3D-printed degradable hydroxyapatite bioactive ceramics for skull regeneration
Abstract
Hydroxyapatite (HA) bioceramics have been extensively employed as bone tissue scaffolds owing to their biodegradability and osteoinductivity. In our work, HA, a significant component of natural bone tissue used as the raw material to produce porous scaffolds employing three-dimensional (3D)-printing technology. Physical and chemical properties, porosity, and compression resistance of the scaffolds were investigated in vitro. The scaffold was confirmed to have a large number of interconnected pore structures on the surface and inside HA scaffolds showed good cell compatibility and cell adhesion in cell text. To analyze the effect of the scaffold on bone repair and regeneration in vivo, the large-size defect of beagle skull was repaired with a 3D printing group and an autologous bone group (ABG) for 8 months. Images and histological analysis of the 3D printing group indicated better integration with adjacent tissues. However, there were obvious gaps in the ABG, which indicates weak bone regeneration ability of this group due to unmatched implant dimension. Immunohistochemistry and immunofluorescence results showed that 3D-printed scaffolds had a highly vascularized structure. This study indicates that 3D-printed bioceramics scaffolds that are osteoinductivity and biodegradable have great potential in maxillofacial bone regeneration.
1 INTRODUCTION
Craniomaxillofacial trauma is on the rise as a result of rising rates of car accidents and other forms of unintentional injury.1, 2 When exposed to mild trauma, bone tissue has an innate ability to heal itself. When the bone defect is too extensive, however, a transplant is necessary to restore the tissue.3, 4 Craniomaxillofacial injuries vary widely in severity and complexity.5-13 In recent years, several novel approaches and materials have been suggested for use in bone repair.14-17 Both artificial calcium phosphate ceramics and natural bone tissue are biocompatible, and both exhibit bone conductivity and osteogenic induction similarly.18, 19 Calcium phosphate ceramics are biodegradable and may decay slowly after implantation into bone defects without impacting the formation of normal bone tissue.20-22 In contrast with conventional inert implant materials. Currently, calcium phosphate ceramics have become a popular material for bone repair.23-27
Hydroxyapatite (HA), tricalcium phosphate, and other calcium phosphate composites are different types of calcium phosphate minerals. HA is the most popular researched material for bone regeneration. When it comes to thermodynamic stability, HA is the calcium phosphate crystalline phase with the greatest longevity. Besides other calcium phosphate components, HA most closely resembles the inorganic component of human bone tissues.28 To stimulate bone repair, implanted materials may lead mesenchymal stem cells and osteoblast precursors from the surrounding connective tissue to differentiate into osteoblasts.29 Bone-inducing materials implanted into bone tissue may hasten bone healing and reduce repair time. HA ceramics were used to prepare bone-induced implants with degradability and osteogenic inducibility.30 Bone induction implants and their most salient characteristics as described in the current literature were studied. Firstly, the implant should have a biomimetic material composition. The fundamental component should mimic natural bone tissue. The calcium and phosphorus ions found in HA ceramics are crucial for the induction of bone tissue repair.20 Secondly, the implant should be designed with biomimetic trabecular-like micropores. In this study, scaffolds with multilevel porous structures were shown to improve nutrient transfer and stimulate bone and tissue regeneration. The optimal pore size for bone tissue development is between 300 and 800 μm, thus researchers should aim for this range while creating the macropore. After the scaffold has been implanted into the faulty tissue, blood should be able to permeate the material more easily via the micropore design, which should be less than 10 μm.31, 32 After all these steps, the implant interface should be touching the patient's own tissue. As new bone forms, it is crucial that the regenerating material stays in close contact with the autologous tissue. This may prevent bone resorption by stimulating the material under stress, allowing bone to develop. Bone tissue is better able to develop into the implant because the new tissue is more strongly connected with the autologous tissue.33-36
It is still a challenge to prepare HA ceramics with accurately porous structures. Many technologies, including gas foaming, phase separation method and freeze casting were proposed to prepare the accurate porosity and pore size of scaffolds, however, none of these methods are ideal for the preparation of biomimetic porous bone scaffolds.37, 38 In recent years, with the development of manufacturing technologies, three-dimensional (3D) printing have been introduced to prepare accurately porous structures in the bone scaffold.39, 40 3D printing involves many technologies, including extrusion molding, light curing molding, and so forth. In extrusion molding, the print nozzle is guided by a computer along a predetermined route to fabricate components.41, 42 The technology can create complex, custom-made porous structures. The conventional bone graft cannot accurately match the defect site of the patient. Intraoperative manipulation of prostheses can prolong the operation time, increase the risk of surgical failure, and ultimately lead to unsatisfactory surgical results. In contrast, the use of computer-aided design (CAD) and personalized customization 3D printing has been shown to more closely mimic the form of a patient's lesion, save surgical time, and provide significantly better clinical outcomes.43, 44 In the preparation of the HA implant, CAD was used to design macropores of the HA scaffold. The pore size was adjusted within the appropriate range of bone tissue growth. At the same time, sacrificial materials are introduced into 3D printing ink. In the sintering process of the scaffold, secondary pores are produced by removing sacrificial materials. The size of secondary pores is controlled by adjusting the sintering temperature.45, 46
In this study, HA ceramic scaffolds with multilayer pore structures were precisely prepared using 3D printing technology, according to the key factors of bone-induced scaffolds. The size of secondary micropores in the scaffold was precisely controlled by a two-step sintering method. Then, the effects of scaffolds on cell activity and cell adhesion were studied. Finally, bone-induced biodegradable ceramic was used to repair the beagle skull to investigate the effect of bone-induced materials on osteogenesis and vascularization expression associated with the long-term repair of large bone defects.
2 RESULTS
2.1 Characterization of 3D-printed HA scaffolds
The 3D-printed HA scaffolds showed multilevel porous structures. The multilevel pore characterization of 3D-printed scaffolds (3DP) is shown in Figure 1. The orthogonal HA scaffold was prepared by continuous extrusion. The 3D porous structure can provide mechanical support and biological properties. The size of the pores in the scaffolds was designed by modeling software. Macropores of the scaffolds were designed to be 400 μm according to bone tissue ingrowth conditions. The porosity of the scaffolds is related to the pore size. And the porosity of the 400 μm scaffolds is about 65%. After the preparation of the scaffold, the two-step sintering method was adopted. The scaffold was held at 600°C for 10 h to remove organic polymers and held at 1150°C for 2 h to grow grains for sufficient mechanical support and forming secondary micropores on the surface. The optical and micrograph of macropores of sintered scaffold showed that the scaffold has an orthogonal pore structure (Figure 1A–C). The sintering shrinkage rate was about 10%. After sintering, lots of secondary micropores are generated on the surface of the scaffold, as shown in Figure 1D–F. The size of these micropores was around 1–5 μm. Micropores could be observed in the enlarged view of the truncated surface of inter strut of the scaffold (Figure 1G–I).
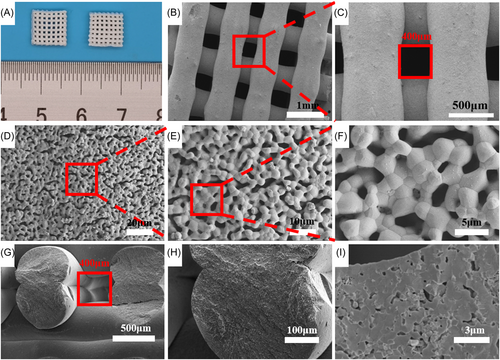
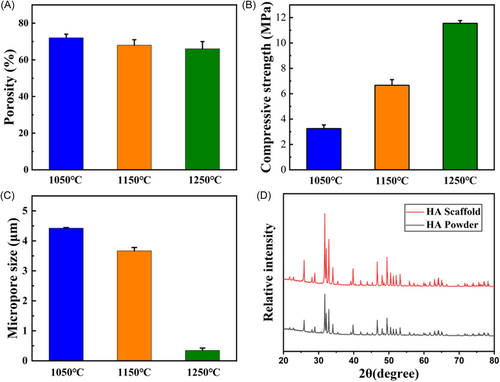
The mechanical properties and porosity of the scaffold were changed at different sintering temperatures. At sintering temperatures of 1050°C, 1150°C, and 1250°C, the porosity of the scaffolds decreased gradually (Figure 2A). With the decrease of porosity, the mechanical properties of the scaffold will increase gradually (Figure 2B). The size of secondary micropores on the surface of the scaffold decreased with the increase of sintering temperature (Figure 2C). And there were almost no secondary micropores on the surface of the scaffold at 1250°C. The composition and crystal phase of 3D-printed HA scaffolds were further analyzed by X-ray diffraction (XRD; Figure 2D). First, the XRD peaks of HA powder were 31.8°, 32.9°, and 32.2°, corresponding to (211), (300), and (112) of HA (JCPDS 09-0432), respectively. Then, we compared the test results of the scaffolds with the diffraction peaks of HA powder. The result showed that the main diffraction peaks matched the HA powder. The main component of the scaffold was HA.
2.2 In vitro assessment of HA scaffolds
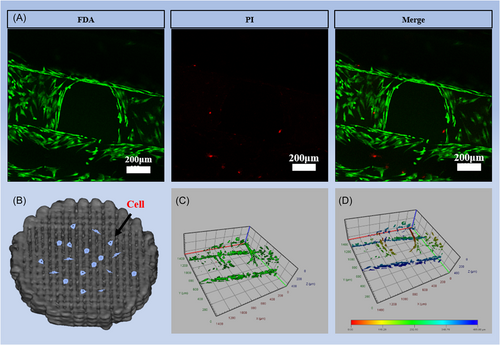
The biocompatibility of HA scaffolds was tested in vitro using MC-3T3 cells. After the MC-3T3 cells were implanted on HA scaffolds for 4 days (Figure 3B), the scaffolds were stained with fluorescein diacetate (FDA) and propidium iodide (PI). The staining results showed that the cells adhered well to the scaffold. A small number of dead cells were found on the scaffold, which belongs to normal cell growth metabolic behavior, reflecting the good biocompatibility of the bioceramic scaffold (Figure 3A). In the visualization of cell staining, cells grew uniformly along the outline of the scaffold (Figure 3C,D).
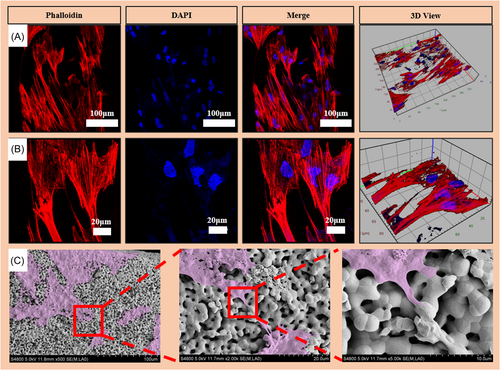
The nucleus and cytoskeleton were stained with 4′,6-diamidino-2-phenylindole (DAPI) and phalloidin, respectively. The cytoskeleton is clearly red and the nucleus is blue (Figure 4). The results showed that the cells adhered well on the scaffold, and the cell pseudopods had multiple binding sites on the surface of the material (Figure 4A,B). The cells on the scaffold surface were observed by spectroscopic electron microscopy (SEM) after gradient dehydration and critical point drying. The SEM images of scaffolds were in accordance with the corresponding fluorescence images (Figure 4C). The cells can be seen growing pseudopodium (purple) at high resolution.
2.3 Angiography assessment of new vessel formation in scaffolds
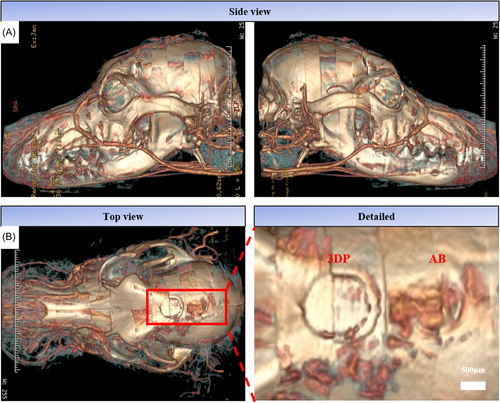
After 2 months of implantation, the angiogenesis at the defect site was detected by microcontrast agent perfusion (Figure 5). The reconstructed 3D model showed many new blood vessels around the 3DP and the autogenous bone material (AB).
2.4 In vivo assessment of HA scaffolds
X-rays were used to analyze beagles bone tissue integration at 2, 5, and 8 months as shown in Figure 6A–C. At 2 months, there was an obvious interface between the material and the bone tissue in the 3D-printed groups. The bone tissue was not integrated obviously. The gap between the autogenous bone group material and the host bone tissue was small. The overall combination was good. At 5 and 8 months, the interface between the scaffold and the bone tissue in the 3D printing group was blurred. And the bone tissue was well fused. However, there was a large gap and obvious boundary between AB and host bone tissue.
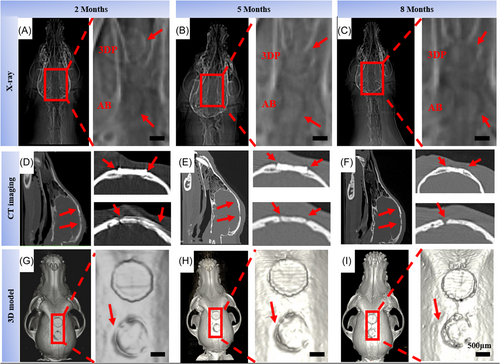
The bone regeneration was further analyzed, combining the vivo computed tomography (CT) imaging of 2, 5, and 8 months. Screenshots of the CT scan results showed large holes in the autogenous bone group over time (Figure 6D–F). However, there were no obvious defects in the 3D printing group indicating the material had a good bone integration effect over time. In the 3D reconstruction model, a large gap between AB and host bone tissue was found at 8 months (Figure 6G–I). The X-ray results were consistent with the CT in vivo, the large holes appeared in the autologous group, while the 3D printing group maintained good integration over time.
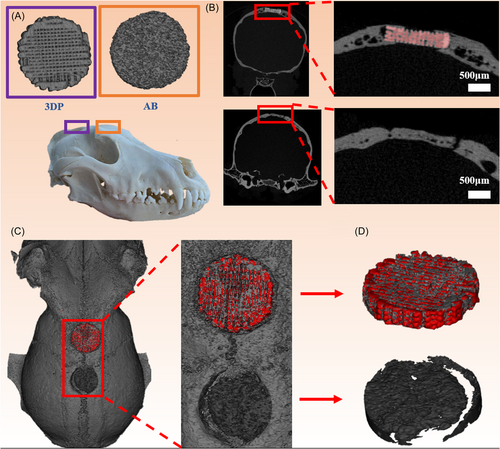
The samples of beagles were taken for further analysis at 8 months. Micro-CT was used to analyze the new bone tissue in all groups (Figure 7A). The threshold for bone (gray) was 1500–6000. And the threshold for scaffold (red) was 6000–10,000. In the 3D-printed group, bone tissue grew along the scaffold from the edge toward the inside. In the autogenous group, there is a clear boundary between the material and the host bone tissue (Figure 7B). The 3D reconstruction model showed that the bone tissue (gray) had grown into the 3DP (red). In addition, there were large holes around the material of the autogenous bone group (Figure 7C,D). The HA scaffold served as the bridge and provided a suitable environment for the new bone tissue crawling to the middle, which could prevent loosening in animal movement. Whereas, since the autologous bone (AB) was difficult to customize, it formed an obvious gap with the host bone after implantation and then caused bone absorption.
2.5 Histological assessment of HA scaffolds
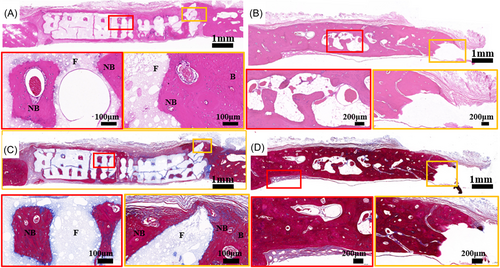
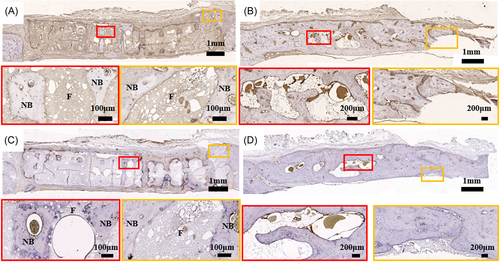
Hematoxylin and eosin (H&E) and Masson's trichrome staining were used to analyze the new bone of HA scaffolds and the bone resorption of AB (Figure 8). The HA scaffolds stimulated extensive bone regeneration at 8 months. The new bone tissue grown along the pore wall of the HA scaffold, and is tightly attached to the scaffold surface. The pores of the scaffold were also filled with bone tissue and blood vessels as shown in the enlarged image of H&E staining (Figure 8A). In the autogenous bone group at 8 months, there were many lacunae in the materials and large defects in the interface between the material and the host bone tissue (Figure 8B). In Masson trichrome staining, the new bone tissue was dyed red, indicating that it is mature bone (Figure 8C). In the autogenous bone group, fibrous tissue appeared at the junction of materials and host bone tissue. Many lacunae tissue appeared in materials, resulting in decreased bone density and mass (Figure 8D).
Immunohistochemical (IHC) staining was used to analyze the expression of osteogenesis-related proteins (Figure 9). In the osteocalcin (OCN) staining results, new bone tissue inside the scaffold was positive in the 3D printing group (Figure 9A). It can be found that OCN-positive expression was better in autologous bone group (Figure 9B). Bone morphogenetic protein-2 (BMP-2) staining showed positive expression of new bone tissue in the scaffold (Figure 9C,D).
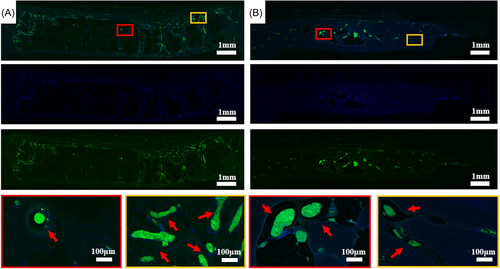
Immunofluorescent staining was used to analyze angiogenesis in the tissue (Figure 10). Staining results showed that new blood vessels appeared at the interface between the scaffold and bone tissue. Many clusters of differentiation 31 (CD31)-positive tissues could be found in the inner pores of the scaffold (Figure 10A). Many large vessels with positive expression can also be found in the AB group (Figure 10B).
3 DISCUSSION
Cranial and maxillofacial bone defect repair is usually complicated and needs to be customized. At present, a wide variety of artificial substitute materials are used in skull regeneration surgery,47-49 including bioactive glass, titanium (Ti), polyether ether ketone, and polymethylmethacrylate, and so forth. However, none of these traditional biomaterials are satisfactory. For example, Ti, which is the most commonly used repair material, may produce significant image artifacts in CT. In addition, the mechanical properties of Ti are higher than those of natural human bone, resulting in severe deformation or atrophy of the skull several years after implantation.50 Most biomaterials used in clinical practice are bioinert or nonbiodegradable, which means that these materials can neither induce bone regeneration nor be replaced by new bone tissue. As a result, the osseointegration between the cranioplasty material and the surrounding skull is poor. This study proposed a personalized skull repair method using 3D printing technology to prepare porous structure bone-induced HA ceramic scaffold.
In this study, 60% of the printing ink was prepared with HA powder. HA ceramics have good bone inducibility and degradability,21 which can continuously degrade and release calcium and phosphorus ions in vivo. The calcium and phosphorus ions released help rebuild bones. The process of bone regeneration requires the assistance of a multilayer pore structure. In this study, the macropores of scaffolds were prepared by combining computer-aided modeling and 3D printing. And the pore size was set to 400 μm according to the requirement of bone growth. In ceramic fabrication, defatting and sintering are relatively independent processes. Increasing the temperature directly to the sintering temperature will lead to the rapid decomposition of organic matter and produce a large number of gases, which will irreversibly damage the ceramic embryo body. Therefore, we chose two sintering methods in this study, first, slow defatting was carried out at 600°C, and then the ceramic embryo body was sintered at 1150°. Two-step sintering method was used for the 3D printing of scaffolds. The first step was sintering for degreasing, which was heated to 600°C in a muffle furnace and held for 10 h. Sacrificial materials in 3D printing ink were removed, and the remaining pure HA scaffolds contained a large number of internal pores. The second step is sintering for grain growth, holding at 1050°C, 1150°C, and 1250°C for 2 h. SEM pictures demonstrated that an increase in the final sintering temperature led to a larger grain size of HA and a smaller size and fewer secondary micropores. The formation of micropores is dependent on the sintering procedure. In the first phase of sintering, the scaffold was cleaned of excess oil. A significant number of pores are left in the scaffold when the glue is removed as a sacrificial material. The residual ceramic grains eventually melt as the temperature rises. The porosity of a ceramic decreases gradually as its density increases. The sintering temperature controls the size of the micropores. The growth between the grains could not fuse at 1050°C and 1150°C, and there were many holes in the scaffold. Grain fusion and a significant reduction in internal porosity occur at 1250°C. Scaffolds sintered at three distinct temperatures were examined for their compression characteristics and porosity. As the sintering temperature was raised, the mechanical characteristics of the scaffolds improved while the porosity was reduced. Due to the low force of implants, the biological activity of scaffolds is prioritized above their mechanical properties in craniomaxillofacial defect repair. For this purpose, the sintering at 1150°C scaffold was used in further characterization experiments. Firstly, SEM was used to analyze the sintered scaffold's morphology. Images taken at a low magnification level confirmed that the sintered scaffold maintained its intended architecture and pore size. The high-magnification picture indicated that there were a substantial number of secondary micropores on the surface of the scaffold. The micropores' sizes varied from 1 to 5 m, and they were scattered uniformly. The scaffold has micropores in the cross-section as well. Micropores improve the surface roughness of scaffolds and stimulate cell attachment. The capillary effect allows the scaffold to take in oxygen, nutrients, and proteins from the body through these pores. In the scaffold, the optimal conditions were created for the development of bone-forming cells called osteoblasts. After being sintered, the scaffold was ground into powder and XRD was used to compare the raw components of the powder to those of the HA powder. As can be seen in Figure 2D, the findings confirmed that the sintered scaffold was composed entirely of HA. Lastly, the sintering temperature effect on the mechanical properties and porosity of scaffolds was measured, and it was shown that when the sintering temperature was raised, the mechanical qualities improved while the porosity decreased. Scaffolds with less porosity are less effective biologically, according to the researchers.51 When it comes to fixing cracks in skulls, biological activity is more crucial than mechanical qualities. For this reason, the scaffolds sintered at 1150°C were selected for further cell and animal tests due to their optimal combination of biological activity and mechanical qualities.
Bone remodeling relies on cells adhering to and proliferating on the surface of the substance. Staining for cell viability indicated that cells proliferated effectively over the scaffold's surface, confirming the HA scaffolds have high biocompatibility. Cell adhesion was analyzed using actin staining. The staining analysis revealed typical cell morphology, including a pristine nucleus and an extensively expanded cytoskeleton. On the scaffold's surface, more healthy cells were found. After a period of gradient dehydration and critical point drying, the morphology of cells on the scaffold surface was examined using SEM images which showed that the cells successfully attached to the scaffold surface. Cells were able to adhere to the scaffold because of its numerous secondary micropores. Based on our findings, 3D-printed HA scaffolds may be noncytotoxic and useful for boosting cell adhesion and proliferation.
In clinical bone grafting, AB is the preferred material. To test the efficacy of in situ repair using HA scaffold and AB prosthesis, we developed a defect model in a beagle skull. Two months after implantation, the scaffold's influence on angiogenesis was examined using microcontrast perfusion. CT reconstruction showed many vascular regenerations around the defect in both the 3D-printed HA scaffold group and the autogenous bone group. These results indicated the potential of 3DPs to get a blood supply and nutrients during the early stages of healing, paving the way for tissue regeneration and growth later. The beagles were scanned using X-ray and in vivo CT at 2, 5, and 8 months postimplantation. X-rays revealed that there were fissures inside the autogenous bone cluster, and those fissures grew with time. Both the CT cross-section and CT reconstruction model revealed sizable holes between the AB and the matrix bone tissue. The 3D printing group combined well with the matrix bone tissue. Eight months after implantation, samples were taken for micro-CT detection. The CT cross-section and reconstruction model showed that new bone tissue from the 3D-printed group crawled into and fused through pores on the surface and inside the scaffold. The inside and edge of the 3D printing group and the AB group were selected as representatives for observation. New bone tissue was seen in the inside pores of the 3DPs when we stained beagle skull slices with H&E and Masson staining. In the autogenous bone group, there were large pores between the autogenous bone prosthesis and host bone tissue. BMP-2 and OCN are important indicators of the functional expression of bone tissue. The IHC staining showed that the BMP-2 and OCN in the internal bone tissue of the AB group were similar to the native bone tissue. And there were also positive expressions in 3D printing which was weaker than that of the AB group. This indicated that 3DPs had the ability of osteoinduction, but the effect was weaker than that of autogenous bone. Furthermore, the evaluation of bone regeneration was also greatly aided by angiogenesis. Numerous tissues with positive expression of CD31 in the interior pore of the scaffold were seen using CD31 and DAPI staining, showing that many angiogenesis occurred in the 3D printing group, while a considerable number of angiogenesis were also observed in the autogenous bone group.
The findings demonstrated that the AB tissue did not adhere well to the matrix in the irregular shape defect. Bone resorption may occur if the autogenous bone prosthesis becomes loose after extensive healing. The 3D printing subgroup successfully integrates with the patient's natural bone tissue and can adjust to the defect's accurate dimensions. In addition, the porous internal structure is conducive to the growth of bone tissue, and the new bone tissue can better strengthen the combination of scaffold and host bone tissue.
Although the 3D-printed degradable HA ceramic scaffold is effective for cranial and maxillofacial repair, its poor mechanical qualities and excessive brittleness limit the repair of load-bearing bone defects. As a result, improving the compressive and flexural properties of ceramic materials by integrating a third phase inside the ceramic material or sintering the ceramic at ultrahigh temperatures to make it dense inside may be the key to solving the clinical transformation of 3D-printed HA ceramics in the future.
4 CONCLUSION
In this study, a biodegradable porous HA scaffold with bone inductivity was prepared by 3D printing technology. The scaffold has a large number of interconnected secondary micropores. After 8 months, a 15-mm diameter beagle skull defect was repaired using this scaffold. The 3D printing specimens group was restored well. Immunohistochemistry staining and immunofluorescence (IFC) showed that the new bones grew into the scaffold and which was similar to the natural bone tissue. The degradation rates of the scaffolds can be controlled by adjusting the composition of the printing ink to match the regenerative progress of the skull. These results indicated that 3D-printed bioceramics scaffolds have great potential in maxillofacial bone regeneration.
5 MATERIALS AND METHODS
5.1 Materials
HA powders were supplied from the Engineering Research Center for Biomaterials at Sichuan University. The diameter of these HA powders was around 50 μm. Polyvinyl butyral (PVB, Mn = 70,000–90,000) was purchased from Sigma. The PVB solution was prepared by mixing 3 g PVB with 30 mL ethanol. The printing ink was prepared by mixing HA powders (60%) and PVB (40%) solution. The ink was stirred in an open environment to maintain powder distribution well before printing.
5.2 3D printing of HA scaffolds
The scaffold model was designed with 3D modeling software (Solidworks, Dassault Systemes). The model was set to STL format. The thickness of the printed layer, the printing speed, and the filling gap were set by the slicing software (simplified 3D). The diameter of the 3D printer nozzle was 400 μm, the printing speed was set at 8 mm s−1, the printing layer thickness was 320 μm and the filling gaps were 1.0–1.2 mm. Then, the HA scaffolds (3DS) were prepared by an inkjet 3D printing device (ALPHA-BP11; Beijing Sunp Biotech).
5.3 Sintering
The HA scaffold green bodies were sintered in muffle furnace. Sintering was a two-step process. Firstly, specimens were heated to 600°C with a rising speed of 5°C/min and hold for 10 h to evaporate the polymer. Then, specimens were individually heated to 1050°C, 1150°C, and 1250°C with the same heating rate and hold for 2 h. Finally, specimens were cooled down to room temperature in the furnace. The shrinkage after sintering was measured by Vernier Caliper.
5.4 Characterization of hierarchical porous HA scaffolds
The morphology of the printed scaffolds was visualized using scanning electron microscope (SEM; JSE-5900LV). The scaffolds were sputter-coated with gold before observation. The mechanical properties of the printed specimens were tested with an electronic universal testing machine (AGS-500NX; Shimadzu). Standard test specimens that have different sintering temperatures were fabricated as a cylinder with Ф5 × 7.5 mm. The force was loaded along the vertical direction of the pores. The stress–strain curve of the scaffolds was performed at a crosshead speed of 1 mm/min. And the compressive strength was calculated according to the stress–strain curve. The phase composition of the scaffolds was analyzed using XRD (Philips X'Pert1Xray diffractometer).
5.5 In vitro cell behavior assay
5.5.1 Cell culture and seeding
The mouse embryo osteoblast precursor (MC3T3-E1) cells were cultured in 10 mL of α-minimum essential medium (Gibco) containing 10% fetal bovine serum (Gibco) and 1% antibiotic (purchased from Gibco) streptomycin at 37°C in 5% CO2. Cells were seeded on the scaffold with a density of 1 × 105 cells/mL in 24-well plates, while the medium was replaced every 48 h.
5.5.2 Cell viability
After 4 days of culture, the cell on HA scaffolds viability was detected by fluorescent dye. The dye contains 0.1% FDA (Sigma) of phosphate-buffered saline (PBS) solution and 0.1% PI (Sigma) of PBS solution. The results of cell death and live staining were observed by laser confocal microscopy (Carl Zeiss) The FDA stained the living cells in green and PI stained the dead cells in red.
5.5.3 Cell adhesion
After 4 days of culture, the cell on HA scaffolds adhesion was detected by fluorescent dye. The dye contains 5% rhodamine−phalloidin (Invitrogen) and DAPI (Invitrogen). The cytoskeleton was stained with phalloidin (red), and the nucleus was stained with DAPI (blue).
The samples were fixed with 4% paraformaldehyde for 2 h at room temperature and washed with PBS three times. Then, the scaffolds were dehydrated with an ethanol gradient (45%, 55%, 65%, 75%, 85%, 95%, and 100%) and supercritical drying. Finally, the cells on the scaffold were observed by SEM (JSE-5900LV).
5.6 In vivo experiments
5.6.1 Animal surgery
Beagles were obtained from the Sichuan University Laboratory Animal Center (body weight: 10–11 kg; gender: male). The beagles underwent surgery under anesthesia by intraperitoneally injecting 0.02 g/mL pentobarbital sodium before experimentation (40 mg/kg body weight; Sigma Chemical). After the beagles were anaesthetized, two Φ15 mm circular defects were created on each beagle's skull. The bone tissue from one defect was used as AB, and the other one defect used the 3DP. The 60% HA solid scaffold with a diameter of 15 mm and height of 3 mm was sintered at 1150°. At least triple parallel specimens did the mechanical test for optimal selection before animal model implantation The experiment consisted of two groups of samples: one was implanted with HA scaffolds and the other was AB. The weight of the experimental beagles was measured before each implantation. After surgery, the beagles were continuously injected with penicillin at 105 U/kg/day for 3 days to prevent infection. The specimens were harvested after 8 months of implantation. All the experiments were approved by the Animal Care and Use Committee of Sichuan University (Approval No. KS2021544).
5.6.2 Angiography analysis
Contrast-enhanced angiography was performed at 2 months after surgery to assess vascularization within the bone defects. After the beagles were anaesthetized, contrast medium was injected into the vessel and a CT scan was performed in vivo.
5.6.3 Imaging analysis
X-ray film and small animal in-vivo micro-CT was used to assess the repair of beagle skull at 2, 5, and 8 months point. The animals were anesthetized by intraperitoneally injecting 0.02 g/mL pentobarbital sodium before the examination (40 mg/kg body weight; Sigma Chemical). 3D data of the surgical area of the skull were obtained by CT. Bone tissue analysis was performed using medical image control system (Mimics, Materialise).
5.6.4 Micro-CT analysis
High-resolution micro-CT imaging (Optima CT680; GE Medical) was used to assess new bone formation within the micro/nano hierarchical porous HA scaffolds and ABs. Samples were taken at 8 months after surgery. The implants were fixed in 4% buffered formaldehyde before CT. The micro-CT results were analyzed using a medical image control system (Mimics, Materialise). The test conditions of micro-CT were as follows: layer thickness of 22 μm and medium resolution. The threshold for bone (gray) was 1500–6000. And the threshold for scaffold (red) was 6000–10,000.
5.6.5 Histology analysis
The implanted specimens were extracted from the surrounding tissues and washed with PBS three times. The specimens were decalcified using 10% ethylenediaminetetraacetic acid solution (pH 7.4). Then the samples were processed with paraffin for sections. The section's thickness was about 5 μm. Next, the sections were processed with H&E staining and Masson's trichrome staining. Sections were dewaxed three times with xylene for 15 min. Then the sections were treated with 100% ethanol, 95% ethanol, 85% ethanol, 75% ethanol, and 50% ethanol for 5 min each. Next, the specimen sections were cleaned with deionized water twice for 3 min each. The H&E staining and Masson staining were used by Solarbio Kit.
For IHC analysis, BMP-2 and OCN secreted by cells were evaluated using IHC staining. The sections were cleaned with deionized water, and the stained areas were circled with an oil marker to prevent leakage of the dye. Protease K was added to repair the antigen and incubated at 37°C in the dark for 10 min. The sections were washed with TBS-T (50 mL TBS with 50 μL 10% Tween 20) twice, and then were blocked with goat serum (10%) for 30 min before incubation with antibody overnight at 4°C. Finally using Polink-2 plus Polymer HRP Detection System and Rabbit Specific HRP-DAB (ABC) to detect with IHC kit. The sections were observed by an Olympus BX60 microscope equipped.
For IFC analysis, CD31/DAPI in the tissue was evaluated using IFC staining. The basic procedure was similar to IHC. The stained sections were observed using a Pannoramic MIDI pathological section scanner (3DHISTECH Ltd.). All antibodies were diluted 1:100 with antibody thinners.
5.7 Statistical analysis
Statistical analysis was conducted with GraphPad Prism Software (GraphPad Software Inc.), and all the numerical results are presented as mean ± standard deviation. Three independent experiments were performed for mechanical properties studies, and three samples were analyzed per group in each test. A value of p < 0.05 was accepted as statistically significant.
AUTHOR CONTRIBUTIONS
Xingyu Gui: Conceptualization (equal); data curation (equal); methodology (equal); writing—review and editing (equal). Boqing Zhang: Software (equal); writing—original draft (equal). Zixuan Su: Supervision (equal); visualization (equal). Zhigang Zhou: Validation (equal). Zhihong Dong: Writing—review and editing (equal). Pin Feng: Validation (equal). Chen Fan: Visualization (equal). Ming Liu: Resources (supporting). Qingquan Kong: Formal analysis (equal). Changchun Zhou: Supervision (equal); writing—review and editing (equal). Yujiang Fan: Project administration (equal); writing—review and editing (equal). Xingdong Zhang: Project administration (equal).
ACKNOWLEDGMENTS
This work was partially supported by the National Natural Science Foundation of China (Grant Nos. 81171731 and 31971251). Sichuan Science and Technology Program (Grant Nos. 2022YFG0066, 2023YFQ0053). Science and Technology Project of Tibet Autonomous Region (XZ201901-GB-08, XZ202202YD0013C), and the 1·3·5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYGD21001, ZYJC21026, ZYJC21077). Some icons in Figures 3 and 5 were created with BioRender.com.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
All the experiments were approved by the Animal Care and Use Committee of Sichuan University (Approval No. KS2021544).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




