The osteogenic effect of mesenchymal stem cells regulated by photo-crosslinked hydrogels with tunable elastic modulus
Haifu Sun, Chen Qian, Kai Chen, and Yu Wang contributed equally to this study.
Abstract
Biomimicry is the enduring pursuit in the field of bone implants, wherein bio-materials with adjustable elastic modulus and porosity, the same as natural bone, offer a novel strategy for developing and applying new bone repair materials. Conventional biomaterials are often used to repair bone defects without complete consideration of structural and functional osseointegration, leading to interface repair failure. In this study, organic-inorganic interpenetrating network technology was employed using varying amounts of nano-hydroxyapatite (nHAP) and methacrylated gelatin (GelMA) and osteogenic growth peptide (OGP) to construct biomimetic bones with low, medium, and high nano-hydroxyapatite content (GelMA-c-OGP/nHAP). As the concentration of nano-hydroxyapatite increases, comprehensive evaluations of the biomimetic materials were conducted using osteogenic ability tests, Micro-CT scans, nanoindentation tests, and mechanical tests. The developed biomimetic structural material exhibits well-controlled mechanical properties. Compared to natural bone trabeculae, this biomimetic material not only maintains the organic and inorganic ratio of natural bone but also demonstrates exceptional mechanical load-bearing capabilities. Additionaly,this scaffold exhibits good porosity and mechanical properties. It enhances cell adhesion, integrates perfectly with bone tissue, and demonstrates excellent osteogenic ability both in vitro and in vivo. This study lays the foundation for constructing biomimetic scaffolds with adjustable mechanical properties, presenting high prospects for applications in the field of tissue engineering.
1 INTRODUCTION
A biological bone serves as a mechanical barrier against external stimuli and environmental injuries for living organisms. Biological skeletons are typically multifunctional, serving roles such as hematopoiesis, movement, connection. In addition, natural skeletons also have excellent mechanical properties and self-healing ability, but the self-healing ability of bones is limited.1-4 Despite advances in bone tissue engineering, challenges still exist in repairing bone defects larger than those naturally healed.5 In the past, researchers have used growth factors to impart biological activity to biomaterials, but this approach can only promote partial tissue regeneration, such as integration in areas like tendons or bones.6 To address this challenge, in recent years, tissue engineering scaffolds based on biomimetic strategies have received widespread attention in tissue regeneration research. These scaffolds can enhance their regenerative potential in complex tissues by altering the composition of the scaffold material.7-9
Biomimetic scaffolds must be designed using a thorough understanding of natural bone's composition, structure, biomechanical characteristics, and biochemistry. A combination of these qualitative and quantitative parameters can be used to engineer high-quality biomimetic scaffolds. Generally, living bones in the human musculoskeletal system consist of approximately 10%–20% collagen, 60%–70% bone minerals, and 9%–20% water by weight. The fundamental component of bone mineral composition can be approximately defined as hydroxyapatite (HA) with a chemical formula of Ca10(PO4)6(OH)2.10, 11 Collagen fibers, the primary component forming sheets at the microscopic level, constitute the major portion of the skeletal system, mostly type I collagen and a small amount of type V collagen. Collagen fibrils are organized by assembling original collagen molecules along the fibrils, arranged in a 3/4 staggered and parallel configuration.12 In addition to collagen, the content of bone minerals significantly influences the mechanical properties of the skeletal system, with the Young's modulus values increasing significantly with the increase in Ca content. When preparing bone biomaterials, it is important to consider mimicking the structure and composition of the bone matrix. Biomimetically preparing bone scaffold materials enables them to possess excellent bone conductivity, bone inductivity, and bone integrativity from a biomimetic perspective, promoting bone regeneration.13-16
Methacrylated gelatin (GelMA) is prepared by modifying gelatin with methacrylic anhydride. It has strong hydrophilicity and high side-chain reactivity, as well as advantages such as good biocompatibility and biodegradability. GelMA retains the arginine-glycine-aspartic acid (RGD) sequence, similar to components of the extracellular matrix (ECM), which promotes cell adhesion, differentiation, and proliferation, exhibiting biological properties similar to collagen.17, 18 The physical properties of GelMA can be customized by adjusting the concentration to form a three-dimensional structure with a certain strength. Compared to collagen, GelMA has better plasticity and degradability. Its mechanical properties are adjustable, providing a variety of viscoelastic characteristics. Therefore, it can be widely used in various biomedical fields such as bone and vascular regeneration.19-22 In our previous study, a GelMA-c-osteogenic growth peptide (OGP) hydrogel was prepared by co-crosslinking to induce bone formation. However, after the complete release of OGP, the hydrogel could not continue to provide an environment conducive to osteogenesis.23 Moreover, as GelMA is derived from gelatin, it lacks any inorganic components. Also, due to the limitations of the material itself, the co-crosslinking form, while enhancing the hydrogel's mechanical properties, is still insufficient to support the pressure at the bone defect site. Therefore, further research is necessary. The inorganic component in the bone matrix is mainly hydroxyapatite (HAP). Artificially synthesized nano-hydroxyapatite materials have excellent biocompatibility and bioactivity, making them a promising biomaterial. Nano-hydroxyapatite has certain osteoconductive and osseointegration properties, and can directly form bone bonding under specific conditions, but lacks osteoinductive ability.24 Therefore, it is possible to address the drawbacks of HAP by combining it with other materials.
There have been efforts to composite nano-hydroxyapatite with other materials to create composite materials. For example, Zhou Fang and colleagues grafted polylactic acid oligomers onto the surface of nano-hydroxyapatite, enhancing its dispersibility and stability in aqueous solutions. This composite material exhibited increased mechanical strength when combined with polylactic acid hydroxyethyl acetate.25 In another instance, the Quinlan team employed a freeze-drying method to manufacture collagen-hydroxyapatite scaffolds combined with recombinant human bone morphogenetic protein 2 (rhBMP-2). This combination displayed excellent healing performance in vivo. GelMA-nHAp composite hydrogels with increased pore size exhibited improved mechanical properties, and biological results demonstrated the effective osteogenic potential of GelMA-nHAp hydrogels.26 Additionally, Wang and colleagues utilized 3D printing to create a porous nano-hydroxyapatite scaffold with controlled release of BMP-2, effectively improving the condition of bone defects in vivo.27 But much of the past research has not been detailed to regulate the ratio of inorganic components to make it more similar to natural bone.
To solve the problems of using traditional materials for bone defect repair, our research has developed an elastic pore size and elastic modulus-adjustable biomimetic structural material. The inspiration for this innovative material comes from the composition of natural bone. By adjusting parameters such as the concentration of nano-hydroxyapatite and the proportion of GelMA-c-OGP, we have introduced an effective strategy to regulate the component ratios, achieving natural bone-like composition ratios, mechanical strength, and elastic modulus. This study aims to develop a systematic way to control biomimetic materials' mechanical properties by design. We have demonstrated that these materials can be used for cranial implants, where composite scaffold materials with moderate elastic modulus and pore size show promising prospects as implants.
2 RESULTS
2.1 Surface morphology of biomimetic hydrogels
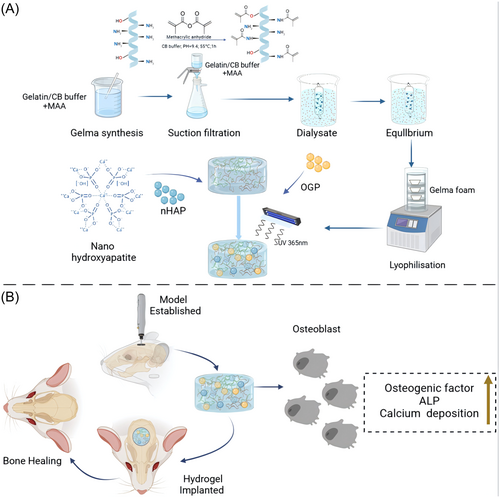
We synthesized a GelMA monomer using gelatin and MA. Based on this, we further produced GelMA-C-OGP. As shown in Table S1,GelMA-c-OGP with a concentration of 10% was mixed with different mass fractions (0, 0.5, 1, 3, and 5 wt%) of nHAP, followed by photocrosslinking to obtain biomimetic hydrogels with varying nHAP concentrations (GO, GOH0.5, GOH1, GOH3, and GOH5) (Figure 1).
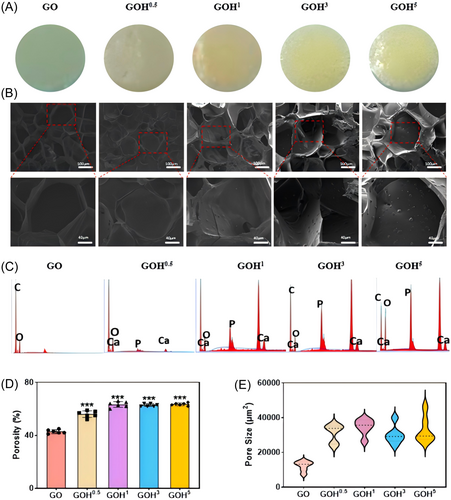
GelMA-c-OGP hydrogel is transparent; after adding nHAP, the biomimetic membrane appears milky white, and with increasing nHAP concentration, the transparency of the biomimetic hydrogel decreases, with visible particle aggregation within the hydrogel. Increasing the number of particles can increase the roughness of the pore walls, which is beneficial for promoting cell mineralization28 (Figure 2A). This indicates that we uniformly mixed nHAP into the hydrogel. Scanning electron microscopy (SEM) analysis revealed that all hydrogel materials exhibited a porous scaffold structure. Nano-sized mesopores were visible inside the hydrogel, with most pore sizes around 80 μm in the nHAP-free group. As nHAP increased, pore sizes expanded, and the material surface became rougher. With higher concentrations, the sidewalls thickened significantly, with a pore size of 150 ± 15 μm (Figure 2B). Simultaneously, the porosity of the hydrogel increased with rising nHAP concentration. The porosity was about 42.6% in the GO group and 56.6% in the GOH0.5 group, and thereafter remained at about 63% with increasing nHAP concentration (Figure 2D). Using energy dispersive spectrometer (EDS) experiments, it was shown that Ca2+ and P+ content increased as nHAP concentration increased (Figure 2C).
2.2 Physical and chemical properties of biomimetic hydrogels
Fourier-transform infrared spectroscopy was performed on synthesized GelMA and Gelatin. The absorption peak around 3074 cm−1 is located in the amide B band, which are characteristic peaks of GelMA. The absorption peak at 1537 cm−1 corresponds to the C-N vibration, indicating the presence of OGP in the GelMA hydrogel and the successful preparation of GelMA-c-OGP. With the addition of nano-hydroxyapatite (0.5%), the C = O absorption peak at 1743 cm−1 disappears, and the amide band and hydroxyl absorption peak at 3284 cm−1 shift significantly. This suggests the existence of hydrogen bonding and chemical crosslinking between gelatin C = O and amide groups and nano-hydroxyapatite, where the carbonyl group C = O is a critical chemical crosslinking site. When the proportion of nano-hydroxyapatite is increased (3%), the characteristic peak of gelatin amide band remains clear, indicating that GelMA-c-GOP can encapsulate nano-hydroxyapatite well. However, with a continuous increase in the proportion of nano-hydroxyapatite (5%), the relative intensity of P-O absorption peaks at 1023, 600, and 560 cm−1 significantly increases, and GelMA-c-GOP absorption peaks almost disappear, indicating that a large amount of nano-hydroxyapatite is exposed and locally aggregated, leading to a significant decrease in the uniformity and an increase in the roughness of the hydrogel (Figure 3D). This further confirms that the GOH5 group has high roughness, which is conducive to the mineralization of bone tissue.
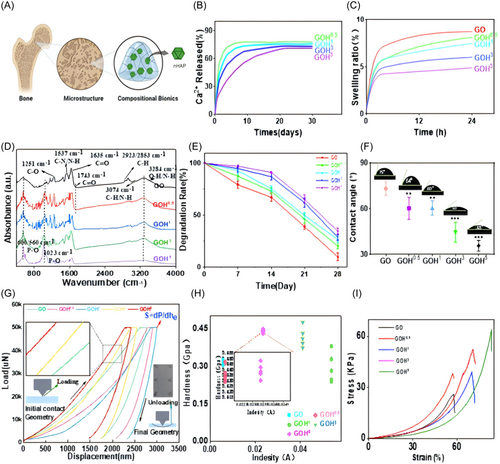
Swelling and degradation tests indicated that the swelling rates of all biomimetic hydrogel groups are lower than that of the control group GO and tend to stabilize after 12–24 h (Figure 3C). The degradation rates of all biomimetic hydrogel groups are less than that of the control group GO and tend to stabilize after 25 days (Figure 3E).
The Ca2+ release curves of various biohybrid hydrogel groups within 30 days are depicted in Figure 3B. Materials in three groups released Ca2+ steadily within 30 days. The release reached its peak within 48 h, followed by a decline, and stabilized after 72 h. The release of Ca2+ in the hydrogel is positively correlated with the nHAP content, and Ca2+ is released slowly, benefiting from the hydrogel scaffold structure.29 Contact angle measurement is commonly employed to assess the wetting state of a material surface and the wetting properties of a pure fluid on a flat surface. It serves as a qualitative method to evaluate whether a surface exhibits hydrophobic or hydrophilic characteristics. In this study, the hydrophilicity of the GOH group is considerably higher than that of the GO group, possibly due to nHAP itself being a hydrophilic material within the GOH group, resulting in a contact angle of approximately 10° (Figure 3F).30
2.3 Mechanical properties of biomimetic hydrogels
The implantation of biomimetic hydrogels into bone defects is subject to compression by adjoining bone and soft tissues, and merit mechanical properties are a prerequisite for their clinical use.31 To validate the compressive capability of this material, mechanics and nanoindentation experiments were conducted. GOH5 (65.23 ± 3.14 kPa) and GOH3 (58.55 ± 4.68 kPa) exhibited higher compressive abilities than the GO group (28.2 ± 2.39 kPa) (*p < 0.05). Good compressive strength can provide support in the bone defect area, facilitating the growth of BMSCs. The elastic modulus of the GOH group is more higher than the GO group (Figure 3I). This indicates that the GOH5 group hydrogel is more flexible, with better deformability.
Elastomeric loading at the beginning in the experimentally measured nanoindentation data set has a relatively short distance (indentation depth is usually no more than a few tens of nanometers). Literature review reveals that most studies focus on applying Hertzian theory (Equations 1 and 2) to the unloading segments of load-displacement data obtained in measurements. The figure displays all the data obtained during the depth-sensing nanoindentation experiment, with the testing process divided into three main stages: loading, constant loading, and unloading (both sides of the figure show the loading and unloading stages). During the constant loading stage, the sample exhibits creep, and enlarged images of the imprints on the sample surface suggest the presence of plasticity. After comparing GOH3 and GOH5 with the GO group, it was observed that their toughness gradually strengthened, and the elastic modulus of the fifth group manifested the highest among the five groups (Figure 3G). This scatter plot further confirms the increased hardness of the fifth group, 10% GelMA +5% HAP. We conducted tests at five different points for each of the five hydrogel groups, and a scatter plot of absorbance and hardness was created (Figure 3H). From the figure, we observed that groups GO0.5 and GO1 did not show a significant increase in hardness compared to the control group. However, groups GOH3 and GOH5 exhibited a noticeable improvement in hardness. By zooming in on the images, we can observe that the hardness values of 10% GelMA +5% HAP are relatively higher and concentrated. The achieved results align with our expectations. Therefore, increasing the concentration of nHAP appropriately contributes to enhancing the mechanical performance of the hydrogel. The GOH5 group hydrogel exhibits relatively good toughness and hardness. The mechanical tests indicate that the GOH5 group hydrogel has excellent mechanical properties, capable of withstanding pressure at the site of bone defects, making it a promising material for bone regeneration engineering.
2.4 Biocompatibility of biomimetic hydrogels
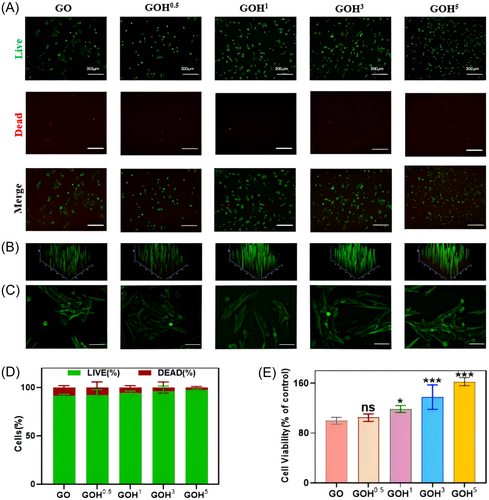
CCK-8 assays, live/dead staining experiments, and calcein-AM staining were conducted. The status of live cells (green fluorescence) in each group was good, accounting for the most of the cells. A very small number of dead cells (red fluorescence) were observed, indicating a high cell survival rate (Figure 4A,B,D). The results of the CCK-8 experiment indicated that the proliferating cell amount in the GOH group was considerably higher than that in the GO group (Figure 4E). Cell attachment was detected for different cryogels, and the results are shown in the figure. The cell attachment status was good in each group, with clear cell morphology and evident cell elongation, indicating that the addition of HAP did not change the adhesive properties of GelMA (Figure 4C).
2.5 Evaluation of in vitro osteogenic activity
Alkaline phosphatase (ALP) staining showed that the ALP activity in the GOH0.5, GOH1, GOH3, and GOH5 groups was considerably higher than that in the GO group, and the higher the concentration of nHAP, the higher the ALP activity (Figure 5A,D). Furthermore, on Day 21, Alizarin Red S (ARS) staining for calcium-binding proteins in the mineralized matrix showed that the count and size of mineralized nodules in the GOH5 group were more greater than those in the other groups (Figure 5B,E).
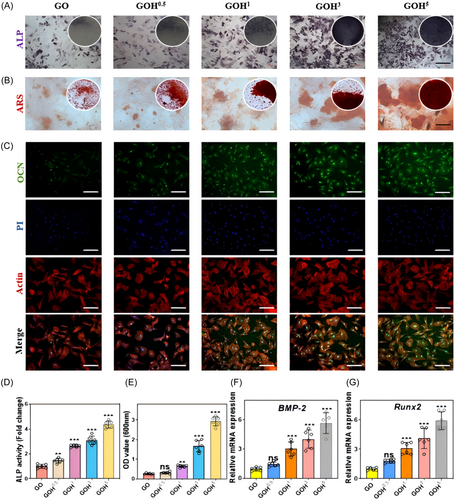
A quantitative analysis of the osteogenic ability of each group was conducted. Osteocalcin (OCN) staining showed that the GOH5 group expressed significantly more OCN than the GO, GOH0.5, GOH1, and GOH3 groups, indicating a significant enhancement of osteogenic differentiation in the GOH5 group (Figure 5C). The study also detected the expression levels of other genes associated to osteogenesis (Runx2 and BMP-2) (Figure 5F,G), and the expression levels of osteogenic marker genes in the GOH5 group were higher than those in the GO group and other GOH groups.
2.6 RNA-seq validation of bone immunomodulation by GOH5
Principal component analysis and sample-to-sample correlation testing showed good reproducibility for each group of samples. In comparison with the control group, there were 881 inhibited genes and 1235 activated genes in the GO group. Compared to the GO group, the GOH5 group exhibited approximately 1652 upregulated genes and 2112 downregulated genes (Figure 6A,B). Thousands of genes with significant changes were found between the control group and the GO group, with an enhancement of osteoblast signaling pathways in the GO group compared to the control group (Figure 6C). The volcano plot and DESeq. 2 variance analysis demonstrate that GO group exhibited an increase in 1329 genes and a decrease in 960 genes compared to the control group. The genes involved in osteoblastogenesis were mostly upregulated in the GO group compared with the control group (Figure 6D). Gene Ontology (GO) analysis revealed that the changes induced by GO were mainly associated with osteoblastogenesis. The GO group primarily expresses upregulation of osteogenic signaling and the generation of osteogenesis-related molecular mediators (Figure 6E–G). According to the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis, the bubble representing the Rap1 signaling pathway is relatively large and deep red in color. Additionally, its enrichment value is relatively high. It can be seen that,compared to the control group, there is a strong interaction between the GO group and the Rap1 signaling pathway (Figure 6H).
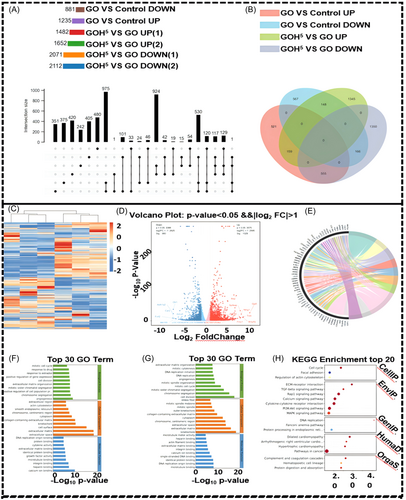
There were also thousands of significantly changed genes between the GO group and the GOH5 group (Figure S1A). Based on volcano plots and DESeq. 2 variance analysis, the GOH5 group had 2289 genes upregulated and 1793 genes downregulated compared to the GO group (Figure S1B). GO analysis revealed that the changes induced by GOH5 mainly involved osteogenesis (Figure S1C–E). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis further indicated that, compared to GO group, the GOH5 group showed a strong interaction with pathways related to the PI3K-Akt signaling pathway (Figure S1F).
2.7 In vivo bone regeneration efficiency of biomimetic hydrogels
Establishment of a rat calvarial defect model The overall procedure and operational flow are illustrated in Figure 7A. We collected calvariae at 12 weeks post-surgery and assessed osteogenesis using microcomputed tomography (Micro-CT). Bone defects were reconstructed in the GOH5 group at a significantly higher rate than the other groups. By the 12th week, the new bone tissue had almost completely filled the defect. These images were also used to quantify bone microstructure parameters, such as bone mineral density (BMD), bone volume/total volume (BV/TV), and trabecular number (Tb.N). The BMD, BV/TV, and Tb.N in the GOH5 group were much higher than those in the control group, while Tb.Sp was significantly lower. The GOH5 group exhibited superior bone repair capability compared to the other groups.
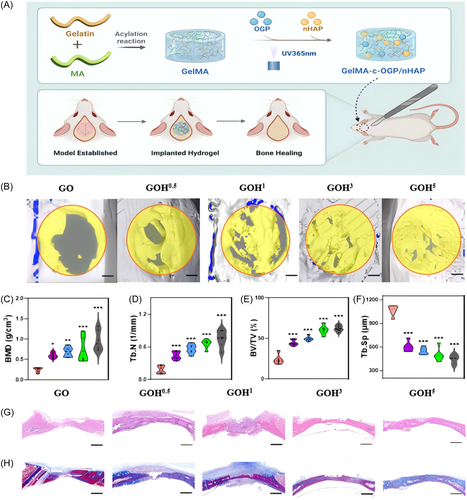
To further evaluate the effect of hydrogels on promoting calvarial defect repair, hematoxylin and eosin (H&E) staining and Masson's trichrome staining were took place on calvarial specimens from each group. Histological observations showed abundant bone matrix and evident bone-like granules in the GOH5 group at 12 weeks postimplantation, indicating significant bone regeneration. In contrast, the control group exhibited mostly loose fibrous tissue with minimal bone formation even at 12 weeks postsurgery. Masson's trichrome staining reveal minimal collagen fibers and osteoid tissue formation during the defect citation in the control group, while there was more fibrous tissue and bone-like formation in the GOH group. Besides, the density of collagen tissue was much higher in the GOH5 group.
3 DISCUSSION
Hydrogel is a biocompatible biomaterial that not only serves as a platform to act as a good slow-release drug delivery system, but also provides local mechanical support. As an osteogenic biomaterial, the ability to have a structure similar to that of natural bone trabeculae is the ultimate goal of research. However, there are few previous studies involving the modulation of the elastic modulus of hydrogels. In this experiment, by increasing the proportion of nHAP in GelMA-c-OGP. The pore area of the hydrogel also increased with higher nHAP concentrations. Large pore area is beneficial for encapsulating cells and promoting nutrient exchange.32 And the porous nature of the hydrogel is suitable to the adhesion, proliferation, and differentiation of osteoprogenitor cells, as well as vascular regeneration, promoting bone repair.20, 33 Besides, EDS experiments has shown that Ca2+ and P+ content increased as nHAP concentration increased. As reported, Ca2+ and P+, as bioactive ions, play roles in promoting the proliferation and differentiation of osteoblasts, regulating the synthesis and mineralization of bone matrix, and other functions.34
By magnifying the scaffold surface, granular protrusions can be seen. Cell adhesion was facilitated by the rough surface, and Ca2+ release was sustained more easily with the scaffold structure. These results indicate that with the increase in nHAP concentration, the hydrogel exhibits higher porosity and pore area, as well as higher release of Ca2+ and P+, all of which are beneficial for bone tissue regeneration. As soon as artificial materials are implanted, they are immersed in bodily fluids, and they require some structural stability in bodily fluids to maintain functionality. Swelling and degradation tests can indicate structural stability of biomimetic hydrogels. The experimental results indicate that the GOH5 group do not undergo excessive weight loss or expansion in bodily fluids, exhibiting the best structural stability. The hydrogel will integrate well with the surrounding bone tissue at an appropriate swelling rate, without causing damage. This characteristic will allow it to remain in the body for a long period of time, which is a critical condition for promoting bone defect regeneration.
Good cell compatibility is a precondition for the use of artificial biomaterials,35 and GelMA hydrogels typically exhibit good biocompatibility.36 We co-cultured bone marrow mesenchymal stem cells with each hydrogel group and conducted CCK-8 assays, live/dead staining experiments, and calcein-AM staining. Each group of nano-hydroxyapatite biomimetic hydrogels exhibited some proliferative influence on cell growth, with no significant hazardous effects, and demonstrated greatly biological compatibility. And the results of the CCK-8 experiment indicated that the proliferating cell amount in the GOH5 group was considerably higher than that in oother group. This may be due to the sustained release of Ca from the hydrogel, which promotes the proliferation of BMSCs.37 During the process of bone regeneration, bone marrow mesenchymal stem cells are recruited to the location of injury and then assist with the repair of bone tissue through osteogenic differentiation. To investigate the effect of an osteogenic differentiation-improving nano-hydroxyapatite hydrogel, Runx-2 transcription factor initiates early osteogenic differentiation, and ALP (alkaline phosphatase) is an enzyme that breaks down organic phosphates and regulates calcium deposition in bone matrix. ARS (Alizarin red staining) is a common practice for evaluating osteogenic differentiation.38 This study indicated that the ALP、Runx-2 and so on levelsin the GOH5 group were higher than those in the GO group and other GOH groups.
These results suggest that compared with other groups, the GOH5 group has stronger osteogenic differentiation ability and the hydrogel of the GOH5 group has a beneficial effect on the osteogenic differentiation of BMSCs, which is because nano-hydroxyapatite promotes osteogenic differentiation by enhancing the gene expression profile and protein deposition of bone-specific markers during BMSCs differentiation, promoting mineralization, and thereby accelerating osteogenic differentiation.39
To further explore the mechanism by which GOH5 promots osteoblastogenesis, we conducted RNA sequencing, revealing that GOH5 can satisfactorily promoting the expression of osteogenic-related genes. Thus, we decided to elucidate the potential mechanisms and associated biological events underlying GOH5-mediated of osteoblastogenesis. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis further indicated that, compared to GO group, the GOH5 group did not express the TGF-β pathway, possibly due to the presence of GOH5, which inhibited osteoclastogenesis. It also showed a strong interaction with pathways related to the PI3K-Akt signaling pathwayPI3K, a heterodimeric enzyme, plays a role in cell growth and programmed cell death, whereas AKT is a serine-threonine kinase that transmits signals promoting cell survival from growth factors. Pathways and their downstream signaling molecules are crucial for osteogenesis and closely associated with mesenchymal stem cell osteogenic differentiation.40, 41 We established a rat cranial bone defect model to observe the effect of bionic hydrogel on osteogenesis in vivo. the GOH5 group almost completely repaired the defect at Week 12, showing excellent bone defect repair capability. These results confirm the significant promoting effect of biomimetic hydrogels on in vivo osteogenesis, and the rate of calcification and maturation of bone-like tissue is accelerated with increasing n-HAP concentration, thereby accelerating bone healing.
We did not further validate the relevant pathways due to the limitation of conditions. Meanwhile, the animal model of rat cranial defects cannot perfectly represent the human bone regeneration situation.
4 CONCLUSION
In summary, we have designed a GelMA-c-OGP/nHAP hydrogel biomimetic scaffold with adjustable elastic modulus. This scaffold has a composition similar to natural bone and, by adjusting the nHAP content, it exhibits good porosity and mechanical properties. It enhances cell adhesion, integrates perfectly with bone tissue, and demonstrates excellent osteogenic ability both in vitro and in vivo. Mechanistically, the GelMA-c-OGP/nHAP scaffold can significantly enhance osteogenic activity through PI3K/AKT signaling, increasing the expression of osteogenic proteins such as BMP-2, OCN, and RUNX2. Our research results demonstrate the potential clinical application of GelMA-c-OGP/nHAP hydrogel biomimetic scaffolds and reveal that increasing the nHAP content in hydrogels to produce porous scaffolds with excellent biomechanical properties is a promising bone regeneration strategy, providing new inspiration for future tissue engineering and regeneration. In summary, we designed a bioactive hydrogel (GelMA-c-OGP/nHAP) with good porosity as well as mechanical properties, which showed the best osteogenic activity in the GOH5 group. However, this study still has some limitations.
5 MATERIALS AND METHODS
5.1 Cell isolation, culture, and treatment
Obtain femurs and tibias from the hind limbs of rats, remove surrounding muscle tissues, immerse femurs and tibias in alcohol, and then rinse them twice with PBS containing 1% penicillin/streptomycin. Cut both ends of the bone epiphyses with tissue forceps, rinse the marrow cavity with α-MEM culture medium containing 10% fetal bovine serum until the wash color changes from brown to orange. After filtering the bone marrow suspension through a 200 μm mesh sieve, centrifuge it. Discard the supernatant, and plant the cells in a 100 mm culture dish with α-MEM containing 10% fetal bovine serum. Incubate the culture dish in a 37°C, 5% CO2 incubator, change the culture medium every 3 days, and perform a 1:3 subculture.
5.2 Preparation of GelMA-c-OGP
Place 20 g of gelatin into 200 ml PBS, maintain a temperature of 60°C, and stir continuously for 2 h. Slowly add 1 mL of methyl methacrylate through a water-based membrane to the gelatin mixture, repeating this process 16 times, and continue stirring for 2 h. During this process, GelMA has already formed. Dilute this mixture in 800 ml preheated PBS solution and stir slowly for 15 min. Place the diluted solution in a dialysis bag (10,000 molecular weight) and change the dialysis bag twice a day to remove any unreacted methyl methacrylate, continuing the dialysis for a week. After dialysis, store the solution in a −80°C freezer, transfer it to a freeze dryer after 2 days, collect the dried samples, and place them in 50 mL centrifuge tubes with lids. Fifty milligrams lyophilized GelMA and 20 µg OGP (Methylacrylamide-GGGGG-YGFYY; Qiangyao Company) dissolved in 1 mL PBS and mixed evenly. Photoinducer 2-hydroxy-4’-(2-hydroxyethoxy)- 2-methylpropiophenone was added (1 wt-%; Sigma) the solution. By exposed to UV for 30 s, the GelMA/OGP hydrogel was formed. In addition, the photocrosslinkable OGP was produced by mixing OGP with methylacrylamide. In the same way, GelMA and photocrosslinkable OGP was in situ photocrosslinked to form a GelMA-c-OGP co-crosslinked hydrogel.
5.3 Physicochemical characterization of GelMA-c-OGP/nHAP
5.3.1 Fourier transform infrared spectroscopy (FTIR)
ATR-FTIR spectra were recorded using a FTIR spectrometer (Thermo Scientific Nicolet iS20) to analyze the coating's chemical composition and structure. The resolution is set at 4 cm−1, with 32 scans, and the tested wavenumber range is 520–4000 cm−1.
5.3.2 In vitro degradation performance
Weigh the scaffold W0, place it in a sealed 50 mL PBS centrifuge tube, and incubate it in a 37°C shaking bed at 60 rpm. Measure the remaining weight inside the centrifuge tube at designated time points (1, 3, 5, 7, 14, 21, 28, 35, 42 days) and denote it as Wt. Degradation rate = (Wt-W0)/W0 × 100%.
5.3.3 Scanning electron microscope (SEM)
After freeze-drying GelMA-c-OGP/nHAP in various ratios, employ the Quorum SC7620 sputter coater to gold-sputter for 45 s at 10 mA. Utilize a scanning electron microscope (FEI Scios 2 HiVac) to observe the microstructure and pore morphology of the scaffold material.
5.3.4 Swelling ratio measurement
5.3.5 Mechanical performance evaluation
Mechanical testing was conducted using a universal testing machine (INSTRON 3343). The GelMA-c-OGP/nHAP scaffolds, prepared in various ratios, were shaped into discs with a diameter of 12 mm and a thickness of 52 mm. Subsequently, compression tests were performed using the testing machine, and compression curves were generated.
5.3.6 Water contact angle
Employing the Theta Lite contact angle instrument (Biolin Science Company), place the crafted 1 × 1 cm flat samples on the contact angle testing platform. Utilize the device's automated titration system to drop water droplets and analyze the surface wettability of different samples.
5.3.7 Calcium ion release
To investigate the release behavior of Ca2+, specimens (n = 3) were immersed in 2 mL PBS and soaked in the dark at 37°C for 28 days. Collect the entire volume of the solution and refill with fresh PBS at specified time points (1, 2, 5, 7, 10, 14, 21, and 28 days). Subsequently, use inductively coupled plasma mass spectrometry (ICPMS; Agilent 7700) to measure the release spectrum and rate of Ca2+.
5.3.8 Nanoindentation
In Equation (1), P is the elastic indentation depth and a is the radius of the contact boundary. He is the elastic indentation depth. Reff and Eeff respectively denote the effective radius and effective modulus of the indenter and sample system. In Equation (2), v and E represent the Poisson's ratio and Young's modulus, with subscripts s and i indicating the sample and indenter. For consistency, we define Es as the sample material's Young's modulus. Sample modulus for indentation or simply modulus for indentation, or Eeff, stands for effective indentation modulus, and Es = 1n2s stands for indentation sample modulus. A rigid indenter produces the same effective indentation modulus as the indentation modulus. Under elastic load, as Rs approaches infinity, Reff = Ri.
5.4 Biocompatibility testing of GelMA-c-OGP/nHAP
First, place the scaffold into a 24-well plate, then resuscitate, centrifuge, and count BMSCs, planting them onto the scaffold in the 24-well plate (2 × 104 cells per well) (the control group directly plants BMSCs on the culture plate). Each group has three replicate wells. Add prepared α-MEM culture medium (containing 10% fetal bovine serum and 1% antibiotics), and incubate in a CO2 incubator (37°C, 5% CO2) for 24 h until the cells adhere and grow sufficiently. Change the α-MEM culture medium every 2 days, while the control group only changes the α-MEM culture medium on the culture plate. Perform live/dead staining and fluorescence microscopy observations after 1 and 3 days of cultivation.
5.5 Morphological observation of BMSCs implanted on the scaffold
Place five sets of materials into a 96-well plate, then resuscitate, centrifuge, and count BMSCs, planting them onto the scaffold in the 96-well plate (2 × 103 cells per well). Incubate the cells in a CO2 incubator (37°C, 5% CO2) for 24 h until they adhere and grow sufficiently. Change the α-MEM culture medium every 2 days, while the control group only changes the α-MEM culture medium on the culture plate. On the third day, perform cell staining with Hoechst 33342 and observe under fluorescence microscopy.
SEM: Following the Hoechst 33,342 staining steps, seed cells, and on the third day, remove the culture medium, fix cells with 4% paraformaldehyde for 30 min; dehydrate with ethanol gradient (10%, 20%, 35%, 50%, 70%, 80%, 90%, 100%) and critical point dry for 4 h; sputter coat with gold for 100 s. Accelerating voltage is set to 10 kV.
5.6 Proliferation activity assessment of BMSCs
Use the CCK-8 method to determine cell viability and proliferation activity. First, resuscitate, centrifuge, and count BMSCs, then plant them into a 96-well plate (2 × 103 cells per well). Add the prepared α-MEM culture medium (with 1% antibiotics and 10% fetal bovine serum) and the extracts from the five groups after 3 days of cultivation. The control group only receives α-MEM culture medium. Incubate in a constant-temperature incubator (37°C, 5% CO2) for 24 h until the cells adhere and grow sufficiently. Perform CCK-8 tests on Days 1, 3, 5, and 7 after cell adhesion.
5.7 ALP and ARS assays
BMSCs were cultured at a density of 2 × 104 cells/well in α-DMEM for 12 h. Subsequently, the culture medium was replaced with osteogenic induction medium containing bone-forming components (10 mM β-glycerophosphate, 0.1 μM dexamethasone, and 0.25 mM ascorbic acid), as well as the extracts from five groups cultured for 3 days. Further cultivation was carried out for 14 and 21 days. Cells were fixed with 4% paraformaldehyde and stained for ALP and ARS using BCIP/NBT working solution and Alizarin Red S (ARS) staining solution (pH 4.2; Beyotime). ALP activity was detected using an alkaline phosphatase assay kit (Beyotime), and semiquantitative analysis of ARS was performed by dissolving it in hydrochloric acid.
5.8 Reverse transcription PCR (RT-PCR)
RT-PCR: Total RNA was isolated from the BMSCs according to the standard protocol. The mRNA concentrations and purity were assessed using NanoDrop-2000 (Thermo Fisher Scientific). The mRNA expression was calculated using the method. Primer sequences for the target genes can be found in Table 1, Supporting Information.
| Gene | Primer Sequences (5′–3′) |
|---|---|
| BMP-2 | Forward primer: AACGAGAAAAGCGTCAAGCC |
| Reverse primer: AGGTGCCACGATCCAGTCAT | |
| RUNX2 | Forward primer: TCACAAATCCTCCCCAAGTGG |
| Reverse primer: GAATGCGCCCTAAATCACTGA | |
| GAPDH | Forward primer: ACTCCCATTCTTCCACCTTTG Reverse primer: CCCTGTTGCTGTAGCCATATT |
5.9 Immunofluorescence
Immunofluorescence staining was used to assess bone-related proteins. In brief, the cells were fixed with 4% paraformaldehyde, and 0.2% Triton X 100 was used to permeabilize them, 2% BSA was used to block them, and a primary antibody (RUNX2, 1:200; Abcam) was used to incubate them. Subsequently, cells were incubated with secondary antibodies, fluorochrome, and DAPI. Finally, observe the cells under a fluorescence microscope (Axio Imager M1).
5.10 RNA sequencing (RNA-seq) and bioinformatic analysis
BMSCs were cultured as described in 4.1 above. Three kinds of sterile scaffolds, cylindrical pore structures (C), gyroid curved surface structures (G), and diamond pore structures (D), were inserted into the 48-well plate and immersed in the medium for 0.5 h (n = 3). Then, 50 μL of cell suspension at a cell density of 2 × 106 cells/mL was added to each well. The culture medium was collected after 4 days of culture. The scaffolds were rinsed with PBS solution, and Trizol (Invitrogen) was added. Finally, three parallel samples were collected. The number and integrity of RNA were assessed using FastQC and RseQC software. Then, according to the protocol of Illumina (mRNA-SEQ Sample Preparation Kit), the cut RNA fragment was reverse-transcribed to generate the final cDNA library. Transcriptome sequencing and analysis were conducted by OE Biotechnology. The paired clean sequences were mapped onto the genome. The number of fragments per kilobase (FPKM) of the exon model was used to measure mRNA abundance using Hisat software. Log2 (Fold change) > or Log2 (Fold change) < −1 and was statistically significant (p < 0.05) covered by the edge. Gene ontology (GO) (http://www.geneontology.org) analysis supported the interpretation of the genes and the functions of the gene products. Biological information analysis was performed using the Sangerbox tool at http://vip.sangerbox.com/home.html.
5.11 Establishment of skull defect model
Thirty SD rats were anesthetized with Zoletil®50 (Virbac), and after removing the fur, circular defects with a diameter of 5 mm were prepared. The defects were then rinsed repeatedly with sterile saline two to three times, dried with sterile gauze, and the preprepared, UV-sterilized bone graft material was filled into the bone defect. Subcutaneous tissues and skin were sutured layer by layer. Postoperatively, a 3-day course of intramuscular penicillin injection (160,000 units of penicillin diluted in 5 mL saline, subcutaneously injected at 0.5 mL per rat, approximately 160,000 units per rat) was administered for prophylactic anti-infection treatment. The postoperative environment for rat growth was kept as a constant temperature of 25°C. Adequate feeding, prevention of cannibalism, and timely changing of bedding were ensured while closely monitoring the general condition of the rats. After 8 weeks postoperatively, samples were taken for Micro-CT detection and histological analysis.
5.12 Micro-CT analysis of isolated rat skulls
Perform Micro-CT scanning analysis on the isolated rat skulls 8 weeks postoperatively. The Micro-CT machine parameters are set as follows: voltage: 65 KV, current: 385 μA, resolution: 18 μm, rotation angle: 0.7°. Use the software on the workstation for three-dimensional reconstruction of the skull surface. In the bone defect area of the skull, select a cylindrical region with a diameter of 3 mm and a height of 150 layers for CT analysis. Record parameters such as BV (bone volume), TV (tissue volume), BV/TV (bone volume/tissue volume ratio), and conduct statistical analysis.
5.13 HE (Hematoxylin-eosin staining) and Masson's staining
After removing the samples 4 weeks postimplantation, fix them in 4% paraformaldehyde for 48 h, and decalcify them in a 10% ethylenediaminetetraacetic acid (EDTA) solution at indoor temperature for 1 week. Dehydrate the samples through a graded series of alcohols and embed them in paraffin blocks. Cut central sections of the embedded samples at a thickness of 5 µm, then stain with HE and Masson's trichrome to assess the bone formation area.
5.14 Statistical analysis
For an analysis of variance (one- or two-way) to determine the differences between groups, Tukey's multiple comparison test was used. Unless otherwise noted, data were presented as means and standard deviations. A p < 0.05 was considered statistically significant.
AUTHOR CONTRIBUTIONS
Haifu Sun: Writing—original draft; formal analysis; data curation; conceptualization. Chen Qian: Writing—original draft; formal analysis; data curation; conceptualization. Kai chen: writing—original draft; formal analysis; data curation. Yu Wang: Writing—review & editing; formal analysis; data curation. Yuqing Yang: Writing—review & editing; data curation. Yonggang Li: Writing—review & editing; data curation. Fan Xu: Writing—review & editing; data curation. Liang Chen: Writing—review & editing; methodology. Kun Li: Writing—review & editing; methodology; funding acquisition. Youzhi Hong: Writing—review & editing; supervision; investigation; funding acquisition. Yusen Qiao: Writing—review & editing; writing—original draft; supervision; methodology; investigation; conceptualization. Dechun Geng: Writing—review & editing; writing—original draft; supervision; resources; project administration; methodology; investigation; formal analysis; conceptualization. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
Thank you for all the support from the First Affiliated Hospital of Soochow University. We would like to thank BioRender.com for providing the tools used to create the figures in this manuscript. The illustrations generated through BioRender significantly enhanced the clarity and visual presentation of our findings. This work was supported by the National Natural Science Foundation of China (82072425, 82072498, 82272567), the Natural Science Foundation of Jiangsu Province (BK2021650).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Approval was obtained from the ethics committee of Soochow University. Approval No. SUDA20220925A01. All authors approved the manuscript and its publication.
Open Research
DATA AVAILABILITY STATEMENT
The data sets used and/or analyzed during the current study available from the corresponding author on reasonable request. We have uploaded the RNAseq data to the CNCB database. GSA: CRA019039.




