Biomimetic photosynthetic system: Shedding light on the restoration of cell metabolism
Graphical Abstract
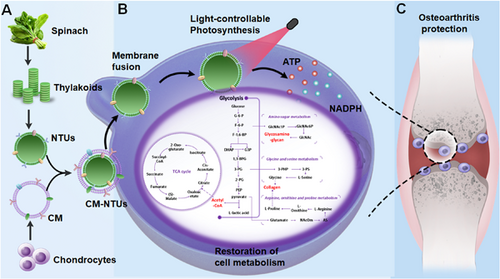
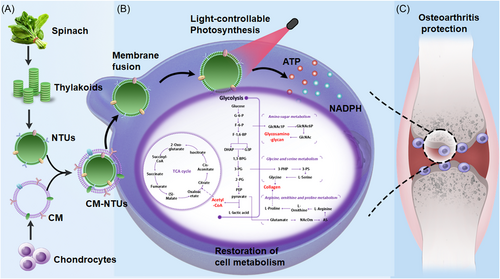
Specific mature cell membrane camouflaging facilitated the internalization of nanothylakoids through homologous targeting and membrane fusion. Under red light irradiation, the biomimetic nanothylakoids (cell membrane-camouflaged nanoparticulate thylakoids, CM-NTUs) would provide sufficient ATP and increase nicotinamide adenine dinucleotide phosphate (NADPH) to correct energy imbalance to improve metabolic homeostasis through enhancing anabolism in degenerated cells. Consequently, CM-NTUs promoted cartilage homeostasis and remarkedly ameliorated osteoarthritis progression in animals, offering therapeutic avenues for various degenerative diseases.
Chen et al.,1 in a paper published in Nature, leveraged the capacity of nanoparticulate thylakoids to restore cell metabolism after exposure to light. Specific mature cell membrane camouflaging facilitated the internalization of nanothylakoids through homologous targeting and membrane fusion (Figure 1A). Under red light irradiation, the biomimetic nanothylakoids (cell membrane-camouflaged nanoparticulate thylakoids, CM-NTUs) would provide sufficient ATP and increase nicotinamide adenine dinucleotide phosphate (NADPH) to correct energy imbalance to improve metabolic homeostasis through enhancing anabolism in degenerated cells (Figure 1B). Consequently, CM-NTUs promoted cartilage homeostasis and remarkedly ameliorated osteoarthritis progression in animals (Figure 1C), offering therapeutic avenues for various degenerative diseases.
Two crucial components of any living organism were considered energy generation and substance metabolism, together power their internal machinery. Restoration of cellular metabolism holds great promise to treat various degenerative diseases. In this context, the rectification of dysregulated supply of intracellular energy and reducing power under pathological conditions might offer new therapeutic options. Although ATP and NADPH were key energy carriers and reducing power for cell anabolism, respectively, a direct supplement of exogenous metabolites by biomaterials failed to regulate cellular metabolism due to the uncontrolled delivery, expensive cost, and severe adverse effects. Thus, delivering key metabolites to degenerated cells in a controllable, indirect, and precise fashion to achieve efficient and safe therapeutic outcomes, remains a great challenge.
Nature brings evolutionary advances for designing artificial systems that can be sophisticatedly applied to science, engineering, and technology. The limitations of synthetic materials in metabolite delivery motivated scientists to seek inspiration from harnessing natural biosystems to produce active metabolites for fruitful directions.2 Thylakoids were compartments in green plants and bacteria where the light reaction of photosynthesis took place. In such a scenario, sunlight is employed only for the splitting of water into oxygen through a redox reaction, to produce a large amount of ATP and NADPH for further light-independent fixation of carbon dioxide into carbohydrates.3 Despite potent success of transplanting thylakoids or their fragments to mammalian cells for photocatalysis-based therapeutics,4 the possibility of employing thylakoid-derived biomaterials that could produce ATP and NADPH from light to reshape the cellular metabolism was very alluring yet remained to unrealize.
Chen et al. proposed the use of nanoparticulate thylakoids as a light-activatable “energy bar” for restoring metabolic homeostasis in degenerated cells. Nanoparticulate thylakoids (NTUs) were isolated from spinach (Spinacia oleracea), including 77 proteins closely related to photosynthetic membranes. Natural NTUs components were mainly involved in ATP metabolism and NADPH regeneration further catalyzed the production of ATP from ADP and the reduction of NADP+ to NADPH after exposure to light. To improve the stability and delivery efficacy, NTUs were then encapsulated within cell membrane-derived vesicles to form biomimetic core-shell nanosystems, termed by CM-NTUs. Importantly, CM-NTUs were more efficiently internalized by homologous cells compared with NTUs, lipid-camouflaged NTUs, and NTUs coated with other membranes. Similar to the invasion of the enveloped viruses, biomimetic NTUs entered cells predominantly through a membrane fusion mechanism rather than cellular endocytosis to avoid further lysosome degradation. Additionally, some internalized NTUs were secreted out again for facilitating active penetration, which was confirmed by the “infection” process between neighboring cells.
After optimizing light intensity (80 µmol photons m−2 s−1), irradiation time (30 min), and encapsulated ferredoxin concentration (25 µM), the photosynthetic apparatus of CM-NTUs was stable and gradually increased ATP and NADPH in the first 8 h, but not excessed reactive oxygen species (ROS) in interleukin-1β-challenged chondrocytes upon illumination. Correspondingly, the addition of CM-NTUs restored the balance of intracellular ATP/ADP after irradiation and promoted the expression of SIRT-1, PGC-1α, TFAM, NRF1, and NRF2, which represented improved mitochondrial biogenesis and corrected energy metabolism. To explore the versatile possibility of light-controllable photosynthetic nanosystems, various membrane-coated NTUs enhanced cell anabolism in the corresponding degenerative cells (muscle cells, nucleus pulposus cells, and endothelial cells) after exposure to light. To expand such a specific strategy toward the homologous cell membrane coating method, authors have facilitated the cross-species transplantation of biologically active systems in a versatile and efficient way.
Beyond improving cell anabolism, the integration of transcriptomics and metabolomics data demonstrated that CM-NTUs reprogrammed cell metabolism, which was illustrated by the balance of energy (e.g., glycolysis, tricarboxylic acid cycle, and oxidative phosphorylation) generation and substance (e.g., collagen and glycosaminoglycan) metabolism in degenerated chondrocytes after illumination. Encouraged by these in vitro findings, intra-articular injection of the CM-NTUs and subsequent exposure to red-light irradiation with high penetration of knee joint cavity have greatly suppressed the progression of surgery-induced osteoarthritis in young mice, particularly performing substantial amelioration on aged mice. The outperforming therapeutic effect was further confirmed by increased production of ATP and NADPH, decreased ROS level, and promoted cartilage homeostasis. Notably, CM-NTUs alleviated the right hind limb and reduced osteoarthritis pain in the surgery-challenged mice with good biosafety profiles following exposure to light. Collectively, the robust and safe protection against the pathological progression of osteoarthritis was contributed by biomimetic photosynthetic nanosystems, which provided specific advantages for the treatment of various degenerative or mitochondrial-related diseases.
Potential future challenges in this area included the immunogenicity that was essential to maintain bioactivity. Employing biosynthetic technologies to edit and reconstruct existing photosynthetic nano-thylakoids would create sophisticated and stable systems with maximal biofunction and minimal immunogenicity. Additionally, coupling this photosynthetic nanosystem with existing active pharmaceutical ingredients, including nonsteroidal anti-inflammatory drugs and glucocorticoids, would be a step toward a multifunctional platform powered by integrating the restoration of cell metabolism and regulating inflammation. Using microfluidics or flash nanocomplexation methods would enable cell membrane-coating manufacturing in a simple, effective, reproducible, and scalable manner,5 resulting in a better therapeutic performance of biomimetic nanosystems. Compared to a red light or the near-infrared (NIR)-I region, light in the NIR-II region had much less scattering within biological tissues and dramatically improved penetration depth, allowing two-photon NIR-II fluorescence excitation to activate photosynthetic nanosystem for treating degenerative diseases in the central nervous system.
Chen et al. demonstrated a major advancement in biomedical engineering and a crucial milestone toward the application of a biomimetic photosynthetic nanosystem for reprogramming cell metabolism. The ability to convert light energy to sufficient energy carriers and reducing powers created an essential foundation for the transplantation of photosynthetic systems that will find use in many other areas, from active ingredients synthesis in situ to the development of a multifunctional platform for combined modulation of degenerative diseases. As we are gaining the ability to rethink and redesign photosynthetic systems in more depth, there would be many great leaps, such as what has been achieved by Chen and colleagues, in both understanding basic biomedical concepts as well as developing biomimetic innovations from integrating science and engineering to medicine.
AUTHOR CONTRIBUTIONS
Jiayi Hu and Fangman Chen conducted the literature review and drafted the manuscript. Fangman Chen and Dan Shao revised the manuscript. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors acknowledged Xing Meng for scheme preparation. This work was supported by the National Natural Science Foundation of China (32271388).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
No new data were created or analyzed in this highlight. Data sharing is not applicable to this highlight.