Tunable Properties of Inclusion Complexes Between Amylose and Polytetrahydrofuran
Rachmawati Rachmawati
Department of Polymer Chemistry, Zernike Institute for Advanced Materials, University of Groningen, Nijenborgh 4, 9747AG Groningen, The Netherlands
Search for more papers by this authorAlbert J. J. Woortman
Department of Polymer Chemistry, Zernike Institute for Advanced Materials, University of Groningen, Nijenborgh 4, 9747AG Groningen, The Netherlands
Search for more papers by this authorCorresponding Author
Katja Loos
Department of Polymer Chemistry, Zernike Institute for Advanced Materials, University of Groningen, Nijenborgh 4, 9747AG Groningen, The Netherlands
Department of Polymer Chemistry, Zernike Institute for Advanced Materials, University of Groningen, Nijenborgh 4, 9747AG Groningen, The NetherlandsSearch for more papers by this authorRachmawati Rachmawati
Department of Polymer Chemistry, Zernike Institute for Advanced Materials, University of Groningen, Nijenborgh 4, 9747AG Groningen, The Netherlands
Search for more papers by this authorAlbert J. J. Woortman
Department of Polymer Chemistry, Zernike Institute for Advanced Materials, University of Groningen, Nijenborgh 4, 9747AG Groningen, The Netherlands
Search for more papers by this authorCorresponding Author
Katja Loos
Department of Polymer Chemistry, Zernike Institute for Advanced Materials, University of Groningen, Nijenborgh 4, 9747AG Groningen, The Netherlands
Department of Polymer Chemistry, Zernike Institute for Advanced Materials, University of Groningen, Nijenborgh 4, 9747AG Groningen, The NetherlandsSearch for more papers by this authorAbstract
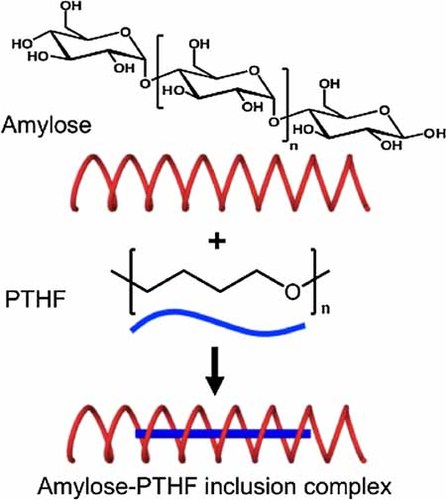
Amylose and polytetrahydrofuran (PTHF) are mixed in an aqueous solution to form inclusion complexes. DSC shows that immediate mixing results in complexes having lower melting temperatures compared with complexes prepared with longer mixing times. The washed complexes melt at higher temperatures compared with the corresponding unwashed complexes. XRD indicates that amylose–PTHF complexes diffract similar to amylose–fatty acids complexes (V6I-amylose helices), with additional diffractions correlating with amylose–alcohol complexes (V6II-amylose helices). This suggests that the structure of amylose–PTHF complexes is an intermediate or a mixture between V6I- and V6II-amylose. This shows that, besides residing inside the amylose helices, some PTHF chains are located in between the amylose helices.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| mabi_201300022_sm_suppdata.pdf303.9 KB | suppdata |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 J. R. Katz, Recl. Trav. Chim. Pays-Bas. 1937, 56, 766.
- 2
- a) S. Immel, F. W. Lichtenthaler, Starch/Stärke 2000, 52, 1;
- b) M. Tusch, J. Krüger, G. J. Fels, Chem. Theory Comput. 2011, 7, 2919.
- 3 Y. Kanekiyo, R. Naganawa, H. Tao, Angew. Chem., Int. Ed. 2003, 42, 3014.
- 4 G. Wulff, G. Avgenaki, M. S. P. Guzmann, J. Cereal Sci. 2005, 41, 239.
- 5 S. Ahmadi-Abhari, A. J. J. Woortman, R. J. Hamer, A. A. C. M. Oudhuis, K. Loos, Carbohydr. Polym. 2013, 93, 224.
- 6 S. Zabar, U. Lesmes, I. Katz, E. Shimoni, H. Bianco-Peled, Food Hydrocolloids 2010, 24, 347.
- 7
- a) G. F. Fanta, C. L. Swanson, W. M. Doane, J. Appl. Polym. Sci. 1990, 40, 811;
- b) R. L. Shogren, R. V. Greene, Y. V. Wu, J. Appl. Polym. Sci. 1991, 42, 1701;
- c) R. L. Shogren, A. R. Thompson, R. V. Greene, S. H. Gordon, G. Cote, J. Appl. Polym. Sci. 1991, 42, 2279;
- d) R. L. Shogren, Carbohydr. Polym. 1993, 22, 93.
- 8 J. Kadokawa, A. Nakaya, Y. Kaneko, H. Tagaya, Macromol. Chem. Phys. 2003, 204, 1451.
- 9
J. Kadokawa,
Y. Kaneko,
S. Nagase,
T. Takahashi,
H. Tagaya,
Chem. Eur. J.
2002,
8,
3321.
10.1002/1521-3765(20020802)8:15<3321::AID-CHEM3321>3.0.CO;2-E CAS PubMed Web of Science® Google Scholar
- 10 T. Kida, T. Minabe, S. Okabe, M. Akashi, Chem. Commun. 2007, 1559.
- 11
- a) J. Kadokawa, Y. Kaneko, A. Nakaya, H. Tagaya, Macromolecules 2001, 34, 6536;
- b) J. Kadokawa, Y. Kaneko, H. Tagaya, K. Chiba, Chem. Commun. 2001, 449;
- c) Y. Kaneko, J. Kadokawa, Chem. Rec. 2005, 5, 36;
- d) Y. Kaneko, K. Beppu, J. Kadokawa, Macromol. Chem. Phys. 2008, 209, 1037.
- 12
- a) J. van der Vlist, M. P. Reixach, M. van der Maarel, L. Dijkhuizen, A. J. Schouten, K. Loos, Macromol. Rapid Commun. 2008, 29, 1293;
- b) J. van der Vlist, I. Schonen, K. Loos, Biomacromolecules 2011, 12, 3728;
- c) J. v. d. Vlist, M. Faber, L. Loen, T. J. Dijkman, L. A. T. W. Asri, K. Loos, Polymers 2012, 4, 674;
- d) J. Ciric, J. Oostland, J. W. de Vries, A. J. J. Woortman, K. Loos, Anal. Chem. 2012, 84, 10463;
- e) J. Ciric, K. Loos, Carbohydr. Polym. 2013, 93, 31.
- 13 R. Rachmawati, A. J. J. Woortman, K. Loos, Biomacromolecules 2013, 14, 575.
- 14 J. Nuessli, B. Sigg, B. Conde-Petit, F. Escher, Food Hydrocolloids 1997, 11, 27.
- 15 H. F. Zobel, A. D. French, M. E. Hinkle, Biopolymers 1967, 5, 837.
- 16 W. Helbert, H. Chanzy, Int. J. Biol. Macromol. 1994, 16, 207.
- 17 M. B. Cardoso, J. L. Putaux, Y. Nishiyama, W. Helbert, M. Hÿtch, N. P. Silveira, H. Chanzy, Biomacromolecules 2007, 8, 1319.