Multiple biomaterials for immediate implant placement tissue repair: Current status and future perspectives
Xiaoqi Su and Shasha Jia contributed equally to this work.
Abstract
Immediate oral implant placement is a widely accepted technique, known for its efficacy in reducing treatment duration, surgical visits, and overall healing time. One of the primary challenges associated with immediate implant placement is the attainment of initial stability. The inevitable loss of bone and soft tissue after extraction poses a risk to implant osseointegration in both vertical and horizontal dimensions. Guided tissue regeneration/guided bone regeneration (GTR/GBR) is a well-established method for periodontal regeneration. However, current GTR/GBR membranes lack tissue inherent regeneration properties and necessitate combination with grafts to enhance tissue recovery. In this context, biomaterials have emerged as a promising option due to their good biocompatibility, biodegradability, and bioactive properties. They present a potential alternative to standard autologous/allograft procedures. The field of biomaterials for bone regeneration has rapidly evolved, developing new guiding materials and engineering techniques. These advances have become integral in addressing tissue defects at the immediate implant site. Various materials such as bioceramics, natural polymers, and synthetic polymers have been used for tissue repair. This article undertakes an etiological examination of tissue deficiency associated with immediate implant placement. Additionally, it reviews the advantages and disadvantages of a variety of biomaterials, aiming to provide references for clinical treatment and areas for further investigation.
1 INTRODUCTION
Immediate implant placement involves the insertion of an artificial implant directed into a recently extracted tooth. This technique is widely accepted for its advantages in reducing the number of procedures and duration of healing time.1-3 It plays a crucial role in maintaining bone volume and soft tissue morphology in the extraction site, addressing common issues arising from tooth extraction.4, 5 For example, Aimetti et al.6 used three-dimensional cone beam computed tomography (CBCT) to investigate changes in bone tissue immediately postextraction and 1 year later in patients with chronic periodontitis. This study demonstrates that significant defects in the buccal bone wall of the alveolar fossa undergo dramatic three-dimensional volume changes in the natural healing state. The shape and size of the alveolar ridge can be improved by early use of slow-resorbing implants, reducing the need for bone augmentation during dental implant placement.
The loss of bone and soft tissue after extraction is inevitable and poses challenges to implant stability in both vertical and horizontal dimensions.7, 8 Trauma-induced tooth fractures9 and common oral diseases, like chronic apical periodontitis and periodontitis, contribute to this issue. Patients often delay seeking treatment due to a lack of obvious clinical symptoms. Guided tissue regeneration/guided bone regeneration (GTR/GBR) has been used for periodontal regeneration for many years.10 GBR promotes bone regeneration through the use of a barrier membrane, which serves to inhibit epithelial growth to the root surface, allowing space for the formation of alveolar bone and promoting the activity of osteoblasts.11 The main materials used clinically to promote peri-implant bone regeneration are autogenous, allograft, and xenograft bone. While autogenous bone is regarded as the benchmark, its disadvantages, such as requiring a second operative site, increasing risk of infection, and limitations based on the natural size and structure, make allograft and xenograft bone convenient alternatives.12, 13
Biomaterials, such as ceramics, natural polymers, and synthetic polymers, have gained attention for their biocompatibility, biodegradability, and bioactivity.14-16 These materials have been incorporated into GBR membranes to improve efficacy and success rate.17 They also enhance calcium phosphate (CaP) precipitation, stimulate osteogenic differentiation, and provide antimicrobial properties.14, 18 Bioceramics have the advantage of increased osteoconductivity and osteoinduction,19 but are relatively brittle materials with low flexural strength19, 20 necessitating additional scaffolds.21 Natural polymers, including chitosan, collagen, gelatin, and silk fibroin, exhibit excellent biocompatibility, are biodegradable, and can be used individually or in combination for tissue regeneration.22, 23 Their main disadvantage is lower physical and mechanical properties, which has led to the development of synthetic polymers.24 The increased width and thickness of the keratinized mucosa facilitates peri-implant health. Clinical methods such as connective tissue grafts(CTG), free gingival grafts (FGG), acellular dermal matrix (ADM), and growth factor concentrate (CGF) have proven effective in increasing the width of keratinized mucosa.25
This article analyzes the tissue deficiency faced by immediate implant placement from an etiological point of view. It also reviews the main characteristics of several biomaterials and their advantages and disadvantages. The aim is to provide a practical clinical reference for utilizing biomaterials in immediate implant tissue restoration.
2 ETIOLOGY OF TISSUE DEFECTS IN IMMEDIATE IMPLANT PLACEMENT
Tooth loss is a common disease in dentistry and is usually associated with accidents, dental caries, periodontal disease, and congenital absence of permanent embryos.26, 27 Microorganisms and their metabolites play a key role in the onset, development, and establishment of periapical and periodontal lesions.28 When microorganisms invade the pulp and periodontal tissues, they can either directly poison the tissue cells or indirectly cause tissue damage by triggering nonspecific inflammatory responses and specific immune responses.29
2.1 Chronic apical periodontitis
Chronic apical periodontitis (CAP) is a chronic inflammation of the periapical tissues caused by the prolonged presence of infection in the root canals, which is manifested by the formation of inflammation and destruction of the alveolar bone.30 Bone loss in apical periodontitis (AP) is inexorable and is caused by microbial factors and immune defense responses.31, 32
Microorganisms may directly contribute to the formation of AP or trigger an immune response in the host, causing tissue destruction (Figure 1).33 The most common bacteria found in periapical lesions are Fusobacteria (4.2%), Proteobacteria (9.1%), Bacteroidetes (12.1%), Actinobacteria (14.0%), and Firmicutes (62.9%).34 Anaerobic Gram-negative bacteria dominate the root canals of periapical lesions.35 Endotoxins such as lipopolysaccharide (LPS) from the cell walls of Gram-negative bacteria, may cause local tissue swelling and bone resorption. Meanwhile, high LPS levels are positively correlated with the degree of bone damage.36, 37 LPS is a TLR4 ligand that stimulates the receptor activator of nuclear factor κB ligand (RANKL) through TLR4 signaling.38 RANKL is a vital cytokine in bone resorption and consistently promotes periapical inflammation.39 Lipoteichoic acid (LTA) constitutes a significant element of the cell wall in numerous Gram-positive bacteria. LTA prompts the differentiation of osteoclasts and is crucial for promoting the viability of mature osteoclasts. Hence, these actions collaboratively trigger alveolar bone loss through inflammation. LTA also stimulates the production of prostaglandin E2 (PGE2).40 It has been demonstrated that arachidonic acid metabolites, including PGE2, mediate alveolar bone resorption by Porphyromonas gingivalis and Fusobacterium nucleatum.41, 42 Phosphoglycerol dihydroceramide produced by P. gingivalis promotes osteogenesis induced by RANKL via interaction with nonmuscle myosin II-A, an osteoclast cell fusion.43 Continuous stimulation with Enterococcus faecalis induces the pre-resolution polarization of macrophages into an M2 phenotype.44 E. faecalis induces secretion of interleukin (IL)-1β and RANKL, thereby exacerbating the severity of bone resorption.45 Osteoclast activity in CAP is affected by T cell regulation.46 IL-17 and tumor necrosis factor alpha (TNF-α) secreted by Th17 cells promote RANKL expression and osteoclast differentiation.47 Pathogen-associated antigen triggers a macrophage polarization switch toward M1.48 Macrophage polarization may be associated with alveolar bone loss.49 Mast cells promote bone damage in periapical periodontitis by secreting the pro-inflammatory factor TNF-α.50
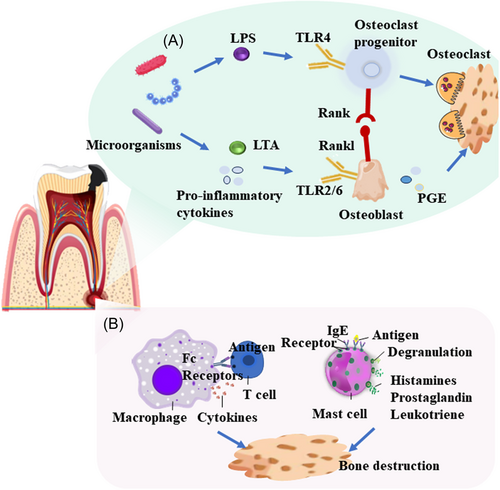
Most periapical infections are asymptomatic and untreated inflammation may develop gradually and persist. An uncontrolled infection usually leads to significant bone loss around the root tip, which eventually leads to tooth loss.
2.2 Chronic periodontitis
Periodontitis is a chronic inflammatory disease associated with plaque biofilm accumulation. It is characterized by progressive destruction of the tooth support apparatus, including the periodontal ligament and alveolar bone (Figure 2).51, 52 A specific group of gram-negative anaerobic bacteria known as the red complex causes chronic inflammation in subgingival dental biofilms.53, 54 These bacteria include P. gingivalis, Tannerella forsythia, and Treponema denticola, which are predominantly found in deep periodontal pockets of patients with periodontitis.55, 56 LPS and virulence factors produced by bacteria stimulate the secretion of pro-inflammatory cytokines, such as TNF-α, IL-1β, and PGE2, by host macrophages and dendritic cells as well as periodontal ligament cells.57-59 The presence of pro-inflammatory cytokines promotes the production of matrix metalloproteinases (MMPs) by macrophages, fibroblasts, and neutrophils. In periodontal tissue, these MMPs mediate the destruction of collagen fibers.60 Oral bacteria and osteoclast-derived proteases may degrade osteoprotegerin (OPG) and promote osteoclastogenesis in vitro.61, 62 In addition, pro-inflammatory cytokines induce osteoblasts and T helper cells to express RANKL, leading to osteoclastogenesis and maturation.59, 63 Mature osteoclasts mediate alveolar bone destruction. In advanced cases, periodontitis leads to tooth loss.
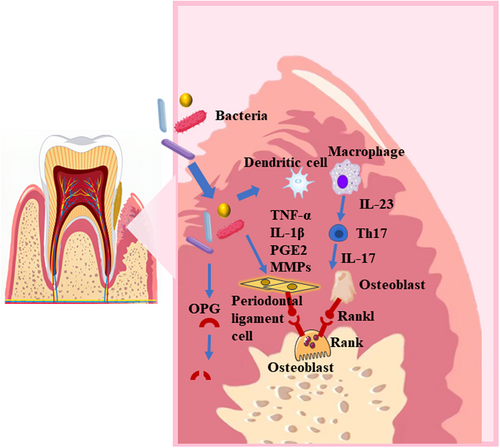
2.3 Others
Dental injuries caused by trauma occur frequently and can result in displaced tooth fractures, jaw fractures, and soft tissue injuries.64 For affected teeth that cannot be retained, immediate implant placement can be performed after tooth extraction to preserve bone volume. Oligodontia is a type of dental hypoplasia that is defined as the absence of six or more permanent teeth, excluding the third molar.65 Oral manifestations in patients with hypodontia may include malocclusion or microdontia, alveolar bone hypoplasia, and retained primary teeth.66, 67 Immediate implant placement is an ideal option for adult patients with hypodontia to achieve the desired esthetic results and chewing function.68, 69
3 BIOMATERIALS FOR TISSUE DEFECTS
Bone loss may cause early instability of the immediate implant placement. Insufficient soft tissue is also a challenge. On one hand, it depends on the shape of the extraction cavity after extraction, and on the other hand, inflammatory infiltration often leads to soft tissue swelling and deformation and carries more bacteria, which must be removed intraoperatively to avoid germs damaging healthy periodontal tissue. This can interfere with postoperative wound closure, thus not providing desirable healing space for the bone tissue. Additionally, to effectively guarantee the immediate implant placement effect and increase the success rate, it is essential to properly repair the bone and soft tissue deficiencies surrounding the implant during this procedure.
3.1 Bone tissue
Bone alternative biomaterials are key to guided bone regeneration (GBR), and the selection of the appropriate biomaterials is critical to the success of immediate implant placement in patients with bone defects.70 The bone alternative biomaterials currently used in the oral and maxillofacial region are autologous bone, allogeneic bone, xenogeneic bone, bioceramic materials, growth factor-based materials, natural polymers, and synthetic polymers (Figure 3) (Table 1).
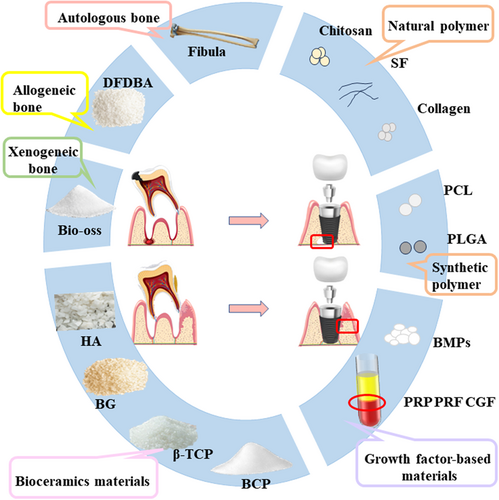
| Graft | Advantages | Disadvantages | Reference | |
|---|---|---|---|---|
| Autologous bone | Biocompatibility, osteoconductivity, osteoinductivity, and osteogenic characteristics, with a fast-healing rate | Limited bone extraction, additional surgical sites, and easy infection at the extraction site | [71-73] | |
| Allogeneic bone | Not limited in size or shape, preserve the host bone, BMPs | Potential risks of immune rejection and disease transmission | [74-79] | |
| Xenogeneic bone | Antigen-free, osteoconductive, ability to regenerate bone similarly to autologous bone | Antigenicity from different species, high disease transmission | [80-85] | |
| Bioceramics materials | HA | Bioactivity, biocompatibility | High degradability | [86] |
| n-HA | Tiny particle size and large surface area | Low mechanical properties | [87] | |
| BG | Osteoconductive, osteoinductive, biocompatible, and highly bioactive | Low mechanical properties | [88-90] | |
| β-TCP | Malleable, easy to handle, formability, self-degradation, induce bone regeneration | Low mechanical properties | [91, 92] | |
| Growth Factor-based Materials | PRP | High concentration of growth factors | Low preparation and lack of harmonization | [93] |
| PRF | Three-dimensional mesh structure, growth factors | [94] | ||
| CGF | Larger, higher concentration of fibrin clumps | [95] | ||
| Natural polymer | Chitosan | Excellent biocompatibility, biodegradability, and antimicrobial | Low mechanical properties | [96-100] |
| SF | Advantageous strength, biodegradability, elasticity, and low tissue reactivity | Lack the potential to induce osteogenesis | [101-103] | |
| Collagen | Induce bone regeneration, hydrophilicity, high processability, and excellent physical properties | High degradation, low mechanical properties | [104-107] | |
| Synthetic polymer | PCL | Biocompatibility, superior toughness, and mechanical strength | Slow degradation rate, hydrophobic nature of PCL inhibits cell adhesion | [108-111] |
| PLGA | Biocompatibility, biodegradability, and controlled degradation rate | Low mechanical properties | [112-116] | |
- Abbreviations: BG, bioactive glass; β-TCP, β-tricalcium phosphate; CGF, growth factor concentrate; HA, hydroxyapatite; PCL, polycaprolactone; PLGA, poly lactic-co-glycolic acid; PRF, platelet-rich fibrin; PRP, platelet-rich plasma; SF, silk fibroin.
3.1.1 Autologous bone
Autologous bone grafting is considered the gold standard for repairing bone defects.71 It has good biocompatibility, osteoconductivity, osteoinductivity, and osteogenic characteristics, with a fast healing rate. However, there are drawbacks, including limited bone extraction, additional surgical sites, and susceptibility to infection at the extraction site.72, 73
3.1.2 Allogeneic bone
Allografts have no size or shape limitations and can restore bone defects. In addition, they are a safeguard for the host's bone reserve, with no risk of damage to the donor site.74 Decalcified freeze-dried bone allografts (DFDBA) have both osteoconductive and osteoinductive properties. The amount of bone morphogenetic proteins (BMPs) remaining after commercial processing is related to the osteoinductive potential of DFDBA.75 BMPs play an important role in communication between osteoblasts and osteoclasts and are a family of multifunctional growth factors involved in numerous molecular and signaling pathways that have a major impact on bone remodeling. By stimulating the mineralization, differentiation, and survival of osteoblasts, BMPs help to maintain healthy bone.76 Despite these advantages, the potential risks of disease transmission and immune rejection limit the use of allograft bone grafts in clinical practice.77-79
3.1.3 Xenogeneic bone
Xenogeneic bone is derived from the bone matrix of animals or the bone-like matrix of calcified coral.80 Bio-Oss is a natural protein derived from the mineral fraction of bovine bone in the form of granules or porous bone masses with a macroscopic and microscopic conformation very similar to that of human bone.81 The main advantages of Bio-Oss are:(i)it can be dehisced by a proprietary extraction process, making it antigen-free82; (ii) it is osteoconductive and may contribute to the formation of periodontal bone defects83; and (iii) it has the ability to regenerate bone similarly to autologous bone.84 Undesirable outcomes of allogeneic bone grafts are due to antigenicity from different species and high disease transmission.85
3.1.4 Bioceramic materials
Bioceramics are biocompatible ceramic materials that have good biocompatibility, stiffness, and compressive strength. The most common bioceramics are hydroxyapatite (HA), bioactive glass (BG), and calcium phosphate (CP). HA is a ceramic material with a long history of clinical use and positive bioactivity and biocompatibility properties.86 The porosity and degradability problems associated with HA have limited its further development, so scholars nanosized HA and nano-hydroxyapatite (n-HA) emerged. N-HA has a tiny particle size and a large surface area, which is rapidly absorbed and replaced by vital bone in a matter of weeks.87
BG comprises synthetic allosteric reactive materials with silicate substrates, possessing the remarkable capacity to establish connections with mineralized hard tissues—for example, bone—in a physiological setting.88 PerioGlas is an injectable bioactive glass composed of silica, sodium oxide, calcium oxide, and phosphorus pentoxide, which is malleable, easy to handle, and can better adapt to the morphology of bone defects, bind closely to bone tissue, and induce bone regeneration through self-degradation.89 However, the mechanical properties of bioactive glasses make them brittle and weak.90
Calcium phosphate cement (CPC) has calcium phosphate salt as the main ingredient is cured and molded at low temperatures, and has no cytotoxicity, good biocompatibility, and high osteoconductivity.117 The FDA has approved CPC as a replacement material for the clinical repair of bone defects.118 β-TCP has proven to be one of the most attractive bone graft substitutes available today, and its osteoconductive properties, its osteoinductive properties, and its cell-mediated resorption properties. The main clinical use is as a scaffolding material to guide bone regeneration.91 A study doped Lithium (Li) into β-TCP and demonstrated its important role in regulating the proliferation and differentiation of osteoblasts by detecting alkaline phosphatase (ALP) activity and collagen Type-I (Col I⍺).92 Li et al.119-121 is a micronutrient that facilitates bone defect repair by upregulating the expression of osteogenic markers in adipose-derived stem cells (hASCs). Bone cement is capable of regulating the setting time through the manipulation of the concentration of Li that is incorporated.92 Biphasic calcium phosphate (BCP) consists of HA and TCP, the combination of which is more osteoinductive. It combines the advantages of HA and β-TCP. BCP is obtained when synthetic or bio-deficient calcium apatite is sintered at temperatures of 700°C and above.122
3.1.5 Growth factor-based materials
Platelet-rich plasma (PRP), platelet-rich fibrin (PRF), and growth factor concentrate (CGF) are the three-phase products of plasma extraction.123 PRP is a first-generation platelet concentrate, consisting of plasma with elevated platelet concentrations attained by centrifuging whole blood in a specified manner. It has a positive effect on both bone regeneration and wound healing. It contains high levels of growth factors that support the regeneration and repair of bone and soft tissue.93 PRF is a second-generation platelet concentrate based on PRP and was first proposed by Choukroun et al.94 The manufacturing protocol was simplified and animal-derived thrombin was replaced with calcium to coagulate and produce fibrin. The three-dimensional structure of the mesh allows growth factors to be released gradually, extending the duration of action and ultimately improving soft and hard tissue repair. CGF is a third-generation platelet concentrate with a heightened level of fibrin clumps, prepared through blood samples and specialized centrifugation equipment.95 PRP, PRF, and CGF all contain high levels of growth factors and are often used in combination with bone replacement materials for immediate oral implant sites, thereby promoting bone and soft tissue regeneration.124
3.1.6 Natural polymer
Chitosan is a natural biopolymer derived from the deacetylation of chitin with the molecular formula (C6H11O4N)n. The available amino group in chitosan allows it to chemically bind to DNA, RNA, lipids, proteins, and metal ions. Chitosan is the sole natural cationic polysaccharide that can undergo chemical modifications to create derivatives suited to specific functional and application purposes.125-127 Chitosan and its derivatives have desirable biocompatibility, biodegradability, and nontoxicity, as well as a broad spectrum of antimicrobial activity against both Gram-positive and Gram-negative bacteria.96, 97 The degree of deacetylation during chitosan preparation affects cell bioactivity, proliferation, and attachment.97 Chitosan-based scaffolds have the potential to facilitate bone regeneration. Nevertheless, the bioactivity and mechanical properties of chitosan as a scaffold are quite limited. Blending chitosan with polymers and/or biomaterials has the potential to significantly enhance its mechanical properties, as well as improve its biological activity.98-100
SF is a protein material of natural origin that has been utilized in cartilage and bone repair procedures in a clinical setting. SF has a complex molecular structure consisting of disulfide-linked light chains (molecular weight 26 kDa) and heavy chains (molecular weight 390 kDa). The chemical composition and structure of SF provides excellent biocompatibility and biodegradability.101 SF possesses favorable mechanical properties and can adequately support the adhesion and growth of cells.102 Its β-sheet structure makes it easy to process. It can be transformed into various structures such as hydrogels, fibers, membranes, and microspheres.103
Collagen constitutes a protein that comprises the extracellular matrix (ECM) of the majority of our tissues. Of the several types of collagens in the body, types I–IV hold the highest prevalence.128 Collagen induces bone regeneration and remodeling by stimulating the metabolic activity of osteoblasts.104 The hydrophilic nature of collagen, its ease of processing, and its physical properties allow collagen to be used in a wide range of products, such as injectable gels, films, meshes, and fibers. However, the degradation rate is high, and the mechanical properties are low, which can be mitigated by adding other biomaterials.105-107
3.1.7 Synthetic polymer
Compared with natural polymers, synthetic polymers lack biocompatibility, hydrophilicity, and cell adhesion, which may lead to aseptic inflammatory reactions. However, their mechanical and processing properties provide notable benefits. Polylactic acid (PLA), polycaprolactone (PCL), and poly lactic-co-glycolic acid (PLGA) are representative synthetic polymers that have been prepared to obtain a variety of scaffold materials.129, 130
PCL is an aliphatic and semi-crystalline polymer that displays remarkable toughness and mechanical strength, and it exhibits sufficient biocompatibility. However, its degradation products may contribute to the spread of degradation rates and inflammatory responses.108 A hydrolysis reaction drives the biodegradation of PCL-based scaffolds. It does not require the incorporation of enzymes or catalysts.109 Therefore, the addition of osteogenic and inorganic compounds, such as titanium dioxide, HA, or BG, is necessary to improve the mechanical and biological properties of PCL-based scaffolds.110, 111 The properties of PLGA include Biocompatibility, biodegradability, and controlled degradation rate.112 PLGA lacks sufficient mechanical strength for bone tissue regeneration.113 Therefore, many ceramic nanoparticles were doped into PLGA structures to fabricate PLGA nanocomposite biomaterials.114-116
3.2 Soft tissue
The level of the peri-implant soft tissues and, in particular, the thickness of mucosa are essential for the esthetic outcome of the implant restoration.131, 132 Avila-Ortiz et al.133 defined the peri-implant phenotype as the “morphologic and dimensional features characterizing the clinical presentation of the tissues that surround and support osseointegrated implants.” The peri-implant phenotype is constituted by the peri-implant keratinized mucosa width (KMW), the mucosa thickness (MT), and the suprarenal tissue height (STH). Clinically, autologous tissue grafts, allografts, xenografts, and growth factor-based biomaterials are used to enhance soft tissue thickness (Figure 4) (Table 2).
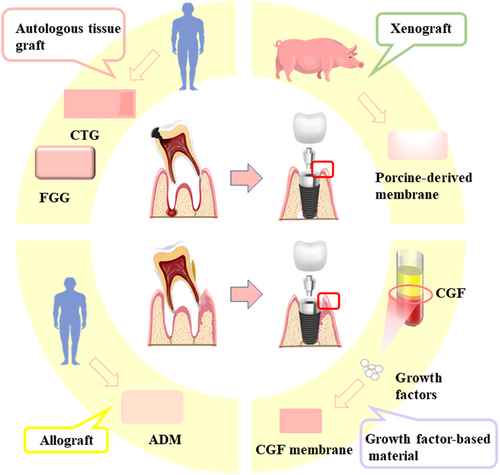
| Graft | Advantages | Disadvantages | Reference | |
|---|---|---|---|---|
| Autogenous tissue grafts | CTG | Prevent a postoperative gingival recession, good esthetic outcome | A second surgical area, long surgery time, difficult operation, postoperative shrinkage | [134-136] |
| FGG | ||||
| Allograft | ADM | Good esthetic outcome | Low long-term stabilization | [137] |
| Xenograft | Easily accessible, good esthetic outcome | Antigenicity from different species, high disease transmission | [138] | |
| Growth Factor-Based materials | PRP | High concentration of growth factors | Low preparation and lack of harmonization | [139] |
| PRF | Three-dimensional mesh structure, growth factors | |||
| CGF | Larger, higher concentration of fibrin clumps | |||
- Abbreviations: ADM, acellular dermal matrix; CGF, growth factor concentrate; CTG, connective tissue grafts; FGG, free gingival grafts; PRF, platelet-rich fibrin; PRP, platelet-rich plasma.
The use of autogenous tissue grafts, including CTG and FGG, remains the gold standard for a postimplant soft tissue defect. In 1955, Reikie134 used free connective tissue flaps to provide soft tissue coverage around the healing abutment during implant surgery, which was thought to be effective in preventing postoperative gingival recession. Lee et al.135 observed changes in soft tissue levels after immediate implant placement and CTG of 11 maxillary anterior teeth associated with gingival atrophy defects and showed significant improvements in peri-implant soft tissue levels and keratinized gingival width, which significantly improved the esthetic outcome. However, autogenous tissue grafts have the following disadvantages: (i) this method requires the opening of a second surgical area, which increases the patient's pain and may have complications such as donor area infection and scarring; (ii) this method increases the time as well as the difficulty of the procedure; and (iii) the free mucosal flap will shrink to varying degrees after the procedure.136
ADM has been produced as an alternative option for plastic periodontal and implant surgery. Clinical results showed that ADM and CTG have comparable clinical efficacy and lead to satisfactory esthetic results, but the long-term stabilization of results still needs to be improved.137 To overcome these drawbacks, xenografts were developed for soft tissue augmentation. A study evaluating porcine-derived membranes found that the mean thickness increase of the soft, thin tissue was 1.8 ± 0.13 mm. The results showed that the porcine membrane can be used for vertical thickening of soft tissue with a significant increase in the height of the tissue.138
CGF is another option for immediate postimplantation wound closure due to its rich growth factor content and its simple, low-cost production. To close the wound with CGF, the film is first pressed into either a jelly-like hydrogel or applied to gauze and then fixed to the surface of the wound using sutures.139 Because CGF shrinks in volume due to dehydration 1–2 days after surgery and tends to fall off, suture fixation is critical.
4 APPLICATIONS
4.1 Bone tissue
A recent controlled study evenly divided 40 patients into group A (Bio-Oss) and group B (PerioGlas). Gingival index, probing depth, and bone resorption were compared at different time intervals in both groups. The results showed no statistical differences in any of the parameters recorded (medial, distal, buccal, and lingual). Bone formation was comparable in both groups.140 In a randomized controlled trial, DFDBA and Bio-Oss covered with collagen membranes had positive effects on alveolar ridge preservation in extraction sockets. Histomorphometrically, the percentage of new bone formation was higher in the DFDBA group than in the Bio-Oss group. Bio-Oss was associated with more residual graft particles.141 Another study compared the effects of DFDBA and modified HA granules in patients requiring immediate dental implants. A nonsignificant difference was observed.142 A fully synthetic BCP consisting of 60% HA with 40% β-TCP was used to elevate the floor of the upper frontal sinus. After 6 months of healing, histology and imaging demonstrated mature bone tissue formation and adequate bone volume in the maxillary sinus.143, 144
The combination of PRF and DFDBA was evaluated in a prospective study for its effect on immediate implant survival in extraction sites with periapical lesions. The 12-month implant survival rate was 91.67%. This combination resulted in a significant reduction in bone resorption, an acceleration of bone healing, and a significant improvement in gingival esthetic scores on the interproximal and midfacial surfaces.145 ArRejaie et al.146 conducted a prospective clinical trial comparing a test group (Bio-Oss + PRP gel) and a control group (Bio-Oss alone) in immediate implant placement. The results showed a 94.30 ± 2.58% defect fill rate in the +PRP gel group, approximately 10% higher than the control. A clinical case by Zhou et al.147 demonstrated the successful use of a PRF fibrin clot combined with Bio-Oss at the immediate implant placement bone defect site. Six months postoperatively, good osseointegration and new bone production around the implant neck were observed. Yang et al.148 compared CGF and Bio-Oss in the implant-bone gap in two groups of 10 cases. Results 1 year postoperatively showed that the change in buccal bone width was 0.85 ± 0.25 mm in the CGF group and 0.35 ± 0.25 mm in the Bio-Oss group. The conclusion was that CGF alone has no significant effect on promoting new bone regeneration. The same conclusion was reached in another clinical study149 in which 40 patients were divided into a test group (CGF + Bio-Oss) and a control group (Bio-Oss alone) and placed in the jaw defect. Five months after surgery, bone density in the defect area was significantly higher in the test group than in the control group.
Recent advances that have not yet been tested in humans include chitosan gels and scaffolds and modified silk fibrinogens. One animal study found that the newly formed bone was stronger in the occlusal area when rhBMP-2 was used in combination with chitosan gel in rats.150 In another study, plasmid DNA/c-my conjugated with chitosan gold nanoparticles (Ch-GNPs/c-my) was shown to promote osteogenesis and inhibit osteoclastogenesis in MC-3T3 E1 cells.99 Chitosan/Biphasic Calcium Phosphate (CS/BCP) scaffolds loaded with Arg-Gly-Asp (RGD) and BMP-2 have been developed, and the resulting scaffold closely resembles the extracellular matrix (ECM) of natural bone in composition and structural characteristics. The findings indicated that the scaffolding provided a favorable environment for bone formation and could be used as a potential bone regeneration material.100 The SF matrix was modified with tannic acid (TA), which significantly improved the mechanical properties of the SF membranes and promoted the proliferation and bone differentiation of MC3TC cells.151 Bone morphogenetic protein-2 loading of human mesenchymal stem cells into poly(ethylene oxide) nanofibre composite scaffolds regenerates bone-like tissue.152 Furthermore, the incorporation of hydroxyapatite nanoparticles into SF has been shown to improve bone regeneration in animals.153
Due to the limitations of single materials, composite biomaterials offer improved biological, physical, and chemical properties, as well as versatility for bone regeneration. These composite materials include bioceramics and polymer materials, preparation technologies and materials, and technologies and materials for tissue engineering. The integration of these materials and technologies opens new directions for the development of the next generation of immediate implant bone restoration materials.
4.2 Soft tissue
In an animal experiment in dogs, a CGF film was placed over the alveolar ridge surface in following immediate implant placement surgery.154 The gingiva was sutured, and after 1 month, immunofluorescence staining revealed positive expression of vascular endothelial cells, indicating neurovascular regeneration. Results demonstrated better soft tissue wound healing in the test group compared to the blank control group.
Zhou et al.155 conducted a clinical study involving 48 patients with immediate implant placement in the inflammatory sites. Patients were divided into two groups: the observation group, where Bio-Oss + CGF membrane was implanted, and the control group, where only Bio-Oss was implanted. The control group experienced one case of mucosal congestion and pus overflow 1 month after surgery, with the implant well stabilized. All cases in both groups achieved final restoration with a 100% implant retention rate. The experimental results suggested that CGF-rich cytokines may reduce the inflammatory response and decrease postoperative infection. While these studies demonstrate that CGF applied to immediate implant placement results in good soft tissue closure and reduced postoperative infection rates, more clinical data are needed to further support these findings.
The height and attachment of the soft tissue on the bone around the implant influence the health of the hard and soft tissue surrounding the implant. Currently, no biomaterial can regenerate soft tissues well, and with the advancement and development of technology, there must be a better solution for peri-implant soft tissue closure in the future.
5 PROSPECTS
Currently, immediate implant placement is widely used in clinical applications, and its applications continue to broaden with the advancement of immediate implant technology. At the same time, new biomaterials are constantly being developed for bone regeneration and soft tissue closure, which can better improve the survival rate of immediate implant placement. To treat damaged tissues and organs, tissue engineering is an effective alternative to traditional methods. Many pathologically damaged tissues can be regenerated using tissue scaffolds made from suitable biomaterials. The combination of composite materials with metal ions, bone proteins, or mesenchymal stem cells is under investigation. Although artificial bone repair materials are constantly being modernized, there are still many unanswered questions. The key problem is that the degradation of the material in vivo is not perfectly adapted to the rate of new bone production, and the resulting biomaterial-bone hybrid does not completely rebuild the physiological bone tissue. Meanwhile, the osteoconductivity, osteoinductivity, and vaso-inductive activity of the material cannot be well balanced, leading to serious limitations in the timeliness and regionality of the material to repair bone defects. Advanced tissue engineering technologies could break through the limitations of biomaterials used to replace bone tissue. New implants that better mimic the dynamic properties of the microenvironment during the development of regenerating bone tissue could be efficiently and reproducibly produced by combining appropriate biomaterials with tissue engineering techniques. The natural processes of bone regeneration, such as the coupling between angiogenesis and osteogenesis, should be better modeled in new research directions.
AUTHOR CONTRIBUTIONS
Xiaojing Wang, and Guowei Wang offered direction and guidance for the manuscript. Xiaoqi Su and Shasha Jia drafted the initial manuscript and prepared the figures. Xueya Wang revised the figures for the entire manuscript. Xiaojing Wang and Baodong Zhao revised the framework for the manuscript. All authors have checked the manuscript and agree to be published.
ACKNOWLEDGMENTS
We apologize to colleagues whose important work could not be cited due to space constraints. The authors thank Prof. Sarah Zingales for providing language support. The authors also thank Lilan Zhu and Jiabei Zhang for figure production. Thanks for the support by Natural Science Foundation, Shandong, China (grant numbers ZR2022MH266); Medical and Health Science and Technology Development Plan, Shandong, China (grant numbers 202108020661); Clinical Medicine +X Project of The Affiliated Hospital of Qingdao University (grant numbers. QDFY + X2021002); Shinan District Sci-Tech Plan (grant numbers 2022-2-016-YY); Qingdao Demonstration and Guidance Project of Science and Technology to Benefit the People (Grant No. 23-2-8-smjk-6-nsh).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
No ethical approval was required for this study.
Open Research
DATA AVAILABILITY STATEMENT
The data in this study are available upon reasonable request.




