Antioxidant Natural Plant Constituents for Diabetic Wound Repair
ABSTRACT
Diabetes mellitus is a prevalent metabolic disorder characterized by a prolonged hyperglycemic state, which can result in complications affecting multiple organ systems. Among these complications, impaired wound healing in diabetic patients poses a significant challenge, leading to considerable suffering and economic burden. This issue has emerged as one of the major challenges in global healthcare, where oxidative stress, bacterial infections and chronic inflammation are critical contributing factors. Antioxidant compounds derived from natural plants are increasingly being explored in diabetic wound healing research due to their beneficial biological properties. These botanical components effectively scavenge excessive reactive oxygen species and mitigate cellular damage associated with oxidative stress. By controlling bacterial infections, inhibiting pro-inflammatory cytokines, and enhancing the activity of antioxidant enzymes, these compounds not only improve the wound microenvironment but also directly promote the proliferation and migration of fibroblasts and keratinocytes, thereby facilitating tissue regeneration. This paper examines the modulation of oxidative stress, control of bacterial infections, the impact on inflammatory responses, and the promotion of wound repair, with a focus on the application of specific antioxidant plant components in diabetic wound healing, delivery systems, and clinical applications, as well as challenges and future directions.
1 Introduction
Diabetes mellitus is a chronic endocrine metabolic disorder characterized by elevated blood glucose levels resulting from relative or absolute insufficiency and dysfunction of insulin secretion [1]. With economic development and changes in lifestyle, the global incidence of diabetes has markedly increased over the past few decades, currently affecting an estimated 537 million adults and projected to rise to approximately 783.2 million by 2045, posing a significant health concern. As the disease progresses, the hyperglycemic state impacts the cerebrovascular, cardiovascular, and other vascular systems, as well as leading to nephropathy and retinopathy. Impaired wound healing, due to tissue necrosis, inflammation, and ulceration, is particularly concerning [2-4]. According to the World Health Organization, diabetes could become the seventh leading cause of death worldwide by 2030, with impaired healing of surgical wounds and diabetic foot ulcers as major contributing factors [5]. Approximately 20% to 25% of diabetic patients experience impaired wound healing, and diabetic wounds are a leading cause of non-traumatic lower limb amputations, accounting for up to 85% of cases [6]. The economic burden is substantial, with diabetics receiving care for peripheral vascular disease accounting for 50 per cent of total American healthcare expenditure in the United States alone [7]. Beyond financial costs, diabetic wounds significantly impair patients’ quality of life, leading to prolonged hospitalization, disability, and psychological distress [8]. Current treatments for diabetic wounds primarily include glycemic control, surgical debridement, negative pressure drainage, skin grafting, wound dressing, anti-infective measures, stem cell transplantation, growth factors, and hyperbaric oxygen therapy. However, these interventions primarily aim to cleanse the wounds and reduce the risk of infection, rather than effectively accelerating the healing process [9-11].
Diabetic wounds are characterized by complex pathophysiology, including hyperglycemia, chronic inflammation, microbial infection, impaired angiogenesis, and oxidative damage [12]. Persistent hyperglycemia disrupts the normal inflammatory response, resulting in prolonged and excessive inflammation. This is driven by the sustained activation of pro-inflammatory cytokines and impaired resolution of inflammation [13]. Chronic hyperglycemia induces excessive production of reactive oxygen species (ROS), leading to oxidative stress [14]. At the same time, high glucose levels and impaired immune function create an environment conducive to bacterial colonization and biofilm formation, further complicating wound healing [15]. This damages cellular components such as lipids, proteins, and Deoxyribonucleic Acid (DNA), impairing cellular function and delaying wound healing [16]. In addition, due to endothelial dysfunction and reduced levels of proangiogenic factors, such as vascular endothelial growth factor (VEGF), diabetic wounds exhibit reduced angiogenesis and impaired cytogenesis, including reduced fibroblast proliferation and keratinocyte migration, which further impedes tissue repair [17, 18].
Oxidative stress is one of the key factors in impaired wound healing in diabetes [19]. It stems from an imbalance between the production of ROS and the body's antioxidant defense mechanisms [20]. Diabetic wound repair is influenced by several factors, among which oxidative stress plays a crucial role in altering the local microenvironment [21, 22]. ROS are non-radical derivatives of oxygen produced by oxygen radicals, including superoxide anion radicals, hydroxyl radicals, and other reactive oxygen species such as hydrogen peroxide and perchloric acid. ROS are continuously generated by tissue cells and are essential for normal cellular metabolism and biological processes [23]. At normal levels, ROS stimulate wound closure, enhance epidermal cell migration and differentiation, and induce local angiogenesis. They also play a critical role in preventing bacterial invasion and promoting fibroblast proliferation and collagen formation [24]. In diabetic patients, chronic hyperglycemia drives excessive ROS generation through multiple pathways, including the polyol pathway, advanced glycation end products (AGEs) formation, protein kinase C (PKC) activation, and mitochondrial dysfunction [25]. This oxidative stress damages cellular components such as lipids, proteins, and DNA, impairing the function of cells essential for wound healing, including fibroblasts, keratinocytes, and endothelial cells [26, 27]. Oxidative stress is not merely a byproduct of diabetic wound pathology but a central driver of its chronicity [28]. It exacerbates inflammation, impairs angiogenesis, and disrupts extracellular matrix (ECM) remodeling, creating a hostile microenvironment that hinders tissue repair [29]. Addressing oxidative stress is, therefore, a key therapeutic target for improving diabetic wound healing outcomes.
Under normal conditions, cells secrete antioxidant components to mitigate ROS levels. However, the high glucose environment characteristic of diabetes diminishes the body's antioxidant capacity, primarily through reduced activity of antioxidant enzymes such as superoxide dismutase and catalase. Additionally, levels of antioxidants like glutathione may also decrease, rendering the body more susceptible to oxidative stress damage [30]. Consequently, mitigating oxidative stress and restoring antioxidant homeostasis are considered effective strategies for promoting diabetic wound healing. The application of exogenous ROS scavengers is essential for reducing ROS-induced damage to skin tissue [31]. Approaches such as overexpression of antioxidant enzymes (e.g., superoxide dismutase) and stem cell therapy aim to enhance endogenous antioxidant defenses [32, 33]. Exogenous antioxidants, such as vitamin C, vitamin E, and N-acetylcysteine, have been used to scavenge ROS and restore redox balance [34, 35].
Notably, recent advances have highlighted the therapeutic potential of natural plant-derived antioxidants, particularly polyphenols (such as curcumin, quercetin) and flavonoids [36]; Compared with synthetic drugs, natural plant antioxidants have the advantages of multi-target, low toxicity, and wide range of sources. Antioxidants from natural plant sources are generally proven for long-term consumption or medicinal use and have relatively few side effects [37, 38]. Moreover, natural plant antioxidants often have a variety of active ingredients and can act on multiple physiological and pathological targets. In addition, plant resources are abundant, and many plants can be obtained by artificial cultivation or reasonable collection, which is sustainable. These compounds not only neutralize ROS but also modulate inflammatory signaling pathways, promote angiogenesis, and enhance cellular functions [39]. For example, curcumin has been shown to inhibit Nuclear Factor kappa-B (NF-κB) and reduce inflammation, while Centella asiatica scavenges free radicals and enhances cellular antioxidant capacity by upregulating the expression of antioxidant enzymes [40, 41]. Flavonoids found in Datura metel extracts also demonstrate strong free radical scavenging abilities and can mitigate cellular damage caused by oxidative stress [42]. In diabetic wound repair, various antioxidant herbs promote fibroblast proliferation and collagen synthesis, thereby accelerating wound healing [43]. Compounds such as luteolin and quercetin enhance cell proliferation and migration through multiple pathways to facilitate tissue repair [44, 45]. Naturally sourced apigenin not only improves the hyperglycemic state but also alleviates pancreatic cell damage through oxidative stress-related signaling, while increasing collagen content in epidermal wound tissues to promote wound repair [46]. Additionally, many plant antioxidants exhibit antimicrobial properties, addressing the infection susceptibility of diabetic wounds [47]. Their natural origin, low toxicity, and multifunctional effects make them attractive candidates for diabetic wound repair.
2 Diabetes and Diabetic Wounds
The occurrence of diabetes and its complications is due to the disorder of metabolic regulatory network caused by the interaction of genetic susceptibility and environmental factors. The pathogenesis of diabetes is complex, mainly related to insufficient insulin secretion or insulin resistance [48]. In terms of etiology, type 1 diabetes is mainly caused by selective destruction of islet beta cells mediated by autoimmunity, while type 2 diabetes is closely related to insulin resistance induced by obesity and sedentary lifestyle, such as reduced sensitivity of skeletal muscle, liver and adipose tissue to insulin, forcing the pancreas to secrete compensatory insulin. Eventually, the beta cells fail [49, 50]. Long-term hyperglycemia can lead to metabolic disorders, including abnormal glucose metabolism, fat metabolism, and protein metabolism, which can lead to a variety of complications, such as diabetic foot ulcers [51]. Through metabolic abnormalities such as polyol pathway, hexosamine pathway and the generation of AGEs, hyperglycemia induces oxidative stress and inflammatory cascade reaction, damaging vascular endothelial cells and nerve fibers. This pathological process is particularly prominent in the occurrence and development of diabetic wounds (such as diabetic foot ulcers) [52-54].
The manifestations of diabetes mellitus and its wounds are varied; Systemic manifestations include polydipsia, polyuria, polydipsia, weight loss, and other typical symptoms, as well as fatigue and blurred vision [55]. Local wound manifestations include redness, swelling, pain, exudate increase, necrotic tissue formation, and so forth, which can lead to tissue gangrene in severe cases, threatening limbs and even life [56]. The characteristic manifestation of diabetic wound is the stagnation of repair process, including: long-term wound healing, exudate increase, pigmentation or fibrosis of surrounding tissue, and purulent secretion and odor when combined with infection [57]. In terms of treatment strategy, the basic treatment of diabetes should take into account blood glucose control and complication prevention, including lifestyle intervention (diet control, regular exercise), oral hypoglycemic agents (such as metformin, SGLT2 inhibitors), and insulin replacement therapy [58]. For diabetic wounds, traditional treatments include debridement, negative pressure aspiration, and antibacterial dressings, but with limited effectiveness [59]. Emerging therapies focus on precise intervention of pathological mechanisms: Antioxidants (such as Edaravone and nano-selenium particles) reduce oxidative damage by scavenging ROS, recombinant human platelet-derived growth factor (rhPDGF) promotes fibroblast proliferation and collagen synthesis, and mesenchymal stem cells (MSCs) improve angiogenesis and immune microenvironment by paracrine HGF, TGF-β, and other cytokines [60-62]. Breakthroughs in the field of biomaterials have provided innovative vectors for wound repair, such as alginate hydrogels loaded with curcumin sustainably release antioxidant components, pH-responsive chitosan dressings for bacteriostasis in acidic wound environments, and electrospinned nanofiber membranes that promote keratinocyte migration by simulating extracellular matrix structures [63-65]. It is worth noting that targeted therapies (such as anti-RAGE antibodies) based on the AGEs-RAGE pathway have entered the experimental stage, which is expected to break the vicious cycle of metabolic disorders and tissue damage by blocking AGES-mediated inflammatory signals [66]. The future direction of treatment will integrate multi-omics data and develop personalized and precise programs, moving from single-target intervention to systematic regulation of the metabolism-inflammation-repair network.
3 Pathophysiology of Diabetic Wounds
The chronic development of diabetic wounds (such as diabetic foot ulcers) is a pathological process in which the regulatory networks of metabolism, inflammation, blood vessels, nerves, and extracellular matrix (ECM) are cascade failures in a hyperglycemic microenvironment. The core pathological process begins with metabolic disorders caused by sustained hyperglycemia, and the chronic mechanism involves multiple pathological links (as shown in Figure 1) [67]. Abnormal accumulation of AGEs activates NF-κB through AGE-RAGE signaling pathway and drives excessive release of pro-inflammatory factors such as TNF-α and IL-6, which not only inhibits fibroblast migration and VEGF transcriptional expression, but also inhibits fibroblast migration and vascular endothelial function. The uncontrolled inflammatory state further induces hyperactivation of neutrophils, forming neutrophils extracellular snare NETs rich in DNA-protease complexes, which release myeloperoxidase (MPO) in coordination with ROS to degrade ECM and damage surrounding normal tissues [68, 69]. In addition, the activation of the polyol pathway leads to the conversion of glucose into sorbitol by aldose reductase, resulting in NADPH depletion and the decrease of glutathione level, breaking the REDOX balance, triggering the explosive generation of mitochondrial derived reactive oxygen species (ROS), aggravating oxidative stress, and further damaging the cell membrane and DNA, forming a vicious cycle of oxidative stress [70, 71]. Moreover, this metabolic disorder and inflammatory disorder form a bidirectional interaction: the polarization imbalance of macrophages makes M1 type pro-inflammatory phenotype dominate, while the function of repair M2 macrophages is suppressed, resulting in the continuous release of inflammatory factors such as IL-1β and TNF-α. Neutrophils further disrupt the tissue microenvironment by releasing proteases and reactive oxygen species (ROS) through excessive NETosis, prolongs the inflammatory response and impedes the repair process [72, 73].
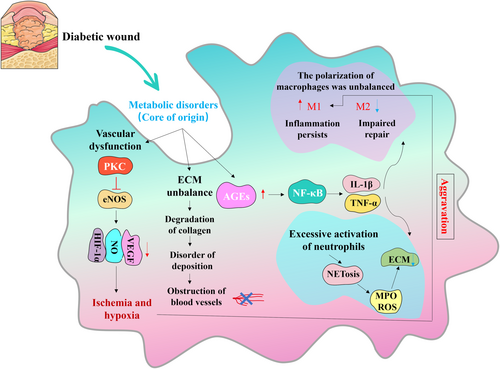
In addition, the microvascular system is subjected to multiple attacks in the metabolism-inflammatory cascade; Hyperglycemia inhibits endothelial nitric oxide synthase (eNOS) activity through PKC pathway, reduces nitric oxide (NO) production, and leads to microvasomotor dysfunction. At the same time, hypoxia-inducing factor-1α (HIF-1α) was abnormally ubiquitination degraded under normal oxygen partial pressure, which blocked VEGF gene expression and lost angiogenesis ability [74, 75]. Platelets accumulate abnormally at the site of endothelial injury mediated by AGEs and form fibrin microthrombus, which aggravates tissue ischemia and hypoxia, and the hypoxia environment in turn promotes the polarization of M1-type macrophages, forming a positive feedback loop of “ischemic inflammation” [76, 77]. The thickening of the basement membrane of microvessels and the apoptosis of endothelial cells caused tissue ischemia and hypoxia, and the expression of proangiogenic factors such as VEGF was downregulated, while the NETs formed by excessive activation of neutrophils released protease and ROS, continued to degrade the extracellular matrix and prolong the inflammatory period.
The degenerative changes of the nervous system are manifested by the loss of neurotrophic factors NGF and BDNF, which leads to the absence of pain caused by sensory nerve degeneration and makes the wound not be detected in time. The autonomic nervous dysfunction causes the skin to be cracked through abnormal secretion of sweat glands, which builds an invasion channel for pathogens such as Staphylococcus aureus, and forms biofilm wrapped by polysaccharide matrix by using anoxic microenvironment. Lipoteichoic acid (LTA) binds to Toll-like receptor 2 (TLR2) on its surface, continuously activates the innate immune response, depletes reserves of antimicrobial peptides (such as defensins), and induces fibroblast senescence [78, 79]. These pathogens form drug-resistant biofilms by secreting polysaccharide matrix, which not only escapes immune clearance, but also inhibits host defense mechanism through TLR signaling pathway. The formation of pathogenic biofilms leads to refractory infections, which further worsens the local microenvironment [80, 81].
Finally, at the ECM level, the imbalance of MMP-9/TIMP-1 leads to excessive degradation and deposition disorder of collagen. Under the combined action of oxidative stress and inflammatory factors, the overexpression of matrix metalloproteinases such as MMP-9 degrades collagen fibers. However, the relative insufficiency of tissue metalloproteinase inhibitors (TIMP) disrupts the ECM synthesis-degradation balance, and the disregulated collagen deposition not only impedes fibroblast contraction, but also adsorbs and inactivates residual growth factors [82]. The aging of fibroblasts was accelerated and the migration of keratinocytes was blocked, which severely inhibited the wound contraction and reepithelialization process. It is worth noting that this pathological network composed of metabolic disorders, inflammatory storms, vascular nerve damage, and microbial invasion forms a chronic process that is difficult to reverse through a cascade amplification effect [83]. Age-induced oxidative stress enhanced NF-κB activity and promoted the polarization of M1 macrophages. The inhibition of eNOS activity by inflammatory factors aggravated vascular endothelial injury; Ischemia and hypoxia induce MMPs expression through HIF-1α independent pathway, and finally constitute a five-element pathological network of metabolic disorders, inflammatory disorders, vascular ischemia, neurodegeneration, and ECM disintegration, resulting in an irreversible stagnation of wound repair characterized by “inflammation, ischemia, and degradation”.
4 The Role of Antioxidant Natural Plant Components in Diabetic Wound Repair
4.1 Oxidative Stress Modulation
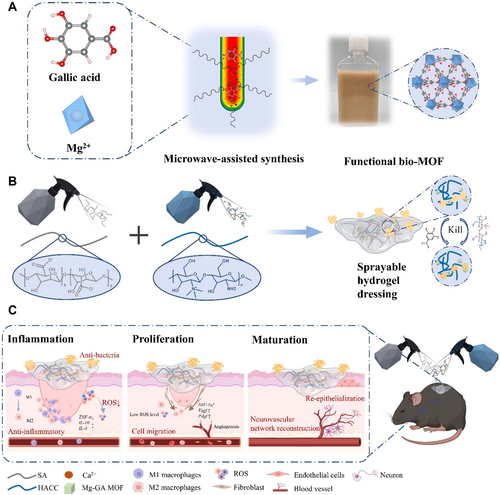
Oxidative stress plays a crucial role in the occurrence and development of diabetes. Due to insufficient insulin secretion or insulin resistance in diabetic patients, glucose and lipid metabolism are abnormal, resulting in the production of a large number of ROS, such as hydrogen peroxide, hydroxyl radicals, and superoxides [84]. At the same time, people with diabetes usually have low levels of antioxidants in their bodies and cannot effectively remove these excess ROS, leading to the occurrence of oxidative stress [85]. Oxidative stress can directly damage islet beta cells and affect insulin secretion [86]. It can also affect the insulin signaling pathway, leading to insulin resistance, forming a vicious cycle that keeps blood sugar levels high [87]. In addition, ROS activates the NF-κB and Mitogen-activated Protein Kinase (MAPK) pathways, enhancing the inflammatory response [88]. More seriously, oxidative stress is the main culprit of diabetic complications. It can damage the endothelial cells of blood vessels, promote atherosclerosis and thrombosis; Attacks retinal cells, leading to diabetic retinopathy; Affect kidney function, lead to diabetes kidney disease and so on [89-93]. Consequently, the removal of excess ROS is an effective strategy to accelerate diabetic wound healing [94]. Antioxidant herbal ingredients can effectively regulate oxidative stress by scavenging excessive ROS and enhancing the body's antioxidant capacity [95]. Many natural plant components, such as quercetin, Huangbai Liniment, gallic acid, and sinapic acid, exhibit strong antioxidant activity and can mitigate tissue damage from oxidative stress through direct scavenging of free radicals and modulation of antioxidant enzyme activity (e.g., superoxide dismutase, catalase, and glutathione peroxidase) [96]. As a central regulator of the cell's oxidation–reduction reaction (REDOX) state, Nuclear Respiratory Factor 2 (Nrf2) is responsible for regulating the transcription of cell protection and antioxidant genes, thereby affecting wound healing in diabetic patients [97]. Activation of Nrf2 or inhibition of its repressor Keap1 can reduce the production of ROS and reduce oxidative damage [97]. Huangbai Liniment can attenuate oxidative damage in skin wounds of diabetic rats by inhibiting oxidative stress-related signaling pathways, such as Nrf2, enhancing Transforming Growth Factor-β 1 (TGF-β1) levels, and reducing matrix metalloproteinase 9 (MMP9) levels, thereby promoting wound healing [98]. A hydrogel dressing containing gallic acid, which facilitates ROS scavenging, reduces oxidative stress levels and enhances vascularization and nerve regeneration during the early stages of wound repair, significantly modulating the microenvironment and promoting chronic diabetic wound repair in later stages (as shown in Figure 2) [99]. Additionally, sinapic acid, a compound extracted from natural plants, significantly increases nitric oxide (NO) and glutathione (GSH) levels while decreasing malondialdehyde (MDA) levels in a streptozotocin-induced diabetic rat wound model. The sinapic acid-loaded gel increases collagen content and promotes re-epithelialization, angiogenesis, and diabetic wound healing [100].
4.2 Inflammatory Responses Regulation and Controls Infection
The inflammatory response is a critical factor in the wound healing process; under normal conditions, it helps clear pathogens and damaged tissue to promote healing [101]. However, in diabetic patients, excessive and prolonged inflammatory responses triggered by high glucose levels and microenvironmental abnormalities lead to tissue damage and delayed wound healing [102]. Chronic inflammation obstructs the conversion of macrophages from pro-inflammatory M1 to repair M2 [103]. Inflammatory markers such as Resistin and Defensin 3 are upregulation, which in turn exacerbates tissue damage [104, 105]. In addition, the reduction of the enzyme SET Domain Bifurcated Histone Lysine Methyltransferase 2 further promotes the release of inflammatory cytokines such as Interleukin-6 (IL-6) and Tumor necrosis factor-α (TNF-α) [106]. The release of inflammatory factors such as TNF-α, IL-6, etc., can not only lead to insulin resistance, but also activate immune cells, trigger inflammatory responses, and further damage tissues and organs [107]. Inflammation also plays a key role in the complications of diabetes. For example, in diabetic nephropathy, the activation of inflammatory mediators can lead to the proliferation of mesangial cells, the accumulation of extracellular matrix, and eventually lead to renal function injury. In diabetic retinopathy, inflammatory responses promote the release of cytokines such as VEGF, leading to retinal angiogenesis and leakage, affecting vision [108, 109].
Diabetes patients are in a state of high blood sugar for a long time, which provides a favorable environment for the growth and reproduction of bacteria [110]. At the same time, hyperglycemia inhibits the chemotaxis, phagocytosis, and bactericidal activity of neutrophils, reduces the expression of antimicrobial peptides (such as psoriasis), weakens the innate immune barrier, and weakens the resistance of patients to bacterial infection [111]. Due to changes in the microenvironment, bacteria easily adhere to the surface of diabetic wounds. High glucose concentrations and the presence of extracellular matrix components provide favorable conditions for bacterial adhesion. Bacteria coordinate biofilm formation and virulence factor secretion through Quorum Sensing (QS) system to enhance infection persistence. Advanced glycation end products (AGEs) bind to the receptor RAGE, activate the NF-κB pathway, induce the overexpression of pro-inflammatory factors (TNF-α, IL-6), and inhibit the production of antimicrobial peptides. ROS and AGEs synergically activate NF-κB and MAPK pathways, amplifying inflammatory signals and forming a vicious cycle of “inflammation-oxidative stress”.
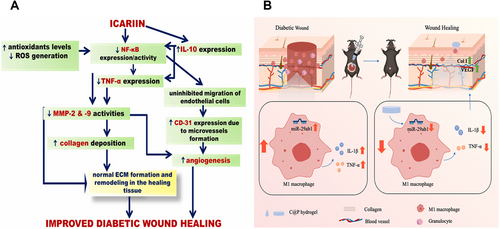
In addition to scavenging free radicals and delaying the oxidation reaction, many antioxidant natural plant ingredients also showed significant inflammatory regulation and antibacterial activities. The growth of pathogenic microorganisms was inhibited by destroying bacterial cell membrane, inhibiting enzyme activity, and interfering with nucleic acid synthesis. The inflammatory response is regulated by inhibiting inflammatory enzymes and regulating the production and release of inflammatory mediators. The NF-κB signaling pathway is an important transcription factor involved in regulating the expression of numerous inflammatory factors [112]. Icariin reduces the inflammatory response in diabetic wounds by inhibiting the NF-κB signaling pathway, decreasing the production of pro-inflammatory cytokines such as tumor necrosis factor (TNF-α), and increasing interleukin-10 (IL-10) expression (as shown in Figure 3A) [113]. Puerarin accelerates skin repair by inhibiting miR-29a/b1 expression and macrophage M1 polarization while reducing interleukin-1 beta (IL-1β) and TNF-α production (as shown in Figure 3B) [114]. Resveratrol can be inserted into the double helix structure of bacterial DNA, affect the replication and transcription of DNA, and inhibit the proliferation of bacteria. It can also regulate the fluidity of the bacterial cell membrane, destroy the integrity of the cell membrane, and make the substances in the cell leak out [115]. Curcumin, the main active component of turmeric, can act on the cell wall and cell membrane of bacteria and increase the permeability of the cell membrane. It can also inhibit the DNA topoisomerase of bacteria, interfere with the replication and transcription process of bacterial DNA, and thus inhibit the growth and reproduction of bacteria [116]. In addition, Curcumin-containing nanomaterials effectively reduce the inflammatory response, promote wound epithelialization and granulation tissue formation, and significantly facilitate wound repair [117].
4.3 Promoting Wound Healing
Diabetic wound healing is a complex process that involves multiple cellular and molecular events and is influenced by multiple factors. In addition to oxidative stress, inflammatory response, and susceptibility to infection, vascular lesions caused by diabetes cause insufficient local blood supply to the wound, and it is difficult for nutrients and immune cells to effectively reach the wound site, which also brings challenges to wound healing [118]. During the proliferative phase, the angiogenesis capacity of diabetic patients is impaired, resulting in wound ischemia and hypoxia, which affects the migration and proliferation of fibroblasts and endothelial cells, which in turn affects the formation of granulation tissue [119]. At the same time, hyperglycemia and inflammatory environment inhibited the migration and proliferation of keratinocytes and fibroblasts, and delayed wound re-epithelialization and granulation tissue formation [120]. Beyond regulating oxidative stress and inflammatory responses, many antioxidant natural plant components can promote cell regeneration and functional recovery, accelerating wound healing through various mechanisms, including promoting cell proliferation and angiogenesis [121, 122]. For example, Pien-Tze-Huang, a common Chinese medicinal preparation, activates the Nrf2 pathway in wound tissues, upregulates the expression of growth factors VEGF-A, Platelet-derived growth factor, and Epidermal Growth Factor, and promotes fibroblast proliferation and angiogenesis to enhance wound healing [123]. Gingerol can promote the proliferation of keratinocytes and fibroblasts, accelerate wound re-epithelialization and granulation tissue formation [124]. Resveratrol enhances antioxidant enzyme activity by activating Silent Information Regulator 1 (SIRT1) pathway, improves mitochondrial function, and promotes angiogenesis [125]. In addition, Epigallocatechin gallate (EGCG), the main component of tea polyphenols, has been found to significantly inhibit the skin inflammatory target signaling pathway, macrophage accumulation and inflammatory response in diabetic wounds by targeting membrane receptors, and improve local blood circulation in wounds to promote diabetic wound healing [126]. Myricetin-loaded hydrogels accelerate diabetic wound healing under inflammatory conditions by activating the Nrf2 pathway to alleviate oxidative stress and promote angiogenesis in the wound immune microenvironment [127]. These plant components not only directly clear ROS, but also improve the microenvironment of diabetic wounds through multi-target effects, promote angiogenesis, ECM remodeling, and cell proliferation, and provide a new strategy for diabetic wound treatment.
5 Application of Antioxidant Natural Plant Constituents in Diabetic Wound Repair
Diabetic wound healing disorder is closely related to oxidative stress, inflammation, infection, impaired angiogenesis, and dysfunction of cell repair induced by hyperglycemia. Natural plant-derived antioxidants, such as resveratrol, catechin gallate, puerarin, quercetin, asiatica, curcumin, mangiferin, proanthocyanidins, etc., have shown great potential in the field of diabetic wound repair due to their antioxidant, anti-inflammatory, antibacterial, and angiogenic biological activities. As shown in Table 1, the source, chemical structure, mechanism, and application of natural antioxidants such as resveratrol and curcumin in diabetic wound repair are shown.
| Name | Chemical structure | Source | Molecular mechanism | Apply | Cite |
|---|---|---|---|---|---|
| Resveratrol | 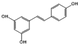 |
Grape seeds, knotweed root, peanuts, etc | Antioxidant; Anti-inflammatory; Promote angiogenesis; Improvement of insulin resistance. Enhancement of glucose uptake and metabolism; Preservation of islet β-cells and release of insulin from β-cells. |
A silk-based nanocomposite hydrogel loaded with resveratrol was used for wound healing in diabetic rats. | [125, 128-132] |
| Epigallocatechin gallate | 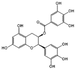 |
Green tea | Antioxidant; Anti-inflammatory; Anti-infection; Regulate metabolism; Promote angiogenesis; Promotes epithelial regeneration. |
Multifunctional hydrogel containing epigallocatechin gallate accelerates the healing of diabetic wounds in rats. | [133-139] |
| Puerarin | 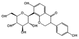 |
Pueraria lobata | Antioxidant; Anti-inflammatory; Improve microcirculation; Regulation of cell signaling; Lower blood sugar levels; Improvement of insulin resistance; Facilitating epidermal regeneration and collagen deposition. |
Chitosan based hydrogels containing puerarin effectively promote diabetic wound healing. | [114, 140-143] |
| Quercetin | 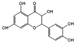 |
Apple peel, onion, grape, broccoli, tea, etc | Antioxidant; Anti-inflammatory; Anti-infection; Promote angiogenesis; lowering blood sugar; Increasing insulin sensitivity; Improve mitochondrial function; Prevention and improvement of diabetes complications; Enhances barrier function. |
Quercetin loaded carboxymethyl chitosan/tannic acid dynamic hydrogel promotes diabetic wound healing by regulating cell function. | [44, 144-150] |
| Asiaticoside | 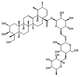 |
Centella asiatica | Anti-inflammatory; Antioxidants; Anti-allergy; Antibacterial; Neuroprotection; Immune regulatory activity; Promote angiogenesis; Promote collagen synthesis; Anti-fibrosis. |
Asiaticoside hydrogels promote diabetic wound healing by regulating the Wnt/β-Catenin signaling pathway. | [151-154] |
| Curcumin |  |
Turmeric | Antioxidant; Antibiosis; Antiviral; Anti-inflammatory; Promote autophagy; Promote angiogenesis. |
The curcumin-loaded thermosensitive hydrogel group showed higher collagen content and wound repair in the incision model. | [155-160] |
| Mangiferin |  |
Mango | Antioxidant; Anti-inflammatory; Antibacterial; Lower blood sugar levels; Promote angiogenesis; Promotes collagen synthesis; Regulation of immunity; Anti-fibrosis. |
A carrier free hydrogel containing mangiferin enhances cell and angiogenesis to accelerate wound healing. | [161-165] |
| Proanthocyanidins |  |
Grape seeds, blueberries, cranberries, pine bark, etc | Antioxidant; Anti-inflammatory; Promote angiogenesis; Bacteriostatic; Neuroprotective effects; Regulate blood sugar levels; Regulate intestinal flora; Protect extracellular matrix. |
Chitosan hydrogels containing anthocyanins significantly improved wound repair in diabetic mice. | [166-169] |
5.1 Resveratrol
Resveratrol is a polyphenolic compound found mainly in grape skins, knotweed, peanuts and other plants [170]. During the winemaking process, the resveratrol in the grape skin is partially transferred to the wine [171]. As a traditional Chinese medicine, knotweed has high resveratrol content in its roots [172]. Common extraction methods include solvent extraction method (such as using organic solvents such as ethanol and methanol), supercritical fluid extraction technology and microwave-assisted extraction method are also gradually applied [173]. It possesses various biological and pharmacological properties, such as vascular protection, anticancer effects, anti-aging, and skin whitening, along with strong antioxidant and anti-inflammatory activities [132]. Resveratrol is applied in diabetic wound repair due to its ability to regulate tissue regeneration, improve microcirculation, promote peripheral nerve recovery, regulate cytokines, and improve insulin resistance [129, 174, 175]. In diabetic wound repair, immune dysregulation and ROS regulation play crucial roles. Resveratrol effectively removes intracellular ROS, protects mitochondrial function, restores redox homeostasis, promotes adenosine triphosphate (ATP) synthesis, and reduces oxidative stress damage (as shown in Figure 4) [131]. Controlling chronic inflammation is also vital for diabetic wound repair. Resveratrol promotes wound healing by activating the PI3K/Akt pathway, reducing the secretion of inflammatory factors such as TNF-α, Inducible Nitric Oxide Synthase (iNOS), and Interleukin-1β (IL-1β), and inducing M2 macrophage polarization to reduce inflammation [130]. Recent studies have shown that Silent Information Regulator 1 controls the angiogenic activity of endothelial cells, participates in regulating vascular endothelial homeostasis and remodeling, and is highly expressed during vascular growth. It has a protective effect on endothelial dysfunction by preventing stress responses, making it a target for treating diabetic complications. Resveratrol effectively activates Silent Information Regulator 1 and promotes the degradation of Forkhead Box O1, improving endothelial function and promoting angiogenesis [125]. A hydrogel containing resveratrol nanoparticles and platelet derivatives exhibits good biocompatibility and promotes angiogenesis and diabetic wound repair by reducing TNF-α and iNOS expression [176]. A resveratrol-loaded dermal matrix, which sustains the release of resveratrol, significantly improves the oxidative status of diabetic wound tissues, reduces local inflammatory responses, and promotes wound repair in a diabetic rat model of total skin excision [175].
5.2 Epigallocatechin Gallate
Epigallocatechin gallate (EGCG) is a natural polyphenol derived primarily from tea, especially green tea, and is known for its anti-inflammatory, antioxidant, and antibacterial properties [134]. The traditional hot water extraction method uses the solubility of EGCG in hot water to extract, but there are more impurities. Further purification can be achieved by column chromatography, where the extract is separated through a column filled with a specific adsorbent according to the difference in adsorption capacity between EGCG and other components [177]. It is used in diabetes treatment to alleviate insulin resistance, oxidative stress, and mitochondrial dysfunction, as well as to reduce endoplasmic reticulum (ER) stress, lower blood glucose levels, and modulate intestinal function [133, 139]. In dermal diabetic wound repair, epigallocatechin gallate regulates macrophage polarization through its antioxidant, antimicrobial, and anti-inflammatory effects. It induces nuclear displacement of Nrf2 to promote the activation of epidermal keratinocytes via cytokeratin 16 (K16) expression, enhancing wound re-epithelialization through the K16/NRF2/KEAP1 signaling axis, and improving wound re-epithelialization in diabetic mice by inhibiting macrophage aggregation and inflammatory responses [137, 178]. Injectable hydrogels containing epigallocatechin gallate effectively mitigate excessive inflammation by modulating macrophage phenotype. In a diabetic total cutaneous wound model, these hydrogels promote diabetic wound healing through anti-inflammatory effects, angiogenesis, and collagen fiber production [179]. The emergence of bacterial biofilms in diabetic wound repair increases resistance to antibiotics, leading to ineffective conventional treatments. Nano preparations containing epigallocatechin gallate and berberine can inhibit and eliminate biofilms of drug-resistant bacteria, accelerating the repair of diabetic wounds infected with resistant bacteria [135].
5.3 Puerarin
Puerarin mainly comes from puerarin root, and the roots of puerarin and Puerarin are rich in puerarin [180]. The active ingredients are extracted by alkaline extraction and acid precipitation, which is based on the properties of puerarin dissolved under alkaline conditions and precipitated under acidic conditions. Ultrasonic-assisted extraction method can improve the extraction efficiency, accelerate the dissolution of puerarin from plant cells, and shorten the extraction time [181]. It has anti-inflammatory, antihypertensive, anti-apoptotic, and antioxidant properties [114]. It reduces ROS production, upregulates superoxide dismutase (SOD) and glutathione peroxidase 1, lowers MDA levels, and mitigates oxidative stress by mediating the phosphoinositide 3-kinase (PI3K)/Akt pathway. Puerarin protects pancreatic β-cell function and improves glucose tolerance and insulin secretion in diabetic animal models. It enhances glucose uptake in diabetic models by increasing glucose transporter 4 (GLUT4) levels, activating peroxisome proliferator-activated receptors (PPAR), and promoting fatty acid oxidation in skeletal muscle cells and adipocytes [182]. Additionally, puerarin induces M2 polarization of macrophages by downregulating inflammatory cytokine expression through inhibition of the nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways, reducing the release of inflammatory mediators by inhibiting Toll-like receptor 4 (TLR4), p38 MAPK, and Extracellular Regulated Protein Kinases 1/2 activation [141, 183]. Hybrid hydrogels containing puerarin effectively reduce ROS levels, increase SOD and glutathione peroxidase activities, promote cell proliferation, and accelerate the regeneration of diabetic full-thickness skin wounds [140].
5.4 Quercetin
Quercetin is a kind of flavonoid compound, widely found in fruits (such as apples, pears), vegetables (such as Onions, broccoli), tea and some medicinal plants (such as sophora rice), sophora rice content of quercetin is relatively high, is an important raw material for extraction [184]. Solvents such as methanol, ethanol, and acetone are often used for heated reflux extraction, which is known for its antioxidant, antibacterial, anti-inflammatory, and anti-infection activities [185]. It scavenges ROS, inhibits lipid peroxidation, improves re-epithelialization, and promotes granuloma formation and collagen deposition [150]. Quercetin effectively prevents and ameliorates diabetic complications, including diabetic nephropathy, cardiovascular issues, neuropathy, delayed wound healing, and retinopathy, by lowering blood glucose and increasing insulin sensitivity via signaling pathways such as TNF-α, NF-κB, AMPK, AKT, and Nrf2 [148]. In diabetic wound repair, quercetin promotes the elevation of catalase, glutathione peroxidase, superoxide dismutase, and total thiol levels, decreases malondialdehyde levels, improves the antioxidant status of diabetic rat wounds, and stimulates cell proliferation to accelerate wound healing [145]. Importantly, quercetin promotes vascular endothelial cell migration, reduces renal tubular dysfunction, and enhances angiogenesis, while also promoting fibroblast growth and inhibiting scarring and fibrosis to improve wound healing [44, 144]. A smart delivery system loaded with quercetin effectively mitigates oxidative stress and promotes macrophage M2 polarization via the Akt/STAT6 signaling pathway, facilitating wound repair in chronic infectious diabetes [149]. When combined with gentamicin, quercetin effectively promotes epithelial regeneration, regulates biofilms, and facilitates the wound repair process [146]. A quercetin-containing nanodressing not only exhibits the inherent antioxidant and anti-inflammatory activities of quercetin but also inhibits bacterial growth, restricts the release of bacterial toxins, reduces inflammatory responses, and accelerates wound repair in bacterial-infected diabetic mice wounds (as shown in Figure 5) [147].
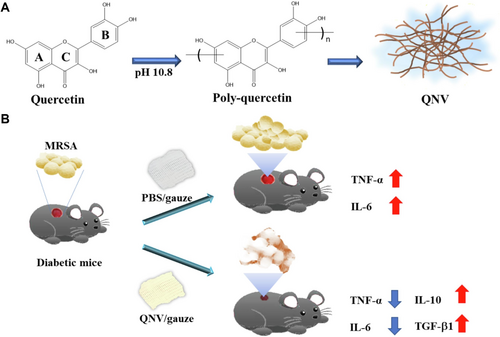
5.5 Asiaticoside
Asiaticoside is mainly derived from Asiaticoside, and its main active ingredients include asiaticoside and hydroxyasiaticoside [186]. Ethanol reflux extraction is often used, and the active components are dissolved by crushing and heating with ethanol reflux. Macroporous adsorption resin purification technology can further improve the purity of the extract, and the target components can be separated by the adsorption and desorption characteristics of different components of the resin [187]. Asiaticoside has antioxidant, antitumor, neuroprotective, cardiovascular, skin protective, fat protective, collagen synthesis, and angiogenesis properties [188]. It is primarily used in wound healing, skin care, neurological injuries, Alzheimer's disease, Parkinson's disease, tumors, lung injuries, and cardiovascular diseases [151]. In diabetic wound repair, asiaticoside promotes fibroblast proliferation, reduces scar formation, and accelerates wound healing [152]. C. asiatica glycosides improve diabetic wound healing by increasing miR-21 activity and the expression of Mitogen-Activated Protein Kinase 1 (MAPK1), Receptor Tyrosine Kinase Like Orphan Receptor 1 (ROR1), Ten-eleven Translocation (TET2), and 5-Hydroxymethylcytosine, promoting angiogenesis, collagen production, and DNA methylation regulation in the wound bed [154]. The combined application of asiaticoside and nitric oxide (NO) effectively inhibits bacterial growth on wound surfaces, alleviates inflammatory responses, increases the expression of VEGF, iNOS, Endothelial Nitric Oxide Synthase (eNOS), and CD34, and promotes angiogenesis, thereby accelerating diabetic wound repair by regulating the Wnt/β-Catenin signaling pathway [152].
5.6 Curcumin
Curcumin is mainly derived from the rhizome of the ginger plant turmeric. As a commonly used spice and traditional medicine, turmeric is widely cultivated in Southeast Asia and India [189]. Curcumin is mostly extracted with organic solvents such as ethanol and acetone at a certain temperature. In recent years, enzymolysis-assisted extraction has emerged, using cellulase and pectinase to destroy plant cell wall, promote curcumin release and improve extraction rate [190]. Curcumin is known for its antioxidant, anti-inflammatory, antibacterial and angiogenesis promoting activities. It effectively blocks the production of pro-inflammatory cytokines and reduces inflammation by inhibiting NF-κB, a transcription factor that initiates inflammatory responses, and activating the PI3K/AKT/NF-κB pathway. Curcumin also scavenges ROS and enhances antioxidant enzyme activities [156]. In diabetic wound repair, curcumin stimulates TGF-β1, enhances re-epithelialization, increases granulation tissue formation, collagen deposition, and promotes wound healing by improving local re-epithelialization [159, 160, 191]. Fibrillin-1 (FBN1) and TGF-β have significant roles in diabetic wound repair. Curcumin activates the FBN1/TGF-β pathway by inhibiting miR-152-3p, inhibiting fibroblast apoptosis, and promoting fibroblast proliferation, migration, and angiogenesis, thereby accelerating wound repair in diabetic rats [157]. Curcumin-containing multifunctional hydrogels promote angiogenesis by scavenging ROS, ameliorating oxidative stress, upregulating CD31 expression levels, and promoting M1 to M2 macrophage polarization, which enhances wound re-epithelialization, granulation tissue formation, alleviates inflammatory responses, and facilitates the formation of new blood vessels and hair follicles [155]. Curcumin-containing hydrogels made from hyaluronic acid and chitosan, synthesized via the Schiff base reaction, exhibit good antioxidant, anti-inflammatory, and cell migration-promoting effects in vitro, with rapid curcumin release alleviating wound inflammation and oxidative stress, improving the wound microenvironment, and promoting wound repair (as shown in Figure 6) [156].
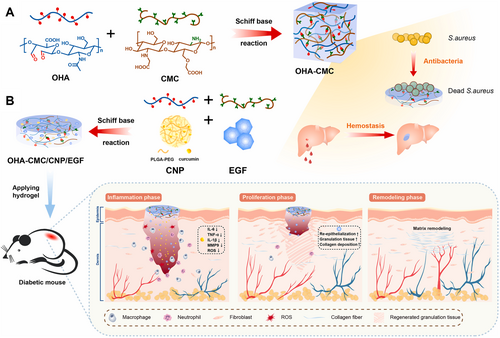
5.7 Mangiferin
Mangiferin is a natural polyphenol C-glucoside flavonoid, which is mainly derived from mango leaves and fruit. As a common fruit, mangiferin is an important raw material for extracting mangiferin. At present, the method of hot water extraction is used to extract mangiferin by its solubility in hot water. High-purity mangiferin can be obtained by column chromatography and high-performance liquid chromatography [192]. Mangiferin has anti-inflammatory, antioxidant, anti-apoptotic, anticancer, and antiviral effects. It effectively reduces oxidative stress and apoptosis by inhibiting Nox4 protein expression, stabilizing mitochondrial membrane potential, and decreasing ROS production [163]. Recent studies indicate that mangiferin significantly reduces fasting blood glucose levels in tetracycline-induced diabetic rats, affects glycogen synthesis in diabetic rats by increasing glycogen levels in muscle and liver, and promotes pancreatic β-cell proliferation and islet regeneration [162]. It also attenuates diabetes-induced pulmonary fibrosis and renal interstitial fibrosis through the Adenosine 5'-monophosphate (AMP)-activated protein kinase (AMPK)/FoxO3/SIRT3 and PTEN/PI3K/Akt pathways, respectively [164, 193]. Additionally, mangiferin-loaded hydrogels effectively promote epithelial cell migration, neovascularization, and endothelial cell proliferation for diabetic wound healing through inflammation modulation, reduction of intracellular ROS levels, neovascularization, and collagen deposition [161].
5.8 Proanthocyanidins
Proanthocyanidins are polyphenolic compounds with various biological activities, mainly derived from grape seeds, pine bark, peanut bark, cranberry, etc. Grape seeds are produced in large quantities in the wine industry, and are ideal raw materials for extracting proanthocyanidins [194]. Proanthocyanidins can be effectively extracted from common mixed solvents such as acetone-water and ethanol-water. Ultrahigh pressure extraction technology can improve the extraction rate of proanthocyanidins by destroying plant cells under instantaneous high pressure, and has little effect on its structure [195]. Proanthocyanidins have anti-oxidation, anti-tumor, antihypertensive, hypolipidemic, anti-inflammatory, anti-allergy, anti-radiation, anti-mutagenesis and antiviral effects [196]. They exhibit antioxidant, anti-tumor, anti-hypertensive, hypolipidemic, anti-inflammatory, anti-allergy, anti-radiation, anti-mutagenic, and anti-viral effects. They can regulate blood glucose levels and reduce diabetes risk, preventing cardiovascular and cerebrovascular diseases, as well as Alzheimer's disease, making them widely used in medicine and health foods [166]. Proanthocyanidins fine-tune macrophage function in inflammatory environments, promote M2-type macrophage polarization, regulate the wound microenvironment, and improve wound repair in diabetic mice by upregulating anti-inflammatory factors and reducing inflammatory responses [168]. Zhao Nuoya et al. synthesized a nanocomposite hydrogel containing proanthocyanidins, which exhibited good antimicrobial and antioxidant activity, reduced local oxidative stress in wounds, induced skin tissue remodeling, promoted blood vessel and hair follicle regeneration, and accelerated wound healing [197].
6 Conclusion and Outlook
The wound repair process in diabetic patients is influenced by several factors, including chronic inflammation, oxidative stress, and microangiopathy. Oxidative stress not only damages cells but also decreases cellular function, affecting all stages of wound healing. Antioxidant natural plant components, such as polyphenols and flavonoids, effectively remove reactive oxygen species from the body and reduce oxidative damage, thereby creating a favorable microenvironment for wound healing. They promote the proliferation and migration of fibroblasts and keratinocytes, collagen synthesis, and angiogenesis, enhancing tissue regeneration and improving wound healing capacity. Antioxidant natural botanical ingredients typically have a higher safety profile and fewer side effects than synthetic drugs, and their multi-targeted effects allow them to modulate multiple biological pathways simultaneously during the complex diabetic wound healing process, providing a comprehensive solution for diabetic wound treatment.
However, the clinical application of these natural plant compounds is often limited by low bioavailability, poor stability, and insufficient targeted delivery, and needs to be combined with biological materials to optimize efficacy. For example, the solubility of Resveratrol in water is only 0.03 mg/mL, but its solubility can be increased by more than 20 times by loading nanoparticles. The inclusion of biomaterials not only solves the solubility problem, but also protects the active ingredient from enzymatic or oxidative degradation, extending its half-life. At the same time, nanoparticle surface modification targeting ligands can specifically bind endothelial cells or inflammatory cells to enhance the accumulation of drugs on the wound. Pegylated liposomes reduce the binding of proanthidins to serum proteins and extend the wound retention time to 24 h. In addition, biomaterials can form synergistic effects with antioxidants. For example, Quercetin loaded in the electrospinning fiber membrane not only promotes slow drug release, but also absorbs wound exudates through the porous structure of the material, reducing the risk of bacterial colonization. At the same time, certain biomaterials, such as collagen scaffolds, can mimic extracellular matrix, provide physical support for antioxidants, and promote fibroblast migration and angiogenesis. In the future, multi-dimensional intelligent response systems such as pH/ROS/glucose can be developed to achieve accurate drug release; Or simulate the natural bionic delivery mechanism of extracellular vesicles (EVs), and construct bionic skin scaffolds containing antioxidants through 3D bioprinting to integrate blood vessel networks for precise wound treatment.
In addition, antioxidant natural plant ingredients face problems such as extraction and standardization of active ingredients, bioavailability, lack of clinical evidence, and individual differences, so it is necessary to optimize the extraction process, large-scale preparation, optimization cost and stability, and ensure the stability and activity of active ingredients. At the same time, the degradation products and residues of biological materials may cause immune reactions, and it is necessary to conduct multiple clinical trials and further long-term safety research. In addition, appropriate materials and drug combinations should be selected according to the patient's blood sugar level and wound type to provide personalized design schemes to improve the efficacy. Future directions include developing smart responsive materials (such as photocontrolled or enzyme-controlled release systems), incorporating Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) technology to regulate antioxidant metabolic pathways, and using Artificial Intelligence (AI) algorithms to optimize material-drug compatibility for precision wound treatment. Through continuous exploration and research, it is expected to promote the wide application of these natural ingredients in diabetic wound repair, and provide better solutions for the health of diabetic patients.
Author Contributions
Lele Meng: conceptualization (equal), investigation (equal), methodology (equal), resources (equal), formal analysis (equal), writing – original draft (equal). Xueying Zhang: conceptualization (equal), visualization (equal), formal analysis (equal). Li Sun: conceptualization (equal), methodology (equal), writing – review and editing (equal), funding acquisition (equal), project administration (equal), supervision (equal). Long Chen: conceptualization (equal), methodology (equal), writing – review and editing (equal), funding acquisition (equal), project administration (equal), supervision (equal). All authors have read and approved the final manuscript.
Acknowledgments
We confirm that there are no additional contributors or funding sourcesto acknowledge for this work.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.




