples, it was found that the long rod calcium sulfate had sharper characteristic peaks at 11.6° and 20.7°. However, there was no distinct difference for the three different solidification methods, except that citric acid as solidification liquid had higher peak intensity.
2.2.3 Setting time determination
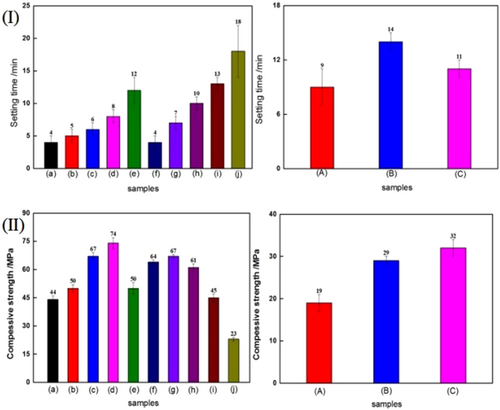
Figure 3I gives the setting time of samples. For pure calcium sulfate bone comment, the setting time was only 4 min, while the corresponding setting time of the calcium sulfate/chitosan composites bone cement was relative longer than that of pure calcium sulfate bone comment, whether the short rod or long rod calcium sulfate. Moreover, the setting time was prolonged with the increase of chitosan solution concentration. Comparing with the different solidification methods, it could be found that the S-III method possessed the longest setting time. However, for the same solidification method, the long rod calcium sulfate/chitosan bone cement displayed longer setting time than the short rod calcium sulfate/chitosan bone cement. Similarly, for the same long rod calcium sulfate bone comment, the setting time of S-I method was a little longer than that of S-II method.
2.2.4 Compressive strength test
Figure 3II shows the compressive strength of calcium sulfate-based bone cement obtained with different morphology of calcium sulfate and different solidification methods. As shown in the figure, for the short rod calcium sulfate-based bone cement, the compressive strength of calcium sulfate/chitosan composite bone comment obtained by S-I or S-II mode increased firstly and then declined with the increase of chitosan solution concentration, and S-I mode endowed the calcium sulfate/chitosan composite bone cement with higher compressive strength than S-II mode did, especially, the addition of 1 wt% chitosan solution in the S-I mode exhibited the highest improvement, which was increased by 75%. For the long rod calcium sulfate-based bone cement, the addition of chitosan could also improve the compressive strength, and S-II mode presented higher compressive strength. However, the compressive strength of the long rod calcium sulfate-based bone cement was by far lower than that of the short rod calcium sulfate-based bone cement.
2.2.5 SEM observation
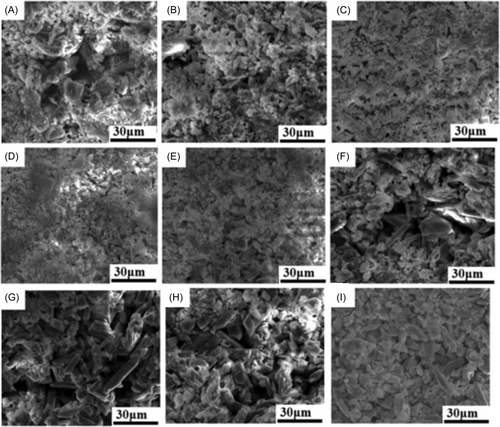
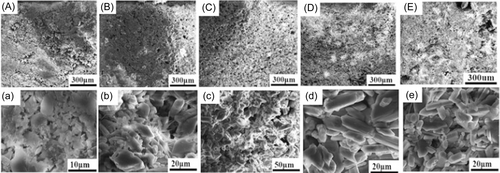
The micro-morphologies of different calcium sulfate-based bone cements are shown in Figure 4. It could be seen that they displayed different microstructures. For the pure CSC, there existed more hollow. When chitosan was added, pore number became less for the short rod calcium sulfate/chitosan composite bone cements. However, in contrast with the three different solidification methods, it was obvious that S-III mode showed more pores than S-I and S-II mode, and the lower chitosan content was good for the dense structure for S-II mode. In addition, the long rod calcium sulfate-based bone cements also exhibited relatively looser microstructure than the short rod calcium sulfate-based bone cements.
2.3 Degradation behavior investigation
2.3.1 pH value change
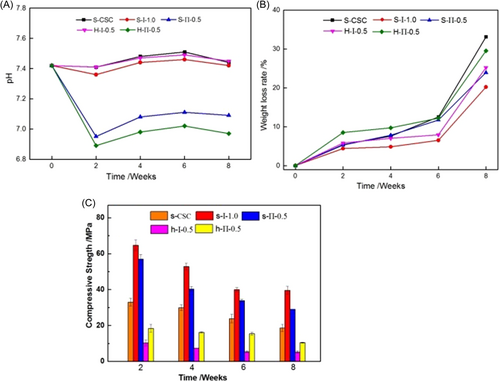
Figure 5A shows the pH value of the samples immersed in SBF for 2, 4, 6, and 8 weeks. Based on the previous discussion, it could be concluded that the calcium sulfate/chitosan bone cements obtained by S-III mode existed a large number of loose structures, so it was completely collapsed after degradation for 2 weeks. Therefore, here, we only studied the degradation for calcium sulfate/chitosan bone cements obtained by S-I and S-II modes. As can be seen from the curve, the pH value of the samples all displayed a decreasing first and then increasing tendency. However, comparing the S-I and S-II modes, the pH value of the calcium sulfate/chitosan bone cements obtained by S-I mode was higher than that of the bone cements obtained by S-II mode. Moreover, the pH value of calcium sulfate/chitosan bone cements was nearly as high as that of pure calcium sulfate. For the different aspect ratios of calcium sulfate-based bone cement, the long rod calcium sulfate-based bone cement exhibited lower pH value than the short rod calcium sulfate-based bone cement. However, the pH value change was not very remarkable, which was still about 7, so it could meet the requirement of human body environment.
2.3.2 Weight loss rate
Figure 5B exhibits that the weight loss of samples during the immersion process. As can be seen from the figure, as expected, the weight loss rate of all samples increased as the soaking time prolonged. Comparing the different calcium sulfate-based bone cements, we found pure CSC possessed the higher weight loss rate than calcium sulfate/chitosan bone cements. Especially, the S-І mode endowed the composite bone cement with the lowest weight loss rate, which was only 20% after soaking 8 weeks, while calcium sulfate/chitosan bone cement with long rod calcium sulfate obtained by S-ІI mode reflected higher weight loss.
2.3.3 The compression strength reduce
Figure 5C gives the compressive strength reduce of the samples during 2, 4, 6, and 8 weeks soaking. There is no doubt about the compressive strength reduce with the extension of soaking time. After soaking for 4 weeks, the compressive strength of the bone cements was still 60% or so. However, after soaking for 8 weeks, the strength of the bone cements decreased significantly, which was decreased by 57.5%, 46.4%, 56.5%, 68.9%, and 68.7% for S-CSC, S-І−1.0, S-П-0.5, H-І-0.5, and H-П-0.5, respectively. Obviously, the calcium sulfate/chitosan composite bone cements obtained by S-I mode displayed the slowest degradation rate, while the long rod calcium sulfate/chitosan bone cements exhibited faster degradation than the short rod calcium sulfate/chitosan bone cements, which was accord with the results of pH value and weight loss rate.
2.3.4 Microstructure observation
Figure 6 is surface micrograph of the bone cement soaking for 8 weeks. It could be found that the voids of the bone cement became larger after soaking, and the crystal surface became smoother. Comparing the five bone cement samples, the long rod calcium sulfate/chitosan composite bone cements had a little more cavities than the short rod calcium sulfate/chitosan bone cements. In addition, it could be seen that there was a large number of apatite deposition on the surface, and there was no obvious difference among the five samples.
2.4 In vitro cytocompatibility
2.4.1 In vitro cell proliferation
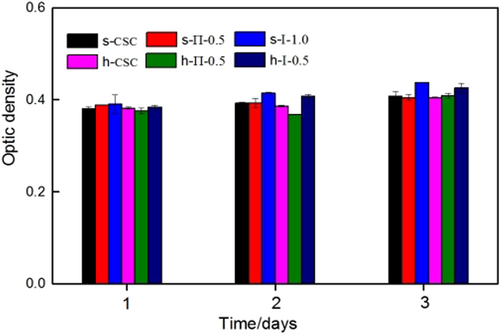
The cell proliferation of samples was determined by MTT (3-{4,5-dimethylthiazol-2yl}-2,5-diphenyl-2H-tetrazoliumbromide) assay, shown in Figure 7. It can be found that the optical densities (OD) values of all samples were almost the same when they were cultured after 1 day. With the extension of culture time, the OD values were also increasing, and there was no obvious difference for the calcium sulfate with different aspect ratios. However, for the two solidification modes, it could be found that the calcium sulfate/chitosan composite bone cement obtained by the S-I solidification mode exhibited better cell proliferation than S-II solidification mode.
2.4.2 In vitro cell attachment
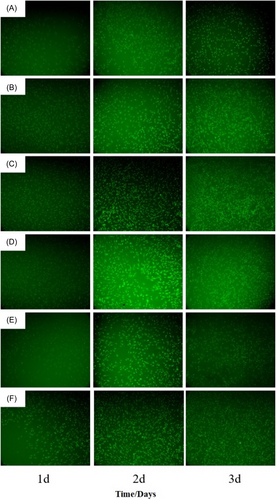
The fluorescence staining photos of cell culture with pure CSC and calcium sulfate/chitosan bone cements formed in different ways are shown in Figure 8. Obviously, the adhered cells number was gradually increased with the prolonging of culture time. At the same culture time, the cells number for the composite bone cement samples was significantly increased than that for pure CSC. Particularly, on the third day, the cells in the calcium sulfate/chitosan composite bone cement began to connect. For the different solidification methods, it can be found that S-I mode produced more adhered cells, and the cells connection was more obvious, however, the different morphologies of calcium sulfate did not affect the cytocompatibility.
3 DISCUSSION
Calcium sulfate bone cement has been used in bone defect repair for several decades, however, its properties is expected to be further improved, and the morphology of calcium sulfate is a very important factor to affect its bone comment properties. In the preparation process, the (111) crystal surface of calcium sulfate was composed of Ca2+, which could selectively adsorb anions and changed the surface free energy, and then hindered the growth of ions in the solution on the crystal surface and reduced the growth rate of the crystal surface. Citric acid, a multicomponent weak acid, was used as the crystal agent, where the acid radical ion was attached on the different crystal surfaces of calcium sulfate, so as to change the relative growth rate and obtain calcium sulfate with different morphologies. When the pH value was 2, the ionization of citric acid was inhibited, and the complextion between acid radical ion and Ca2+ was not strong, so the calcium sulfate crystal was long rod. When the pH increased to 3.5, citric acid was mostly ionized and there would be relatively more acid radicals in the solution were complexed with Ca2+, which hindered its growth on the crystal surface, resulting in the decrease of the aspect ratio, and formed short rod calcium sulfate hemihydrate crystal.30
To clarify the combination mode and solidification process of calcium sulfate/chitosan bone comment, IR and XRD spectra of different samples were used to study the effect of two different morphologies of calcium sulfate and three different bone cement solidification methods containing different chitosan concentrations. IR analysis showed that calcium sulfate hemihydrate with different morphologies was hydrated and transformed into calcium sulfate dihydrate after reacting with distilled water. However, chitosan did not react with calcium sulfate but a simple physical mixing, so the introduction of chitosan did not change the solidification procession. However, the long rod calcium sulfate-based bone cement had lower hydration degree and longer solidification time. Moreover, XRD results also indicated that hemihydrate calcium sulfate reacted with water and transformed into dihydrate calcium sulfate, but it did not completely transformed into dihydrate calcium sulfate, which was accord with the relative literature.31 Moreover, the long rod calcium sulfate had higher crystallinity, and the hydration reaction of calcium sulfate in Mode III was the most thorough, because calcium sulfate was first hydrated in water and then mixed with chitosan solution, and the chelation of citric acid with Ca2+ made the solidification completely, so the characteristic peak was the strongest, suggesting that the solidification was more difficult, and the results were consistent with the discussion of IR.
Setting time is crucial in evaluating the orthopedic surgery feasibility. It is well known that rapid solidification would cause inconvenience for the clinical operation. In our study, the higher concentration of chitosan solution prolonged the setting time, which was caused from the increase of the solution viscosity, resulting in the extension of the hydration process of bone cement. Moreover, the calcium sulfate/chitosan composite was difficult to be dissolved in citric acid solidification liquid, because the surface of calcium sulfate was covered by chitosan, so it would take longer time to solid, which was agreement with the speculation of XRD analysis. Similarly, the long rod calcium sulfate was also difficult to be dissolved by chitosan solution due to higher crystallinity, so it would need longer time to solid, especially for citric acid as solidification liquid. According to the reference,32 the setting time of the calcium sulfate/chitosan composite bone cement would meet the requirements for actual bone surgery operation. Initial mechanical properties plays an important role in bone defect application. In this work, it could be found that the compressive strength of sample obtained by S-I−1.0 solidification method was the highest, which was 75% higher than pure calcium sulfate, and it could meet the mechanical strength requirements according to the relative literature.33 However, the calcium sulfate/chitosan composite bone cement obtained by S-III mode had the worst compressive strength, because the calcium sulfate/chitosan composite was more difficult to be dissolved in citric acid, and it was consistent with the previous analysis. Likewise, the long rod calcium sulfate-based bone cement had poor mechanical property, which was attributed to the more cavity existed because of the accumulation of the larger powder size, where dihydrate calcium sulfate was formed from hemihydrate calcium sulfate during the reaction with water and releasing certain heat, and the crystals were produced with an interwoven and interlinked network structure during calcium sulfate dihydrate continuous precipitation process.34 However, the compressive strengths were still higher than the results of relative literature of calcium phosphate/calcium sulfate bone cement increased with citric acid/sodium alginate.35 The micro-morphology of the composite bone cement is a critical factor to affect the compressive strength, and the difference of micro-morphologies was relative to the solidification methods. The dense micro-morphology of the calcium sulfate/chitosan composite bone cement indicated that chitosan solution was appropriate to be solidification liquid for calcium sulfate powder, while citric acid solution was difficult to dissolve the calcium sulfate/chitosan composite, so it would cause more pore structure, and the larger aspect ratio of calcium sulfate would cause more pore structure, which was consistent with the explanation of compressive strength.
For the degradation of the bone cements, the calcium sulfate/chitosan bone cements obtained by S-II mode displayed lower pH value, larger weight loss, faster compressive strength reduce, and more cavity than S-I mode, which was caused by the addition of poor citric acid solution, and the more cavity micro-structure would be easy to collapse because of the release of citric acid, and the loose structure of long rod calcium sulfate accumulation also would lead to faster degradation. However, the addition of chitosan could form a protective layer around the calcium sulfate, so slowed down the bone cements degradation rate. From the previous analysis, it could be summarized that the bone cement degradation was interfered by the solidification method and aspect ratio of calcium sulfate, and short rod calcium sulfate powder was solidified by chitosan solution was the best solidification method, which produced appropriate degradation. In addition, the bone-like apatite deposition predicted that the calcium sulfate/chitosan bone cements might have good in vitro bioactivity, which was originated from the excellent osteoconductivity of calcium sulfate.36
In vitro cytocompatibility evaluation indicated that all bone cements were nontoxic,37 and chitosan could improve cell activity. Especially, the calcium sulfate/chitosan composite bone cement obtained by S-I solidification mode displayed better biocompatibility than that by S-II mode, and the reason was that the S-II mode might possess strong acidity, which was unfavorable for cell adhesion, and the result was in accordance with the degradation.
4 CONCLUSION
In this study, calcium sulfate hemihydrate with different aspect ratios were prepared by using citric acid as crystal agent, and the long rod calcium sulfate was produced at pH = 2 and short rod at pH = 3.5.
For the calcium sulfate/chitosan composite bone cement, there was no chemical bond between chitosan and calcium sulfate. However, the short rod calcium sulfate bone cement presented shorter setting time, better compressive strength, and slower degradation than the long rod calcium sulfate bone cement. In addition, for the three different solidification modes, S-I mode was better than S-II mode, and S-III mode was the worst solidification mode because the surface of calcium sulfate covered by chitosan would be difficult to be completely dissolved in citric acid. Accordingly, the poor adhesion between chitosan and calcium sulfate caused the lowest compressive strength of composite bone cement.
In vitro degradation results showed that the S-I mode displayed the slowest degradation rate than that obtained by S-II mode, and the S-III mode reflected the fastest degradation. Furthermore, in vitro cell culture results proved that there was no remarkable difference of the biocompatibility for the two calcium sulfate powders with different aspect ratios, but S-I mode displayed a little better biocompatibility than S-II mode.
In a word, an effective, simple, and low-cost preparation method was obtained, where the short rod calcium sulfate powder was solid phase and 1 wt% chitosan solution was liquid phase, which endowed calcium sulfate/chitosan composite bone cement with appropriate setting time, high compressive strength, proper degradation, and good biocompatibility, displaying a promising application in bone defect field. However, in vitro osteogenic activity and in vivo bone defect repair animal experiments are in progress, whose relevant results would be reported in next paper in the future.
5 MATERIALS AND METHODS
5.1 Materials
CaCl2 (AR), Na2SO4 (AR), citric acid (AR), NaOH (AR), and ethanol (AR) were all provided by Tianjin Fengchuan Chemical Reagent Technologies Co., Ltd. Dimethyl sulfoxide (DMSO), acridine orange (AO), and thiazolyl blue tetrazolium bromide (MTT) were all purchased from Sangon Biotech (Shanghai) Co., Ltd. DMEM/F-12 and FBS were bought from Thermo Fisher (Suzhou) Instrument Co., Ltd. Other agents were all of analytical grade.
5.2 Preparation of calcium sulfate with different morphologies
The preparation process was given according to the relative literature.38 Briefly, a certain amount of anhydrous sodium sulfate was dissolved with the adding of citric acid and calcium chloride solution, and the pH value was adjusted to 3.5 with 10% NaOH solution, keeping the solution at 96°C for 4 h, washed with hot water and ethanol, and the white powder was put into the oven at 130°C, and the produce was short rod-shaped calcium sulfate. Similarly, the long rod-shaped calcium sulfate was prepared by the same preparation process, except that pH value was adjusted to 2.0.
5.3 Preparation of calcium sulfate/chitosan composite bone cement by different methods
Calcium sulfate/chitosan composite bone cement was prepared by adopting three different methods, which were given as following.
I. Short rod calcium sulfate powders were mixed with chitosan solution (dissolved in 2 wt% acetic acid) with different concentrations of 0.2, 0.5, 1.0, and 2.0 wt%, and the dough shape mixture was added into a syringe of 2.5 mL to solidify in air mold.
II. Calcium sulfate powder and chitosan powder were uniformly mixed, which contained 0.5 wt%, 1.0 wt%, and 2.0 wt% chitosan, then, 5 wt% citric acid solution was added as the liquid phase, and the dough shape mixture was formed and put into the mold to solidify in the air.
III. Calcium sulfate powder was dispersed with distilled water and kept stirring, and chitosan solution was slowly added to the calcium sulfate dispersion, kept stirring for 1 h, and calcium sulfate/chitosan composite was prepared, which was put in a 40°C oven for 24 h. The dry lumps were ground and sieved with a 100-mesh sieve to obtain a composite powder containing 0.5 wt% chitosan, then 5 wt% citric acid solution was added and as the liquid phase, and the dough shape mixture was formed and put into the mold to solidify in the air.
5.4 Characterization of calcium sulfate and properties of calcium sulfate/chitosan composite bone cements
SEM (S-520; Hitachi) was used to observe the morphology of calcium sulfate powders with different aspect ratios and bone cements, after being sprayed with gold.
An infrared spectrometer was used to analysis by pressing powder samples with KBr, after the solidified bone cement was ground (4000–500 cm-1).
X-ray powder diffraction (XRD; Bruker AXS) analyses of bone cements were performed with a current of 45 mA and an operating voltage of 40 kV, and the diffraction patterns were obtained at a range (2θ) of 5°–40° with the scanning rate 5°/min, and phase analysis was obtained using JADE software.
5.5 Setting time determination of bone cements
The setting time of the bone cements were recorded according to ISO-9597-1989E.39 After the solid and liquid were fully mixed, the dough-like substance was put into a de-headed modified syringe molded, then the dough-like substance was pushed out from other end after 2 min. The sample was pressed every 1 min until the sample did not move, and the time was recorded. Three parallel experiments were conducted, and mean value and standard error were given.
5.6 Compression strength of bone cements
The compressive strengths of samples were tested using the universal testing machine (Changchun New Experiment Co.), whose size was Ф 9 mm × 18 mm, with the compression ratio of 50% and compression speed of 1 mm/min. The average value of three specimens for each sample was given.
5.7 In vitro degradation of bone cements
Where W1 and W2 were noted for the initial weight and the ultimate dry weight of the sample, respectively. The compression strengths of samples soaked for different periods were tested according to the previous description, and the surface microstructures of samples were observed by SEM after being soaked for 8 weeks.
5.8 In vitro cell culture of bone cements
In vitro cells viability was evaluated by coculture of samples with bone mesenchymsal stem cells (BMSCs), which was isolated from SD rat (a week-old). After three passages, the red blood cells and hematopoietic stem cells were removed, and the pure BMSC was finally obtained and removed into the culture medium.42 About 0.1 g sample was immersed in serum-containing medium at 37°C for 24 h after it was cleaned with 75% ethanol solution and sterilized with ultraviolet lamp, then the supernatant was centrifuged, and extract of 200 μL was used for cell culture. Next, BMSCs was added into the 96-wells plate with the density of about 5 × 103 cells, when the cells attached to the wall, the initial culture medium was poured out and different samples extracts were added, then co-incubated at 37°C in the incubator with a humidified 5% CO2 atmosphere to estimate the cell proliferation by MTT assay.43, 44 After the well plates with cell and samples extracts were cultivated for 24, 48, and 72 h, threw off the medium and washed by adding phosphate buffer saline (PBS) twice, and dropped into every well with 200 μL MTT solution (3 mg/mL), incubated in an air atmosphere with 5% CO2 at 37°C for 4 h, and dissolved the formazan crystals with 200 μL of DMSO. Finally, added 200 μL solution into a 96-well plate with enzyme-linked immunosorbent assay, and measured the OD values at least 3 parallel wells for each sample with an Elisa microplate reader (Synergy HTX; BIOTEK) at 492 nm, which was given as the mean value and standard deviation for each sample.
Additionally, after culturing for 24, 48, and 72 h, washed the well plate with PBS twice, and dropped 0.1 mg/mL 200 μL acridine orange solution to wells plate. After half an hour, washed with PBS twice again, fluorescence microscope (Olympus IX71) was used to observe cell morphology and growth.
5.9 Statistical analysis
Mean ± standard error was shown for each group of samples (N = 3). One-way analysis of variance followed by a Tukey post hoc test was adopted to assess statistical significance. All statistical tests were performed with a confidence level of 95% (p < 0.05).
AUTHOR CONTRIBUTIONS
Liuyun Jiang: Conceptualization (equal); supervision (equal); visualization (equal); writing—original draft (equal); writing—review and editing (equal). Chunyan Tang: Data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); writing—original draft (equal). Shuo Tang: Data curation (equal); funding acquisition (equal); methodology (equal); writing—review and editing (equal). Yuqing Wang: Data curation (equal); investigation (equal); writing—review and editing (equal). Qi Ouyang: Investigation (equal); methodology (equal). Xiang Hu: Investigation (equal); resources (equal); writing—review and editing (equal). All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
We are grateful for the test support by large-scale instrument management platform, Hunan Normal University. The present study is supported by Hunan Provincial Innovation Foundation For Postgraduate (CX20230518).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The authors have nothing to report.
Open Research
DATA AVAILABILITY STATEMENT
Data used for this research is available upon reasonable request to the corresponding author.




