Evaluation of CRISPR/Cas9 exon-skipping vector for choroideremia using human induced pluripotent stem cell-derived RPE
Funding information: B.R.C. acknowledges support through gifts from Claire Giannini Fund, That Man May, the Roddenberry Foundation, Pauline and Thomas Tusher and the Gladstone Institutes. This works is supported by JSPS KAKENHI (Grant Number 20K09823 to T. Iwagawa) and TaNeDS Daiichi Sankyo Co., Ltd. B.R.C. was supported by the National Institutes of Health, R01-EY028249 and R01-EY027789, R01-HL130533, R01-HL13535801, P01-HL146366. B.R.C.
Funding information: JSPS KAKENHI, Grant/Award Number: 20K09823; National Institutes of Health, Grant/Award Numbers: P01-HL146366, R01-HL13535801, R01-HL130533, R01-EY027789, R01-EY028249
Abstract
Background
Exon-skipping is a powerful genetic tool, especially when delivering genes using an AAV-mediated full-length gene supplementation strategy is difficult owing to large length of genes. Here, we used engineered human induced pluripotent stem cells and artificial intelligence to evaluate clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR associated protein 9-based exon-skipping vectors targeting genes of the retinal pigment epithelium (RPE). The model system was choroideremia; this is an X-linked inherited retinal disease caused by mutation of the CHM gene.
Methods
We explored whether artificial intelligence detected differentiation of human OTX2, PAX6 and MITF (hOPM) cells, in which OTX2, PAX6 and MITF expression was induced by doxycycline treatment, into RPE. Plasmid encoding CHM exon-skipping modules targeting the splice donor sites of exons 6 were constructed. A clonal hOPM cell line with a frameshift mutation in exon 6 was generated and differentiated into RPE. CHM exon 6-skipping was induced, and the effects of skipping on phagocytic activity, cell death and prenylation of Rab small GTPase (RAB) were evaluated using flow cytometry, an in vitro prenylation assay and western blotting.
Results
Artificial intelligence-based evaluation of RPE differentiation was successful. Retinal pigment epithelium cells with a frameshift mutation in exon 6 showed increased cell death, reduced phagocytic activity and increased cytosolic unprenylated RABs only when oxidative stress was in play. The latter two phenotypes were partially rescued by exon 6-skipping of CHM.
Conclusions
CHM exon 6-skipping contributed to RPE phagocytosis probably by increasing RAB38 prenylation under oxidative stress.
1 INTRODUCTION
The retinal pigment epithelium (RPE) is a monolayer of cells adjacent to the photoreceptor layer. The RPE plays a pivotal role in vision, maintaining photoreceptor health and function principally by phagocytosis of shed photoreceptor outer-segments and recycling of visual molecules.1 If the RPE dies or becomes dysfunctional, the photoreceptors degenerate and vision is lost. Inherited retinal diseases (IRDs) such as retinitis pigmentosa trigger photoreceptor cell degeneration. In addition to the photoreceptor specific genes, several RPE specific genes, such as RPE65, have been identified to be causal of IRD by their mutations.2 Therefore, RPE is being sought as a therapeutic target,3 and several treatments are under development. A recombinant adeno-associated virus (rAAV)-based gene therapy for biallelic RPE65 mutation-associated retinal dystrophy was approved by the US Food and Drug Administration in 2017; this was the first such therapy approved worldwide.4, 5 Although rAAV-mediated gene transfer is promising, the limitations include the development of neutralizing antibodies against the AAV capsid proteins6 and the small packaging capacity (less than 5 kb). Thus, it is difficult to deliver large genes such as EYS and MYO7A to retina or RPE.
When seeking to develop therapeutic tools, manipulation of the human RPE is essential. It is important to understand the pathophysiology in great detail and to screen the actions of pharmacological drugs. The major sources of human RPE cells are embryonic stem cells (ESCs) and human induced pluripotent stem cells (hiPSCs);7, 8 several protocols that cause ESCs/iPSCs to differentiate into RPE have been reported.9 Accurate evaluation of differentiation is critical. Here, we established a rapid and simple evaluation platform using clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR associated protein 9 (Cas9)-based exon-skipping of hiPSCs. We chose choroideremia (CHM) as the model system.
Choroideremia is an X-linked IRD characterized by degeneration of the choroid, retina and RPE. Choroideremia is caused by mutations in the CHM gene, which encodes Rab escort protein 1 (REP-1). About 30% of all mutations are nonsense mutations; at least one nonsense mutation has been identified in each exon.10 CHM is essential for prenylation of Rab small GTPases (RABs) involved in secretion, phagocytosis and intracellular tracking.11 In choroideremia patients, CHM dysfunction triggers the accumulation of unprenylated RABs, which are thought to be related to the degeneration of the RPE, retina and choroid.12 A gene therapy (provision of full-length CHM via rAAV transfection) is in phase III clinical trials.13, 14
Here, we studied a recently established hiPSC line, the human OTX2, PAX6 and MITF (hOPM) cell line.15 In this line, expression of exogenous OTX2, PAX6 and MITF, which are inserted in the AAVS1 locus, is induced by doxycycline; RPE differentiation is then rapidly and effectively induced. We established a non-invasive artificial intelligence (AI)-based cell evaluation system. As CHM lies in the X-chromosome, we used only male hiPSCs because manipulation of only one CHM gene was required. Because the CRISPR/Cas9-mediated exon-skipping allows the use of the endogenous promoter and removal of the unprenylated RABs, and would be a permanent therapy, we explored whether exon-skipping induced by CRISPR/Cas9 gene editing could serve as a choroideremia therapy. We established a clonal hOPM cell line with a frameshift mutation in exon 6 and differentiated into RPE. CHM exon 6-skipping was induced, and the effects of skipping on phagocytic activity, cell death, RAB prenylation and RAB localization were evaluated.
2 MATERIALS AND METHODS
2.1 hiPSC culture, the establishment of the hOPM cell line, RPE differentiation and induction of exon skipping using the CRISPR/Cas9 system
WTC11 cells16 were cultured according to the protocol provided at the following site: https://labs.gladstone.org/conklin/protocols.html. The establishment of the hOPM cell line and differentiation of hOPM into RPE were done following the previous report.15 After reaching confluence on a 12 well plate coated with Matrigel (Coring, 356231), hOPM cell lines were cultured with the RPEMM115 without nicotinamide. After 7 weeks the culture medium was changed to X-VIVO 10 (Lonza, 04-380Q). We confirmed doxycycline-dependent differentiation of hOPM to RPE by morphological observation (Supplemental Figure S1A) and gene expression (Supplemental Figure S1B). To induce exon 6-skipping, hOPM RPE cells were transfected with the plasmid described below using the Neon Transfection System (ThermoFisher Scientific) and cultured for 7 weeks with X-VIVO 10. To induce oxidative stress, RPE cells were incubated with X-VIVO 10 containing 2 mm sodium iodate (Sigma Aldrich, S4007) for 1 day.
2.2 Genome edition using the CRISPR/Cas9 system and the establishment of clonal cell lines
The target site (Supplemental Table S1) of guide RNA (gRNA) was designed using CRISPRdirect (https://crispr.dbcls.jp/). Oligo DNAs were hybridized and inserted into the BbsI sites of pSpCas9(BB)-2A-GFP (PX458, Addgene, 48,138). To construct PX458 expressing the Cas9 VQR variant (D1135V/R1335Q/T1337R), each mutation was introduced by an inverse PCR-based mutagenesis kit (Toyobo, SMK-101). hOPM cells were transfected with the plasmids by the Neon Transfection System. After 2 days of culture, GFP-expressing cells were sorted using FACSAria II (BD). For the establishment of clonal cell lines, 5E3 cells were seeded on a six-well plate. Genomic PCR was carried out using PrimeSTAR GXL DNA polymerase (Takara, R050).
2.3 Reverse transcription and semi-quantitative or quantitative PCR (RT-qPCR), droplet digital PCR (ddPCR), immunocytochemistry
Total RNA was extracted using Sepasol RNA I Super G (Nacalai tesque, 09379) and cDNA was synthesized using ReverTra Ace (Toyobo, FSQ-301). Semi-quantitative PCR or qPCR was performed using PrimeSTAR GXL DNA polymerase or THUNDERBIRD SYBR qPCR Mix (Toyobo, QPS-101) and LightCycler 96 (Roche), respectively. The relative mRNA expression level was normalized by the expression level of ACTB and GAPDH. Quantitative ddPCR was carried out using the QX200 ddPCR system (BioRad) and ddPCR Supermix for Probes (BioRad, 1863024). The same amounts of cDNA were used for templates. Primer sequences are available in Supplemental Table S1.
Retinal pigment epithelium cells derived from hOPM cell lines were seeded on a chamber slide glass (Corning, 354118) coated with Matrigel. Immunostaining was done as previously described17 using antibodies indicated in Supplemental Table 2. Nuclei were visualized with 4′, 6-diamidino-2-phenylindole dihydrochloride. Fluorescence images were obtained using Zeiss Axio Imager M2 (ZEISS).
2.4 Phagocytosis assay
Porcine eyes were obtained from Tokyo Shibaura Zouki, and photoreceptor outer segment (POS) was prepared as previous reported.18 Fluoresbrite YG Microsphere latex beads (Polysciences, 17154–10) were mixed with 1.5 mg of POS in DMEM medium with 2.5% sucrose and rotated at room temperature for 1 h for opsonization of latex beads with POS to enhance the phagocytic activity.19 After washing with 0.9% NaCl solution, beads were resuspended with X-VIVO 10 containing 30% fetal bovine serum and incubated with RPE cells which were preincubated with X-VIVO 10 containing 30% fetal bovine serum for 1 h to prime cells.20 After 24 h of culture, RPE cells were resuspended with X-VIVO 10 containing propidium iodate and analyzed by using FACSAria II.
2.5 Building of neural network and training
We made a model based on VGG16,21 which is a convolutional neural network model containing five blocks, the convolutional layer, the ReLU activation function and the Max pooling layer.22 We used the initial parameters of the five blocks from results obtained by ImageNet training. Then, stacked information in the blocks was further stacked to two dimensions. Finally, the probability of each class was returned by the softmax formula.
2.6 Construction of an automated model to choose differentiated regions
We compared total non-pigmented (undiff) (1,847 blocks), mixed (1,227 blocks) and pigmented (diff) blocks (1,387 blocks) using the model, and cross-segregation of the matrix was performed. We found that undiff and diff were identified with about 80% accuracy, but lower accuracy was observed with mixed blocks, which was predictable because the mix contains mingled diff and undiff regions (Supplemental Figure 2A). Only less than 50% of accuracy was obtained with the mixed category (Supplemental Figure 2A), which is predictable since the category contains both undiff and diff regions. Based on the results, we then generated a model to classify the blocks to diff and undiff automatically. We used undiff (322 blocks) and diff (358 blocks) for building a model for automated classification of diff and undiff blocks. Since we used the softmax formula, the output of the neural network model is total 1 probability, i.e. {Diff:0;9, Undiff:0.1}. The differentiation classification model was applied to a total of 1,907 blocks, and output is shown in Supplemental Figure 2B. Then, based on the probability of judgment as “diff”, we adjusted the threshold to make the Youden index the maximum. The judgment of either “mix” or “undiff” was also made by using the same method. The identification of “undiff”, “mix”, and “diff” patches was used for the judgment of the maturation of hOPM RPE cells, and mixed populations were used in the experiments for the evaluation of the effects of a CHM mutation and exon-skipping.
2.7 In vitro prenylation assay and western blotting
The in vitro prenylation assay was performed following previously reports.23 In brief, RPE cells were resuspended with cold prenylation buffer (25 mm HEPES pH 7.2, 50 mm NaCl, 2 mm MgCl2, 2 mm DTE, 20 μm GDP) containing a protease inhibitor cocktail (Nacalai tesque, 25,955). After cellular debris was removed, the supernatant was ultracentrifuged at 80,000 rpm for 60 min at 4°C. The supernatant was concentrated using Amicon Ultra (Millipore, UFC200324) and used for the in vitro prenylation assay or as a cytoplasm fraction. The supernatant was incubated with 1 μm RabGGTase (JenaBioscience, PR-103), 1 μm REP-1 (JenaBioscience, PR-105) and 5 μm biotin-geranyl pyrophosphate (JenaBioscience, LI-105) for 6 h at room temperature. Biotin-labeled RAB protein was detected using streptavidin-HRP (Invitrogen, 434323) and Amersham Imager 600 (Cytiva). In western blotting analysis of the cytoplasm fraction, mouse IgG anti-ACTIN (Sigma Aldrich, A4700), anti-RAB27A (Santa Cruz, sc-81,924), anti-RAB38 (Santa Cruz, sc-390,176), anti-REP-1 (Santa Cruz, sc-23,905) and HRP-linked anti-mouse IgG antibody (Cytiva, NA-9310) were used. The signal quantification was carried out by using ImageQuant TL (Cytiva).
2.8 Statistical analysis
Statistical analysis was done using Python Scipy (https://scipy.org/). The p-values were calculated Tukey's test as indicated in the figure legends with R software (The R Foundation for Statistical Computing, Vienna, Austria).
3 RESULTS
3.1 AI-based evaluation of cell differentiation into RPE
We established the hOPM cell line by introduction of the plasmids as previously described.15 In the hOPM cell line, RPE differentiation is not homogeneous; pigmented and non-pigmented regions are intermingled (Figure 1A). We first confirmed that OTX2 and MITF were expressed only in pigmented cells (Figure 1B), and the pigmented and non-pigmented regions are referred as “diff” and “undiff”, respectively. We explored whether deep learning could be used to identify the developmental transition into hiPSC-derived RPE. Photographs were taken using a conventional microscope (Nikon Eclipse, TE300) and camera (Nikon, D3300) (Figure 1A), and then divided into small squares; an expert separated the pigmented and non-pigmented areas. The former were dark, the cells were hexagonal, and the cell edges were visible (Figure 1A, C). Each “diff” block contains seven or eight hexagonal cells (Figure 1D, E). Blocks with both “undiff” and “diff” regions were classified as mixed (Figure 1D). The population of “diff” blocks increased linearly for 7 weeks, and was then maintained at about 30% of the total (Figure 1F).
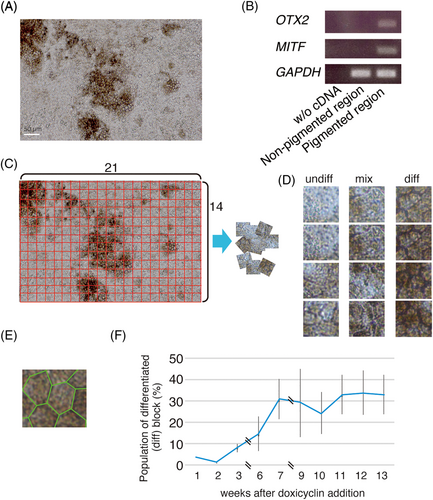
We examined whether “undiff”, “mixed” and “diff” blocks could be separated by AI. K-Fold cross validation (k = 5) was used to create a model based on the VGG16 convolutional neural network. Cross-segregation consistently afforded 70–80% accuracy in terms of “diff”/“undiff” separation at 4/5, 6/7 and 8/9 weeks (Table 1, 2, and 3). Artificial intelligence distinguished “diff” regions at these times with remarkable accuracy (> 99%) (Table 4).
| True predict | Undiff 483 block | Mix 400 blocks | Diff 587 blocks | All 1,470 blocks |
|---|---|---|---|---|
| undiff | 405 | 151 | 50 | 606 |
| mix | 54 | 122 | 80 | 256 |
| diff | 24 | 127 | 457 | 608 |
| All | 483 | 400 | 587 | 1,470 |
| accuracy | 0.84 | 0.30 | 0.78 | 0.67 |
| True predict | Undiff 624 blocks | Mix 449 blocks | Diff 597 blocks | All 1,670 blocks |
|---|---|---|---|---|
| undiff | 525 | 154 | 52 | 731 |
| mix | 73 | 203 | 134 | 410 |
| diff | 26 | 92 | 411 | 529 |
| All | 624 | 449 | 597 | 1,670 |
| accuracy | 0.84 | 0.56 | 0.69 | 0.68 |
| True predict | Undiff 280 blocks | Mix 378 blocks | Diff 663 blocks | All 1,321 blocks |
|---|---|---|---|---|
| undiff | 239 | 90 | 1 | 330 |
| mix | 41 | 217 | 63 | 321 |
| diff | 0 | 71 | 599 | 670 |
| All | 280 | 378 | 663 | 1,321 |
| 0.85 | 0.57 | 0.90 | 0.80 |
| True predict | 4/5 wks | 6/7 wks | 8/9 wks | All |
|---|---|---|---|---|
| 4/5 wks | 581 | 2 | 6 | 589 |
| 6/7 wks | 3 | 595 | 0 | 598 |
| 8/9 wks | 3 | 0 | 657 | 660 |
| All | 587 | 597 | 663 | 1847 |
| accuracy | 1.00 | 1.00 | 0.99 | 0.99 |
3.2 AI-based identification of various developmental stages of hiPSC-derived RPE cells
Using a fresh culture, we took 10 photographs daily in weeks 1, 4, 8 and 13 after RPE differentiation commenced and prepared small blocks (14 × 21 blocks from one photo). “Diff”/“undiff” classification was based on the softmax model described in the Materials and Methods. Cross-validation revealed that the accuracy was 90–100% (data not shown). Then, we prepared a new series of photographs (the test data) and evaluated predictions of culture-weeks. The accuracy was very high in weeks 1, 4 and 8, but only about 50% in week 13 (Table 5), suggesting that later samples exhibited similar profiles. We used the system to confirm maturation of hiPSCs into RPE in the following experiments.
| Sample stage | |||||
|---|---|---|---|---|---|
| True predict | 1 week | 4 weeks | 8 weeks | 13 weeks | Total |
| 1 week | 74 | 3 | 3 | 0 | 80 |
| 4 weeks | 0 | 326 | 0 | 4 | 330 |
| 8 weeks | 0 | 0 | 807 | 303 | 1,110 |
| 13 weeks | 0 | 39 | 76 | 312 | 427 |
| Total | 74 | 368 | 886 | 619 | 1,947 |
| Accuracy | 1.0 | 0.886 | 0.911 | 0.504 | 0.780 |
3.3 Construction of exon-skipping vectors and evaluation thereof in Y79 cells
We chose CHM as a model for evaluation of exon-skipping in hOPM cells. CHM mutations of the patients are found in most of coding regions of CHM (Supplemental Figure 3A). As the numbers of nucleotides of exons 6, 9, and 10 are multiples of 3, the exon 6, 9, or 10 skipping is in-frame. We designed gRNAs targeting the splice acceptor (SA) or splice donor (SD) sites and found that Cas9 cut close to the SD sites of exons 6, 9 and 10 (Supplemental Figure 3B). Before expressing the skipping vector in hOPM cells, we checked whether the vector worked in a conventional cell line Y79 retinoblastoma line24 for the first screen in various ways. After electroporation of the vector into Y79 cells, we sorted GFP-positive cells. The RT-PCR and droplet digital PCR (ddPCR) results indicated reduced expression of CHM mRNA containing exon 6, 9 or 10 and also induction of CHM mRNA synthesis in the absence of exon 6, 9 or 10 (Supplemental Figure 4A, B); ddPCR quantitated the exon-skipping rate (Supplemental Figure 4B). The decomposition (TIDE) assay25 predicted high indel efficiency around the SD site of exon 6, 9 or 10 explaining the high level of CHM exon 6, 9 or 10 skipping (Supplemental Figure 4C), meaning that the first screening using Y79 identified the promising vectors. We then focused on the hiPSC line.
3.4 CHM exon 6-skipping was associated with expression of truncated REP-1 in hiPSCs
Plasmids expressing Cas9, gRNA, and GFP were introduced to WTC11 cells, and RT-PCR of purified GFP-expressing cells revealed bands corresponding to CHM-encoding mRNA without the target exons (Figure 2A). We confirmed exon skipping by sequencing the RT-PCR products, and the genomic DNA regions around the target SD sites (Figure 2A, B, Supplemental Figure 3B). The molecular weights of the truncated REP-1 were as expected in all cases (Figure 2C). REP-1 lacking exon 9 or 10 exhibited faint bands, but the expression level of REP-1 lacking exon 6 was much stronger (Figure 2C). The result indicated that REP-1 lacking exon 6 is more stable than REP-1 lacking exon 9 or 10 and exon 6-skipping is more effective. Thus, we focus on CHM exon 6-skipping below.
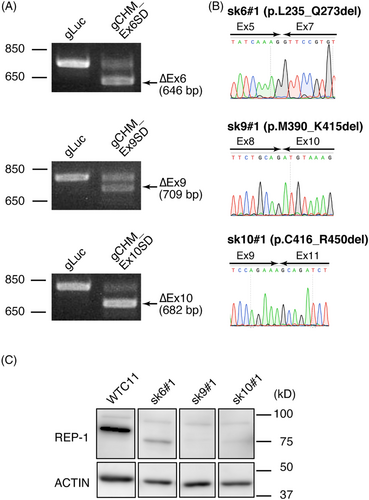
3.5 CHM exon 6-skipping was induced in RPE cells with a frameshift mutation in that exon
We established a clonal hOPM cell line (CHM#1) with a 17 nt deletion in exon 6 and a premature stop codon (Figure 3A). Plasmids expressing Cas9 and gRNA targeting the CHM exon 6 SD site (gCHM_Ex6SD) or control luciferase (gLuc) were introduced into CHM#1 RPE cells via electroporation. Immunocytochemistry confirmed the RPE differentiation; OTX2 and PAX6 were expressed in the nuclei, and ZO-1 in the cell membranes, of all three samples (Figure 3B). We used the AI-based staging method to evaluate whether the mutation affected the maturation of RPE cells over 14 weeks. Artificial intelligence judged that the cells were at 13 weeks of culture period among the 1, 4, 8 and 13 week options with >90% prediction in all three samples (Figure 3C). This suggested that neither the frameshift mutation in CHM exon 6 nor the CHM exon 6-skipping adversely affected differentiation into an RPE.
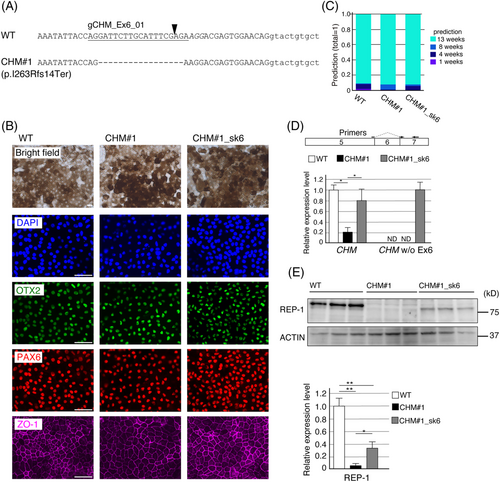
Exon 6-skipping was confirmed in CHM#1_sk6 RPE cells (Figure 3D). We found that the CHM mRNA level was reduced (compared with that in WT RPE cells), probably owing to nonsense mediated decay in CHM#1 RPE cells, but this deficit was rescued in CHM#1_sk6 RPE cells (Figure 3D). Finally, we examined REP-1 expression levels using western blotting. In CHM#1 RPE cells, REP-1 expression was minimal (Figure 3E); however, we observed truncated REP-1 expression in CHM#1_sk6 RPE cells (Figure 3E). Because the REP-1 expression level in CHM#1_sk6 was not restored as the CHM mRNA level, REP-1 lacking exon 6 was probably unstable compared with full-length REP-1.
3.6 CHM exon 6-skipping rescued the reduced phagocytic activity of CHM#1 RPE cells under oxidative stress
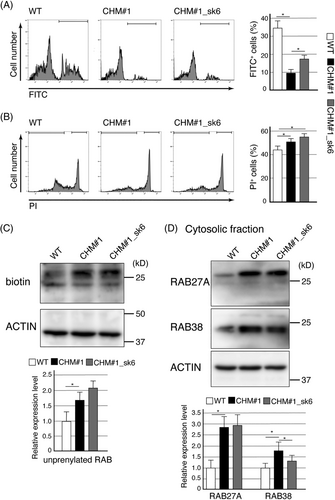
We then evaluated phagocytic activity via flow cytometry. We observed no decrease in the percentage of FITC-positive cells among CHM#1 RPE cells (Supplemental Figure 5). In addition, the cell death levels of CHM#1 RPE and control cells were comparable (Supplemental Figure 5). Since CHM dysfunction is associated with high levels of oxidative stress, we employed sodium iodate, which induces oxidative stress to RPE cells.26, 27 In WT RPE cells, treatment with sodium iodate for 1 day decreased phagocytic activity and increased cell death (Supplemental Figure 5 and Figure 4A, B); both phenomena were accelerated in CHM#1 RPE cells (Figure 4A, B). In CHM#1_sk6 RPE cells, although the level of (accelerated) cell death was the same as that of as CHM#1 RPE cells (Figure 4B), the phagocytic activity was significantly restored (Figure 4A). This suggested that REP-1 lacking exon 6 was partially functional and contributed to the phagocytic activity of RPE cells under oxidative stress conditions.
3.7 REP-1 lacking exon 6 is likely to contribute to prenylation of RAB38 but not RAB27A
REP-1 controls cellular RAB localizations via prenylation; we thus examined the prenylation status of RABs in RPE cells under oxidative stress using an in vitro prenylation assay, which labels unprenylated RABs with biotin. CHM#1 RPE cells showed significantly higher levels of unprenylated RABs than did WT RPE cells (Figure 4C). The high levels of unprenylated RABs were maintained in CHM#1_sk6 RPE cells (Figure 4C). Most RABs are prenylated and then localized to cellular membranes; unprenylated RABs reside in the cytosol. Unprenylated RAB27A accumulates in choroideremia patients; the cytosolic levels of RAB27A increases in RPE cells differentiated from hiPSCs derived from a choroideremia patient.28, 29 In addition to RAB27A, RAB38 has been suggested to contribute to choroideremia.30 The cytosolic levels of RAB27A and RAB38 were significantly increased in CHM#1 RPE cells, probably reflecting REP-1 dysfunction (Figure 4D). Although high RAB27A levels were also observed in CHM#1_sk6 RPE cells, the RAB38 level was significantly decreased (compared with that in CHM#1 RPE cells; Figure 4D). Thus, REP-1 lacking exon 6 may regulate the prenylation status of certain RABs, including RAB38, and the phagocytic activity of RPE cells.
4 DISCUSSION
In this study, we sought to establish a platform by which non-specialized laboratories can evaluate whether gene therapy vectors repair mutations in RPE-expressed genes. Here, mutations that mimicked those of patients were introduced (using the CRISPR/Cas9 system) into hiPSCs. The host cells, those of the hOPM line, exhibit fast and efficient differentiation into RPE.15 Experimental mutation of hOPM cells not only reduces the time required to establish hiPSC lines for each case but also allows the use of cells with identical genetic backgrounds, for comparison of the various vectors.
Several methods have been used to differentiate ESCs/iPSCs into RPE.9 Accurate evaluation of differentiation of these methods is critical. While microscopic evaluation of hexagonal morphology and pigmentation (characteristic features of RPE cells) has been employed, the in vitro manipulations associated with biochemical/molecular biological analyses are time-consuming and it is necessary to harvest cells. Furthermore, cells in tissue do not always differentiate homogeneously; any analysis of cell mixtures may yield inaccurate judgment. As a non-invasive reproducible test, combinations of quantitative bright-field absorbance microscopy with deep neural network evaluations has been used to predict tissue function and the cellular donor identities of iPSC-derived RPE.31 We have taken a similar approach to using an AI-based assay that can be implemented in many laboratories. We used photographs taken by a standard camera attached to a standard laboratory microscope without any lighting adjustment; AI accurately distinguished cells.
We evaluated how a CHM exon-skipping vector affected RPE cells with a frameshift mutation in CHM exon 6. RPE cells with a frameshift mutation in exon 6 did not exhibit any change (compared with normal cells) in terms of phagocytic activity or cell death Although choroideremia patients exhibit symptoms such as nyctalopia from the first decade of life, the disease progresses slowly and loss of central vision occurs only in the fifth-to-sixth decades.32 Thus, eye changes on aging, perhaps created by increased oxidative stress, may play critical roles in disease progression.
The RAB family members orchestrate membrane trafficking; in particular, the RAB5 and RAB7 subfamily members are essential for phagosome maturation.33, 34 Although we found that CHM exon 6-skipping did not affect the overall level of RAB prenylation, changes in the prenylation levels of certain RABs may have been in play. RAB38 plays important roles in melanogenesis and ensures appropriate targeting of tyrosinase-related protein 1 to the melanosomes of RPE cells.35 Rab38-deficient mice exhibited fewer and smaller mature melanosomes and a thinner RPE than did control mice.36 CHM exon 6-skipping reduced the cytosolic RAB38 level; thus, the effects of CHM exon 6-skipping on melanosomes should be further explored.
The resolved structure of Rab7 in complex with REP-1 indicated that the Rab binding platform, the C-terminus binding region and helices D and E are crucial for the interaction among REP-1, Rab7 and RabGGTase.37, 38 REP-1 lacking exon 6 loses a part of the C-terminus binding region involved in the interaction with Rab7, while amino acids important for the interaction between REP-1 and RabGGTase remain in REP-1 lacking exon 6. Therefore, although REP-1 lacking exon 6 might show weaker interaction with RAB proteins, it is plausible that the interaction with RabGGTase remains unchanged, leading to the partially functional REP-1 protein. Compared with REP-1 lacking exon 6, REP-1 lacking exon 9 or 10 showed very low protein expression levels, indicating that exon 9 or 10 skipping largely decreased the REP-1 stability. A machine learning tool for the prediction of protein stability would give a rational explanation for this point.39
In order to consider the off-target effect resulting from the CRISPR/Cas9 system, we made a search of target sites using CRISPRdirect (https://crispr.dbcls.jp/) with the following criteria: a sequence is matched with more than 12mer of the 3′ region of the target sequence adjacent to the PAM; and a sequence is localized on a coding sequence. Genes ETV4 and OXSR1 are potential off-targets of gCHM_Ex6_01 and gCHM_Ex6SD, respectively. We performed sequencing of these two genes with gCHM_Ex6_01 or gCHM_Ex6SD expressing samples, and no mutation was identified. CHML, which encodes a homologous protein to CHM, was not included in the highly possible targets. Therefore, we concluded that the off-target effects are less plausible. We evaluated the effects of a CHM mutation and exon-skipping using only one clone, CHM#1. Although we performed preliminary experiments using transiently transfected cell population several times and obtained essentially the same results, we also cannot exclude the possible effects of clonal variation40 on the phenotypes observed in this study. Thus, further analysis using choroideremia patient-derived hiPSCs would be valuable to assess the effects of CHM exon-skipping.
Most IRD mutations are recessive; patients are often compound heterozygotes. In such cases, allele-specific design of repair of the mutations is necessary if the mutations are in the different exons. Here, we designed gRNAs to target exon-SA and -SD sites. We previously constructed a plasmid contains tandem-two gRNAs, and this worked well.41 The two gRNAs remove exons by targeting both ends of an intron. If single-nucleotide polymorphism data are available, it is possible to target a single-nucleotide polymorphism to one allele when designing an allele-specific exon-skipping vector; this would effectively and safely “cure” mutations. In summary, in this study, we showed that CHM exon-6 skipping partially rescued phenotypes appeared in RPE cells with a frameshift mutation in CHM exon 6 under oxidative stress probably by increasing RAB38 prenylation.
ACKNOWLEDGEMENTS
We thank Yutaka Morizaki for fruitful discussion, Miho Nagoya, and Erika Mitsui for excellent technical assistance and Kousuke Saita for proofing the manuscript. B.R.C. acknowledges support through gifts from Claire Giannini Fund, That Man May, the Roddenberry Foundation, Pauline and Thomas Tusher and the Gladstone Institutes. This works is supported by JSPS KAKENHI (grant number 20K09823 to T. Iwagawa) and TaNeDS Daiichi Sankyo Co. Ltd. B.R.C. was supported by the National Institutes of Health, R01-EY028249 and R01-EY027789, R01-HL130533, R01-HL13535801, P01-HL146366. B.R.C.
CONFLICT OF INTEREST
The authors have no conflict of interest regarding this work.
AUTHOR CONTRIBUTIONS
T. I. and H. M. performed the research. T. I., H. M., and S. W. designed the research study. H. T., K. T., and B. R. C. contributed essential reagents or tools. T. I. and S. W. wrote the paper.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of the present study are available from the corresponding author upon reasonable request.




