Infrared attenuated total reflection spectroscopic surface analysis of bovine-tail intervertebral discs after UV-light-activated riboflavin-induced collagen cross-linking
Funding information: European Union's Horizon 2020 research and innovation program, Grant/Award Number: 780598
[Correction added on 19 October 2020, after first online publication: Boris Mizaikoff was designated as cocorresponding author.]
Abstract
The tensile strength of the intervertebral disc (IVD) is mainly maintained by collagen cross-links. Loss of collagen cross-linking combined with other age-related degenerative processes contributes to tissue weakening, biomechanical failure, disc herniation and pain. Exogenous collagen cross-linking has been identified as an effective therapeutic approach for restoring IVD tensile strength. The current state-of-the-art method to assess the extent of collagen cross-linking in tissues requires destructive procedures and high-performance liquid chromatography. In this study, we investigated the utility of infrared attenuated total reflection (IR-ATR) spectroscopy as a nondestructive analytical strategy to rapidly evaluate the extent of UV-light-activated riboflavin (B2)-induced collagen cross-linking in bovine IVD samples. Thirty-five fresh bovine-tail IVD samples were equally divided into five treatment groups: (a) untreated, (b) cell culture medium Dulbecco's Modified Eagle's Medium only, (c) B2 only, (d) UV-light only and (e) UV-light-B2. A total of 674 measurements have been acquired, and were analyzed via partial least squares discriminant analysis. This classification scheme unambiguously identified individual classes with a sensitivity >91% and specificity >92%. The obtained results demonstrate that IR-ATR spectroscopy reliably differentiates between different treatment categories, and promises an excellent tool for potential in vivo, nondestructive and real-time assessment of exogenous IVD cross-linking.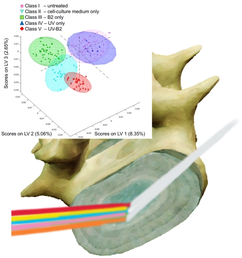
1 INTRODUCTION
Collagen constitutes the main component of soft tissues, such as ligaments, cartilage and intervertebral discs (IVDs) [1]. The tensile strength of those tissues is mainly ensured by cross-links of the collagen matrix. However, age-dependent degenerative changes lead to a loss of cross-links, and consequently, of biomechanical stability. Therefore, efforts have been made in ophthalmology, dentistry and orthopedics to artificially enhance the biomechanical properties of collagen by efficiently inducing exogenous cross-linking [2-4].
Specifically, sufficient collagen cross-links are crucial for maintaining the biomechanical stability of the IVDs with respect to the pronounced mechanical loads. Endogenous augmentation of cross-linking constitutes a physiological response to degenerative changes [5]. However, this regenerative capacity is limited, and progressive degenerative changes may lead to protrusion or even herniation of discs, which causes local and/or radicular pain. Therefore, exogenous cross-linking strategies may augment endogenous regenerative processes.
In fact, exogenous cross-linking of IVDs has shown promising results, and constitutes an interesting therapeutic approach to improve the biomechanical properties of IVDs [6, 7]. Chemical cross-linking processes are based on the formation of bonds via amino or imine functionalities using nucleophilic substitution reaction mechanisms, or via radical polymerization. Covalent bonds within monomers or intermediate molecular strands commonly provide more resilient materials vs physical bonds based on hydrogen bonds or ionic interaction.
Conventional methods for the determination of the degree of cross-linking in tissues include high-performance liquid chromatography (HPLC) and diffusion scanning calorimetry after enzymatic digestion of collected samples. However, both methods are time consuming, require sample blocks for analysis and are destructive preventing subsequent analysis with alternative techniques [8-11].
Spectroscopy in the mid-infrared spectral regime (MIR; 3-15 μm) is a powerful optical tool for examining deviations in molecular composition of analyzed materials. MIR spectroscopy in the condensed phase probes well-pronounced fundamental vibrational modes of molecules, and thus, enables direct insight into molecular changes upon chemical reactions. Characteristic transitions of collagen in the MIR include the amide I band of the CO stretching vibration around 1670 cm−1. The amide II band occurs around 1550 cm−1, and represents NH and CN stretching vibrations. Upon altering the secondary structure of proteins, the amide I band is pronouncedly affected. Shape and position of this band allows tracing back the orientation of the protein chain including, for example, α-helix or β-sheet confirmations. Typically, shifts toward longer wavelengths of this band are attributed to a higher levels of organization of the protein molecular structure [12]. Apart from conventional transmission spectroscopy, internal reflection elements are frequently used as signal transducers in the MIR via single or multiple total internal reflections. The established evanescent field at the high-refractive-index-waveguide/analyte interface penetrates few micrometers into the adjacent sample enabling recording evanescent field absorption spectra. Since water is a strong absorber in the MIR regime, the correspondingly called attenuated total reflectance (ATR) spectroscopy benefits from that limited penetration depth of the evanescent field. Infrared ATR (IR-ATR) spectroscopy using appropriate Fourier transform infrared (FTIR) spectrometers is nowadays considered a powerful routine tool for monitoring structural changes upon, for example, chemical reactions or other alterations of the analyzed molecules. Using ultrafast infrared spectroscopy, even reaction intermediates or transients of photoexcited states may be directly monitored [13]. When evaluating complex matrix changes, Mizaikoff et al have exemplarily shown that alterations induced, for example, by fungal infections on commodities may be monitored via FTIR- and quantum cascade laser-based IR sensing technologies. Evaluation of the resulting matrix variations upon fungal activity, such as digestion, has been shown to be sensitive to indicate the presence of low-concentrated target species— such as in that case mycotoxins— rather than screening directly for those molecules. In this context, multivariate data evaluation algorithms, such as principal components analysis and partial least squares discriminant analysis (PLS-DA), have been used along with machine learning strategies for training highly sensitive classification schemes [14-17].
The present study has for the first time utilized IR-ATR spectroscopy for the assessment of exogenous ultraviolet (UV)-light-activated riboflavin-induced collagen cross-linking (UVA-CXL) on ex-vivo IVDs. The aim was to demonstrate that a nondestructive analytical approach via IR-ATR spectroscopy along with multivariate classification algorithms may reliably differentiate treated vs untreated samples, and even different treatment procedures.
2 EXPERIMENTAL
Thirty-five caudal discs (C2–3- C6–7) were isolated from nine bovine tails (age: 18-24 months old) within 3 hours postslaughter in the local slaughterhouse (Fleischmarkt Donautal, Ulm), as described by Saggese et al [18]. Bovine coccygeal discs have been proposed as a prime physical, biomechanical and biological model for the study of human lumbar discs [19, 20]. Bovine tail discs have a large size (area around 430 mm2, volume around 3376 mm3) and similar aspect ratio, diffusion distance and resting pressure (0.2-0.3 MPa) to human lumbar discs [19]. Bovine caudal discs have also been found to be compositionally similar to human lumbar discs, with comparable hydration, collagen and proteoglycan profiles and similar rate of proteoglycan synthesis [21]. Finally, several studies investigating enzyme-induced IVD degeneration models, verify that in young ages, bovine coccygeal discs present no degeneration [22, 23].
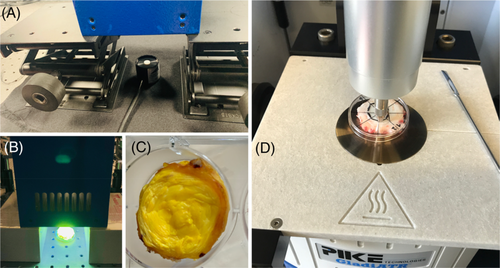
The samples were macroscopically segmented into annulus fibrosus (AF) and nucleus pulposus (NP) facilitating a spatial orientation of the performed analysis (Figure 1D).
Riboflavine-5′-phosphate was dissolved in Dulbecco's Modified Eagle's Medium (DMEM). Riboflavin solutions were used at a concentration of 8 mM. All chemicals were acquired from Merck (Merck KGaA, Darmstadt, Germany) and used without further purification. For purging purposes, deionized water (Millipore, resistivity @ 25°C: 18.2 MΩ cm) was used.
UV irradiation was realized via an UV-LED module (Opsytec Dr Gröbel GmbH, Ettlingen, Germany). The UV-LED module produced a homogenous field of UVA light at a wavelength of 365-370 nm. The UV-LED module was mounted at a distance of 35 cm from the samples such that the irradiance at the surface of the samples— as determined using a radiometer (Radiometer RM-12, (Opsytec Dr. Gröbel GmbH)— was held constant at 3 mWcm−2 (Figure 1(A)). Prior to measurements, riboflavin-treated samples were soaked for 15 minutes in 8 mM riboflavin solutions (Figure 1C). The irradiation time was set for all samples to 15 minutes, which corresponds to a dose of 2.7 Jcm−2. All riboflavin-treated sample measurements were performed in a “dark-mode” setting, that is, eliminating natural light sources to avoid unintended light activation of riboflavin prior to UVA-CXL.
After soaking, riboflavin residues at the surface of the samples were removed by rinsing with distilled water in order to avoid attenuation of the UVA radiation.
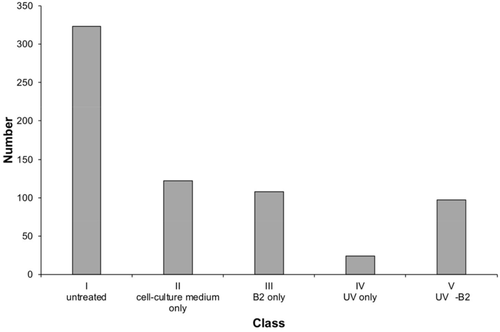
To generate representative reference samples, (a) untreated controls, (b) IVDs soaked only in cell culture medium DMEM were generated. Additionally, (c) samples soaked in cell culture medium treated only with UV-light, (d) IVDs soaked in cell culture medium treated only with B2 and (e) IVDs soaked in cell culture medium treated with UV-light and B2 were analyzed via IR-ATR spectroscopy using the same procedures. From each IVD, at least 12 measurements were performed from the AF, and NP, respectively. The freshly prepared samples were labeled as 'untreated' (n = 323). The samples were split into two groups with (a) one group immersed in cell culture medium DMEM only (ie, labeled “cell culture medium only”, n = 122) and (b) the other group immersed in a riboflavin 5′-phosphate-containing cell culture medium DMEM solution (CRiboflavin = 8 mM) (ie, labeled “B2 only”, n = 108) for 15 minutes. Afterward, the soaked samples were treated with UV radiation for 15 minutes (ie, labeled “UV-light only”, n = 24, and “UV-light B2”, n = 97). IR-ATR spectra were recorded at each step. The population of each class is displayed in Figure 2.
A single-bounce IR-ATR assembly (GladiATR, PIKE Technologies, Wisconsin) was used for collecting IR spectra. The system comprises a single-reflection diamond ATR crystal, which provided excellent inertness toward biomedical samples. Furthermore, an adjustable pressure clamp facilitated determining the optimum contact pressure for reproducibly acquiring IR-ATR spectra. Spectra were recorded using a Bruker Vertex 70 FTIR spectrometer (Bruker Optics GmbH, Ettlingen, Germany), which was operated with the Bruker OPUS 8.1 (Bruker Optics GmbH, Ettlingen, Germany) software package. For each spectrum, 128 scans were averaged vs air as background. Spectra were recorded within a spectral window of 4000-600 cm−1 at a spectral resolution of 2 cm−1. Each IVD was divided into 24 sections, whereby 12 sections were defined at the AF and 12 sections were defined at the NP.
The recorded IR spectra were converted into data point tables via the Essential FTIR software package (Operant LLC, Madinson, Wisconsin), and then analyzed via Matlab (Version R2019b, The MathWorks Inc., Natick, Massachusetts) and the PLS_Toolbox add-in comprising a PLS-DA function (Version 8.7, Eigenvector Research Inc., Manson, Washington). Analysis of the recorded spectra was performed using PLS-DA. The region of interest was limited to the fingerprint regime ranging from 900 to 1850 cm−1. Furthermore, spectra were preprocessed using an auto-weighted least squares (WLS; 2nd order) baseline correction, a generalized least squares weighting (alpha = 0.019) filter and mean-centering. The resulting model was built using six latent variables (LVs). The number of LVs was limited to six capturing approximately 27% of the variance by maximizing the prediction performance while suppressing interference by noise and artifacts. Cross-validation was performed via the venetian- blinds-routine with 10 splits and at a blind thickness of 1.
For creating the validation data set, the initial dataset was split into two groups via the Kennard-Stone approach, thereby keeping 66% of the dataset as the training data, and using the remaining 33% of the dataset as validation data. From the total number of 674 samples, the training set contained 449 samples, and the validation set contained 225 samples. Data used for validation were deliberately excluded from the training data. The “per class” classification error was calculated as follows: Class Err. = average of false positive rate and false negative rate for class = 1−(sensitivity+specificity)/2, and is listed along with other parameters statistically characterizing the quality of the established classification scheme in Table 1.
| Class | V (UV-B2) | IV (UV only) | III (B2 only) | II (cell culture medium only) | I (untreated) |
|---|---|---|---|---|---|
| Sensitivity (Cal) | 1.000 | 1.000 | 1.000 | 1.000 | 0.955 |
| Specificity (Cal) | 1.000 | 0.969 | 1.000 | 0.995 | 0.987 |
| Sensitivity (CV) | 0.947 | 0.917 | 1.000 | 0.938 | 0.950 |
| Specificity (CV) | 0.996 | 0.939 | 0.976 | 0.962 | 0.921 |
| Sensitivity (Pred) | 1.000 | 1.000 | 1.000 | 1.000 | 0.965 |
| Specificity (Pred) | 1.000 | 0.973 | 1.000 | 1.000 | 0.980 |
| Class Err (Cal) | 0.000 | 0.017 | 0.000 | 0.003 | 0.024 |
| Class Err (CV) | 0.028 | 0.072 | 0.012 | 0.050 | 0.065 |
| Class Err (Pred) | 0.000 | 0.013 | 0.000 | 0.000 | 0.028 |
| RMSEC | 0.132 | 0.148 | 0.163 | 0.141 | 0.213 |
| RMSECV | 0234 | 0.193 | 0.290 | 0184 | 0.299 |
| RMSEP | 0.130 | 0.132 | 0.184 | 0.193 | 0.206 |
- Bold values are statistical significance; RMSEC, root mean square error of calibration; RMSECV, root mean square error of cross-validation; RMSEP, root mean square error of prediction
3 RESULTS AND DISCUSSION
In Figure 3A, unmodified IR spectra of (a) untreated samples, (b) samples soaked only in cell culture medium DMEM, (c) samples soaked in cell culture medium treated only with UV-light, (d) samples soaked in cell culture medium treated only with B2 and (e) samples soaked in cell culture medium treated with UV-light and B2 are exemplarily shown. As expected, spectra of fresh samples show strong water absorption features. In addition, distinct molecular absorption features are evident in the fingerprint region (1800-900 cm−1) corresponding to well-pronounced transitions characteristic of collagen (protein) components, and of hydrocarbon and lipid components. Noteworthy, differences between pristine and altered, that is, cross-linked tissues are particularly evident in this spectral region.
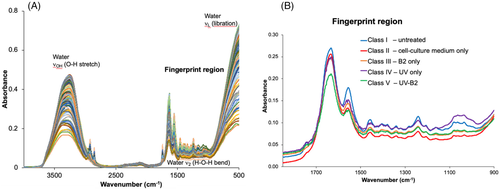
For better differentiation, average spectra of the individual classes are shown in Figure 3B. Spectral differences within the individual classes (Figure 3B) are minimal and distinct shifts are not as well pronounced as for degeneration effects as presented in other studies [30, 31]. Hence, the trained PLS-DA model is a necessity for facilitating computer-aided interpretation of highly complex patterns of the spectral signatures upon treatment as represented by the different classes that are not evident by human evaluation.
The classification results along with their statistical characterization are summarized in Table 1. Notably, in the calibration dataset a sensitivity and a specificity >0.95 was achieved. For the cross-validation, a sensitivity and a specificity of >0.91 was obtained, whereas the data subset that was used for the prediction lead to a sensitivity and a specificity of >0.96. Furthermore, the average classification error within the different classes of 0.009, 0.045 and 0.008 for calibration, cross-validation and prediction, respectively, were calculated. The established PLS-DA model further resulted in a root mean square error of calibration (RMSEC) within 0.132 and 0.213 for the different classes. A root mean square error of cross-validation (RMSECV) within 0.184 and 0.299, and a root mean square error of prediction (RMSEP) within 0.130 and 0.206 was derived.
For the untreated samples (class I), slightly poorer class statistics were obtained, thereby indicating an elevated intra-class variance of the untreated samples. As the untreated samples in class I represent freshly harvested specimen, minor differences in, for example, water content of the individual samples is expected giving rise to this observation.
In Figure 4, the spectral covariance of the individual samples of the prediction data subset is represented by the first three LVs. Evidently, the individual classes along with the surrounding 95% confidence ellipsoids appear well separated. The slightly poorer class statistics of the untreated samples (class I) is also evident from the more broadly spaced datapoints within the scores plot indicating a reduced correlation due to less similar weights. Noteworthy, samples treated with UV and B2 are exceedingly narrowly spaced, which indicates that a high correlation within this dataset is captured by the PLS-DA model. Most important for the future utility of the developed strategy, the established PLS-DA model enables the clear discrimination between untreated samples and even different treatment steps until complete treatment (ie, cross-linking of the tissue via UV-B2) is achieved based on the data shown in Figure 3. Most importantly, the model enables elucidation of successful cross-linking of the tissue, which is highly crucial for therapeutic success, yet not directly visible by the naked eye during surgery.
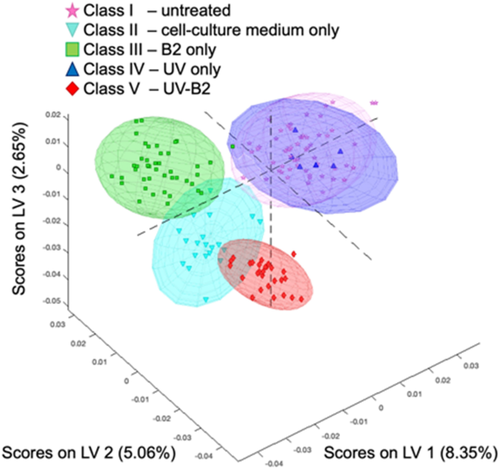
The processed spectral dataset in Figure 4 indicates that IR-ATR spectroscopy serves as a nondestructive analytical method, which indeed facilitates rapid and reliable differentiation among treated and untreated bovine tail disk samples, and even differentiates different treatment regime.
The classification results obtained in the present study correlate well with previously published studies evaluating structural alterations of collagenous tissues via IR spectroscopic tools. Structural changes of sheep menisci after meniscectomy have been evaluated via IR-ATR spectroscopy revealing absorbance changes and peak shifts of the amide I band, the amide II band, the CH bending band and sugar band region discriminating between healthy samples and meniscectomy samples [24]. In that study, a decrease of a sub-peak at 1645.5 cm−1 of meniscectomy samples was related to a decrease in the triple helical structure of the contained collagen. In another study, the degeneration of human meniscus samples via IR-ATR spectroscopy was evaluated [25]. A shift of the peak maximum of the amide I band from 1635 cm−1 (healthy) to 1639 cm−1 (degenerated with calcification) was deduced, which was correlated with a loss of fibril structure of the collagen network.
Besides its utility as an ex vivo technique, it is envisaged translating IR-ATR spectroscopy into an endoscopic intraoperative technology for in vivo investigating exogenous cross-linking of the IVD. Such an intervention (ie, in vivo cross-linking in patients) requires a diagnostic tool that reliably verifies whether the treatment was activated at the desired location of interest without affecting the tissue itself. It should be noted that, miniaturized IR-ATR with the potential of integration into arthroscopic devices has been demonstrated by the team of Mizaikoff in the mid-infrared [26], and has lately been shown also for near- and mid-infrared devices [27-31] facilitating real-time intraoperative assessment of tissue and cartilage condition. This technology paves the way for a technology that may assist spinal surgery allowing for the determination as to whether the IVD has been sufficiently reinforced by exogenous cross-linking. Future experiments aim at a direct comparison of IR-ATR data with conventionally obtained HPLC results for potentially also quantifying the extent of cross-linking via UVA-CXL based on IR data. Last but not least, aim at combining IR spectroscopic data with biomechanical experiments for assessing more precisely the UVA-CXL of the IVD. The resulting strategies and data would significantly aid in creating more effective and clinically relevant UVA-CXL protocols.
4 CONCLUSIONS
The results of the present study show that IR-ATR spectroscopy provides a nondestructive diagnostic tool for investigating and differentiating untreated, riboflavin-treated, UVA-treated and UVA-B2- treated IVD samples. The obtained data support the hypothesis that IR-ATR spectroscopy could evolve in the future into an in vivo applicable optical diagnostic tool enabling real-time monitoring of exogenous collagen cross-linking of the IVD and other binding tissues.
ACKNOWLEDGMENTS
This study was in part supported by the European Union's Horizon 2020 research and innovation program (H2020-ICT-2017-1, MIRACLE project grant agreement No 780598).
AUTHOR CONTRIBUTIONS
Conception and design: All authors. Acquisition of data: Ioannis Vasilikos, Julian Haas, Graciosa Q. Teixeira, Julia Nothelfer, Boris Mizaikoff. Analysis and interpretation of data: Julian Haas, Ioannis Vasilikos, Ulrich Hubbe, Boris Mizaikoff. Drafting the manuscript: Ioannis Vasilikos, Julian Haas, Boris Mizaikoff. Critically revising the manuscript: All authors. Final approval of the manuscript: All authors.
FINANCIAL DISCLOSURE
The authors declare no financial interests.
CONFLICTS OF INTEREST
The authors declare no potential conflicts of interest.
AUTHOR BIOGRAPHIES
Please see Supporting Information online.