Recent progress in thermosensitive hydrogels and their applications in drug delivery area
Abstract
The scientific community has widely recognized thermosensitive hydrogels as highly biocompatible material with immense potential in drug delivery systems. When the temperature of these hydrogels approaches that of human body, a phase change occurs, enhancing their usefulness in a range of medical scenarios. This review article highlighted the background of thermosensitive hydrogels, their properties, and their applications in transdermal, oral, ophthalmic, intravaginal, nasal, rectal, cancer therapy, and cell-loaded drug delivery systems. The literature suggests numerous advantages of these hydrogels over conventional drug delivery systems and find applications in various fields, such as therapeutic systems, filling processes, and sustained drug delivery systems. One of their key benefits is the ability to eliminate invasive procedures like surgery, providing a noninvasive alternative for drug administration. Moreover, they streamline the formulation process for both hydrophilic and hydrophobic drug delivery systems, simplifying the development of effective treatments. The thermosensitive hydrogels have been found to be green materials with negligible side effects and desirable drug delivery properties. The thermosensitive hydrogel's sustained-release characteristics, immunogenicity, and biodegradability have also gained increased interest. Some of the disadvantages of thermosensitive hydrogels include delayed temperature response, weak mechanical characteristics, and poor biocompatibility, which limits their potential use in drug delivery applications.
1 INTRODUCTION
Hydrogels are three-dimensional polymeric networks that can contain a substantial quantity of water in their cores while remaining insoluble.1 The hydrogel network promotes bonding with other macromolecules, allowing cage formation in the form of a matrix.2 Hydrogels are classified based on their properties including bonding nature, physical appearance, mechanical behavior, and swelling ability.3 Smart hydrogels are a type of traditional hydrogel that has the same properties as traditional hydrogels with the addition of stimuli sensitivity behaviors, which makes them the more potential type of hydrogel for biomedical applications.4 Smart hydrogels respond to various stimuli including temperature, pressure, PH, bio signals, light, and enzyme reactions.5
Thermosensitive hydrogels are a type of smart hydrogel that responds to temperature stimuli and alters its shape from sol to gel phase at a specific range of temperature. In general, thermosensitive hydrogels are made from natural and derived thermosensitive polymers using traditional synthesis methods such as crosslinking, polymerization, and so on.6 It is a promising candidate for use in the fields of drug delivery due to its potential properties as good mechanical stability, excellent biocompatibility, thermos-transition behavior, and ease of formulation.7-9 Furthermore, thermo-sensitive hydrogels contain thermosensitive groups which have different gelation temperatures depending on their nature and material.10 They are classified into two types; upper critical solution temperature polymers (UCST), those that respond positively to the temperature, and lower critical solution temperature (LCST), those that react negatively to temperature.11, 12 The LCST polymer-based thermosensitive hydrogel is hydrophobic while UCST polymers based is hydrophilic, while, we can change the solubility nature of both types of the hydrogel by varying the quantity of LCST and UCST polymers during the synthesis phase.13, 14
Oral and intravascular routes of drug administration are common in the field of drug delivery due to their effectiveness and convenience. However, both routes have several limitations, including low sustained release, poor targeted drug delivery, and side effects.9-11 To overcome these challenges, researchers attempted trials on thermosensitive hydrogels in the drug delivery area, Whereas the literature highlighted that thermosensitive hydrogels are green materials with low side effects with desired drug administration characteristics.12, 13 Due to their sol-gel qualities, thermosensitive hydrogels may be applied anywhere in the human body. While at body temperature, it may store different hydrophilic and hydrophilic drugs in the matrix for prolonged release.14-16 The sustained and targeted release of the bioactive component from the thermosensitive hydrogel overcome the challenges faced by the traditional drug administration routes and can be used as a promising candidate in drug delivery applications.17-20
Thermosensitive hydrogels are currently attracting global researchers due to their exceptional properties and biocompatibility. As a result, this review article focused on the background of thermosensitive hydrogels, their properties, and their applications in transdermal, oral, ophthalmic, cancer therapy, intravaginal, and cell-loaded drug delivery systems. It also highlighted recent studies of thermosensitive hydrogel in drug delivery applications. In the first section of this review paper, we demonstrated the material, classification, and potential properties of the thermosensitive hydrogel, while in the second section, we highlighted the most recent findings of thermosensitive hydrogel applications. At last, we listed our potential remarks based on the highlighted studies. We believe that this review article will give researchers a thorough understanding of thermosensitive hydrogel for applications in drug delivery areas.
2 MATERIAL AND CLASSIFICATION OF THERMOSENSITIVE HYDROGEL
Hydrogels are made up of natural polymers and derived polymers depending on their applications, but thermosensitive hydrogels are only made up of thermosensitive polymers that are either natural or derived in origin.21, 22 Natural thermosensitive polymers have peptide and glucose ring structures, whereas derived thermosensitive polymers are formed through a series of chemical modifications.23 The potential thermosensitive materials identified in the literature are listed in (Table 1). Furthermore, natural thermosensitive polymers have several limitations24 when compared to derived polymers because natural polymers have very low chemical diversity, which causes problems with thermal transition properties,25 whereas derived polymers have a wide range of chemical diversity, making them more potential than naturally occurring thermosensitive polymers.26, 27
| Sources | Name | Characteristics | Application | References |
|---|---|---|---|---|
| Naturally derived polymer | Cellulose | Most abundant natural polymers and lack thermosensitive properties, due to that it need always thermosensitive agents in the formulation | Injectable, transdermal, wound healing | [32] |
| Chitosan | Most suitable and 2nd most abundant natural polymer and have very lower negligible thermosensitive properties | Transdermal, filling, and sustainable drug delivery | [33] | |
| Hyaluronic acid | Good thermosensitive properties | Intravesical administration | [34] | |
| Gelatin | Biocompatible and biodegradable have poor thermos sensitivity | Bioprinting, transdermal | [35] | |
| Collagen | Poor thermo sensitivity | Oral, intraocular, intravascular | [36] | |
| Agrose | Poor thermosensitivity | Intravascular | [37] | |
| Synthetic polymers | PNIPAAM | Good thermos sensitivity and nondegradable | Tumor treatment, Cell growth | [38] |
| PCL-PEG | Biocompatible, biodegradable, good thermo sensitivity, good mechanical strength | Injectable for tumor treatment | [39] | |
| POEGMA | Biocompatible, biodegradable, good thermo sensitivity, | Tuning of cell interaction | [40] | |
| PCL-PEG-PCL | Biodegradable, good thermo sensitivity | Drug delivery model | [41] | |
| Polyphosphazene | Biodegradable, good thermo sensitivity, good mechanical strength | Tumor treatment | [42] | |
| Pluronic | Good thermosensitivity, low mechanical strength, nondegradable | Drug delivery models | [43] |
- Abbreviations: PCL, poly(ε-caprolactone); PEG, polyethylene glycol; PNIPAAM, poly (N-isopropyl acrylamide); POEGMA, poly (oligo ethylene glycol methacrylate).
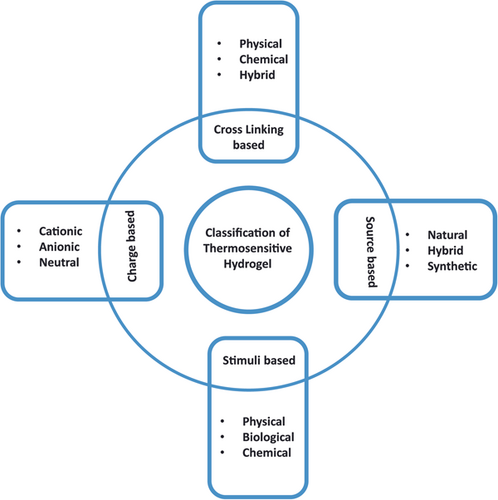
Thermosensitive hydrogels are classified based on the type of stimuli, source, crosslinking, and charge as illustrated in (Figure 1). There are various types of stimuli such as physical response, which can come from the external environment, biological response, which almost comes from the internal body, and chemical response, which occurs because of chemical reactions in the body.28, 29 While based on the sources, it can be natural, derived, or hybrid. Also, thermosensitive hydrogel can be anionic, cationic, or neutral according to its polymeric charge and synthesis mechanism. Lastly crosslinking distinguishes thermosensitive hydrogel into physical, chemical, and hybrid bonding-based hydrogels.30 Moreover, thermosensitive hydrogels have been classified and characterized based on their basic material composition, which is essential to select the thermosensitive hydrogel for desired drug delivery applications.31
3 PHYSIOCHEMICAL PROPERTIES OF THERMOSENSITIVE HYDROGEL
3.1 Mechanical property
Hydrogels are polymeric materials that exhibit two types of mechanical behaviors (viscoelasticity and rubber elasticity). These properties are determined by the hydrogel's swelling ratio; for example, if the hydrogel has a low swelling ratio, it exhibits rubber elastic properties, whereas a high swelling ratio demonstrates viscoelastic properties.44, 45 Naturally, polymers have low mechanical strength, but researchers are experimenting with different types of matrix and reinforcement materials to tune the mechanical properties of thermosensitive hydrogels to use them in drug delivery applications.46 Mechanical properties studies by various researchers, among them Bin Imran et al. reported the highly stretchable thermosensitive hydrogel which demonstrated extraordinary mechanical properties. The synthesized thermosensitive hydrogel was prepared by the introduction of ionic groups into the polymeric network of the polyethylene and α-cyclodextrin. The reported hydrogel demonstrated extraordinary results and visually highlighted in (Supplementary Figure 1). Furthermore Supporting Information: Figure S1A–C parts demonstrated the elongated, compressed, coiled states of thermosensitive hydrogel whereas Supporting Information: Figure S1D highlighted the mechanical credibility against knife.44 This work confirmed the tuning of mechanical capability of thermosensitive hydrogels by implementing suitable reinforced materials. So, in drug delivery systems the mechanical property of thermosensitive hydrogels matters more than other characteristics.
3.2 Adhesion
Adhesion is a fundamental property of materials that makes them more compatible with living organisms. It depends on the internal structure and chemical bonding of the materials whereas hydrogels contained the excessive quantity in their core which make them weaker in terms of adhesive characteristics due to hydrogen bonding. Excessive water absorption in the core of the hydrogel causes weak bonding with the substrate, resulting in a gap between the real surface of the hydrogel and the substrate.46 Furthermore, water molecules form hydrogen bonds with the hydrogels' sticky spots, reducing interfacial interaction between the hydrogels and the target substrate. Because most surfaces (human organs and tissues) are moist and sensitive, utilizing hydrogels in biomedicine makes the problem far more challenging.47, 48
Researchers attempted different techniques to overcome these issues and considered the target texture before the synthesis of the hydrogels.49, 50 Because almost all medical applications require surface texture contact with cells and tissues. Thermosensitive hydrogels demonstrated good adhesion properties as compared to traditional hydrogels because thermo polymers are naturally sticky.51, 52 So, to overcome these issues of adhesion in traditional hydrogels, thermosensitive hydrogel can be considered as a potential candidate instead of other traditional materials.
3.3 Phase transition
Phase transition is the material mechanism in which materials change their state on the application of stimuli. It is one of the most important characteristics of thermosensitive hydrogels, which makes them a more promising material than traditional hydrogels. The basic mechanism involves the dissociation of weak bonding (hydrogen bonding, Vander-Waals forces, ionic interaction, and hydrophobic bonding), resulting in a phase shift from sol to gel and vice versa.51, 52 Furthermore, copolymers' functional groups and system negative energy also affect the phase change of the hydrogels.51, 53 Phase transition characteristics of thermosensitive hydrogel determined by their type of polymers used like UCST and LCST. The graphical representation of the phase transition is shown in Supporting Information: Figure S2, which demonstrated the transition behaviors of UCST and LCST-based thermosensitive hydrogels.
3.4 Drug releasing property
Traditionally the oral and intravenous drug delivery routes were used to treat different diseases while these methods are believed to be effective till today, but they also cause the severe issues including sustained drug release, which resulted the fatal consequences. To overcome these issues thermosensitive hydrogels demonstrated excellence characteristics for their sustained drug-releasing properties. The drug-releasing mechanism of thermosensitive hydrogels is classified into three types: diffusion-based releasing, swelling-based releasing, and erosion-based releasing as demonstrated by Supporting Information: Figure S3B. The movement of particles from a high concentration to a lower concentration is referred to as diffusion. According to Ficks' first law of diffusion, hydrogels almost release drugs via diffusion.54 The diffusion of drugs in smart hydrogels is affected by a variety of factors such as matrix structure, chemical bonding, releasing surface, and stimuli type. In general, the temperature stimulates the hydrogel bonding to release the drug,55 and the drug molecule size influences the diffusion property because the matrix and drug molecules must be of the appropriate size to hold for a long time and release at the time of stimulation.56 However, it has been highlighted in the literature that the swelling behavior of smart hydrogels also plays a role in the drug's sustained release.57 Swelling mechanisms involve the dissociation of three-dimensional macromolecule chains, which occurs before swelling-based release.58, 59 Furthermore, in some cases, erosion effects occur on the account of the sustained drug release.60 In the erosion mechanism, reversible and irreversible reactions occur between the ions of polymers on the application of the stimuli.61
The researchers are investigating different materials and technique to enhance the discussed properties (mechanical properties, adhesion, phase transition, and drug-releasing properties) of thermosensitive hydrogel to make it a more promising material for innovative drug delivery systems.
4 APPLICATIONS
4.1 Transdermal drug delivery system
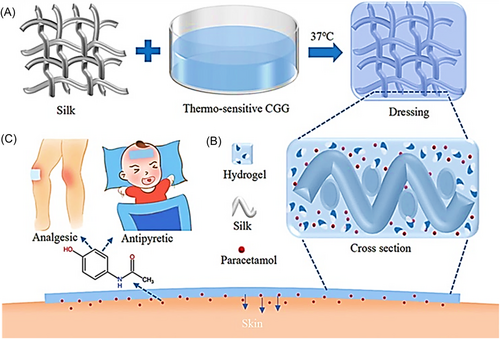
Thermosensitive hydrogels play a significant role in transdermal drug delivery systems because of their excellent biocompatibility, improved mechanical properties, simple formulation, and sustained drug delivery. Many researchers used thermosensitive hydrogel in smart patches and creams to treat dermal infections.62, 63 Nirmayanti et al reported a thermos responsive hydrogel for the sustained release of valsartan to treat hypertensive patients through a transdermal drug delivery system. The proposed gel is composed of poloxamer 407 and Poloxamer 188 in varying amounts and their characteristics were found to be altered by poloxamer concentrations. The reported hydrogel subjected to characterization and in vivo testing, which demonstrated the enhanced characteristics including bioavailability in comparison to oral tablet.62 Qiao et al. synthesized a paracetamol-encapsulated thermosensitive hydrogel and silk-based medical dressing to prevent the gastrointestinal first-pass impact of drugs and short oral interval administration as illustrated in Figure 2. Genipin, glycerol-phosphate disodium salt, and chitosan were utilized to tune the gelation while skin permeation was enhanced by a silk covering. Moreover in-situ phase transition enabled a sustained release of paracetamol from the hydrogel for 12 h. The gel-coated silk dressing was found to be compatible with direct skin application and had a higher water vapor transfer rate. There was temperature-dependent rapid-release behavior from the medical dressing during the first 2 h of use. The Korsmeyer-Peppas model of non-Fickian diffusion provides a good match for the drug's prolonged release profile. Furthermore, Figure 2: demonstrated the illustration of thermosensitive hydrogel-based medical dressing, while Figure 2A demonstrates the sol-gel decorating technique for the transdermal drug delivery medical dressing. First, silk fabric was dip-coated in a chitosan and glycerophosphate disodium salt thermosensitive solution, followed by a heat treatment at 37°C to achieve in situ hydrogel curing. The sol-gel transition characteristic under moderate temperature conditions enables the introduction of a genipin natural cross-linker for boosting cross-linking efficacy and lowering pore size to prolong drug release. Figure 2B highlighted the crosslinked illustration of the medical dressing containing silk fabric penetrated with thermosensitive hydrogel. Moreover, Figure 2C demonstrated the illustration of the transdermal application of thermoresponsive medical dressing for analgesic and antipyretic conditions.64 S. KHAN et al used microneedle-assisted thermos-responsive hydrogel for improved drug delivery. Various poloxamer grades (PF127 and P87) were employed in the present investigation to construct unique in situ synthesized depots in skin micropores formed by microneedles treatment. To establish the phase conversion property at skin temperature, rheological studies on poloxamers were undertaken (32°C) before the application. The stability, Pressure forces, in situ solid dispersion, and moisture contents of the microneedles were also measured. The nonsoluble cross-linked character of the improved microneedles was tested using dissolution kinetics. OCT and dye binding studies validated the pores development and pig skin permeation of microneedle arrays. The in vitro permeation analysis indicated that curcumin permeability occurred in a sustained and protracted manner across microneedle-treated skin samples in contrast to microneedle's untouched skin. Confocal laser microscopic examination revealed a higher content of curcumin in the incorporated epidermal layer. Overall, it was determined that the phase conversion characteristic of pluronics may be employed to establish in situ microneedle aided depots in the porated site at the skin and would enable regulated administration of curcumin incorporated in poloxamers formulation for a long time.63
4.2 Oral drug delivery system
Oral drug delivery is thought to be a promising route to cure diseases effectively, but it has certain limitations including low bioavailability. It causes severe side effects through the gastrointestinal tract because the liver digests the oral formulations before it reaches the target sites, so to overcome these issues researchers formulated the oral drugs with thermosensitive material vehicles to prevent the drug from being digested.65, 66 Ortega et al. synthesized curcumin-infused lipid-core thermoresponsive hydrogel nano capsules containing chitosan covering for improved muco-adherence and overcoming different limitations of oral drug delivery in the treatment of malignant cells. The reported formulation is composed of poloxamer 407, hydroxypropyl methylcellulose, and curcumin lipid core. Moreover, curcumin lipid-core nano capsules demonstrated an average diameter of (173 ± 22 nm) while chitosan curcumin lipid-core nano capsules demonstrated an average diameter of (179 ± 48 nm). Last, the in vitro model demonstrated the enhanced reduction in cell viability across all treatment groups as compared to the oral squamous cell carcinoma cell line.65
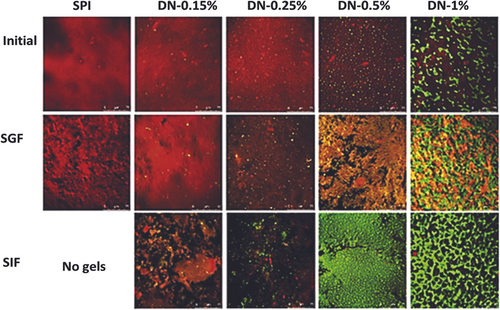
Cheng et al. reported a modified thermosensitive formulation for sustained release of calcitonin. The synthesized formulation was composed of Methoxy poly (ethylene glycol) propionic acid (mPEG-PA) and lysine amino acid. The biocompatibility & calcitonin release profile of the reported hydrogel particles were investigated over a range of pH values. The drug release kinetics demonstrated the sustained release of calcitonin over 1 day.66 Thouvenin et al. developed (S)-ketamine thermosensitive formulation for oral buccal administration. The reported smart thermosensitive formulation was synthesized by crosslinking poloxamer 407 with sodium alginate, both components enhanced the absorption of the (S)-ketamine in the buccal region. The selection of appropriate thermosensitive polymer for gelling temperature (32°C) based on the rheological studies of poloxamer 407. Furthermore, the synthesized formulation showed a promising in vitro drug-releasing profile and demonstrated high bioavailability with the fast release.67 Yan et al. reported the hybrid hydrogel of corn fiber gum and soya bean protein to deliver the thermosensitive bioactive components orally. The thermosensitive model component was riboflavin (Vitamin B2). The pharmacokinetics of the proposed hydrogel were investigated in the simulated gastric and intestinal fluid. The sample with 0.25% of corn fiber gum demonstrated the highest biocompatibility in the oral administration of thermosensitive biocomponents. Furthermore, Figure 3 demonstrated the confocal images of the hydrogel at the initial, SGF, and CFG phases. Initially, the red color demonstrated the protein phase while the green color represents the CFG phase. In conclusion, all phases confirmed the enzymatic action of the proposed hydrogel.68
4.3 Intravaginal drug delivery system
The intravaginal route is almost used for HIV and gynecological disorders. Due to physiological barriers, drugs are poorly absorbed through the vaginal mucosa. Furthermore, the large surface area of the vaginal mucosa is also a barrier to the drug delivery route,69 so researchers investigated biocompatible thermosensitive hydrogel trails on the vaginal surface to overcome these issues. Isa et al first-time designed polyethylene glycol (PEG) as a permeability promoter in thermo-responsive mucoadhesive hydrogels containing Nile as a paradigm of hydrophobic composites for vaginal distribution. Because administering hydrophobic medicines using traditional preparation techniques is difficult. Pluronic® F127 (P127) and Pluronic® F68 (P68) were utilized as temperature-sensitive agents in the proposed study, whereas HPMC was used as a mucoadhesive agent. The gelation temperature, rheological behavior, Ph restoration, and mucoadhesive characteristics of the hydrogels were all unaffected by the Polyethylene glycol (PEG) concentration. When PEG was employed, Nile red's ex-vivo permeability and retention were increased up to 15 and 20 times, respectively.69 Salehi et al. reported a thermo-responsive Pluronic F127 gel with curcumin (CRC) and thiolated chitosan (TC) nanoparticle (NPs) (PF127-CRC-TCNPs) for vaginal administrations. The influence of two important factors (CRC–TCNPs) on the release of curcumin was also examined. To overcome a normal non-mucoadhesive vaginal dosing approach, large dosages are typically required, which limits patient compliance and raises the risk of unwanted effects. The authors used a combination of nanoparticle and gel technologies, which yielded promising results. It was determined via estimations of drug entrapment and loading efficiency that both increased with increasing curcumin concentrations. The curcumin release kinetics indicated a reduction with rising pH when the release media was tested at three distinct PH values (4.5, 5, and 5.6). The low dependence on the viscoelastic modulus data, which indicates good gel elasticity, contributed to the formulation's longer retention period. The HeLa cell line was examined using MTT assays. According to the results of this investigation, the planned technique is a promising option for cervical carcinoma prevention and might be included in parenteral formulations.70 Argenta et al. proposed a way for preventing the systemic side effects of oral secnidazole formulations. To minimize the drug's systemic effects, researchers placed secnidazole into mucoadhesive thermo-responsive hydrogel structures. Fast gelation times (100–115 s) and favorable sol-gel transition temperatures (>30°C) were shown by formulation comprising of 20% poloxamer 407 and 1% poloxamer 188, with 1.5% or 2.5% chitosan (100–115 s). Hydrogels have also shown a tendency, relative to the control, to slow the rate of delivering drugs via mucosa. The mucosa was modified by adding mucin to improve the realism of the retention simulation. In comparison to a control group, the drug's retention was reduced by around 2.0 times when hydrogels containing chitosan were used. Since the hydrogels may persist in the vaginal area. Therefore, this study demonstrated that trichomoniasis might be efficiently treated locally with proposed formulation.71
4.4 Ophthalmic drug delivery system
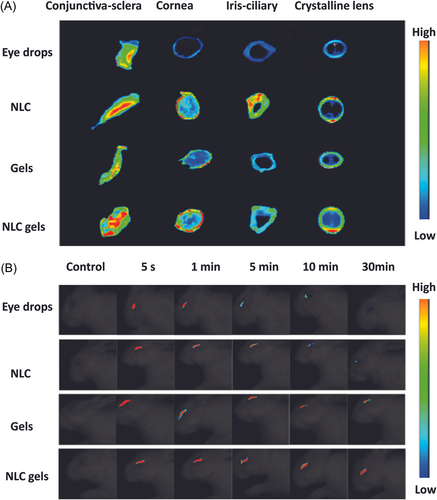
The eye is a major organ of the body that is very soft and sensitive. In general, doctors and professionals recommended eye drops for various ophthalmic diseases such as glaucoma, amblyopia, and so on which could not reach the target positions due to the eye's biological barrier layers. The frequent use of eye drops can cause vision loss, so researchers developed smart hydrogels to address these problems.72 Xu et al. developed a thermosensitive Poly (DHSe/PEG/PPG Urethane) hydrogel for long-term delivery of remdesivir medicine. The investigated hydrogel demonstrated the excellent sol-gel transition at 35°C, as well as in vivo and drug pharmacokinetics showed the sustained and long-term release of remdesivir. Furthermore, in rabbit eyes, the remdesivir incorporated in situ gel demonstrated high ocular tolerance and biocompatibility.72 Annala et al. reported an injectable self-sustaining thermosensitive hydrogel technology for long-term dexamethasone release. The smart hydrogel is synthesized from ABA triblock co-polymers by reversible addition-fragmentation chain transfer (RAFT) polymerization. The developed hydrogel demonstrated excellent self-sustaining characteristics and good gelation temperature (37°C). The system was injectable because disulfide connections were present in the cystamine cross-links. Dexamethasone was released from the hydrogel over 430 days at 37°C by ester hydrolysis in an aqueous phase at 7.4 PH. Simulations showed that 100 mg of hydrogel is enough to maintain therapeutic dexamethasone levels in the vitreous for at least 500 days. Notably, the LC-MS data demonstrated that dexamethasone was liberated in its original form from the hydrogel. According to cytocompatibility investigations, adult retinal pigment epithelium (ARPE-19) cells tolerated both the polymer and the cross-linker at clinically relevant dosages. Furthermore, the hydrogel had little effect on ARPE-19 cells.73 Chen et al. developed in-situ thermos gellable poloxamer (P407)-based hydrogels by combining hypromellose (HPMC) to lower P407 concentration and hyaluronic acid to increase muco-adhesion of the resultant hydrogels for sustained ocular administration of HPCD-solubilized testosterone. Solubilization was achieved by adding 10% HPCD to a 0.5% testosterone solution. In-situ gelation was achieved by adding 2.0%–2.5% HPMC to a non-gellable 13% poloxamer-407 sol, and muco adhesibility was increased further by adding 0.3% HA-L (low MW) or HA-H. (high MW). Based on 0.5% HPCD-solubilized TES P407, optimized 13% P407, 2.5% HPMC, and 0.3% HA-L or HA-H thermos gellable hydrogels with enhanced muco-adhesion for longer ocular administration. According to rheological characterizations using simulated eye blinking, the nonthixotropic features of improved hydrogels may spread evenly and hold a higher number of drug-loaded hydrogels on the ocular surface for a longer period. Ex vivo and in vivo investigations demonstrated that hydrogels had longer residence times than standard eye drops. By adding 2.5% HPMC and 0.3% HA to 13% P407, it was determined that P407-based thermosensitive hydrogels with reduced P407 concentration and improved muco-adhesion might achieve successful clinical therapy of dry eye disease.74 Ye et al. synthesized the hybrid PH and thermosensitive hydrogel for delivery of the quercetin. The formulated hydrogel was prepared by crosslinking poloxamer 407 and carboxymethyl chitosan with nanostructured lipid carriers using crosslinker genipin. The prepared hydrogel offered excellent biocompatibility and lower ocular irritation. Additionally, the ex vivo study and fluorescence imaging as shown in Figure 4A,B demonstrated that nano lipid carriers enhanced the trans corneal penetration of the drug as well as residence time. Lastly, the pharmacokinetic profile showed that the area under the curve of the formulation was 4.4-fold than the eye drops groups so due to excellent and enhanced characteristics the proposed formulation can be the best candidate for ocular prolonged drug delivery.75
4.5 Nasal drug delivery
Nasal administration is almost preferred by professionals to deliver proteins and peptides due to the large surface of the nasal mucosal membrane and it provides greater absorbance to the therapeutics as compared to oral administration. However, there are some challenges including proteolytic enzyme attack, macromolecule absorption, and so on, that need to be overcome if the choice of administration is nasal route.76 Cunha et al. synthesized the thermosensitive in situ nasal hydrogel loaded with an acetylcholinesterase inhibitor (rivastigmine) along with nano lipid carriers and nano emulsifiers. The reported hydrogel showed excellent biocompatibility, long-term stability, muco-adhesion, prolonged drug release, and spherical shape from a morphological perspective. The pharmacokinetic studies revealed that 4% of the drug was present in the olfactory regions. The prepared hydrogel can be considered a good candidate for nasal drug delivery.77 Chen et al. reported a nano thermosensitive hydrogel loaded with the albiflorin drug to treat Parkinson's disease. In the synthesis mechanism, the glycyrrhizic acid was used as a crosslinker as shown in Supporting Information: Figure S3 and bound the drug tightly to avoid burst release. The prepared nano gel offered excellent bioavailability, lower degradation, and prolonged drug release. Additionally, the nano system exhibited excellent antioxidants, selective brain distribution, and anti-inflammation effects. Supporting Information: Figure S4 demonstrated the benefit of the hydrogen bond as stabilizing agent within the nano gel system and helped the system to enhance prolonged release. The Poloxamer 188 and 407 were used to synthesize the nano thermosensitive hydrogel while albiflorin and glycyrrhizic acid encapsulate the nanogel. The pharmacokinetic effects studied in the Parkinson's disease model. The reported work demonstrated the best compatibility of nano gels with intranasal administration route and open the doors further for nano gels application in intranasal drug delivery system.78
4.6 Rectal drug delivery
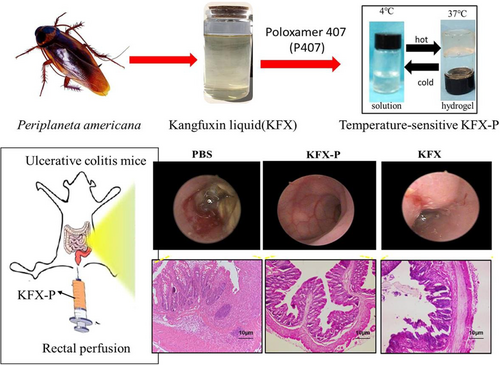
A rectal drug delivery system is a potential alternative administration route to the parenteral and oral routes. This route administers local as well as systematic drug therapies. However, the traditional techniques and formulation of the rectal dosage produced discomfort in the patients due to low effectiveness and poor biocompatibility. The introduction of sensitive, smart, and biocompatible materials increases development in the rectal administration system.79 Din et al. reported as alternative reversible nanocarrier thermosensitive system for rectal administration. The nano system was fabricated by dispersing nano solid particles in a poloxamer solution. The pharmacokinetics profile of the system confirmed the sustained release of the Irinotecan. Additionally, the nano system can be injected through the anus easily and effectively as compared to the conventional hydrogel. So, this investigation can be implemented effectively in rectal administration.80 Xue et al. developed in situ thermosensitive hydrogel loaded with Chinese medicine (Kangfuxin liquid) for rectal administration to repair the colon mucosal barrier as shown in Figure 5. The hydrogel was synthesized from the poloxamer P-407 thermosensitive polymer. Moreover, the reported hydrogel screened for mechanical strength, and gelation time by varying the concentration of poloxamer 407 (25%, 20%, and 25%). Increasing the concentration of P-407, the gelation time and phase transition temperature decreased, while the mechanical characteristics enhanced. Last, the hydrogel was tested on the mice models, which confirmed the effective repairing of the mucosal membrane of the colon, Figure 5 highlighted the description of the Poloxamer (P-407) thermosensitive hydrogel loaded with Chinese medicine for the treatment of colonization inflammation and mucosal membrane healing by rectal administration. However, the first section depicts the extraction of Kang Fuxin liquid from the Periplaneta americana insect, followed by its loading in poloxamer to synthesize the hydrogel with a gelation temperature range of (4°C–37°C). There is also an illustration of rectal perfusion in a mouse model with ulcerative colitis. Finally, the therapeutic properties of the formulation were emphasized. So, the integration of Chinese medicines in thermosensitive hydrogel can be the best candidate for repairing the colonic mucus membranes and their inflammations.81
4.7 Cancer therapy drug delivery system
Cancer is a deadly disease that affects the entire globe. Chemotherapy is often used to treat cancer across the world. However, it has several adverse effects including skin irritation, hair loss, apoptosis, and so on. To combat these side effects, the world needs smart drug delivery methods with fewer adverse effects, so different researchers investigated thermosensitive materials to cure the oncogene cells with lesser side effects.82 Zhang et al. developed a unique method to avoid using conventional substrates so if photo-thermal drug transporters try to maintain persistent fixation at a specific tumor site, typically this causes toxic side effects due to uncontrolled drug discharges as well as adverse effects on neighboring healthy tissues. This work created an injectable near-infrared induced ferrimagnetic chitosan thermally responsive hydrogel technology for light hyperthermia and controlled drug administration to the tumor site ablation. The hydrogel produced exhibited long-term stability in phosphate buffer solution as well as gelation capabilities at 37°C. The securely embedded photothermal component iron oxide nano cubes enabled the chitosan hydrogel's exceptional hyperthermia performance under near-infrared triggering, as well as the accurate release of loaded medicines. Ferrimagnetic chitosan hydrogel packed with doxorubicin Hydrochloride revealed synergistic efficacy and prolonged dealing in tumor cell termination when compared to light hyperthermia and doxorubicin therapy.82 Lu et al. developed a new synergistic breast cancer therapy method combining honokial nanosuspensions encapsulated thermo-responsive injectable hydrogels (HK-NS-Gel) for local injection with systemic paclitaxel (PTX). The created composites' gelation temperature and time were enhanced. Drug retention experiments conducted in vitro and in vivo revealed that HK-NS-gel may release the drug steadily and gradually over 12 days. Furthermore, following intra-tumoral injection of HK-NS-Gel, there was increased PTX deposition in the tumor. To demonstrate the synergic effects of free PTX mixed with HK-NS-Gel against 4T1 cells, in vitro cytotoxicity and cell death experiments were employed. An in vivo anticancer study performed on 4T1-bearing mice revealed that combining HK-NS-Gel with PTX significantly reduces tumor growth, with an inhibition tumor rate of up to 72.51%.83 S. KHAN et al developed poloxamer 407 and carboxymethyl chitosan (CMCS), patched thermogels for sustained drug release were created utilizing glutaraldehyde as a crosslinking agent loaded with 5-Fluorouracil. Swelling studies with changing pH buffers and temperature ranges validated thermogels' adjustable responsiveness. Maximum swelling & drug release were observed at a pH (7.4) and temperature of 25°C. According to pharmacokinetics, drug release followed a zero-order release process including pore diffusion and degradation. In vitro cytotoxicity investigation revealed that 5FU-loaded thermogels may trigger cell death in HeLa and MCF7 cancer cell lines. The computed IC50 values demonstrated that 5FU-loaded thermogels needed less inhibitory concentration than free 5FU solution. The effective grafting and creation of a new structure among PF127 and CMCS were validated by FTIR analysis. DSC and TGA validated the phase transition at hemodynamic temperature, while SEM examination revealed that chemically grafted thermogels had interconnected pores that allow solvent & drug particles to diffuse through the gel network. Last, the reported hydrogel demonstrated effective therapy against cancer cells.84
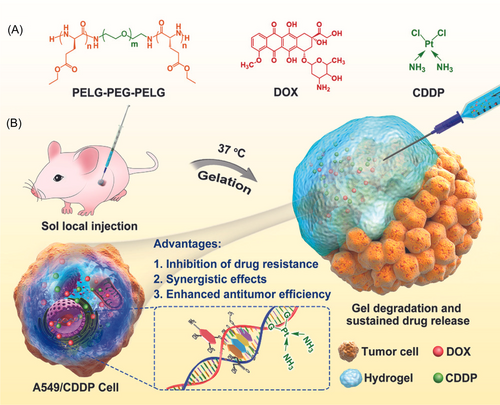
Ghomi et al. synthesized the bio-responsive nanocomposite loaded hydrogel to treat breast cancer with the help of bioimaging. The reported gel contained the creatinine functionalized carbon dots to employ bioimaging While, Herceptin and PEGylation loaded nanogel enhanced the bio fate and treat HER2-positive breast cancer. Furthermore, assessment of cellular uptake demonstrated the inhibition proliferation of cancer cells, so reported work can be considered a promising theragnostic for breast cancer.85 Lv et al synthesized a thermosensitive hydrogel using a new approach of multidrug resistance strategy, in which authors used hybrid drugs (doxorubicin and cisplatin) to investigate the tumor therapy. The thermosensitive hydrogel contained the PELG-PEG-PELG polymers as shown in Figure 6A. The reported formulation tested on the A549 and CDD persistent A549 tumor models and demonstrated that the dual drugs combinedly inhibited both models and inhibited the expression of multidrug resistance. The presented study demonstrated an excellent strategy to cure the drug-resistant tumor and an overview of the reported work is highlighted in Figure 6A,B.86
4.8 Cell-loaded drug delivery system
The world is paying close attention to cell-mediated drug delivery systems these days because of their excellent efficacy and biocompatibility. However, the techniques remain to be developed, and many researchers are investigating the appropriate techniques to apply cell-mediated drug delivery systems on living organisms to combat deadly diseases.87
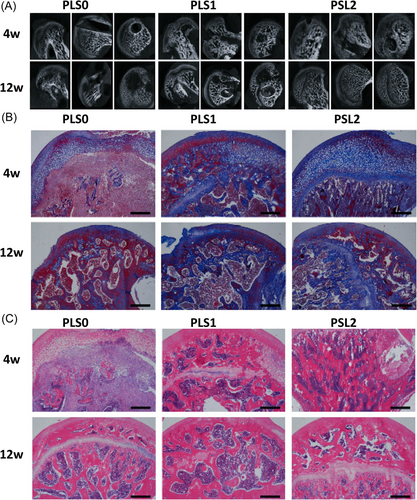
Ji et al. reported a thermosensitive hydrogel loaded with the mesenchymal stem cell to heal bone defects. They investigated the efficiency of the hydroxypropyl chitin-based thermosensitive hydrogel for mesenchymal cell delivery and the response of the immune system against the introduction of hydrogel, Lastly, they hypothesized that the hydroxypropyl chitin hydrogel effectively delivers the mesenchymal cells and enhanced the bone healing.87 Zhou et al. synthesized a poly(ε-caprolactone)–poly (ethylene glycol)–poly(ε-caprolactone) thermosensitive hydrogel loaded with the TGF- β1 cells to repair the cartilage. The thermosensitive hydrogel was subject to surface characterization, rheological testing, in vitro degradation, phase transition, and TGF- β1 releasing rates. The reported hydrogel demonstrated excellent biocompatibility and sustained drug release. Furthermore, in vivo results confirmed the cartilage repair for 8 weeks which was induced in the rat model knees. Micro-CT & histological studies confirmed the novelty and higher optimal capacity of hydrogel.88 Huang et al. synthesized a thermosensitive hydrogel loaded with super-activated platelet lysate to treat femoral head necrosis. The hydrogel was prepared from PLGA thermosensitive polymers, hydroxyapatite, and SrCl2. Moreover, hydrogel demonstrated excellent growth factor with sustained release properties and higher biocompatibility, also hydrogel was subjected to in vivo testing by inserting into the necrotic femoral head of the rat model for 4 and 12 weeks. CT micro scans, Histopathology Masson staining, and HE staining results as shown in Figure 7A–C confirmed healing and regeneration of the femoral head which were treated with reported hydrogel. Figure 7A demonstrated the micro-Ct scan images in which three groups were formed (PLS0, PLS1, and PLS2), whereas the PLS2 showed an enhanced therapeutic effect as compared to PLS0 and PLS1. Figure 7B,C highlighted the Histopathology of Masson staining, and HE is staining of femoral head necrosis after 4 and 6 weeks. The images of both staining's showed the presence of collagen I and osteogenic ALP factor in all groups, but a positive staining degree was only demonstrated by PLS implanted groups. So, this work can be potentially considered for bone regeneration and tissue engineering.89
In the above application, all are recent and high-impacted works are discussed, Further major formulated hydrogels for respective areas are summarized below in (Table 2).
| Application | Material | Drug | Targeted disease | Reference |
|---|---|---|---|---|
| Transdermal DDS | Poloxamer 407 and critical micelle concentration (CMCs) | Cortex moutan (CM) | Atopic dermatitis (AD) | [90] |
| Nano particles | Benzocaine (BZC) | Topical pain | [91] | |
| Poly(N-isopropylacrylamide), co-butyl acrylate. | Poly (N-isopropylacrylamide) (85%) | Wound management | [92] | |
| Genipin coupled with chitosan and glycerol-phosphate disodium salt | Paracetamol | Long-term healthcare monitoring | [93] | |
| PLGA-PEG-PLGA | Metformin and levofloxacin hydrochloride | Corneal neovascularization | [94] | |
| Poloxamer 407 (P407) | Naltrexone (NTX) | Skin treatment in vitro | [95] | |
| Chitosan-β-glycerophosphate | Paclitaxel | Tumor | [95] | |
| Poloxamer 407 (P407) | Silver sulfadiazine (Ag-SD) | Wound healing infection | [94, 96] | |
| Oral DDS | Pectin-based superabsorbent polymers (SAP) | Nonsteroidal anti-inflammatory drugs (NSAIDs) for example, ibuprofen | Infection | [97] |
| PECE copolymer | Cisplatin | Cell carcinoma | [98] | |
| pNIPAAM, Poloxamer, (PEG/PLGA) & chitosan | Model drug | Oral diseases | [99] | |
| N-Isopropylacrylamide (NiPAAm) | Acetaminophen and dyphylline | GI track | [100] | |
| HTCC and PEG | HTCC–PEG–GP | Nasal infection | [101] | |
| Poloxamer 407 (P407) | Pentaerythritol | Periodontitis treatment | [102] | |
| Poly (DL-lactide-co-glycolide) and Poly-caprolactone | Prednisolone | Tumor | [103] | |
| Poloxamer 407, poloxamer 188 and xanthan gum | Methyl-Cyanine 5 | Buccal diseases | [104] | |
| Intravaginal DDS | Pluronic acid | Metroni dazole | bacterial vaginosis | [105] |
| Chitosan/β-glycerophosphate (β-GP) | clotrimazole | Infection | [106] | |
| F127/(hydroxypropyl methylcellulose) HPMC | M48U1 | Anti-HIV-1 microbicide | [107] | |
| Chitosan | Polymeric micelle | Sustained-release vaginal drug | [108] | |
| Poloxamer | Carboplatin | Cervical tumor | [109] | |
| Poloxamer 407, Poloxamer 188 and Chitosan | Secnidazole (SEC) | Trichomonas vaginalis | [71] | |
| Chitosan and Poloxamer 407 | caspofungin (CSP | Vulvovaginal candidiasis | [110] | |
| Poloxamer 407 | Lactobacillus gasseri | enhance vaginal flora | [111] | |
| Ophthalmic DDS | Solid lipid nanoparticles (SLNs) | Resina draconis | Ophthalmic diseases | [112] |
| Glycol chitosan | Levofloxacin | Ocular irritation | [113] | |
| Chitosan | Latanoprost | Ocular hypertension | [114] | |
| Poloxamer 407: Poloxamer 188 | Diclofenac sodium (DS) | Corneal penetrability | [115] | |
| Poloxamer 407 and Carbopol 940 | Tea polyphenols (TPs) | Sustained ophthalmic delivery. | [116] | |
| Poloxamer 407, Chitosan and MC | l-carnosine | Corneal wounds relieve | [117] | |
| Poloxamer 407-HPMC K50M | Betaxolol hydrochloride | Glaucoma | [118] | |
| Chitosan disodium α-d-Glucose 1-phosphate (DGP) | Levocetirizine dihydrochloride | Sustained ophthalmic delivery | [119] | |
| Nasal DDS | Chitosan | Insulin | Intranasal | [120] |
| Chitosan | Ibuprofen | Neurological disorder | [121] | |
| Chitosan microspheres | NSAID | Pain therapy | [122] | |
| Thiolate polymers | Proteins | Nasal Delivery | [123] | |
| Chitosan, β‑glycerophosphate, and HPMC | Ropinirole | Dopamine agonist | [124, 125] | |
| Chitosan and glycerophosphate | Ellagic acid | Brain cancer | [125] | |
| Poloxamer 407, hydroxypropyl cellulose | Ondansetron hydrochloride | Nausea and Vomiting | [126] | |
| Poly (N‑isopropylacrylamide) | Insulin | Nasal absorption | [127] | |
| Rectal DDS | P407, PVP, HEC, HPMC | Metoclopramide | Vomiting | [128] |
| P407, Chitosan | Candesartan | Antihypertensive | [129] | |
| P407, P108, HPMC | Ondasetron | Emesis | [130] | |
| P407, PVP, Sodium Alginate, Methylcellulose | Ondasetron | Emesis | [131] | |
| P407, P108, HPMC | Promethazine | Antiallergic | [132] | |
| P407, P108, HPMC, Carbopol | Lidocaine | Hemorrhoids | [133] | |
| P407, Carbopol, PCP, PVP | Chloroquine phosphate | Antiallergic | [134] | |
| P188, Menthol | Ibuprofen | Analgesic | [135] | |
| Cancer therapy DDS | PTX-NC-PECT | Paclitaxel PTX | Breast Cancer | [136] |
| PEG-PCL-PEG | Paclitaxel PTX | Breast cancer | [137] | |
| Chitosan/Glycerophosphate | Indocyanine | tumor | [138] | |
| Chitosan/Glycerophosphate | Doxorubicin DOX | Hepatoma | [139] | |
| Poly (lactic-co-glycolic acid) PLGA | Paclitaxel PTX | Mammary tumor | [140] | |
| Hydroxyapatite/Pluronic F127 | Doxorubicin DOX/Docetaxel DOC | Colorectal carcinoma | [141] | |
| Chitosan | Disulfiram (DSF) | Cancer cells | [142, 143] | |
| PLGA-PEG-PLGA | Doxorubicin (DOX) & cyclodextrin CD- curcumin | Osteosarcoma | [143, 144] | |
| Cell loaded DDS | Pluronic and PLGA-PEG-PLGA | Salinomycin | glioblastoma | [144, 145] |
| PLGA-PEG-PLGA | Bee venom peptide | inflammation | [145] | |
| PEG-PLGA-PEG | TGF-b1 gene | Diabetic wound healing | [146] | |
| PECT copolymer | Nano micelles | Cancer | [147] | |
| PLGA–PEG–PLGA | Periodontal stem cells | Alveolar bone defects | [148] | |
| Multiple polymers | liposome | Sustained drug delivery | [149] | |
| Poloxamer 407 and Hyaluronic Acid | Ginsenoside Rg3 | Skin Wound Healing | [150] | |
| Chitosan-SA sodium alginate- α, β-glycerophosphate. | Polymeric microspheres | Vaginal drug delivery | [108, 151, 152] |
- Abbreviations: CM, cortex moutan; DOX, doxorubicin; DS, diclofenac sodium; DSF, disulfiram; PEG, polyethylene glycol; PLGA, poly (lactic-co-glycolic acid); PTX, paclitaxel, PVA, poly Vinyl Alcohol; TGF-b1, transforming growth factor-beta.
5 CONCLUSION
This review paper highlighted the most recent progress in thermosensitive polymeric materials and their physiochemical properties including swelling, mechanical stability, releasing behavior, sensitivity, and adhesion. Moreover, the potential applications including transdermal, oral, ophthalmic, intravaginal, nasal, rectal, tumor, and cell loaded are demonstrated. It was emphasized that thermosensitive hydrogels are green materials with negligible side effects and desirable drug-delivery properties. It can be used everywhere in the human body because of their sol-gel properties. It may store various hydrophilic and hydrophilic medicines in the matrix for sustained release while at body temperature. Continuous and tailored release of the bioactive component from the thermosensitive hydrogel overcomes the limitations of standard drug administration methods, making it a desirable choice for drug delivery applications. Furthermore, thermosensitive hydrogels demonstrated the promising properties and applied in many applications of life sciences and health care sector but in some perspectives these hydrogel faced limitations including a delayed temperature response, weak mechanical characteristics, and poor biocompatibility, which limits their potential use in drug delivery applications.
The future of thermosensitive hydrogel needs advanced techniques with the discovery of green materials which must be cost-effective, nontoxic, and biologically degradable Meanwhile, detailed research with sophisticated methodologies is required to properly turn the hydrogels into practical applications.
AUTHOR CONTRIBUTIONS
Bangul Khan: Conceptualization (equal); writing—original draft (equal); writing—review and editing (equal). Areesha Arbab: Writing—original draft (equal). Samiullah Khan: Writing—review and editing (equal). Hajira Fatima: Writing—original draft (equal). Isha Bibi: Writing—original draft (equal). Narinder P. Chowdhry: Supervision (equal); writing—review and editing (equal). Abdul Q. Ansari: Supervision (equal); writing—review and editing (equal). Ahsan A. Ursani: Writing—review and editing (equal). Sanjay Kumar: Writing—original draft (equal). Jawad Hussain: Writing—review and editing (equal). Saad Abdullah: Supervision (equal); writing—review and editing (equal). All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors would like to thank Health @ InnoHK (Hong Kong Centre for Cerebro-Cardiovascular Health Engineering (COCHE), Shatin, Hong Kong, SAR China, Mälardalen University Sweden, and Mehran University of Engineering and Technology Jamshoro, Pakistan, for providing conductive work environment in documentation of the data.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Four figures are supplementary figures.




