Precise tumor treatment: pH-responsive nanoparticles for modulating and real-time monitoring tumor microenvironment
Graphical Abstract
The tumor microenvironment (TME) presents a unique and complex milieu, characterized by spatial and temporal heterogeneity that significantly hinders cancer treatment strategies. Nanoparticles emerge as a versatile solution, potentially overcoming these challenges by enabling targeted therapy and real-time, concurrent assessment of therapeutic effectiveness under abnormal physiological and biochemical conditions. A recent study by Lu et al. introduces a significant innovation in the form of pH-responsive companion diagnostics (CDx) reagent, comprising core-satellite semiconducting polymer nanoparticles @ cobalt hydroxide oxide (SPNs@CoOOH). This novel structure demonstrates a specific affinity for TME targeting, while effectively combining chemiluminescence with reactive oxygen species (ROS)-mediated therapy. This synergy facilitates efficient tumor treatment alongside real-time monitoring, using only water as a resource in the body. As research in nanoparticle-based strategies progresses, the incorporation of pH-responsive systems heralds new possibilities for personalized and more effective cancer therapies.
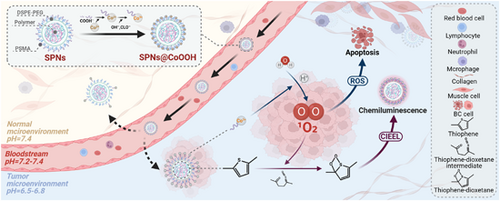
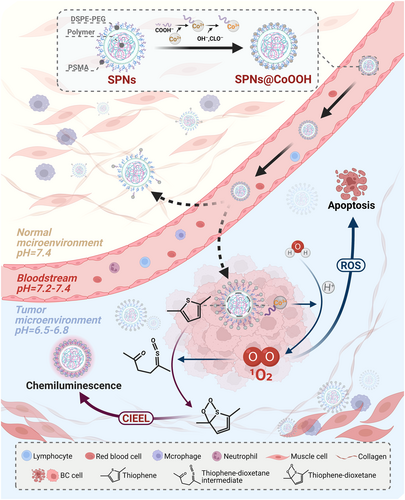
In a recent publication featured in Chem, Lu et al. have introduced a pioneering companion diagnostic (CDx) reagent tailored to exploit the pH-responsive nature of the tumor microenvironment (TME).1 This innovative agent, named core-satellite semiconducting polymer nanoparticles @ cobalt hydroxide oxide (SPNs@CoOOH), emerges as a potent tool for both CDx and precise tumor treatment. Capitalizing on the pH modulation, SPNs@CoOOH effectively homes in on the TME, harnessing the cobalt (III) (Co3+)-mediated reaction with endogenous H2O to generate singlet oxygen (1O2). This integration seamlessly merges chemiluminescence (CL) emission and reactive oxygen species (ROS)-based therapy, culminating in remarkable antitumor effects and the concurrent real-time monitoring of the therapeutic process.
The TME encompasses the immediate surroundings of tumor cells, characterized by abnormal physiological and biochemical attributes like hypoxia, acidity, and elevated glutathione levels.2 In recent years, the progress of nanomedicine has led to widespread utilization of nanomaterials to enhance targeted drug delivery for tumors. Nonetheless, numerous nanoformulations predominantly emphasize cancer treatment while neglecting the crucial aspect of therapeutic monitoring, resulting in compromised accuracy in gauging drug effects and treatment efficacy. Within the realm of precision medicine, the emerging concept of theranostics merges treatment and diagnosis. Leveraging specific molecular probes, theranostics facilitates disease localization, imaging, and treatment based on individual patient biomarkers.3 The realization of theranostics hinges upon CDx, an array of medical tools linked to targeted medications. CDx furnishes insights into patient responses to specific treatments by measuring biomarker levels within the body. However, the separation of imaging and therapeutic agents in most in vivo CDx tests may potentially compromise assessment accuracy. Therefore, the realm of nanomedicine holds promise in cultivating targeted and pragmatic cancer therapeutics and interventions, grounded in the principles of theranostics and CDx.
Building upon the achievements of prior Mn-oxide (MnOx) studies,4 the researchers harnessed copolymer poly(styrene-co-maleic anhydride) (PSMA), sodium hypochlorite (NaClO), sodium hydroxide (NaOH), and cobalt(II) nitrate (Co(NO3)2) as foundational constituents. Employing a tailored hydrothermal method, SPNs@CoOOH nanostructures were synthesized through a two-step process. In this design, semiconducting polymer-based semiconducting polymer nanoparticles (SPNs) were enveloped as cores via hydrophobic interactions, encapsulated within 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000) and PSMA matrices. The satellite entities emerged as CoOOH particles resulting from the swift oxidation of Co(NO3)2 by NaClO. Notably, the interaction between cobalt (II) (Co2+) ions and COO− groups stemming from the PSMA hydrolysis onto the SPNs' surface facilitated the adsorption of both core and satellite.
Building upon this intricate framework, the authors adeptly addressed challenges associated with targeted delivery, efficient action, and real-time monitoring of nanoparticles. To tackle specificity concerns, they opted for a chemodynamic therapy (CDT) strategy, specifically targeting the abnormally downregulated pH within the TME. The efficacy of pH-responsive nanomaterials in localizing to acidic TME has been previously validated, underscoring promising application prospects.5 Through conducting a comprehensive analysis of the correlation between cobalt ions (encompassing Co2+ and Co3+) and the generation of 1O2 within the CoOOH nanoparticles, the authors innovatively proposed an “accelerator/brake” strategy tailored to the TME. This ingenious approach leverages the acid-driven acceleration of Co3+ release within the CoOOH nanoparticles as the accelerator, while simultaneously utilizing the pH-suppressed 1O2 generation stemming from Co3+ as the brake. Ingeniously, 1O2, functioning as a ROS, exhibits a biphasic paradigm. At lower concentrations, it catalyzes the malignant transformation of cancer cells, whereas at elevated concentrations, it exerts a marked cytotoxic effect, leading to the demise of cancer cells. This orchestrated release of oxygen enhances the precision and effectiveness of targeting the TME, thereby augmenting therapeutic outcomes. Moreover, leveraging the generation of 1O2, the author innovatively employed it for signal release from thiophene-based organic semiconductor polymers, enabling real-time monitoring of nanoparticle effects. Drawing from the insights gleaned through prior research, the authors introduced a CL mechanism for SPNs@CoOOH nanoparticles, aptly termed as chemically induced electron exchange luminescence (CIEEL). Specifically, under acidic conditions, SPNs@CoOOH facilitated the oxidation of thiophene groups by oxygen molecules, engendering the formation of thiophene-dioxetane intermediates. These intermediates underwent rapid and spontaneous degradation, furnishing chemical energy crucial for activating SPNs via the CIEEL mechanism, thus resulting in the emission of near-infrared light upon returning to the ground state. This approach enables the dynamic evaluation of treatment effects in real-time and furnishes a dependable imaging reference for fine-tuning treatment parameters.
In summary, building upon a robust theoretical foundation and previous achievements, Lu and collaborators have further advanced the progress by realizing a mature fusion of pharmaceutical and diagnostic elements, resulting in the development of SPNs@CoOOH employing cobalt ions (Figure 1). Compared with their earlier investigations and recent studies of similar nature, the foremost strength of SPNs@CoOOH lies in integration. This quality ensures the colocalized distribution of imaging reagents and therapeutic agents within the living body, thereby guaranteeing both efficient action and precision in evaluating efficacy. In comparison to their previous achievement MnOx-SPNs,4 SPNs@CoOOH exhibit notable enhancements, optimizing the pH range in alignment with the distinctive traits of the TME. Notably, SPNs@CoOOH obviate the necessity for hydrogen peroxide (H2O2), instead relying solely on H2O as a substrate to generate 1O2 efficaciously under nonilluminated conditions. This judicious design mitigates challenges stemming from light penetration limitations in conventional photodynamic therapy and circumvents the gamut of side effects stemming from H2O2 depletion within the TME. Furthermore, the generated 1O2 displays dual versatility: it not only permeates inward to induce CL emission in SPNs, but also diffuses outward to trigger tumor cell demise via ROS, thereby exhibiting heightened adaptability across diverse scenarios. Of particular note, the core-satellite nanostructure of SPNs@CoOOH, established via an injection-based drug delivery approach, configures a minute spherical morphology. This design departure from the sheet-like structure of MnOx-SPNs4 reduced adhesion to biological barriers' components (e.g., blood vessels) during delivery. The net result is an amplified uptake rate by tumor cells and a refinement of pharmacokinetic parameters, signifying a consequential improvement in drug delivery efficacy.
However, avenues for refining the SPNs@CoOOH design persist. Despite its commendable attributes such as heightened sensitivity and resolution, CL does grapple with inherent limitations. Variables such as tissue depth and light transmission can exert influences on imaging efficacy. Future progress hinges upon the cultivation of novel contrast agents, harnessing cutting-edge imaging modalities, and synergizing CL with complementary imaging or diagnostic tools (including genomics and proteomics) to amplify the reproducibility and dependability of outcomes. Simultaneously, it's crucial to consolidate widely adopted standard curves and formulas, furnishing the groundwork for impending clinical applications. Moreover, the inherent transience of 1O2, predicates its swift annihilation following its activation, thereby circumscribing its temporal and spatial reach. Consequently, this necessitates frequent and prolonged administration, entailing further exploration of the associated toxicity.
The intricacy and diversity inherent in tumors underscores the necessity for patient screening and the formulation of tailored therapeutic approaches. Collectively, Lu and colleagues' innovative platform not only facilitates real-time assessment of therapeutic efficacy during treatment but also furnishes a dependable imaging benchmark to calibrate treatment parameters. Consequently, it effectively circumvents the potential pitfalls arising from ambiguous drug dosages, avert adverse reactions, and refine the precision of cancer therapy. In essence, as research delves deeper, this precision-driven cancer treatment paradigm is poised to yield heightened therapeutic efficacy and an improved quality of life for individuals grappling with cancer.
AUTHOR CONTRIBUTIONS
Xinming Su: Conceptualization (equal), data curation (equal), formal analysis (equal), visualization (lead), writing—original draft (lead); Zehua Wang: Visualization (equal); Shiwei Duan: Conceptualization (equal), funding acquisition (equal), project administration (equal), writing—review and editing (equal). All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors would like to thank PubMed for the valuable information. Figure was created by BioRender (biorender.com). In addition, we would like to thank Ming Pan for her suggestions on the figure and text. This study was supported by the Qiantang Scholars Fund in Hangzhou City University (No. 210000-581835).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.