Recent advances and perspectives of multifunctional nanogels in biomedical applications
Abstract
Nanogels (NGs) are considered as a kind of nanoscale hydrogels (<200 nm) endowing with the functions of both nanomaterials and hydrogels. In the last 20 years, NGs have garnered significant attention due to their versatility and adaptability. Herein, a comprehensive overview of the latest advancements and current research status of NGs is provided, with a particular focus on the synthesis strategies involving physical and chemical cross-linking methods, as well as the advantages of NGs in drug loading and responsive release. Based on the diverse design strategies of NGs, four key biomedical applications, including inflammation therapy, regenerative medicine, bioimaging and tumor therapy are further summarized and discussed. Moreover, the existed inherent challenges facing NGs are proposed, while highlighting their potential to revolutionize therapeutic and diagnostic approaches. Finally, we look forward to the further development and promising potentials of NGs in biomedical applications. This review aims to serve as a valuable reference for researchers, providing some insights into the evolving landscape of NGs and their potential in advanced biomedical applications.
1 INTRODUCTION
In 1999, Akiyoshi et al.1 pioneered the synthesis of a nanoscale three-dimensional polymer mesh system synthesized from polyethyleneimine (PEI) and polyethylene glycol (PEG), which was defined as the nanogels (NGs), and the concept of NGs has gradually become known to the general public since then. After more than two decades of development, NGs are now normally defined as nanoscale (usually tens to hundreds of nanometers) hydrogel particles formed from hydrophilic or amphiphilic polymer chains by physical or chemical cross-linking.2-4 Different from the conventional hydrogels, the size, electronegativity, amphiphilicity, flexibility, and degradability of NGs can be easily adjusted by altering the chemical makeup of NGs. Moreover, the structural diversity of NGs allows them to encapsulate different kinds of guest molecules (e.g., inorganic nanoparticles, plasmids, small interfering RNA [siRNA], pharmaceutical small molecules, and biomacromolecules) for flexible applications in different biomedical fields.5 Meanwhile, NGs usually have good safety, biocompatibility and degradability and low toxicity due to their composition of generally natural polymers (e.g. hyaluronic acid, chitosan, gelatin, etc.).
Compared to other NG drug delivery system, NGs are often favored by researchers, mainly due to their unique advantages. Firstly, the soft structure of NGs mimics the extracellular matrix (ECM), and their ease of modification ensures high stability in the bloodstream and internal aqueous environments of organisms without triggering unwanted immune reactions.6, 7 Besides, appropriate size (<200 nm) of NGs evades the reticuloendothelial system (RES), which is conducive to improving their active and passive targeting performance and greatly increasing the drug enrichment rate in the target region8-10; The last but not the least, the size, refractive index, permeability and hydrophilicity of certain NGs could vary depending on environmental stimulation such as heat, temperature, light and pH and so forth, which would be beneficial in regulating drug loading and release.11-14
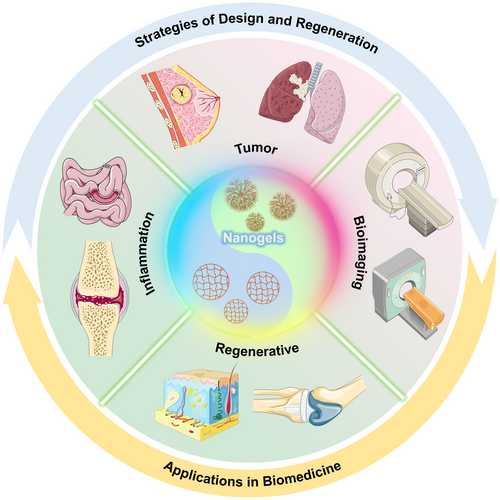
Herein, we summarize the recent research advances in multifunctional NGs utilized in disease diagnosis and treatment over the last few years, focusing on inflammation therapy, regenerative medicine, bioimaging, and oncology (Figure 1). The main content involves in the examination of fundamental preparation techniques, innovative NGs fabrication methods, various influences on the physicochemical characteristics of NGs, and recent advancements in biomedical uses, and so forth. The NGs discussed in the review include poly (methacrylic acid) (PAA), PEG, oxidized hyaluronic acid and hyaluronic acid (HA), and so forth. At end, we address the challenges and promising advancements in multifunctional NGs materials and anticipate that it will contribute new reference ideas for the design and application of multifunctional NGs materials.
2 SYNTHESIS STRATEGY OF NGS
With the growing interest of researchers in the field of NGs, numerous synthesis strategies have emerged, which can be mainly classified into noncovalent crosslinking (physical crosslinking) and covalent crosslinking (chemical crosslinking) based on the cross-linking mechanism.15-19 It should be noting that both covalent and noncovalent interactions play unique roles in NGs synthesis (Figure 2).
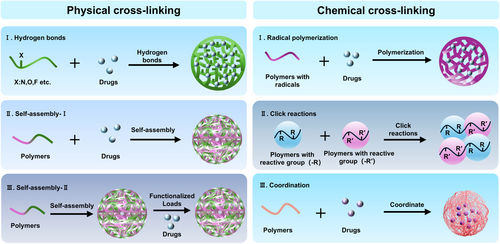
2.1 Physical crosslinking
Physically crosslinked NGs are synthesized through noncovalent interconnections, therein, the crosslinking bonds are typically formed by weak forces like hydrogen bonds, van der Waals forces, or electrostatic forces. These interactions generally occur during the gel synthesis process or between NGs and their cargo. Thanks to these noncovalent interactions, NGs excel as carriers for loading with various payloads, including small molecule drugs, nucleic acids, and proteins, and so forth.20-22 More importantly, this synthesis method is straightforward to implement, and the obtained NGs can disassemble in response to changing external conditions due to the relatively weak noncovalent bonds, facilitating the release of the encapsulated drug.
Hydrogen bonding occurs predominantly between atoms that are highly electronegative and have small atomic radii, such as oxygen, fluorine, and nitrogen, with at least one of these atoms bonded to a hydrogen atom.23, 24 Fan et al.25 developed a supramolecular polymer named PECH–PEG–Cy that includes cytosine pendant groups held together by hydrogen bonds, hydrophilic side chains made of poly (ethylene glycol), and a hydrophobic backbone of poly (epichlorohydrin), which allows cytosine molecules to target cancer cells, enhance drug delivery and release, and increase the safety and effectiveness of chemotherapy (Figure 3A). By physically linking together, these polymers come together to create reversible network structures and held together by cytosine, resulting in the formation of spherical NGs in water. NGs with a high concentration of hydrogen bonding networks exhibited exceptional stability in cell culture matrices containing serum over extended periods, while those with a lower concentration were unable to remain intact.25 Additionally, by utilizing the layer-by-layer (LbL) hydrogen bonding binding force of gelatin and tannic acid (TA), Lei et al.26 designed a system of single-cell NGs with externally modified borate esters attached to triiodothyronine (T3). Gelatin can on the one hand provide noncovalent forces for primary encapsulation of the nanoparticles by electrostatic forces and provide TA-binding sites through hydrogen bonding, on the other hand, prevent TA from directly interacting with the cell membrane thus avoiding cell death.26
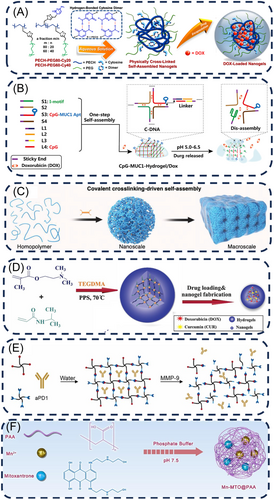
Furthermore, DNA nanostructure-based drug delivery systems (DDS) have gained prominence for their editability, biodegradability, and multifunctionality.32 Wei et al.27 reported a DNA-loaded NGs DDS by a one-step self-assembly strategy. They combined pH-sensitive I-motif sequences with targeted MUC1 inducer-immunoadjuvant CpG fusion sequences (CpG-MUC1) to form cross-shaped DNAs (C-DNAs) with the help of a DNA linker, and the size of the NGs could be controlled by modifying the DNA linker composition. Following that, Doxorubicin (DOX) was incorporated into the nucleotide pairs of the NGs to create CpG-MUC1-NGs/DOX, which disintegrated to release DOX and CpG in acidic environment (Figure 3B).27
Traditional noncovalent self-assembly suffers from drawbacks such as low stability, process complexity, and intricate fabrication techniques.33-37 Unlike conventional noncovalent self-assembly mechanisms, Bai et al. proposed a novel approach to synthesizing NGs through a self-assembly strategy (Figure 3C).28 By precisely adjusting the covalent cross-linking rate, they synthesized NGs with a novel covalent polymer self-assembly mechanism to achieve the particles sizes ranging from below 10 nm to over 100 nm, which is anticipated to create multi-scale materials for various uses such as drug delivery and tissue engineering.
2.2 Chemical crosslinking
The formation of covalent bonds by chemical cross-linking during the synthesis of NGs is usually more stable and compact than those synthesized by physical methods.38, 39 The chemically synthesized NGs generally are classified based on their reaction types and bonding modes, including radical polymerization,40 click reactions,41 ring-opening polymerization,42 coordination,43 disulfide formation,44 enzymatic crosslinking,45 carboxyl–amine reactions, carboxyl–hydroxyl reactions, and so forth, and each of these reactions is described in detail below.
Radical polymerization stands as one of the most prevalent principles in NGs synthesis strategies. Free radical polymerization has wide applications in NGs synthesis owing to its benefits of mild reaction condition, facile reaction control, and broad temperature regulation. Employing the mechanism of free radical polymerization enabled the realization of multi-system synthesis strategies for NGs, such as emulsion, mini emulsion, microemulsion, and precipitation.46 In recent years, researchers have synthesized plenty of functionally diversified and size-adjustable NGs through free radical polymerization.47, 48 For instance, Qi et al.49 proposed a method named ATRPase that uses enzymes to catalyze atomic-transfer radical polymerization, resulting in the creation of biocompatible polymer brushes through interfacial polymerization of N-acryloyl-l-lysine amino-acid monomers, followed by using ferric ions as crosslinkers to strengthen the hydrogel structure, resulting in polymeric NGs with effective multienzyme-like functions (e.g., SOD and POD).49 Abedi et al.29 also synthesized nontoxic pH/thermal dual-responsive NGs through free radical polymerization. In their synthesis procedure, the water-soluble cationic monomer N,N′-dimethylaminoethyl methacrylate (DMAEMA), containing a tertiary amine group, undergoes free radical polymerization with the thermosensitive polymer PNIPAAm (Figure 3D). Additionally, silver/poly(3-aminophenylboronic acid)/sodium alginate NGs (Ag@PABA-SA) were created using green in situ chemical oxidative polymerization by Titilope John Jayeoye et al. Boronic acids could effectively binding with cis-diol compounds through covalent bonding to form boronate esters.50 Thus, the interaction between sodium alginate (SA) and 3-aminophenyl boronic acid (APBA) occurs through covalent interaction, facilitated by the numerous hydroxyl groups present in SA.51
Click chemistry, introduced by Sharpless, encompasses simple, efficient, and selective reactions that proceed rapidly and yield high products. These reactions are tolerant of diverse functional groups, with the copper-catalyzed azide-alkyne cycloaddition (CuAAC) being a prominent example.52 As a novel and practical approach to the formation of carbon-heteroatom-carbon bonds in aqueous environments, it has wide applications in the chemical and biological fields.53 Afterwards, due to the toxicity of copper catalysts, researchers later developed copper-free click reactions, including the Diels–Alder (DA) reaction, the strain-promoted azide-alkyne cycloaddition (SPAAC) reaction, the thiol-alkene coupling reaction, the antielectron demand DA reaction, and the tetrazolium-alkene photo-click reaction.54 For instance, Zhao et al.30 devised a method to synthesize reactive poly(ethylene glycol) (PEG) NGs through linking four-armed PEG using copper-free click chemistry (Figure 3E). In this material, the use of PEG with outstanding biocompatibility eliminates the requirement for possibly harmful catalysts or linking agents and enables high amounts of immune checkpoint inhibitors (ICIs) to be enclosed in situ, with the three-dimensional mesh structure of the NGs guaranteeing exceptional colloidal stability of the substance during atomization.30 Additionally, Nagel et al.55 employed surfactant-free reverse nanoprecipitation to prepare NGs using strain-promoted click chemistry, in which the degree of functionalization of dendritic polyglycerol (dPG) with cyclooctyne groups and the peptide crosslinker component modulated the sizes and cross-linking density of NGs. They utilized the intrinsic crosslinker reporter fraction to explore the effect of NGs composition on the degradation profile.55 Furthermore, Fan et al.56 synthesized biocompatible and degradable NGs via thiol-click chemistry using a hyperbranched dendrimer-linear-dendrimer copolymer (HBDLD) system, and achieved the modulation of NGs particle size and weight by varying the length or dry weight percentage of PEG.
Coordination reaction constitutes a class of reaction in which compounds with a specific chemical structure formed by the interaction of a central atom (or ion, collectively referred to as the central atom) with surrounding molecules or ions (known as ligands), and this interaction occurs through wholly or partial coordination bonds.57 Compared to other chemical cross-linking methods, coordination offers several distinct advantages. First, the straightforward and convenient coordination of metal ions with ligands enables rapid preparation of large quantities of NGs using a one-pot method.58 Second, since metal ions and their corresponding ligands have multiple types, enabling construction of various types of NGs through metal particle coordination.59 Finally, the action of ligands is reversible, allowing for the breaking of ligand bonds within the organism under specific conditions, thus achieving controllable drug release.60 For instance, Zhu et al.31 formed NGs through co-assembly of manganese, the chemotherapeutic drug Mitoxantrone (MTO), and PAA via ligand interactions under neutral condition (Figure 3F). The obtained material underwent decomposition in the weakly acidic tumor microenvironment (TME), thereby facilitating the release of drugs and metal ions.
3 NG DRUG DELIVERY SYSTEMS
In recent years, drug delivery systems have undergone significant advancements, particularly the “nanoparticle drug delivery system” that utilizes nanomaterials as carriers, has attracted considerable attention from researchers, focusing on the construction of nanoscale particles (1–1000 nm) to enhance drug loading and encapsulation efficiency.61, 62 Up to date, various types of nanoparticle drug delivery systems have been developed, including inorganic/metal-based systems such as silicon-based nanoparticles,63 quantum dots,64 metal oxide nanoparticles,65 and so forth, lipid-based systems like liposomes,66 lipid nanoparticles (LNPs)67 etc., and organic material-based systems, such as polymersomes,68 micelles,69 and dendrimers,70 and so forth. Although these nanoparticle drug delivery systems have demonstrated excellent delivery capabilities, there are several challenges remaining. For example, those issues related to cost, preparation processes, drug loading efficiency, nanoparticle stability, drug controlled release mechanisms, degradability of the nanoparticle delivery systems, as well as biocompatibility and biosafety still needed to be addressed.71
As considered a new generation of nanodrug delivery systems,72 NGs enable to simplify the complexity of current nanodrug delivery systems and address possible limitations of conventional methods. First of all, NGs are relatively easy to prepare, exhibit excellent biocompatibility, hydrophilicity, and responsiveness to various stimuli (such as temperature, pH, light, ultrasound and electro, etc.),73 and offer tunable sizes. As discussed above, NGs are nanoscale versions of hydrogels, combining the benefits of both nanomaterials and hydrogels. The small size, large specific surface area, and complex three-dimensional mesh structure of NGs allow for the formation of hydrophobic pockets during polymerization, which enhances the solubility of hydrophobic drugs and facilitates their high loading and efficient transport. Besides, like hydrogels, NGs possess surfaces rich in multifunctional groups such as –OH, –COOH, –NH2, and –SO3H,74 which are endowing with a unique balance of hydrophilicity and hydrophobicity, as well as multiple reactive sites.
The typical drug loading capacity of conventional nanoparticle drug delivery systems ranging from 5% to 25%.75, 76 At the same drug dosage, a higher drug loading in nanocarriers means fewer carriers are needed, which in turn can reduce the overall toxicity to the body. Compared to other nanoparticle-based delivery systems, NGs can achieve a drug loading capacity of up to 10%–30%,3, 4, 77 significantly higher than that of common nanocarriers. This superior drug loading capacity is primarily due to the excellent swelling properties of NGs, which enable them to absorb large amounts of the aqueous phase.
Drugs are loaded into NGs using various methods, which are generally categorized into two main approaches, that is, incorporation during synthesis and loading after synthesis.78 The former typically involves chemical interactions, such as covalent coupling, while the latter primarily relies on physical interactions, such as electrostatic adsorption. Physical adsorption is a straightforward and efficient method for drug encapsulation, where drugs are adsorbed onto the surface or within NGs through electrostatic interactions, hydrogen bonding, or van der Waals forces. Zhu et al.79 synthesized DNA dendrimer NGs (DNGs) via one-step self-assembly of dendrimer DNA polymers. Due to the negative charge of DNA molecules and the positive charge of DOX molecules, they can be bonded together through electrostatic interactions, which allows DOX to be either adsorbed onto the surface of the DNA NGs or embedded within their internal structure. Authors quantified the loading efficiency of DNGs on DOX using the ratio of DOX molecules to the number of molecules per base pair (RDOX = NDOX/Nbp). Their results demonstrated that RDOX could reach up to 154 DOX molecules adsorbed per base pair, with a drug loading capacity (DLC) as high as 98.7%.79 Additionally, Chen et al. synthesized a quercetin-alginate NG (QU-Nanogel) via emulsion polymerization, utilizing alginate and quercetin as the primary components. In this process, the antioxidant drug quercetin was predominantly incorporated into the NG structure through hydrogen bonding. Interestingly, the o-hydroxyl group in quercetin exhibits a stronger reactive oxygen species (ROS) scavenging ability compared to the meso-hydroxyl group, thereby enhancing its antioxidant effect. This is because the hydrogen atom in the o-hydroxyl group facilitates the formation of a more stable DPPH-H molecule from DPPH, allowing quercetin to establish a resonance-stable semiquinone radical structure. The drug loading and encapsulation efficiencies of the NGs were determined to be (0.92 ± 0.02)% and (97.7 ± 1.2)%, respectively, by analyzing the mass of the lyophilized NGs in comparison with the initial mass of quercetin.80
Compared to physical adsorption, chemical cross-linking offers greater stability, and most NGs synthesized through this method can release the loaded drug in response to specific conditions. Wang et al. developed a cross-linking agent, DBHD, synthesized by coupling two 4-nitrophenyl chloride (NPC) molecules via disulfide bonds, which are sensitive to glutathione (GSH). Utilizing DBHD, along with DOX and 5-aminolevulinic acid (ALA), they synthesized DSA NGs (DOX and PS precursor ALA NGs). Upon accumulation at the tumor site, the DSA NGs are decomposed by the elevated levels of GSH in the tumor microenvironment, leading to the release of DOX, which effectively kills the tumor cells.81 Additionally, Yao et al.82 developed a versatile prodrug NG platform utilizing PEG-tethered methacrylic acid (PEGMA) and bis(ethylene methacrylate) disulfide (DSDMA). This platform was designed to activate the drug via GSH reduction, specifically targeting the treatment of malignant tumors that are resistant to paclitaxel-based therapies, which could increase drug concentration at the tumor site while reducing systemic toxicity. The hydrophobic drugs cabazitaxel (CTX) and PI3K inhibitor (PI103) were loaded into the NG, where they were covalently coupled through disulfide bonds. This coupling prevents premature drug leakage during blood circulation and allows for targeted activation within cancer cells. The final drug encapsulation efficiency, determined by high-performance liquid chromatography, was over 95% for both drugs.82
4 RELEASE MECHANISMS OF DRUGS FROM NANOGELS
Due to the diverse composition and properties of NGs, the encapsulated drugs can be released through various mechanisms mainly depending on the characteristics of the polymers used. First, from the perspective of raw materials, the type of polymer, functional groups,83 swelling behavior,84 and hydrophilicity85 or hydrophobicity85, 86 all significantly influence drug release. Additionally, during the synthesis process, the degree of cross-linking also plays a crucial role.87 On one hand, cross-linking greatly affects the mechanical properties of NGs, such as hardness, stiffness, and elastic modulus.88 Generally, higher cross-linking increases the rigidity and stability of the gel, enhancing its resistance to deformation and external forces. However, this may also hinder its circulation in the body, making it more prone to clearance by the RES.10 Conversely, lower cross-linking results in a softer NG with better deformability, but this may impede cellular uptake. Therefore, when designing NGs, the cross-linking degree should be tailored to the specific characteristics of the target lesion. On the other hand, the degree of cross-linking could influence the swelling behavior of NGs. Higher cross-linking, characterized by more cross-linking points, restricts the mobility of polymer chains, resulting in a denser network structure within the NG.78 This reduces the space for water molecules to penetrate the gel, thereby decreasing its swelling capacity and subsequently affecting drug release. Typically, higher cross-linking may slow down drug diffusion, thereby extending the release time, while lower cross-linking may accelerate drug release.
Shah et al.72 summarized the release mechanisms of NGs, which mainly include the following five aspects: (1) diffusion, (2) erosion of the NG matrix, (3) changes in the surrounding pH, (4) release via displacement by counterions present in the external environment, and (5) triggered release by external energy sources such as magnetic fields or light. The current NG designs are focused primarily on the latter three mechanisms. Table 1 summarized some typical design strategies of responsive NGs. For example, NGs are tailored to respond to the pH environment of specific pathological regions by using polymers with different functional groups that undergo protonation or deprotonation reactions under varying pH conditions. In details, carboxyl groups are protonated to form neutral molecules (–COOH) at low pH and deprotonated to form negatively charged ions (–COO–-) at high pH.89 Besides, smart responsive NGs containing disulfide bonds are designed for the high-GSH environment of tumor microenvironments. The disulfide bond (–S–S–) is a reducible chemical bond that can be cleaved by GSH through its thiol groups (–SH), resulting in the formation of two –SH groups.90 This process leads to the depolymerization or dissolution of the material, thereby triggering drug release or other responsive behaviors. In our previous work, a novel functional chitosan polymer (CS-FTP) was designed using thioketal (TK) as a ROS-responsive linker. Meanwhile, pazopanib (PAZ) was conjugated to the CS backbone and a hypoxia-activated prodrug AQ4N was physically encapsulated into polymer network during sol–gel transition. In the tumor microenvironment with over expressed ROS, the structure of CS-FTP was broken via TK moiety to achieve a stimuli responsive drugs release, eventually contributing to the synergistic starvation therapy with AQ4N-based chemotherapy in an orthotopic 4T1 breast tumor model. This study offers a precise approach of drug delivery through responsive polymer design to achieve improved therapeutic efficacy and inhibiting cancer metastasis.91
| Responsive condition | Responsive components | Ref. |
|---|---|---|
| pH | Poly (N-isopropylacrylamide) (PNIPAM) and poly (N-Acryloyl-l-phenylalanine) (PAphe) | [82, 92] |
| Poly[methacrylic acid-co-(poly(ethylene glycol) methyl ether methacrylate)-co-dopamine-co-N,N-bis(acryloyl)cystamine], poly(MAA-co-PEGMA-co-DAA-co-BACy) | [93] | |
| Nanogel gold nanorod@poly(n-isopropylacrylamide)-vinyl acetic acid (AuNR@PNIPAM-VAA) | [94] | |
| 5-Methyl-5-pentafluorophenyloxycarbonyl-1,3-dioxan-2-one (MTC-PFP) | [95] | |
| Oxidized dextran (Dex) forming aldehyde groups, hydroxylated generation five (G5) dendrimer (Den) with 25% amines | [96] | |
| GSH | Poly[methacrylic acid-co-(poly(ethylene glycol) methyl ether methacrylate)-co-dopamine-co-N,N-bis(acryloyl)cystamine], poly(MAA-co-PEGMA-co-DAA-co-BACy) | [93] |
| P(NIPAAm-co-DMAEMA) | [29] | |
| β-Cyclodextrin-conjugated hyaluronic acid (HA-βCD) and polyethyleneimine (PEI), | [97] | |
| Poly(ethylene glycol)−poly(l-glutamic acid-co-l-cystine) | [98] | |
| Poly(N-vinylcaprolactam) (PVCL) | [99] | |
| Granzyme B (GrB) and pore-forming peptide (GALA) | [100] | |
| Thermo-responsive | Poly(N-isopropylacrylamide-co-acrylic acid) (PNA) | [11] |
| Bile acid and β-cyclodextrin | [101] | |
| (2-((2-(Methacryloyloxy)ethyl) dimethylammonio)acetyl) (phenylsulfonyl) amide (MEDAPA), poly(MEDAPA)-based (PMEDAPA), ethyleneglycol dimethacrylate (EGDMA) and bis(acryloyl)cystamine (BAC) | [102] | |
| ROS | Mannose (MAN), indocyanine green (ICG) and cross-linker (PSe-Se) | [103] |
| Organometallic photosensitizer (TIr3) and hyperbranched polyglycerol (PG) | [104] | |
| Cyclodextrin (CD) and adamantane (ADA) | [105] | |
| Pituitary adenylate cyclase-activating polypeptide (PACAP) and hyaluronic acid (HA), | [106] |
Generally, NGs exhibit stability in both kinetic and thermodynamic aspects, providing sustained or responsive release characteristics, which can achieve zero-order release kinetics for drug delivery. The fundamental kinetic models used to evaluate drug release from NGs include zero-order kinetics, the Higuchi model,107 the Korsmeyer–Peppas model,108 and the Weibull model.109 Among these, most systems follow and Peppas kinetics. In NGs, the Higuchi model describes the slow release of drugs from the gel matrix through diffusion, with the release amount proportional to the square root of time.110 The Peppas model, however, is more versatile and applicable to various release mechanisms in NGs, especially when NGs possess complex structures or functionalities.111 Based on the release exponent in the Peppas model, it is possible to determine whether drug release from NGs is primarily controlled by diffusion or other mechanisms, such as swelling or environmental stimuli.
5 APPLICATIONS OF MULTIFUNCTIONAL NGS
As discussed above, Nanogels have received extensive attention from a wide range of researchers because of their excellent drug loading and release capabilities. In the past decade, researchers have achieved remarkable strides in the design, synthesis and functional optimization of NGs. This section offers an overview of some typical applications of NGs in inflammation therapy, regenerative medicine, bioimaging and oncology.
5.1 Inflammation therapy
Inflammation is the body's natural response to internal or external stimuli, often presenting as redness, swelling, warmth, discomfort, and impaired function.112 The inflammation can be categorized into infectious inflammation and noninfectious inflammation. Moreover, inflammatory disease microenvironments generally have high levels of inflammatory cell subsets and generate large amounts of lactic acid through active metabolism produced by stromal fibroblasts and infiltrating immune cells. NGs have endowed with following advantages in inflammation therapy. For example, NGs can be used as carriers to deliver anti-inflammatory agents such as antibiotics,113 nucleic acids,114 and other compounds with anti-inflammatory properties to the site of the lesion, thereby achieving therapeutic effects. Besides, due to the diverse and multifunctional composition of NGs themselves, some NGs inherently possess the ability to modulate inflammation.115
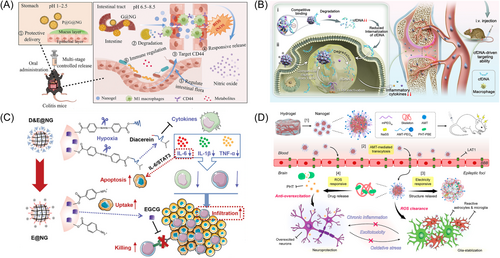
Very recently, Fu et al.116 designed a kind of NGs based on responsive release of nitric oxide (NO) by targeting inflammatory tissue to alleviate inflammatory bowel disease (IBD). The aldehyde moiety of altered oxidized hyaluronic acid reacted with two amino groups of l-arginine through a Schiff base reaction, which, results in the cleavage of the Schiff base and subsequent release of NO upon activation at the site of inflammation. Following this, gelatin microspheres were utilized for microencapsulation and modification to create gelatin@nanogel (G@NG) microcapsules, which were then coated with polydopamine to form PDA@gel@nanogel (P@G@NG) microcapsules (Figure 4A).116 They found that the proportion of beneficial bacteria Mycobacterium and Prevotella-UGG-001 increased, whereas the proportion of harmful bacteria Enterobacteriaceae and Aspergillus decreased in the gut microbiome of DSS mice that received P@G@NG as a preventive measure.
For the treatment of osteoarthritis, Sun et al.119 devised a self-enhancing NGs (KZIF@HA) by incorporating hyaluronic acid (HA) onto a ZIF-8 surface loaded with kartogenin (KGN) in which leveraging the inherent hydrophilicity of HA, the NGs spontaneously formed, ensuring prolonged drug release within the osteoarthritic (OA) microenvironment. Besides, the controlled release of KGN from KZIF@HA stimulated the secretion of cartilage extracellular matrix, thus fostering hyaline cartilage regeneration. Transcriptome sequencing validated that KZIF@HA induced M2-type macrophage polarization, leading to the secretion of IL-10 to inhibit the JNK and ERK pathways, thereby promoting chondrocyte recovery and enhancing ECM remodeling.119
Apart from aforementioned therapeutic strategy for osteoarthritis, Dai et al.120 developed an injectable NGs aimed at achieving sustained release of siRNA targeting Cd61-integrins (RGD-NG/siRNA Cd61). RGD-NG demonstrated exceptional biocompatibility both in vitro and in vivo, along with precise targeting capabilities, and local administration of RGD-NG/siRNA Cd61 significantly mitigated cartilage degeneration and reduced subchondral bone mass in osteoarthritic mice.120 Furthermore, beyond siRNA delivery for osteoarthritis, elevated levels of free DNA (cell-free DNA, cfDNA) were observed in the serum and joint cavity of rheumatoid arthritis (RA) patients, with levels approximately three orders of magnitude higher than those found in healthy individuals. The circulating cell-free DNA, which is released by cells that have been damaged, has the ability to trigger the Toll-like receptor-9(TLR-9) signaling pathway, a key player in the development of RA.113, 119 To address this problem, Zhu et al.117 proposed a strategy involving cationic peptide dendritic macromolecular NGs conjugated with deoxyribonuclease I (DNase I) for RA treatment. Notably, by optimizing the surface charge density and size of the cationic polymers, these NGs superior targeting ability, enhanced accumulation, prolonged duration, and improved DNA clearance in inflamed joints (Figure 4B). Leveraging these characteristics, the researchers demonstrated that these organelle-mimicking cationic NGs effectively downregulated the TLR-9 signaling pathway and alleviated RA symptoms in mice with collagen-induced arthritis.117
Besides directly modulating inflammatory lesions, NGs also offer an indirect approach to curing other diseases by improving the inflammatory environment. Luo et al.114 designed a hypoxia-responsive NGs to enhance the delivery and infiltration of epigallocatechin gallate (EGCG) and Diacerein in tumors. Upon injection into the bloodstream, diacerein was initially released in the tumor microenvironment, effectively blocking the entry of regulatory T cells (Treg) and myeloid-derived suppressor cells (MDSCs) by changing the tumor inflammatory environment, which also led to tumor cell death by blocking the IL-6/STAT3 signaling pathway, resulting in strong antitumor effects (Figure 4C).114
For the realm of epilepsy therapy, Zhou et al.118 engineered a NGs that respond to ROS by using phenytoin (PHT) as a precursor drug (Figure 4D). Upon tail vein injection, NGs could enrich at the lesion site by passive targeting, and released PHT centered on epileptic foci to deplete environmental ROS in the lesions, thereby reducing oxidative stress and alleviating the inflammatory microenvironment. They demonstrated that the NGs ultimately inhibited the over-excited circuits through target accumulation, remodeling the epileptic circuits and microenvironments, thereby improving the therapeutic efficacy for epilepsy.118
5.2 Regenerative medicine application
Regenerative medicine means the use of biological and engineering techniques and technologies to create lost or functionally impaired tissues and organs with the structure and function of normal tissues and organs, restoring physiology to a state similar to the original state.121 For example, researchers can use three-dimensional (3D) printers could create tissues and organs.122-124 Advances in stem cell technology also offer the possibility of treating chronic diseases.125 Additionally, scientists were taking inspiration from the animal world, with species such as salamanders having the ability to regenerate limbs.126 A deeper understanding of the cellular mechanisms behind this ability could lead to the development of new technology. In addition to the advantages of controlled release of active substances and adjustable mechanical strength, NGs are found to have the advantage of resembling ECM. Consequently, NGs are frequently utilized by researchers in the field of bone tissue regeneration and wound repair, and have achieved great research progress. This section offers an overview of the development and application of NGs in regenerative medicine.
5.2.1 Bone tissue regeneration
The primary components of bone include extracellular matrix, hydroxyapatite, calcium phosphate, and cells such as osteoblasts and osteoclasts, of which osteoblasts and osteoclasts were mainly responsible for the formation, resorption, and repair of bone tissues, and therefore play a vital role in the regeneration process of bone tissues.127, 128 Currently, NGs primarily influence bone tissue regeneration by constructing suitable scaffolds and delivering bioactive substances.129, 130 For example, Tang et al.131 produced heparin/PEI NGs by self-assembling on multi-hollow BCP protein ceramics made with 3D printing using digital light processing as the base material (Figure 5A). The NGs were filled with BMP2 and attached to the substrates using a layer of dopamine and dihydroxyphenylacetic acid to create a BCP/layer/NP/BMP2 scaffold that could release BMP2 continuously, resulting in a bioactive scaffold with strong osteogenic abilities. The loaded heparin/PEI NGs synergistically enhances the delivery of bioactive factors such as BMP2.131 Furthermore, Fan et al.132 synthesized a novel magnetic NGs containing Fe3O4, chitosan, and heparin, through a specific nucleobase pairing, which was found to achieve high adsorption efficiency of BMP2 protein. When subjected to a magnetic field, the substance exhibited directional release of BMP2 protein, significantly enhancing the survival of MG-63 cells.132
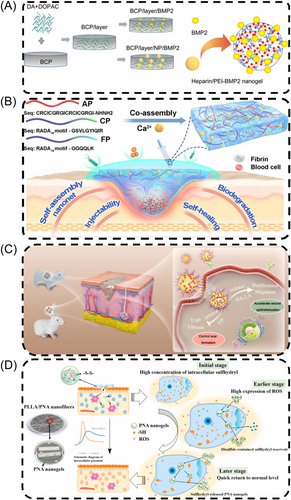
5.2.2 Wound repair
The process of wound healing is intricate and well-coordinated, involving four distinct biological stages: wound hemostasis, generation of inflammation, cell proliferation, and wound remodeling, with the ultimate goal of restoring damaged tissue.135, 136 Hydrogels have traditionally been used in wound healing for their capacity to conform to different shapes, high drug loading capabilities, and compatibility with the body's natural ECM.137-139 NGs, as a class of hydrogel, also exhibit similar properties and have recently gained prominence in wound healing applications due to their multifunctionality. Chen et al.133 engineered verteporfin (VP)-loaded NGs using hyaluronic acid and polylactic acid (PLA), which exhibited targeted delivery to fibroblasts when topically applied to wounds. Afterwards, NGs release HA and LA to promote the growth and movement of fibroblasts, while NGs loaded with VP prevent fibrosis by reducing the expression and nuclear localization of specific proteins (Figure 5C). Notably, traditional hydrogels usually help to minimize scar formation but do not completely eliminate scars in wound repair, whereas VP-loaded NGs controlled scar formation by accelerating wound re-epithelialization, promoting scarless wound healing.133 Moreover, certain wounds, such as chronic wounds, cannot heal within a short timeframe due to external conditions or underlying diseases.140 Inspired by humanized defensin nanonets, recently, Yuan et al. created a combination of RADA16 hydrogelators with θ-defensin peptide, namely ACFP, which exhibited rapid coagulation, strong antibacterial effects, and promotion of wound healing. The ACFP has also been demonstrated to be biocompatible, biodegradable, and injectable, further enhancing its therapeutic potential (Figure 5B).141
ROS play a crucial role in controlling cell growth, vascular development, and skin cell migration, all of which are critical in the wound healing process.142, 143 Zhang et al.134 synthesized a novel redox-responsive poly(N-isopropylacrylamide-acrylic acid) (PNA) NGs through free radical emulsion polymerization with disulfide-bond-containing compounds as cross-linkers, which was integrated into poly(l-lactic acid) (PLLA) nanofibrous membranes to create bioresorbable membranes with balanced levels of ROS and sensitive to redox reactions. In comparison to individual PLLA layers, this substance greatly improved cell attachment and growth, with the addition of PNA boosting durability and enhancing water absorption, indicating promise for more advanced wound healing uses (Figure 5D).134
5.3 Bio-imaging application
With the rapid advancements in imaging technology, increasingly multifunctional and diverse NGs have been applied in the field of bioimaging.144, 145 NGs generally provide two main approaches to enhance imaging capability, that is, they can serve as carriers for various imaging agents, or imaging components can be incorporated into the synthesis process of NGs themselves. Compared to traditional imaging materials, NGs offer several advantages including higher biocompatibility, responsiveness to multiple stimulation, heightened sensitivity, and increased signal intensity.146 This section provides a summary of NGs for multimodal imaging, encompassing techniques such as magnetic resonance imaging (MRI), positron emission tomography (PET), computed tomography (CT), and fluorescence imaging (FI).
Currently, nanostructured sensitizers based on high-Z elements such as gold (Au), manganese (Mn), and hafnium (Hf) are widely employed to externally enhance photoelectric and Compton effects.147 Zhang et al.148 developed a NGs platform by loading Au and MnO2 nanoparticles via precipitation polymerization using poly(N-vinyl caprolactam) (PVCL) as template, enabling dual-mode imaging of tumors using both CT and MR, owing to the strong X-ray absorption of Au nanoparticles and the generation of Mn2+ by MnO2 in response to the TME (Figure 6A). Similarly, Carniato et al.149 demonstrated the imaging capabilities of high-Z elements. Unlike conventional NGs that utilize Gd (III) complexes as contrast agents, they developed an innovative chitosan-based material that facilitated the formation of chelates coordinated with Mn (II). This process involves the reaction between the NH2 group of chitosan and the anhydride functionality of the ligand, leading to the formation of an amide bond, thereby stabilizing the NG network. The obtained material showed a high peak relativity of approximately 30 mM−1·s−1 at 30 MHz, which is comparable to MRI contrast agents (CAs).149
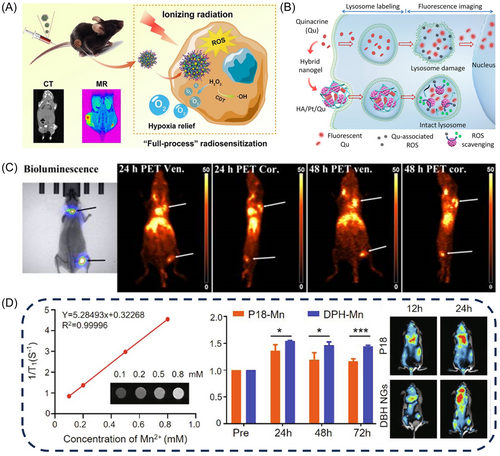
Besides, Lux et al.151 constructed metal-crosslinked NGs as a contrast agent of PET imaging by doping 64Cu as a metal chelator into three ligands: diethylenetriaminepentaacetic acid (DTPA), 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA), and 1,4,7-triazacyclononane-1,4,7-triacetic acid (Figure 6C). The NGs gather at the cancerous area due to the enhanced permeability and retention effect, enabling PET imaging with high sensitivity.151 Using a similar approach, He et al. developed a NG containing manganese dioxide and poly(N-vinylcaprolactam) (PVCL-MnO2 NGs), which broke down into Mn2+ with five unpaired 3d electrons in the cancerous environment, enabling manganese dioxide to achieve T1-enhanced MR imaging.153
In recent years, fluorescence imaging has garnered significant interest from researchers and has emerged as a widely embraced tool for measuring pathophysiological microenvironments.154-156 For example, lysosomal fluorescence imaging stands as an effective means to explore the physiological characteristics of lysosomes within living cells. Nonetheless, persistent exposure to light inevitably induces lysosomal damage and triggers phototoxicity. To address this problem, Zhang et al.150 devised a composite NG embedding both platinum nanoparticles (Pt NPs) and the fluorescent dye Quinacrine (Qu) (Figure 6B). Leveraging the superoxide dismutase-like property of Pt NPs, the NG could efficiently scavenges ROS produced by photoexcited Qu upon lysosomal labeling, which also contributed to the active mitigation of phototoxicity during fluorescence imaging of living cells, enabling prolonged lysosomal tracing while minimizing photodamage.150 Ma et al.152 engineered bio-responsive DPH precursor NGs (DPH NGs) by conjugating purine 18 (P18) with 10-hydroxycamphorin. By utilizing the powerful near-infrared fluorescence (NIRF) imaging capability of P18 and T1-weighted MRI of P18-Mn2+ complex ions, the DPH NG to exhibit enhanced MRI/NIRF dual-modality imaging depth and high sensitivity (Figure 6D).152 In addition, ROS-responsive fluorescence imaging NGs can also be synthesized through enzyme-catalyzed reaction. Qi et al.49 synthesized MPG (metal-coordinated polymeric NG) with highly efficient superoxide dismutase (SOD) and peroxidase (POD)-like enzyme activities via biocatalytic ATRPase and metal coordination technology.49
5.4 Tumor therapy
With the three-dimensional structure, excellent hydrophilicity, rheological properties, mechanical strength, and stability, NGs have been found extensive applications in tumor therapy,157 including in chemotherapy, chemodynamic therapy (CDT), sonodynamic therapy (SDT), immunotherapy, and more.158-160 These multifunctional NGs have emerged as versatile and promising platforms for various tumor therapy modalities, offering precise targeting, controlled release, and enhanced therapeutic efficacy while minimizing side effects, representing a significant advancement in cancer treatment.
For CDT, typical transition metal ions or compounds catalyze the transformation of endogenous hydrogen peroxide (H2O2) into extremely harmful hydroxyl radicals (·OH) in the TME, resulting in apoptosis of tumor cells.157 For example, Su et al.161 synthesized DOX@Mn-Alg NGs using alginate (Alg), Mn, and DOX via microfluidic technique. Due to the acidic feature of the TME, pH-sensitive DOX@Mn-Alg NGs could rapidly release Mn2+ and DOX, resulting in a synergistic effect for CDT-immunotherapy. Similarly, Xiao et al.99 prepared redox-sensitive poly(N-vinylcaprolactam) (PVCL) NGs using a precipitation polymerization, containing MnO2 and cisplatin inside for Fenton-like reactions, further coated with macrophage membranes to facilitate blood brain barrier (BBB) penetration (Figure 7A).99
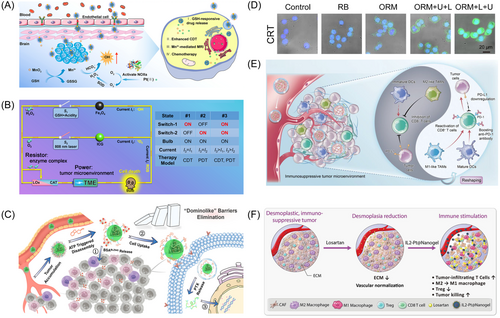
PDT generates endogenous ROS through light exposure to kill tumor cells.167 Qin et al.162 synthesized a novel NGs (FIGUREs-LC) with multi-enzyme activity by loading lactate oxidase (LOx), Fe3O4 nanoparticles and catalase (CAT) into NGs synthesized from polystyrene-block-poly (acrylic acid) (PS-b-PAA), indocyanine green (ICG) and 3-mercaptopropyltrimethoxysilane. Therein, LOx catalyzes the production of H2O2 from endogenous lactate and the generation of ·OH with GSH-activated Fe2+, while CAT reduces H2O2 to O2, and ICG converts O2 to 1O2 under 808 nm laser irradiation, resulting in synergistic effects of CDT and PDT (Figure 7B).162
As a noninvasive strategy for cancer diagnosis and treatment, SDT utilizes ultrasound (US) with deep tumor tissue penetration to activate acoustic sensitizers loaded in the NGs to generate large amounts of ROS, which in turn induces apoptosis of the tumor cells, thereby offering an effective and minimally invasive approach to tumor treatment.38, 168, 169 Li et al.164 designed a new green nano-modulator, ORM, using self-assembly of oleanolic acid (OA), Rose Bengal (RB), and Methylene Blue (MB), which was found to effectively penetrate tumors and modulate TME. Thereinto, MB disintegration facilitated PTT, leading to collagen degradation and remodeling of the ECM, thereby enhancing material penetration. Besides, ultrasound facilitated deep penetration of RB post-release, enabling effective SDT, presenting a promising approach to tumor treatment (Figure 7D).164
Immunotherapy has emerged as a revolutionary tumor treatment strategy that achieves clarity of tumor lesions through activation of the immune system, which is common to combine with other treatment strategies to achieve synergistic therapeutic effects.170-172 Generally, NGs can be engineered to carry immunomodulatory agents, antigens, or immune checkpoint inhibitors to enhance the efficacy of immunotherapy by promoting immune cell activation and tumor-specific immune responses. Due to variations in the immune activation status within the tumor immune microenvironment (TIME), tumors are categorized as “Cold” or “Hot”, each having distinct implications for immunotherapy. “Cold” tumors typically exhibit immune ignorance and low immunogenicity, rendering them less responsive to immunotherapy. In contrast, “Hot” tumors, also known as immunoinflammatory tumors, are characterized by high expression of immune checkpoints, intrinsic immunogenicity, elevated tumor mutational load (TMB), and increased activity in the interferon (IFN) signaling pathway. Immunotherapy tends to be more effective in “Hot” tumors due to their higher immunogenicity and greater responsiveness to immunomodulatory interventions.173, 174 Zhang et al.163 engineered an ATP-responsive NGs, BBLZ-945@PAC-PTX, which underwent decomposition triggered by ATP upon delivery to the tumor site, releasing BLZ-945 conjugated albumin (BBLZ-945) to deplete tumor-associated macrophages (TAMs), suppresses CXCR4 expression linked to metastasis, and enhances immune cell infiltration, collectively referred to as the “Dominolike” barriers elimination strategy (Figure 7C).163 Consequently, the BBLZ-945@PAC-PTX effectively alleviates the “Cold” TME, reversing the low response rates to immunotherapy.163 In addition, Dai et al. designed a novel responsive NGs (PMI NGs) employing a drug repurposing strategy (Figure 7E). PMI NGs encapsulated with imiquimod (Imi) and metformin (Met) as immunomodulators were designed to reshape the TIME including: promoting dendritic cell maturation, polarizing tumor-associated macrophages from M2-type to M1-type, and downregulating PD-L1 expression, demonstrating much potential of PMI NGs as a combination therapy to enhance antitumor immune responses.165 Similarly, Mu et al.166 engineered a chemo-immunotherapeutic NGs (IL2-Pt@NGs) capable of dual delivery of IL-2 and the type II immunogenic cell death inducer Pt-NHC, which was found to reduce the immunosuppressive phenotype of tumor-associated macrophages and decrease the infiltration of regulatory T-cells by inducing the production of Pt-NHC (Figure 7F).166
6 CONCLUSION REMARKS AND PERSPECTIVES
With the evolution of nanotechnology over the past two decades, NGs have emerged as a versatile material with applications spanning materials science and biology. This review initiated by delineating the developmental trajectory, provides a brief overview of the preparation methods of NGs, and the drug control properties of the NGs are also described in detail, followed by focusing on the design of multifunctional smart-responsive NGs materials and their current status of research and progress in the fields of inflammation, regenerative medicine, bioimaging and tumor treatment, with a view to give a useful guidance and reference for the functionalized design and application of NGs delivery systems.
Nanogels can be created through either physical or chemical cross-linking processes. To be specific, physical cross-linking relies on noncovalent interactions such as hydrogen bonding, van der Waals forces, and electrostatic interactions, which facilitate efficient self-assembly, nucleic acid hybridization, and drug delivery. In contrast, chemical cross-linking involves covalent bonds such as radical polymerization, ring-opening polymerization, and click reactions, allowing for the adjustment of characteristics such as strength and sensitivity. Overall, both synthesis techniques come with their own set of pros and cons, and in general, scientists combine covalent and noncovalent interactions to create multifunctional NGs. NGs can be served as versatile platforms for drug delivery and/or multimodal imaging, which are applied in such as inflammation therapy, regenerative medicine, bioimaging, and oncology. Moreover, NGs can encapsulate drugs and imaging agents by utilizing a pore structure, enabling efficient healing as well as integration of diagnostics and therapy. Particularly in cancer therapy, NGs could provide the integration of diagnostic and therapeutic owing to their tunable mechanical properties and high loading capacity.
Nanogels, as emerging biomaterials, hold tremendous potential in biomedical fields such as drug delivery, gene therapy, and tissue engineering, and offer significant advantages in terms of biosafety. First, the materials commonly used in NGs, such as biocompatible polymers like PEG or natural macromolecules, exhibit minimal immune rejection within the body. These materials are biodegradable, and their degradation products are nontoxic, thus reducing the risk of long-term side effects associated with retention in the body. Second, NGs provide excellent tunability. By carefully adjusting their structure and surface properties, scientists can engineer smart nanomaterials that respond to specific stimuli, such as temperature, pH, or enzymes, ensuring that therapeutic agents are released precisely at the target site. This significantly lowers toxicity to nontarget tissues. Moreover, with typical sizes ranging from tens to hundreds of nanometers, NGs can circulate for extended periods within the body and be efficiently cleared through the kidneys, reducing the risk of accumulation in nontarget organs. Additionally, NGs can be surface-modified with multifunctional molecules, such as antibodies, peptides, or carbohydrates, to achieve specific targeting, further enhancing their safety profile. This targeted design not only increases therapeutic efficacy but also minimizes harm to healthy cells. Taken together, the superior biosafety of NGs positions them as a promising candidate for next-generation biomedical applications.
Despite the significant progress made by NGs and their advantages, various research findings have also found certain unresolved issues or limitations in real-world scenarios. For example, NGs exhibit good stability on their own, however, this stability diminishes once they interact with guest molecules. Thus, how to effectively bind to other components while maintaining NG stability was an ongoing challenge that requires further research. Additionally, many research works demonstrated the in vivo safety of multifunctional NGs in mice, however, their long-term biosafety, in vivo clearance, and biodegradability necessitate further validation.
Furthermore, the development of subcellular localization as well as diagnostic and therapeutic integrated materials with NGs as a focus requires further research. Besides, it should be mentioned that current drug delivery modalities are predominantly confined to intravenous or subcutaneous injection, urging the exploration of alternative, less invasive delivery mechanism. Notably, the size of NGs greatly affects their use in biology. For example, in bioassay applications, proper pore structure helps to improve diagnostic specificity, and in tumor therapy, the size of NGs affects the efficiency of phagocytosis and uptake by organisms. Nevertheless, a comprehensive investigation into the correlation between size, pore structure of NGs, and their potential biological uses was still not enough. All in all, NGs stand as a highly promising frontier for future applications though there still are numerous scientific challenges required resolution by researchers. The purpose of this review hopes to make a valuable contribution to the continued progress of research on NGs and their various uses.
AUTHOR CONTRIBUTIONS
Bicheng Han: Writing—review & editing; writing—original draft; visualization; resources; project administration; methodology; investigation; conceptualization. Zideng Dai: Writing—review & editing; writing—original draft; visualization; validation; methodology; investigation; formal analysis; data curation. Hangrong Chen: Writing—review & editing; revision; supervision; resources; project administration; funding acquisition. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
This work was supported by the National Key Research and Development Program of China (grant no. 2021YFB3801000); National Natural Science Foundation of China (grant no. 32030061); and Shanghai International Cooperation Project (No. 23490712900).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The authors have nothing to report.
Open Research
DATA AVAILABILITY STATEMENT
The data in this paper are available upon resonable request.




