A hydrogel miscible azelaic acid-ionic liquids for the treatment of acne vulgaris: Enhanced solubility and skin retention
Abstract
Azelaic acid (AzA) is a natural dicarboxylic acid used to treat acne vulgaris but is greatly limited by poor aqueous solubility. This study aims to enhance the solubility and skin retention of AzA by ionic liquids (ILs). AzA-ILs were synthesized by a decomposition reaction with amine compounds. AzA-ILs synthesized with Tris-(hydroxymethyl)-aminomethane ([AzA][Tris]) and meglumine ([AzA][Meg]) at a molar ratio of 1:2 were liquid at room temperature and miscible with water. 1H-NMR and FT-IR confirmed the synthesis of AzA-ILs. [AzA][Tris] got higher transdermal transport and skin retention of AzA than [AzA][Meg]. ZEN has a lower viscosity and better spreadability than Carbomer and thus was adopted as the gel matrix. [AzA][Tris] was also miscible with the ZEN matrix at any concentration. Hydrogels containing 10% (w/w) AzA exhibited the highest transdermal transport and skin retention among hydrogels with higher or lower concentrations of AzA. AzA-IL hydrogel (10%, w/w) obtained similar therapeutic efficacy but lower skin irritation than the Finacea® (a marketed hydrogel of 15% AzA). In conclusion, ILs greatly enhanced the aqueous solubility of AzA to develop transparent hydrogel and skin retention to achieve good treatment for acne vulgaris.
1 INTRODUCTION
Acne vulgaris is a disease of the sebaceous glands that occurs at any age. The clinical manifestations include acne, pimples, pustules, and nodules. Four main pathogenic mechanisms are involved in acne vulgaris: increased sebum production, excessive keratinization, Propionibacterium acne infection in the hair follicles, and inflammation.1 Due to the long duration of the disease and the constant exposure to the lesion site, acne vulgaris seriously deteriorates the life quality of patients.2, 3 Since the largest group of acne patients is teenagers, providing them with good compliance may be challenging.4 Topical preparations may have greater advantages over oral, injectable, and other preparations.
Azelaic acid (AzA) is a nine-alkyl straight-chain dicarboxylic acid with the appearance of colorless to light yellow crystal or powder. Due to its anti-keratosis, antibacterial, anti-inflammatory, and inhibitory effects on melanin generation, AzA can act on various stages of acne development.5-7 Besides, AzA is safer than other antiacne drugs and can be used in pregnant women.8 AzA has become one of the most promising active pharmaceutical ingredients in treating acne vulgaris. Commercially available AzA preparations include 20% AzA cream (Skinoren®) and 15% AzA gel (Finacea®). However, AzA is poorly soluble in water (~0.24/100 g at 25°C). Although many surfactants are added to the formulation, most AzA is suspended in the matrix, greatly limiting its efficacy. Besides, the surfactants lead to potential irritation.
Various nanoparticles have been developed to improve the solubility and permeability of AzA, such as ethosome,9 microemulsion,10, 11 liposomal,12 nanocrystals,13, 14 and polymeric nanoparticles.15 Due to the limited loading capacity, few nanoparticles meet the dose requirements of AzA. The large quantity of carrier materials induces safety concerns.16, 17 Nanocrystals are advantageous in high drug loading and low carrier-associated toxicity,18, 19 and several new stabilizers have been used to stabilize the crystals.20, 21 However, nanocrystals may dissolve in topical preparations' matrix during storage and increase particle size due to Ostwald ripening. Solubilization with fatty alcohols, polyols, surfactants, and cyclodextrin inclusions is another strategy used to improve the solubility of AzA.22-24 Similarly, even a large amount of the solubilizer cannot dissolve AzA to the dose of 15%–20% in the preparation but causes significant irritation.
Ionic liquids (ILs) are salts composed of anions and cations with melting points lower than 100°C.25, 26 Some ILs are liquid at room temperature and show great solubilizing capability comparable to organic solvents. The nonvolatile, nonflammable, and low-toxic properties endow ILs with “green solvent.”27 In recent years, ILs have shown broad application prospects in enhancing drug solubility and permeability.28, 29 The ionic drug can also be transformed into ILs to improve their physicochemical and biological properties.30 For example, ILs composed of etodic acid and lidocaine enhance etodic acid's solubility and lidocaine's skin permeability by 9.3 times.31 Given this, transferring AzA into an IL may significantly improve its solubility and permeability.
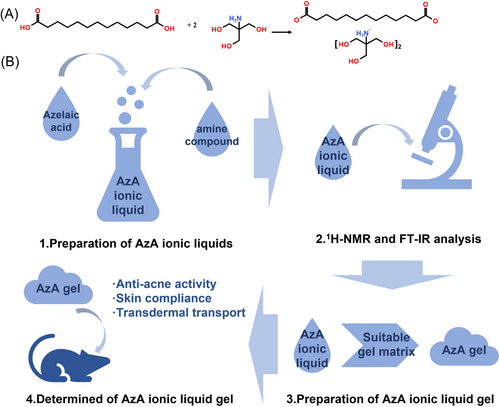
Figure 1A shows the chemical reaction equation between AzA and Tris-(hydroxymethyl)-aminomethane (Tris). The synthesis of ILs was confirmed by 1H-NMR and FT-IR, while solubility and permeability were adopted as indicators to screen amine compounds; then the gel matrix and concentration of AzA were determined by hydrodynamics and the in vitro transdermal study; finally, the antiacne activity and skin compliance of the gel were evaluated (Figure 1B).
2 RESULTS
2.1 Synthesis of AzA ILs
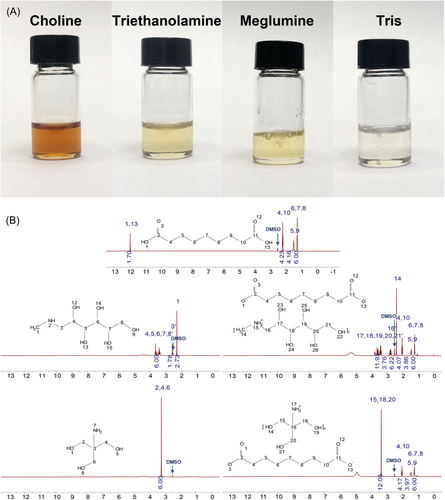
To screen the appropriate AzA-IL, we have selected over 20 amine compounds to synthesize AzA-ILs. AzA can only form liquid salts with meglumine, choline hydroxide, triethanolamine, and Tris at room temperature. However, the aqueous solubility of the ILs with a molar ratio of 1:1 was all lower than 5%. When the molar ratio of AzA to amine compounds was increased to 1:2, all ILs can be miscible with water. Figure 2A shows the appearance of the ILs (AzA/amine compounds=1:2). However, the ILs of AzA and triethanolamine (1:2) transformed into a semisolid state during storage. Choline salts and their esters are prohibited in skincare products. Therefore, the AzA-ILs composed of Tris ([AzA][Tris]) and meglumine ([AzA][Meg]) at a molar ratio of 1:2 were subjected to follow-up studies.
2.2 1H-NMR analysis
The synthesis of AzA-ILs was determined by 1H-NMR analysis and FT-IR analysis. The 1H-NMR spectrogram of AzA, the amine compounds, and the corresponding ILs are shown in Figure 2B. Compared with AzA, the main change in the 1H-NMR spectra of AzA-ILs lies in the disappearance of the characteristic peak of the carboxyl group (No.1 and No.13), indicating the formation of the ionic bond.32
2.3 FT-IR analysis
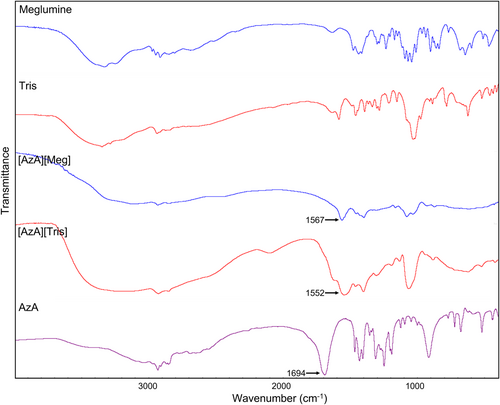
The FT-IR spectrogram of AzA, the amine compounds, and the corresponding ILs are shown in Figure 3. The stretching vibration peak of carboxyl group C=O at ~1690 cm−1 for AzA shifted to ~1560 cm−1 after the formation of ionic liquid. These FT-IR spectra showed that the carboxylic acid was converted to a carboxylic anion, confirming the successful synthesis of ILs.
2.4 In vitro transdermal transport
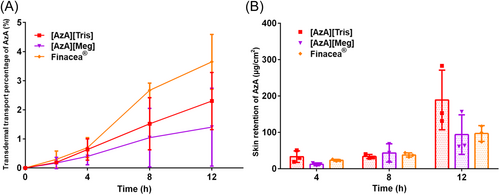
To identify the suitable amine compound for AzA-ILs, the transdermal transport and skin retention capacities of each AzA-ILs were measured. The transdermal transport and skin retention of AzA via administration of the ILs and Finacea® are shown in Figure 4. At the first 4 h following administration, the three preparations didn't show significant differences in the transdermal transport; however, Finacea® showed superior transdermal transport ability than the two ILs in the later stage (Figure 4A). Since acne vulgaris is a local skin disease, we further compared the skin retention ability of AzA from the three preparations (Figure 4B). At the first 8 h postadministration, the three preparations didn't show significant differences in the skin retention of AzA. However, [AzA][Tris] achieved higher skin retention of AzA than Finacea® at 12 h. The higher skin retention may be due to the ionized state of AzA in [AzA][Tris], because the ionic substances cannot effectively permeate the skin in comparison with the free one.33
2.5 Screening of gel matrix
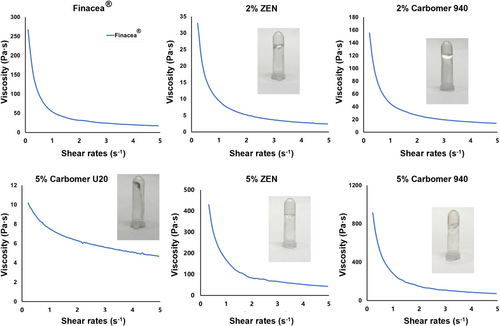
To screen suitable gel matrix, the rheological properties of each gel was measured. The appearance of hydrogels prepared with different matrices and the rheological curves are shown in Figure 5. [AzA][Tris] was miscible with all hydrogels without precipitation. Based on the rheological curves, all hydrogels showed a thin shear of non-newtonian fluid. As far as Carbomer U20 is concerned, the hydrogel can only be formed at a concentration higher than 5% (w/w). Nevertheless, the hydrogel is still too fluid. The hydrogel prepared with Carbopol 940 had a higher viscosity than the Carbomer U20. The viscosity of the hydrogel increased with the concentration of Carbopol 940. At a concentration of 2% (w/w), the viscosity of the Carbopol 940 hydrogel was comparable with that of Finacea®.
2.6 Screening of AzA concentration in hydrogel
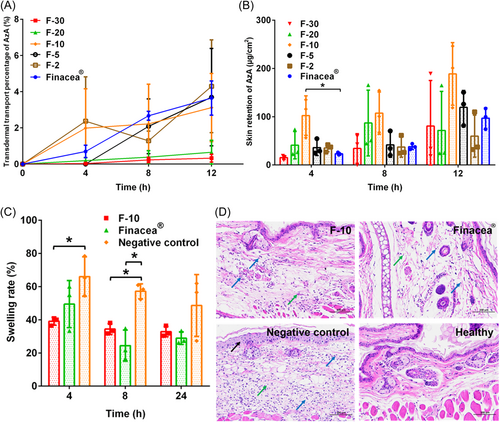
The concentration of AzA in the hydrogel may affect their transdermal transport capacity, the proper concentration of AzA was determined by the transdermal study. [AzA][Tris] was miscible with the hydrogel at all AzA concentrations. The transdermal transport capacity and skin retention of each hydrogel are shown in Figure 6A and B. F-10 is a watershed among the preparations, which achieved the highest transdermal transport and skin retention of AzA. It is well acknowledged that a high concentration gradient facilitates transdermal transport. Thus, although the transdermal transport percentage of F-2 and F-5 was similar to that of F-10 and Finacea®, the actual transdermal transport was lower. The low transdermal transport and skin retention from F-2 and F-5 are due to the low concentration of AzA in the preparation. Ionic liquid per se is viscous. The higher concentration of [AzA][Tris] inevitably increases the viscosity of the preparation and hinders the transfer of AzA from the hydrogel to the skin.
2.7 Antiacne activity
The antiacne ability of AzA hydrogel was studied in an acne mice model induced by croton oil. Figure 6C shows the variation in ear swelling percentage of mice treated with F-10, Finacea®, and normal saline (negative control). At 4 h postadministration, F-10 got a significantly lower swelling percentage than Finacea® and the negative control, indicating faster onset and better effectiveness. Although the swelling percentage for all groups continued to decrease at 24 h, the rats treated with F-10 and Finacea® had lower swelling percentages than the negative group.
Figure 6D shows the HE-stained pathological sections of the ears. The pathological analysis showed that the pathological indicators such as the degree of epidermal edema in the F-10 and Finacea® groups were reduced compared with the negative control group. In conclusion, although there was no significant difference in the swelling percentage at 24 h, the pathological analysis showed that the negative control group still had significant epidermal swelling and inflammatory cell infiltration, both F-10 and Finacea® could effectively relieve the swelling and inflammation.
2.8 Skin compliance study
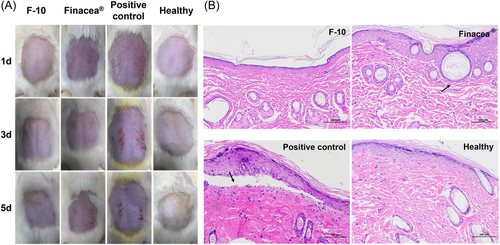
The skin compliance of each gel was evaluated by back skin model of healthy rats. Figure 7A shows the back skin of rats treated with F-10, Finacea®, 4% paraformaldehyde (positive control), and normal saline (healthy) for 5 days, respectively. The positive treatment and Finacea® led to inflammation of the rat's skin. Severe inflammatory reactions appeared on the skin of rats treated with 4% paraformaldehyde on the first day. Inflammatory reaction was mild in the rats treated with Finacea® on the first day, which became more severe over time. Conversely, F-10 did not cause any inflammatory reactions. The skin of the rat treated with F-10 remained normal on the fifth day, which was similar to the skin of the healthy rat. Analysis of the pathological section confirmed the results (Figure 7B). The skin treated with 4% paraformaldehyde showed serious pathological lesions such as the separation of the epidermis from the dermis (black arrow). Only slight pathological changes were induced by Finacea® such as slight dilation of hair follicles and flattening of the epithelium (black arrow). Conversely, F-10 didn't induce impairment to the skin, which was similar to healthy skin.
3 DISCUSSION
In the in vitro transdermal transport study, Finacea® showed similar transdermal transport ability in first 4 h but superior transdermal transport ability than the two ILs in the later stage, which may due to the difference relates to the state of AzA in the preparation. AzA presents a free form in Finacea®, while an ionized state in ILs. It is well known that undissociated molecules have higher transdermal permeability than ionized ones.34 Polysorbate 80 is another reason, which is a good permeation enhancer in transdermal drug delivery by fluidization and extraction of stratum corneum lipids.35 However, due to the poor solubility, most of AzA is suspended in Finacea®, leading to slow transdermal transport in the initial stage. With the dissolution of the suspended AzA during the transdermal process, Finacea® achieved higher transdermal transport than the two ILs after 4 h. Ionic liquids per se are permeation enhancers, being capable of disruption of cellular integrity, fluidization, creation of diffusional pathways, and extraction of lipid components in the stratum corneum.36, 37 Therefore, the ionized AzA also permeated across the skin, though surfactants were absent in the preparation. [AzA][Meg] didn't enhance the skin retention of AzA than Finacea®. The difference between the two ionic liquids lies in the cationic part. The interaction between the ions may affect the transdermal transport. The exact mechanisms are yet to be explored. Considering the higher skin retention and the lower permeation than Finacea®, [AzA][Tris] was selected for further study.
Compared with solution, hydrogel have advantages in drug delivery on skin,38 so it is necessary to select a suitable gel matrix. The viscosity of the Carbopol 940 hydrogel was comparable with that of Finacea®, but Carbopol 940 hydrogel has an uneven texture and is difficult to apply evenly in use. The hydrogel prepared with ZEN as the matrix has a comfortable feeling and is less viscous than Finacea®, making it easier to apply. The viscosity of the ZEN hydrogel also increased with the matrix concentration. However, the ZEN hydrogel at a lower concentration was more plastic than the one at a higher concentration, making it more suitable for various containers, and more comfortable to handle. Therefore, ZEN was selected as the gel matrix, and the concentration was set to 2%. In addition, conventional hydrogel substrates were poorly resistant to electrolytes. The hydrogel viscosity is irreversibly impaired at a high electrolyte content. This explains that Carbomer U20 could hardly form a sufficiently viscous hydrogel with the ILs. ZEN was developed to get electrolyte resistance and thus could maintain sufficient viscosity in high electrolyte concentrations.39
The screening of AzA concentration in hydrogel showed F-10 may get a balance between the transdermal concentration gradient and the hydrogel viscosity, producing optimal transdermal transport and skin retention. In addition, although F-10 got similar transdermal effects to [AzA][Tris] at 12 h postadministration, the hydrogel preparation got higher transdermal transport and skin retention at the first 4 and 8 h. Diluting ionic liquid with water to an appropriate concentration enhanced the skin penetration ability of AzA. The result is similar to our previous study that a lower concentration of tretinoin-ionic liquid in water had stronger skin penetration ability.40 However, the specific reasons for this phenomenon need further exploration. Based on the results, F-10 was adopted for further investigation.
The antiacne study showed the antiacne activity of F-10 and Finacea®. Due to the acute inflammatory effect, croton oil can quickly induce the acne model but cannot maintain a long-term effect. The swelling rate of the negative control group subsided with no treatment. Therefore, although F-10 and Finacea® still showed lower swelling percentages than the negative group at 24 h postadministration, no significant differences were observed among the groups. The pathological analysis showed that F-10 and Finacea® have similar treatment effects. In addition, skin compliance study showed that the ILs have better safety than Finacea®, being due to the absence of irritating solubilizing excipients. Therefore, F-10 showed great application prospects for the treatment of acne vulgaris in this study.
However, there are also some shortcomings in this study, such as the lack of research on ionic liquids with other molar ratio, and the lack of further screening and optimization of gel matrix and gel preparation process, which need to be supplemented in the future studies.
4 CONCLUSION
AzA-ILs can be synthesized by the metathesis reaction. Combination with Tris or meglumine led to the liquid AzA-ILs at room temperature that were miscible with water. [AzA][Tris] showed higher transdermal transport and skin retention than [AzA][Meg]. ZEN was compatible with [AzA][Tris] due to its resistance to ions and provided good spreadability. ZEN hydrogels with 10% (w/w) AzA exhibited the maximum transdermal transport and skin retention than those with other concentrations. AzA-IL hydrogel (10%, w/w) obtained similar antiacne efficiency to Finacea® which contains 15% AzA. However, the hydrogel showed no skin irritation. The IL-based AzA hydrogel has the potential to treat acne.
5 MATERIALS AND METHODS
5.1 Materials
AzA was purchased from SIGMA-ALDRICH Trading Co., Lt. Choline hydroxide was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. Tris, triethanolamine, meglumine, methyl tert-butyl ether, and croton oil were purchased from Sinopharm Chemical Reagent Co., Ltd. Carbomer U20 and 940 were purchased from Shanghai Macklin Biochemical Co., Ltd. SEPIMAX ZEN™ (ZEN) was purchased from Seppic Chemical Specialities Co., Ltd. Finacea® was purchased from LEO Pharma Trading Co., Ltd. Ultrapure water prepared by Millipore was used throughout the study.
BALB/c mice and Sprague-Dawley (SD) rats used in this study were purchased from SLAC Lab Animal Co., Ltd.
6 METHODS
6.1 Preparation of AzA ionic liquids
The amine compounds included meglumine, triethanolamine, choline, and Tris. AzA-ILs were prepared by a metathesis reaction. 0.01 mol AzA and 0.01/0.02 mol amine compound were dissolved in a 20 mL mixed solution of ethanol and water. The solution was stirred at 500 rpm for 24 h. Then ethanol in the mixture was removed by rotary evaporation at 60°C for 2 h. Following freezing at −80°C for 24 h, the residual water was removed by freeze-drying for 48 h to obtain AzA-ILs.
6.2 1H-NMR analysis
AzA, the ILs, and the amine compounds were dissolved in d6-DMSO at a concentration of 20 mg/mL. Spectra were recorded on a 400 MHz Varian spectrometer operating at 25°C. The data were subsequently processed using MestReNova software.
6.3 FT-IR analysis
AzA, the ILs, and the amine compounds were also analyzed by Nicolet Avatar 360 FT-IR. For solid samples, 2 mg sample was mixed with 200 mg of KBr to prepare a tablet under 25 MPa pressure for 20 s for the analysis. For liquid samples, dripping 2 mg sample onto KBr blank tablet for analysis. The wavelength for the FT-IR scan ranged from 400 to 4000 cm−1, and the obtained spectra were analyzed by Omnic software.
6.4 In vitro transdermal transport studies
The abdominal skin hair of the SD rats was removed 1 day before the experiment. Following the sacrifice of the rats, the abdominal skin was taken from the body. The subcutaneous fats and tissues were removed. The skin with a diffusion area of 1.77 cm2 was then fixed between the donor and the receptor chambers of a vertical Franz diffusion cell. 7.7 mL phosphate buffer was adopted as the receptor fluid. The temperature of the receptor chamber was maintained at 32 ± 1°C. The samples, 0.3 mL, were applied to the donor chamber.41 At predetermined time intervals, 200 μL receptor fluid was withdrawn and an equal volume of fresh media was immediately added. The fluid was mixed with 1050 μL methanol via vortex. The mixed solution was then centrifuged at 12,000 rpm for 5 min. The supernatant was analyzed by HPLC for the cumulative permeating amount of AzA.
The skin retention of AzA was investigated using the same procedure. However, the skin was collected at each time interval. The residual preparations were removed by normal saline. Then, the skin was cut into pieces. The AzA permeated into the skin was extracted with 1 mL methanol under vortex for 10 min and 5 mL tert-butyl methyl ether under ultrasonication for 30 min sequentially. The methanol and tert-butyl methyl ether were collected and mixed. The organic solution was removed by nitrogen flow. The residue was dissolved in an appropriate amount of mobile phase under vortex for 5 min. Following filtration through a 0.22 μm membrane, the solution was analyzed by HPLC for the retention of AzA in the skin.
6.5 Determination of AzA
The analysis of AzA was conducted by an Agilent 1100 HPLC system (Agilent) at 210 nm. A ZORBAX SB-C18 column (150 × 4.6 mm, 5 µm) was used for the separation which was maintained at 40°C. The mobile phase consisted of 84% of phosphate buffer (containing 1 g/L H3PO4 and 6.8 g/L NaH2PO4) and 16% of acetonitrile. The flow rate was maintained at 1 mL/min. Fifty microliters sample was injected for analysis.
6.6 Screening of gel matrix
The investigated gel matrix included Carbomer U20, Carbomer 940, and ZEN. The AzA-ILs and glycerol (humectant) were dissolved in distilled water first. Then the gel matrix was added to the solution, stirring overnight for full swelling. The final concentration (w/w) of AzA and glycerol in the hydrogel was 15% and 10%, respectively. The concentration (w/w) of the gel matrix was set to 2% and 5% for comparison.
The rheology of the hydrogels was tested for screening of the matrix. The rheological curves were determined by a rotational rheometer. A rotating circular plate with a diameter of 20 mm was adopted, while the gap was set to 1000 µm.
6.7 Screening of AzA concentration in hydrogel
A series of hydrogels with different AzA content were prepared according to the composition listed in Table 1. The preparation method was the same as that used in the screening of the gel matrix. The in vitro transdermal transport of each hydrogel was compared to screen the AzA concentration.
| Formulation code | AzA | Tris | Glycerol | ZEN | H2O |
|---|---|---|---|---|---|
| F-30 | 9.0 | 11.6 | 1.5 | 0.6 | 7.3 |
| F-20 | 6.0 | 7.7 | 3.0 | 0.6 | 12.7 |
| F-10 | 3.0 | 3.9 | 3.0 | 0.6 | 19.5 |
| F-5 | 1.5 | 1.9 | 3.0 | 0.6 | 23.0 |
| F-2 | 0.6 | 0.8 | 3.0 | 0.6 | 25.0 |
6.8 Antiacne activity study: Croton oil-induced rosacea disease model
The mice were euthanized at 24 h, while the ears were excised for histological examination.
6.9 Skin compliance study
The hair on the back of SD rats was shaved off 1 day before the experiment. The rats were divided into four groups: one positive control group treated with 4% paraformaldehyde,42 one healthy group treated with normal saline, and two test groups treated with F-10 and Finacea®, respectively. One milliliter of each preparation was applied to the back of the rats every day for 5 days. The back skin of the rats was recorded every other day. On the fifth day, the rats were euthanized, while the back skin was withdrawn for pathological analysis.
AUTHOR CONTRIBUTIONS
Zhezheng Fang and Xianzi Zheng experimented. Zhezheng Fang and Yi Lu wrote the manuscript. Yanyun Ma and Wei Wu read and revised this paper. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
This research was funded by the Science and Technology Committee of Shanghai Municipality, grant numbers 23S11901500 and 23ZR1413100.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The animal studies were performed in the central animal house of the School of Pharmacy, Fudan University, following the institutional and governmental regulations concerning the ethical use of animals (protocol number 2024-03-YJ-LY-42).
Open Research
DATA AVAILABILITY STATEMENT
Data of this study is available from the corresponding author on reasonable request.




