Emerging Trends in Injectable Stimuli-Responsive Hydrogel Microspheres: Design Strategies and Therapeutic Innovations
Jiacheng Liu, Chengcheng Du, and Senrui Liu contributed equally to this study.
ABSTRACT
Hydrogels, as three-dimensional hydrophilic polymer networks, have been widely utilized in biomedical applications due to their excellent biocompatibility, high water content, and tunable physicochemical properties. However, traditional bulk hydrogels often suffer from limitations such as inadequate mechanical strength, slow response to external stimuli, and restricted diffusion efficiency, which hinder their performance in dynamic biological environments. To overcome these challenges, hydrogel microspheres (HMs) have emerged as a promising alternative, which offers advantages such as injectability, high surface-area-to-volume ratio, and tunable functionality. By integrating natural and synthetic materials with advanced fabrication techniques, including microfluidics and emulsification, researchers have achieved precise control over the morphology, size, and bioactivity of HMs. In recent years, stimuli-responsive HMs have attracted significant attention for their ability to respond intelligently to environmental cues such as pH, reactive oxygen species (ROS), enzymes, and temperature. This enables controlled drug release, enhanced therapeutic precision, and spatiotemporal regulation in biomedical applications. This review systematically summarizes the materials, fabrication strategies, and functional mechanisms of stimuli-responsive HMs, highlighting their applications in drug delivery, disease treatment, and tissue engineering. Furthermore, key challenges and future perspectives are discussed, which provides insights into how these intelligent HMs can advance personalized medicine and clinical translation.
1 Introduction
Hydrogels are three-dimensional (3D) hydrophilic polymer networks formed through chemical or physical crosslinking, characterized by high water content and soft mechanical properties [1-3]. Since their first report in 1894, hydrogels have emerged as significant materials in the biomedical field due to their excellent biocompatibility, extracellular matrix (ECM)-mimicking structure, and tunable physicochemical properties [4-7]. Based on their material composition, hydrogels can be categorized as natural, synthetic, or hybrid types, which endows them with broad application potential in tissue engineering and drug delivery [8-12]. For instance, hydrogels can provide mechanical support for tissue regeneration or serve as delivery vehicles for transporting drugs, cells, and bioactive molecules [13-19]. The historical development of hydrogels can be divided into three stages [8, 20]: The first-generation hydrogels were primarily composed of simple water-soluble polymers and fabricated using chemical crosslinking techniques. The second-generation hydrogels introduced stimulus-responsive properties, enabling them to respond to environmental changes such as pH and temperature. The third-generation hydrogels integrated intelligent and injectable features while enhancing mechanical strength and degradation properties through dynamic crosslinking. Currently, with advancements in organic chemistry and emerging technologies, scientists continue to develop “smart hydrogels” to address increasingly complex biomedical needs.
Despite the numerous breakthroughs achieved with traditional hydrogels in the biomedical field, their inherent limitations have restricted their broader application [21]. Firstly, traditional hydrogels are typically prefabricated into fixed shapes, which makes them unsuitable for filling irregular cavities formed after tissue defects, such as complex bone defects or voids left by the resection of solid tumors [22-24]. Secondly, the nanoscale porous structures within bulk hydrogels limit the diffusion efficiency of macromolecules; when the hydrogel diameter exceeds 200 μm, the diffusion of proteins and polysaccharides is significantly impeded [25]. Furthermore, the insufficient mechanical strength and limited dynamic responsiveness of traditional hydrogels constrain their use in certain complex applications [26]. To overcome these challenges, hydrogel microspheres (HMs) have emerged as an innovative solution. These microspheres, typically on the micron scale, are capable of encapsulating drugs, cells, or biomacromolecules, enabling precise delivery through localized injection while significantly enhancing diffusion efficiency [27-31]. Advanced fabrication techniques, such as microfluidics, further endow HMs with superior structural stability and functional diversity, making them flexible, convenient, and highly efficient tools for biomedical applications [32-34]. Additionally, the spherical structure of HMs imparts excellent rolling capability and dispersibility, which allows them to easily reach deep lesions and perfectly fill irregular tissue cavities [35-40]. These features not only enhance their potential applications in tissue regeneration and drug delivery, but also establish a foundation for minimally invasive therapeutic approaches [41-45].
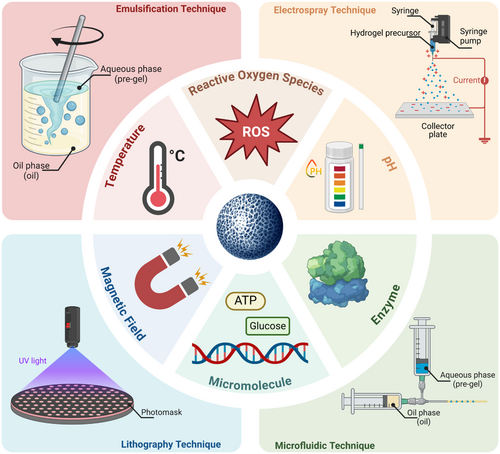
With the advancement of biomedical research, the incorporation of stimuli-responsive properties into HMs has become a major research focus in recent years [46]. These properties enable HMs to intelligently adjust their performance in response to external stimuli such as pH, temperature, redox agents, enzymes, glucose concentrations, or magnetic fields, thereby achieving more precise therapeutic effects [8, 47]. For instance, pH-responsive HMs can release drugs in response to the acidic conditions of the tumor microenvironment, which enhances the targeting of tumor therapy [48, 49]; Temperature-responsive HMs can rapidly alter their shape or release drugs upon local heating, which provides therapeutic options for inflammation or thermosensitive diseases [50, 51]; reactive oxygen species (ROS)-responsive HMs can respond to high concentrations of ROS at inflammation sites or in tumors, which then release antioxidant or anti-inflammatory drugs [52, 53]. These stimuli-responsive features not only improve the efficiency and safety of drug delivery, but also enable precise spatiotemporal control of therapeutic effects, offering new solutions for the treatment of complex diseases [54-57]. Furthermore, the development of these smart HMs has opened up unprecedented possibilities for personalized therapy, targeted drug delivery, and tissue regeneration.
Given the vast potential of stimuli-responsive HMs in the biomedical field, we have written this review to provide researchers with a comprehensive understanding of the latest developments in this area. Distinct from earlier reviews on injectable or stimuli-responsive hydrogels, this review comprehensively catalogs the materials commonly utilized in the fabrication of HMs. Moreover, it further explores advanced fabrication technologies, such as microfluidics and lithography, and elaborates on methodologies for endowing HMs with various stimuli-responsive properties. These include responsiveness to pH, temperature, ROS, enzymes, glucose, and magnetic fields, along with their mechanisms and practical applications (Figure 1). Furthermore, we discuss in detail the current applications of these HMs in disease treatment, drug delivery, tissue repair, and regenerative medicine, and also analyze their advantages and limitations. Finally, we provide an outlook on the future directions for the development of stimuli-responsive HMs, with the hope that this review will serve as a reference for research in the field and contribute to the development of innovative technologies and their clinical translation.
2 Material Selection and Design Strategies
HMs can be classified into two main categories based on the source of materials (Figure 2): natural materials and synthetic materials [8]. Natural materials primarily include polysaccharides and proteins derived from animals, plants, and microorganisms, which possess good biocompatibility and biodegradability, while also promoting cell adhesion and proliferation, making them highly attractive in the biomedical field [58-61]. However, their mechanical strength is relatively low, and they are easy to degradation under environmental conditions, which limits their application range. In contrast, synthetic materials, due to their high mechanical strength, non-immunogenicity, good structural stability, and ease of customization and large-scale production, have gained increasing importance in recent years [62-64].
2.1 Natural Materials
2.1.1 Alginate
Alginate is a natural anionic polysaccharide extracted from brown algae, composed of α-l-guluronic acid and β-d-mannuronic acid units linked by glycosidic bonds (Figure 2A) [65]. It can rapidly crosslink with polyvalent cations (such as Ca2+, Ba2+) under mild conditions to form gels, which thus makes it commonly used as an injectable hydrogel matrix material [66, 67]. Alginate-based hydrogels exhibit excellent biocompatibility, biodegradability, and low toxicity, and their soft consistency and good water retention make them suitable for use as drug and gene delivery carriers [58, 68]. Although alginate-based hydrogels have relatively low mechanical strength and lack cell adhesion capability, these limitations can be addressed by combining them with other polymers or through chemical modification [65, 69, 70]. In recent years, optimized extraction processes have further reduced their immunogenicity, which enhances their potential applications in tissue engineering, cartilage repair, and gene therapy [67]. For example, the combination of chemical crosslinking and composite materials technology can significantly improve the mechanical properties and stability of the hydrogel, providing more reliable solutions for complex biomedical needs [71]. Landa et al. developed a calcium-crosslinked alginate hydrogel that undergoes a phase transition to form a gel when injected into the infarct area of a rat myocardial infarction model [72]. The results showed that the hydrogel increased scar thickness, limited left ventricular expansion, and improved both systolic and diastolic heart function in two rat myocardial infarction models (a 7-day acute infarction model and a 60-day chronic infarction model). This indicates that calcium-crosslinked alginate hydrogel is an effective acellular strategy for improving cardiac remodeling and function after myocardial infarction.
2.1.2 Hyaluronic Acid
Hyaluronic acid (HA) is a natural linear polysaccharide composed of d-glucuronic acid and D-N-acetylglucosamine, widely present in the ECM of vertebrates (Figure 2B) [13, 73, 74]. Its excellent biocompatibility, biodegradability, and non-immunogenicity make it an ideal material for hydrogel preparation [75-79]. As one of the main components of the human ECM, HA possesses various biological properties. HA can regulate cell proliferation, differentiation, migration, and adhesion, and it can bind with growth factors to synergistically promote angiogenesis and tissue regeneration [75, 80-82]. However, HA itself cannot form a stable hydrogel network and requires chemical modification to introduce crosslinking points to enhance its mechanical strength and stability [75, 83]. In recent years, HA-based hydrogels prepared through methods such as dynamic covalent bonding and physical crosslinking have not only demonstrated self-healing properties, but also meet the diverse needs of tissue engineering, drug delivery, and wound healing [13, 75, 84, 85]. Lei et al. developed a shear-responsive HA-based hydrogel (CLX@Lipo@HA-gel), which incorporates liposomes and the anti-inflammatory drug celecoxib through dynamic covalent bonds [86]. Under shear force, the hydrogel can reconstruct its structure, expose liposome micro-reservoirs, form a stable lubricating layer, and continuously release the anti-inflammatory drug. Experiments showed that this hydrogel could reduce cartilage wear, suppress inflammatory responses, and promote the synthesis of cartilage matrix, and finally effectively delay the progression of osteoarthritis.
2.1.3 Chitosan
Chitosan is a natural cationic linear polysaccharide composed of glucosamine and N-acetylglucosamine, with a structure similar to glycosaminoglycans in the ECM (Figure 2C) [58, 87]. Chitosan exhibits good biocompatibility, antimicrobial properties, and biodegradability, and its mechanical properties and degradation characteristics can be optimized by adjusting the deacetylation degree and molecular weight [58, 88-91]. Its pH and temperature-responsive properties make it an ideal material for the preparation of smart hydrogels, with widespread applications in hemostasis, drug delivery, and tissue engineering [58, 65, 67]. However, the mechanical properties of chitosan are relatively weak, but they can be enhanced by composite formulations with other materials (such as gelatin or calcium phosphate) or through chemical crosslinking [76, 92, 93]. Xu et al. developed a dual-antimicrobial hydrogel (CS-PA/CNP) loaded with curcumin nanoparticles by crosslinking chitosan with antimicrobial peptide-modified polyethylene glycol (PEG) [94]. In a mouse model of periodontitis combined with hypertension, CS-PA/CNP demonstrated excellent therapeutic effects, significantly exerting immunomodulatory and anti-inflammatory actions by inhibiting the accumulation of lymphocytes and bone marrow cells, as well as enhancing the antioxidant capacity of macrophages.
2.1.4 Collagen
Similar to HA, collagen is also one of the major components of the ECM and plays an important role in tissue repair and regeneration (Figure 2D) [16, 58]. As a natural biomaterial, collagen exhibits excellent biocompatibility, biodegradability, and the ability to promote cell adhesion, migration, and proliferation, which makes it widely used in hydrogel preparation [58, 95, 96]. Collagen-based hydrogels have low immunogenicity and good cell adhesion and growth-promoting properties, particularly demonstrating significant effectiveness in fields such as myocardial infarction repair and skin wound healing [97-100]. However, collagen hydrogels also have some limitations, including low physical strength, poor mechanical properties, and high synthesis costs [58, 96]. To overcome these drawbacks, researchers have attempted to obtain high-purity, low-immunogenic collagen through recombinant technology and composite it with other polymers (such as alginates and cellulose) to improve its mechanical properties and expand its application range [16, 101-105]. Hu et al. developed an injectable hydrogel combining curcumin and customized recombinant human type III collagen (rhCol III) for myocardial infarction repair [106]. Curcumin exhibits antioxidant and anti-inflammatory effects, while rhCol III promotes cell proliferation, migration, and angiogenesis. The experiments showed that this hydrogel significantly improved heart function, demonstrating its potential in myocardial infarction treatment, especially the important role of recombinant collagen in promoting myocardial repair.
2.1.5 Gelatin
Gelatin is a natural biopolymer derived from the hydrolysis of collagen, and is now widely used for hydrogel preparation due to its excellent biocompatibility, biodegradability, and ability to promote cell adhesion and proliferation (Figure 2E) [87, 107, 108]. Through modification with methacrylic anhydride (MA), methacrylated gelatin (GelMA) can be synthesized, which possesses photopolymerizable properties, allowing rapid gelation and imparting tunable mechanical properties [109, 110]. GelMA hydrogels, due to their similarity to the ECM, provide an ideal microenvironment for cell growth, promoting cell migration, differentiation, and tissue regeneration, and have been widely applied in cartilage repair, bone tissue engineering, and drug delivery [109, 111-114]. However, pure gelatin has poor mechanical properties and degrades rapidly under physiological conditions because its triple helix structure is disrupted during preparation [67, 107, 115]. These deficiencies can be improved through crosslinking modifications or by combining gelatin with other materials [107, 116-118]. Wang et al. developed a photo-crosslinked GelMA injectable hydrogel containing hydroxyapatite microspheres for bone tissue repair [116]. The composite hydrogel, at concentrations of 10% GelMA and 3% hydroxyapatite microspheres, demonstrated high structural stability, viscosity, and mechanical properties, while also exhibiting good antimicrobial activity, significantly reducing the risk of infection after implantation. Cell studies showed that the Ag-hydroxyapatite/GelMA hydrogel had good cell compatibility and low toxicity. The research suggests that this novel antimicrobial photothermal hydrogel is a promising material for minimally invasive bone repair.
2.1.6 Silk Fibroin
Silk fibroin is a natural biomaterial derived from insects such as silkworms (Bombyx mori), and it its widely used in hydrogel preparation due to its excellent biocompatibility, biodegradability, low immunogenicity, and superior mechanical properties (Figure 2F) [76, 119, 120]. The molecular structure of silk fibroin consists of light and heavy chains, which can self-assemble by forming β-sheet structures, imparting excellent mechanical properties and tunable physical characteristics [67, 76, 107, 119, 120] The advantages of silk fibroin-based hydrogels include their good hydrophilicity, biocompatibility, and outstanding mechanical strength, which make them highly promising for applications in tissue engineering, drug delivery, and the design of stimulus-responsive hydrogels [76, 119, 121, 122]. However, despite its good mechanical strength and self-healing ability, the inherent biological activity of silk fibroin is relatively weak, and thus limits its performance when used alone [123-125]. Therefore, it is typically combined with other highly bioactive materials to enhance its biological function [76]. For instance, combining silk fibroin with other natural polymers such as HA and chitosan can improve the bioactivity of silk fibroin hydrogels, thereby enhancing their application in cartilage repair, bone regeneration, and other areas [124, 126]. Additionally, silk fibroin-based hydrogels can accelerate the gelation process through external stimuli (such as pH, ultrasound, or temperature), giving them promising applications in smart drug delivery systems [8, 76].
2.1.7 Other Natural Materials
In addition to the mentioned materials above, many other natural materials, such as agarose, cellulose, chondroitin sulfate, peptides, ECM, and DNA, have also emerged as potential candidates for hydrogel preparation in recent years [127, 128]. For example, Zareei et al. developed an ultrasound platform based on agarose hydrogel for real-time monitoring of ECM stiffness [129]. This platform demonstrated the ability to detect stiffness in the range of 4.3 to 308 kPa by adjusting the polymerization density of agarose. Chen et al. prepared an injectable hydrogel using modified cellulose (CMC-NH2 and CMC-CHO), combined with pH-responsive micelles, to achieve sustained and stimulus-driven drug release, providing an efficient carrier for drug delivery [130]. Yang et al. designed a bilayer scaffold based on chondroitin sulfate (CS) hydrogel and porous pure zinc (Zn), which not only promoted cartilage regeneration but also provided bone support, achieving synchronized regeneration of osteochondral tissue and excellent host integration in a pig model [131]. Notably, DNA hydrogels, which self-assemble according to the Watson-Crick base pairing rules, have gained attention due to their outstanding advantages in precisely tuning molecular structure and function, high specificity in molecular recognition, unique genetic functions, and convenient programmability. As a result, DNA hydrogels have played an important role in various biomedical applications [132-136].
2.2 Synthetic Materials
2.2.1 Polyethylene Glycol (PEG)
PEG is a synthetic hydrophilic polymer widely used in hydrogel preparation due to its biocompatibility, low toxicity, and non-immunogenicity (Figure 2G) [137, 138]. By modifying its terminal hydroxyl groups, PEG can be functionalized with various bioactive groups to enhance hydrogel functionality [67]. PEG hydrogels exhibit excellent injectability and tunable mechanical properties, which makes them promising as drug carriers or tissue engineering scaffolds for applications in bone and cartilage repair, cancer therapy, gene therapy, and nerve regeneration [65, 139-144]. However, due to its inherent bioinertness [145], PEG hydrogels require combination with other materials (e.g., growth factors or natural polymers) to provide an ideal microenvironment for cell adhesion and tissue formation [146-149]. This combination strategy enhances cell adhesion while maintaining the excellent mechanical properties and controllability of PEG hydrogels, demonstrating significant potential for biomedical applications [8]. Park et al. developed a biodegradable elastic hydrogel scaffold based on hydrophilic PEG and hydrophobic poly(caprolactone) (PCL) for rabbit chondrocyte delivery and the formation of neocartilage [150]. Using a salt-leaching method to fabricate porous scaffolds, they showed that different PEG–PCL ratios significantly influenced the physicochemical properties of the scaffolds and cell interactions. The hydrophilicity of PEG promoted cell growth within the scaffold, while the combination with PCL allowed for modulation of mechanical properties. Experiments identified a PEG–PCL ratio of 14:6 as optimal, significantly enhancing the expression of cartilage-specific genes such as type II collagen and SOX9, accelerating cartilage tissue regeneration. This highlights the critical role of PEG in cartilage tissue engineering and its importance in optimizing scaffold performance.
2.2.2 Polyvinyl Alcohol (PVA)
PVA is a water-soluble long-chain polymer prepared by polymerizing and hydrolyzing vinyl acetate. It exhibits excellent tissue compatibility, non-toxicity, and low irritability, making it widely used in hydrogel preparation (Figure 2H) [67, 151, 152]. PVA hydrogels, due to their high water content and good mechanical properties, have found significant applications in drug delivery, wound dressings, and the treatment of intervertebral disc degeneration [153-155]. However, PVA hydrogels inherently suffer from insufficient mechanical performance, including low elongation at break and poor fatigue resistance [67, 154, 156]. Fortunately, these shortcomings can be significantly improved by emerging techniques such as dual physical and chemical crosslinking, which enables them to meet the high mechanical demands of applications such as cartilage and intervertebral disc repair [157, 158]. Additionally, incorporating other materials, such as sodium alginate or nanoparticles, into PVA hydrogels is a common strategy to enhance their strength [159, 160]. Jiang et al. fabricated PVA/sodium alginate composite hydrogels using a freeze/thaw method and further improved their strength and conductivity by immersing them in a saturated sodium chloride solution [159]. The study revealed that immersion time influenced the mechanical properties and conductivity of the hydrogel. Under optimal conditions, the hydrogel exhibited a tensile strength of 1.32 MPa, an elongation at break of 400%, and a conductivity of 3.62 S/m, along with excellent antifouling properties. This nontoxic fabrication method provides a novel approach for developing high-strength, conductive, and biocompatible hydrogel electrolytes, suitable for applications in bioelectrodes and artificial tissue engineering.
2.2.3 Poly Lactic-Co-Glycolic Acid (PLGA)
PLGA is also a synthetic biomaterial widely used in hydrogel preparation due to its tunable degradation rate and excellent biocompatibility (Figure 2I) [65, 161]. The mechanical properties and degradation characteristics of PLGA can be adjusted by altering the ratio of lactic acid to glycolic acid, which allows it to meet the specific demands of various tissue engineering and drug delivery applications [67, 93]. For example, PLGA hydrogels can load bioactive substances such as growth factors and proteins, which effectively promotes cartilage repair and cell proliferation [162]. Although PLGA exhibits good mechanical performance and biodegradability, its strong hydrophobicity and poor hydrophilicity often necessitate combination with other hydrophilic materials, such as HA, to enhance its bioactivity and cellular affinity [163, 164]. Furthermore, the hydrogel properties of PLGA enable controlled release of drugs and genes, which highlights its significant potential in drug delivery systems. Liang et al. investigated the therapeutic effects of PLGA microspheres seeded with adipose-derived stem cells (ADSCs) on degenerated intervertebral discs in rats [162]. X-ray, magnetic resonance imaging (MRI), histological, and gene expression analyses revealed that after 24 weeks of treatment, both the PM (PLGA microsphere treatment group) and PMA (ADSCs-PLGA microsphere treatment group) groups exhibited significant restoration of disc height and MRI signal intensity. Notably, the PMA group demonstrated superior repair outcomes, underscoring the potential of ADSC-seeded PLGA microspheres in the treatment of intervertebral disc degeneration.
2.2.4 Poly(N-Isopropylacrylamide) (PNIPAAm)
PNIPAAm is a synthetic polymer typically synthesized through free radical polymerization of N-isopropylacrylamide (NIPAAm) monomers with an initiator such as ammonium persulfate in an appropriate solvent (Figure 2J) [156, 165]. Additionally, the properties of PNIPAAm hydrogels, such as hydration, mechanical strength, and temperature responsiveness, can be tailored by adjusting the degree of polymerization, crosslinking, and other reaction conditions to meet various biomedical application requirements [166, 167]. Due to its unique temperature-responsive behavior, PNIPAAm has become the most prominent example of thermoresponsive polymers, capable of undergoing a liquid-to-solid phase transition at 32°C [165, 168, 169]. As a result, PNIPAAm is widely used for the preparation of temperature-responsive hydrogels, and plays a crucial role in applications such as cell encapsulation, drug delivery, and bone tissue engineering [168, 170, 171]. Chen et al. developed a novel temperature-responsive hydrogel by combining sodium alginate, N-isopropylacrylamide (NIPAAm), and calcium chloride to create a calcium alginate/poly(N-isopropylacrylamide) (CAPH) hydrogel [172]. Due to the temperature-responsive properties of PNIPAAm, this hydrogel demonstrated rapid drug release (e.g., mupirocin) at 34°C, effectively preventing infections and promoting wound contraction. In a mouse model of second-degree deep burn wounds, CAPH exhibited faster healing, enhanced collagen restoration, well-aligned collagen bundles, and significantly reduced scarring.
2.2.5 Other Synthetic Materials
In addition to the aforementioned synthetic materials, other synthetic polymers such as PCL, poly(urethane) (PU), poly(alanine) (PA), poly(amide) (PAM), poly(oxyethylene) (PEO), and poly(propylene oxide) (PPO) are also commonly used in hydrogel preparation. PCL, with its ease of fabrication, biocompatibility, flexibility, and extended biodegradation period, is currently a popular candidate for tissue engineering scaffold materials [173-175]. In addition, due to the chemical diversity of isocyanates and polyols, PU hydrogels, synthesized from the reaction of isocyanates with polyols, can be readily tuned in mechanical properties by modifying the chemical structures of these reactants [176, 177]. However, since most synthetic materials share similar characteristics, we will not elaborate further on each. Although synthetic materials are often criticized for their limited biodegradability and lack of bioactivity, their remarkable controllability and customizable physicochemical properties make them excellent complements to natural materials. They can enhance the mechanical strength, gelation rate, and adjustable biodegradation time of hydrogels. Recent perspectives suggest that combining synthetic materials with natural materials can effectively improve the overall performance of hydrogels [178, 179]. For instance, such combinations can enhance the mechanical properties of 3D networks, which provides a promising strategy for fabricating HMs with superior properties [17, 180-182].
3 Preparation Methods of Hydrogel Microspheres
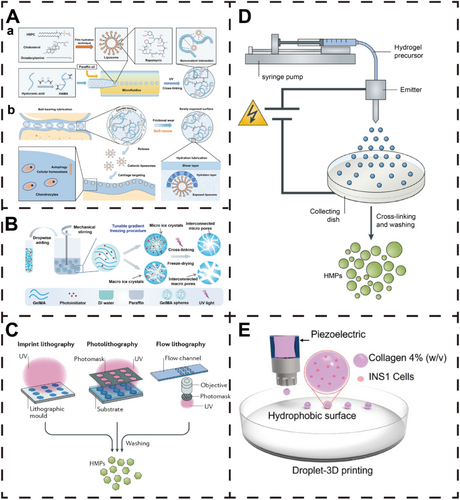
There are various methods for preparing HMs, with microfluidic technique, emulsification technique, lithography technique, and electrospray technique being the four most common approaches (Figure 3). Microfluidic technique enables the fabrication of uniform and precisely shaped microspheres by accurately controlling droplet formation within microchannels, making them suitable for high-precision applications. However, the complexity of the equipment and high costs limit its use to small-scale production. Emulsification technique involves dispersing the hydrogel solution into an oil phase to form emulsion droplets, which are subsequently crosslinked and solidified. This method is relatively simple and suitable for large-scale production but yields microspheres with lower uniformity and stability, and potential residual oil contamination. Lithography technique leverages photolithographic techniques to generate precise templates for microsphere production. It is particularly advantageous for creating complex microstructures or microspheres with specific shapes, although the process is labor-intensive and the cost of template fabrication is high. Electrospray technique utilizes an electric field to atomize solutions into microdroplets, which allows effective control over microsphere size and shape. It is compatible with various materials but requires strict control over solution viscosity and conductivity, and the equipment is complex. In brief, microfluidic technique and emulsification technique are advantageous for producing uniform microspheres and large-scale applications, respectively. Lithography technique excels in the design of complex microstructures, while electrospray technique balances uniformity with material versatility. Different strategies suit different application scenarios; thus, researchers must carefully consider material properties, microsphere size requirements, production costs, and application goals when selecting a preparation method.
3.1 Microfluidic Technique
Microfluidic technique is a method for generating microdroplets by precisely controlling fluid flow, which is already widely used in the preparation of HMs (Figure 3A) [8, 185]. The basic principle involves merging the aqueous phase (containing the hydrogel precursor solution) and the oil phase (typically oil with surfactants) within the microchannels of a microfluidic chip. At the junction of the microchannels, microdroplets are formed in the oil phase due to shear forces and hydrophobic interactions [186]. In this process, surfactants play a crucial role in stabilizing droplet formation and preventing droplet coalescence, resulting in uniform microspheres.
HMs prepared via microfluidic technique generally exhibit excellent dispersity, uniform size, and a wide size-adjustment range [28, 186]. The unique advantage of this approach lies in its ability to achieve precise control at the microscale, which produces highly uniform microspheres. Additionally, the shape and structure of the microspheres can be adjusted by designing different chips and controlling operational parameters [8]. The size of microspheres can be easily modulated by altering various parameters, primarily including the flow rate ratio, oil/water phase ratio, and the dimensions and geometry of the microchannels [25, 187-190]. Due to its ability to precisely control droplet formation, microspheres fabricated via microfluidics demonstrate high consistency and reproducibility, which makes this technique suitable for applications with strict requirements, such as cell encapsulation and drug delivery systems [191-194]. Moreover, microfluidic techniques are suitable for producing complex functional microspheres, such as multilayer structures or composite material microspheres, meeting diverse functional requirements [195-197].
However, microfluidic technique also has some limitations. First, the equipment is expensive, and the operation is complex, requiring technical support, which restricts its use in large-scale production. Second, while microfluidic techniques produce high-precision microspheres, the yield is relatively low, making it more suitable for small-scale or high-value-added product manufacturing rather than high-throughput, batch production. Additionally, the design and maintenance of microfluidic chips involve significant costs and technical demands.
3.2 Emulsification Technique
Emulsification technique is a classic method for preparing HMs. Its basic principle involves dispersing a hydrogel solution as the aqueous phase into a continuous oil phase, where shear forces generated by stirring or ultrasonication break the aqueous phase into microdroplets. These droplets are then solidified into HMs through chemical crosslinking (e.g., heating or adding a crosslinker) or physical gelation (e.g., cooling or solvent evaporation) (Figure 3B) [8, 183, 198, 199]. To prevent droplet coalescence, surfactants are typically added to the oil phase to stabilize the emulsion system, thereby enabling the formation of uniform microspheres.
The emulsification technique offers several distinct advantages, including simplicity of operation, low equipment requirements, and suitability for large-scale microsphere production [27]. Additionally, this method is compatible with a wide range of hydrogel materials, such as alginate and gelatin, and performs particularly well with natural materials [200-202]. It also provides flexibility in controlling microsphere size by adjusting parameters such as stirring speed, oil-to-water ratio, and surfactant concentration [8].
However, the emulsification technique has limitations. First, it provides less control over the uniformity of the resulting microspheres, often producing microspheres with a broad size distribution, which may not meet the requirements of high-precision applications. Second, the use of oil phases may lead to residual organic solvents, potentially affecting the biocompatibility of the microspheres, particularly for biomedical applications where additional purification may be necessary. Furthermore, the emulsification and solidification processes can cause the inactivation or partial loss of active substances, such as drugs or proteins, limiting the application of this technique as a drug delivery carrier.
3.3 Lithography Technique
Lithography technique is a precise method for fabricating HMs using photolithography to define their shape and size with high accuracy (Figure 3C) [27, 203]. The process begins by creating a template with specific patterns on a silicon wafer or polymer substrate using photolithographic techniques. The hydrogel precursor solution is then introduced into the microcavities of the template, where ultraviolet (UV) irradiation or other chemical crosslinking methods are applied to solidify the hydrogel into microspheres within the cavities. The microspheres are subsequently retrieved from the template via mechanical demolding or solvent washing.
The lithography technique offers distinct advantages in terms of precision and flexibility. It enables the production of HMs with highly uniform shapes and sizes, making it ideal for designing microspheres with special shapes or complex functionalities [204, 205]. Additionally, the method provides excellent control over microsphere size and shape, allowing customization of geometric features by modifying the template pattern. This makes it particularly advantageous for applications requiring precise structures, such as biosensing, drug delivery, and tissue engineering [110, 206-208].
However, the lithography technique also has limitations. First, the cost of template fabrication is high, the steps are complex, and the production cycle is lengthy, making it unsuitable for large-scale industrial production. Second, the reliance on template cavity dimensions limits the yield, restricting the method to producing small quantities of high-value functional microspheres. Additionally, in certain applications, templates may require chemical treatment to enhance demolding efficiency, which can increase operational complexity and pose potential contamination risks.
3.4 Electrospray Technique
Electrospray technique is a method that utilizes an electric field to atomize liquid into microdroplets, widely used for preparing uniform HMs (Figure 3D) [209, 210]. The principle involves injecting a hydrogel precursor solution through a high-voltage syringe nozzle, where the applied electric field forms a conical liquid jet [210, 211]. The jet breaks into microdroplets under electrostatic forces, and the droplets are subsequently solidified (via crosslinkers or temperature modulation) to form HMs [209, 210]. The crosslinked microspheres are typically stabilized in a liquid collection medium, resulting in uniform hydrogel particles.
The unique advantage of the electrospray technique lies in its ability to produce microspheres with uniform and controllable particle sizes. By adjusting parameters such as voltage, solution flow rate, nozzle diameter, and collection distance, the size and morphology of the microspheres can be precisely controlled [210, 211]. In addition, the method is compatible with various hydrogel materials and is particularly suited for incorporating active substances such as live cells, bioactive molecules, and therapeutic agents to create functionalized microspheres [8, 212].
However, the electrospray technique also has limitations. Its primary drawback is the low production yield, making it unsuitable for large-scale manufacturing. The method imposes strict requirements on the physical properties of the solution, such as viscosity and conductivity, which limits the range of applicable materials. Furthermore, the high-voltage operation may damage sensitive bioactive molecules, such as proteins and cells, restricting its applications in certain biomedical fields.
3.5 3D Printing Technique
3D printing technology has emerged as a revolutionary approach in modern manufacturing, which enables the direct construction of 3D entities from digital designs. Utilizing computer-aided design (CAD) models, 3D printing facilitates the fabrication of highly accurate and personalized HMs (Figure 3E) [184].
Currently, 3D printing exhibits numerous advantages in the preparation of HMs. First, this technology allows for the precise control of the size and structure of HMs based on optical and fluid channel parameters, along with providing a uniform pore distribution, which meets the needs for highly customized therapeutic applications [213]. Second, the rapid crosslinking immediately after printing ensures that the HMs produced have a uniform texture, which allows drugs loaded within the 3D spherical space to be released in all directions, and caters to various drug release and cell culture requirements [214]. Additionally, 3D printing supports the mixing of multiple materials, and thus enables the integration of multiple functionalities such as cell/drug-loading capacity and bioactive molecules within a single structure, thereby enhancing its applications in biomedicine [215, 216]. This technology is extensively used for preparing HMs that possess high bioactivity and satisfactory mechanical properties, suitable for applications including drug delivery, wound healing, and cell culture [217].
However, there are still several challenges and technical issues associated with 3D printing of HMs fabrication. The cost of high-resolution 3D printers and specialized bio-inks is relatively high, which limits their application in broader fields. Furthermore, the stability and reproducibility of 3D-printed biomaterials remain critical issues in ongoing research, particularly in ensuring the mechanical integrity and functionality of the printed items while maintaining bioactivity. Moreover, while 3D printing offers precise structural control, optimizing the biocompatibility and long-term stability of materials still requires further research.
4 Common Stimuli-Responsive Strategies
4.1 pH Responsiveness
The pH plays a crucial role in the functionality of biological systems, affecting various aspects from enzyme activity and cellular processes to the overall homeostasis of the organism [218]. Different tissues in the body have varying pH values, and the tissue regions affected by disease or injury are often associated with changes in pH [219, 220]. Therefore, pH-responsive HMs can control characteristics such as targeting and controlled release in response to changes in tissue pH [221]. Generally, pH-responsive polymers are those that contain basic or ionizable acidic residues, with their ionization dependent on the pH of the solution. These polymers belong to a class of stimuli-responsive polymers that are specifically triggered by environmental pH (by accepting or releasing protons), resulting in changes in their physicochemical properties, such as solubility, chain conformation, surface activity, and configuration [222-224]. The pH responsiveness of HMs is typically achieved through ionizable functional groups present within the polymer network. Depending on the type of group, pH-responsive HMs can be classified into anionic, cationic, and covalent bond-based pH-responsive hydrogels [23, 225]. Common anionic groups include carboxyl (–COOH), sulfonic (–SO3H), phosphate (–PO4H2), and phosphonic (–PO3H2) groups, while common cationic groups include amino (–NH2), quaternary ammonium (–NR3+), and imidazole groups (e.g., histidine derivatives). Well-designed pH-responsive HMs have broad applications in various biomedical fields, such as the controlled delivery and release of drugs (therapeutic small molecules, RNA, DNA, proteins, etc.) at specific pH values [226-228].
When designing pH-responsive HMs, it is essential to consider the mechanism of action of the responsive groups to suit different application scenarios. These groups function through different mechanisms of pH responsiveness. For example, carboxylic acid groups are weak acids that can donate protons to the surrounding environment. When the pH is above their acid dissociation constant (pKa), typically around 4–5, they deprotonate to form carboxylate anions (–COO−). When these groups ionize, they increase the negative charge density of the hydrogel, which leads to electrostatic repulsion within the polymer network and causing the hydrogel to expand. Polymers such as polyacrylic acid (PAA) and poly(methyl methacrylate) (PMAA), which contain carboxylic acid groups, are widely used in pH-responsive HMs [229, 230]. For example, Zhang et al. used photoinduced crosslinking to prepare pH-responsive hydrogels made of acrylic acid and polyethylene glycol diacrylate, which can release drugs or bioactive substances In Situ in response to tissue pH changes [231]. In addition, sulfonic acid groups, which have a stronger acidity than carboxylic acids, typically have pKa values around 1-2. These groups can deprotonate in various pH environments to form sulfonate anions (–SO3−), causing the hydrogel to expand. Phosphate and phosphonic groups can gradually ionize, with each ionizable hydrogen having a different pKa value, leading to the formation of various anionic species (e.g., –PO4H−, –PO42−). Therefore, the degree of ionization of these hydrogels depends on the pH and the specific structure of the phosphate or phosphonic groups [232]. In addition to acidic groups, hydrogels with basic groups, such as amino (–NH2) groups, will protonate under low pH (acidic conditions), forming –NH3+ groups [233]. Similar to anionic hydrogels, when water enters the hydrogel to balance charges, the ionization leads to increased osmotic pressure and expansion. Amino groups, being basic, accept protons in acidic environments (with pH values below their pKa, typically around 8–10) and carry a positive charge (–NH3+). On the other hand, quaternary ammonium groups remain charged regardless of pH and do not deprotonate, meaning they retain a positive charge in all pH environments [234-236]. In histidine residues, the imidazole group has a pKa of around 6–7, enabling it to acquire a proton and carry a positive charge in slightly acidic conditions, such as in the pH of inflamed or tumor tissues. For instance, Hu et al. developed a smart aminoglycoside hydrogel by crosslinking chitosan with tripolyphosphate or citric acid. This hydrogel has tunable gel degradation, on-demand drug release, and high antimicrobial activity. The swelling ratio of the hydrogel in gastric fluid (pH 1–4) was higher than in intestinal fluid (pH 5–7), and the drug release rate was significantly faster at gastric pH than at intestinal pH [237]. In addition to the commonly used ionic groups mentioned above, metal cations and noncovalent intermolecular interactions can also provide pH responsiveness. Metal cations such as Fe3+, Al3+, and Ca2+ play a crucial role in forming coordination bonds with negatively charged ligands, and these coordination bonds are highly sensitive to changes in pH. Alterations in these bonds can lead to changes in the hydrogel's structure and properties. For example, Fe³⁺ can form coordination complexes with imine and carboxyl groups. Under weak acidic conditions, protonation of the imine bonds and proton competition can break these coordination bonds. A typical example is protocatechuic aldehyde (PA), which forms a stable ternary complex with Fe³⁺ in a solution with pH > 8.5, exhibiting reversible formation and breaking of the complex based on pH changes [238, 239].
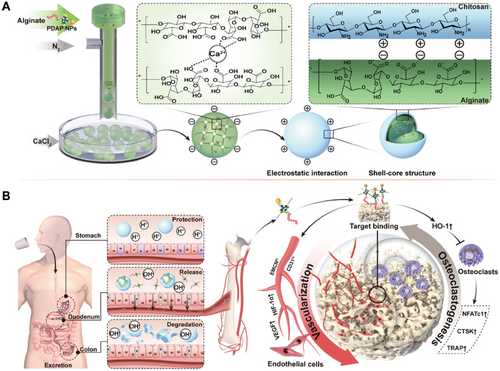
By selecting different types of pH-responsive strategies, the properties of hydrogel precursor solutions can be tailored to meet the specific needs of the final HMs. pH-responsive HMs can enhance drug release efficiency [195]. For example, Malihe et al. prepared pH-sensitive Fe3O4/GQDs@G microspheres by emulsification, which, compared to pure gelatin microspheres, exhibited higher drug loading and release at pH 5.0 [240]. Li et al. developed a pH-responsive core-shell structured micro/nano-HM (PDAP@Alg/Cs) loaded with polyhedral oligomeric silsesquioxane (POSS) for oral administration and bone tissue targeting [195]. This system enables pH-controlled drug release, promotes angiogenesis, and inhibits bone resorption, effectively slowing down bone loss (Figure 4). It provides a novel strategy for the treatment of postmenopausal osteoporosis. The pH of different tissues in the body varies significantly. For instance, the pH of various sections of the gastrointestinal tract differs substantially, and the extracellular tumor microenvironment is more acidic than healthy tissues (pH = 6.5–7.2), with pH differences also present between organelles within cells (e.g., lysosomes have an acidic environment with a pH of 5–6.5) [241-243]. Therefore, pH-responsive strategies are equally applicable to complex tissue and cellular environments. For instance, using gelatin HMs as the base, Javanbakht et al. developed Cu-MOF/IBU@GM microspheres that enable controlled drug release in the gastrointestinal tract. Using ibuprofen as a model drug, 72% of the drug was released in a controlled manner from Cu-MOF/IBU@GM microspheres at pH 1.2 (2 h), pH 6.8 (2 h), and pH 7.4 (4 h) in in vitro simulated gastrointestinal media [244]. Amide bonds (-CONH) are pH-sensitive chemical bonds formed when the hydroxyl or carboxyl group of a carboxylic acid molecule reacts with an amino or carbonylamine group. Hydrogels containing amines are easily modified to form amide bonds. Amide bonds are acid-unstable and can hydrolyze in the acidic microenvironment of tumors to produce positively charged primary amines, leading to a charge reversal. Amphiphilic prodrugs containing drugs and exhibiting charge reversal have precise chemical structures that can be tuned for targeting tumor cell membranes, improving drug bioavailability and reducing side effects [245, 246].
4.2 ROS Responsiveness
ROS are highly reactive oxygen-containing molecules that play dual roles in biological systems, both beneficial and harmful [247, 248]. ROS include free radicals, such as superoxide and hydroxyl radicals, as well as non-radical species like hydrogen peroxide and singlet oxygen. ROS are involved in regulating cellular signaling pathways that control processes such as cell growth, apoptosis (programmed cell death), and immune responses [249]. At low to moderate levels, ROS act as signaling molecules that help maintain cellular homeostasis, while immune cells such as macrophages and neutrophils generate ROS during immune responses to destroy invading pathogens [250]. However, an imbalance in ROS levels can trigger pathological processes. For example, when ROS levels exceed the antioxidant defense capacity of cells, oxidative stress occurs, which then leads to damage to proteins, lipids, and DNA. This damage is associated with various diseases, including cancer, cardiovascular diseases, neurodegenerative disorders (such as Alzheimer's and Parkinson's diseases), and aging [105, 251-253].
ROS are primarily generated from endogenous and exogenous sources. Endogenous ROS are mainly produced by mitochondria within cells. During cellular respiration, a small portion of electrons leak from the electron transport chain and react with oxygen to form superoxide. Other endogenous sources of ROS include peroxisomes, cytochrome P450 enzymes, and NADPH oxidase [254]. In addition, external stimuli such as UV radiation, pollution, smoking, and certain chemicals or drugs can induce ROS production [255, 256]. Therefore, developing and utilizing ROS-responsive smart HMs offers the potential to harness naturally occurring oxidative stress signals for targeted and controlled therapeutic, diagnostic, and environmental applications. This approach can help address challenging medical and environmental issues. The construction of ROS-responsive HMs involves several key steps, including selecting suitable materials, designing crosslinking mechanisms, incorporating ROS-responsive elements, and ensuring controllable release or degradation upon ROS response.
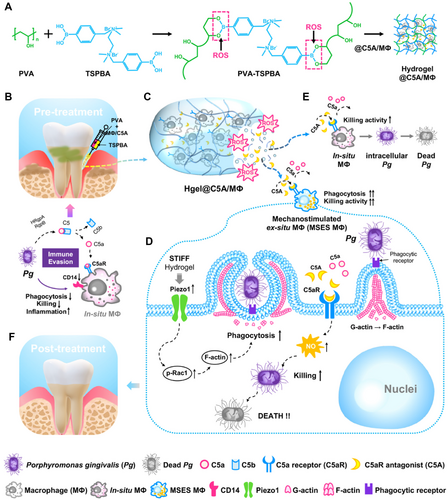
Common ROS-responsive elements include thioketones, boronic esters, and selenium-containing compounds, which can undergo cleavage or chemical transformation in the presence of ROS, leading to hydrogel degradation or structural changes [257, 258]. These groups function through different mechanisms of ROS responsiveness. For example, thioketone linkages contain sulfur atoms bonded to carbon atoms, forming a –C(S)–C– group, which can be cleaved by ROS, particularly hydroxyl radicals and hydrogen peroxide. Oxidative cleavage of the C–S bond results in polymer chain breakage, causing the hydrogel network to degrade [259]. Materials such as polyethylene glycol diacrylate (PEGDA) and PLGA are commonly used as carriers or scaffold materials modified with thioketone groups for HMs construction. Boronic esters have a general structure of –B(OR)2, where R is an organic group. They react with ROS to form phenols and boric acid. This reaction is highly specific to hydrogen peroxide, which makes boronic esters suitable for environments with elevated hydrogen peroxide levels. Phenylboronic acid (PBA) derivatives, PVA, and PNIPAAm can be used to construct HMs based on the responsive principles of boronic esters [260]. For instance, Gan et al. developed an ROS-sensitive hydrogel based on PBA, capable of releasing active bone marrow–derived macrophages and the C5a receptor antagonist (C5A) into the gingival sulcus [261]. By modulating the stiffness of the hydrogel, they significantly enhanced the phagocytic activity of macrophages and prevented adverse receptor activation caused by Porphyromonas gingivalis (Pg) through C5A. Meanwhile, the hydrogel was able to reduce ROS levels in the periodontal microenvironment, alleviating periodontal inflammation and mitigating bone loss (Figure 5). In addition, selenium-containing linkages typically involve selenides (–Se–) or diselenides (–Se–Se–) as part of the polymer backbone. These selenium linkages are oxidized by ROS to form selenium oxides or selenones, which are unstable and lead to polymer backbone cleavage. Thiomethyl groups with a general structure of –C–S–C–, where sulfur is bonded to two carbon atoms, can be oxidized by ROS to form sulfoxides (–SO−) and sulfones (–SO2−) [262]. For instance, polyethylene glycol dimethacrylate (PEGDMA), a HM precursor material, can be crosslinked with thiomethyl groups, while methacrylate-modified gelatin (GelMA) is often used as a matrix material functionalized with thiomethyl groups. PBA, with the structure Ph-B(OH)2 (where Ph represents a phenyl group), reacts with hydrogen peroxide to form phenols and boric acid. This reaction is reversible and highly selective, which enables controlled responses to ROS. Disulfide bonds (–S–S–) consist of two sulfur atoms bonded together, connecting two parts of a molecule or polymer. These disulfide bonds can be cleaved by ROS, causing the polymer network to break, which then leads to hydrogel degradation or drug release. Tetrasulfides contain four sulfur atoms in a chain, with a general structure of –S–S–S–S–. Similar to disulfides, tetrasulfides can also be cleaved by ROS, but they provide multiple interaction sites, making them highly sensitive to oxidative environments. The cleavage of these bonds causes significant structural changes in the hydrogel matrix.
4.3 Enzyme Responsiveness
Enzymes play a critical role in nearly all biological activities, including catalyzing biochemical reactions, participating in metabolism, DNA replication and repair processes, and protein synthesis and degradation. They enable organisms to efficiently perform complex reactions, maintain internal balance, respond to environmental changes, and ensure the proper functioning of cells and tissues. The development of enzyme-responsive HMs allows for responsive changes in the presence of enzymes associated with certain physiological or pathological conditions [263-265]. Enzyme-responsive HMs are composed of polymers that can be degraded or crosslinked by specific enzymes, which then leads to changes in the gel structure, such as expansion, degradation, or release of encapsulated drugs. This responsiveness is typically achieved by incorporating enzyme-sensitive peptide sequences or specific chemical groups within the polymer network [266-268].
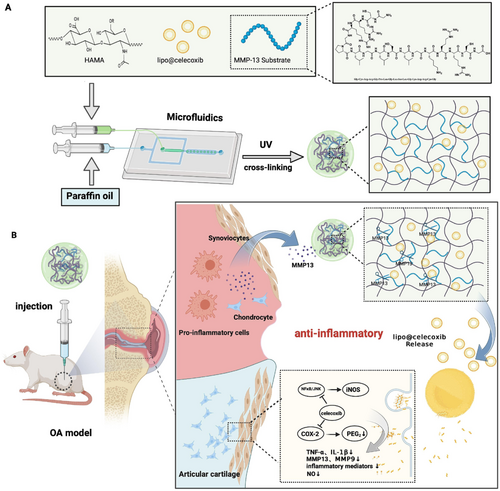
Matrix metalloproteinases (MMPs) are a class of zinc-dependent endopeptidases that play a crucial role in the remodeling of the ECM and are involved in various physiological and pathological processes. MMPs can degrade all components of the ECM, including collagen, elastin, gelatin, and proteoglycans. Under normal conditions, their activity is tightly regulated, but in disease states, they may become dysregulated, leading to tissue damage and other pathological effects. For example, MMPs are typically upregulated in tumors, where they play a key role in cancer progression by degrading the ECM, enabling cancer cells to invade surrounding tissues and metastasize. Chronic inflammatory diseases, such as rheumatoid arthritis and osteoarthritis, are associated with elevated MMP levels, and excessive MMPs contribute to the degeneration of cartilage and joint tissues. One common approach to achieve MMP responsiveness in hydrogel polymer networks is the incorporation of MMP-cleavable peptide sequences. These sequences remain stable under normal physiological conditions but degrade when MMP levels are elevated. MMPs typically recognize and cleave specific sequences, which often include hydrophobic amino acids at the P1′ position and proline residues at the P3 position. For example, Gly-Pro-Leu-Gly-Leu-Trp-Ala-d-Arg (GPLGLWA-d-Arg) is a peptide sequence recognized and cleaved by several MMPs, including MMP-2 and MMP-9. Another example is Pro-Gln-Gly-Ile-Ala-Gly-Gln (PQGIAGQ), a specific target sequence for MMP-3 (stromelysin-1). Li et al. developed an MMP-13 responsive micro-nanoscale HM system, which degrades through the action of the MMP-13 substrate peptide (MMP13sp), promoting the accelerated release of drug-loaded liposomes, improving the inflammatory microenvironment, and facilitating rapid treatment of osteoarthritis (Figure 6) [269]. Gao et al. prepared an injectable HM system (HAM-SA@HCQ) for hypoxic-inflammatory joints by using microfluidic devices and photochemical crosslinking techniques. This system, based on methacrylate-modified sulfonated azobenzene (SAC4A-MA), methacrylated hyaluronic acid (HA-MA), and disulfide-terminated MMP-13 sensitive peptides, encapsulated the anti-inflammatory drug hydroxychloroquine (HCQ) through host–guest interactions. The HMs exhibited strong drug loading capacity, significant ROS scavenging ability, and specific MMP-responsive drug release properties [270]. In addition to MMPs, many other proteases also serve as inputs for stimuli-responsive biomaterials, including hyaluronidase, cathepsins, phospholipases, thrombin, and azo-reductases. For example, an intelligent drug delivery system incorporates heparin via a thrombin-sensitive peptide linker into a PEG hydrogel. These PEG molecules are covalently linked by peptide units containing the sequence (D)Phe-Pip-Arg-Ser, which is highly selectively cleaved by thrombin after the arginine residue. This system adjusts anticoagulation through a self-regulating mechanism [271].
4.4 Micromolecule Responsiveness
The development of bioresponsive materials sensitive to small molecules such as glucose, adenosine triphosphate (ATP), and nucleic acids represents a significant advancement in the field of smart biomaterials and drug delivery systems. These materials can detect and respond to specific biological signals, which then enables targeted and controlled therapeutic interventions, thereby providing a solid foundation for the construction of small molecule-responsive HMs.
Glucose plays a crucial role in physiological processes and the development of various pathological conditions. First, glucose is the primary energy source for most cells in the body. It produces ATP through glycolysis, the citric acid cycle, and oxidative phosphorylation, providing energy for cellular activities. Second, it plays a key role in metabolic homeostasis. Under physiological conditions, insulin lowers blood glucose by promoting cellular uptake of glucose, while glucagon increases blood glucose by stimulating glycogenolysis in the liver. In pathological conditions such as diabetes, chronic hyperglycemia can lead to microvascular complications, including retinopathy, nephropathy, and neuropathy, as well as macrovascular complications such as cardiovascular diseases and stroke. Glucose-responsive HMs represent a promising approach for developing smart drug delivery systems, particularly for the treatment of diabetes and its complications [272]. The key to their development lies in selecting appropriate glucose-sensitive components for achieving responsiveness. One of the most commonly used glucose-responsive components in hydrogels is PBA. PBA can form reversible covalent bonds with diols (such as glucose). When glucose levels are high, glucose binds to PBA, causing the hydrogel to swell and release its encapsulated drug [273, 274]. In addition, glucose oxidase (GOx) and Concanavalin A (Con A) can also be used to construct glucose-responsive HMs. Theoretically, GOx catalyzes the oxidation of glucose to gluconic acid, lowering the local pH. This pH change, combined with pH-sensitive hydrogels, can trigger drug release for responsiveness [275]. For example, Anderson et al. prepared monodispersed HMs (256 ± 18 μm) by electrospraying, using a pH-responsive chitosan matrix, enzyme nanocapsules, and recombinant human insulin. Glucose-specific enzymes were covalently encapsulated in the nanocapsules. As glucose is converted to gluconic acid by the enzyme, the chitosan network undergoes protonation, causing the HMs to swell under hyperglycemic conditions and release insulin [276]. Con A is a glucose-binding lectin that can form crosslinks within hydrogels. When glucose binds to Con A, it disrupts these crosslinks, leading to hydrogel swelling and the release of its contents [277]. For example, Zhang et al. used an inverse emulsion crosslinking method to prepare glucose-responsive HMs using chitosan derivatives as Con A polymer ligands. The insulin release inside these microspheres was influenced by blood glucose levels and exhibited the desired pulsatile release behavior [278].
ATP is produced through cellular respiration, primarily in the mitochondria, via glycolysis, the citric acid cycle, and oxidative phosphorylation. It provides energy for various cellular processes, including muscle contraction, nerve impulse transmission, and chemical synthesis. In addition to serving as an energy currency, ATP also functions as a signaling molecule in various cellular pathways. For instance, it participates in purinergic signaling, binding to purinergic receptors on the cell surface, and influencing processes such as neurotransmission, muscle contraction, and inflammation. Under physiological conditions, the intracellular concentration of ATP (1–10 mM) is approximately 1000 times higher than the extracellular environment (< 5 μM), making ATP an attractive candidate for intracellular targeted delivery [279]. In pathological processes, ATP production can be altered. For example, many cancer cells exhibit metabolic changes known as the Warburg effect, where they become more reliant on glycolysis for ATP generation, even in the presence of oxygen. Meanwhile, due to mitochondrial dysfunction, ATP generation is impaired in neurodegenerative diseases such as Alzheimer's and Parkinson's disease, and the resulting energy deficiency exacerbates the progression of these diseases. Therefore, responsive HMs targeting ATP changes can be designed to treat these related conditions. The ATP-responsive mechanism is primarily based on ATP aptamers, which are short DNA or RNA sequences that specifically bind to ATP. ATP binding induces conformational changes in the aptamers, leading to drug release or other reactions [280-282]. For example, Gu et al. functionalized polymer nanocarriers with ATP-binding aptamer DNA motifs, which could selectively release the inserted doxorubicin upon conformational switching. This carrier demonstrated promising effects in chemotherapy to inhibit tumor growth [283].
Nucleic acids as triggers for stimuli-responsive biomaterials have not been fully explored, but they hold significant potential for the future of personalized medicine, diagnostics, and gene therapy [284, 285]. A common strategy for nucleic acid-sensitive materials is the use of DNA/RNA aptamers that bind to specific nucleic acid sequences, triggering conformational changes, which in turn release drugs, emit diagnostic signals, or enable targeted gene editing or silencing based on CRISPR systems [286-288]. For example, Chan et al. assembled a colloidal nanoparticle system using DNA as a molecular key. The system consists of a core nanoparticle surrounded by small satellites, where the conformation of the satellites can be altered by DNA in a displacement-based mechanism. The conformational changes affect the optical properties and biological interactions of the assembled nanoparticle system. Changes in the distance between fluorescence-modified particles alter photoluminescence signals, and by modifying the surface display of targeting ligands, cell targeting efficiency can be enhanced by 2.5 times. This system provides a new strategy for designing nucleic acid-responsive HMs [289].
4.5 Temperature Responsiveness
Temperature-responsive HMs are a type of smart material that undergo physical or chemical changes in response to temperature variations. These changes typically include swelling, contraction, or phase transitions, which can be utilized in drug delivery, tissue engineering, and other biomedical applications [126, 290-292]. The temperature-responsive mechanisms primarily include the lower critical solution temperature (LCST) and the upper critical solution temperature (UCST) [293]. LCST refers to the temperature below which a polymer is soluble in water and above which it becomes insoluble. Polymers exhibiting this behavior are hydrophilic at lower temperatures and become hydrophobic when the temperature exceeds the LCST [294]. Below the LCST, the HMs expand as the polymer chains hydrate and elongate. When the temperature rises above the LCST, the polymer chains undergo dehydration and collapse, causing the hydrogel to shrink or release its contents. PNIPAM is one of the most commonly used polymers in temperature-responsive hydrogels [295]. PNIPAM is a polymer of the N-isopropylacrylamide monomer, with each monomer containing a hydrophilic amide group and a hydrophobic isopropyl group. Its LCST is approximately 32°C. Below this temperature, hydrogen bonds between the amide group and water molecules dominate, leading to expansion and a hydrated state. Above this temperature, these hydrogen bonds are disrupted, causing the polymer chains to collapse into a hydrophobic state, which leads to hydrogel contraction and water expulsion [296]. UCST is the temperature above which the polymer is soluble in water, and below this temperature, the polymer is insoluble. Polymers exhibiting this behavior are hydrophobic at lower temperatures and become hydrophilic when the temperature exceeds the UCST. Below the UCST, the polymer is in a collapsed state, and as the temperature increases, the polymer hydrates and expands, absorbing water and dissolved substances [297, 298].
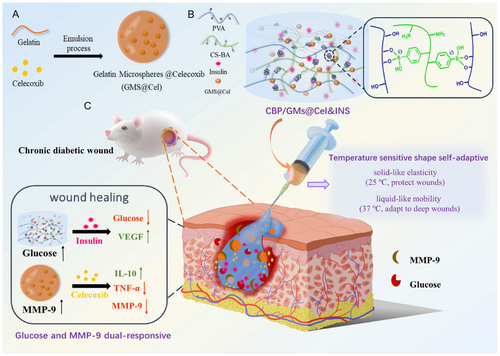
The LCST of temperature-responsive hydrogels can be adjusted through various methods. Adding more hydrophilic monomers, such as hydroxyethyl acrylamide (HEAm), acrylamide (AAm), and acrylic acid (AAc) to PNIPAM, can increase its LCST [299]. In contrast, adding hydrophobic monomers like t-butyl acrylamide (TBAM), N-t-butyl acrylamide (NT), or butyl acrylate (BA) can lower the LCST of PNIPAM and form stiffer gels [300, 301]. Moreover, introducing ionic groups (e.g., carboxyl or sulfonic acid groups) into the polymer chain generally increases the LCST [302]. Although PNIPAM is widely used due to its unique thermal-responsive properties, it has some drawbacks, including a lack of biodegradability, poor mechanical strength, and slow response time [165]. Fan et al. designed a glucose and MMP-9 dual-responsive hydrogel with temperature-sensitive shape-adaptive behavior for the treatment of chronic diabetic wounds. This hydrogel was constructed from phenylboronic acid-modified chitosan (CS-BA) and PVA through phenylboronic ester bonds, with celecoxib-loaded gelatin microspheres (GMs@Cel) and insulin uniformly dispersed in the CS-BA-PVA hydrogel (CBP). The mechanism of action is as follows: when the hydrogel is exposed to chronic diabetic wounds, its temperature-sensitive adaptability ensures that the hydrogel provides mobility and elasticity on demand to quickly conform to and protect the wound. Meanwhile, the glucose and MMP-9 dual-responsive system promotes the on-demand release of insulin and anti-inflammatory drugs (celecoxib) (Figure 7) [303].
4.6 Magnetic Field Responsiveness
Magnetic field-responsive HMs are a type of smart material capable of responding to external magnetic fields. These materials combine the unique properties of hydrogels with the magnetic responsiveness provided by embedded magnetic nanoparticles. Magnetic responsiveness enables fast, controllable, and remote manipulation of these HMs, which makes them suitable for applications in targeted drug delivery, hyperthermia, tissue engineering, and biosensing [304]. Magnetic-responsive HMs typically consist of a hydrogel matrix and magnetic nanoparticles, where the hydrogel matrix is often made from synthetic polymers such as PVA, PEG, or natural polymers like alginate and chitosan, which provide swelling, biocompatibility, and drug-loading capacity. Common magnetic materials include iron oxide nanoparticles (Fe3O4 and γ-Fe2O3), transition metal ferrites (CoFe2O4, MnFe2O4, etc.), and nickel nanoparticles (Ni) [305]. Among these magnetic nanoparticles, iron oxide nanoparticles, particularly magnetite (Fe3O4) and maghemite (γ-Fe2O3), exhibit superparamagnetism, meaning they are magnetized only in the presence of an external magnetic field and do not retain magnetization once the field is removed, while also possessing excellent biocompatibility. As a result, they are the most widely used magnetic materials in the fields of biomedicine and pharmaceuticals.
These magnetic-responsive materials primarily exert biological functions through mechanisms such as magnetic hyperthermia, magnetic targeting, magnetic driving, and magnetorheological effects. In specific applications, the type, size, distribution, and concentration of iron oxide nanoparticles significantly affect the response of HMs to magnetic fields [306, 307]. Magnetic hyperthermia refers to the heat generation of magnetic nanoparticles when exposed to an alternating magnetic field. The mechanism of heat generation occurs as the magnetic moment of the nanoparticles fluctuates and aligns with the alternating magnetic field, and the energy dissipated during these fluctuations is released as heat. The efficiency of heat generation by magnetic nanoparticles directly influences the effectiveness of magnetic hyperthermia, often measured by the Specific Absorption Rate (SAR), which quantifies the magnetic heat conversion capacity of a given mass or volume of nanoparticles over a set period, with units in W/g or W/cm3. A higher SAR value indicates stronger magnetic-induced heating capability. Ma et al. studied the SAR values of iron oxide nanoparticles with varying sizes (7.5–416 nm) under an applied alternating magnetic field (80 kHz, 32.5 kA/m). Their results showed a strong size-dependent SAR value. For ferromagnetic nanoparticles larger than 46 nm, the SAR value decreased with increasing particle size. The maximum SAR value of 75.6 W/g was observed for nanoparticles with a size of 46 nm [308]. Along with size, the morphology of magnetic nanoparticles is also a critical factor influencing their magnetic thermal performance. It directly affects the atomic arrangement on the particle surface, altering surface anisotropy and magnetic domain structure, which in turn alters the magnetic thermal performance. Noh et al. found that spherical magnetic nanoparticles exhibited higher magnetic surface anisotropy, but their magnetization was lower than that of cubic nanoparticles with the same magnetic moment. A simulation study on surface magnetic spin structures revealed that cubic nanoparticles exhibited less surface disorder (4%) compared to spherical nanoparticles (8%) because the curved morphology of spherical nanoparticles leads to a larger surface effect. Experimental results showed that Zn0.4Fe2.6O4 cubic nanoparticles had a higher saturation magnetization (MS = 165 emu/g) compared to spherical nanoparticles (MS = 145 emu/g) [309]. Magnetic hyperthermia can be applied for controlled drug release, as magnetic nanoparticles generate heat under the influence of high-frequency external magnetic fields due to Brownian and Néel relaxation effects. The increase in temperature of magnetic hydrogels accelerates the motion of drug molecules, promoting faster diffusion and release from the hydrogel network. Additionally, the temperature increase accelerates the degradation of the hydrogel network, allowing for faster drug release [310, 311]. Magnetic-responsive hydrogels or HMs with magnetic hyperthermic effects also hold great potential in tumor therapy. Within the temperature range of 42°C–46°C, tumor cells undergo increased susceptibility to radiation and chemotherapeutic agents due to protein and DNA denaturation, leading to cell death. Therefore, the temperature range of 42°C–46°C is widely used clinically as an effective heating dose for tumor treatment [312-314].
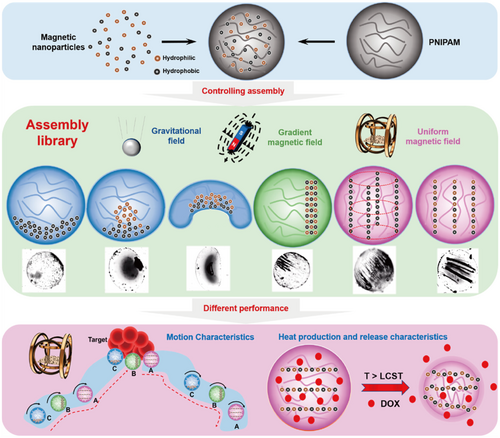
Magnetic-responsive HMs can serve as drug carriers, loading drugs into the hydrogel network structure and improving drug targeting to diseased tissues under the influence of an external magnetic field, thereby achieving precise, localized drug release and reducing systemic side effects [315, 316]. Additionally, the external magnetic field can act as a “switch” for drug release. In the absence of an external magnetic field, the drug release rate from the hydrogel network gradually slows down. However, when a magnetic field is applied, the drug release rate increases. This “switch” effect is primarily due to the rotation and vibration of magnetic nanoparticles under the influence of the magnetic field, which causes relaxation of the polymer chains, ultimately leading to expansion and loosening of the hydrogel network, thus enhancing drug diffusion. On the other hand, the orientation of the magnetic nanoparticle dipoles under the applied magnetic field induces mechanical vibrations and deformations in the hydrogel itself, creating localized stretching and compressive stresses that stimulate drug release [317, 318]. Under low-frequency longitudinal magnetic fields (< 100 Hz), magnetic nanoparticles can convert magnetic energy into mechanical energy through dipole-dipole interactions. The resulting mechanical force can deform the magnetic hydrogel and provide necessary stimuli for cells/tissues, thereby inducing specific biological effects. Magnetic driving effects can enable the hydrogel to deform, move, and change shape under magnetic field control. Wang et al. constructed magnetic nanorobotic swarms using temperature-sensitive HMs as carriers to ensure the distribution and stability of the swarm structure [319]. Three different assembly strategies (gravitational field, gradient magnetic field, and uniform magnetic field) were used to explore the performance of six different magnetic nanoparticle (MNP) assembly modes in motion execution, magnetothermal effects, and the release of DOX drugs (Figure 8). The study found that the symmetric chain assembly with the magnetic chain distributed on the outside exhibited the best performance, achieving a speed of up to 600 μm/s and a temperature rise rate of 1.5°C/min. This study provides an excellent solution to the problem of poor MNP cluster distribution stability and enriches the assembly control strategies for microrobots in medical, catalytic, and 3D printing applications. Furthermore, Cezar et al. reported a biphasic iron hydrogel containing a gradient of iron oxide, which can induce significant deformation and trigger drug release even in small-sized gels [320]. For example, Zhou et al. reported a magnetic chitosan hydrogel that can remotely switch the drug release from passive to pulsatile release under a low-frequency alternating magnetic field (intensity of 0.4 T, frequency of 2 Hz). Compared to passive release, the cumulative release and fraction release of the drug increased by 67.2% and 31.9%, respectively [321]. Pulsatile drug release can also be achieved through a reversed external magnetic field (EMF) switch mode. In short, in the absence of an external magnetic field, the drug molecules in the magnetic-responsive HMs are released through free diffusion. When a magnetic field is applied to the hydrogel, the magnetic nanoparticles aggregate, causing the hydrogel to contract, reduce pore size, and restrict drug release. However, once the magnetic field is removed, the molecular free diffusion in the hydrogel network resumes, returning to the previous expanded state and releasing the drug [322]. Lu et al. reported a simple one-pot synthesized magnetic β-cyclodextrin (β-CD)/cellulose HM that exhibits rapid swelling-dissolving properties under EMF. The drug release can be remotely controlled from passive release to gradual release. When the electromotive force is switched to the “ON” state, the swelling rate of the magnetic hydrogel decreases, while it increases and nearly restores to the original state when the electromotive force is switched to the “OFF” state [323]. In addition to the switching mode of the magnetic field, the direction of the magnetic field can also be used to regulate the drug release behavior. By manipulating the direction of a uniform magnetic field provided by two electronic magnets, magnetic nanoparticles can self-assemble either vertically or parallel to the direction of drug diffusion [318]. This artificially controllable magnetic targeting can effectively mimic the pulsatile release of hormones and specific peptides in the body, which is especially suitable for the treatment of metabolic diseases (such as diabetes) and cancer [324].
5 Current Biomedical Applications of Injectable Stimuli-Responsive Hydrogel Microspheres
At the molecular level, the fundamental mechanism of “stimuli-responsive HMs” lies in the dynamic alterations of their internal cross-linked networks in response to specific stimuli, such as temperature or pH. These alterations typically involve the breaking and reformation of intermolecular bonds, which result in the swelling or shrinking of the hydrogel matrix. Such structural transitions directly affect the physical state of the HMs—manifesting as dissolution, aggregation, or collapse—and subsequently trigger the release of encapsulated therapeutic agents, including drugs, cells, cytokines, or other bioactive substances. In this manner, the microspheres enable controlled or even “smart” release of therapeutics. This highly regulated release behavior is particularly advantageous in treatment strategies requiring precise dosage and targeted delivery, such as chemotherapy involving cytotoxic agents, where enhanced efficacy and minimized systemic toxicity are crucial. During disease progression, the local microenvironment of affected tissues often undergoes significant pathophysiological changes. For instance, the pH level within solid tumors is markedly lower than that of healthy tissues, while elevated levels of ROS are commonly observed in osteoarthritic cartilage. These abnormal physicochemical characteristics create favorable conditions for stimuli-responsive HMs to be activated at specific pathological sites, thereby improving the selectivity and efficacy of targeted therapies.
5.1 Drug Delivery and Controlled Release
One of the most common and effective strategies for stimuli-responsive HMs to achieve “smart” drug delivery is based on the physiological pH differences between different tissues or organs. For example, a team led by Li developed an organic–inorganic hybrid nanoparticle (PDAP NPs) based on a polyhedral oligomeric silsesquioxane (POSS) nanoplatform, loaded with deferoxamine (DFO) and a bone-targeting peptide Asp8 [195]. These nanoparticles were encapsulated in a pH-responsive core-shell structured micro/nano HM composed of sodium alginate/chitosan (Alg/Cs) (PDAP@Alg/Cs). The hydrogel remained stable in the gastric acidic environment, and upon reaching the intestine, it rapidly expanded and released the drug, achieving oral delivery, sustained release in the intestine, and bone tissue targeting. In vitro and In Vivo experiments demonstrated that this system promoted Type H angiogenesis by activating the HIF-1α/VEGF pathway, while upregulating HO-1 expression to inhibit osteoclast activity, effectively alleviating osteoporosis. The combined effect of pH-dependent release and targeting made it a potential clinical strategy for the treatment of osteoporosis. Similarly, Zheng et al. developed a pH-responsive HM based on the EDTA-Ca-Alginate (EDTA-Ca-Alg) system for the oral delivery of the probiotic Lactobacillus rhamnosus ATCC 53103 [325]. This system utilized the reversible chelation of Ca2+ by EDTA, maintaining the solution state under neutral conditions, while rapidly releasing Ca2+ in the gastric acidic environment (pH < 4.0), inducing the In Situ formation of calcium alginate gel. This enabled a smart delivery strategy for protection in the stomach and release in the intestine. The prepared HMs exhibited a dense and stable structure in acidic environments, effectively protecting the encapsulated probiotics from gastric acid degradation; while in the neutral intestinal environment, they quickly disintegrated and released the probiotics for efficient intestinal-targeted release.
Additionally, due to the pH differences in the local microenvironment of certain diseased tissues compared to normal physiological tissues, this pH disparity also paves the way for the “smart” controlled release of drugs. For example, Ma and colleagues developed a pH-responsive HM based on aldehyde-modified hyaluronic acid methyl acrylamide (AHAMA) and stabilized bovine serum albumin nanoparticles (BNP) loaded with IL-1 receptor antagonist (IL-1Ra) on its surface via a Schiff base reaction, forming a smart drug release system (Modified MS) [326]. This system utilized the acidic microenvironment caused by lactate accumulation in the intervertebral disc degeneration region to trigger the targeted release of IL-1Ra, effectively inhibiting the expression of inflammatory factors, reducing ROS and NLRP3 activity, restoring ECM metabolic balance, and promoting the survival and repair of nucleus pulposus cells. Similarly, in the field of anticancer drug delivery, Dadsetan et al. developed a pH-responsive HM system based on the copolymerization of fumaric acid poly(ethylene glycol) oligomer (OPF) and sodium methacrylate (SMA) for controlled release of doxorubicin (DOX) [327]. The system introduced SMA to impart an adjustable negative charge density to the microspheres, enabling efficient DOX loading and pH-dependent release via an ion exchange mechanism. Results showed that with increasing SMA content, the DOX loading capacity and release half-life of the HMs were significantly enhanced, while burst release was significantly reduced. Further studies indicated that in solutions simulating different pH environments, the HMs exhibited significant pH-responsive release behavior, particularly under acidic conditions (e.g., pH 3), where the DOX release rate increased substantially, aligning with the characteristic acidic microenvironment of solid tumor tissues. Mathematical modeling demonstrated that the DOX release process could be accurately fitted to a stretched exponential model, with the release rate being linearly correlated to SMA concentration. In Vitro experiments confirmed that the released DOX retained significant anticancer activity in chordoma cells (UCH-1), reducing cell survival to below 10% under sustained treatment. This study demonstrated that by regulating the ionic monomer ratio in the hydrogel, pH-controllable release kinetics were achieved, significantly prolonging the action time of DOX and effectively combating chemotherapy-resistant tumor cells, highlighting the broad application potential of OPF-SMA HMs as an intelligent tumor drug delivery carrier.
ROS is one of the key signaling molecules mediating intracellular and intercellular communication. Notably, under pathological conditions, the abnormal elevation of ROS levels can lead to oxidative damage in local tissues and cells, promoting the onset and progression of diseases. However, this also provides a unique biological basis and design opportunity for the development of ROS-responsive smart HMs. For example, Zhang et al. developed a core-shell structured HM (Mg@PLPE MS) based on PLGA and ROS-responsive polymer poly(PBT-co-EGDM), with magnesium microparticles encapsulated inside for antioxidant therapy in intervertebral disc degeneration (IVDD) [328]. The system utilized the response characteristics of poly(PBT-co-EGDM) to hydrogen peroxide (H2O2), transitioning from hydrophobic to hydrophilic in an oxidative stress microenvironment, thereby disrupting the shell structure and initiating the reaction of magnesium with water to generate hydrogen gas, continuously scavenging ·OH. Results have shown that Mg@PLPE MS exhibited significant ROS-responsive hydrogen gas release in the presence of H2O2 and efficiently scavenged ·OH, significantly reducing ROS levels and inflammatory factor expression in LPS-induced macrophages and nucleus pulposus cells. Compared to free magnesium particles, Mg@PLPE MS provided a more stable release, avoiding local alkalization and tissue irritation caused by burst release. In Vivo experiments further confirmed that the microspheres effectively inhibited ROS accumulation, inflammation, and apoptosis in intervertebral disc tissues, alleviating ECM degradation and maintaining disc height and structural integrity. Similarly, Chung and colleagues developed an ultra-sensitive ROS-responsive gas-generating hollow microsphere (RRHMs) for the targeted release of anti-inflammatory drugs in an inflammatory microenvironment [329]. The system used PLGA as the shell material, containing dexamethasone phosphate (DEX-P), an ethanol-Fe2+ acid precursor, and sodium bicarbonate (SBC) as a gas-generating agent. H2O2 in the inflammatory region oxidized ethanol to acetic acid through the Fenton reaction, which induced the decomposition of SBC to generate CO2 bubbles, disrupting the microsphere shell and achieving rapid drug release. Results showed that RRHMs were an ROS hypersensitive drug delivery platform that could be activated at physiologically relevant concentrations of H2O2, combining acid activation and gas generation mechanisms for on-demand drug release in lesion areas. It exhibited high local anti-inflammatory efficacy, significantly reducing ROS levels in the joints, restoring the expression of proteoglycans, aggrecan, and type X collagen in cartilage tissue, and inhibiting inflammation and cartilage matrix degradation. A team led by Han et al. developed a ROS-responsive HM (KGN/Dex-TSPBA@WHMs) based on gelatin methacrylamide (GelMA) for the localized and precise treatment of osteoarthritis (OA) [330]. The system used microfluidics combined with ultraviolet polymerization to embed ROS-responsive nanoparticles (KGN/Dex-TSPBA) loaded with dexamethasone (Dex) and kartogenin (KGN) along with a type II collagen-targeting peptide (WYRGRL) into the GelMA network, forming a smart drug delivery system with “bee-like” chemotactic behavior. In an oxidative stress environment, TSPBA reacted with ROS, causing nanoparticle disaggregation and triggering In Situ drug release, significantly clearing intracellular ROS, inhibiting the expression of inflammatory factors such as IL-6 and TNF-α, and promoting BMSC differentiation toward a chondrocyte phenotype. Animal experiments demonstrated that KGN/Dex-TSPBA@WHMs could achieve long-term drug retention In Vivo, maintain joint space width, repair cartilage defects, and significantly delay OA progression. This study established a dual-release system combining ROS responsiveness and cartilage targeting functions, achieving “on-demand release + targeted repair” in the OA inflammatory microenvironment, which provided an efficient and translatable new strategy for the localized treatment of degenerative joint diseases.
In addition, as key mediators of physiological reactions, enzymes play a critical role in the onset and progression of diseases. For instance, specific enzymes like MMPs are significantly active during the pathological processes of diseases such as tendon adhesion and OA, promoting tissue remodeling and inflammatory responses. Based on this, recent studies have led to the development of a series of “smart” stimuli-responsive HMs, which target enzymes that are abnormally overexpressed under these pathological conditions, achieving precise drug delivery and therapeutic modulation. For example, Cai et al. developed an MMP-2-responsive hydrogel-electrospun composite membrane (siRNA@MS@HA) that integrates self-healing properties, macrophage modulation, and responsive gene silencing for the prevention and treatment of postoperative tendon adhesion [331]. This system utilized dynamically covalently crosslinked hyaluronic acid self-healing hydrogel as a matrix, loaded with GelMA microspheres that degrade in MMP-2-enriched environments, and encapsulated Smad3-siRNA nanoparticles, enabling on-demand release at the adhesion lesion site. This study showed that the composite membrane significantly reduced local inflammatory responses, inhibited M2 macrophage polarization, and effectively reduced adhesion tissue formation while inhibiting fibroblast proliferation and scar matrix deposition without interfering with tendon repair, leading to significant improvements in postoperative sliding function. Furthermore, Zhou and colleagues developed an injectable HM system (HAM-SA@HCQ) that combined ROS scavenging, MMP-13 degradation, and hypoxia responsiveness for the microenvironmental regulation and combination therapy of OA [270]. The system ultilized methacrylate-modified sulfonated azobenzene[4]arene (SAC4A-MA) as the core material, crosslinked with HA-MA and MMP-13-sensitive peptides to form a microsphere network, efficiently loading the anti-inflammatory drug hydroxychloroquine (HCQ). In the inflammatory hypoxic environment typical of OA joints, the system achieved precise drug release through the reduction-responsive azobenzene group of SAC4A-MA, and the network degradation triggered by MMP-13 overexpression improved drug delivery efficiency and retention time. In Vitro and In Vivo experiments showed that HAM-SA@HCQ significantly scavenged ROS, inhibited the expression of inflammatory factors such as HIF-1α and TNF-α, restored cartilage structure, and significantly slowed the progression of OA. Similarly, the group of Miao et al. developed a dual-layer HM (ChsMA+CLX@Lipo@GelMA) based on chondroitin sulfate methacrylate (ChsMA) for the precise treatment of OA [332]. This system featured a GelMA outer layer encapsulating celecoxib liposomes (CLX@Lipo) and a ChsMA HM core, simulating a “chocolate-wrapped peanut” structure. The GelMA outer layer is sensitive to the high expression of MMP-13 in OA joints, rapidly degrading to release the anti-inflammatory drug CLX for early anti-inflammatory and analgesic effects. Subsequently, the ChsMA core gradually degrades, continuously releasing chondroitin sulfate to activate chondrocyte anabolic metabolism and promote cartilage repair. In Vitro and In Vivo experiments showed that the HMs exhibited excellent injectability, biocompatibility, and joint lubrication properties, significantly reducing the expression of inflammatory factors TNF-α and IL-6, inhibiting MMP-13 and iNOS activity, and simultaneously upregulating Aggrecan (Agg) and Collagen II (Col2) expression to repair cartilage structure. This study innovatively combined pH/MMP responsiveness, sequential release, and cartilage repair functions into a single platform, proposing a targeted, intelligent, and long-lasting OA microsphere therapy strategy, providing a new material platform and therapeutic approach for clinical intervention in degenerative joint diseases.
In recent years, strategies based on abnormally elevated glucose levels, specific temperatures in different tissues and organs, and externally applied alternating magnetic fields have also become common approaches for preparing “smart” HMs with specific stimuli-responsive characteristics. For example, Shao et al. developed a wound microenvironment adaptive hydrogel system (DFO@G-QCSFP) based on borate ester dynamic bonds for the treatment of chronic diabetic wounds [333]. This system incorporated gelatin microspheres (DFO@G) loaded with the angiogenesis-promoting drug DFO into an injectable self-healing hydrogel crosslinked with fluorinated phenylboronic acid-modified chitosan (QCSF) and polyethylene glycol (PVA), achieving multiple responses to critical pathological signals in wounds, such as hyperglycemia, hydrogen peroxide, and MMP-9. In the early stages of diabetic wounds, the borate ester bonds in the hydrogel were sensitive to hyperglycemia and ROS, rapidly degrading and releasing DFO@G microspheres to effectively alleviate oxidative stress. Subsequently, the DFO@G microspheres continued to degrade in an MMP-9-enriched environment, achieving controlled release of DFO, which upregulated HIF-1α and VEGF expression, significantly promoting angiogenesis and collagen deposition, and accelerating tissue regeneration. This system not only exhibited good self-healing and shear-thinning properties, adapting to complex wound shapes, but also completely degraded in low pH conditions after wound healing, preventing residuals. Additionally, the team of Zhao et al. developed an injectable, temperature-responsive nanofiber gel microsphere system (NF-GMS) to enhance the retention rate and therapeutic effects of cardiomyocyte (CM) transplantation after myocardial infarction [334]. This system was based on the self-assembly of PLLA-PEG-PNIPAm triblock copolymers, which formed nanofiber structures similar to the (ECM of myocardium and exhibited temperature-induced gelation. At room temperature, the system was a fluidic liquid, while at body temperature (37°C), it rapidly formed a 3D hydrogel, effectively mimicking the structure and function of natural ECM. The results showed that NF-GMS not only significantly improved the survival rate and antioxidant stress capacity of human embryonic stem cell-derived CMs, but also achieved a 10-fold higher cell engraftment efficiency compared to traditional CM injections in a rat myocardial infarction model, significantly reducing infarct size, enhancing angiogenesis, and improving left ventricular ejection fraction and fractional shortening. Compared to traditional hydrogels or microspheres alone, this system demonstrated unprecedented advantages in cell adhesion, tissue integration, and functional recovery. Similarly, Schilling and colleagues developed a temperature-responsive PNIPAAm hydrogel and PLGA microsphere-based local drug delivery system (TEMPS) for long-acting corticosteroid release treatment in sinonasal diseases [335]. The system embedded PLGA microspheres loaded with mometasone furoate (MF) into PNIPAAm hydrogel, achieving rapid gel formation at body temperature, tightly adhering to the sinonasal epithelium, and continuously releasing the drug for up to 4 weeks.
Furthermore, externally applied stimuli such as magnetic fields have also become common strategies for preparing stimulus-responsive HMs for drug targeting and controlled release. For example, Xue and colleagues developed a magnetic-responsive hydrogel microsphere (DM-ACMSs) based on sodium alginate-chitosan (AC) to achieve localized, multimodal combined therapy for postoperative breast cancer [336]. The system utilized superparamagnetic iron oxide nanoparticles (SPIONs) and DOX, employing the thermal effect of SPIONs under an alternating magnetic field (AMF) both as a tumor hyperthermia agent and to drive directional drug release within the microspheres. The reported results showed that DM-ACMSs achieved “on-demand release” under AMF stimulation, with 22.5% of DOX released within 10 min, far higher than under water bath heating or without stimuli. In Vitro experiments demonstrated that the combination therapy led to a 95.5% cell death rate in MCF-7 breast cancer cells, significantly superior to single magnetic hyperthermia or chemotherapy. In animal experiments, the combination therapy group showed complete tumor regression within 12 days and no recurrence within 40 days, while the single treatment group had tumor recurrence after 25 days, further confirming its outstanding synergistic therapeutic advantage. This study innovatively constructed a dual-modal magnetic hyperthermia-chemotherapy treatment platform controlled by AMF, proposing a “heat-driven + concentration gradient” synergistic controlled release mechanism for the precise elimination of postoperative residual tumor cells and suppression of recurrence, providing an efficient, safe, and controllable strategy.
As research has progressed, many scholars have begun to explore the integration of various stimuli-responsive strategies, successfully applying them in the biomedical field to create more complex multi-stimuli responsive mechanisms. This innovative mechanism provides a solid “double assurance” for precise drug delivery and controlled release, significantly enhancing treatment accuracy and efficacy, and paving the way for the development of personalized medicine. For example, Ciarleglio et al. developed a dual-responsive HM (DRAP microspheres) based on sodium alginate and PNIPAM, prepared using electrospray and high-energy ultrasonic emulsification techniques, aimed at achieving precise release of lipophilic drugs (Ozoile) in response to both temperature and pH changes [337]. The system was stable at low temperatures and neutral pH, and at physiological temperature (~37°C) and alkaline environments, it could trigger volume phase transition (VPTT ≈ 37.5°C–40°C) and network disassembly, promoting drug release. The results showed that the HMs exhibited significant pH responsiveness in the range of pH 4–7.4, with degradation rates increasing in more alkaline environments, and higher temperatures further accelerating the swelling–shrinking transition and drug release. The chitosan coating enhanced the structural stability and hydration properties of the microspheres while also delaying degradation to some extent. Additionally, inspired by the dual stimuli of elevated MMPs and ROS in the osteoarthritis (OA) microenvironment, Xia and colleagues used microfluidic technology to anchor PBA and methacrylated gelatin (GelMA) onto a HA-MA scaffold, cleverly constructing a ROS/MMP dual-responsive HM (DMY@HGP) loaded with dihydromyricetin (DMY) [338]. The system provided PBA for specific ROS response, while GelMA contributed MMP sensitivity, achieving on-demand drug release in the OA-specific pathological environment. In Vitro and In Vivo experiments demonstrated that the microspheres achieved an “early ROS-triggered + later MMP-responsive” sequential release model, significantly enhancing the stability and bioavailability of DMY in the joint cavity. DMY, as a SIRT3 agonist, inhibited mitochondrial apoptosis (mt-Apoptosis) mediated by the BCL2/BAX pathway and activated PINK1/Parkin-LC3B-dependent mitophagy, maintaining mitochondrial homeostasis in chondrocytes, regulating the balance of ECM synthesis and degradation, thereby delaying OA progression.
In the field of tuberculosis drug delivery, the team of Kajjari developed a pH and temperature dual-responsive HM based on sodium alginate (NaAlg) and poly(N-isopropylacrylamide)-grafted guar gum (PNIPAAm-g-GG) for controlled release of isoniazid (INH) [339]. The system was prepared by emulsion crosslinking using glutaraldehyde as a crosslinking agent, overcoming the limitations of weak PNIPAAm responsiveness and NaAlg's rapid swelling at high pH, significantly improving the microsphere's structural stability and controlled release performance through complementary dual components. The HMs exhibited significant pH and temperature sensitivity in acidic (pH 1.2) and alkaline (pH 7.4) buffers, as well as at 25°C and 37°C. NaAlg provided the microspheres with pH-dependent volume expansion behavior, while PNIPAAm-g-GG maintained hydrophilicity below its LCST and became hydrophobic at 37°C, thus regulating drug release. In addition, Rodkate et al. developed a multi-responsive HM system based on PNIPAAm, carboxymethyl chitosan (CMC), and magnetic nanoparticles (MNPs) for smart drug-controlled release in response to environmental stimuli (temperature, pH, and magnetic field) [316]. The system used PNIPAAm for temperature-responsive release, with rapid contraction “expelling” the drug when the temperature exceeded the LCST. The carboxyl groups in CMC provided enhanced swelling under alkaline conditions, enabling pH-sensitive release. Additionally, the embedded Fe3O4 magnetic nanoparticles allowed for magnetic field manipulation, achieving targeted accumulation and rapid retrieval. In Vitro release experiments showed that the microspheres exhibited significantly higher release rates under pH 11 or 50°C conditions (26% and 87%, respectively), far exceeding the release under acidic or low-temperature conditions. The drug release behavior followed a non-Fickian diffusion model, demonstrating excellent stimulus-responsive characteristics and controlled release capabilities.
5.2 Tissue Engineering
In addition to drug delivery and controlled release, these advanced stimuli-responsive smart HMs have also become a research hotspot in the fields of tissue engineering and wound repair in recent years, demonstrating broad application prospects. For example, a hyperglycemic microenvironment often leads to chronic local inflammation, impaired angiogenesis, cellular dysfunction, and increased susceptibility to infections, causing diabetic wounds to remain in the inflammatory phase for a prolonged period and preventing them from entering the proliferative repair stage, ultimately resulting in chronic non-healing ulcers. To accelerate diabetic wound healing, Liu and colleagues developed a hydrogel based on Dioscorea opposita polysaccharide (DOP)-doped calcium carbonate (CaCO3) microspheres (PL−PVA/DOP-CaCO3) [340]. The hydrogel was formed via borate ester bonds between poly-l-lysine-phenylboronic acid (PL−PBA) and polyethylene glycol (PVA), with DOP-modified CaCO3 microspheres providing good mechanical properties and swelling ability, enhancing the hydrogel's biocompatibility and anti-inflammatory properties. This hydrogel system responded to the hyperglycemic environment and released insulin, thereby regulating hyperglycemia in diabetic wounds and promoting healing. In Vitro experiments showed that the PL−PVA/DOP-CaCO3 hydrogel exhibited excellent cell compatibility and anti-inflammatory activity, promoting fibroblast proliferation and migration. More importantly, In Vivo experiments indicated that insulin-loaded PL−PVA/DOP-CaCO3 hydrogel effectively regulated blood glucose levels, reduced inflammation, promoted angiogenesis, and accelerated tissue repair. In diabetic rat models, INS@PL−PVA/DOP-CaCO3 hydrogel significantly improved wound healing rates, reduced infection and inflammation, and promoted epithelial cell regeneration. Similarly, the group of Shao et al. developed an adaptive hydrogel system based on borate ester bonds (DFO@G-QCSFP) [333]. This system responded to high glucose and hydrogen peroxide environments, releasing the loaded DFO@gelatin microspheres on demand. The hydrogel could scavenge ROS, promote angiogenesis, and alleviate oxidative stress through DFO, stimulating HIF-1α and VEGF expression to accelerate wound healing. Both In Vitro and In Vivo experiments demonstrated that DFO@G-QCSFP significantly enhanced the healing speed of diabetic wounds, promoted angiogenesis, and collagen deposition. Additionally, Liu et al. developed a drug delivery system based on MMP9-responsive and temperature-sensitive hydrogel [341]. The team encapsulated self-assembled curcumin nanoparticles (CNPs) in gelatin microspheres (GMs) and then loaded them into a temperature-sensitive hydrogel to form an intelligent drug release system (CNPs@GMs/hydrogel). The system utilized the MMP9 overexpression in diabetic wound environments, where upon contact with the wound site, the hydrogel triggered the MMP9 enzyme to degrade the microspheres, enabling the targeted release of curcumin and accelerating wound healing. In Vitro and In Vivo experiments showed that the CNPs@GMs/hydrogel system effectively increased curcumin bioavailability and promoted cell migration and proliferation through antioxidant and anti-inflammatory effects. Notably, in diabetic mouse models, this system significantly accelerated wound healing, promoted angiogenesis and collagen deposition, and reduced oxidative stress at the wound site.
Due to the challenges of insufficient osteogenic potential, difficult vascular reconstruction, loss of mechanical support, and imbalanced biological microenvironments in defect areas, bone defect repair often faces slow new bone formation or even non-union, frequently requiring the assistance of biomaterials or tissue engineering strategies to promote bone regeneration. Notably, in the field of bone defect repair, stimuli-responsive HMs have also demonstrated immense potential. For example, Segredo-Morales et al. developed a thermo-responsive hydrogel based on Tetronic 1307 (T-1307) and alginate composites for bone repair in osteoporosis patients [342]. This system involved loading 17β-estradiol (βE) and bone morphogenetic protein-2 (BMP-2) into PLGA microspheres to create a localized drug delivery system. The study found that this hydrogel system could continuously release the drugs at simulated bone defect sites, exhibiting a synergistic effect. In Vivo experiments in osteoporotic rat models showed that the T-A-βE-BMP combination significantly improved bone repair, with the repair rate being twice as high as that of the single treatment group, especially under the influence of BMP-2. Similarly, Hu and colleagues developed a multifunctional microsphere scaffold based on temperature-responsive shape-memory hydrogels for repairing irregular bone defects [24]. This system combined a poly(lactic acid-trimethylene carbonate-hydroxyacetic acid) (PLLA-TMC-GA) ternary copolymer with dopamine-modified nano-hydroxyapatite (PHA) to construct a shape-memory bone repair scaffold. The study showed that the composite scaffold exhibited excellent biocompatibility and mechanical properties both In Vitro and In Vivo, adapting to the complex shapes of bone defects. By loading sodium alginate hydrogel coatings containing Icaritin (Ica), the system promoted osteocyte growth and differentiation, significantly enhancing bone defect repair. In Vivo experiments in a rat cranial defect model demonstrated that the PTG/PHA/Ica scaffold showed superior osteogenesis capability, with significant new bone formation and increased bone density after 12 weeks of treatment, particularly in the PTG/20PHA/Ica group, where bone bridging was evident, showing strong bone-inductive ability. Additionally, the group of Chen et al. developed a dual-crosslinked self-healing magnetic injectable biopolymer hydrogel for bone repair and drug delivery [343]. This system used CMCS and oxidized gelatin (OGG) crosslinked through a Schiff base reaction to form the hydrogel, and magnetic hydroxyapatite/gelatin microspheres (MHGMs) were prepared via emulsion crosslinking. The antimicrobial drugs tetracycline hydrochloride (TH) and silver sulfathiazole (AgSD) were loaded into MHGMs to further enhance the hydrogel's drug release and antimicrobial properties. Experimental results indicated that the composite hydrogel containing 6% hydroxyapatite (HAp) and 10 mg/mL MHGMs exhibited excellent magnetic responsiveness, self-healing properties, and injectability. Compared to pure hydrogels, the composite hydrogel showed significant improvements in gelation time, swelling ratio, degradation performance, and compressive strength. In particular, antimicrobial tests revealed that the hydrogel exhibited potent inhibitory effects against Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) and showed good cytocompatibility. The sustained drug release properties of this composite hydrogel demonstrated a slower drug release rate in simulated biological environments, with the ability to control drug delivery through an external magnetic field. This hydrogel not only exhibited excellent mechanical properties and biocompatibility for bone repair but also provided a novel intelligent platform for bone defect repair and drug delivery through its self-healing and magnetic-responsive characteristics.
Notably, recent studies have begun to explore the integration of multiple stimuli mechanisms such as pH, temperature, and enzyme responsiveness to develop advanced multifunctional HM systems for bone regeneration and repair. For example, the team of Wan et al. developed a dual-responsive hydrogel composite material, combining a temperature-responsive hydroxybutyl chitosan (HBC) hydrogel with near-infrared (NIR) responsive polydopamine (PDA)-modified magnesium calcium carbonate microspheres for drug delivery in bone regeneration [344]. This system rapidly released the anti-inflammatory drug aspirin (Asp) to alleviate inflammation in the early stages of bone repair, followed by the release of bone morphogenetic protein-2 (BMP-2) under NIR light to promote osteogenesis. In Vitro and In Vivo experiments demonstrated that this composite hydrogel significantly promoted new bone formation, especially in the skull defect model of SD rats, achieving optimal bone repair by controlling the release timing of BMP-2. The dual-response mechanism of the system optimized the temporal release of drugs, providing precise treatment for different stages of bone healing, and showed broad application potential in bone tissue engineering. Similarly, Lin and colleagues developed a multi-responsive HM system (ABOHA@ZnO) based on HA [345]. This system combined antimicrobial peptides (AMPs) and zinc oxide nanoparticles (ZnO-NPs), and microspheres were fabricated using 3D printing technology. The system intelligently responded to changes in the local microenvironment, including pH, enzyme activity, and ROS levels, thereby promoting bone repair. In Vitro experiments showed that ABOHA@ZnO hydrogel microspheres released AMPs in acidic environments and under the action of hyaluronidase, effectively inhibiting the growth of S. aureus and displaying significant antibacterial and antibiofilm activity. Additionally, the HMs promoted osteogenic differentiation of bone marrow stromal cells (BMSCs) and enhanced the expression of osteogenic markers such as ALP, OCN, OPN, and COL-1. In a rat cranial defect model, ABOHA@ZnO hydrogel microspheres significantly promoted bone regeneration, enhanced angiogenesis, and modulated immune responses, demonstrating excellent multifunctional capabilities in antibacterial activity, promoting bone regeneration, and regulating immune responses.
5.3 Tumor Treatment
The tumor microenvironment (TME) exhibits unique characteristics such as acidic pH, elevated ROS, and overexpression of MMPs, which create significant differences from normal tissues. Stimuli-responsive HMs designed based on these features can intelligently recognize these pathological signals, achieving precise drug controlled release and targeted accumulation, providing an innovative solution to the challenge of selective tumor treatment. Among them, pH-responsive HMs are the most commonly used design strategy, as they specifically respond to the acidic microenvironment of tumor tissues for targeted drug delivery [48]. For example, Dadsetan and colleagues developed a pH-responsive HM (OPF-SMA) based on OPF and SMA copolymer for controlled release of the anticancer drug doxorubicin (DOX) [327]. The HMs effectively loaded DOX through charge interactions and regulated drug release through an ion exchange mechanism in response to changes in pH. The study found that as the concentration of SMA increased, the negative charge density of the microspheres also increased, causing them to expand in acidic environments, thereby accelerating the release of DOX. In Vitro experiments showed that DOX release was influenced by pH and ionic strength, with a significant acceleration of DOX release in acidic environments (e.g., pH 3), while slower release occurred at physiological pH (pH 7.4). Mathematical modeling further indicated that the release of DOX followed a stretched exponential function, and the microsphere's SMA concentration was linearly related to the maximum release amount and half-life of DOX. Moreover, after release, DOX exhibited significant anticancer effects, showing clear cytotoxicity in the human chordoma cell line UCH-1. Similarly, Liu et al. developed an innovative HM vaccine aimed at enhancing the immune efficacy after tumor ablation [346]. The vaccine consisted of a combination of FLT3L and CD40L cytokines, designed to release in response to the acidic tumor microenvironment, particularly for pancreatic cancer. This system utilized the acidic pH of the TME to promote the recruitment and activation of CD103+CD11b− conventional dendritic cells (cDC1s). Activated dendritic cells then migrated to the tumor-draining lymph nodes (TdLN) and initiated a cascade of antigen cross-presentation, amplifying the response of specific CD8+ T cells. The study showed that the HM vaccine significantly increased the infiltration of CD8+ T cells into the tumor, inhibited tumor growth, and reduced metastasis, with particularly notable results in a pancreatic cancer mouse model. Additionally, this hydrogel system effectively reduced liver damage caused by systemic cytokine delivery, demonstrating its safety and clinical application potential. When combined with irreversible electroporation (IRE) ablation therapy, the hydrogel vaccine significantly enhanced the antitumor immune response, with survival rates markedly higher than those of mice treated with IRE alone. This hydrogel-based strategy addressed key challenges in tumor immunotherapy, enhancing local immune responses while minimizing systemic side effects.
Temperature-responsive HMs have also started to show promise in the treatment of solid tumors. For example, Wen et al. developed a dual-drug delivery system based on temperature-responsive Pluronic F127 HMs for the treatment of hepatocellular carcinoma (HCC) with ascites [347]. This system combined resveratrol (RES) microspheres and cisplatin (DDP) into F127 HMs, with temperature changes controlling the drug release. The F127 HM system remained in a liquid state at low temperatures and could undergo gelation at body temperature (37°C), triggering a slow release of the drugs. Experimental results showed that the F127 HMs effectively controlled the delivery of RES and DDP, and demonstrated significant antitumor effects in a hepatocellular carcinoma ascites mouse model. This microsphere system reduced systemic side effects by delivering drugs locally, significantly inhibiting tumor cell proliferation and reducing ascites volume. Immunohistochemical analysis indicated that the HM system effectively inhibited tumor angiogenesis and prolonged the survival of the mice, demonstrating its excellent therapeutic efficacy. Similarly, Ma and colleagues developed a composition based on temperature-responsive Pluronic F-127 (PF-127) HMs (PF-127@MnCl2/ALG-MS@MgCl2) to enhance cancer immunotherapy [348]. This hydrogel combined the rapid release of manganese ions (Mn²⁺) with the sustained release of magnesium ions (Mg²⁺), optimizing the tumor microenvironment to enhance the cytotoxic activity of CD8+ T cells and natural killer cells, thereby significantly improving the effectiveness of programmed death-ligand 1 (PD-L1) immune checkpoint inhibitors. Through this hydrogel, the research team successfully converted “cold tumors” into “hot tumors,” greatly enhancing the efficacy of immunotherapy. Furthermore, the PF-127@MnCl2/ALG-MS@MgCl2 hydrogel showed excellent antitumor effects in a mouse tumor model, effectively inhibiting tumor growth, reducing tumor metastasis, and promoting the tumor infiltration of T cells and natural killer cells. Single-cell RNA sequencing (scRNA-seq) analysis revealed that the hydrogel significantly enhanced the infiltration of immune effector cells by modulating the immune microenvironment and altered the expression of PD-L1, thus improving the effectiveness of immune checkpoint inhibitor therapy.
The elevated levels of ROS in solid tumors not only drive tumor malignancy progression, but also provide a specific activation target for ROS-responsive HMs, enabling precise drug delivery and treatment triggered by the TME. For example, Gao and colleagues proposed an innovative postoperative therapeutic strategy using an environment-responsive camphor polysaccharide hydrogel (MnP@DOP-Gel), which achieved this by embedding pectin microspheres (MnP) rich in manganese ions (Mn²⁺) into the hydrogel [53]. This system could respond to oxidative stress (ROS) stimulation in the postoperative environment, gradually releasing Mn²⁺, which enhanced the antitumor immune response by activating immune cells such as dendritic cells and macrophages, as well as inducing immunogenic cell death (ICD) in tumor cells. The design of MnP@DOP-Gel took full advantage of the high oxidative stress environment in the postoperative local area and triggered the hydrogel's degradation through this environment, thereby releasing manganese ions and its microspheres. In In Vivo experiments in a mouse melanoma model, the hydrogel significantly inhibited the growth of residual tumors, and when combined with anti-PD-1 antibodies, it showed excellent synergistic therapeutic effects in preventing tumor metastasis and brain metastasis. Furthermore, the study demonstrated that MnP@DOP-Gel was not only nontoxic, but also effectively activated the cGAS-STING signaling pathway, promoting the production of interferons and thereby inducing immunogenic cell death (ICD) in tumor cells.
Similar to the applications in drug delivery and tissue engineering, researchers have integrated multiple stimuli-responsive characteristics into single HM systems through various design strategies to enhance their multifunctionality in tumor therapy. For example, the group of Dai et al. developed a pH and temperature-responsive drug release system based on NaYF4:Yb³⁺/Er³⁺ upconversion fluorescent microspheres (NaYF4:Yb³⁺/Er³⁺@SiO2@P(NIPAM-co-MAA)), which were used as multifunctional drug carriers [49]. The system exhibited strong upconversion fluorescence under 980 nm laser irradiation, providing real-time imaging In Vivo. Additionally, by coating the outer layer of the microspheres with a temperature and pH-responsive polymer P(NIPAM-co-MAA), the system could trigger controlled drug release under different pH environments. Experimental results showed that the encapsulated doxorubicin (DOX) exhibited pH-responsive characteristics during release. At normal physiological pH 7.4, the drug was released slowly; however, in acidic environments (such as the TME or acidic environments in endosomes), the drug was rapidly released. The system demonstrated significant antitumor effects In Vitro, effectively killing SKOV3 ovarian cancer cells. Furthermore, NaYF4:Yb³⁺/Er³⁺@SiO2@P(NIPAM-co-MAA) microspheres not only served as drug carriers but also enabled cellular imaging, providing a unique method for real-time monitoring of the drug release process through changes in upconversion emission intensity. Similarly, Wu and colleagues attempted to introduce magnetic nanoparticles into a pH-responsive HM system to impart additional magnetic responsiveness [349]. They developed a magnetic/pH-responsive hydrogel microsphere system (MTX@Fe₃O₄-Chitosan) based on Fe₃O₄ nanoparticles and chitosan hydrogel for controlled release delivery of methotrexate (MTX). The system was prepared using a simple gelation process, exhibiting a uniform porous structure and superparamagnetism. MTX was successfully encapsulated in magnetic chitosan HMs, with a drug entrapment efficiency of 93.8% and a drug loading capacity of 6.28%. In Vitro drug release studies showed that MTX@Fe₃O₄-Chitosan HMs exhibited significant pH responsiveness, releasing 90.6% of MTX within 48 h in an acidic environment (pH 4.0), while the release rate was lower (20.4%) under physiological pH conditions (pH 7.4). Moreover, the magnetic properties of the HMs enabled targeted localization and accelerated drug release under an external magnetic field. When a magnetic field was applied locally, it enhanced drug accumulation and release efficiency at the target area. Cell culture experiments demonstrated that these HMs exhibited good cell compatibility and antitumor activity in HepG2 liver cancer cells, effectively inhibiting tumor cell proliferation.
6 Clinical Translation and Challenges
Stimuli-responsive HMs are a class of emerging intelligent biomaterials with highly tunable properties and advanced responsiveness to various stimuli. By integrating multiple stimuli-responsive mechanisms (e.g., pH, temperature, ROS, enzymes, and small molecules), these HMs can dynamically adapt to specific changes in physiological and pathological environments. With their unique 3D network structures and highly controllable physicochemical properties, these HMs demonstrate remarkable precision in drug delivery, efficiency in tissue repair, and advantages in targeted therapy. This review systematically summarizes the preparation materials, construction strategies, stimuli-responsive mechanisms, and current applications of HMs in drug release, disease treatment, and regenerative medicine. It also provides an in-depth analysis of the key challenges and emerging trends in this field. Studies indicate that leveraging the dynamic regulatory capabilities of these HMs can significantly enhance therapeutic efficacy while reducing side effects, offering new opportunities for personalized medicine and regenerative therapies. By integrating the design of HMs with emerging technologies such as microfluidics and nanotechnology, researchers have been able to develop “smart” stimuli-responsive HMs with higher sensitivity, greater stability, and broader adaptability. These advancements present innovative solutions for the diagnosis and treatment of complex diseases.
- 1.
Biocompatibility and safety: Ensuring that HMs are biocompatible and safe for In Vivo applications is crucial. This involves minimizing potential cytotoxicity and immunogenic responses. Incorporating natural biopolymers, such as gelatin or collagen, or integrating cell-binding domains like adhesive peptides (e.g., RGD sequences), has been shown to promote long-term cell viability and compatibility. Comprehensive evaluations of biodegradability and long-term effects are essential to ensure that HMs do not induce adverse reactions over time.
- 2.
Performance limitations: Addressing the mechanical strength and stability of HMs is vital for their practical application. Many current HMs lack the robustness required for certain biomedical uses. Developing HMs with enhanced mechanical properties, such as dynamic covalent bonds or supramolecular interactions, can improve their durability and functionality. Furthermore, optimizing the swelling kinetics and responsiveness of HMs to specific stimuli is essential to achieve precise control over drug release profiles and therapeutic outcomes.
- 3.
Developing multi-stimuli-responsive properties: Future research should focus on exploring multi-stimuli-responsive mechanisms, such as designing HMs capable of simultaneously responding to multiple environmental signals, including pH and ROS, or temperature and enzymes. Such advancements would not only enhance the application potential of HMs in treating complex diseases (e.g., tumors and inflammatory conditions) but also open new possibilities for personalized medicine. Further optimization is required to ensure that drug release processes triggered by different stimuli do not interfere with each other. For instance, precise molecular design of materials, combined with dynamic crosslinking networks and the synergistic effects of block copolymers, can enable hierarchical responses to various stimuli, thereby achieving precise control over targeted therapy.
- 4.
Optimizing fabrication techniques and performance: Advancing fabrication technologies will be key to enhancing the performance of HMs. Microfluidic techniques can further enable high-precision production of microspheres, achieving greater consistency in shape, size, and internal structure while meeting the demands of large-scale production. Additionally, 3D printing and photolithography technologies can facilitate the fabrication of HMs with complex shapes and multifunctional layered structures. Furthermore, the development of dynamic crosslinking network materials that enable rapid formation and offer higher mechanical strength is essential. These innovations will improve the mechanical properties and durability of HMs, ensuring their reliability for long-term applications in the biomedical field.
- 5.
Exploring new biomaterials: Material innovation is fundamental to the development of stimuli-responsive HMs. On one hand, the combination of novel natural and synthetic materials should be explored, such as integrating the biocompatibility of natural materials with the mechanical stability of synthetic materials to develop hybrid materials with dynamic crosslinking and self-healing properties. On the other hand, research on functional nanomaterials (such as metal-organic frameworks, graphene, and silica nanoparticles) should be strengthened. Combining these materials with HMs can impart additional functions, such as photothermal therapy, magnetic targeting, and multimodal imaging, thereby expanding their application range [91, 350-352].
- 6.
Accelerating clinical translation: The ultimate goal of HMs is to achieve clinical application. To this end, their safety, immunogenicity, biodegradability, and long-term efficacy need to be systematically evaluated in animal models and clinical trials. Additionally, efforts should focus on optimizing cost-effectiveness by developing manufacturing processes that meet industrial production requirements, thereby reducing the cost of HMs production and lowering application barriers. For specific disease applications (such as cancer, diabetes, and osteoarthritis), researchers must collaborate across clinical medicine, pharmacology, and engineering to establish standardized evaluation systems, thereby accelerating the translation of HMs from the laboratory to clinical settings.
- 7.
Scalability and regulatory hurdles: Translating HMs from the laboratory to clinical settings requires scalable manufacturing processes that comply with regulatory standards. Achieving reproducibility, consistency, and cost-effectiveness in large-scale production is challenging. Additionally, navigating the complex regulatory landscape necessitates thorough documentation of safety, efficacy, and quality control measures to meet the stringent requirements set by health authorities.
7 Conclusions and Perspectives
In conclusion, with the ongoing intersection of biomaterials science, nanotechnology, and medical needs, stimuli-responsive HMs are poised to drive further innovations in the biomedical field and open up new therapeutic avenues for addressing a variety of complex diseases. Beyond their established applications in medicine, these microspheres also hold great potential in emerging fields such as soft robotics and environmental monitoring. In soft robotics, stimuli-responsive HMs can be engineered to mimic biological motions and functions, thereby enhancing the versatility and adaptability of robotic systems. In environmental monitoring, these microspheres can act as sensitive sensors that detect and respond to chemical or biological stimuli, offering real-time data and insights into environmental health and safety. Looking ahead, deeper interdisciplinary collaboration will further advance the development of this versatile technology, enabling broader applications across diverse fields.
Author Contributions
Jiacheng Liu: conceptualization (lead), investigation (lead), methodology (lead), project administration (lead), writing – original draft (lead). Chengcheng Du: conceptualization (equal), investigation (equal), methodology (equal), project administration (equal), writing – original draft (equal). Senrui Liu: conceptualization (equal), investigation (equal), methodology (equal), project administration (equal), writing – original draft (equal). Junyan Liu: investigation (equal), methodology (equal), project administration (equal). Xuefeng Luo: investigation (equal), methodology (equal). Jingdi Zhan: investigation (equal), methodology (equal). Zhuolin Chen: investigation (equal), methodology (equal). zhenglin zhu: supervision (equal), writing – review and editing (equal). Zhong Alan Li: supervision (equal), visualization (equal), writing – review and editing (equal). Wei Huang: funding acquisition (equal), supervision (equal), writing – review and editing (equal). Yiting Lei: conceptualization (lead), funding acquisition (lead), supervision (lead), visualization (lead), writing – review and editing (lead).
Acknowledgments
J. Liu, C. Du, and S. Liu contributed equally to this study. Jiacheng Liu wants to thank Yuanyuan Wang for her love and company. Figure 1 is created with the help of BioRender.com. This study was financially supported by the National Natural Science Foundation of China (82302755, U22A20284), the Qian De Outstanding Young Talent Program of The First Affiliated Hospital of Chongqing Medical University (2025QDYQ-09), the Chongqing Medical Youth Top-notch Talent Program (YXQN202490), the China Postdoctoral Science Foundation (2024M753873), the Hong Kong Scholars Program (XJ2024017), and the Natural Science Foundation of Chongqing (CSTB2024NSCQ-MSX1200).
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.




