Amphiphilic photosensitizer polymer as a nanocarrier of cytotoxic molecule for carrier-free combination therapy
Abstract
Various nanocarriers have been explored to deliver drugs for combination therapy. However, most nanocarriers are composed of inert materials without contribution for improving the cancer therapeutic effect. Herein, a hydrophobic photosensitizer is conjugated to poly (ethylene glycol) to form an amphiphilic polymer, which further self-assembles into nanomicelle. The generated nanomicelle can act as a nanocarrier to encapsulate a cytotoxic molecule, IR-775, for combination therapy. The yielded nanodrug is totally composed of pharmacologically active ingredients to avoid any possible toxicity resulted from carrier materials. The nanodrug performs enhanced therapeutic effect compared with any monotherapy and exhibits negligible hemolysis, indicating good biocompatibility for further in vivo applications.
1 INTRODUCTION
Photosensitizer (PS)-dependent photodynamic therapy (PDT) can selectively induce cancer death under light irradiation with minimized cytotoxicity to normal tissues, making it an attractive cancer therapy modality.1 Nevertheless, the treatment effect of PDT is limited for the limited penetration depth of light, the strong dependence on oxygen level, the short lifetime of reactive oxygen species, and poor tumor-targeting ability of PS.2, 3 Therefore, the combination of chemotherapy and PDT has been explored to achieve enhanced synergistic therapeutic effect and offer a new strategy for tumor with drug resisitance.4, 5 To achieve photo-chemo combination therapy, many nanodrug delivery systems have been designed with various advantages, including co-loaded drugs, improved tumor accumulation and less side effect to the healthy tissue.6-10 However, most nanodrug delivery systems with complicated structures suffer from the problem of complicated fabrication process, and are difficult to achieve the clinical translation.
Recently, simply self-assembled nanodrug has been explored by integrating hydrophobic photoagents and chemotherapeutic drugs within self-assembled polymeric nanocarriers for tumor-targeted delivery.11-13 For example, ICG and DOX coloaded-based theranostic nanoplatform was developed for imaging-guided chemo-photothermal combination cancer therapy. The nanoplatform prepared by a facile method exhibits a superior ability to accumulate in tumor by enhanced permeability and retention (EPR) effect and a chemo-photothermal synergistically enhancd therapeutic effect.14 However, the low drug loading capabilities prevent the nanodrug from effective treatment with lower dosage.15-17
Herein, an amphiphilic PS-based polymer was synthesized by coupling the hydrophobic PS, pyropheophorbide-a (PhA), to poly (ethylene glycol) (PEG), which further self-assembles into nanomicelle and loads with a cytotoxic molecule, IR-775, for photo-chemo combination therapy (Figure 1A). The yielded nanodrug is totally composed of pharmacologically active ingredients to avoid any possible toxicity resulted from carrier materials, making the nanodrug an extremely high drug loading of both chemotherapeutic drug and PS.18 The synthesized nanodrug performs enhanced therapeutic effect compared with any monotherapy and exhibits negligible hemolysis, indicating good biocompatibility for further in vivo applications.
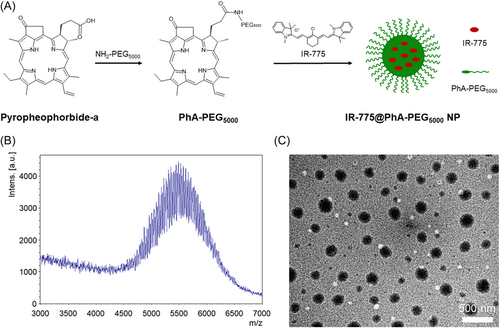
2 RESULTS
2.1 Preparation and characterization of IR-775@PhA-PEG5000 NPs
The PhA-PEG5000 was synthesized by conjugating PhA with PEG5000-NH2 under the catalysis of EDC·HCl and HoBt. The MALDI-TOF MS spectrum of yielded PhA-PEG5000 exhibits a molecular weight of ~5500 (Figure 1B), which is the sum of the molecular weights of PhA and PEG5000-NH2. The critical micelle concentration (CMC) of amphiphilic PhA-PEG5000 in water is determined to be 63.8 nM. The relative low value of CMC facilitates the self-assembly and stability of PhA-PEG5000 NPs in water (Supporting Information: Figure S1).
To prepare IR-775@PhA-PEG5000 NPs, IR-775 and PhA-PEG5000 were dissolved in CH2Cl2, which was further added into deionized water and formed an emulsion after ultrasonication. The IR-775@PhA-PEG5000 NPs formed after removing the CH2Cl2 by overnight evaporation. As characterized by TEM, IR-775@PhA-PEG5000 NPs had a diameter of ~150 nm and exhibit a spherical morphology (Figure 1C). Due to the cavitation caused by ultrasonication, IR-775 that was dissolved in CH2Cl2 droplets was encapsulated with amphiphilic PhA-PEG5000 in water and further shrunk into NPs after complete evaporation of CH2Cl2.
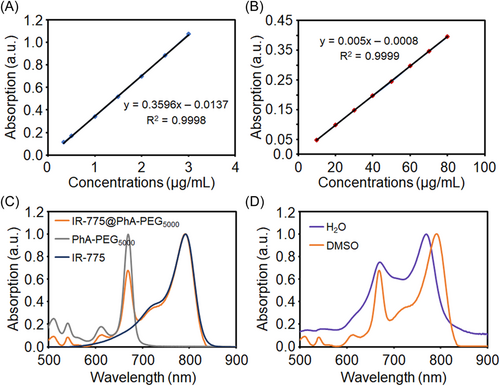
The standard curves of IR-775 and PhA-PEG5000 NPs were established to obtain the correlation between their concentrations and the corresponding absorbance values, benefiting the further calculation of IR-775@PhA-PEG5000 NPs. As shown in Figure 2A, the fitting straight line of IR-775 has a coefficient of determination of R2 = 0.9998 and the equation of the straight line of y = 0.3596x−0.0137, where y is absorbance and x is concentration (μg/ml). As for PhA-PEG5000 NPs, the fitting straight line has a coefficient of determination of R2 = 0.9999 and the equation of the straight line of y = 0.005x−0.0008 (Figure 2B). Furthermore, the ultraviolet-visible-near-infrared (UV-vis-NIR) absorption spectrum of IR-775@PhA-PEG5000 NPs is measured in dimethyl sulfoxide (DMSO) to calculate the encapsulation efficiency and drug loading. To investigate the characteristic absorption, the UV-vis-NIR spectra of IR-775 molecules, PhA-PEG5000 NPs and IR-775@PhA-PEG5000 NPs are normalized as shown in Figure 2C. Further results show that IR-775@PhA-PEG5000 NPs had the similar absorption spectra in deionized water and DMSO. The absorption spectrum of IR-775@PhA-PEG5000 NPs in water is partially distorted, therefore, the absorption spectrum in DMSO is chosen for calculation (Figure 2D). According to the absorbance of PhA-PEG5000 at 669 nm and IR-775 at 791 nm, the encapsulation efficiency and drug loading of IR-775@PhA-PEG5000 NPs are determined to be 41.09% and 3.95%, respectively.
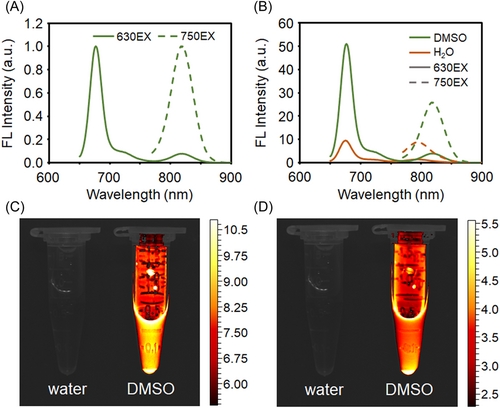
The fluorescence spectra of IR-775@PhA-PEG5000 NPs were recorded in water and DMSO, respectively. As shown in Figure 3A and Figure S2, IR-775@PhA-PEG5000 NPs exhibit strong fluorescence emission at 675 nm in both solvents (Ex = 630 nm), corresponding to the characteristic fluorescence emission of PhA. While under 750 nm excitation, the NPs exhibit intense fluorescence at 818 nm in DMSO and 794 nm in water, corresponding to the fluorescence emission of IR-775. According to the fluorescence spectrum shown in Figure 3B and the fluorescence photos shown in Figures 3C,D, IR-775@PhA-PEG5000 NPs exhibit superior fluorescence intensities in DMSO to that in water. The phenomenon is ascribed to the fluorescence quenching resulting from the self-assembly of IR-775@PhA-PEG5000 NPs in water, and the fluorescence recovery resulting from disassembling of the NPs triggered by the lipophilic DMSO.
2.2 In vitro release of IR-775 from IR-775@PhA-PEG5000 NPs
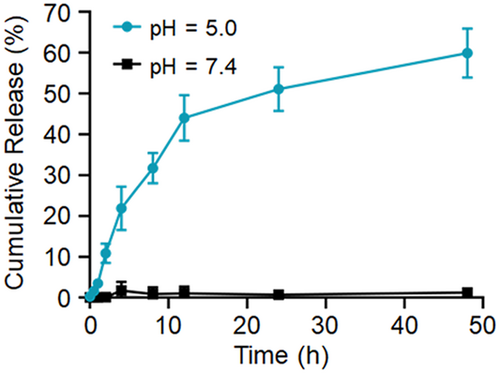
To clarify the release process of IR-775, the release profiles of IR-775@PhA-PEG5000 NPs in different pH buffers were investigated (pH = 5.0 and 7.4), respectively. The cumulative release of IR-775 was measured at scheduled time points (0, 0.5, 1, 2, 4, 8, 12, 24, and 48 h). As shown in Figure 4, approximately 60% of IR-775 molecules is released at pH 5.0, while seldom release occurred at pH 7.4, indicating accelerated release in the acidic lysosome environment inside the cells.
2.3 The cellular endocytosis and in vitro cytotoxicity of IR-775@PhA-PEG5000 NPs
As shown in the fluorescence images of Figure 5A, the PhA fluorescence and the IR-775 fluorescence distribute in the cytoplasm after 24 h incubation. On the one hand, the result indicates that a large amount of IR-775@PhA-PEG5000 NPs entered Hela cells and were enriched in the cytoplasm, confirming efficient cancer cell endocytosis of these NPs. On the other hand, the result indicates that the NPs disassembled inside cells, thus the PhA-PEG5000 and IR-775 were released, resulting the fluorescence recovery of both PhA and IR-775.
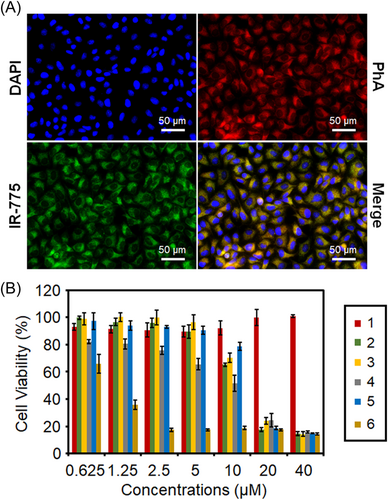
The in vitro cytotoxicity of IR-775@PhA-PEG5000 NPs is evaluated by standard MTT assay. As shown in Figure 5B, PhA-PEG5000 NPs exhibit negligible cytotoxicity without light irradiation but obvious cytotoxicity under light exposure. IR-775 exhibits obvious cytotoxicity (IC50 = 16 µM) in darkness as a chemotherapeutic drug. IR-775@PhA-PEG5000 NPs show similar cytotoxicity (IC50 = 17 µM) to IR-775 in the darkness, indicating the dark cytotoxicity of the NPs was mainly ascribed to IR-775. Remarkably, IR-775@PhA-PEG5000 NPs exhibit much stronger cytotoxicity under 670 nm light irradiation than that in darkness, demonstrating the significantly improved cell-killing efficiency of photodynamic/chemo-combination therapy compared to their monotherapy couterparts (Figure 5B). To further confirm the enhanced therapeutic effect of IR-775@PhA-PEG5000 NPs, the combination index (CI) was calculated to be 0.9187 < 1 (IC = 50%), demonstrating a synergistically enhanced therapeutic effect of the designed NPs.
2.4 The cell uptake of IR-775@PhA-PEG5000 NPs
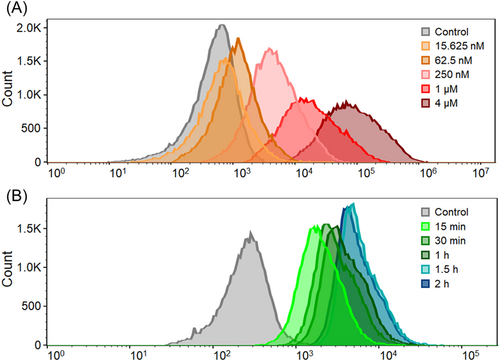
To study the impact of time and concentration on the cell uptake of IR-775@PhA-PEG5000 NPs, flow cytometry experiments were performed. According to Figure 6A, the result shows that the concentration did have an important impact on the cellular uptake of IR-775@PhA-PEG5000 NPs. Increased concentration of cell incubation can significantly promote the uptake of IR-775@PhA-PEG5000 NPs. In addition, cell uptake of IR-775@PhA-PEG5000 NPs was also affected by incubation time (Figure 6B). Increased incubation time can also mildly promote the cell uptake, indicating the longer incubation time might have better therapeutic outcome.
2.5 Hemolysis tests of IR-775@PhA-PEG5000 NPs
Hemolysis test is also conducted to examine the biocompatibility of the prepared IR-775@PhA-PEG5000 NPs. As shown in Figure S3, the RBCs retains to be intact and precipitates at the bottom but hemolysis occurs for the RBCs in water as the control, verifying the biocompatibility of the prepared of IR-775@PhA-PEG5000 NPs.
3 DISCUSSION
Currently, frequently used anticancer drugs are confronted with the situation of ineffectiveness and severe side effects. New strategies for cancer treatment are extremely desired, especially for those with drug resistance. Photo-chemo combination therapy has been extremely attractive for cancer treatment, due to its synergistically enhanced therapeutic effect and reversal of drug resistance. Both chemotherapeutic drugs and photo agents (PS and photothermal conversion agents) are simultaneously delivered into tumor cells by being co-loaded into nanodrug delivery systems. Many studies focus on designing self-assembling nanodrug delivery systems by taking amphiphilic polymers with self-assembly properties as the nanocarrier (e.g., DSPE-PEG).11-14 Integrating photo agents and chemotherapeutic drugs within polymeric nanocarriers has been demonstrated to be a good strategy to guarantee the simultaneous effect of chemotherapy and PDT. However, such nanodrug delivery systems generally have a drawback of low drug loading.15-17 In this research, the cytotoxic IR-775 and the amphiphilic PS polymer, PhA-PEG5000, self-assembled into NPs for enhanced photo-chemo combination therapy. The design of the amphiphilic PS polymer PhA-PEG5000 is to greatly improve the total drug (chemotherapeutic drug + PS) loading of the nanodrug delivery systems, which further enhance the therapeutic effect.
The prepared IR-775@PhA-PEG5000 NPs, IR-775-loaded PhA-PEG5000 nanomicelles, are nanospherical with diameters of about 150 nm. The suitable size of the IR-775@PhA-PEG5000 NPs is beneficial to the tumoral accumulation by EPR effect. Furthermore, IR-775@PhA-PEG5000 NPs exhibit intense fluorescence emission under 675 nm excitation and 750 nm excitation, severally corresponding to the characteristic fluorescence emission of PhA and IR-775. The efficient fluorescence emission at respective excitation wavelengths guarantees efficient fluorescence imaging at cellular level in vitro and small animal fluorescence imaging for noninvasive in vivo tracking, respectively. The phenomenon that IR-775@PhA-PEG5000 NPs exhibit strong fluorescence in DMSO while weak in water, suggests the fluorescence quenching in water, due to IR-775 and PhA-PEG5000 aggregate as NPs in aqueous solution. However, the fluorescence dequenches when the NPs enter cells, where is abundant with lipophilic microstructures to induce the disassembling of the NPs and fluorescence recovery. The release profiles of IR-775@PhA-PEG5000 NPs in different pH buffers indicate that IR-775 can be released efficiently at the lysosome (pH 5.0) after endocytosis into the cells, while maintained intact during blood circulation.
The fluorescence images of Hela cells exhibit efficient cell endocytosis of IR-775@PhA-PEG5000 NPs, confirming the simultaneous entry of both PhA and IR-775 into cancer cells. On the other hand, the fluorescence recovery of PhA and IR-775 indicates the disassembly and drug release of the NPs inside cells. MTT and hemolysis tests reveal that IR-775@PhA-PEG5000 NPs can synergistically enhance the cancer cell death via photo-chemo combination therapy under light irradiation, but are safe in the blood without irradiation. To further investigate the factors that impact cell uptake, the flow cytometry experiments were carried out. The results suggest that the cell uptake of IR-775@PhA-PEG5000 NPs is time-dependent and concentration-dependent. The cell uptake can be promoted by increasing the incubation concentration or time. In addition, after 1 h incubation, cells that incubated with the NPs at a concentration of 250 nM exhibited significantly enhanced fluorescence compared to that in control group, indicating that IR-775@PhA-PEG5000 NPs were internalized into cells and presented discernible fluorescence under low concentration and short time incubation. The results demonstrated that IR-775@PhA-PEG5000 NPs were suitable to be tracked by fluorescence imaging. At an incubation concentration of 250 nM, the cells exhibited little distinction at time points of 1.5 and 2 h, suggesting that it has limited impact on cell uptake. In sum, the prepared IR-775@PhA-PEG5000 NPs have a synergistic effect for enhanced photo-chemo therapy in cancer therapy.
However, there are some limitations in this study. The main limitation is the lack of animal experiments, which can better reveal the synergistic therapeutic effect of IR-775@PhA-PEG5000 NPs. In the future, we are supposed to design and suppliment the animal experiment. The noninvasive in vivo fluorescence imaging will be presented to investigate the metabolism and tumor accumulation of IR-775@PhA-PEG5000 NPs by IR-775 fluorescence tracking. Furthermore, the in vivo antitumor activity of IR-775@PhA-PEG5000 NPs should be investigated to better reveal the PDT-chemo combination therapeutic effect of NPs. In addition, some analogues of IR-775 with ultrahigh mass extinction coefficients are confirmed as highly efficient NIR-sensitive photothermal agents for photothermal therapy, such as indocyanine green and IR-797. Accordingly, IR-775 is supposed to be a photothermal agent possessing a high photothermal conversion efficiency. With the capability to catalyze PTT, IR-775@PhA-PEG5000 NPs can exhibit an excellently synergistic therapeutic effect for PTT-PDT-chemo tri-modal combination therapy. Thus, the design of the IR-775@PhA-PEG5000 NPs is meaningful, and the further exploration of the IR-775@PhA-PEG5000 NPs is deserved to be in progress.
4 MATERIALS AND METHODS
4.1 Preparation and characterization of PhA-PEG5000 NPs
PEG5000-NH2 (0.12 mmol, 600.0 mg), PhA (0.1 mmol, 53.4 mg), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl, 0.5 mmol, 96.5 mg), 1-hydroxybenzotriazole (HoBt, 0.12 mmol, 16.2 mg) and pyridine (320 µl) were dissolved in 20 ml N,N-dimethylformamide (DMF). After overnight stirring, the mixture was dialyzed with dialysis bag (cutoff = 14 kDa) against water. Fresh water was changed every 4 h. After 24 h, DMF, pyridine, EDC·HCl, HoBt, excessive PEG5000-NH2, and free PhA were removed to obtain the purified PhA-PEG5000 NPs. Lyophilized PhA-PEG5000 NPs were stored at 4°C for further use and dissolved in methanol for Bruker autoflex speed MALDI-TOF (Germany) characterization. PhA-PEG5000 NPs can be self-assembled in water for their amphiphilicity. The CMC value of PhA-PEG5000 NPs was determined by the curves based on the Pyrene 1:3 ratio data, which were measured in the presence of the PhA-PEG5000 NPs at various concentrations.
4.2 Preparation of IR-775@PhA-PEG5000 NPs
IR-775 (5 mg; full name: (2Z)-2-[(2E)-2-[2-chloro-3-[(E)-2-(1,3,3-trimethylindol-1-ium-2-yl)ethenyl]cyclohex-2-en-1-ylidene]ethylidene]-1,3,3-trimethylindole; chloride; Tokyo Chemical Industry Co., Ltd.; purity >90.0%) and PhA-PEG5000 (50 mg) were dissolved in 2 ml CH2Cl2 to form a stock solution, which was added into deionized water (15 ml) under ultrasonication. The emulsified mixture was then stirred overnight without capping to remove CH2Cl2 by slow evaporation.
4.3 Characterization of IR-775@PhA-PEG5000 NPs
IR-775 molecules and PhA-PEG5000 were dissolved in DMSO to prepare the stock solution. Then, IR-775 solution (1 mg/ml) was diluted by DMSO into different concentrations (0.333, 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 μg/ml). The PhA-PEG5000 solution (10 mg/ml) was diluted into different concentrations (10, 20, 30, 40, 50, 60, 70, and 80 μg/ml). All the solutions of known concentrations were determined to obtain their corresponding absorbance. The plots were drawn to investigate the correlation between concentrations and relevant absorbance.
UV-vis-NIR absorbance in DMSO and deionized water were measured with a Lengguang Technology 759S spectrophotometer (China), respectively. Fluorescence spectra excited at 630 and 750 nm in deionized water and DMSO were conducted using a Hitachi F-4700 spectrophotometer (Japan), respectively. Fluorescence images in water and DMSO were obtained by Vieworks VISQUE® InVivo Smart-LF in vivo imaging system (Korea) under the Cy5.5 channel (Ex: 630–680 nm; Em: 690–740 nm) and the ICG channel (Ex: 740–790 nm; Em: 810–860 nm), respectively. Transmission electron microscopy (TEM) images were obtained with FEI Tecnai F20 TEM (America) after dropping the NP solution onto a copper mesh.
4.4 The cellular endocytosis of IR-775@PhA-PEG5000 NPs
Hela cells were incubated with 4 μM IR-775@PhA-PEG5000 NPs for 24 h, and then fixed with 4% paraformaldehyde and stained with 4′,6-diamidino-2-phenylindole (DAPI) for fluorescence imaging. In vitro fluorescence imaging was performed using a KEYENCE BZ-X800 fluorescence microscope (America) in the ICG channel (IR-775, Ex = 775/50 nm; Em = 845/55 nm), the Cy5 channel (PhA, Ex = 620/60 nm; Em = 700/75 nm), and the DAPI channel (DAPI, Ex = 360/40 nm; Em = 460/50 nm), respectively.
4.5 Cell cytotoxicity of IR-775@PhA-PEG5000 NPs
The in vitro cytotoxicity was assessed by using standard 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Hela cells were seeded and cultured in 96-well plates at 37°C for 24 h (3000 cells/well). Then, IR-775, PhA-PEG5000 NPs, and IR-775@PhA-PEG5000 NPs with increased concentration gradient were added and incubated for 24 h. Next, the cells were irradiated with xenon lamp with filter centered at 670 nm (100 mW/cm2) for 10 min and further cultured in darkness for another 24 h. Then, standard MTT assay was used to evaluate the cytoviabilities of each group. The dark cytotoxicities of the corresponding samples were measured by the same procedure without light irradiation.
The combination index (CI) was calculated from the cytotoxicity data of PhA-PEG5000 NPs and IR-775@PhA-PEG5000 NPs. The CI was calculated by the formula: CI = D1/Dm1 + D2/Dm2, where D1 and D2 are the concentrations of IR-775@PhA-PEG5000 NPs to produce certain single therapeutic cytotoxicity in combination therapy, while Dm1 is the concentration of IR-775 performing chemotherapy and Dm2 is the concentration of PhA-PEG5000 NPs performing PDT.
4.6 Flow cytometry of IR-775@PhA-PEG5000 NPs
Hela cells were seeded in a six-well plate and cultured overnight. Then, the cells were incubated with increasing concentrations of IR-775@PhA-PEG5000 NPs (0–4 μM) for 1 h. Afterward, the cells were digested by trypsin and washed three times with phosphate-buffered saline (PBS). The cell uptake of IR-775@PhA-PEG5000 NPs was analyzed by flow cytometry. To further investigate the time-dependence of cell uptake of IR-775@PhA-PEG5000 NPs, Hela cells were incubated with IR-775@PhA-PEG5000 NPs (250 nM) for different time periods (0–2 h), then were digested for flow cytometry analysis. In the control group, cells were just incubated with cell culture media.
4.7 The hemolysis test of IR-775@PhA-PEG5000 NPs
Hemolysis tests were conducted to evaluate the biocompatibility of the IR-775@PhA-PEG5000 NPs. Mouse blood was collected and mixed with five drops of EDTA-Na2 anticoagulant and 1 ml PBS. The plasma was removed after centrifugation at 1000 rpm for 3 min, and the red blood cells (RBCs) were washed with 1× PBS (pH 7.4) for three times until supernatant colorless, after which RBCs were diluted with 1 ml PBS to form RBCs suspension. IR-775@PhA-PEG5000 NPs in 800 µl 1× PBS solution was added with 200 µl RBCs suspension. As control, 800 µl deionized water was added with 200 µl RBCs suspension. After 4 h, the two groups of samples were centrifuged to observe and compare the hemolysis of RBCs.
4.8 In vitro release of IR-775 from IR-775@PhA-PEG5000 NPs
A total of 0.4 ml IR-775@PhA-PEG5000 NPs suspensions were injected into a dialysis cartridge with a molecular weight cutoff (MWCO) of 14 kDa for dialysis against 200 ml of pH = 5.0 and 7.4 buffers, respectively. Two hundred millilitres of ethyl acetate was added into each of the buffers to mimic the liphophilic environment inside the cells. Then, the buffers were agitated under 220 rpm shaking at 37°C. Three millilitres of ethyl acetate was collected from each sample for UV-vis-NIR absorption measurement at scheduled time points. Samples of three parallel experiments were collected and measured, calculated and values were presented as means ± SD.
AUTHOR CONTRIBUTIONS
Jingqi Xin: Conceptualization, methodology, formal analysis, writing – original draft, writing – review and editing. Caiting Deng: Formal analysis, writing – review and editing. Meichen Zheng: Formal analysis, writing – review and editing. Feifei An: Writing – review and editing, supervision, project administration, funding acquisition. All authors have given approval to the final version of the manuscript.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (51903201).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
All data generated during the study appear in the submitted article.




