Beyond Conventional Approaches: The Revolutionary Role of Nanoparticles in Breast Cancer
These authors contributed equally: Mohan Liu, Yusi Wang and Yan Li.
ABSTRACT
Breast cancer (BCa) remains a significant health challenge worldwide, with a high propensity for early metastasis and poor prognosis. While surgery, chemotherapy, and radiotherapy are fundamental for managing BCa, severe side effects, such as low patient adherence and suboptimal survival outcomes, cause concern. Therefore, there is a critical need to innovate new approaches that facilitate early detection, accurate diagnosis, and more effective treatment strategies for BCa. Nanotechnological approaches have been introduced for the diagnosis and treatment of various cancers, especially BCa. The current review aims to emphasize and highlight possible applications of nanomedicine in early detection, accurate diagnosis and efficient treatment strategies for BCa. Nanocarriers can deliver chemotherapeutic agents, enhancing cytotoxicity against BCa cells and preventing the development of drug resistance. Nanoparticles also boost the efficacy of gene therapy which promotes their potential for regulating gene expression. The co-delivery of drugs and genes by nanoparticles can have a synergistic effect on BCa and remodel the tumor microenvironment. In this review, we discussed the latest advances in the application of nanomedicines for diagnosing and treating BCa. Current research highlights the potential benefits of nanomedicine over traditional approaches and further efforts to translate these research findings into clinical practice for BCa.
1 Introduction
Breast cancer (BCa) is the most common malignant tumor affecting women. According to the World Health Organization (WHO), there were 2.261 million new cases of BCa patients worldwide in 2020, ranking first among all malignant tumors. Moreover, reports from the National Cancer Center indicate that the incidence and mortality rates of breast cancer in China are increasing rapidly. BCa is a highly heterogeneous disease that includes several molecular subtypes that are broadly defined by the differential expression of cell surface receptors. The common intrinsic molecular subtypes of BCa include luminal A, luminal B, and Her2 overexpression, as well as basal cell tumors, which are further classified into specific subtypes. Thereinto, triple-negative breast cancer (TNBC) refers to breast tumors that lack estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor 2 (HER2) [1]. TNBC accounts for 15% to 20% of all BCa [2]. Gene expression profiling has classified TNBC as a subtype of basal-like BCa [3]. In contrast to other BCa subtypes, TNBC tends to occur in younger women, with a mortality rate as high as 40% in advanced stages within the first 5 years after diagnosis [3]. Approximately 45% of patients diagnosed with advanced TNBC will experience distant metastasis to the brain, with a median survival time of 13.3 months [4]. Due to the lack of expression of targetable proteins, TNBC patients with poorer prognoses than other BCa [2] primarily rely on surgery, radiotherapy, and chemotherapy. TNBC has a recurrence rate of up to 25%, and the presence of residual micrometastatic disease after neoadjuvant chemotherapy is associated with an increased risk of tumor recurrence and mortality, while options for routine postoperative adjuvant chemotherapy are limited [5].
The lack of effective biomarkers for predicting treatment responses in BCa limits the possibilities for personalized treatments [6]. Aside from poly ADP-ribose polymerase (PARP) inhibitors, which have been successfully incorporated into clinical practice for patients with BRCA1/2 mutations, and the checkpoint inhibitor atezolizumab, which was recently approved as a first-line therapy for metastatic patients, traditional chemotherapy continues to be the primary treatment for many BCa patients without biomarker guidance [7-9]. BCa patients commonly develop strong resistance to chemotherapy and radiotherapy drugs, which fail to improve patient prognosis or quality of life [10]. Due to the variability in pathological characteristics among BCa patients, there is an urgent need to develop treatment strategies tailored to the specific conditions of each patient.
In the diagnosis and treatment of BCa, traditional methods have made significant advancements over the past few decades, but they still have several limitations. Common diagnostic techniques include imaging (such as mammography, ultrasound, and magnetic resonance imaging) and tissue biopsies [11, 12]. While these methods can provide initial information about the location and size of tumors, they face challenges in early detection and precise characterization. For instance, mammography and ultrasound can often produce false-positive and false-negative results [13], and tissue biopsies, though accurate, are invasive and can cause patient discomfort and complications [14]. Conventional treatments primarily consist of surgery, radiation therapy, and chemotherapy. Surgery is the main approach for early-stage breast cancer, aiming to remove the tumor and achieve a cure [2]. However, post-surgery, patients often require additional treatments such as radiation therapy and chemotherapy to prevent recurrence [15]. Although these treatments have improved survival rates, they also have notable drawbacks. Radiation therapy can damage surrounding healthy tissue, increasing patient discomfort and recovery time [16]. Chemotherapy, on the other hand, often leads to a range of side effects, including nausea, hair loss, and immune system suppression, significantly impacting the patient's quality of life [17]. Furthermore, these conventional therapies are less effective for advanced or metastatic breast cancer, failing to provide a complete cure in many cases. These shortcomings highlight a critical gap in current clinical practices, particularly the need for more integrated and precise approaches to manage breast cancer. These shortcomings highlight a critical gap in current clinical practices, particularly the need for more integrated and precise approaches to manage breast cancer. Theranostics is an innovative approach that combines diagnostic and therapeutic functions in a single platform to improve the management of BCa, has emerged as a promising solution to these challenges [18]. This concept aims to provide more precise and personalized treatment by offering real-time information about the tumor and the efficacy of the therapy [19]. By integrating imaging agents and therapeutic drugs into a single nanoparticle, these platforms can track the delivery of the drugs to the tumor and assess how well the treatment is working. One of the key advantages of multifunctional nanoplatforms is their ability to enhance the accuracy of diagnosis. By incorporating imaging agents such as magnetic resonance imaging (MRI) or fluorescence markers, these platforms can provide detailed and specific images of tumors. For example, liposomes loaded with chemotherapy drugs and tagged with MRI contrast agents can not only deliver the therapy but also provide high-resolution images of the tumor, helping to identify the exact location and extent of the disease [20-22]. Moreover, these nanoplatforms can improve the efficacy and safety of treatments. Gold nanoparticles, for instance, are often used for photothermal therapy (PTT) because they can convert light into heat, destroying cancer cells [23]. When combined with near-infrared (NIR) imaging, these nanoparticles allow doctors to monitor the heat generation and treatment progress in real-time, ensuring that the therapy is both effective and safe [24-26]. Clinical trials have already shown promising results with multifunctional nanoplatforms. For instance, radiolabeled iron-based nanoparticles used with PET and CT imaging have successfully monitored the distribution and metabolism of chemotherapy drugs in patients, allowing for more precise treatment planning and adjustments [27-29].
The development of nanoplatforms has led to revolutionary innovations in drug delivery, allowing for more precise targeting of drugs to cancer cells and minimizing damage to normal cells [30, 31]. Moreover, the release rate and timing of drugs can be controlled, reducing the systemic side effects of chemotherapy drugs through nanotechnology [32], and can also be used to develop multidrug carrier systems that help overcome multidrug resistance in BCa [33].
In this review, we focus on nanoplatform for the diagnosis and treatment of BCa. A nanoplatform refers to a multifunctional system composed of nanosized particles, which is designed for various biomedical applications, including drug delivery, diagnostics, and imaging [34]. With well engineering, theses platforms can transport drugs to overcome conventional biological barriers and can be guided to specific cell types within target organs using active or passive targeting techniques, enabling applications in BCa imaging, diagnosis and treatment [35]. According to different application, nanoplatforms can be broadly divided into three main types: diagnostic nanoplatforms, therapeutic nanoplatforms and theranostic nanoplatforms, which integrate both diagnostic and therapeutic functions into a single system [36]. Several nanomaterials have shown promise in breast cancer treatment and diagnostics, including carbon-based nanomaterials, silica-based nanomaterials, polymer-based nanomaterials, metal-based nanomaterials and exosomes. Nanoplatforms for clinical applications must be biocompatible and tunable with special targeting and low toxicity. Excellent biocompatibility and biodegradability can help the nanoparticles evade immune clearance and avoid unwanted immune response and inflammation [37]. Tunability means the size, shape, and surface properties of nanomaterials can be tailored to optimize drug delivery, targeting and drug release [38]. Special targeting and low toxicity mean high bioavailability and safety [39]. In summary, nanoplatforms are versatile systems that leverage the unique properties of nanomaterials, which is comprehensively revealed the current development of novel BCa nanomedicine and future trends.
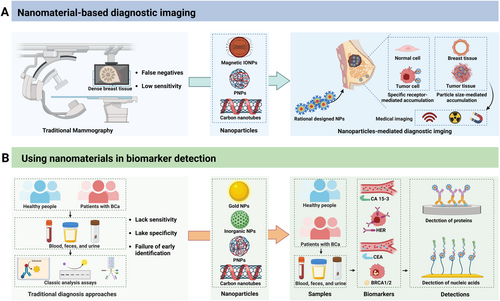
2 Nanomaterial-Based Diagnostic Imaging
For precise treatment, effective screening and diagnostic methods are vital for delivering personalized drugs that can enhance patient outcomes and improve survival rates [40]. Currently, tissue biopsy is the predominant method for detecting, staging, and assessing the prognosis of TNBC patients. However, cancer tissue biopsies are invasive and difficult to obtain, and the molecular and genetic information derived from biopsies offers limited utility for early detection, screening, and monitoring (Figure 1 and Table 1) [50]. Moreover, mammography remains the only clinically validated imaging technique for the early detection of TNBC, although it has a considerable risk of false negatives and low sensitivity in dense breast tissue [51]. For traditional prognostic markers, the serum level of cancer antigen 15-3 (CA15-3) is currently used for monitoring breast cancer; however, CA15-3 levels are not effective for diagnosing this disease, particularly in early-stage patients, and do not aid in therapeutic decision-making for TNBC patients [52]. To address the limitations of traditional techniques used for TNBC screening, nanotechnology provides a revolutionary approach and has multiple benefits [53, 54].
| Nanoparticle composition | Size (nm) | Application | References | |
|---|---|---|---|---|
| Magnetic IONP | Fe3+-based metal-phenolic networks with BSA modification | 158.9 ± 1.8 | Potential T1-weighted contrast agents | [41] |
| IONP/MSN | 3.2 ± 0.5 | T2-weighted contrast ability on a 9.4 T MRI scanner | [42] | |
| DIR/silver sulfide nanoparticles/IONP/PEGylated micelles | 88 ± 22/114 ± 2.7 | Potential CT contrast agent T2-weighted MRI agent |
[43] | |
| Polymeric nanoparticles | SQ1 nanoprobe/DSPE-PEG2000/pentapeptide CREKA | ~20 | Significant enhancement of NIR-II imaging signals | [44] |
| Manganese-containing inner core/phospholipid bilayer shell/fluorescent dye | ~40 | Potential T1-weighted contrast agents | [45] | |
| Peptide-modified SQ/fibronectin-targeting peptide/GFLG peptide/maleimide-DSPE-PEG-modified ultrasmall magnetic IONP | ~20/11–15 | MRI contrast agent light-up fluorescence imaging |
[46] | |
| Targeted probe/QSY21-NHS/PEG | 21.7 ± 2.5 | Fluorescence image-guided breast cancer surgery | [47] | |
| Carbon nanotubes | SWNTs/phospholipid-PEG-amine/Rituxan/Herceptin | ~50–150 | As NIR fluorophors for sensitive and selective biological detections and imaging | [48] |
| Gadolinium doped carbon dots/NIR photothermal agent | 308.8 | MR- guided combination photothermal chemotherapy for TNBC | [49] |
- Abbreviations: BSA, bovine serum albumin; DiR, near-infrared fluorophore; DSPE-PEG2000, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy-(polyethylene glycol)-2000; Gox, glucose oxidase; IONP, iron oxide nanoparticle; MRI, magnetic resonance imaging; MSN, mesoporous silica nanoshell; NIR, near-infrared; PEG, polyethylene glycol; SQ, squaraine-based photosensitizer; SWNT, single-walled carbon nanotube; TNBC, triple negative breast cancer.
2.1 Magnetic Iron Oxide Nanoparticles (IONPs)
Superparamagnetic iron oxide magnetic nanoparticles (MNPs) are molecular-specific imaging agents for magnetomotive optical coherence tomography (MM-OCT) [55]. Molecularly targeted iron oxide nanomaterials (a diameter of 20 nm) were directed to orthotopic breast cancer in rats using anti-Her2/neu antibodies, this in vivo MM-OCT imaging of dynamic functionalized MNPs demonstrated high specificity, showing a signal only when the cancer was targeted by the iron oxide nanomaterials and not in the control group [56]. They are biocompatible, biodegradable, and exhibit low toxicity. Their unique paramagnetic properties produce strong T2, T*2, and T1 contrast effects even at low concentrations [57]. Various forms of IONPs have been preclinically and clinically tested and shown to be safe for use. The incorporation of Fe3+ into metal-phenolic networks (MPNs) with bovine serum albumin (BSA) modification nanoparticle to be potential MRI contrast agents, which effective accumulated NPs at the tumor site in 4T1 tumor-bearing BALB/c mice and favored the precise MR imaging [41]. Du et al. reported core–shell–satellite nanomaces (Au@MSN@IONP) are fabricated by synthesizing ultrasmall IONPs and decorating them on a larger mesoporous silica nanoshell (MSN) with an embedded gold nanorod (Au) for photothermal conversion [42]. The MRI contrast capabilities of these nanomaces were investigated both in vitro and in vivo, showing maximal accumulation in tumor tissue at 6 h postinjection, as indicated by the darkest T2-weighted MRI signals at this time in the MDA-MB-231 tumor-bearing mice [42]. Hsu et al. encapsulated a near-infrared fluorophore (DiR), silver sulfide nanoparticles (Ag2S-NP), and iron oxide nanoparticles (IONP) within PEGylated micelles by a one-pot ultrasonic emulsification procedure [43]. This facilitated in vivo tumor imaging, as demonstrated by the contrast enhancement postinjection in a murine model of breast cancer [43].
2.2 Polymeric Nanoparticles
In recent years, polymeric nanoparticles (PNPs) have gained significant popularity for in vitro and in vivo bioimaging due to their exceptional versatility and optical properties. These include polymeric NPs that encapsulate near-infrared dyes, aggregation-induced-emission fluorogens, cationic dyes doped with bulky hydrophobic counterions, and semiconducting polymers [58]. Thus, fluorescent PNPs have applied as a highly attractive and promising platform for visualizing the complex biological and pathological processes.
A multifunctional nanoparticle with potential for NIR imaging and phototherapy has also been developed. Yao et al. fabricated and synthesized a new NIR-II emissive squaraine photosensitizer 1 (SQ1) nanoprobe through a donor−acceptor−donor (D–A–D) structure, with the NIR-I squaraine dye SQ2 was designed with ethyl-grafted 1,8-naphtholactam as donors and squaric acid as the acceptor [44]. To achieve NIR-II emission, malonitrile was added to the squaric acid acceptor. The SQ1 nanoprobe have the advantages of NIR-II imaging in angiography, effectively showed blood vessel distribution and flow in hind limb arteries with enhanced spatial resolution in mice bearing MDA-MB-231 xenografts [44]. Moreover, in vivo tests revealed significant tumor-targeting imaging capability of the SQ1 nanoprobe, with stronger signals in tumors compared to a nontargeting version. This highlights the feasibility and efficacy of using SQ1 nanoprobe for NIR-II angiography and tumor imaging [44]. A multifunctional nanoplatform (NanoMn-GOx-PTX) featuring a manganese-containing inner core and a phospholipid bilayer shell that co-loads glucose oxidase, paclitaxel, and a NIR fluorescent dye [45]. After intratumoral injection of NanoMn-GOx-PTX in 4T1 tumor-bearing mice, T1-weighted imaging of mice showed clear tumor site visibility at 1–3 h, which weakened over time, possibly due to released manganese ions participating in chemo-dynamic therapy [45].
A versatile nanoprobe (Pep-SQ@USPIO) was designed for fibronectin-targeting MR imaging and Cathepsin B (CTSB)-activatable fluorescence imaging for TNBC [46]. The fluorescence capability of the SQ were quenched by ultrasmall superparamagnetic iron oxide (USPIO). TNBC-derived CTSB selectively cleaved the Gly–Phe–Leu–Gly (GFLG) linker, activating fluorescence [46]. The in vivo MR/NIRF imaging capabilities of Pep-SQ@USPIO was conducted on mice with MDA-MB-231 or MCF-7 tumors, showed a significant T2 contrast effect with MRI signal intensity decreasing in MDA-MB-231 tumor sites after injection and minimal change in MCF-7 sites. T2 relaxation time decreased in MDA-MB-231 tumors up to 4 h postinjection, with a slight increase at 6 h, whereas MCF-7 tumors showed a weaker decrease. Inhibition of CTSB activity in MDA-MB-231 tumors did not affect nanoprobe accumulation, indicating effective targeting and imaging properties [46]. Another smart fibronectin-targeting and metalloproteinase-activatable imaging probe, was developed by Cheng et al. [47]. The MMP-9-cleavable peptide sequence GPVGLIGK (GK8) was used to link the NIR dye Cy5.5, and the fibronectin-targeting peptide CREKA, forming the dual-targeted NIR-I probe (CREKA-GK8-QC). These molecules form uniform nanoparticles in aqueous solutions due to differences in hydrophilicity and lipophilicity [47]. NIR fluorescence imaging showed that CREKA-GK8-QC localized at the breast tumor 30 min postinjection, peaking from 1 to 12 h and decreasing by 48 h in orthotopic 4T1 breast cancer models [47]. These results highlight this imaging probe has tumor-targeting abilities and potential for intraoperative image-guided surgery of breast tumors [47].
Li et al. innovatively constructed a TNBC-targeting nanoplatform (through the strong affinity between HA and CD44), by incorporating ICG and Fe-polydopamine into the HA molecular chain with covalent bonds, which was designed for NIR FI/MRI/PTI guided CDT/PDT/PTT [59]. Once accumulated in tumor tissues, HA-ICG-Fe-polydopamine generated amplified magnetic resonance signals for MRI in MDA-MB-231-tumor xenografted mice [59]. Fluorescence signals peaked at 8 h at the tumor site, with an enhanced T2 signal observed at 8 h postinjection by in vivo MRI, highlighting the potential of HA-ICG-Fe-polydopamine in TNBC precise diagnosis [59].
A rationally synthesized NP loaded with MnFe2O4, DOX, and NO donor l-Arg in PLGA shells (DNMF/PLGA NPs) was used for MRI/PAI dual imaging in 4T1 tumor-bearing mice [60]. It showed excellent photoacoustic imaging (PAI) contrast enhancement in vitro and accumulated at the tumor site through passive targeting with a residence time up to 12 h vivo [60]. Additionally, outstanding T1 signal enhancement at 6 h post-intravenous injection demonstrated DNMF/PLGA NPs) can be used as a fast and accurate MRI-contrast agent [60].
Rutin hydrate (RH) is applied to bind the TNBC promising target glucose transporters (GLUTs) receptors, and Fe3+ biomineralized with RH to form nanoprobe colloidally stabilized by a matrix of poly(vinyl pyrrolidone) (PVP), allowing this Fe(III)–RH/PVP nanoprobe can be recognized by GLUTs receptors on the surface of tumor cell and target 4T1 tumors in vivo [61]. The T1 signal intensity of this novel nanoprobe was significantly stronger compared to the clinical MRI agent Gd-DTPA in 4T1 subcutaneous and orthotopic tumors [61].
2.3 Carbon Nanotubes
The sensitive optoelectronic properties of carbon nanotubes, in response to their surrounding environment, make them highly suitable for selective biosensing applications [62, 63].
At an early stage, biologically inert single-walled carbon nanotubes (SWNTs) functionalized with polyethylene glycol are conjugated to specific antibodies to target CD20 receptors on B-cells and Herceptin to target HER2/neu positive breast cancer cells. This method shows ultralow NIR autofluorescence in various cells, advantageous over high autofluorescence in the visible range. Thus, SWNTs serve as novel NIR fluorophores for sensitive and selective biological detection and imaging in vitro and potentially in vivo [48].
Gadolinium-doped carbon dots (Gd@CDs) were synthesized using 3,4-dihydroxyhydrocinnamic acid (DHCA), 2,2′-(ethylenedioxy)bis(ethylamine), and gadolinium chloride (GdCl3) via a hydrothermal method [49]. Gd@CDs acting as a T1 contrast agent, enhanced the MRI effect by increasing the longitudinal relaxation rate of surrounding water molecules, with brightness increasing with Gd@CD concentration [49]. After photothermal agent IR825 and antitumor drug Dox loaded on the surface of Gd@CDs, Dox@IR825@Gd@CDs showed increasing MR signals at tumor sites over time by in vivo MRI in 4T1 xenograft tumor model [49].
3 Using Nanomaterials in Biomarker Detection
Screening and early diagnosis are crucial for reducing BCa mortality rates and treatment costs. Biomarkers play a critical role in the diagnosis, prognosis, and treatment of BCa, offering insights into the biological characteristics of tumors. These biomarkers can be classified into various categories, including genetic, protein, and cellular markers, each providing valuable information for clinical decision-making. Thus, highly sensitive and selective methods capable of detecting low-abundance cancer biomarkers in biological samples are always in demand [64]. Over the past several decades, various rapid analysis assays for disease markers have been developed, including enzyme-linked immunosorbent assay (ELISA), chemiluminescent immunoassays, electrical chemiluminescent immunosensors. However, these traditional diagnosis approaches of biomarker detection often lack the sensitivity and specificity needed for early-stage identification and monitoring of TNBC. Nano-objects with external dimensions ranging from 1 to 100 nm or nanostructured materials with internal or surface features at the nanoscale made nanomedicine as a highly selective diagnostic method at the molecular level. For example, cardiac troponin I (cTnI) is the optimal biomarker for evaluating myocardial injury in clinic and the accuracy of cTnI measurement depends on the number of cTnI molecules binding to capturers and the intensity of this binding [65]. Nanomaterials offer a promising solution to these limitations due to their physicochemical properties, providing a sensitive and selective method for monitoring myocardial infarction (MI). In this part, we summarize various nanomaterial applications in high precision biomarker detection (Figure 1 and Table 2).
| Target | Nanoplatform | Detection techniques | Linear range | LOD | Sample testing matrix | References |
|---|---|---|---|---|---|---|
| CA 15-3 | An amine-functionalized nanocomposite of Pt and Fe3O4 nanoparticles on multiwalled carbon nanotubes | EIS | 0.0005–100 U/mL | 0.00008 U/mL | Serum samples | [66] |
| The electrochemical responses of anthraquinone-2-carboxylic acid, thionine chloride, and AgNO3(Ag+) on the PEI-AuNPs | EIS CV |
— | 0.10–100 U/mL | Serum samples | [67] | |
| Gold-silver bimetallic nanoclusters with zein and formed a protective shell around Au-Ag BNCs | EIS CV |
0.001–100 U/mL | 0.0003 U/mL | Serum samples | [68] | |
| Ternary silver/titanium dioxide/reduced graphene oxides nanocomposites | 0.1–300 U/mL | 0.07 U/mL | Serum samples | [69] | ||
| HER2 | Gold nanoparticles decorated copper-organic framework and quaternary chalcogenide with platinum-doped graphitic carbon nitride | EIS DPV |
0.01–1.00 pg/mL | 3.00 fg/mL | Plasma samples | [70] |
| Graphene oxide/DNA/AuNPs/GCE | CA | 0.37–10 nM | 0.16 nM ERBB2(HER2) 0.23 nM CD24 |
Spiked real sample | [71] | |
| Laser-scribed graphene electrodes modified with nanostructured gold and molecularly imprinted polymer | EIS CV SWV |
1–200 ng/mL | 0.43 ng/mL | Serum samples | [72] | |
| PEGylated nano-structured cerium oxides | CV | 0.001–20.0 ng/mL | 34.9 pg/mL | Serum samples | [73] | |
| CEA | Aptamer-modified magnetic Fe3O4–Au nanoparticles | — | 2–200 ng/mL | 0.6 ng/mL | Serum samples | [74] |
| Self-polymerized dopamine-decorated Au and coordinated with Fe-MOF | EIS CV |
1 fg/mL–1 μg/mL | 0.33 fg/mL | Serum samples | [75] | |
| Three-dimensional gold nanoparticles/prussian blue-poly(3,4-ethylenedioxythiophene) | EIS CV DPV |
0.05–40 ng/mL | 0.01 ng/mL | Serum samples | [76] | |
Graphene/methylene Blue-chitosan/antibody and bovine serum albumin on indium tin oxide glass electrode |
EIS CV LSV |
0.1–1 and 1–100 pg/mL | 0.04 pg/mL | Serum samples | [77] | |
| Hairpin-shaped oligonucleotide-functionalized gold nanorods, graphene and the avidin-biotin reaction | DPV | 5 pg/mL –50 ng/mL | 1.5 pg/mL | Serum samples | [78] | |
| BRCA1 and BRCA2 | Immobilized capture probe DNA, target DNA and gold nanoparticle conjugated reporter probe DNA | — | 1 fM–100 pM BRCA1 DNA | 100 aM | — | [79] |
| MPA, polyethylene glycol functionalized gold nanoparticle, capture DNA, target BRCA1 DNA and gold nanoparticle labeled reporter DNA on gold electrode | CA EIS STS |
50 aM–1 nM | 50 aM | — | [80] | |
| Reduced graphene oxide-yttria nanocomposite | DPV CA |
10 aM–1 nM | 5.95 aM | Serum samples | [81] |
- Abbreviations: CA, chronoamperometry; CV, cyclic voltammetry; DPV, differential pulse voltammetry; EIS, electrochemical impedance spectroscopy; EQCM, electrochemical quartz crystal microbalance; LOD, limits of detection; LSV, linear sweep voltammetry; MPA, mercaptopropionic acid; STS, scanning tunneling spectroscopy; SWV, square-wave voltammetry.
3.1 CA 15-3
CA 15-3 is a crucial biomarker for assessing disease severity and predicting recurrence in early BCa diagnosis [82]. Human serum typically contains less than 30 U/mL of CA15-3 in normal physiological conditions, while rapid increases in CA15-3 levels occur in 30%–50% of BCa patients [66]. Therefore, monitoring CA15-3 concentration provides accurate data on patient recovery status and facilitates detection of BCa recurrence and metastasis. Carbon nanotubes, with their high surface area and excellent electrical conductivity, are ideal for developing electrochemical sensors. When functionalized with specific biomolecules, these nanotubes can detect TNBC biomarkers through changes in electrical signals. This approach provides rapid and accurate detection, making it suitable for point-of-care diagnostics. An ultrasensitive sandwich-type electrochemical immunosensor was designed for breast cancer biomarker CA 15-3 detection [66]. An amine-functionalized composite of reduced graphene oxide and Fe3O4 nanoparticles (MRGO-NH2) was used as the sensor platform. An amine-functionalized nanocomposite of Pt and Fe3O4 nanoparticles on multiwalled carbon nanotubes (Pt–Fe3O4–MWCNTs–NH2) served as the signal-amplifying label, displaying enhanced peroxidase-like performance. The immunosensor demonstrated a broad detection range of 0.0005 to 100 U/mL with a lower detection limit of 0.00008 U/mL [66].
A label-free multiplex electrochemical biosensor is designed using three redox species-antibody-conjugated PEI coated-gold nanoparticles (PEI-AuNPs) [67]. The screen-printed carbon electrode with a three-working electrode array is modified with PEI-AuNPs conjugates [67]. Multiplex sensing is achieved using the electrochemical responses of anthraquinone-2-carboxylic acid, thionine chloride, and AgNO3(Ag+) on the PEI-AuNPs for detecting MUC1, CA15-3, and HER2, respectively [67]. The detection ranges are 0.10–100 U/mL for CA15-3 and 0.10–100 ng/mL for MUC1 and HER2, with detection limits of 0.21 U/mL, 0.53 ng/mL, and 0.50 ng/mL, respectively, all below clinically relevant cut-off levels [67].
A synergetic-effect-enhanced sandwich-type electrochemiluminescence (ECL) system was constructed to detect CA15-3 [68]. Gold-silver bimetallic nanoclusters (Au-Ag BNCs) with zein as a protective ligand were synthesized, and Zein formed a protective shell around Au-Ag BNCs [68]. The bimetal's synergistic effect enhanced ECL emission, making Au-Ag BNCs ideal ECL probes and achieving a detection range of 0.001–100 U/mL and a detection limit of 0.0003 U/mL, supporting future CA15-3 detection applications [68]. Wang et al. developed a signal amplification strategy of label-free ultrasensitive electrochemical immunosensor for quantifying the breast cancer antigen CA 15-3. Ternary Ag/TiO2/rGO nanocomposites were used as a signal amplification platform and amplificated signal was explored by measuring hydrogen peroxide reduction and evaluating the electrocatalytic current response from the CA 15-3 antibody-antigen immunoreaction on the Ag/TiO2/rGO surface. A linear concentration range of 0.1–300 U/mL with a correlation coefficient of 0.9996 was achieved, and the detection limit was 0.07 U/mL in human serum samples [69].
3.2 HER2
Overexpression of HER2 is closely related to breast cancer malignancy and poor prognosis, and HER2-positive tumors account for about 15%–30% of BCa, making HER2 applied as a valuable prognostic and predictive biomarker [83]. Current clinical methods for determining HER2 expression levels rely on slide-based assays such as immunohistochemistry, fluorescence in situ hybridization and chromogenic in situ hybridization-based assays that require invasively collected primary tumors. Since a persisting challenge in treating a subset that develops late-stage metastasis and shows resistance to targeted therapies, the early detection of breast cancer underscores the need for and potential benefits of more sensitive techniques [84]. The electrochemical immunosensor was developed using Au nanoparticles decorated Cu-organic framework (AuNPs/Cu-MOF) and platinum-doped graphitic carbon nitride (g-C3N4) with quaternary chalcogenide Cu2ZnSnS4 nanoparticles (CZTS NP) (Pt/g-C3N4) [70]. The synthesis of AuNPs/Cu-MOF composite involved an amidation reaction between amino group-functionalized AuNPs and carboxylic acid-containing Cu-MOFs [70]. After conjugation of HER2 antibody primer and HER2 protein antigen to AuNPs/Cu-MOF as the sensor platform, CZTS NPs/Pt/g-C3N4 composite was prepared via one-pot hydrothermal method [70]. The developed immunosensor demonstrated high sensitivity with a HER2 detection limit of 3.00 fg/mL [70]).
A suitable biosensor, Cd2±aptamer@AMNFs@ZIF-67 nanocomposite, has been developed for detecting the HER2 biomarker. Antimonene through adsorb single-stranded DNA-aptamer, allowing HER2 biomarker detection [71]. The aptamer binds metal ions (Cd2+) as signal labels through electrostatic interactions, forming the antimonene@ZIF-67@aptamer composite [71]. allowing for the analytical detachment of aptamer-marker complexes after targeting the biomarker. The detection limit was 4.853 fg/mL within 60 min, with a detection range of 0–1000 pg/mL [71]. A gold-modified molecularly imprinted polymer-based laser-scribed graphene (LSG) biomimetic sensor was reported for Her-2 detection. LSG electrodes were fabricated by irradiating a polyimide sheet with a CO2 laser and nanostructured gold was electrodeposited onto the LSG to enhance sensitivity and facilitate Her-2 immobilization. The sensor detected Her-2 in the range of 1 to 200 ng/mL with a LOD of 0.43 ng/mL [72]. Cerium oxide (CeO2) nanoparticle is a commonly utilized high-performance electrochemical biosensors. PEGylated nano-structured cerium oxides, combined with varying concentrations of anti-HER2 antibodies, were used to form bioconjugates. A screen-printed carbon-gold nanoparticle electrode was employed to design a label-free platform, with the bioconjugates being covalently stabilized on the electrode surface for assaying the HER2 antigen in serum samples [73].
3.3 CEA
Over several decades, research has demonstrated that carcinoembryonic antigen (CEA) from breast ductal secretions holds significant diagnostic value for BCa [85, 86]. Moreover, combined detection of CA15-3 and CEA could offer more comprehensive information for clinical decision-making [87].
Based on the specific affinity between aptamer (Apt) modified nanoparticles and CEA, Apt functionalized magnetic core–shell Fe3O4@Au nanoparticles (Apt-MNPs) were designed as carriers to enable rapid separation of CEA from complex samples through binding and manual magnetic separation [74]. After the magnetic separation process, only magnetic molecules (comprising the CEA–carrier complex and the carrier) remained [74]. The CEA–carrier complex generates deeper current blockade signals, which can be distinctly differentiated from the carrier and quantitatively detect CEA in complex samples [74].
In another way, self-polymerized dopamine-decorated Au nanoparticles, coordinated with Fe metal-organic frameworks (Au@PDA@Fe-MOF), were designed for the detection of CEA [75]. This nanocomposite served as a transducer, displaying strong electrochemical signals [75]. Additionally, the nanocomposite assembly was used to immobilize the recognition element (CEA aptamer) due to the abundant COOH groups embedded in Fe MOFs [75]. Additionally, the aptasensor has a broad CEA detection range, from 1 fg/mL to 1 μg/mL, with a LOD limit of 0.33 fg/mL, presenting high sensitivity and excellent selectivity [75]. Yang et al. presented a novel label-free electrochemical immunosensor for detecting CEA utilizing a three-dimensional (3D) nanocomposite of gold nanoparticles and Prussian blue-poly(3,4-ethylenedioxythiophene) (AuNPs/PB-PEDOT). The synthetic procedure including a simple redox reaction between PB precursors and EDOT in an aqueous solution, followed by the electrochemical reduction of HAuCl4. The AuNPs/PB-PEDOT exhibited a 3D hierarchically porous nanostructure, while PB-PEDOT displayed a core–shell structure and the modified immunosensor demonstrated good linearity for CEA concentrations ranging from 0.05 to 40 ng/mL, with a detection limit of 0.01 ng/mL, making it highly promising for real sample analysis applications [76]. A novel multiplexed label-free electrochemical immunosensor was developed using graphene/methylene blue-chitosan/antibody and bovine serum albumin on an indium tin oxide glass electrode for the simultaneous detection of three tumor markers: CEA, CA15-3, and CA12-5 [77]. This immunosensor was successfully applied to detect the three tumor markers in blood serum samples, yielding good recoveries and its reliability was found to be in good agreement with that of the enzyme-linked fluorescent assay method [77]. A smart triplex signal amplification strategy for sensitive biosensing of cancer biomarkers, leveraging hairpin-shaped oligonucleotide-functionalized gold nanorods (HO-GNRs), graphene, and the avidin-biotin interaction [78]. This approach enhances the electrochemical detection of CEA by using an aptamer as the biosensor's recognition element and HO-GNRs as signal amplifiers [78]. Under optimal conditions, the electrochemical biosensor exhibited a wide dynamic range from 5 pg/mL to 50 ng/mL for CEA standards, with a low detection limit of 1.5 pg/mL [78].
3.4 BRCA1 and BRCA2
Genetic biomarkers, such as BRCA1 and BRCA2 mutations, are well-known for confer a high risk of BCa, offering prognostic values and influencing treatment strategies [88]. Development of genosensors for the sensitive detection of pathogenic gene mutations is of high priority in the diagnosis of complex diseases.
A gold nanoparticle-labeled DNA sensor was explored to amplified BRCA1 gene detection. This sensor employs a “sandwich” detection strategy, involving an immobilized capture probe DNA, target DNA, and a gold nanoparticle-conjugated reporter probe DNA. This DNA sensor could detect DNA targets at concentrations as low as 1 fM (5.896 fg of BRCA1 gene per ml) and demonstrated excellent selectivity against noncomplementary sequences and three-base mismatch complementary targets [79]. Mercaptopropionic acid (MPA) and functionalized gold nanoparticles (AuNPPEG) were immobilized on a gold electrode surface (Au electrode) before the immobilization of the capture DNA probe [80]. This “sandwich” hybridization scheme involves capture probe DNA on the AuNPPEG binding to one half of the target DNA, while the reporter probe DNA labeled with gold nanoparticles binds to the other half [80]. This sensor has high sensitivity and very low detection limit 50attomolar DNA target (294.8attogram BRCA1gene/mL) [80].
Additionally, a reduced graphene oxide-yttria nanocomposite has been applied as an electrochemical genosensor platform for the ultra-sensitive detection of the BRCA1 gene [81]. This sensor utilizes a sandwich assay where a gold nanoparticle cluster-labeled reporter DNA hybridizes with the target DNA [81]. A glassy carbon electrode modified with rGO-yttria serves as the immobilization platform for the capture probe DNA [81]. As expected, the sensor demonstrated excellent capability in sensing the BRCA1 gene, with a linear detection range from 10 attomolar (aM) to 1 nanomolar (nM) and a detection limit of 5.95 attomolar [81].
4 Nano-Based Therapy for Breast Cancer
4.1 Potential Therapeutic Targets in Breast Cancer
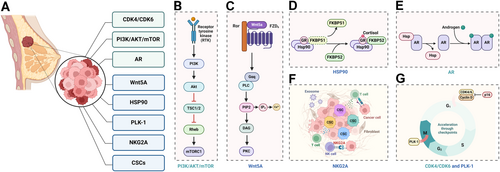
Identifying potential therapeutic targets is crucial for developing effective treatments for BCa. Massive cohort studies, clinical trials, and in-depth mechanism research have uncovered various molecular alterations and revealed emerging potential therapeutic targets in BCa [89]. Several molecular targets have been identified that offer promising avenues for therapy, particularly in the context of targeted and personalized medicine. The first approved drug for treating ER+ advanced BCa patients was tamoxifen, which reduces tumor recurrence by approximately 40%–50% [90]. In this section, we highlighted the critical findings and promising therapeutic targets in BCa (Figure 2 and Table 3).
| Target | Mechanism of action | Related therapies | Clinical trials | References | |
|---|---|---|---|---|---|
| Under active clinical evaluation | CDK4/6 | Cell cycle regulation, promotes cell proliferation | Palbociclib Ribociclib Abemaciclib |
NCT01684215 NCT06075758 NCT04681768 |
[91-94] |
| PI3K/AKT/mTOR pathway | Cell proliferation and survival signaling pathway | Alpelisib Everolimus Temsirolimus |
NCT05038735 NCT01272141 NCT00062751 |
[95-97] | |
| AR | Precursors to estrogens, breast cell growth and proliferation | Enzalutamide Bicalutamide | NCT04142060 NCT02348281 |
[98, 99] | |
| FGFRs | Survival and proliferation of postnatal mammary luminal and basal epithelial cells | Erdafitinib | NCT03238196 | [100] | |
| ESR1 | Estrogen receptor, promotes estrogen-dependent tumor growth | Tamoxifen Aromatase inhibitors | NCT02806544 | [101] | |
| HER2 | Overexpression, promotes tumor growth | Trastuzumab | NCT00542451 | [102] | |
| PD-1/PD-L1 | Immune checkpoint, inhibits immune response | Nivolumab Atezolizumab Pembrolizumab |
NCT03650894 NCT02530489 NCT05159778 |
[103-105] | |
| Under experimental evaluation | WNT5A | Noncanonical Wnt signaling, involved in cell movement and polarity | Sequence-specific siRNAs | Inhibit breast cancer cell migration and invasion through an EMT-independent mechanism | [106] |
| HSP90 | Molecular chaperone, stabilizes many proteins required for tumor growth | Gambogic acid | PEGylated two-dimensional boron nanosheets were loaded with gambogic acid, administrate mild photothermal therapy and chemotherapy for breast cancer | [107] | |
| PLK1 | Regulates cell cycle | PLK1 siRNA PLK1-inhibitor |
PLK1 siRNA is loaded last and shielded under the PEG layer, it delays the onset of mortality and enhances overall survival in metastasis mouse models. GSK461364 was used to treat the MDA-MB-231-derived xenograft mouse model. |
[108, 109] | |
| NKG2A | Immune checkpoint receptor on NK cells, inhibits immune response | Anti-NKG2A antibody | TNBC-specific phenotype drives NK-cell infiltration leading to alternative checkpoint blockades | [110] | |
| Vitamin D3 | Modulates cell growth, apoptosis, and differentiation | Vitamin D3-coated micelles | Enhanced breast cancer apoptosis, reduced angiogenesis, invasion and autophagy, | [111] | |
| Cancer Stem Cells | Subpopulation of cells with self-renewal and differentiation properties | Targeting CSC markers (e.g., CD44, ALDH1) | — | [112] |
4.1.1 Targets Under Clinical Application
The critical role of cyclin-dependent kinase 4 (CDK4) and CDK6 is mediate cellular transition into S phase and are necessary for cellular proliferation of various cancer types [113, 114]. CDK4/6 forms a complex with cyclin D, which subsequently phosphorylates the retinoblastoma (Rb) protein. Phosphorylated Rb releases E2F transcription factors, leading to the transcription of genes necessary for S phase entry and cell proliferation [115, 116]. In HR+ BCa, the CDK4/6-cyclin D-Rb pathway is often dysregulated, leading to uncontrolled cell proliferation [117]. The cyclin-dependent kinase (CDK) 4/6 inhibitors (CDK4/6i), palbociclib, ribociclib, and abemaciclib, currently become the standard first-line treatment for patients with advanced HR+ BCa [91-94]. By inhibiting CDK4/6, these drugs prevent the phosphorylation of Rb, thereby halting the cell cycle in the G1 phase and effectively reducing tumor growth [91, 118].
The PI3K/AKT/mTOR pathway is another important therapeutic target in BCa. Mutations and aberrations in this pathway are common in various subtypes of breast cancer, driving tumor growth and survival. Targeted inhibitors of PI3K, AKT, and mTOR are being explored in clinical trials, showing potential for improving outcomes, especially when combined with other therapies. The aberrant activation of the PI3K/AKT/mTOR pathway in BCa can result from mutations in PIK3CA (the gene encoding the p110α catalytic subunit of PI3K), loss of function of PTEN (a tumor suppressor that dephosphorylates PIP3), or overactivation of receptor tyrosine kinases (RTKs) upstream of PI3K [119]. These alterations lead to persistent activation of the pathway, driving tumor growth and resistance to apoptosis [119]. Therapeutic targeting of this pathway includes the use of PI3K inhibitors, AKT inhibitors, and mTOR inhibitors. PI3K inhibitors, such as alpelisib, specifically target the mutated p110α subunit, reducing PIP3 levels and subsequent AKT activation [95, 120, 121]. AKT inhibitors directly inhibit AKT kinase activity, while mTOR inhibitors like everolimus and temsirolimus inhibit mTORC1, thereby reducing protein synthesis and cell growth [96, 97, 120, 121].
Previous studies have shown that androgens and the androgen receptor (AR) is a are key drivers to the invasion and metastasis of BCa, particularly a subset of triple-negative breast cancer (TNBC) [122, 123]. Additionally, dihydrotestosterone (DHT) can induce epithelial-to-mesenchymal transition in BCa cells through an AR-dependent, ER-independent mechanism [124]. This is particularly significant in TNBC, which lacks the expression of ER, PR, and HER2, making it more challenging to treat with conventional hormonal therapies [125, 126]. Targeting the AR pathway in AR-positive breast cancer involves the use of AR antagonists or anti-androgens, drugs such as enzalutamide and bicalutamide are examples of AR antagonists that have shown promise in preclinical and clinical studies [98, 99, 125, 127].
Fibroblast growth factor receptors (FGFRs) are a family of receptor tyrosine kinases that are activated by binding to fibroblast growth factors (FGFs). This binding induces receptor dimerization and autophosphorylation on tyrosine residues within the intracellular domain. The phosphorylated tyrosines serve as docking sites for various downstream signaling proteins, initiating multiple pathways such as the RAS-MAPK, PI3K-AKT, and PLCγ pathways. These pathways collectively contribute to cell proliferation, survival, migration, and angiogenesis [128]. Aberrant FGFR signaling mediates progression and characteristics in BCa [129]. For instance, FGFR1 amplification is commonly observed in HR+ BCs and FGFR2 mutations have been implicated in driving aggressive tumor behavior in certain BC subtypes [130, 131]. Clinical therapeutic targeting of FGFR involves the use of small molecule inhibitors such as erdafitinib presented favorable outcomes [100].
4.1.2 Experimental Targets for Breast Cancer Under Evaluation
Exploration in BC is continuously evolving, with numerous experimental targets under evaluation. These targets aim to improve therapeutic efficacy, and overcome resistance mechanisms associated with conventional treatments.
Wnt family member 5A (WNT5A) is a member of the WNT family and is involved in noncanonical WNT signaling pathways, which are independent of β-catenin and are essential for various cellular processes that contribute to BC progression and metastasis [106, 132]. WNT5A binds to receptors such as Frizzled (FZD) and receptor tyrosine kinase-like orphan receptor on the cell surface, activating downstream signaling cascades: planar cell polarity pathway for cytoskeletal dynamics and cell polarity regulation, promoting migration and invasion; Ca2+ pathway for gene expression and cell adhesion regulation [133-135].
Using specific siRNAs to transiently knocked down WNT5A in HB2 mammary epithelial cells induced EMT-like alterations and decreases invasive, partially counteracted by adding recombinant WNT5A. These findings imply that WNT5A could potentially suppress migration and invasion of BC cells through a mechanism involving EMT reversal [106]. Moreover, plasmid DNA encoding for the Wnt5a trap was delivered to the tumor by using cationic lipid-protamine-DNA nanoparticles, and significantly reshaped the immunosuppressive tumor microenvironment to enhance immunogenic cell-death-mediated immunotherapy [136].
Heat shock protein 90 (HSP90) functions as a molecular chaperone, assisting in the folding of newly synthesized proteins and maintaining the stability of unfolded or misfolded proteins under stressful conditions, and further implicated in key cancer events like signal transduction, cell cycle regulation, and apoptosis [137]. HSP90 inhibitors are synthesized to disrupt interactions between HSP90 and co-chaperones, leading to the degradation of client proteins involved in oncogenic pathways [138].
NCT-547, a novel C-terminal HSP90 inhibitor designed to overcome trastuzumab resistance, induced significant apoptosis without eliciting the heat shock response in both trastuzumab-sensitive and -resistant cells [138]. Additionally, NCT-547 effectively inhibited tumor growth and angiogenesis in trastuzumab-resistant JIMT-1 xenografts, suggesting that NCT-547 could be beneficial for addressing trastuzumab resistance in HER2+ BCa [138]. Nanoplatform also introduced to inhibit Hsp90, such as PEGylated two-dimensional boron nanosheets (B-PEG) were loaded with gambogic acid, which can decrease Hsp90 production and improve antitumor effects [107].
Polo-like kinase 1 (PLK1) plays a pivotal role in regulating cell division and maintaining genomic stability through its involvement in multiple stages of mitosis [139]. The activation of PLK1 is tightly controlled through its phosphorylation by upstream kinases and interaction with regulatory proteins, ensuring that PLK1 exerts its functions at precise times and locations within the cell, orchestrating the complex events of mitosis [140]. In BC biology, PLK1 is frequently overexpressed in tumor cells [141, 142]. Inhibiting PLK1 activity disrupts mitotic processes in cancer cells, leading to mitotic catastrophe or cell death. Thus, targeting PLK1 holds promise as a therapeutic strategy to selectively inhibit cancer cell proliferation while minimizing toxicity to normal cells [143, 144].
Hu et al. screened effective PLK1 siRNA in a genome-wide human kinase siRNA library to combat the TNBC cell line SUM149 for growth inhibition [144]. Subsequently, a nanoparticle comprises an MSNP core coated layer-by-layer with bioreducible cross-linked PEI and PEG polymers, conjugated with an antibody, and PLK1 siRNA is loaded last and shielded under the PEG layer, was rationally designed [108]. This platform significantly postponed mortality onset and improved the overall survival in metastasis mice models [108]. Another research performed PLK1-inhibitor, GSK461364 to treat MDA-MB-231 derived xenograft mouse model and PLK1 inhibition was shown to increase the radiosensitivity of breast cancer cells by suppressing radiation-induced autophagy [109].
Natural killer group 2A (NKG2A) acts as an inhibitory receptor on the surface of immune cells [145, 146]. It interacts with its ligand, HLA-E, on target cells, leading to the suppression of NK cell cytotoxicity and CD8+ T cell activity, modulating immune responses and preventing excessive immune reactions [145, 147]. NKG2A can form heterodimers with other receptors such as CD94, allowing for a more refined control of immune surveillance by integrating signals from multiple receptors [145].
The combination therapy targeting tumor-infiltrating NK cells with anti-NKG2A and anti-PD-L1 antibodies overcame resistance to anti-PD-L1 treatment and prolonged survival in heterogeneous MHC-I murine mammary tumors, providing evidence that NKG2A represents a therapeutic vulnerability in immunotherapy resistant MHC-I heterogeneous TNBC [110]. Monalizumab (anti-NKG2A) has emerged as a focal point in numerous clinical trials across different cancer types (NCT04307329, NCT04590963, NCT05221840, NCT02671435, NCT05061550, and NCT03833440).
Vitamin D3 is a widely recognized anticancer agent that suppresses the growth of various cancers [148]. The most biologically active metabolite of vitamin D, 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3), is a high-affinity ligand for the transcription factor vitamin D receptor (VDR) [149]. Through VDR, 1,25(OH)2D3 induces changes in the epigenome of both healthy and cancerous cells, thereby influencing their transcriptome [149].
Circulating 25(OH)D and breast cancer (BC) have independent inverse associations with poor prognoses in two subtypes—distant disease and triple-negative disease—in an international pooling project involving 17 cohorts [150]. Vitamin D3 (Vit.D3)-coated micelles were developed to encapsulate the cytotoxic drug etoposide (ETP), and drug-loaded micelles were further coated with a Vit.D3/phospholipid complex envelope to exploit Vit.D3 receptors (VDRs) which overexpressed on breast cancer cell surfaces [111]. These micelles accumulated in tumor tissue and induced increased tumor apoptosis, indicated a promising actively targeted BC delivery and administration system [111]. A generalized strategy, Gemini-like homotypic targeting nanoparticles (NPs) are engineered to transform cancer-associated fibroblasts (CAFs) and eliminate cancer cells simultaneously, remodeling vitamin B3 metabolism [151]. In mouse model of resistant breast cancer, a single dose of hydrogel containing Gemini-like NPs restores chemosensitivity, activates the immune system, and promotes tumor regression, induces durable T cell memory for long-lasting protection against tumor recurrence, presenting a promising strategy to overcome BC chemoresistance [151].
Except specific targets, cancer stem cells (CSCs) are a subpopulation of cancer cells with the ability to self-renew and drive tumorigenesis, which is an emerging therapeutic objective [152, 153]. CSCs eradication could prevent tumor recurrence and improve long-term outcomes for cancer patients [154]. Expectations are focused on identifying markers specific to CSCs and developing agents that selectively target these cells. A notable characteristic of CSCs is their reduced sensitivity to drugs and radiation compared to non-CSCs [155]. Studies are investigating interventions options to eradicate breast CSCs, including pathways and factors which regulating Wnt, Notch, and hedgehog pathways are targeted [156]. The antidiabetic drug metformin shows promise in blocking mTOR through AMPK activation, particularly targeting the CSC population in breast cancer cell lines [112].
4.2 Challenge in Breast Cancer Therapy
BC encounters significant biological obstacles that influence treatment approaches.
4.2.1 Tumor Heterogeneity
Breast cancer is a complex disease characterized by significant inter- and intra-tumoral heterogeneity, and the heterogeneity of BC presents a big challenge in its therapeutic management [157-159]. Tumor stratification is crucial for improving clinical outcomes and a personalized approach is essential to optimize treatment responses in patients [89, 159]. Moreover, existing evidence suggests the presence of distinct subtypes within BC tumors and the potential for these discrete subtypes to transition between each other through cell state plasticity. Molecular subtyping is applied in BCa, which has improved outcomes by guiding targeted therapies such as hormonal (e.g., tamoxifen) and HER2-directed therapies (e.g., trastuzumab) [102, 160]. Histological classification remains standard practice, while emerging technologies have uncovered additional complexities, identifying at least five distinct molecular subtypes (Luminal A, Luminal B, Her2-enriched, Basal-like, and Normal-like) through gene expression profiles [161, 162]. Furthermore, integrated genomic and transcriptomic analyses have identified ten distinct breast cancer subtypes, including efforts to refine subtypes within ER-negative and triple-negative breast cancers [163]. However, there remains a gap between basic research methodologies and current clinical practices that need to be addressed,
4.2.2 Tumor Microenvironment and Tumor Metastasis
The components of the tumor microenvironment (TME), including altered extracellular matrix (ECM), soluble factors, immunosuppressive cells, epigenetic modifications, and reprogrammed fibroblasts, collectively hinder the antitumor response and promote the progression and metastasis of BCa [164]. Cancer cells within the TME interact closely with surrounding stromal cells, influencing each other's behavior and contributing to tumor growth and invasion. Although breast cancer primarily originates in breast epithelial cells, increasing evidence confirms the significant role of breast stromal cells in tumor metastasis [165, 166]. The heterogeneous interplay between cancer cells and stromal cells drives the proliferation and spread of malignant cells [167]. Due to extensive connective tissue hyperplasia in breast tissue, cancer-associated fibroblasts (CAFs) make up about 80% of the tumor mass and are the main stromal cells in the breast tumor microenvironment (TME) [168]. CAFs interact with the TME to worsen tumor progression and are crucial in cancer advancement [169]. If overactivated, CAFs shift from tissue repair to promoting fibrosis or tumor growth [169]. They enhance tumor survival and spread by secreting paracrine factors, cytokines, and exosomes, and by remodeling the ECM, which increases cancer cell motility and facilitates metastasis.
Notably, TNBC is distinguished by a unique TME that sets it apart from other subtypes, characterized by higher proportions of regulatory T cells (Tregs) and exhausted CD8+ T cells, along with increased plasma cell abundance [170]. Unlike HER2+ or luminal-like breast cancer, TNBC exhibits a higher proportion of cytotoxic NK cells and B cells in TNBC tend to differentiate into plasma cells [170].
4.2.3 Drug Resistance in Breast Cancer
The frequent emergence of drug resistance limits the success of current BC therapy, resulting in disease recurrence and relapse [89]. Drug may be intrinsic, referring it is pre-existing in tumor cells before chemotherapy exposure, or acquired, meaning it is induced by anticancer drugs during or after treatment [171]. The mechanisms deregulated in drug-resistant BC mainly containing activation of mitogenic signaling pathways, alterations in transcriptional factors and chromatin remodeling complexes, metabolism disruption and TME disorder [172-174]. Overcoming drug resistance requires a multifaceted approach, combining diverse treatment modalities, targeting multiple pathways simultaneously, and personalizing treatment based on the genetic profile of the tumor are promising strategies.
4.3 Advanced Nanocarriers in Breast Cancer Therapy
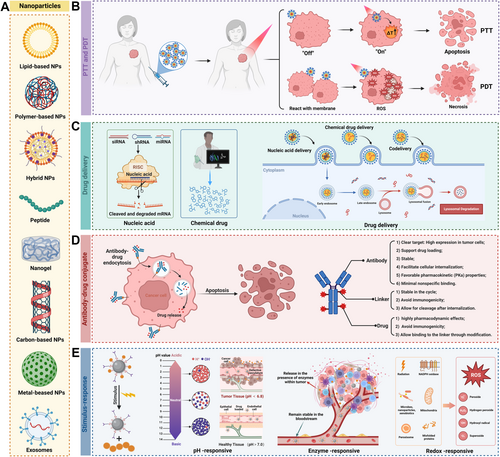
Next-generation cancer treatments aim not only to target cancer cells directly but also modulate immune cells against cancer, reshape the immune-suppressive environment of tumors and induce a robust and lasting response with a minimum of detrimental side effects [175]. Advanced nanotechnology represents a significant innovation in BC therapy, offering improved permeability, retention effect, and targeting over conventional treatments [176]. One of the key advantages of nanocarriers is their ability to improve drug solubility and stability, though encapsulating these drugs enhances their solubility and protects them from degradation before they reach the target cells [177]. Stimuli-responsive nanocarriers, such as those sensitive to pH, redox conditions, and light, can facilitate targeted suppression of breast tumors. By functionalizing the surface of nanocarriers with ligands or antibodies, they can specifically bind to receptors on cancer cells, increasing the concentration of the drug at the tumor site while reducing systemic side effects [178]. Furthermore, nanoplatform offers the capability of controlled and sustained drug release, allowing for the gradual release of the therapeutic agent over time, maintaining optimal drug levels in the tumor location [179, 180]. Recently, certain nanoparticles can co-deliver multiple therapeutic agents simultaneously, which include a combination of chemotherapy drugs, gene therapy, or immunotherapy, providing a multifaceted attack on the cancer cells and potentially overcoming drug resistance [181]. This part, we review latest progress in nano-therapy development, focusing on breast cancer (Figure 3 and Table 4).
| Nanovector | Particle size Zeta potential | Cargo | Remarks | References | |
|---|---|---|---|---|---|
| Nucleic acid delivery | iRGD/LPSA | ~130 nm 28.2 ± 5.4 mV |
VEGF siRNA | Significantly inhibit angiogenesis in zebrafish and tumor growth in nude mice bearing breast cancer without obvious toxicity. | [182] |
| mPEG-SS-PLGA/cationic lipid G0-C14 | 90 nm −19 mV - |
APOC1 siRNA | Effectively deliver siRNA to breast cancer cells, inhibit TNBC growth and metastasis in vivo. | [183] | |
| LPEI/disulfide bonds/HA-SH | ~ 200 nm −20 mV - |
TGF-β siRNA | Enhance the efficacy of anti-PD-L1 against stroma-rich TNBC. | [184] | |
| PEG/mannose doubly modified trimethyl chitosan/poly (allylamine hydrochloride) | ~130 nm 14 mV |
VEGF and PIGF siRNA | Robust suppression of breast tumor growth and antitumor immunity activation of the TME. | [185] | |
| SWCNT/PEI/PEG | 60–100 nm 6.3 and 30.8 eV |
Bcl-xL shRNA | Efficiently and selectively transfer plasmid shRNA to MUC1 positive cells. | [186] | |
| Tween 85/PEI 2 K | ~140 nm (in water)/~120 nm (in NaCl) ~50 mV (in water)/~15 mV (in NaCl) |
p65 shRNA | Effectively deliver p65 shRNA into metastatic tumor cells, leading to remarkable inhibition of cell invasion and suppression of tube formation. | [187] | |
| PLGA/PLL | 122 ± 8 nm ~32 mV |
miR-34a | A platform for miR-34a delivery to treat TNBC and improve therapeutic outcomes. | [188] | |
| Peptides/LNP | 107.5–115.7 nm — |
PTEN mRNA | Specifically deliver PTEN mRNA to a PTEN-deficient 4T1 TNBC model | [189] | |
| Drug delivery | Mannose nanogels | 86.08 ± 2.87 nm −8.35 ± 0.57 mV |
DOX | A powerful and synergistic breast cancer treatment in vitro and in vivo by disrupting glucose metabolism in glycolysis and the TCA cycle. | [190] |
| FeIII–TA/PLGA | 146.9 ± 14.3 nm −21.0 ± 0.2 mV |
DOX | Accumulated in the tumor site of 4T1-bearing nude mice and reached a tumor inhibition. | [191] | |
| Fe-MOF/hollow MON | ~ 100 nm — |
DOX | Activated antitumor immune responses in both in situ tumors and metastatic encephaloma for enhanced immunotherapy. | [192] | |
| DSPE-PEG/DPPC/AIPH/targeting aptamer | ~100–130 nm | Gambogic acid | Effective suppression of deep-seated TNBC with negligible side effects. | [193] | |
| PLGA | — | 3′3′-cGAMP | Inhibited tumor growth, and prolonged survival as effectively as multiple soluble doses. | [194] | |
| MSN/PEG/ammonium-based cationic molecule | 26.7 ± 4.8 nm ~28 mV |
c-di-GMP | Address the challenges that bare cdG encounters in the TME. | [195] | |
| Co-delivery | PLGA/PEG/PEI | ~100 nm −35 mV |
NGF siRNA and Dox | Enhanced immunochemotherapy outcomes. | [196] |
| MSN/PEG | 222.9 ± 16 nm 22.87 ± 1.29 mV |
Cav3.2 siRNA and Dox | Conquered the drug-resistant breast cancer | [197] | |
| PEI/liposomes/dendrimers/nanogels/cell membrane | 129.14 nm 32.6 mV |
CD47 siRNA and DTX | Enhanced macrophage phagocytic effects through the collaborative therapeutic effects. | [198] | |
| DSPE-PEG/RGD peptide | 150–200 nm −28.2 mV |
Fe catalyst, chemotherapeutics and RSL3 | Overcome the chemoresistance and improve the therapeutic effect by synergistic effects of ferroptosis and apoptosis. | [199] |
- Abbreviations: AIPH, 2, 2′-azobis[2-(2-imidazolin-2-yl)propane] dihydro-chloride; DOX, doxorubicin; DPPC, 1,2-dipalmitoyl-sn-glycero-3-phosphorylcholine; DTX, docetaxel; HA-SH, thiolated hyaluronid; LPEI, low molecular polyethylenimine; LPSA, Lipopolysaccharide-amine copolymer; MOF, metal organic framework; MON, mesoporous organosilica nanoparticles; MSN, mesoporous silica nanoparticles; PEG, poly(ethyleneglycol); PLGA, poly(lactic-co-glycolic acid); PLL, poly-l-lysine; SWCNT, single-walled carbon nanotubes; TA, tannic acid; TCA, Tricarboxylic acid; TME, tumor microenvironment; TNBC, triple-negative breast cancer.
4.3.1 The Unique Properties of Nanotechnology in BCa Treatment
Nanomedicines possess several unique advantages in the treatment of BCa, which are primarily associated with the specific characteristics of BCa lesions, treatment requirements, and the inherent properties of nanotechnology [200].
Targeted drug delivery: The key advantage is the ability to achieve specific targeting through surface modifications such as antibodies, peptides, and small-molecule ligands. This allows the drugs to be directly delivered to cancer cells or tumor tissues, significantly reducing adverse effects on healthy cells and enhancing treatment safety, as well as improving the quality of life for patients. For example, antibody-conjugated nanoparticles can specifically deliver chemotherapeutic agents to HER2-overexpressing cancer cells in HER2-positive BCa, thereby minimizing toxicity to normal cells [201].
Mechanisms of active and passive targeting: While significant progress has been made in the development of nanoplatforms for various cancers, there remains a critical gap in understanding the specific mechanisms of active and passive targeting in BCa. The active targeting, which involves attaching specific molecules, such as antibodies, to the surface of nanoparticles [202, 203]. These molecules bind to receptors on cancer cells, allowing the nanoparticles to be absorbed more efficiently by the tumor. Passive targeting is another important mechanism. This approach leverages the enhanced permeability and retention (EPR) effect, where nanoparticles accumulate in tumor tissues due to their leaky blood vessels and poor lymphatic drainage [204]. By taking advantage of these physical characteristics, nanoparticles can naturally concentrate in the tumor, increasing the drug impact on cancer cells. Both active and passive targeting mechanisms play crucial roles in overcoming multidrug resistance (MDR), a common issue in breast cancer treatment. MDR occurs when cancer cells develop resistance to multiple drugs, rendering traditional chemotherapy less effective.
Reduction of drug side effects: The treatment of BCa often involves the use of highly toxic chemotherapeutic drugs [205]. Although these drugs are effective in killing cancer cells, they can also damage rapidly dividing normal cells, leading to a range of adverse effects including nausea, immune system suppression, and so on [206]. Due to their high targeting efficiency, nanomedicines can deliver higher drug doses to tumor sites with minimal impact on normal tissues, thereby reducing unnecessary side effects [206]. Additionally, some nano-carriers are pH-sensitive or enzyme-sensitive, allowing them to release drugs in the acidic tumor microenvironment, further enhancing drug selectivity and improving therapeutic outcomes [207, 208].
Overcoming drug resistance: Prolonged use of certain chemotherapeutic drugs can lead to drug resistance in BCa cells, which is a major impediment to treatment efficacy. Nanomedicines can overcome this issue through multi-mechanism actions. For instance, liposomes and polymeric nanoparticles can simultaneously carry multiple drugs, enabling multi-drug combination therapy [209, 210]. This not only increases the intensity of treatment but also reduces the likelihood of cancer cells developing resistance. Moreover, nano-carriers can alter the drug delivery process, facilitating cellular entry via endocytosis and bypassing efflux pumps on the cell membrane [211]. This increases the intracellular accumulation of drugs, further enhancing their effectiveness.
Enhancing Pharmacokinetic Properties: The pharmacokinetics (PK) of nanomedicines, encompassing absorption, distribution, metabolism, and excretion, is pivotal in the treatment of BCa. Effective delivery and accumulation of therapeutic agents at tumor sites are crucial and can be significantly influenced by modulating the PK properties of nanoplatform-based medicines [212]. For instance, a recent study reported that an optimal nanoconstruct with a hydrodynamic size of 100 nm demonstrated selective targeting and treatment of HER2-positive breast cancer cells over normal cells [213]. In another research, Hu et al. also degraded their multistage-responsive nanoparticle from about 330 nm to a smaller size that was in a size range of 35 to 150 nm with hyaluronidase (HAase) incubation for a better tumor targeting consequence [214]. Amini et al. developed a polymer-lipid hybrid nanoparticle (PLN) system capable of undergoing time-dependent size reduction and morphological transformation from a spherical to a spiky shape. This unique system demonstrated a significant enhancement in doxorubicin (DOX) penetration and retention compared to conventional non-transformable liposomal DOX particles [215]. Additionally, many effective drugs used for BCa treatment have short half-lives in the body, requiring frequent dosing. This not only increases treatment costs but also adds to the burden on patients. Nanomedicines can extend the circulation time of drugs in the body, improving pharmacokinetics and biodistribution [216]. The size and surface properties of nanoparticles can be designed to bypass certain physiological barriers, such as rapid clearance by the liver, or to maintain stability in the bloodstream for longer periods.
Penetrating tumor tissues: Tumor tissues exhibit unique features, such as high interstitial pressure and irregular vascular systems, which hinder the effective penetration of traditional drugs [217]. The small size of nanomedicines allows them to more easily penetrate these barriers and reach deeper into the tumor tissue [218]. Furthermore, certain nano-carriers can respond to specific tumor microenvironment conditions, such as low pH and high oxidative stress, leading to the targeted release of drugs at the tumor site [219, 220]. This increases the drug concentration in the treatment area and significantly improves therapeutic efficacy.
Supporting Multimodal Therapy: Nanotechnology also supports the development of multifunctional nanomedicines that integrate multiple therapeutic modalities, such as chemotherapy, phototherapy, and immunotherapy [221, 222]. This multi-modal or combination therapy strategy can address the diverse biological characteristics and treatment stages of breast cancer, providing more personalized and effective therapeutic options. By combining different mechanisms of action, these nanoplatforms can synergistically enhance treatment outcomes and potentially overcome the limitations of single-agent therapies.
4.3.2 Hyperthermia and Photothermal Therapy
Nanoplatforms have significantly advanced the therapeutic options for breast cancer, particularly through the use of hyperthermia and photothermal therapy. These treatments leverage the unique properties of nanoparticles to generate heat and destroy cancer cells, offering a noninvasive and targeted alternative to conventional therapies [223]. Moreover, this comprehensive approach enables real-time monitoring of treatment efficacy and dynamic therapy adjustments, leading to more personalized and effective cancer treatments [224].
Hyperthermia therapy involves heating the tumor to a temperature that can damage or kill cancer cells [225]. Nanoparticles, such as iron oxide and gold nanoparticles, can be engineered to absorb specific types of energy, such as radiofrequency or near-infrared light, and convert it into heat [225, 226]. When these nanoparticles are injected into the body and accumulate in the tumor, they can be activated externally to heat the cancer cells without harming surrounding healthy tissue [225, 227]. Nanoparticles were synthesized using DOX, l-arginine (l-Arg), ultrasmall spinel ferrites (MnFe2O4), and a PLGA shell [60]. The distribution of nanoparticles in the tumor was accurately monitored in real-time through highly enhanced MRI and PAI [60]. NIR irradiation of tumor cells showed that MnFe2O4 catalyzes ROS production from H2O2, leading to l-Arg cascade catalysis and triggering NO production [60]. The resulting NO improves vascular endothelial cell integrity and pericellular contractility, promoting vessel normalization and facilitating efficient nanoparticle delivery and DOX penetration [60]. Moreover, the chemotherapeutic effect of DOX and the photothermal effect of MnFe2O4 serve as a chemo-hyperthermia synergistic therapy against TNBC [60]. To achieve more precise and effective treatment of HER2-positive BCa, a team proposed a multifunctional bimetallic nanoplatform (PPAPH) capable of targeted delivery of multiple therapeutic effects, including chemotherapy, photothermal ablation, oxidative stress, and immune activation [228]. This platform integrates NIR-absorbing hollow gold-silver nanoshells (AuAg HNSs), the small-molecule tyrosine kinase inhibitor Pyrotinib (PYR), and the targeting molecule Herceptin (HCT) [228]. In vitro studies showed that HCT-modified nanoparticles specifically recognize and are effectively internalized by HER2-positive cells [228]. NIR laser application induces photothermal effects and intracellular ROS bursts, leading to tumor cell apoptosis and ferroptosis [228]. A very recently study proposed a strategy to induce structural transformations in vanadium-based MXene enzymes (TVMz) using TME characteristics [229]. TVMz protected by a hyaluronic acid coating, shows excellent stability and generates a thermal effect under NIR-II laser irradiation [229]. This effect combined with TME features, promoting its transformation into ultra-small vanadium oxide nanozymes and significantly increases ROS generation, further enhancing oxidative stress [229]. Collectively, these effects accelerate tumor cell apoptosis and ferroptosis and synergistically promote cell death, providing new research directions for the TNBC treatment [229].
Photothermal therapy is a specific form of hyperthermia that uses light to generate heat [230]. Nanoparticles are particularly effective in photothermal therapy because they can be designed to absorb near-infrared light, which can penetrate deep into the body [231, 232]. Chen et al. explored a novel ROS-responsive nanomedicine for copper-diethyldithiocarbamate (CuET) delivery, through simple operations [233]. CuET was stabilized with hydroxyethyl starch (HES) to form CuET@HES, enhancing aqueous stability and dopamine polymerization under alkaline conditions forming CuET@PDA/HES, improving pharmacokinetics. Further modification with folic acid and mercapto groups created CuET@PHF, which releases copper ions to induce cuproptosis in 4T1 cancer stem cells (CSCs) [233]. Using the photothermal capabilities of CuET@PHF, hypoxia can be mitigated. The combination of mild photothermal therapy and CuET@PHF treatment enhances cuproptosis and amplifies immunogenic cell death (ICD) to boost antitumor immune responses [233]. Yuan et al. reported a tumor-targeting therapeutic strategy using semiconducting polymeric nanoagonists (DPTT-Mn Lipo NPs), which exert efficient photothermal effects and self-catalyze hydroxyl radical production, promoting tumor repression [234]. Released DNA from dying tumor cells activates the cGAS-STING pathway in TME macrophages, reprogramming the TME and enhancing the anti-PD1 antitumor efficacy [233]. To design multifunctional theranostic nanoparticles for activatable “OFF–ON” dual-modality imaging and synergistic CDT/PTT cancer treatment, perfluorocarbon (PFC)-encapsulated fluorescent polyepinephrine (PEPP) nanoshells chelated with Fe²⁺ (PFC@PEPP-Fe) was synthesized [224]. Both in vitro and in vivo experiments demonstrated that PFC@PEPP-Fe enables effective bimodal imaging and exhibits significant anticancer efficacy through the synergistic effects of photothermal therapy (PTT) and chemodynamic therapy (CDT). Near-infrared (NIR) laser irradiation increased the temperature, enhancing the release of O2 and the production of H2O2, which in turn intensified the CDT effect [224].
4.3.3 Photodynamic Therapy
Photodynamic therapy (PDT) is a noninvasive method that combines light-sensitive drugs, known as photosensitizers, with light to destroy cancer cells [235]. Photosensitizers are light-activated molecules (visible/near-IR) that generate ROS to damage and kill cancer cells [236]. However, the challenge with traditional photosensitizers is ensuring they reach the tumor in sufficient concentrations and remain there long enough to be effective. Nanoparticles can enhance the delivery and efficiency of photosensitizers in BCa therapy. The nanoparticles can encapsulate photosensitizers and protect them from degradation in the bloodstream. Moreover, gold nanoparticles enhance light delivery to tumors, serving as both delivery vehicles and light-enhancing agents to boost photosensitizer activation, improving therapy effectiveness and targeting [237, 238]. In addition to improving drug delivery and light activation, nanoplatforms in PDT reduce side effects by targeting photosensitizers to tumors, minimizing exposure to healthy tissues and lowering toxicity [239, 240]. Moreover, to screen and optimize cyanine-carborane photosensitizers (PSs) for PDT in BCa, Amir Roshanzadeh et al. developed a clinically relevant orthotopic mouse model to test counterion-tuned PSs in a physiologically relevant TME. In vitro, PDT efficacy of [Cy + ] paired with five carborane anions was assessed in mouse and human cell lines, and in vivo using the orthotopic model. Optimized PSs effectively eliminated tumors in vivo, demonstrating a potent therapeutic strategy for aggressive breast cancer with minimal side effects on healthy cells [241].
To optimize photodynamic immunotherapy, a self-amplifying nanoplatform co-delivers a photosensitizer (verteporfin, VER), a hypoxic regulator (ATO), and an anti-inflammatory drug (CXB) was rationally designed [242]. The platform ATO alleviates hypoxia and enhances VER-induced PDT, boosting tumor immunogenicity, while CXB inhibits the COX-2/PGE2 pathway, reducing inflammation-associated immunosuppression [242]. This approach addresses the immunosuppressive microenvironment in TNBC, potentially improving patient responsiveness to immunotherapy [242]. On the other hand, a simple assembly strategy optimized reaction temperature, feeding concentration, and Cu2 + /ICG ratio to prepare carrier-free, water-dispersible CuET/ICG NPs for systemic administration, overcoming poor aqueous solubility of CuET in vivo [243]. CuET/ICG NPs disrupt mitochondria, reduce oxygen consumption, enhance PDT efficacy, and induce ICD under hypoxia, while mitochondrial dysfunction activates the AMPK pathway, downregulating PD-L1 on tumor cells [243]. A similar study also exploited a ROS-sensitive, near-infrared light-activated photodynamic antimetastatic nanomedicine (NP2) to achieve primary tumor containment and prevent metastasis, demonstrating excellent tumor inhibition and antimetastatic effects [244]. The formulation involved synthesizing a ROS-sensitive NIR PDT polymer (P1), conjugating the antimetastasis ruthenium complex NAMI-A to P1 to form polymer P2, and self-assembling it into nanoparticles (NP2), which generated significant ROS under 808 nm laser irradiation, leading to nanoparticle degradation and NAMI-A release [244].
4.3.4 Nucleic Acid Delivery
Nucleic acid delivery represents a promising frontier in BCa therapy, offering the potential to address underlying genetic causes and molecular mechanisms driving disease [245]. By introducing genetic material into cancer cells, this approach aims to correct or modify defective genes, inhibit oncogenes, or enhance the expression of tumor suppressor genes. By introducing DNA, RNA, or other genetic material into cancer cells or TME, this approach aims to modify gene expression, silence oncogenes, or enhance the function of TME. These nanoscale systems enhance the stability, targeting, and controlled release of nucleic acids, making this approach more effective.
siRNA: siRNA holds significant promise in BCa therapy by targeting specific genetic pathways crucial for tumor growth and progression. Utilizing siRNA to silence oncogenes which involved in its progression and malignancy, thereby altering the genetic makeup of cancer cells. For example, a nanostructure with favorable biocompatibility and specific organ-targeting ability was reported [246]. Through circular DNA synthesis, including long linear DNA complementary to siRNA and short linear DNA with T7 promoter, subsequently formatted size-tuned polymeric siRNA microparticles via controlled rNTP and DTT concentrations in rolling circle transcription [246]. The engineering size/surface chemistry-tuned PRNs through condensation and layering with functional biopolymers via electrostatic interactions [246]. In vivo experiments indicate that the polymeric PLK1 siRNA nanoparticle size mediates efficient polymeric siRNA delivery to the targeted tumors, resulting in high RNAi-induced therapeutic efficacy [246]. A study aimed to develop a novel method for incorporating iRGD (is a cyclic peptide containing a tumor/neovascular-specific RGD motif) into nano-delivery systems via electrostatic and hydrophilic/hydrophobic interactions [182]. The authors utilized Lipopolysaccharide-amine copolymer (LPSA) as a base material for constructing targeted vectors, selected for its structural compatibility with iRGD. Since the amphoteric and amphiphilic structure, LPSA self-assembled into nanopolymersomes in water, either independently or with anionic nucleic acids like pDNA and siRNA [182]. Thus, iRGD-NPs efficiently deliver VEGF siRNA to significantly inhibit angiogenesis and tumor growth in nude BCa mice [182]. Apolipoprotein C1 (APOC1) was upregulated in the primary tumors of BCa patients, and elevated APOC1 levels in BCa patients were significantly correlated with poorer overall survival (OS) and shorter relapse-free survival (RFS) [183]. A glutathione (GSH)-responsive nanoplatform (mPEG-SS-PLGA) for in vivo delivery of APOC1 siRNA successfully inhibits TNBC growth and metastasis [183].
Remodeling the TME is a key focus for BCa treatment strategies. Transforming growth factor (TGF)-β, is a multifunctional cytokine produced by cancer-associated fibroblasts (CAFs) and tumor cells that plays a key role in the immunosuppressive TME [247]. A hyaluronidase (HAase) and glutathione (GSH) dual-targeting nanosystem was established to deliver TGF-β siRNA to CAFs and tumor cells. This strategy enhanced the efficacy of anti-PD-L1 therapy, and facilitated nanomedicine penetration for profound TME remodeling [184]. VEGF and PIGF are overexpressed in M2-TAMs and BCa cells, attracted much attention in the BCa immune regulation [248, 249]. A cationic PEG and mannose-modified trimethyl chitosan conjugate and an anionic poly-(allylamine hydrochloride)-citraconic anhydride were synthesized to prepare siRNA-loaded nanoparticles via ionic gelation [185]. This dual-stage pH-sensitive carrier for co-delivering VEGF siRNA and PIGF siRNA to TME via active and passive targeting, resulting in TME remodeling and antitumor immunity, finally slowing the growth and lung metastasis of BCa [185].
In summary, recent studies successfully applied nanocarriers loaded siRNA as a BCa therapeutic tool. Unfortunately, despite promising preclinical results, there is no BCa-related siRNA drug progression into clinical trials and in market. The intricate nature of siRNA-based therapies requires comprehensive preclinical validation and optimization before advancing to human trials.
shRNA: Except for siRNA, another RNA interference (RNAi) tool in BCa therapy is shRNA. Previous strategies for constructing shRNA nanomedicines primarily rely on chemical conjugation or physical complexation of shRNA with chemotherapeutics. However, these approaches have limited clinical translation due to challenges such as low drug loading efficiency and poor biostability [250]. Single-walled carbon nanotubes (SWCNTs) were covalently attached to PEG and PEI, to create efficient gene delivery carriers [186]. The most effective vector, SWCNT-PEG attached to modified PEI 10 kDa with 10% 10-bromodecanoic acid, effectively and selectively transferred plasmid Bcl-xL shRNA to MUC1 positive cells [186]. Subsequently, a study established a gene-hyperthermia therapeutic system including three MUC1-C shRNA plasmids (MUC1-C shRNA) and Fe3O4 magnetic nanoparticles (MNPs), which have demonstrated effective therapeutic outcomes in TNBC without notable side effects [251]. Similar studies were performed in six Tween 85-PEI conjugates with varying lengths of flexible hydrophobic polymethylene spacers, and the most effective nanoparticles were then used to down-regulate p65 expression through effective delivery of p65 shRNA, inhibiting tumor growth and lymphatic metastasis in MDA-MB-435 tumor-bearing mice [187]. Although there is relatively less research on nanomaterial-mediated delivery of shRNA in BCa treatment compared to siRNA, these nano-based delivery systems offer new possibilities to improve treatment strategies for BCa patients.
MicroRNAs: miRNA is a posttranscriptional regulator of gene expression that intricately linked with onset and progression of disease [252, 253]. The major challenges of microRNA-based gene therapy are to ensure safe and efficient delivery of the miRNAs to tumor sites [254]. Nanoparticle has a distinct advantage in packaging multiple anti-miRNAs or premiRNA molecules.
In early phase, exosomes modified with the GE11 peptide or EGF on their surfaces were employed for delivering miRNA to cancer tissues expressing EGFR [255]. After these modified exosomes were intravenously injected to target EGFR, they specifically delivered let-7a miRNA to xenograft breast cancer cells in RAG2–/– mice [255]. Recently, to meet the need for a robust miRNA delivery vehicle to combat BCa, Manisha Ahir et al. developed tailored mesoporous silica nanoparticles (MSNs) for co-delivery of miR-34a mimic and antisense-miR-10b [188]. MSNs were functionalized with a cationic basic side chain and loaded with the dual combination to upregulate miR-34a and simultaneously downregulate miR-10b [188]. Subsequently, the loaded MSNs were coated with hyaluronic acid-appended PEG-PLGA polymer for specific targeting [188]. In vitro and in vivo studies demonstrated high specificity in targeting TNBC tumors, resulting in efficient inhibition of tumor growth and metastasis retardation [188]. Another research designed layer-by-layer (LbL) assembled poly(lactic-co-glycolic acid) (PLGA) nanoparticles for delivering miR-34a. The LbL nanoparticles (NPs) are created through successive deposition of oppositely charged polymers onto a spherical NP core. They possess several attributes that render them advantageous as carriers for RNA delivery.
mRNA: mRNA can direct cells to produce specific proteins, thereby eliciting an immune response or interrupting pathways that promote tumor growth. This makes mRNA a highly promising therapeutic tool. One study employed Pep LNPs, using PD-L1 binding D-peptides to design LNPs that target cancers overexpressing PD-L1, to deliver PTEN mRNA specifically to a PTEN-deficient 4T1 triple-negative breast cancer model in vivo. The activation of PTEN in TNBC induced autophagy-mediated immunogenic cell death, resulting in strong antitumor immune responses [189]. Also, a strategy to restore Lcor expression by delivering Lcor mRNA into tumor cells and combining it with immune checkpoint blockade. Mice treated with EV-based Lcor mRNA and anti-PD-L1 antibodies showed significantly longer survival and complete eradication of lung metastasis compared to those receiving only EV-control and anti-PD-L1 therapy [256]. Moreover, several mRNA-based therapies for triple-negative breast cancer have entered clinical trials. The “TNBC-MERIT” trial initiated by Roche/BioNTech is a Phase I trial (NCT02316457) designed to evaluate the feasibility, safety, and immunogenicity of intravenous administration of a liposomal mRNA vaccine encoding various classes of tumor antigens in TNBC patients after surgery and (neo)adjuvant chemotherapy. The results showed that immunogenicity data were generated in all 14 patients who received BNT121 treatment. A high magnitude of T-cell responses targeting individual neo-epitopes was induced, with response intensity reaching up to 10.3% of peripheral blood CD8+ T cells. Furthermore, in one patient, responses were observed in both CD4+ and CD8+ T cells against 10 neoantigens. After completing the vaccination schedule, T cells were able to maintain a high level of response for at least 6 months. TriMix is an mRNA-based adjuvant composed of mRNA molecules encoding the costimulatory molecules CD70, CD40L, and the TLR4 activation molecule [257]. In cancer vaccine research, the TriMix adjuvant has been shown to enhance dendritic cell maturation and cytotoxic T lymphocyte (CTL) responses [258]. A Phase I study using TriMix alone is underway for breast cancer (NCT03788083), administered intratumorally, and it is currently ongoing.
4.3.5 Drug Delivery
Chemotherapy drugs: Chemotherapy remains a cornerstone in the BCa treatment, but its efficacy is limited by systemic toxicity and drug resistance. Nanocarriers offer an alternative solution to these challenges by enhancing the targeted delivery of chemotherapeutic agents, even achieving controlled and sustained release of chemotherapeutic agents. Doxil, the first FDA-approved nanodrug, is a PEGylated liposomal formulation of doxorubicin, with a diameter of approximately 85 nm. Doxorubicin has historically been a crucial chemotherapy agent for BCa treatment [259]. Compared to the conventional doxorubicin, doxil was equally effective combated both low- and high-growth fraction tumors [260]. In a Phase 3 trial, Doxil offered comparable efficacy to doxorubicin, while significantly reducing cardiotoxicity, myelosuppression, vomiting, and alopecia in first-line therapy for metastatic BCa [261]. Notably, Abraxane also represents a significant milestone in the field of BCa nanomedicine, offering a safer and more effective alternative to conventional chemotherapy. Abraxane, also known as paclitaxel albumin-bound nanoparticles, had been approved by the FDA as the first line therapy for metastatic BCa [262, 263]. Abraxane utilizes albumin, a naturally occurring protein, as a carrier to deliver paclitaxel directly to tumor cells [262, 263]. Unlike conventional paclitaxel, which requires solvents like Cremophor EL that can cause severe allergic reactions, Abraxane is solvent-free, making it safer and more tolerable for patients [262-264]. The albumin-bound nanoparticles exploit the natural pathways of albumin transport, enhancing drug accumulation in tumors through mechanisms such as the EPR effect and albumin receptor-mediated uptake [262-264]. Abraxane has demonstrated superior efficacy and safety in multiple randomized controlled trials (RCTs) across different BCa settings, including metastatic, early-stage, and TNBC. For example, a phase 3 clinical trial (NCT01583426) compared the efficacy and safety of nab-paclitaxel (Abraxane) with solvent-based paclitaxel in patients diagnosed with early BCa. The results showed a significant increase in pathological complete response rates by substituting solvent-based paclitaxel with nab-paclitaxel after anthracycline-based chemotherapy, potentially shifting the preferred taxane to nab-paclitaxel in primary BCa treatment [265]. Researchers have investigated the efficacy and safety of combining Abraxane with other conventional anticancer agents. For instance, in HER2-positive breast cancer patients, a regimen combining Abraxane with carboplatin and trastuzumab demonstrated significant therapeutic effectiveness and a favorable safety profile [266].
Nanogels are nano-sized 3D hydrogels composed of Water-soluble polymers, exhibiting high water content and unique properties, enabling efficient and site-specific delivery of anticancer drugs, especially chemotherapy drugs including DOX, cisplatin (CP), paclitaxel (PTX) and docetaxel (DTX), among others [267, 268]. A universal and versatile strategy was employed to engineer DOX-based nanogels (NGs) by conjugating hydroxyl-containing DOX with hydroxyl-containing drugs using a GSH-sensitive disulfide bond as a linker [190]. These NGs release their payload upon internalization into the tumor microenvironment (TME) where high GSH concentrations enable combined chemo-immunotherapy [190]. Biodegradable poly(lactic-co-glycolic acid) nanoparticles (PLGA NPs) are widely employed for delivering chemotherapy drugs [191]. Through a PEGylated-PLGA inner core and a FeIII-TA complex outer shell to prevent premature DOX release. This novel doxorubicin (DOX)-loaded FeIII-TA complex-coated PLGA NPs (DOX-TPLGA NPs) were prepared using the double-emulsion solvent evaporation technique followed by rapid coating with coordinated TA and FeIII and effectively accumulated at tumor sites in TNBC mice and achieve high tumor inhibition rate [191].
Using p-toluenesulfonate as a removable core, tetraethoxysilane (TEOS)/bis[3-(triethoxysilyl)propyl] tetrasulfide (TESPTS) as a disulfide bond-involved organosilica precursor, and cetyltrimethylammonium bromide (CTAB) as a mesostructure directing agent, uniform nanospheres (HM) of about 100 nm with 30 nm hollow cavities were synthesized [192]. Fe-MOF (MIL-100(Fe)) was grafted onto HM via a solvothermal reaction between FeCl3·6H2O and trimesic acid, forming core-shell nanoparticles (HM@FM) of around 150 nm [192]. DOX loading did not change the hydrated particle size, maintaining dispersity, allowing HM@FM to form a nanosuspension for further experiments [192].
Small-molecule inhibitor/agonist: The emergent of small-molecule inhibitors and agonists has opened new frontiers in the BCa treatment, providing precise targeting of key signaling pathways and cellular processes within cancer cells [269, 270]. However, the effectiveness of these agents is often hindered by poor solubility, rapid metabolism, and drug resistance [271, 272]. Nanoplatforms can encapsulate small-molecule inhibitors and agonists, shielding them from degradation in biological fluids and enabling them to cross biological barriers that would otherwise limit their distribution [273, 274]. This protection results in a higher concentration of the active compound reaching the target site, enhancing therapeutic effectiveness while reducing the required dosage [273, 274].
The multifunctional phototheranostic nanomedicine Lips(PTQ/GA/AIPH) was formulated by co-encapsulating thiadiazoloquinoxaline semiconductor polymer (PTQ), gambogic acid (GA) as an HSP inhibitor, and PTDT prodrug (2,2′-azobis[2-(2-imidazolin-2-yl)propane] dihydrochloride) into thermosensitive liposomes [193]. These tumor-targeted liposomes were modified with nucleolin-specific aptamers), effectively suppress deep-seated TNBC with minimal side effects [193]. A versatile AuAg hollow nanocarrier system was developed for targeted multimodal synergistic therapy of HER2+ BCa. AuAg HNSs were synthesized via electro displacement, yielding a unique hollow porous structure for efficient pyrotinib loading [228]. The nanoshell surfaces were modified with lipoic acid-polyethyleneimine (LA-PEI) and SH-PEG, further modification with Herceptin enabled precise targeting of HER2-overexpressing tumor cells [228]. Intratumoral photocatalysis by AuAg HNSs significantly resulted in superior therapeutic efficacy, markedly reducing tumor size in a HER2+ BCa mouse model [228]. STING agonist-loaded liposomes enhance antigen-presenting cells-mediated adaptive immune response against multifocal metastases models [275, 276]. MSNs with fluorescent RhodamineB isothiocyanate (RITC) were synthesized using a soft-templating method and modified with PEG and TA to create RMSN-PEG-TA nanoparticles with an average diameter of 26.7 ± 4.8 nm [195]. To enhance cellular uptake and STING drug cyclic diguanylatemonophosphate (cdG) loading, a cationic molecule was introduced, resulting in a Z-potential of +28 mV at pH 7.4 [195]. PLGA microparticles were also engineered for sustained release of the STING agonist 3′3′-cGAMP for cancer management [194].
Immune checkpoint inhibitors: Immune checkpoint molecules are expressed on the tumor-cell or immune-cell populations and can suppress anticancer immune responses [277]. Immune checkpoint inhibitors (ICIs)–immunomodulatory agents are widely applied to relieve tumor-mediated immune-cell suppression [278].
To reshape the immunosuppressive TME to enhance ICI response, a tumor microenvironment-responsive nanoassembly based on self-assembled aptamer-polymer conjugates for targeted delivery of the glucose transporter 1 inhibitor BAY-876 (DNA-PAE@BAY-876) [279]. Poly β-amino ester (PAE)-modified aptamers antagonizing PD-L1 (aptPD-L1) and CTLA-4 (aptCTLA-4) are synthesized and co-assembled into supramolecular nanoassemblies for BAY-876 encapsulation [279]. The acidic TME triggers nanoassembly dissociation via PAE protonation, releasing BAY-876 and aptamers. BAY-876 selectively inhibits TNBC glycolysis, suppressing uridine diphosphate N-acetylglucosamine and downregulating PD-L1 N-linked glycosylation, enhancing PD-L1 recognition by aptPD-L1 for improved anti-PD-L1 therapy [279]. DNA-PAE@BAY-876 effectively remodels the immunosuppressive TME in TNBC mouse models, offering a promising strategy for TNBC immunotherapy in clinical settings [279]. An albumin nanoparticle encapsulating the PI3Kγ inhibitor IPI-549 and PTX (Nano-PI) was developed. PTX synergized with IPI-549 to promote M2 to M1 macrophage repolarization, facilitating stable nanoparticle formation [280]. Nano-PI was assessed for dual-drug delivery to macrophages in lymph nodes and tumors. Its efficacy in combination with anti-PD-1 was evaluated in spontaneous breast cancer transgenic mice and mouse xenograft breast cancer models [280].
4.3.6 Antibody-Drug Conjugates
Antibody-drug conjugates (ADCs) are designed to bind specifically to antigens expressed on the surface of cancer cells [281]. Once bound, the conjugate is internalized, and the cytotoxic drug is released directly inside the cancer cell, leading to cell death. This precise targeting enhances the drug's efficacy and minimizes collateral damage to normal cells. Recent studies have shown that ADCs can improve drug stability and solubility, protecting the drug until it reaches the target site, thereby maximizing its therapeutic impact [177]. Furthermore, advancements in ADC technology have led to the development of stimuli-responsive conjugates. These ADCs can be engineered to respond to specific triggers in the tumor microenvironment, such as pH changes or redox conditions, ensuring that the drug is released only in the presence of cancer cells [178]. This targeted release mechanism further enhances the specificity and effectiveness of the treatment, reducing the likelihood of adverse side effects.
The integration of ADCs with nanoplatforms offers additional benefits, including controlled and sustained drug release. This ensures that therapeutic levels of the drug are maintained over extended periods, improving overall treatment outcomes [179, 180, 282].
One prominent ADC, T-DM1, combines trastuzumab, an anti-HER2 antibody, with the microtubule inhibitor DM1 via a non-cleavable thioether linker. In the EMILIA study, T-DM1 significantly improved progression-free survival (PFS) and overall survival (OS) compared to lapatinib and capecitabine in patients with advanced HER2-positive breast cancer who had previously received taxanes and trastuzumab [283]. Specifically, the median PFS and OS were 9.6 months and 30.9 months, respectively [283]. The KATHERINE study further demonstrated that T-DM1 reduced the risk of recurrence or death by 50% compared to trastuzumab alone in early HER2+ BCa patients with residual invasive disease post-neoadjuvant therapy [283].
Another innovative ADC, T-DXd, links trastuzumab to the topoisomerase I inhibitor deruxtecan (DXd) through a cleavable tetrapeptide linker. The DESTINY-Breast03 study revealed that T-DXd significantly reduced the risk of death by 36%, with a median PFS four times longer than that of T-DM1 (28.8 months vs. 6.8 months). Moreover, about 78.5% of patients achieved objective response, and 21.1% attained complete response [102, 284]. The DESTINY-Breast04 study extended the application of T-DXd to HER2-low expressing unresectable/metastatic breast cancer, significantly prolonging both median PFS (9.9 months vs. 5.1 months) and OS (23.4 months vs. 16.8 months) compared to chemotherapy [285].
Sacituzumab govitecan (SG) is another ADC that targets TROP-2 and is conjugated with the topoisomerase I inhibitor SN-38. The ASCENT trial showed that SG markedly improved median PFS (5.6 months vs. 1.7 months) and OS (12.1 months vs. 6.7 months) in patients with unresectable, recurrent, or refractory locally advanced/metastatic triple-negative breast cancer (TNBC) who had received at least two prior standard chemotherapy regimens [286]. The TROPiCS-02 study indicated that SG also demonstrated efficacy in endocrine-resistant metastatic HR+/HER2- breast cancer, with a median OS of 14.4 months compared to 11.2 months with physician's choice chemotherapy [286].
Moreover, some nanoparticles can co-deliver multiple therapeutic agents, such as chemotherapy drugs and immunotherapy agents, providing a comprehensive attack on cancer cells [181]. Trastuzumab emtansine (Kadcyla®) was approved by the Food and Drug Administration in 2013 for the treatment of solid tumors, despite its success, the second-generation ADC faces several limitations, including heterogeneity, limited activity against tumors with heterogeneous antigen expression, and suboptimal tumor penetration [287]. To address these challenges, researchers are increasingly focusing on incorporating nanoplatforms and optimizing linkers. An innovative branched pegylated linker was developed to control the hydrophobicity of monomethyl auristatin E (MMAE) and its cathepsin B-sensitive trigger and then utilized for the efficient generation of internalizing homogeneous ADCs with a drug-to-antibody ratio of 8 and minibody-drug conjugates with a drug-antibody ratio (DAR) of 4, targeting HER2 [288]. Both immunoconjugates were subsequently evaluated in vitro and in vivo on breast cancer models and showed effective tumor size reduction [288]. Most recently, Xia et al. utilized affibody proteins conjugated with hydrophobic drug toxins to prepare ADCs and employ self-assembly strategies to create actively targeted nanomedicines to address the limitations of ADC drugs [289]. Due to the amphiphilic nature of the ADCs ZHER2:342-MMAE (Z-MADCN), it successfully assembles into nanomicelles of approximately 120 nm in aqueous solution, while retaining the high affinity of the affibody protein [289]. In HER2+ BT474 BCa models, Z-MADCN nanomedicine demonstrated excellent antitumor performance in tumor models, with a tumor inhibition rate of 99.8% [289]. The tumors in the mice were completely cured without recurrence, and the nanomedicine exhibited high biosafety in the mice [289].
4.3.7 Codelivery
Although chemoimmunotherapy has seen significant success in cancer treatment recently, curing BCa remains challenging. The unsatisfactory outcomes are likely due to the superimposed factor including low tumor immunogenicity, a highly immunosuppressive TME and tumor metastasis [290]. The complex and changeable features of TME often limits the effectiveness of single treatments, making combination therapy a growing research focus. Combination therapy is expected to overcome multidrug resistance, induce tumor cell apoptosis through various mechanisms, and produce synergistic effects or reduce side effects. The field of codelivery focuses on the combined delivery of multiple therapeutic cargoes within a single delivery system, ensuring they are delivered to the same site and allowing for tailored release kinetics [291, 292].
A combination therapy by co-encapsulating siRNA and DOX in BCa-targeted poly(lactic-co-glycolic acid) (PLGA) nanoparticles to suppress NGF expression post-chemotherapy [196]. PLGA nanoparticles modified with a CD44-targeted aptamer were designed to co-deliver doxorubicin (DOX) and NGF siRNA [196]. By targeting tumors and concurrently delivering DOX and NGF siRNA, the formulated nanodrug delivery system (Ap-DSNPs) efficiently inhibited chemotherapy-induced sympathetic nerve proliferation within tumors, enhancing the synergistic chemo-immunotherapeutic effect [196]. Another strategy using two modules of core–shell tecto dendrimer (CSTD)-based nanomedicines to trigger ICD in the TME and enhance DC maturation in lymph nodes [293]. The CSTDs were assembled through supramolecular self-assembly of generation 5 (G5) poly(amidoamine) dendrimer cores and G3 dendrimer shells [293]. One module loaded DOX for inducing ICD in cancer cells, while another module delivered YTHDF1 siRNA to DCs to stimulate maturation [293]. These CSTD-based nanomedicine formulations enhanced chemoimmunotherapy in breast tumor models by treating cancer cells and DCs, synergistically promoting DC maturation to activate CD8+/CD4+ T cells for tumor eradication [293]. An alternative approach to combat drug-resistant BCa by co-delivering Ca2+ channel siRNA and cytotoxic drugs. The mesoporous silica nanocapsules (MSNCs) with a hollow structure and achieve high drug loading efficiency for simultaneous delivery of siRNA and doxorubicin (DOX). The co-loaded MSNCs exhibited a synergistic therapeutic effect combat drug-resistant BCa cells [197]. A cancer cell membrane-camouflaged nanogel system, loaded with ultrasmall iron oxide nanoparticles, docetaxel (DTX), and CD47 siRNA, inducing ICD and restoring macrophage phagocytosis by downregulating “don't eat me” signals [198]. USIO@PEI nanogels (NGs) were formed using a water-in-oil inverse emulsion method with Michael addition cross-linking and N,N′-bis(acryloyl)cystamine (BAC) containing disulfide bonds. The NGs were then loaded with DTX and siCD47. Finally, the DTX/USIO@PEI NGs/siCD47 was camouflaged with 4T1 cancer cell membrane (CM) to form DTX/USIO@PEI NGs/siCD47@CM [198]. This nanoplatform promotes macrophage M1 polarization, enhancing the combined effects of DTX-mediated ICD and ICB for effective chemoimmunotherapy [198]. A small molecular self-assembling nano-prodrug that not only releases active chemotherapeutics for tumor inhibition but also triggers ferroptosis via an Fe catalyst and GPX4 inhibitor, thereby enhancing chemotherapeutic efficacy [199]. The small molecular prodrug (CPT-SS-Fc) consists of ferrocene (Fc) and camptothecin (CPT), linked by a GSH-responsive disulfide bond. This prodrug self-assembles into stable nanoparticles under physiological conditions, with CPT deactivated by the disulfide bond. The GPX4 inhibitor (RSL3) is incorporated into CPT-SS-Fc during self-assembly via π-π interactions, forming a three-component nano-prodrug (RSL3@CPT-SS-Fc). In the TME, overexpressed GSH cleaves the disulfide bond, disassembling the nano-prodrug and releasing CPT, Fc, and RSL3. CPT induces apoptosis, Fc triggers ferroptosis via ROS generation, and RSL3 enhances ferroptosis by inhibiting GPX4. In various TNBC mouse models, RSL3@CPT-SS-Fc was encapsulated into RGD-targeting phospholipid micelles (DSPE-PEG2000-RGD) to form the active-targeting nano-prodrug, which effectively accumulates in the tumor region for TNBC antitumor and antimetastasis administration [199]. Moreover, to achieve the spatiotemporal control of CPT nano-formulation, Xu et al. designed ROS-responsive nanoparticles by conjugating hydrophobic CPT and hydrophilic 5-fluorouracil (FUDR) via a thioketal (TK) linker, forming CPT-TK-FUDR (CTF) [294]. IR780-based phototherapy was incorporated, with HA-modified IR780 (HAIR). CTF and HAIR self-assembled into HAIR/CTF NPs, targeting tumors via HA-receptor binding [294]. Tumor-overexpressed HAase disassembled the NPs, while 808 nm laser irradiation triggered IR780 to generate ROS, cleaving the TK linker for controlled drug release [294]. ROS-mediated PDT and laser-triggered PTT synergistically eliminated tumor cells, while CPT and phototherapy induced ICD, suppressing TNBC [294]. The HAIR/CTF nano-platform achieves precise drug release, effective tumor eradication, and antimetastasis via combined chemo-photo-immunotherapy [294]. Interestingly, a self-cascaded chemo-PDT strategy (AIE-Pep-DOX, APD) was reported, for pH-responsive drug release, caspase-3-activatable AIE imaging, and repeatable PDT against TNBC [295]. APD, comprising DOX and an AIEgen photosensitizer linked by a caspase-3 substrate (DEVD) and fibronectin-targeting peptide (CREKA), features an acid-labile hydrazone bond for DOX release and caspase-3-triggered AIEgen aggregation [295]. At physiological pH, APD circulates as a hydrophilic molecule, while acidic TME triggers DOX release, activating caspase-3 and cleaving DEVD to induce AIEgen aggregation for prolonged imaging and PDT [295]. The APR effect enables tumor-retained AIEgen for repeatable PDT and ICD induction, enhancing DC maturation and CTL proliferation when combined with aPD-L1, effectively inhibiting TNBC progression and reversing immunosuppression [295].
Emerging research highlights the synergy between targeted drug delivery and immune checkpoint inhibitors. Early-phase trials (NCT04849364), combine the LIV-1-targeted ADC SGN-LIV1A with pembrolizumab in metastatic TNBC, supported by preclinical evidence that ADC payloads (e.g., microtubule disruptors) activate the STING pathway, fostering a pro-inflammatory tumor microenvironment. Another pivotal phase III RCT, TROPION-Breast02 (NCT05374512), evaluates datopotamab deruxtecan (TROP2-targeted ADC) with durvalumab (anti-PD-L1) in advanced TNBC, targeting improved progression-free survival (PFS) and overall survival (OS). The ongoing phase Ib/II DESTINY-Breast07 trial (NCT04538742) investigating trastuzumab deruxtecan (T-DXd) combined with durvalumab has raised concerns regarding overlapping toxicities, particularly cardiorespiratory adverse events such as interstitial lung disease and immune-related pneumonitis, which necessitate rigorous surveillance protocols. Furthermore, emerging data suggest that intratumoral heterogeneity and dynamic alterations in HER2 expression may contribute to therapeutic resistance, highlighting the imperative for dual-targeting strategies and adaptive trial designs incorporating real-time biomarker assessment to optimize patient stratification. In contrast, the MORPHEUS-panBC study (NCT03424005) evaluating sacituzumab goviteca plus atezolizumab in TNBC demonstrated modest progression-free survival (PFS) benefits, underscoring the limitations of combining topoisomerase-I inhibitor-based ADCs with PD-L1 blockade in immune-cold microenvironments characterized by low tumor-infiltrating lymphocytes (TILs) and an immunosuppressive stromal composition. For chemotherapy, liposomal doxorubicin (Doxil) combined with avelumab (anti-PD-L1) in a phase II RCT (NCT03409198) demonstrated a 35% objective response rate in metastatic TNBC, attributed to doxorubicin-induced immunogenic cell death (ICD) enhancing T-cell activation.
The integration of targeted drug delivery systems with immune checkpoint inhibitors presents a transformative paradigm in BCa therapy, yet several challenges must be navigated to realize its full clinical potential. Safety remains a critical concern, as combinatorial regimens may amplify immune-related adverse events, including pneumonitis, hepatitis, and endocrine dysregulation, necessitating optimized dosing schedules and predictive biomarkers for toxicity mitigation. Additionally, therapeutic resistance—driven by tumor antigen loss, compensatory immune checkpoint upregulation (e.g., LAG-3, TIM-3), or immunosuppressive stromal remodeling—demands innovative strategies such as bispecific ADCs or epigenetic modulators to disrupt resistance pathways. The inherent heterogeneity of breast cancer subtypes further complicates universal application, requiring precision approaches tailored to molecular profiles (e.g., HER2 dynamics, PD-L1 status) and immune microenvironmental features (e.g., myeloid cell infiltration, tertiary lymphoid structure density). Future research should prioritize the development of next-generation delivery platforms, including exosome-based or biomimetic nanoparticles engineered for enhanced tumor penetration and organelle-specific payload release, alongside exploration of novel immune checkpoints (VISTA, TIGIT) and dual-targeting inhibitors to overcome adaptive immune evasion. Artificial intelligence (AI)-driven frameworks integrating multi-omics data (spatial transcriptomics, proteomic atlases) and real-world evidence could revolutionize patient stratification and dynamic regimen adaptation. Collectively, while preclinical and early-phase trials demonstrate synergistic antitumor activity, clinical translation hinges on resolving toxicity-resistance trade-offs, validating subtype-specific biomarkers, and advancing adaptive trial designs. By bridging innovations in nanomedicine, immunology, and computational oncology, this dual-modality strategy may ultimately redefine therapeutic landscapes for aggressive breast cancer subtypes, offering durable remission where conventional therapies falter.
4.3.8 Stimulus-Responsive Nanodrug Strategies for Breast Cancer
In the pursuit of advancing BCa therapy, stimulus-responsive nanodrug strategies have been utilized to deliver various pharmaceutical agents. Nanodrugs leverage the unique properties of nanoparticles to deliver therapeutic agents in a controlled and targeted manner, responding to specific stimuli within the TME.
pH-responsive: The acidic environment of tumors, resulting from increased metabolic activity and poor perfusion, can be exploited by pH-responsive nanodrugs. These nanodrugs remain intact in the physiological pH of the bloodstream but undergo structural changes and release their cargo in the acidic tumor microenvironment. This approach not only increases the concentration of drugs at the tumor site but also minimizes systemic toxicity and side effects on healthy tissues.
Mesoporous silica nanoparticles (MSN-COOH) were synthesized and loaded with doxorubicin (DOX) into their pores, forming DOX@MSN-PEI-AA (DMPA), where PEI and anisamide were subsequently surface-modified [296]. DMPA specifically targeted tumor cells via anisamide-mediated receptor-mediated endocytosis. In the acidic environment of cellular lysosomes/endosomes, PEI underwent protonation, causing it to dissociate from the MSN surface and ensuring steady release of DOX from the nanoparticle pores into the cytoplasm of target cells, highlighting its significant potential for BCa therapy [296]. A hydrogel is a three-dimensional cross-linked network of water-soluble polymers that efficiently encapsulates drugs, making it widely utilized in controlled drug release applications [297]. Covalently conjugating metformin (Met) to oxidized hyaluronic acid (OHA) via imine bonds to synthesize OHA-Met, followed by the preparation of carboxymethyl chitosan (CMCS)/OHA-Met drug-loaded hydrogels [298]. After in situ injection of this hydrogel nanodrug, the cumulative release of metformin from CMCS/OHA-Met20 hydrogel was 42.7 ± 2.6% at 6 h, with release reaching equilibrium after 72 h at pH 7.4, and the release remained constant, with a cumulative release rate of 79.3 ± 4.7% at 6 h at pH 5.5, demonstrating excellent pH-responsive behavior [298]. In vivo experiments on breast cancer recurrence indicated that CMCS/OHA-Met20 hydrogel enabled local injection and pH-responsive smart delivery at the tumor resection site, thereby inhibiting BCa recurrence [298]. Another pH-responsive polymeric micelles were synthesized for the co-delivery of PTX and triptolide (TPL), designed to disassemble in acidic TME for targeted drug release and effective eradication of BCa cells [299]. Initially, amphiphilic copolymer mPEG2000-PBAE was synthesized via Michael addition reaction and confirmed through various characterizations [299]. Thin film dispersion method was employed to prepare polymer micelles loaded with TPL and PTX (TPL/PTX-PMs) [299]. The average particle size of TPL/PTX-PMs was 97.29 ± 1.63 nm and zeta potential of 9.57 ± 0.80 mV [299]. This pH-responsive micellar co-delivery of TPL and PTX represen ts a promising strategy for BCa therapy [299].
Enzyme-responsive: Enzymes act as triggers, causing the nanoparticles to degrade and release their therapeutic cargo directly at the tumor site [300]. For example, nanoparticles designed to respond to MMPs remain stable in the bloodstream but break down in the presence of these enzymes within the tumor. This controlled release mechanism ensures that a higher concentration of the drug reaches the cancer cells, enhancing the treatment's effectiveness while minimizing harm to the rest of the body. Enzyme-responsive nanodrug strategies mark a transformative step in BCa treatment.
A Cu-tetra(4-carboxyphenyl)porphyrin chloride(Fe(III)) (Cu-TCPP(Fe)) metal-organic framework (MOF)-based nanosystem has been developed for the efficient incorporation of Au NPs and RSL3 [301]. Specifically, Cu-TCPP(Fe) MOF nanosheets were employed to in situ nucleate Au NPs and were loaded with RSL3 via π−π stacking [301]. The final construct was modified with polyethylene glycol (PEG) and iRGD for targeted drug delivery to tumors, and cumulated in the 4T1 tumor tissues [301]. This system exhibits enzyme-like activities that can broadly suppress antiferroptotic pathways in tumor cells to amplify ferroptotic damage, providing a strong foundation for the clinical application of ferroptosis-based BCa therapies [301]. Furthermore, for second near-infrared (NIR-II) photoactivatable ferroptosis-immunotherapy, a dual-enzyme-decorated semiconducting polymer nanoagents (termed SPHGA), comprise hemoglobin (Hb)-based semiconducting polymer (SP@Hb), adenosine deaminase (ADA), and glucose oxidase (GOx) encapsulated within a thermally-responsive nanoparticle shell, was designed [302]. NIR-II photoactivation of SPHGA induces heat generation, facilitating the on-demand release of ADA and GOx by disrupting the thermoresponsive nanoparticle shells [302]. Within the TME, GOx catalyzes glucose oxidation to produce H2O2, which in turn enhances the Fenton reaction involving iron from SP@Hb, thereby intensifying the ferroptosis effect and triggering immunogenic cell death (ICD). Furthermore, ADA degrades elevated levels of adenosine to counteract the immunosuppressive microenvironment, thereby amplifying antitumor immune responses. SPHGA demonstrates enhanced efficacy in completely eradicating primary tumors and effectively inhibiting tumor metastasis in subcutaneous 4T1 models [302]. Enzyme-responsive nanoparticles are also used for combinational immunotherapy. A tumor cascade-targeted responsive liposome (NLG919@Lip-pep1) has been developed by conjugating a polypeptide inhibitor of the PD-1 signaling pathway (AUNP-12) with a liposome carrier through a matrix metalloproteinase-2 (MMP-2) cleavable peptide (GPLGVRGD) [303]. The GPLGVRGD sequence served as a linker, connecting the N- and C-terminals with two drug modules, I and II, respectively, to create a responsive drug-polypeptide conjugate (I–GPLGVRGD–II) [303]. AUNP-12 functioned as drug module I, linked subsequently to DSPE-PEG to form the lipid component of the liposome [303]. The IDO inhibitor NLG919 was encapsulated within the liposome, resulting in a tumor cascade-targeted responsive liposome drug delivery system (NLG919@Lip-pep1) [303]. The overexpressed MMP-2 at the tumor site triggers the dissociation of AUNP-12, thereby precisely blocking the PD-1 signaling pathway and restoring T cell activity [303]. This process also exposes the secondary targeting module, VRGDC-NLG919@Lip, which targets tumor cells and further alleviates the immunosuppressive TME [303].
Redox-responsive: Redox-responsive nanodrugs have the ability to release drugs in response to the oxidative stress conditions prevalent in tumors [304, 305]. This oxidative imbalance in cancer cells triggers the release of the drug payload directly at the tumor site, optimizing treatment efficacy while minimizing harm to healthy tissues [304, 305]. The integration of redox-responsive mechanisms into nanodrug design exemplifies the synergy between nanotechnology and targeted cancer therapy [306].
A combination of precipitation polymerization and modified sol-gel methods was employed to create novel hyaluronic acid-decorated, pH and redox dual-stimuli responsive poly(methacrylic acid)/mesoporous organosilica nanoparticles with a core–shell structure for controlled drug release [307]. These spherical nanocarriers feature a uniform size and tunable shell thickness, capable of entrapping over 70% of quercetin with a drug loading efficiency of over 10% [307]. Drug release profiles demonstrated good stability under normal physiological conditions, with a significantly increased release in simulated TME, demonstrating pH and redox-dependent release behavior [307]. These findings suggest that the proposed nanocarriers could serve as an advanced drug delivery system, improving the antitumor efficacy of chemotherapeutic agents [307]. Another study to reverse the CAF phenotype for BCa therapy, dithiomaleimide was used as a glutathione (GSH)-sensitive linker to conjugate CTX to the backbone of chitosan, forming a novel CS-DTM-CTX (CDC) conjugate. This conjugate through co-assemble with DAS to create DAS@CDC could accumulate at the tumor site, delivering DAS and CTX into both tumor cells and CAFs, showing distinct tumor growth inhibition in a subcutaneous 4T1 tumor model [308]. Moreover, carboxymethyl chitosan (CMC) can function as a hydrogen-ion sponge, has a pH-dependent surface charge property, and binds with anticancer drugs through stimuli-responsive linkers [309, 310]. Utilizing the superior characteristics of CMC, stimuli-responsive CMC-based nanoplatforms comprises three functional components: a redox-responsive and negatively charged “core” of CMC-DOX, a pH-responsive and positively charged “shell” of oligoethylenimine/siMDR1, and surface-modified HA conjugated with AS1411 aptamer (AHA) and GALA peptide (high affinity and specificity for nucleolin) [311]. This nanoplatform presented the continuous and sustained accumulation in tumor and significantly enhanced antitumor efficacies for drug-resistant BCa models [311].
5 Current Challenges and Limitations
Though nanoparticle-incorporated cancer immunotherapy is a highly promising treatment modality for patients with BCa, with ongoing research and advancements poised to revolutionize treatment paradigms, there are still some challenges and limitations.
5.1 Toxicity and Long-Term Safety
Toxicity and long-term safety of nanoparticles remain a significant concern in the development and application of BCa therapeutics, particularly due to their unique physicochemical properties and biological interactions. First, the potential for nanoparticle accumulation in vital organs such as the liver, spleen, and kidneys may lead to chronic inflammation or organ dysfunction over time [312, 313]. Additionally, the surface chemistry and degradation products of nanoparticles may also contribute to unwanted immune responses and side effects. For example, PEGylation, which is commonly used in nanoparticles to enhance their stability and circulation time, can cause IgG, IgM, or IgE-triggered allergies in the context of repeated administration [314]. In clinical applications, hypersensitivity reactions caused by PEGylated drugs have been frequently reported [315-317]. Furthermore, the lack of standardized protocols for toxicity assessment and the variability in nanoparticle behavior under different physiological conditions complicate the evaluation of their safety profiles.
5.2 Manufacturing and Scalability Issues
The synthesis of nanoplatforms often involves complex and multi-step processes, thus, standardized manufacturing processes and scalable production capabilities are quite essential. The lack of standardized protocols often leads to variability in nanoparticle size, shape, surface chemistry, and drug-loading efficiency, which can significantly impact their therapeutic performance and safety profile [318]. For example, Sykes et al. revealed that different-sized spherical gold nanoparticles possess different tumor-targeting abilities [319]. Besides, complicated synthesis processes can significantly increase the cost of production, making it difficult to scale up [320, 321]. Nevertheless, certain natural nanoparticles with simple and straightforward extraction protocols, such as exosomes and apoptotic bodies, still face challenges including limited raw material availability and time-intensive processing constraints, which collectively hinder scalable manufacturing [322, 323].
5.3 Clinical Translation
Though nanoplatforms have achieved considerable success in BCa in laboratory research, there is a long way for them to go into large-scale clinical application, with lots of obstacles hindering this journal, including cost-effectiveness, accessibility, regulatory requirements and ethical considerations. First, the development of nanoplatforms requires advanced materials, specialized equipment, and highly skilled personnel, resulting in significantly high costs. For example, in research about selective organ targeting (SORT) platform development, multiple classes of lipid-based NPs were systematically constructed to evaluate their target ability [324]. Besides, accessibility remains a critical issue. Many nanoplatforms require sophisticated infrastructure for storage, transportation, and administration, which may not be available in resource-limited settings [325]. In addition, the stringent regulatory approval process necessitates extensive preclinical and clinical trials to ensure the safety and efficacy of candidate nanoplatforms before they can enter the market, resulting in significant expenditures of both time and financial resources [326]. Lastly, ethical considerations cannot be overlooked. Since there are long-term effects of nanoparticles on human health and the environment, it is imperative to address critical ethical considerations such as patient privacy protection and the assurance of informed consent [327].
6 Future Directions for Nanoplatforms In Breast Cancer Diagnosis and Therapy
To achieve greater impact in breast cancer treatment, future development directions of nanoplatform must focus on several key areas with potentially significant influence, bridging the gap between laboratory research and clinical transformation, though it remains a long and challenging journey (Figure 4).
6.1 Precision Medicine
The integration of nanoplatforms with personalized medicine is a major future direction. By tailoring nanoplatforms to the specific genetic and molecular profiles of individual patients, it is possible to optimize therapeutic outcomes [328]. Furthermore, advancements in genomics and proteomics will facilitate the identification of novel biomarkers, further refining the targeting capabilities of nanoplatforms. Nanoplatforms can be designed to target unique biomarkers expressed by a patient's tumor or receptor-ligand interaction, ensuring precise delivery of therapeutic agents. This approach not only enhances efficacy but also minimizes adverse effects, as treatments are specifically directed toward cancer cells while sparing healthy tissues.
Moreover, combined drug-gene delivery systems represent a significant advancement in nanomedicine, offering a synergistic strategy to combat BCa. These systems co-encapsulate chemotherapeutic agents and genetic therapeutics within a single nanocarrier, enabling simultaneous attack on multiple pathways involved in tumor progression. By delivering both agents directly to the tumor site, these systems can increase the concentration of drugs and genes where they are most needed, while reducing exposure to other organs. Also, this dual approach not only enhances therapeutic efficacy but also addresses the issue of tumor heterogeneity, where different subpopulations of cancer cells may respond differently to treatment. By targeting multiple mechanisms simultaneously, combined drug-gene delivery systems can overcome resistance and improve long-term outcomes.
One of the key advantages of nanomedicines is their ability to overcome biological barriers that limit the effectiveness of conventional therapies. The EPR effect allows nanoparticles to accumulate preferentially in tumor tissues due to their leaky vasculature and poor lymphatic drainage. Additionally, surface modifications of nanoparticles, such as the addition of targeting ligands (e.g., antibodies or peptides), can further enhance their specificity for cancer cells. These features enable nanomedicines to deliver higher concentrations of therapeutic agents to the tumor site while sparing healthy tissues, thereby reducing systemic toxicity and improving patient quality of life.
6.2 Multifunctional Nanoplatforms
The development of multifunctional nanoplatforms represents another exciting frontier. These advanced systems can simultaneously deliver multiple therapeutic agents, such as chemotherapeutics, immunotherapies, and gene therapies, in a coordinated manner. By addressing multiple pathways involved in cancer progression, multifunctional nanoplatforms can enhance treatment efficacy and overcome resistance mechanisms. Additionally, these platforms can incorporate diagnostic and imaging agents, enabling real-time monitoring of treatment response and allowing for timely adjustments to therapy. This integrated approach enhances the ability to achieve sustained tumor suppression and improve patient outcomes.
6.3 Overcoming Resistance Mechanisms and Enhanced Tumor Penetration
Overcoming resistance mechanisms is a critical challenge in BCa therapy. Tumors often develop resistance to conventional treatments, leading to disease recurrence and progression [329, 330]. Nanoplatforms offer innovative strategies to address this issue. For example, nanoparticles can be designed to deliver siRNAs or miRNAs that target and silence genes responsible for drug resistance. By reprogramming cancer cells to restore sensitivity to therapy, nanoplatforms can enhance the effectiveness of existing treatments [329, 331]. Furthermore, the ability of nanoplatforms to co-deliver multiple agents allows for synergistic effects, potentially circumventing resistance mechanisms and achieving more robust therapeutic outcomes.
Effective treatment requires that therapeutic agents reach all areas of the tumor, including hypoxic regions and the tumor core [332]. Nanoplatforms can be engineered to enhance penetration through the dense extracellular matrix and reach deeper tumor areas [333]. Strategies such as size optimization, surface modification, and the use of penetration enhancers can improve the distribution of nanoplatforms within tumors. Exosomes have emerged as promising nanocarriers for enhancing tumor penetration due to their unique biological properties and engineerable nature. Ye et al. showed that after embellishing the platelet and neutrophil hybrid cell membrane on a gold nanocage (AuNC), the engineered nanosponges and nanokillers exhibit deeper tumor penetration [334]. Besides, Sun et al. explored artificial cytomembrane nanovesicles as the biomimetics of exosomes, with results showing that these NVs keep the ability to facilitate deep tumor infiltration and remodeling of the immune microenvironment [335]. Enhanced tumor penetration ensures that therapeutic agents can effectively target and eliminate cancer cells throughout the tumor mass, reducing the likelihood of residual disease and recurrence.
6.4 Clinical Translation
The successful translation of nanoplatforms from the laboratory to clinical practice involves addressing regulatory considerations and ensuring safety and efficacy [336]. Rigorous preclinical testing, including in vitro and in vivo studies, is essential to evaluate the biocompatibility, pharmacokinetics, and therapeutic potential of nanoplatforms. Additionally, standardized manufacturing processes and quality control measures are necessary to ensure reproducibility and scalability. Collaboration between researchers and clinicians will be crucial to navigate the regulatory landscape and expedite the clinical translation of nanoplatform-based therapies.
6.5 Emerging Technologies
Emerging technologies such as artificial intelligence (AI) and machine learning (ML) are poised to play a significant role in the future of nanoplatforms for breast cancer therapy [337]. AI and ML can analyze vast data sets to identify patterns and predict treatment responses, guiding the design and optimization of nanoplatforms. These technologies can also assist in patient stratification, ensuring that the most appropriate nanoplatform-based therapies are selected for individual patients. The integration of AI and ML with nanomedicine has the potential to enhance precision medicine approaches and improve clinical outcomes.
Nanoplatforms represent a significant advancement in BCa diagnosis and treatments providing innovative solutions that surpass conventional administration. By enhancing drug delivery, targeting capabilities, and therapeutic efficacy, nanoplatforms have the potential to transform BCa management and improve patient outcomes.
Author Contributions
Mohan Liu: writing – original draft (lead), conceptualization (equal), data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), writing – original draft (equal). Yusi Wang: writing – original draft (equal), conceptualization (equal), data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), writing – original draft (equal). Yan Li: conceptualization (lead), project administration (equal), resources (equal), supervision (equal), validation (lead), writing – review and editing (equal). Yibing Zhang: data curation (equal), formal analysis (equal). Bailing Zhou: data curation (equal), formal analysis (equal). Lei Yang: conceptualization (lead), project administration (lead), resources (lead), supervision (lead), validation (lead), writing – review and editing (lead). Xi Yan: conceptualization (lead), project administration (equal), resources (lead), supervision (equal), validation (equal), writing – review and editing (equal). Li Yang: conceptualization (lead), project administration (lead), resources (lead), supervision (lead), validation (lead), writing – review and editing (lead). All the authors have read and approved the final manuscript.
Acknowledgments
All images, including the graphical abstract image and the figures, were created with BioRender (https://www.biorender.com/). This study was supported by the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (No. ZYGD23008); the Frontiers Medical Center, Tianfu Jincheng Laboratory Foundation (No. TFJC202310005); the National Natural Science Foundation of China (No. 82203016); and the Scientific Research and Innovation Team Program of Sichuan University of Science and Technology (No. SUSE652B003).
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All data generated during the study appear in the submitted article.




