Research progress of hydrogels in the prevention of pelvic inflammatory disease
Yile Xiao, Teng Ma, and Haoming Wu contributed equally to this study.
Abstract
Pelvic inflammatory disease (PID) is a critical global health concern with the potential to lead to adverse outcomes, including infertility and chronic pelvic pain. Since PID is often caused by ascending vaginal infections or urinary tract infections, understanding the treatment of both is critical to preventing PID. Meanwhile, the emergence of drug-resistant and persistently infected strains poses a growing challenge. This review discusses current clinical treatments for the prevention of PID from the physiologic basis of PID, as well as summarizes the advantages and research progress of hydrogels in the prevention of PID. In contrast to conventional treatments, hydrogels serve as excellent vehicles for vaginal drug delivery, maintaining the presence of the drug at the target site and controlling its release. In the context of urinary tract infections (UTIs), hydrogels are employed primarily as coatings on catheters to prevent and treat catheter-associated UTIs. Finally, this review summarizes the limitations of hydrogels in PID prevention and future directions for development with the aim of elucidating avenues for clinical treatment of PID and informing further research.
1 INTRODUCTION
Pelvic inflammatory disease (PID) is an infection of the upper female reproductive tract, including the uterus, fallopian tubes, ovaries, pelvic peritoneum, and internal pelvic region, with a spectrum of clinical manifestations.1 PID mainly includes endometritis, salpingitis, tubo-ovarian abscess, and pelvic peritonitis. Inflammation can be confined to one site or involve several sites at the same time, with salpingitis and tubo-ovarian being the most common. The occurrence of this condition is often due to the ascent of bacteria from the vagina or cervix to the upper regions of the genital tract.2 Symptoms can range from none to severe. Symptomatic PID can be diagnosed by lower abdominal pain and pelvic tenderness, accompanied by inflammation of the cervix and vagina.3 Subclinical pelvic inflammation, characterized by the absence of symptoms, can still result in the spread of infection to the upper genital tract and significantly contribute to tubal infertility.4, 5
Despite limited data on PID in low- and middle-income countries, it remains a global health problem.6, 7 The estimated lifetime rate of self-reported PID among sexually active women of reproductive age (18–44 years) was 4.4%, according to data from the National Health and Nutrition Examination Survey conducted between 2013 and 2014.8 Although PID is rarely life-threatening, long-term infection can have significant adverse effects.9 If not treated appropriately, PID can result in infertility, ectopic pregnancy, and chronic pelvic pain.10, 11 In addition, a history of PID has been associated with an increased risk of developing epithelial ovarian cancer, with a clear dose–response relationship.12
Hydrogels consist of three-dimensional (3D) polymer networks formed by physical and chemical crosslinking of hydrophilic polymers.13-15 Their biocompatibility, cellular interaction capabilities, hydrophilicity, permeability, and biodegradability make them ideal substrates for extracellular matrix (ECM) emulation.16, 17 In addition, hydrogels can absorb water and swell while maintaining a stable structure without being destroyed, making them excellent drug loading materials.18 Compared to conventional drug delivery forms, hydrogels can enhance therapeutic efficacy by maintaining drug presence on the vaginal mucosa and potentially reducing required dosages.19
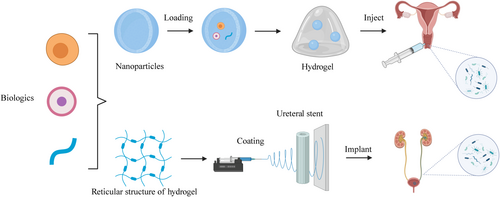
Given the anatomical proximity of the urethra and vagina in women, there is an increased risk of concurrent infections in these regions, meaning that urethral infections can also cause pelvic infections via the vagina. Since PID can originate from ascending infections of the vagina, understanding vaginal and urinary tract infections (UTIs) is crucial for the management and prevention of PID. Because parts such as the fallopian tubes and ovaries are located deep in the pelvis, it is not convenient for the clinical application of hydrogel route delivery, and fewer studies are currently available, so we mainly discuss the hydrogel drug delivery system of the vagina and urethra. At present, the early prevention and treatment of PID is often more important. In this review, we discuss PID infections, especially vaginal and urethral infections and the physiologic basis of PID and the current clinical strategies. Moreover, we introduce the properties of hydrogels as a drug delivery system (Figure 1) and examine the latest developments in the use of hydrogels as drug or nanosystem carriers for vaginal and urethral delivery, hoping to provide direction and a basis for clinical selection of prevention for PID.
2 PHYSIOLOGIC BASES OF PID AND CLINICAL STRATEGIES
2.1 PID in the reproductive system
PID includes salpingitis, endometritis, fallopian tube ovarian cysts, and pelvic peritonitis. PID is one of the main causes of pelvic and fallopian tube-related factors in female infertility. Salpingitis is the most common PID, which is also closely associated with the occurrence of ectopic pregnancy. Approximately 90% of the pathogenic microorganisms in PID ascend from the vagina, with a small portion coming from lymphatic vessels or directly spreading from adjacent tissues, such as peritonitis.1 Based on the origination of pathogens, they can be divided into endogenous and exogenous. The endogenous pathogens mainly indicate the bacterial flora colonized in the vagina. The exogenous pathogens mainly refer to STI, which are Neisseria gonorrhoeae and Chlamydia trachomatis. Some aerobic bacteria, anaerobic bacteria, and viruses are also involved in the occurrence of PID. In clinical practice, the symptoms of PID vary greatly depending on the range of infection and the type of pathogens. Mild PID may be asymptomatic, while severe cases may present with elevated body temperature, abdominal pain, vaginal bleeding, and severe infection symptoms. When combined with peritonitis or abscess compression of the bladder, peritoneal or bladder irritation may occur. The treatment of PID mainly depends on an anti-infection strategy, with oral or intravenous systematic administration. When drug treatment is ineffective or accompanied by continuously enlarged abscesses, surgical treatment is recommended.20 Meanwhile, recently, accumulating studies highlight the role of hydrogels in preventing vaginal ascent infection. In addition to STI, vaginal inflammatory infections also correlate with the onset of PID. These pathogens not only induce the occurrence of PID through vaginal ascent but also increase the risk of STI infection and the probability of PID.
2.2 Microbiology of vaginal infections in PID
Etiologically, vaginal infections are classified as bacterial, fungal, or parasitic.21-23 Bacterial vaginosis (BV), the most common cause of infectious vaginal discharge,24 represents a dysbiosis in which the typically dominant lactate-producing Lactobacillus spp. are replaced by a variety of anaerobic bacteria, including Gardnerella vaginalis and Prevotella spp.25-28 The primary clinical features of BV include grayish-white discharge, pruritus, burning, vaginal pH above 4.5, and a distinctive fishy odor, but notably no leukocytic exudate, erythema, or edema.23, 29, 30 BV represents a significant public health problem for women of reproductive age, as it is associated with adverse reproductive health outcomes such as PID, spontaneous abortion, preterm labor and delivery, miscarriage, postpartum endometritis, increased risk of postoperative infections, and increased susceptibility to both acquiring and transmitting human immunodeficiency virus (HIV).30-33
Trichomoniasis, caused by the parasitic organism Trichomonas vaginalis, is the most common treatable sexually transmitted infection (STI) worldwide, affecting an estimated 156 million people.34, 35 According to 2016 World Health Organization data, the global prevalence is 5.3% (95% uncertainty interval [UI]: 4.0–7.2) in women and 0.6% (95% UI: 0.4–0.9) in men, with regional disparities ranging from 1.6% to 11.7% in women and 0.2% to 1.3% in men.36 In women, T. vaginalis can have a detrimental impact on sexual and reproductive health.37 The infection has been linked to adverse birth outcomes, including preterm delivery, premature rupture of membranes, and low neonatal birth weight.38 In addition, T. vaginalis increases the risk of HIV acquisition39 and predisposes individuals to other STIs. Other health complications associated with the pathogen include PID,40 cervical cancer,41 and infertility.42, 43
Vulvovaginal candidiasis (VVC), a common infectious disease of the lower female reproductive tract, is primarily caused by Candida albicans and results in pathologic inflammation.44 This complex condition is influenced by a number of factors, including host immune mechanisms, genetic polymorphisms, and environmental exposures. Factors such as serum glucose concentrations, antibiotic use, psychosocial stress, hormone levels, and sexual practices may all play a role.45 Although C. albicans is the predominant causative agent of VVC, there is an increasing trend of infections caused by nonalbicans species, including Candida glabrata, Candida tropicalis, Candida krusei, and Candida parapsilosis.46, 47 Typical manifestations include vulvar and/or vaginal itching, erythema, soreness, burning, dyspareunia, and dysuria.48-50 In addition, the consistency of the vaginal discharge may range from thin to flaky,51 often lacking the characteristic odor of BV.50 Recurrent vulvovaginal candidiasis (RVVC), characterized by the occurrence of four or more episodes of VVC annually, adversely affects quality of life, mental health, and sexual function.52, 53 The high prevalence, significant morbidity, and associated economic costs make RVVC a significant global health challenge that requires more effective solutions.54
2.3 Microbiology of urinary system infection in PID
UTIs are the most common genitourinary disease, affecting the urethra, bladder, ureters, and kidneys. In the United States, approximately 13.3% of women, 2.3% of men, and 3.4% of children require treatment for UTIs 55-58 UTIs involving the bladder (cystitis) and kidneys (pyelonephritis) have historically been considered separate conditions. Asymptomatic bacteriuria is characterized by bacterial presence in urine without symptomatic manifestation.59 According to the Infectious Diseases Society of America, asymptomatic bacteriuria affects up to 5% of healthy premenopausal women, 2.8%–8.6% of postmenopausal women, and 1.9%–9.5% of pregnant women.60 In cystitis, although the upper tract may be involved, urinary symptoms are limited to the bladder. In premenopausal women, the most common symptoms are frequency, urgency, and dysuria. When pyelonephritis occurs, people may have symptoms such as back pain, flank pain, fever, and chills with or without urinary symptoms. During pregnancy, pyelonephritis may increase the likelihood of adverse outcomes for both the mother and the newborn, such as preterm delivery, pregnancy-induced hypertension, fetal growth retardation, and low birth weight in the newborn.61
2.4 Current status of clinical treatment of PID
2.4.1 Clinical therapeutic drugs of PID
Antibiotic therapy is the basis of PID therapy. The treatment is mainly broad-spectrum, empirical antimicrobial anti-infection, which should cover all possible pathogenic bacteria of PID, including N. gonorrhoeae, C. trachomatis, mycoplasma, anaerobic bacteria, and aerobic bacteria, and so forth, so the drug treatment regimen of PID is mostly a combination of drugs, and different intravenous and nonintravenous drug regimens have shown good clinical cure and microbiological cure.62, 63 Once the diagnosis is made clinically, empiric therapy does not need to wait for the results of the pathogen testing, and the prevention of long-term sequelae depends on the early application of recommended antimicrobial drugs. Because N. gonorrhoeae and C. trachomatis are highly pathogenic, even a negative cervical etiologic screen cannot rule out upper genital tract infection, so all treatment regimens must cover N. gonorrhoeae and C. trachomatis. The 2021 STD Treatment Guidelines64 published by the CDC (“2021 CDC Guidelines” for short) emphasize the addition of metronidazole to PD regimens to more effectively eradicate anaerobes from the upper genital tract unless there is credible evidence that treatment regimens that do not cover anaerobes are as effective as those that cover anaerobes. Once diagnosed, immediate treatment to prevent long-term sequelae depends on the early use of recommended antimicrobials. For patients with mild to moderate PID, intravenous versus oral regimens are similarly effective. In general, the treatment of intravenous and nonintravenous regimens is mainly cephalosporins, combined with doxycycline to cover atypical pathogenic microorganisms such as C. trachomatis, and metronidazole can be added for patients who do not cover anaerobic bacteria. Antimicrobial therapy was continued for at least 14 days.
Since the release of the CDC guidelines in 2015, antimicrobial use has changed dramatically worldwide, which has also led to a shift in the drug-resistant microbiota. One of the recommended intravenous dosing regimens in the 2021 CDC Guidelines is ceftriaxone 1 g, q12h + doxycycline 100 mg, q12h, po/iv + metronidazole 500 mg, q12h, po/iv. One of the recommended intravenous dosing regimens in the Guideline of PID (2019 revised edition) (hereinafter referred to as “the 2019 China Consensus”)65 is: cefotetan 2 g, q12h/cefoxitin 2 g, q6h/ceftriaxone 1 g, qd, metronidazole 500 mg, q12h, iv; doxycycline/minocycline 100 mg, q12h, po/azithromycin 500 mg, qd, iv, 1 ~ 2d, followed by 250 mg, qd, po, 5–7 days. The bioavailability of oral or intravenous doxycycline or metronidazole is similar. Because intravenous doxycycline is painful, oral therapy is recommended if possible. Metronidazole is well absorbed orally, and oral therapy is recommended for patients with nonsevere or nontubo-ovarian abscesses. Patients with mild and moderate acute PID have similar intravenous and oral regimens, and intramuscular or oral administration may be considered, with similar efficacy.11, 66 If symptoms do not improve after 72 h of intramuscular/oral therapy, the patient should be re-evaluated, diagnosed, and switched to intravenous therapy. Other oral third-generation cephalosporins may be cefdaloxime or cefotaxime, but metronidazole should still be added to intramuscular/oral regimens because they do not cover anaerobes. One of the regimens recommended in the 2021 STD Treatment Guidelines is: ceftriaxone 500 mg as a single dose, im + doxycycline 100 mg twice daily, oral + metronidazole 500 mg, bid, po.
The principles for the use of PID pharmacotherapy are broadly the same in the three editions of the guidelines, but there are differences in the details of the medications. As a third-generation cephalosporin, ceftriaxone is not as active against anaerobes as cefotetan or cefoxitin, but it has stronger antimicrobial activity against N. gonorrhoeae, and metronidazole should be added to intravenous administration. In recent years, the susceptibility of N. gonorrhoeae to ceftriaxone has decreased significantly, and according to pharmacokinetic and pharmacodynamic models, 250 mg of ceftriaxone sodium cannot reach an effective therapeutic dose (minimum inhibitory concentration [MIC] >0.125 μg/mL for a long time). Considering the high and long-lasting effective bactericidal efficacy of the drug, the European and American gonorrhea management guidelines recommend the optimal therapeutic dose of ceftriaxone (human body weight of 80–100 kg) is 500 mg.67, 68 The Sexually Transmitted Diseases Treatment Guidelines, 2015,69 did not emphasize the use of ceftriaxone in the intravenous recommended regimen, and the 2019 Chinese Consensus clearly recommended that ceftriaxone 1 g intravenous infusion once daily. The 2021 CDC guidelines increased the frequency of intravenous ceftriaxone infusion to every 12 h, and increased the intramuscular dose of ceftriaxone from 250 to 500 mg, and indicated that the dose should be increased to 1 g if the patient weighs ≥150 kg. Moreover, the 2021 CDC guidelines emphasize the need to add metronidazole to all intramuscular/oral regimens to enhance antianaerobic activity, and the intravenous gentamicin regimens are no longer recommended because of the severe adverse effects of long-term gentamicin. Furthermore, compared with the 2019 Chinese Consensus, the 2021 CDC guidelines and the 2015 CDC guidelines extended the course of intramuscular/oral azithromycin to 14 days. In 2016, Europe launched a gonococcal antimicrobial surveillance program to monitor NG resistance in 25 European countries, confirming that the high level of NG resistance to azithromycin is becoming more serious worldwide,68 which may be related to the decrease in the susceptibility of the bacterium to azithromycin and the exposure of azithromycin in infected people.70 A multicenter randomized controlled trial confirmed that a short course of azithromycin is less effective in treating mild to moderate PID.71
2.4.2 Drug resistance of microorganisms in PID
In women diagnosed with BV, G. vaginalis is one of the most common and important pathogenic bacteria in the vagina. With the increasing use of antibiotics, the resistance of Gardnerella is becoming a problem that needs to be addressed. In a study conducted in China, Zhang et al.72 isolated 20 Gardnerella strains from vaginal swabs of 31 women. Analysis revealed the presence of macrolide erythromycin resistance genes, specifically ermX and lsaC, and tetracycline resistance genes, tetL and tetM, in 10 of these strains. Notably, 70% of these strains exhibited high levels of resistance to metronidazole, with a threshold of ≥128 μg/mL, which is the primary treatment choice for BV in China. In another work focusing on the clinical management of BV in Northeast China, Ma et al.73 evaluated the causative organisms and their susceptibilities to antibiotics. Of the 24 samples analyzed, 21 (87.5%) were resistant to metronidazole, while 16 (66.7%) were susceptible to clindamycin. These results are similar to those reported by Qin et al.74 Six of the planktonic strains showed resistance to metronidazole, while one exhibited resistance to another drug, clindamycin. The persistent bacterial biofilms appear to be potentially one of the most important factors contributing to the problem. Antibiotics are unable to fully penetrate the negatively charged polysaccharide matrix of the biofilm, which protects the bacteria and allows them to survive in the deeper layers of the biofilm. Overcoming this challenge requires therapeutic interventions that can disrupt the biofilm architecture and facilitate the delivery of effective antimicrobials, thus significantly improving treatment outcomes for BV.
VVC, a prevalent infectious disease caused by Candida species, impacts the quality of life of numerous women each year. Typical manifestations of this condition include itching sensations of the vulva and/or vagina, accompanied by abnormal vaginal discharge.75 However, with the overuse of antifungal drugs, resistance to these drugs is also gradually increasing in C. albicans. Recent research has extensively investigated the mechanisms underlying antifungal resistance, and biofilm formation has been identified as a significant contributing factor. Biofilms are aggregations of fungal cells embedded in self-generated, 3D natural physical extracellular polymeric substances (EPSs). These EPSs create a conducive microenvironment for fungal growth, enabling them to withstand harsh environments and evade host immune defenses. Notably, C. albicans biofilms exhibit inherent resistance to the majority of known antifungal agents, resulting in difficulties in treating these infections.76, 77 Besides C. albicans, other Candida species also have resistance to antifungal drugs. C. glabrata is the most frequently encountered nonalbicans Candida species associated with VVC. In a study of C. glabrata isolates, researchers found that all isolates (20/20; 100%) demonstrated phenotypic resistance to fluconazole, an antifungal drug belonging to the azole class.78
Trichomoniasis, a curable sexually transmitted parasitic infection, is associated with adverse outcomes such as preterm birth and increased risk of HIV acquisition and transmission. Consistent with previous in vitro resistance testing conducted in the United States, T. vaginalis isolates have shown low levels of metronidazole resistance.79 Reported rates of metronidazole resistance among women, primarily those without HIV, vary from 2.2% to 9.6%.80-83 Schwebke et al.81 found that the correlation between in vitro resistance to metronidazole and clinical response to treatment was weak. Additionally, the resistance to tinidazole was significantly less common. The exhibition of low to moderate resistance to metronidazole in the four isolates was characterized by a distinct genotype banding pattern, as revealed by RFLP analysis, in comparison to other 45 metronidazole-sensitive samples.84
Besides the common bacterial, fungal, and parasitic vaginal infections, viral infections are also an important part, with a focus on HIV in recent research. Overall, the microbiota of the vagina is in dynamic fluctuation, and infection by any one of these microorganisms may cause dysbiosis and ultimately lead to PID. We have explicated the usage, advantages, and potential side effects of commonly used vaginal infection drugs in Table 1.
| Diseases | Drugs | Drug administration route | Research type | Advantages | Adverse drug reactions | References |
|---|---|---|---|---|---|---|
| Bacterial vaginosis | Metronidazole | Oral (500 mg bid for 7 days) | Clinical research | High efficiency | Diarrhea, vomiting, metallic taste, headache, and dizziness | [85] |
| Clindamycin | Oral (300 mg bid for 7 days) | Clinical research | Broad-spectrum activity against aerobic and anaerobic gram-positive pathogens | Abdominal cramps, colitis, nausea, vomiting, diarrhea, and elevated liver enzymes | [86] | |
| Metronidazole gel | Topical (0.75% per day for 5 days) | Clinical research | Fewer gastrointestinal complaints | Inconvenience to be used | [87] | |
| Clindamycin cream | Topical (2% per day for 7 days) | Clinical research | Simplicity; Excellent spreadability | Inconvenience to be used | [88] | |
| Secnidazole | Oral (a single 1-g or 2-g oral dose) | Clinical research | Alternative treatment | / | [89, 90] | |
| Lactic acid | Topical (5 mL once per day for 7 days) | Clinical research | Maintain the balance of the gut bacteria (microbiome); Reduce the potential for the development of AMR | / | [91] | |
| Vulvovaginal candidiasis | Oteseconazole | Oral (600 mg on day 1 and 450 mg on day 2, 150 mg per week for 11 weeks, starting on day 14) | Clinical research | Lower risk of drug-drug interactions and side effects compared to other azoles | Headache and nausea | [92, 93] |
| Ibrexafungerp | Oral (300 mg twice for 1 day) | Clinical research | Treat azole-resistant isolates and improve overall clinical cure rates | Abdominal pain, diarrhea, nausea, and vomiting | [94, 95] | |
| Fluconazole | Oral (a single dose of 150 mg) | Clinical research | Highly tolerated; Easily available | Hepatotoxicity, cytochrome P450 interactions, and possible fetal harm in pregnant women recurrence | [96, 97] | |
| Clotrimazole | Topical (1% cream 5 g, 1 time a day, for 7-14 days, or 2% cream 5 g, for 3 days) | Clinical research | Safer for pregnant patients; No systematic effects | Itching, burning, low acceptability, and increased likelihood of the emergence of azole-resistant Candida species | [75, 97] | |
| Miconazole | Topical (2% cream 5 g, 1 time a day for 7 days, or 4% cream 5 g for 3 days) | Clinical research | Safer for pregnant patients | Itching, burning, low acceptability, and certain damage to normal cells and tissues of the vagina | [23, 98] | |
| Trichomoniasis | Metronidazole | Oral (a single 2-g dose) | Clinical research | Effectiveness | Nausea, headache, dizziness, itchy skin, malaise, fatigue | [69, 99] |
| Tinidazole | Oral (a single 2-g dose) | Clinical research | Highly effective for the treatment of Trichomonas vaginalis strains that are resistant to metronidazole | Nausea, headache, dizziness, itchy skin, malaise, and fatigue | [100] | |
| HIV-1 | Dapivirine | Vaginal ring | Clinical research | 31% lower risk of HIV-1 infection compared to the placebo group | / | [101] |
| Tenofovir or TDF-FTC | Oral (once daily) | Clinical research | 67% and 75% protection against HIV-1, respectively, safe and well-tolerated | Poor adherence | [102] | |
| Tenofovir | Topical | Clinical research | Safe and effective | / | [103] | |
| Cabotegravir | Inject intramuscularly every 8 weeks | Clinical research | 88% lower risk of HIV infection compared to TDF-FTC; Convenient and discreet injection with an adherence advantage | / | [104] |
- Abbreviations: AMR, antimicrobial resistance; HIV, human immunodeficiency virus; TDF-FTC, tenofovir-emtricitabine.
Women often suffer from both uncomplicated and complicated UTIs. Acute uncomplicated cystitis and pyelonephritis, categorized as uncomplicated UTIs, are particularly prevalent in healthy individuals and are prone to recur frequently.105 Empiric antimicrobial therapy is generally recommended for uncomplicated UTIs, whereas in complicated cases, this therapy is often initiated before bacterial culture results are available. Subsequently, once the culture results become available, the empiric therapy can be optimized based on antimicrobial sensitivity. However, the continuing rise in antibiotic resistance is observed in both uncomplicated and complicated UTIs. In the treatment of uncomplicated UTIs, efforts are made to prescribe antibiotics specifically tailored to this condition, while the increasing use of broad-spectrum antibiotics has led to reports of higher rates of antimicrobial resistance (AMR) in terms of complicated UTIs.106
According to a study by Li et al.,107 based on the Global Burden of Disease (GBD) 2019 database, there were an estimated 64,900 UTI cases and 255,800 deaths attributable to AMR worldwide in 2019. Among the 21 GBD regions, Southern Latin America and Tropical Latin America reported the highest AMR-related mortality rates, while the four sub-Saharan African regions (Southern, Western, Eastern, and Central) had the lowest rates.
Among different pathogens, Escherichia coli dominates global UTI-related AMR deaths, accounting for approximately 40%, while Klebsiella pneumoniae is the second most common pathogen causing UTI-related AMR deaths, accounting for about 10%. Among different antibiotic classes, fluoroquinolones, third-generation cephalosporins, and carbapenems cause the most deaths due to AMR. Among different drug-pathogen combinations, third-generation cephalosporin-resistant E. coli and fluoroquinolone-resistant E. coli cause the most deaths due to AMR.
Given the significance of E. coli in UTIs, the pathogenic mechanisms of uropathogenic E. coli (UPEC) have been the focus of recent research. The virulence of UPEC is primarily attributed to various factors, including fimbriae, flagella, adhesins, toxins, surface polysaccharides, and iron acquisition systems. Notably, fimbriae plays a pivotal role in the pathogenic mechanisms exhibited by UPEC. These structures have a high affinity for D-mannose-rich polysaccharides on the surface of urothelial cells (such as those found in the bladder), mediating adherence to urothelial cells and facilitating subsequent invasion into these cells. Once inside the cells, bacteria evade the host immune system, undergo extensive replication, and ultimately lead to disruption of the epithelial cells and damage to the host. Moreover, some bacteria exploit the opportunity to further invade transitional cells located beneath the epithelial cells. This deeper invasion helps them more effectively evade host immune surveillance, thereby becoming a crucial mechanism for recurrent UTIs.108
2.4.3 Hydrogels used in PID treatment in the clinic
In the PID, the quest for effective treatment options against vaginal infections and catheter-associated urinary tract infections (CAUTIs) has led to the development of various products, including the Runqingda range for vaginitis and silver-hydrogel catheters for CAUTIs. In the treatment of vaginitis, there are many clinical products such as Bangliean, Nano Silver Women's Inflammation No.2 and Fukangbao. One of the products is Runqingda. The effective active ingredients in Runqingda products can effectively inhibit the reproduction and growth of bacteria, have broad-spectrum antibacterial activity, have antibiotic-like effects, and can effectively inhibit pathogenic bacteria that are prone to drug resistance to general antibiotics, such as Candida and Staphylococcus aureus. Runqingda can play a role in hemostasis, can promote the healing of cervical erosion and other wounds, and is suitable for the treatment of bacterial vaginitis, trichomoniasis vaginitis, fungal vaginitis, mixed vaginitis, cervicitis, and cervical erosion. This product can be used directly to clean the vulva without dilution. Aiqingda (vaginal tamponade cold compress hydrogel) contains a new cold compress care agent composed of grain extract and carbomer, which can inhibit itching caused by a variety of pathogenic bacteria. At the same time, it also has a new drug delivery booster, which is easy to use. It is used for cold compress physiotherapy treatment of bacterial vaginitis, trichomoniasis vaginitis, candidal vaginitis, senile vaginitis. The most relieving symptoms are vaginal and cervical mucosal congestion and edema, vaginal discharge, burning and itching of the vulva and vagina, affecting the urethra, and also relieving symptoms such as urgency and pain in urination.
In the realm of CAUTIs, numerous hydrogel-coated catheter products have emerged, and their antimicrobial efficacy has been extensively examined in various studies. In 1996, the University of Massachusetts Medical Center initiated the utilization of silver-hydrogel catheters (Bardex I.C. Foley catheter; Bard). This catheter features a hydrogel latex Foley design with a single layer of silver metal applied to both the inner and outer catheter surfaces. In 2002, Lai et al.109 conducted a study examining the rate of CAUTIs and associated costs and found that the use of silver hydrogel urinary catheters resulted in a nonsubstantial reduction in UTIs and modest cost savings. In a separate investigation, Thibon et al.110 conducted a randomized, double-blinded, prospective, multicenter trial, comparing hydrogel-coated and silver salt-impregnated urinary catheters with traditional urinary tract catheters. Their findings indicated that insufficient evidence existed to support the claim that silver salt and hydrogel coatings provided superior protection compared to traditional catheters, thus discouraging their widespread use. In 2006, Srinivasan et al.111 undertook a prospective, crossover study to assess the effectiveness of silicone-based Foley catheters, both hydrogel-coated and silver-impregnated, in preventing nosocomial urinary tract infections (NUTIs). Their results revealed that the rate of NUTIs per 1000 Foley catheter days was 14.29 in the silver catheter group, whereas it was 16.15 in the nonsilver catheter group. This equated to an incidence rate ratio of 0.88, with a 95% confidence interval ranging from 0.70 to 1.11, and a p-value of 0.29. Their study further demonstrated that silicone Foley catheters coated with silver alloy and hydrogel did not effectively prevent NUTIs.
Although there are contrasting viewpoints, certain studies highlight a decline in CAUTI occurrences. In 1999, Bologna et al.112 presented the outcomes of a multicenter trial involving silver-hydrogel urinary catheters in ICU patients. Their analysis showed a promising trend in reducing NUTIs, although only one institution achieved a statistically significant reduction. The cost analysis conducted in that particular institution revealed a cost savings of $98,021. In 2000, Karchmer et al.113 reported a 21% reduction in infection risk among wards randomly assigned to silver-coated catheters and a 32% decline among patients utilizing these catheters on the wards. The estimated cost savings ranged from $14,456 to $537,293. More recently, in 2014, Lederer et al.114 conducted a multicenter, nonrandomized cohort study, discovering that the employment of silver-alloy hydrogel urinary catheters led to a significant reduction in symptomatic CAUTI incidences, as defined by both the National Healthcare Safety Network (NHSN) and clinical standards, achieving relative reductions of 47% and 58%.
Since the efficacy of hydrogel-coated catheters in clinical trials of catheter-related UTIs was uncertain, further research and development of hydrogels is needed.
3 THE APPLICATION OF HYDROGELS IN PID
3.1 The properties of hydrogels
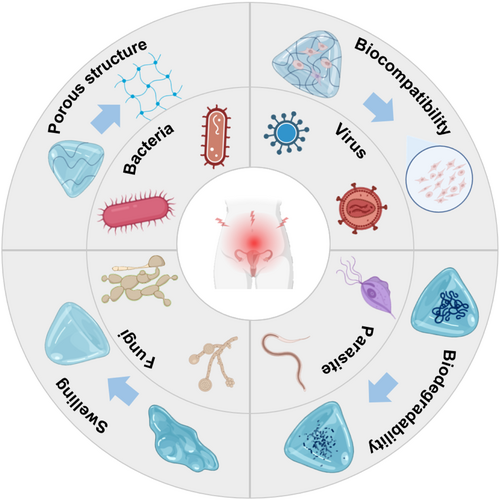
The excellent properties of hydrogels include many key properties such as biocompatibility, biodegradability, swelling, porous structure, mucoadhesion, responsiveness, in addition to the previously mentioned ones, and these special physicochemical properties give them great potential for application in the treatment of PID.
3.1.1 Biocompatibility
Biocompatibility emerged as a focal area of research in the 1940s when researchers began to consider the beneficial and detrimental interactions between medical implants and the body.115 The aim of assessing the biocompatibility of any material is to determine whether it possesses any harmful effects on the body. Thus, it is imperative to evaluate a biomaterial to identify potential biological responses that may cause adverse or undesired side effects in the host. The immunological reaction/immunotoxicity, inflammation, and wound healing constitute the three pivotal responses that ought to be taken into consideration.116 In stem cell and cancer research, cell therapy, tissue engineering, and other fields, hydrogels are required to have bionic features of the ECM in addition to mechanical and chemical versatility.18
3.1.2 Biodegradability
The metabolism and excretion of hydrogels from the body are facilitated by their degradability, which is an advantage when the drug-loaded polymer is intended to be eliminated after the release of its cargo. Another advantageous aspect lies in the polymer's gradual release of the drug at the targeted location, leading to its degradation.117 It is crucial that hydrogel cross-linkers are biodegradable by hydrolysis or enzymatic degradation, as this enables the hydrogel to be replaced by ECM during tissue regeneration.118
3.1.3 Swelling
Hydrogels are exceptional materials comprised of hydrophilic polymers that are crosslinked, exhibiting remarkable capabilities in absorbing and retaining vast quantities of water and biological fluids, while simultaneously preserving 3D structure.119, 120 This ability to swell renders hydrogels an exceptionally suitable material for medical and healthcare applications.121, 122 By controlling their swelling behavior, these hydrogels can regulate drug release, thus enabling prolonged medication delivery.123, 124
3.1.4 Porous structure
Hydrogels, when loaded with drugs and other active substances, can effectively entrap them within the porous structure, allowing for gradual and controlled release.125 The research on hydrogel microspheres found that, in contrast to nonporous microspheres, the porous structure of microsphere scaffolds increased the surface area available for cell adhesion and loading capacity.126 Therefore, by regulating the composition and structural configurations of hydrogels, it is possible to purposefully engineer the release kinetics and time to align with the specific demands of the treatment, thus making hydrogels promising pharmaceutical carriers for therapeutic agents.127, 128 To be specific, hydrogels offer numerous advantages, especially the ability to release drugs over an extended period, which can prolong therapeutic effects,129, 130 reduce dosing frequency,131, 132 and enhance patient adherence.133
3.1.5 Mucoadhesion
Besides the properties discussed above, hydrogels used in the vagina have another important mucoadhesive property. The self-cleaning mechanism of the vagina, which includes mucus secretion, the moist environment of the vaginal lumen, and the peristaltic activity of the vaginal wall, greatly minimizes the duration of conventional vaginal formulations at the intended therapeutic location. Additionally, the mucus layer, epithelial modifications, and potential enzymatic degradation within the vaginal fluid pose further obstacles to effective drug delivery in the vagina.134 The mucoadhesive delivery systems have emerged as a proven approach to address these challenges. By adhering to the vaginal mucosa, these systems offer sustained drug release and accurate cellular targeting, ultimately improving the efficacy of therapeutic interventions.135 Although the exact mechanism is still under investigation, this property makes hydrogels superior vehicles for vaginal drug delivery.
3.1.6 Response to stimuli
Hydrogels can be broadly categorized into two types: traditional hydrogels, which are mostly unresponsive to external stimuli, and their counterparts-intelligent or smart hydrogels, which are sensitive to such factors. These smart hydrogels uniquely adjust their structural conformation, characteristics, or behavior when exposed to specific environmental triggers such as temperature,136, 137 pH levels,138, 139 and light.140 The distinctive physicochemical properties of these materials are particularly promising for their application in the controlled delivery and release of drugs. The benefits conferred by smart hydrogels include the ability to deliver drugs in a targeted manner to ensure the concentrated release at the desired site,141 which can reduce adverse effects that could affect the system as a whole142, 143 and enhance the efficacy of therapeutic intervention.144
Among these hydrogels, temperature-sensitive hydrogels are particularly popular in vagina infections. Torres-Figueroa et al.145 successfully synthesized thermosensitive, bioadhesive semi-interpenetrating polymer networks (s-IPNs) by entrapping chains of poly(methyl vinyl ether-alt-maleic anhydride) (PVME-MA) within the chemically crosslinked poly(N-isopropylacrylamide) (PNIPAAm) network. The in vitro analysis indicated that the s-IPN allowed for the gradual release of antibiotics, with up to 94% release over the course of 48 h, under conditions simulating those of vaginal fluid at a consistent temperature of 37°C. This controlled drug delivery was facilitated by the limited swelling of the hydrogels in this specific release environment. These properties make the PVME-MA/PNIPAAm constructs a promising candidate for a variety of medical and biomedical applications, with particular relevance to the treatment of vaginal infections.
3.2 Hydrogel therapy versus conventional therapy in PID prevention
Oral drug therapies are often effective but are associated with a number of systemic side effects. For instance, the incidence of adverse reactions to oral metronidazole can be as high as 32%, with symptoms including nausea, vomiting, headache, insomnia, dizziness, xerostomia, abdominal discomfort, and even leukopenia and neutropenia.146 In addition, some patients reportedly express aversion to repeated courses of antibiotics, preferring alternative treatments despite their perceived lower efficacy.147 More recently, auranofin, an antirheumatic drug, has been identified as having significant trichomonacidal activity. However, its prolonged plasma half-life of 35 days and notable side effects contribute to sustained systemic exposure despite the short duration of oral administration.148, 149 Another antifungal drug, ibrexafungerp, is associated with diarrhea in 43% of cases, followed by nausea (9%) and abdominal pain (4.1%).150 Notably, oral drug administration poses particular challenges for women with gastrointestinal disorders and pregnant women, as these therapies may be particularly hazardous to the fetus and its development.21
To reduce the risk of systemic drug toxicity, gynecological treatments frequently opt for topical therapies that enable localized drug delivery to the affected region, including formulations like capsules, creams, solutions, gels, and vaginal suppositories. However, the effectiveness of such therapies is often compromised. This is primarily due to uncontrolled leakage, which hinders adequate drug retention in the target tissues.29 Additionally, the exact amount of drug absorbed and the specific tissue area exposed to the drug are often unpredictable, necessitating repeated administrations and consequently causing discomfort for patients. High cumulative doses may further contribute to recurrent infection151 or drug resistance. Ineffective treatment can lead to chronic inflammation, miscarriage, or even infertility. Furthermore, the presence of vaginal fluid facilitates the removal of the formulation, altering both its retention and drug release, thereby requiring numerous applications and resulting in low patient adherence to the treatment regimen.152
Given the constraints of the vaginal route and traditional dosage forms, researchers have explored various pharmaceutical alternatives, notably hydrogels tailored for vaginal drug delivery.153, 154 Hydrogels represent a suitable matrix for the controlled release of biologically active agents, owing to their remarkable compatibility with aqueous environments, biocompatibility, notable porosity, and capability to modulate drug release.155 Additionally, these formulations, unlike solid suppositories, promote prolonged contact with the vaginal mucosa, thereby enhancing the therapeutic efficacy of the administered drug.
3.3 Hydrogels for vagina infections prevent PID
The vagina is part of the connection between the pelvis and the outside world, and early PID is often caused by an infection in the vagina or an upstream infection due to dysbiosis. Therefore, early treatment of PID is often associated with anti-infection of the vagina. Vaginal infections can be divided into bacterial, fungal, and parasitic infections based on their causative pathogens. In terms of viruses, HIV infections are also discussed below. This segment conducts a comprehensive review of vaginal infections and then examines the use of hydrogels, which have been recognized for their efficacy in vaginal drug delivery (Figure 2).
3.3.1 Bacterial vaginal infections
In the United States, BV is the most common condition affecting vaginal health in women of reproductive age. This condition results from an overgrowth of certain bacteria that causes an imbalance in the naturally occurring microbiota of the vagina, potentially causing associated symptoms. Up to 30% of women may resolve BV spontaneously, but most symptomatic women require treatment to avoid severe complications from untreated infections.
The XACIATO™ (clindamycin phosphate) vaginal gel at a 2% concentration, recently unveiled by Organon, is specifically formulated to treat BV. Marketed as a single-dose, colorless vaginal gel, XACIATO can be administered at the patient's convenience and is designed to minimize leakage and maximize retention time.156 The gel's viscosity (thickness and stickiness) increases at body temperature, allowing for a controlled release of clindamycin, as demonstrated in a related in vitro study.157 Previously, the gel was approved by the US FDA in December 2021. This approval was based on data from the Phase 3 DARE-BVFREE study (ClinicalTrials. gov identifier: NCT04370548), which included 307 patients diagnosed with BV aged 12 years and older. Compared to placebo, the percentage of patients treated with XACIATO who experienced clinical cure, bacteriologic cure, and treatment care during the healing test (Days 21–30) visit was statistically significantly higher.158 Individuals who have previously exhibited hypersensitivity to clindamycin or lincomycin should not use XACIATO due to contraindications. The incidence of diarrhea related to Clostridioides difficile-associated diarrhea, a potential consequence of the use of antibacterial agents such as clindamycin, ranges from mild to life-threatening colitis.
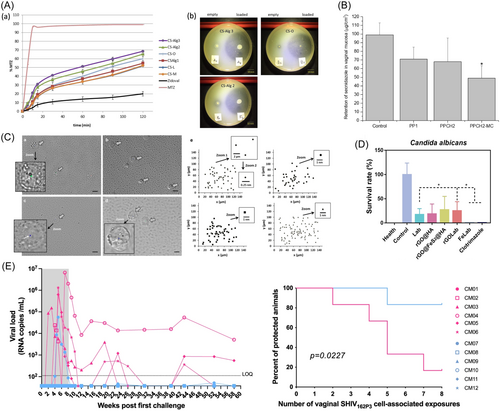
In addition to clindamycin, metronidazole is also commonly used to treat BV. Cirri et al.159 have successfully developed microsphere formulations that serve as mucoadhesive vehicles for the vaginal delivery of metronidazole (Figure 3A). These formulations take advantage of the gelling properties of chitosan and alginate, as well as their recognized compatibility with biological systems, biodegradability, and safety profile. The most effective formulation was prepared by amalgamating chitosan and alginate in a 1:2 weight ratio, incorporating a 10% calcium ion concentration, which resulted in an impressive entrapment efficiency of 70% and facilitated the release of 70% of metronidazole in less than 2 h. The release behavior of the drug from the microspheres was studied using Franz diffusion cells and then compared with that of the commercially available Zidoval® gel, the predominant pharmaceutical form of metronidazole. The results indicated that the microsphere formulation provided a more gradual release, as expected. Specifically, all microsphere formulations exhibited a superior release rate compared to the marketed product, which released only 20% of the drug after 120 min. In addition, in vitro bacterial inhibition studies showed that microspheres configured with both chitosan and alginate exhibited slightly superior antibacterial activity compared to microspheres formulated with chitosan alone. In conclusion, these microsphere formulations can provide extensive coverage within the vaginal tract, thereby increasing the mucoadhesive contact area and improving the precision and control of drug release. They also reduce the risk of detachment and enhance patient compliance.
3.3.2 Parasitic vaginal infections
The adoption of secnidazole (SEC) as a candidate for alternative treatment aims to address the shortcomings of current therapies for combating T. vaginalis, such as side effects and resistance issues. While SEC-based topical formulations are not yet in the market, their integration into thermosensitive bioadhesive systems is targeted to enhance the duration of contact with mucosal surfaces and to minimize systemic drug distribution.
Argenta et al.160 engineered gels containing a mixture of 20% poloxamer 407 and 1% poloxamer 188, supplemented with either 1% or 2.5% chitosan. These gels were characterized by suitable sol-gel transition temperature thresholds above 30°C and accelerated gelation occurring between 100 and 115 s. Significantly, the mucoadhesive capabilities of the gel were reinforced by the integration of chitosan. These hydrogels also demonstrated a reduced rate of SEC transit across the mucosal layer compared to standard preparations. In addition, mucin was added to the mucosa to simulate a more realistic permeability/retention scenario. The chitosan-infused hydrogels showed a reduction in drug permeability/retention in the context of mucin, reducing it by a factor of about two compared to the control (Figure 3B). Overall, the described properties of the hydrogels suggest that they are well suited for the vaginal milieu, thereby underscoring their promise for effective local treatment of trichomoniasis.
Malli et al.161 emphasized the behavior of T. vaginalis motility in a lactate buffer solution at a pH of 4.5. The majority of T. vaginalis cells exhibited motility patterns resembling the “run-and-tumble” motility observed in bacteria. While hydroxyethylcellulose and chitosan hydrogels significantly reduced the motility of T. vaginalis, a portion of the parasites maintained rapid movement through these formulations, thereby causing infection. Remarkably, a thermosensitive hydrogel formulated with pluronic F127 (20 wt%) demonstrated the ability to completely impede T. vaginalis motility (Figure 3C). The overall findings underscored the potential of F127-based formulations to provide a physical barrier against T. vaginalis. This research holds significant promise for reducing the pathogenicity of T. vaginalis and a variety of flagellated microorganisms, whose motility is driven by cilia and flagella. Additionally, the concept of a physical barrier may be applicable to viruses, such as HIV, as previous studies162 on nanoparticles mimicking the size and surface charge of HIV-1 have demonstrated that the F127 hydrogel can also impede particle mobility.
3.3.3 Fungal vaginal infections
VVC, a common fungal inflammatory disease of the external genitalia and vagina, is predominantly caused by C. albicans and affects approximately 75% of women globally. Owing to its widespread prevalence and tendency to recur, VVC exerts a considerable effect on women's physical and emotional well-being. The health and stability of the vaginal microenvironment is highly dependent on the vaginal microbiota from a pathological standpoint. One key bacterium, Lactobacillus, is particularly important in maintaining the balance of the vaginal flora through the production of lactic acid and agents with antimicrobial properties, thereby offering protection against the invasion of harmful microbes. Probiotic therapy, especially for VVC, has garnered significant research interest. While Lactobacillus-based treatments are beneficial for BV, their efficacy against VVC is compromised by the resilience of C. albicans.
Wei et al.163 proposed the conversion of mild H2O2 into toxic hydroxyl radicals using peroxidase-like nanozymes and probiotic Lactobacillus to develop a responsive hyaluronic acid (HA) hydrogel-reduced graphene oxide@FeS2/Lactobacillus@HA system (FeLab), which effectively treats Candida vaginitis and reduces recurrence. In the experimental study, female BALB/c mice were pretreated with estradiol benzoate and then infected with C. albicans to establish a model of vaginitis. After confirmation of the model, intravaginal applications of the treatments were administered daily for 5 days. Vaginal wash samples were analyzed for colony counts and microbiota composition. Compared to Lactobacillus@HA(Lab) treatment, FeLab and clotrimazole treatments significantly reduced C. albicans cell viability and colony formation (Figure 3D). FeLab exhibited antifungal activity in mice with Candida vaginitis, minimizing damage to vaginal mucosal cells and promoting vaginal mucosal repair. The system increased Firmicutes (especially Lactobacilli) and decreased Bacteroidetes, thereby reshaping the vaginal microbiota. This nanobiologic-probiotic combination therapy effectively treated Candida vaginitis, inhibited clinical isolates, and reduced recurrence rates.
In another study, Zimmermann et al.164 aimed to evaluate the efficacy of antifungal treatments using poly(e-caprolactone) nanocapsules loaded with diphenyl diselenide (NC-1) through both in vitro and in vivo studies targeting VVC. NC-1 and control nanocapsules (NC-B) were prepared and tested against Candida strains. NC-1 showed comparable or lower MICs values. A gellan gum hydrogel formulation containing NC-1 was then prepared and tested in a murine model of candidiasis. The hydrogel exhibited acidic pH, robust stability, and strong bioadhesion. Topical treatment with NC-1 hydrogel reduced fungal burden more effectively than nonencapsulated NC-1. Nanoencapsulation maintained antifungal activity, suggesting a promising approach against VVC.
3.3.4 Viral vaginal infections
HIV-1 exists in semen and other body fluids as cell-free virions and infected cells, with both forms contributing to the spread of the virus.165, 166 Historically, the role of infected cells in HIV-1 transmission has been underestimated. Studies in macaques indicate that cell-associated virions are the primary route of simian immunodeficiency virus(SIV) transmission, suggesting that a similar mechanism may be involved in the initiation of some HIV-1 infections in humans.167, 168 To effectively control transmission, prevention strategies must address both cell-free and cell-associated mechanisms. However, cell-associated transmission poses a challenge as it allows the virus to evade antibody neutralization.169 While clinical trials use broadly neutralizing antibodies for passive immunization, their effectiveness against cell-associated transmission often goes overlooked. It is likely that cell-associated HIV-1 transmission, from infected cells in body fluids, dominates infection and helps the virus evade antibody-based immunity.
Suphaphiphat et al.170 developed a topical vaginal application of the anti-HIV-1 bNAb 10-1074 in a microbicide gel that provides protection against vaginal simian-SHIV transmission in nonhuman primates. In the study, animals treated with the gel showed significantly higher protection rates than those receiving placebo (Figure 3E). Kaplan–Meier analysis confirmed a remarkable protective effect in the 10-1074 treated group compared with the control group, with an 80% protection rate (p = 0.0227). Notably, viremia was undetectable in most of the protected animals. Although one treated animal became infected, its viremia was lower than that of the controls. These results underscore the potential of 10-1074 to inhibit cell-associated HIV-1 transmission and support the further development of bNAbs in HIV therapy. They also highlight the need to evaluate passive immunization techniques against cell-associated variants as part of HIV vaccine research.
Before formulating a microbicidal agent containing tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC), Faria et al.171 performed both in vitro and in silico evaluations, examining the physicochemical properties of the drugs and their interactions with lipid models. The preliminary formulation studies indicated that hydrogels would be optimal delivery systems for liposomes encapsulating TDF and for FTC. After analyzing the interactions with mucin, zwitterionic liposomes were selected for their specific properties. These particular liposomes proved to be nontoxic and facilitated the penetration of TDF. The integration of these liposomes with FTC in a Carbomer-based hydrogel resulted in a controlled release pattern of the drug, which fit the parameters of the Weibull model. Moreover, the developed liposomal hydrogels displayed a pseudoplastic flow, making them suitable for vaginal use. Overall, this novel hydrogel composition represents a significant advancement in the vaginal delivery of TDF/FTC.
A variety of hydrogels, specifically designed for the treatment of vaginal infections, are presented in Table 2, which also illustrates a detailed formulation of active pharmaceutical ingredients, polymers, and/or nanocarriers, and their respective advantages.
| Applications | Composition | Active substance | Advantages | References |
|---|---|---|---|---|
| Bacteria | PEG nanocarrier-based hydrogels | Lactic acid | The hydrogels with passively entrapped lactic acid showed retained antimicrobial activity with complete inhibition Gardnerella vaginalis growth within 48 h | [172] |
| Liposomes-in-chitosan hydrogel | Free-drug | It improved the residence time at the vaginal sites and inhibited the growth of Staphylococcus epidermidis and Staphylococcus aureus | [173] | |
| Hydrogel containing 21.5% of poloxamer 407, 1% of sodium alginate, and 9 log10 CFU Lactobacillus crispatus per gel sample (5 g) | Lactobacillus crispatus strain (ATCC 33197) | The ability of Lactobacillus crispatus to inhibit Neisseria gonorrhoeae was still observed with the gel | [174] | |
| Hydrogel based on gelatin and glycerin | Benzydamine | Benzydamine is released at the level of the vaginal mucosa in a slow and gradual manner, which supports the hypothesis of the hydrogel used for the site-specific release | [175] | |
| Nanocomposite hydrogel rings based on polyacrylamide-sodium carboxymethyl cellulose-montmorillonite nanoparticles in the ring-shaped aluminum mold | Montmorillonite | Different antibacterial activities against Escherichia coli were observed for various concentrations of montmorillonite in hydrogels | [176] | |
| Pluronics-based hydrogel (Pluronic F127 and F68); gel flakes system using the combination of gellan gum and chitosan | Metronidazole | It decreased the viability of E. coli and Staphylococcus aureus with reduction percentages of more than 95% after 3 days of treatment, with the healing ability similar to normal vaginal tissue | [177] | |
| AZM-liposomal hydrogels | Azithromycin | It exhibited stronger antibacterial activity than the free drug and achieved prolonged and controlled release of AZM | [178] | |
| Parasite | Hydrogel composed of pluronic®F127 and chitosan | Metronidazole | It allowed a controlled release and anti-T. vaginalis activity of MTZ formulated into F127/- 324 chitosan hydrogel was preserved | [179] |
| PLGA nanoparticles incorporated in thermoresponsive chitosan hydrogel | Auranofin | Nanoparticles-loaded hydrogels were beneficial in enhancing the antiparasitic effect and decreasing side effects | [148] | |
| Hydrogels composed of NC-I3C and gellan gum | Indole-3-carbinol | It has nongreasy and smooth feel, pseudoplastic behavior, low spreadability factor, mucoadhesive potential, controlled release, I3C mucosal retention and nonirritating character | [180] | |
| Hydrogel containing PhSe2-loaded Eudragit® RS100 and coconut oil nanocapsules | Diphenyl diselenide | The hydrogels were obtained by an easy and low-cost process, yielding formulations with suitable spreadability and rheological profiles, high mucoadhesive properties, PhSe2 mucosal retention, and nonirritating potential | [181] | |
| Fungi | Polymeric nanoparticle dispersed in HPMC/Chitosan hydrogel | Metronidazole | The incorporation of metronidazole in the nanoparticles dispersed in the hydrogel promoted an increase in the effectiveness of the drug | [182] |
| Nanostructures dispersed in poloxamer 407 and 108 thermoresponsive hydrogel | Amphotericin B | The dispersion of the drug in both systems increased its effectiveness in vivo and in vitro | [183] | |
| Nanoemulsion dispersed in chitosan hydrogel | Pelargonium graveolens essential oil | In comparison with free oil, its incorporation in both systems increased its in vitro efficacy | [184] | |
| Hydrogels of CTS, P407 and the combination of both | Caspofungin | The CSP-CTS/P407 hydrogel showed better spreadability, extrudability properties and excellent mucoadhesive properties | [185] | |
| Nanocapsules suspension incorporated into the hydrogel formulations | Diphenyl diselenide | The nanoencapsulation maintained the effective antifungal action of diphenyl diselenide and reduced the number of CFU mL−1 in comparison to the negative control (p < 0.001) | [164] | |
| Hydrogel formulation based on chitosan (1.0%) and poloxamer 407 (18%) | C. leptophloeos stem bark extract | It was able to reduce the fungal load and increase the survival rate of G. mellonella larvae infected by C. albicans | [186] | |
| NLC dispersed in hydrogel | Hypericin | The PDT-mediated Hy.NLC-HG system showed a significant difference of p < 0.001 in the number of C. albicans colonies (log) in the vaginal canal | [187] | |
| HIV | Liposomal hydrogels | Tenofovir disoproxil fumarate and emtricitabine | The drug release profile was sustained over time, reaching around 60% for both drugs within 3–6 h, and it featured pseudoplastic profiles that were deemed suitable for topical application | [171] |
| Gum-based hydrogel and sesame oil added Span (R) 60 or Span (R) 60 and Tween (R) 60 | Tenofovir | The system with the lowest guar gum hydrogel/sesame oil proportion and containing Span (R) 60 and Tween (R) 60 (batch ST1) had the highest consistency and adhesion capacity and ST1 showed the longest bioadhesion time and the most controlled release, as well as a low swelling grade | [188] | |
| Hydrogels composed of Pluronics®, hydroxy propyl methyl cellulose, Carbomer and poly(ethylene glycol) 400 | Cabotegravir | The use of PEG in this formulation was able to increase the penetrability of CAB through vaginal tissue with 0.61 ± 0.05 mg and 17.28 ± 0.95 mg of CAB being able to penetrate and localize in the vagina, respectively | [189] | |
| PEG-based hydrogel | Enfuvirtide | The PEG-based hydrogel is found to have suitable physicochemical properties for an intravaginal formulation of the PSA substrate-linked antiretrovirals and is safe toward vaginal epithelium | [190] | |
| HPV | Hydrogel (poloxamer 407) formed with chitosan and hydroxypropylmethylcellulose K4M (M20) | Curcumin | Analysis of results suggests that CUR-SD thermoresponsive hydrogel can be used via the vaginal route for the treatment of mucosal inflammation and infectious disease | [191] |
| HSV | Tannic acid modified silver nanoparticles based hydrogels applying Carbopol 974 P | Tannic acid; silver | The hydrogels with TA-AgNPs possess distinctive ability to prevent both HSV-1 and HSV-2 infection by direct inhibition of viral attachment, penetration and postinfection spread | [192] |
- Abbreviations: AMZ, azithromycin; C. albicans, Candida albicans; C. leptophloeos, Commiphora leptophloeos; CAB, cabotegravir; CFU, colony-forming unit; CSP, caspofungin; CTS, chitosan; CUR-SD, curcumin in solid dispersion form; G. mellonella, Galleria mellonella; HIV, human immunodeficiency virus; HPMC, hydroxypropyl methyl cellulose; HSV, herpes simplex virus; Hy, hypericin; I3C, indole-3-carbinol; MTZ, metronidazole; NC-I3C, nanocapsules of Eudragit® RS100 and rosehip oil containing I3C; NLC, nanostructured lipid carrier; P407, Poloxamer 407; PDT, photodynamic therapy; PEG, polyethylene glycol; PhSe2, diphenyl diselenide; PLGA, poly(lactic-co-glycolic) acid; PSA, prostate specific antigen; T. vaginalis, Trichomonas vaginalis; TA-AgNPs, tannic acid modified silver nanoparticles.
3.4 Hydrogels for UTIs
The conventional strategy for treating UTIs revolves around selecting antibiotics such as beta-lactams, fluoroquinolones, trimethoprim-sulfamethoxazole, amoxicillin-clavulanic acid, or third-generation cephalosporins based on the results of bacterial culture susceptibility.193, 194 However, the treatment of UTI faces significant challenges stemming from the emergence of drug-resistant bacteria and the development of intracellular bacterial reservoirs.108 Specifically, members of the Enterobacteriaceae family, including E. coli and K. pneumoniae, have raised concerns due to their acquisition of plasmids encoding extended-spectrum β-lactamases such as cefotaximases, oxacillinases, AmpC-type β-lactamases, and carbapenemases.195 Furthermore, the use of antimicrobial agents in this context is constrained by adverse drug reactions and potential drug interactions.196 Additionally, emerging research suggests that antimicrobial drugs may disrupt the ecological balance of the gut microbiota, potentially enhancing bacterial infections through modulation of the immune response.197, 198 Therefore, there is an urgent need for novel drugs or drug combinations to treat UTI that have unique modes of action or differ chemically from current therapeutic options.
In the realm of hospital-acquired infections, UTIs represent the most common category. These infections are manifested when invasive pathogens enter the urinary tract through the urethra and multiply in the bladder.199 The susceptibility to UTIs is particularly high in women, who tend to harbor a variety of bacteria in their urinary systems.200, pp.2011–2013 The pathogens responsible for UTIs are showing increasing resistance to conventionally used antimicrobial agents, propelling an urgent demand for novel therapeutic strategies or combination drug therapies to effectively combat these infections.201
Over the past decade, nanoparticles and hydrogels have been increasingly used in the biomedical field.202 In a study, Alshehri et al.203 evaluated the antimicrobial efficacy of three different treatments: the hydrogel alone, hydrogel composites loaded with silver nanoparticles (AgNPs), and a routinely prescribed drug, kanamycin, administered at a 30 μg dose. They assessed the treatments through comparative analyses of the inhibition zone diameters produced against a spectrum of UTI-associated bacteria, including strains of E. coli, K. pneumoniae, Pseudomonas aeruginosa, Proteus vulgaris, S. aureus, and Proteus mirabilis. The inhibition zones caused by hydrogel disks at 1 mg/mL were 8, 7.2, and 9 mm for E. coli, S. aureus, and P. mirabilis, respectively. On the other hand, AgNPs-incorporated hydrogel composites at the same concentration showed greater efficacy, with inhibition zones of 12.6 mm for P. vulgaris, 11.2 mm for K. pneumoniae, and 11.1 mm for S. aureus. Noteworthy is the enhanced effect observed when 5 mg/mL of the AgNPs-hydrogel composite was used; it manifested the most significant zone of 16.6 mm against E. coli. These results indicate that, under comparable test conditions, AgNPs-incorporated hydrogel composites have superior antibacterial activity compared to the hydrogel alone and the well-established standard drug, kanamycin.
Currently, there is no consensus on treatment protocols for CAUTI. Patients are treated similarly to non-CAUTIs (uUTI), despite unique clinical features and causative pathogens. Unlike uUTIs, which are more common in women, CAUTIs show no gender bias. While E. coli is predominant in uUTIs, CAUTI pathogens are diverse, including gram-negative, gram-positive, and fungal species.204 Hydrogel-coated catheters are a useful way to prevent and treat CAUTI (Figure 2).
For the first time, McCoy et al.205 used the increase in urinary pH resulting from pathogenic activity to trigger the release of antimicrobials from innovative hydrogel-based drug carriers. Designed to serve as infection-resistant coatings on urinary catheters, these delivery systems were synthesized from 2-hydroxyethyl methacrylate crosslinked with vinyl modifications of nalidixic acid. When tested at a pH level of 10, which is commonly found in urine during infection, these systems exhibited a significantly accelerated rate of drug release, in contrast to their performance at the neutral pH of 7 (Figure 4A). The pH-responsive drug delivery system achieved up to 96.5% reduction in bacterial adherence in vitro, showing promise in preventing CAUTIs without external intervention.
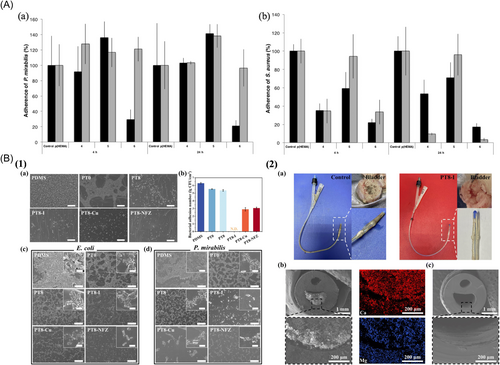
In another study, Miao et al.206 created a hydrogel coating using poly (sulfobetaine methacrylate)-tannic acid (polySBMA-TA) to efficiently carry antimicrobials on urinary catheters. Various antimicrobial agents, such as poly(vinylpyrrolidone)-iodine, copper ions, and nitrofurazone, were successfully incorporated into the polySBMA-TA coating through noncovalent bonds. The initial investigation focused on the adhesion of E. coli to both pristine and modified polydimethylsiloxane (PDMS) surfaces. Notably, a significant population of bacteria adhered to the untreated PDMS, while the PT0-coated surface exhibited a remarkable suppressive effect. Quantitative assessments revealed that the hydrogel coating effectively reduced the number of bacteria on the surface, and the integration of Cu and nitrofurazone further reduced the adherent bacterial population by 3.40 and 3.24 orders of magnitude, respectively. Prolonged incubation of the substrates for over 72 h confirmed the remarkable antibacterial properties inherent in the antimicrobial-loaded hydrogel coatings (Figure 4B(1)). Large urinary catheters coated with PT8-I were fabricated and subsequently implanted in rabbits. After a 7-day implantation period, the catheter was removed from the rabbit for comprehensive analysis (Figure 4B(2)). Both in vitro and in vivo tests validated the exceptional antibacterial properties of the coatings, their ability to prevent incrustation, and their commendable biocompatibility. More importantly, the pH-responsive release of antibacterial agents under alkaline conditions further enhanced the antibacterial activity of the coatings, which was advantageous for killing the urease-producing bacteria and preventing encrustation. The study presents an innovative strategy involving the application of a multifunctional hydrogel coating with antimicrobial agents. This coating features pH-responsive characteristics, which are effective in preventing bacterial adhesion and the formation of encrustations on urinary catheters.
Looking forward to the application of pH-responsive hydrogels in UTIs, we can foresee great potential and development prospects in this field. Current studies have shown that hydrogels with bacterial activation pH and temperature biresponsiveness can release antimicrobial agents in specific environments, thereby effectively killing or inhibiting pathogens in the urinary tract.207 This intelligent hydrogel can not only improve the efficiency of antimicrobial use and reduce unnecessary drug release, but also adaptively adjust the drug release according to changes in the microenvironment of the UTI site, so as to achieve more precise treatment. Table 3 lists the application of hydrogels in UTIs, as well as their active substances and advantages.
| Applications | Systems/compositions | Drug/active substances | Advantages | References |
|---|---|---|---|---|
| CAUTIs | Hydrogel containing copper nanocomposite particles | Copper | The prepared nanocomposite show higher zone of inhibition against these pathogens then that of corresponding hydrogel matrix | [208] |
| P(HEMA) hydrogel | Ampicillin trihydrate (AMP), levofloxacin (LVX) | p(HEMA) impregnated with AMP, LVX, and drug combinations showed significantly increased antimicrobial activity and decreased biofilm-forming ability compared with p(HEMA), in addition to the effects on (HEMA) mechanical properties | [209] | |
| P‑HEMA hydrogel | Rifampicin and cefxime | Drug loaded hydrogel showed better persistence to microbial growth and biofilm formation | [210] | |
| Bacterial cellulose hydrogels | Chitosan | The composites show strong antibacterial activity against strains such as P. mirabilis, E. coli and P. aeruginosa. They have high potential of these composites for successful elimination of bacteria and their promising usage in UTIs and CAUTIs treatments, as well as urinary catheter coating in prevention of encrustation | [211] | |
| Catechol-modified chitosan (Chi-C) hydrogel mixed with poly-(DL-lactic-co-glycolic) acid microspheres | Chlorhexidine (CHX) and triclosan (TCS) | The Chi-C/CHX + TCS microspheres system presented better antimicrobial property for Staphylococcus aureus and P. mirabilis for entire experiment period | [212] | |
| Silver nanoparticles poly(acrylamide)-chitosan-polyvinylpyrrolidone(AgNPs-PAAm-CS-PVP) hydrogel | Ag and CS | Both the outer and inner surfaces of the AgNPs-PAAm-CS-PVP hydrogel-coated urinary catheters had strong antibacterial property against E. coli. | [213] | |
| Hydrogel base layer of poly(vinyl alcohol) | Acetohydroxamic acid or ciprofloxacin hydrochloride) and a fluorescent dye, 5(6)-carboxyfluorescein | It has the potential for theranostic, infection-responsive coatings to combat catheter encrustation and actively delay blockage | [214] | |
| Polydopamine-carboxymethyl cellulose-Ag nanoparticle hydrogel | Ag | The PDA-CMC-AgNPs coated catheters showed both good antibacterial and antiadhesion properties to bacteria, which inhibited the adhesion of live bacteria and dead bacteria by 99.0% and 86.6%, respectively | [215] | |
| PVP-PEGDA-MFP hydrogel | Recombinant mussel protein | In vivo and in vitro experiments showed broad-spectrum antibacterial properties and good cytocompatibility and biocompatibility | [216] | |
| Silver alloy hydrogel | Silver | SAH catheters can effectively inhibit the formation of catheter-related bacterial biofilms in critically ill patients and reduce the incidence of CAUTIs, compared with conventional siliconized latex Foley catheters | [217] | |
| PAAm/SA/TA matrix (PST), AgNPs, and chitosan/bovine serum albumin-AuNPs (CB-AuNPs) | AgNPs and CB-AuNPs | It can reduce biofilms formation and alleviate inflammation degree synergistically, which was monitored in real-time dependent on an on–off variation of fluorescence intensity | [218] | |
| An ultra-low loading of gold/iron co-doped silver peroxide nanoparticles (Au/Fe-Ag2O2 NPs) hydrogel composite | Au/Fe-Ag2O2 NPs | All the results from in vitro and in vivo experiments demonstrate the sustained antimicrobial property, outstanding anti-inflammatory activity, and excellent biosafety of catheter | [219] | |
| Bacterial cystitis | Lev@PADM (combination of levofloxacin and decellularized porcine acellular dermal matrix hydrogel) | Levofloxacin | Lev@PADM effectively prolongs the duration of levofloxacin's action, impedes the retention and invasion of E. coli in the urinary tract, diminishes the infiltration of innate immune cells into infected tissues, and simultaneously preserves the composition of the intestinal microbiota | [220] |
- Abbreviations: AgNPs, silver nanoparticles; AgNPs-PAAm-CS-PVP, silver nanoparticles poly(acrylamide)-chitosan-polyvinylpyrrolidone; AMP, ampicillin trihydrate; CB-AuNPs, chitosan/bovine serum albumin-AuNPs; Chi-C, catechol-modified chitosan; CHX, chlorhexidine; E. coli, Escherichia coli; Lev, levofloxacin; LVX, levofloxacin; MFP-5, recombinant mussel protein; p(HEMA), poly hydroxyethyl-methacrylate; P. aeruginosa, Pseudomonas aeruginosa; P. mirabilis, Proteus mirabilis; PAAm/SA/TA, polyacrylamide/sodium alginate/tannic acid; PADM, porcine acellular dermal matrix; PDA-CMC-AgNPs, polydopamine-carboxymethylcellulose-Ag nanoparticles; PEGDA, polyethylene glycol acrylate; PVP, polyvinylpyrrolidone; SAH, silver alloy hydrogel-coated; TCS, triclosan.
4 CHALLENGES IN THE DEVELOPMENT AND APPLICATION
Currently, most clinical regimens for the treatment of PID are long-term antibiotic therapies, which tend to exacerbate the resistance of pathogenic microorganisms and are often associated with poor tissue penetration, low drug utilization, and low patient compliance. With the growing concern of antibiotic resistance, hydrogels with inherent antimicrobial properties or the ability to add antimicrobial agents offer a solution for the treatment of PID. Most of the studies on hydrogels in the treatment of PID are only in preclinical experiments, such as the rat vaginal infection model, and only some of them have been conducted in preclinical experiments. In nonclinical studies, most studies have investigated the use of hydrogels for topical administration of antibiotics in the treatment of PID. These studies have shown that hydrogels can achieve higher local drug concentrations compared to systemic administration, thereby more effectively inhibiting the progression of PID. For example, hydrogels loaded with doxycycline or metronidazole have been used in animal models to eliminate bacterial populations and inflammatory phenotypes in pelvic organs. Hydrogels containing nonsteroidal anti-inflammatory drugs or corticosteroids have been investigated for the reduction of symptoms associated with PID.
A major advantage of biotissue-engineered hydrogels is their ability to encapsulate and release drugs in a controlled manner, typically including metal nanoparticles, antimicrobial peptides, or other bioactive molecules. For example, both antibiotics and anti-inflammatory drugs can be added to the hydrogel to reduce inflammation while treating an infection. This multipronged approach can improve the overall outcome for PID patients. And hydrogels can be formulated for minimally invasive delivery methods, such as injections or laparoscopic applications. Injectable hydrogels can be injected directly into the site of infection, forming a gel in situ and releasing therapeutic agents over time. This approach reduces the need for extensive surgical procedures and minimizes patient discomfort and recovery time.
However, there are still many limitations in the development of hydrogels. Hydrogels used in PID therapy must be biocompatible and biodegradable to avoid inflammation and can be absorbed or metabolized during normal physiological activities of the body. At the same time, the dynamic environment of the pelvis poses unique challenges for hydrogel design. Hydrogels with adhesive properties adhere to the mucosal surface of the organ and attempt to remain at the site of infection without migrating to other sites, providing sustained release of therapeutic agents. This adhesion facilitates local treatment and minimizes drug loss due to fluid flow. Appropriate mechanical properties also need to be considered. For the pelvis, the hydrogel must have appropriate mechanical properties to withstand the external forces of the pelvis without breaking and thus affecting the efficacy of the treatment.
The pelvic environment is complex, and hydrogel design can begin with gelation conditions, including the development of novel pH-responsive, temperature-responsive, and enzyme-responsive hydrogels for the treatment of PID, while self-healing and shape-memory hydrogels can be delivered via minimally invasive surgery and then conformed to the pelvic anatomy to improve efficacy. Taking into account factors such as PID disease severity, patient-specific microbiota, and individual response to therapy, hydrogel formulations can be tailored to individual patient needs, thereby optimizing therapeutic efficacy. In terms of therapeutics, commercially available antibiotics are currently used. For future development, hydrogels can be combined with antimicrobial peptides, growth factors, stem cells, exosomes or cell derivatives, for example, using liposomes to piggyback on antimicrobial substances to promote PID prevention. In conclusion, a large number of experiments are still needed to confirm the reliability of hydrogels for the treatment of PID.
It should be noted that the use of hydrogel is not limited to vaginal or urethral infections. Hydrogel can also be used to repair the endometrium and restore ovarian function for the entire female pelvic cavity. However, as mentioned above, the pelvis is a complex environment. In terms of PH data of the physiological environment, the PH value of the vagina is acidic, while the uterine environment is alkaline. Therefore, the design of the hydrogel should also be more individualized. In the future, we hope that hydrogels will not only be used for PID but also have the potential to regenerate the endometrium and restore ovarian function, thus restoring the patient's fertility.
5 CONCLUSION
PID can result from ascending vaginal infections, an area particularly susceptible to cross-infection due to its anatomical proximity to the urethra. Despite the prevalence of vaginal infections, effective preventive and therapeutic measures are still lacking. Hydrogels remain a viable approach to address the limitations associated with conventional formulations and vaginal delivery methods. Their exceptional properties are expected to enhance the local therapeutic efficacy for the treatment of such infections. Specifically, stimuli-responsive and mucoadhesive hydrogels play a critical role in advancing this field. In this context, several studies have explored hydrogels encapsulating drugs or nanosystems that offer controlled and prolonged drug release, antimicrobial efficacy, therapeutic benefits, and minimized cytotoxicity. These novel hydrogel formulations represent a significant breakthrough in the treatment of vaginal infections.
Likewise, hydrogels have found utility in UTIs, primarily as coatings on catheters, to prevent and treat CAUTIs. Hydrogel coatings are characterized by their exceptional antibacterial properties, ability to inhibit encrustation, and exceptional biocompatibility. These characteristics make them a compelling solution for protecting urinary catheters from bacterial colonization and encrustation.
In conclusion, multidisciplinary synergistic development has made hydrogels a new approach to PID prevention. There are still many challenges in the preparation of hydrogels and drug piggybacking, such as how to design more personalized hydrogels as well as effective slow-release systems to achieve topical retention while preventing the worsening of infection. With the continuous development of clinical medicine and material science, we believe that hydrogels can be a great initiative for PID prevention.
AUTHOR CONTRIBUTIONS
Conceived and presented the article idea and supervised the whole work: Xulin Hu. Collected the data and wrote the first draft of the manuscript: Yile Xiao. Collected the data and wrote the first draft of the manuscript: Teng Ma and Haoming Wu. Prepared the figures and tables: Qiao Su and Yayu Zhou. Prepared the figures and participated in article revision: Bingnan Zhou. Participated in the revision of articles: Keyi Yang, Zhengguang Pu, and Wanyue Feng. Designed the framework for the entire manuscript and provided detailed guidance and financial support: Xulin Hu, Huili Zhu, and Xin Yong. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
Figures 1 and 2 were drawn with BioRender software, biorender.com. This work was supported by Natural Science Foundation of Sichuan Province(#23NSFSC5880), Chengdu Medical Research Project (#2022004), Natural Science Foundation of Clinical Medical College and Affiliated Hospital of Chengdu University, Natural Science Foundation of China (NSFC, #32200559), China Postdoctoral Science Foundation (#2021M702364), “From 0 to 1” Innovation Research Project by Sichuan University (#2023SCUH0022), Sichuan Science and Technology Program (#2024NSFSC1291).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
No ethical approval was required for this study.
Open Research
DATA AVAILABILITY STATEMENT
The data in this study are available upon reasonable request.




