Molecular imaging for cancer immunotherapy
Abstract
The success of immune checkpoint blockade has reaffirmed the importance of the immune system in cancer treatment. Immunotherapy enables the body's own immune system to fight tumor cells. However, the complex tumor microenvironment and its interaction with the immune system remain a mystery. The efficacy of immunotherapy is often affected by tumor heterogeneity. Molecular imaging techniques, such as single photon emission computed tomography and positron emission tomography, enable noninvasive whole-body imaging of tumor and immune cell signatures. Noninvasive molecular imaging can also be used to monitor the treatment response of tumors, thereby achieving personalized response assessment, which may ultimately lead to improved clinical management, development of individualized treatments, and reliable prognosis. This article reviews recent research in immunotherapy response assessment, immune T-cell imaging, immune checkpoint imaging, and radiomics/radiogenomics in immunotherapy. To date, these studies have primarily comprised exploratory preclinical imaging with preliminary results indicating that biomarker molecular imaging may have a role to play in the assessment of immunotherapy. Therefore, the principle of selecting patients for immunotherapy based on imaging results is feasible.
Abbreviations
-
- 18F-FAC
-
- 1-(2′-deoxy-2′-[18F] fluoroarabinofuranosyl) cytosine
-
- CAR
-
- chimeric antigen receptor
-
- CEST
-
- chemical exchange saturation transfer
-
- CFA
-
- clofarabine
-
- CT
-
- computed tomography
-
- CTLA-4
-
- cytotoxic T lymphocyte associated antigen 4
-
- FDA
-
- Food and Drug Administration
-
- FDG
-
- fluorodeoxyglucose
-
- ICI
-
- immune checkpoint inhibitor
-
- iRECIST
-
- immunotherapy revision of RECIST v. 1.1
-
- MRI
-
- magnetic resonance imaging
-
- NSCLC
-
- non-small cell lung cancer
-
- PD-1
-
- programmed cell death receptor 1
-
- PET
-
- positron emission tomography
-
- RECIST
-
- Response Evaluation Criteria in Solid Tumors
-
- SPECT
-
- single photon emission tomography
-
- SUV
-
- standardized uptake value
-
- TILs
-
- tumor-infiltrating lymphocytes
-
- TME
-
- tumor immune microenvironment
-
- Tregs
-
- regulatory T cells
1 INTRODUCTION
Immunotherapy is playing an increasingly prominent role in tumor treatment [1]. Over the past few years, monoclonal antibody therapies that have been approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) include anti-programmed cell death receptor (PD-1) or its ligand (PD-L) 1 and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) [2-4]. Tumor immunotherapy is the stimulation, enhancement, or improvement of the host's antitumor immune response by targeting and controlling the mechanism of interaction between the tumor microenvironment and immune cells. Additionally, the combined use of immunotherapy with chemotherapy, radiotherapy, and other targeted therapies can enhance its antitumor effect [5].
Not all patients, however, respond to immunotherapy; some patients have severe immunotoxicity, and most immunotherapy drugs are expensive. Hence, the need to optimize patient selection and early prediction of response is imminent. From this point of view, the development and application of biomarkers are critical. The FDA has approved PD-L1 inhibitor treatment based on two biomarkers identified from tumor tissue biopsy: high microsatellite instability and mismatch repair defect status [6]. Research on tumor mutational burden as a predictive biomarker is also gaining traction, and increasingly more evidence shows that a higher mutation burden leads to a better treatment response [7]. However, some patients with a high expression of these biomarkers do not respond to immunotherapy, while others with no or low expression of these biomarkers benefit from immunotherapy [8]. One possible explanation is that the dynamic changes of the immune microenvironment, as well as the heterology of the patient's body and even that of a single tumor, may not be captured by a single biopsy [9]. Therefore, we need a powerful technology that can characterize the immune microenvironment of cancer and simultaneously pinpoint patients with the potential to respond to immunotherapy. Some clinical imaging methods, such as positron emission tomography (PET), single photon emission tomography (SPECT), and magnetic resonance imaging (MRI), have become available for real-time dynamic monitoring of the existence and changes of specific molecular biomarkers using specific molecular targets as tracers. Therefore, the clinical application of new technologies for noninvasive in vivo imaging may become an important tool for the further development of immunotherapy [10]. In this article, we review the current molecular imaging methods of tumor immunotherapy to support the following aspects of decision-making in immunotherapy: response evaluation, imaging of T cells and immune checkpoints, and radiomics/radiogenomics.
2 RESPONSE EVALUATION OF TUMOR IMMUNOTHERAPY
At present, the main evaluation standard for the efficacy of chemotherapeutic drugs is the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [11]. During the assessment of tumor response, we are advised to identify target lesions on baseline images, measure them on consecutive follow-up images, and quantify changes in tumor burden, thereby defining response or progress [11-14]. However, on the basis of the observation of immune-related response patterns, researchers have found that there is the possibility of pseudoprogression in the immunotherapy response, which is based on anatomical false progress with a subsequent tumor shrinkage [15]. Therefore, the RECIST working group revised RECIST v. 1.1 to develop a new efficacy evaluation guide for tumor immunotherapy named iRECIST, which is the latest standard for immunotherapy efficacy evaluation [16]. The main update is with regard to progression; namely, confirmation of disease progression is required within the 4–8 weeks of imaging examinations after the first evaluation, instead of an immediate declaration of progressive disease (PD) when the tumor burden initially increases beyond a threshold [16]. The merit of iRECIST has not been validated by large-scale clinical trials, and some of its content may require further revision. Nevertheless, the introduction of iRECIST is a major advance in the development of an immunotherapy response assessment system.
The functional imaging information from 18F-fluorodeoxyglucose (18F-FDG)-PET scan can provide data that are not available from conventional computed tomography (CT) or MRI anatomical imaging. Although 18F-FDG-PET scan is often used to assess the tumor response to treatment, it was only recommended for the confirmation of disease progression in RECIST v. 1.1. At present, not enough data are available to verify the 18F-FDG-PET reaction standard proposed by the RECIST committee. Some studies have explored the usefulness of 18F-FDG-PET scans to predict the response to immune checkpoint inhibitor (ICI) treatment (Table 1). Such studies evaluated the role of 18F-FDG-PET in patients with melanoma, non-small cell lung cancer (NSCLC), and Hodgkin's lymphoma [17-25], and their results indicate a correlation between metabolic response and clinical outcome (Figure 1). However, a variety of different tumor metabolic response assessment criteria were used in these studies and included criteria from RECIST v. 1.1, imPERCIST, EORTC, PERCIST, and PERCIMT. Additionally, the time points of the 18F-FDG-PET scans after the start of immunotherapy were different. Therefore, comparing the results of these studies is difficult. Furthermore, some studies have reached different conclusions. Wang et al. showed that, while the baseline tumor burden measured by FDG-PET/CT did not affect the treatment response in CD19-targeting chimeric antigen receptor (CAR)-T cell therapy in patients with relapsed/refractory non-Hodgkin's lymphoma (NHL), it was related to serious adverse reactions [26]. Using ipilimumab for immunotherapy of melanoma, the Heidelberg group proved that the prediction of clinical response was more dependent on the number of new lesions on 18F-FDG-PET/CT than it was on changes in a standard uptake value (SUV) [27]. Although the best time for 18F-FDG-PET/CT to evaluate the efficacy of immunotherapy is still uncertain, and the evaluation criteria are not uniform, early response stratification would be desirable to limit the significant toxicity and economic burden of ICIs. In the future, the role of 18F-FDG in monitoring tumor immune reaction needs to be further verified and assessed in large-sample, prospective studies. Furthermore, the increasing indications and strategies for the use of immuno-oncology treatment should be treated with caution. Some recent studies have also shown that 18F-FDG-PET can indirectly reflect the immune status of tumor patients. In a study of 73 patients with advanced head and neck squamous cell carcinoma, systemic FDG-PET/CT parameters were found to be correlated with systemic inflammatory response parameters and M2 macrophage polarization in tumors [28]. Other studies have shown a correlation between 18F-FDG uptake and the expression of multiple immune cells [29, 30], suggesting that 18F-FDG-PET has potential as a predictor for the selection of immunotherapy candidate drugs.
| Treatment | Tumor | Number of patients | Response evaluation criteria | Imaging time node | Findings | Reference |
|---|---|---|---|---|---|---|
| Nivolumab | Non-small-cell lung cancer | 32 | RECIST v1.1 | Baseline | 18F-FDG-PET/CT at baseline can be predictive of response to immunotherapy in patients with metastatic lung cancer | [17] |
| Ipilimumab/pembrolizumab | Melanoma | 56 | Survival/RECIST v1.1/best overall response (BOR) classification | Baseline | For patients with Muc-M treated with ICI, baseline SUVmax was the only prognostic imaging biomarker, whereas for patients with Cut-M, either baseline metabolic tumor burden or bone marrow metabolism was associated with prognosis | [18] |
| Combination treatment with vemurafenib and ipilimumab | Melanoma | 16 | EORTC, PERCIMT | Baseline, after two cycles and after completion of the combined treatment | 18F-FDG PET/CT could evaluate the clinical efficacy of ipilimumab combined with vemurafenib | [19] |
| Ipilimumab, nivolumab, and pembrolizumab | Melanoma | 57 | EORTC, RECIST v 1.1, PERCIMT | Baseline and 12–18 weeks after treatment | 18F-FDG PET/images acquired 3–4 months after immunotherapy can correctly assess treatment response | [20] |
| Ipilimumab | Melanoma | 60 | PERCIST, imPERCIST | Baseline and after the end of ipilimumab treatment | Tumor response according to PERCIST was associated with OS. PMD should not be defined by an increase in the sum of SULpeak | [21] |
| Nivolumab | Hodgkin lymphoma | 45 | Lugano classification and LYRIC | Baseline and treatment of early | Early response assessment in relapsed or refractory HL patients treated with immunotherapy-predicted OS | [22] |
| Sintilimab | Non-small cell lung cancer | 36 | PERCIST | Baseline and after two cycles of sintilimab | 18F-FDG PET-CT can predict MPR of neoadjuvant sintilimab in resectable non-small-cell lung cancer | [23] |
| Pembrolizumab, nivolumab, nivolumab/ipilimumab | Melanoma | 25 | PERCIMT versus EORTC | Baseline and after two cycles treatment | In anti-PD-1 therapy for metastatic melanoma, patients with metabolic benefit according to PERCIMT criteria had significantly higher PFS than those without metabolic benefit | [24] |
| Ipilimumab, BMS-936559, nivolumab | Melanoma | 20 | RECIST v 1.1, immune-related response criteria, PERCIST, and EORTC | Baseline, at days 21–28 and at 4 months after treatment initiation | 18F-FDG PET/CT scans can predict final responses in patients with advanced melanoma | [25] |
| CAR-T cell | Non-Hodgkin lymphoma | 19 | PERCIST | Baseline and 8 weeks after reinfusion of CAR-T cells | Baseline tumor burden was not significantly associated with overall survival, but was positively associated with CRS | [26] |
| Ipilimumab | Melanoma | 41 | iRECIST | Baseline and after two or four cycles of treatment | Number and size of new lesions better predicted clinical response than SUV changes after immunotherapy | [27] |
- Abbreviations: CRS, cytokine release syndrome; EORTC, European Organization for Research and Treatment of Cancer; imPERCIST, immunotherapy-modified PERCIST; iRECIST, revised version of RECIST v1.1 of immunotherapy; LYRIC, Lymphoma Response to Immunomodulatory therapy criteria; MPR, major pathologic response; OS, overall survival; PERCIMT, PET Response Evaluation Criteria for IMmunoTherapy; PERCIST, PET Response Criteria in Solid Tumors; PFS, Progression Free Survival; PMD, progressive metabolic disease; RECIST v1.1, Response Evaluation Criteria in Solid Tumors version 1.1; SULpeak, voxels with the highest average SUL (SUV normalized to lean body mass); SUVmax, Maximum standard uptake value.
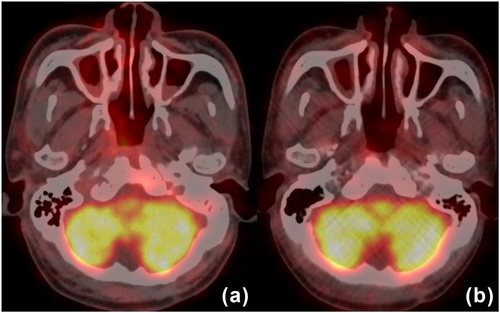
18F-FDG PET/CT images before and after Camrelizumab immunotherapy in a 62-year-old male with recurrent nasopharyngeal cancer. (a) PET/CT images of nasopharyngeal carcinoma patients before treatment showed a higher uptake of 18F-FDG in primary lesions, and SUVmax was 8.1; (b) after two cycles of treatment, there was still a significant uptake of 18F-FDG, and SUVmax was 4.6. Only partial metabolic response (PMR) was achieved according to EORTC criteria.
3 IMAGING OF T CELLS
A common feature of the immunotherapy response is the ability to boost cytotoxic T-cell infiltration and other immune responses to tumors. Therefore, T-cell molecular imaging has great potential for evaluating the response to current and future immunotherapy strategies. To develop molecular imaging biomarkers, our understanding of T-cell metabolism and its changes after T-cell activation urgently needs to improve. Recently, the cancer immune cycle has been used to describe a stepwise series of self-sustaining, essential processes required by the immune system to effectively control cancer growth (Figure 2). Cancer cell death induces the exposure of cancer antigens, and dendritic cells have the ability to recognize and capture tumor-associated antigens. After migration of dendritic cells to lymph nodes, tumor-specific cytolytic CD8+ T cells are primed and activated. Subsequently, T cells migrate and infiltrate the tumor microenvironment, where they recognize and remove cancer cells. The destruction of cancer cells and the release of inflammatory mediators elicit a new immune response that promotes the cancer immune cycle [31, 32]. Markers of the early activation of cytotoxic T cells and altered metabolism are two indicators of the successful induction of immune responses that can be used for T-cell imaging.
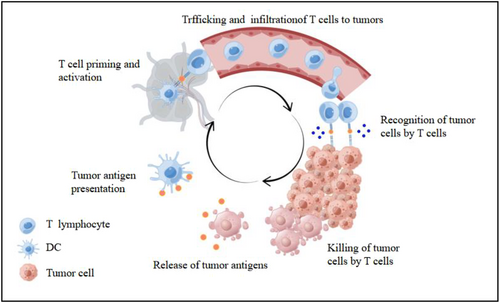
The cancer-immunity cycle. This cycle can be broken down into six major steps, starting with the release of antigens by the cancer cells and ending with the killing of the cancer cells. By Figdraw (www.figdraw.com).
After naive T cells are activated, the increased expression of certain cell surface markers can be targeted for molecular imaging (Table 2) [33-38]. However, the expression of the activation marker itself does not indicate that the cytotoxic effect is functioning, which may limit the usefulness of the imaging signal in tumor regions. Additionally, these cell surface markers are extremely alterable during the immune response; thus, the signal intensity is not directly representative of the number of cells. Studies have used 89Zr-PEGylated VHH-X118 to monitor the CD8+ T-cell response to immunotherapy in the melanoma model. In animal tumor models treated with CTLA-4, as the CD8 PET signal became more uniformly distributed in the tumor, the tumor immune response improved, and as the signal became more heterogeneous, the tumor immune response worsened [39].
| Target | Tracer | Tumor | Summary of findings | Reference |
|---|---|---|---|---|
| OX40 receptor (CD134) | 64Cu-DOTA-AbOX40 | Murine lymphoma | OX40 imaging was able to predict early response to immunotherapy | [30] |
| ICOS receptor (CD278) | 89Zr-DFO-ICOS mAb | Murine lewis lung cancer | 89Zr-DFO-ICOS mAb imaging enabled predict early response to immunotherapy | [31] |
| CD3+T cell | 89Zr-p-isothiocyanatobenzyl-deferoxamine-CD3 | Murine colon cancer | In anti-CTLA-4–treated mice, a higher tracer uptake resulted in smaller tumor volumes | [32] |
| CD25+ T cell | [18F] FB-IL-2 | Murine cervical cancer | [18F]FB-IL-2 uptake was significantly increased in the tumors of mice receiving the combination immunization compared to tumor irradiation alone | [33] |
| CD8+ T cell | 89Zr-malDFO-169 cDb | Murine lymphoma and colon cancer | In preclinical models, anti-CD8 immuno-pet can provide systemic information about the dynamic T cell population changes induced by immunotherapy | [34] |
| CD8+ T cell | 89Zr-Df-IAB22M2C | Human cancer (Melanoma; hepatocellular carcinoma) | 89Zr-IAB22M2C imaging is safe and can show the systemic distribution of CD8+ T cells in tumors and lymphoid organs, predicting early response to immunotherapy | [35] |
After activated T cells migrate and infiltrate the tumor microenvironment, their metabolic activities are altered, allowing them to adapt to the environment to survive and function. The metabolic pathways of activated lymphocytes include glycolysis, glutaminolysis [40], and nucleic acid metabolism [41]. Targeting these metabolic pathways can distinguish between active and inactive T cells. Certain tracers have been developed as key substrates of the deoxyribonucleoside rescue pathway. For example, the 18F-labeled analog of arabinofuranosyl guanine ([18F]F-AraG) has been used as a PET imaging agent to target activated T cell-specific metabolic pathways. This tracer mainly accumulates in activated T cells through the deoxyguanosine kinase (dGK) pathway. PET imaging with [18F]F-AraG displayed early signal differences between anti-PD-1 treatment and control groups in the treatment of rhabdomyosarcoma in mice. The difference in [18F]F-AraG signal between responders and nonresponders to anti-PD-1 therapy was also obvious [42]. Studies have shown that activated CD8+ T cells preferentially take up 1-(2′-deoxy-2′-[18F] fluoroarabinofuranosyl) cytosine (18F-FAC), a deoxycytidine analog. 18F-FAC is a specific substrate of deoxycytidine kinase (dCK), which is an indicator of the active area of immune cells [43]. In a mouse model of malignant glioma, the 18F-FAC-PET signal intensity was directly related to the count of intracranial tumor-infiltrating lymphocytes (TILs). Unlike the situation in mice, however, 18F-FAC breaks down rapidly in the human body. Therefore, the researchers used another dCK PET probe, 18F-clofarabine (18F-CFA). After immunotherapy, 18F-CFA PET probes were found to accumulate in tumors and secondary lymphoid organs (SLO) [44]. Furthermore, studies have reported on successful clinical applications of PET imaging; for example, PET imaging with 3'-deoxy-3'-[18F]fluorothymidine (18F-FLT), a thymidine analog targeting DNA synthesis, monitors the early response to pembrolizumab therapy in patients with melanoma. 18F-FLT-PET at the sixth week was able to predict the response of the CT assessment at the 12th week [45]. 18F-FDG-PET imaging can be an indicator of the level of glycolysis in tumor tissues. Related research [17-27] has been discussed above. One of the biggest challenges in using the tracers discussed above is the lack of specificity. Because they detect the abnormal metabolic processes of tumors and proliferation of immune cells simultaneously, distinguishing between the signals generated by these two conditions is technically difficult. The latest chemical exchange saturation transfer (CEST) method enables MRI of glutamate [46], creatine [47], glucose [48], inositol [49], lactate [50], glycosaminoglycans [51], and other metabolites. Increased glycolysis in activated T cells can lead to the amassing of such metabolites, including lactate, creatine, choline, and glutamate. Monitoring changes in the levels of these metabolites before and after immunotherapy is possible to evaluate the relative changes in effector T-cell density. However, few preclinical studies have evaluated the role of CEST technology in immunotherapy responses. Additionally, the most advanced near-infrared optical imaging technology in cancer immunotherapy has been applied to target various tumor-related immune cells, showing high sensitivity and accuracy [52].
4 IMAGING OF IMMUNE CHECKPOINTS
T cells play a vital role in cancer immunotherapy. The activation of T cells is dependent on the initial antigen-specific signal. Activated T cells also express co-suppressive molecules that can downregulate the immune response [53]. These negative regulators of immune activation serve as immune checkpoints. One of the main immune checkpoint molecules is PD-1, together with its ligands PD-L1 and PD-L2; of the two, PD-L1 has a more significant role. The expression of PD-L1 is upregulated by T-cell activation and cytokine release, and the combination of PD-L1 and PD-1 can hinder T-cell activation. The PD-1/PD-L1 immune checkpoint contributes to the maintenance of immunotolerance, thereby reducing autoimmune reactions as well as healthy tissue damage. By upregulating the expression level of PD-L1, tumor cells can evade immune recognition and attack [54]. While the results of clinical trials of anti-PD-1/PD-L1 immunotherapy have been exciting, not all patients respond to this therapy. CTLA-4 is another major immune checkpoint molecule. It has been shown to have a powerful inhibitory effect on regulation of the T-cell response, but anti-CTLA-4 therapy faces the same dilemma as anti-PD-1/PD-L1 immunotherapy [55]. Therefore, a biomarker that can be used to stratify patients and predict treatment response is urgently needed.
Importantly, the expression of PD-1/PD-L1/CTLA-4 in tissue samples detected by immunohistochemistry requires careful interpretation because the expression of these molecules in tumor tissue changes dynamically with ICI treatment and the release of inflammatory factors [56-60]. Additionally, tumor biopsy does not consider the space heterogeneity of the PD-1/PD-L1/CTLA-4 target protein expression in primary and metastatic lesions [54, 55]. Noninvasive in vivo molecular imaging can overcome some of the limitations of immunohistochemical assays. It can be used to measure the PD-1/PD-L1/CTLA-4 expression in tumor tissues at multiple time points and multiple sites, possibly making it easier and more accurate than immunohistochemical analysis of these molecules. Noninvasive in vivo molecular imaging results may also be used as a biomarker for patients who choose anti-PD-1/PD-L1 or anti-CTLA-4 immunotherapy and allow longitudinal monitoring of PD-1/PD-L1/CTLA-4 expression during treatment.
5 IMAGING OF PD-1/PD-L1
To achieve noninvasive imaging and quantification of the PD-L1 expression, a series of imaging agents, including monoclonal antibodies, monoclonal antibody fragments, and small proteins and peptides, have been tested and discussed in preclinical studies. Some of these drugs are undergoing early clinical studies and have shown good results. Preclinical studies (Table 3) demonstrated the viability of visualizing the biodistribution of PD-L1-targeted molecules [61-74]. Radiolabeled PD-L1-targeting antibodies accumulated in PD-L1-positive tumors specifically and were also able to differentiate tumors with high and low PD-L1 expressions [61, 63-66, 68]. The regulation of PD-L1 expression was observed, and interferon-γ increased the uptake of PD-L1-targeting antibodies in tumors [70].
| Type of imaging | Tracer | Tumor model | Summary of findings | Reference |
|---|---|---|---|---|
| SPECT/CT | 111In-PD-L1.3.1 antibody | Human cell lines (MDA-MB-231, SK-Br-3, SUM149, BT474, and MCF-7) | Efficient and specific uptake in xenografts expressing PD-L1 | [58] |
| SPECT/PET | [131I] SIB-anti-PD-L1M, [124I] SIB-anti-PD-L1 | Mouse cell lines (B16F10,4T1) | Highly specific uptake in tumors expressing PD-L1 | [59] |
| Human cell lines (MCF-7,HEPG2) | ||||
| PDX model of lung adenocarcinoma | ||||
| SPECT | 111In-DTPA-PD-L1 antibody | NT2.5 (mouse mammary tumor) | Significant uptake in tumor, spleen, liver, thymus, heart, and lungs; Excess unlabeled antibody effectively blocked uptake of the tracer by the spleen | [60] |
| SPECT | 111In-PD-L1 antibody | Human cell lines(NCI-H2444, NCI-H1155, MDAMB231,SUM149) | Specific uptake in xenografts and higher uptake in spleen, lungs, liver, and kidneys | [61] |
| CHO | ||||
| PET | [64Cu]-atezolizumab | Human cell lines (MDAMB231 and SUM149) | Specific uptake in tumors with a high PD-L1 expression | [62] |
| 4 T1 (mouse mammary tumor) | ||||
| CHO | ||||
| PET | 89Zr-DFO-PD-L1 mAb | Human cell lines (MDA-MB231) | Specific tumor uptake and higher uptake in spleen, lymph nodes | [63] |
| PET | 89Zr-DFO-6E11 | Human cell lines (H1703, H1993, and HCC827) | Significant uptake in tumors expressing PD-L1 | [64] |
| Murine colon carcinoma cells (CT26 and B16-F10) | ||||
| PET | 89Zr-C4 (recombinant IgG1 mAb) | Human cell lines (H1975, A549, and PC3) | Tracer uptake in tumor, spleen and liver; Acute changes in PD-L1 expression in tumors after treatment were detected | [65] |
| Mouse melanoma(B16F10) | ||||
| PET | 89Zr-atezolizumab | Human cell lines (H1975) | Low specific activity 89Zr-atezo can more accurately measure the PD-L1 expression in the tumor microenvironment | [66] |
| Murine colon carcinoma cells (B16 F10) | ||||
| SPECT/CT | 99mTc-MY1523 | Mouse cell lines(MC-38,A20, 4T1) | SPECT/CT imaging effectively guided the determination of the time window of PD-L1 blockade and improved the therapeutic effect | [67] |
| PET | 68Ga-NOTA-Nb109 | A375 (human malignant melanoma), human PD-L1 gene transfected A375 | Highly specific affinity for PD-L1 and different from the binding epitopes of PD-1 and PD-L1 antibodies | [68] |
| PET | 18F-NOTA-ZPD-L1_1 | Human cell lines (LOX-IMVI and SUDHL6) | Specific differentiation between PD-L1-positive and -negative tumors | [69] |
| PET | 64Cu-WL12 (PD-L1 binding peptide) | CHO cell line expressing hPD-L1 | Specific detection of PD-L1 expressions in tumors | [70] |
| PET | 18F-BMS-986192 | Human cell lines (L2987 and HT-29) | Specific uptake in tumor expressing PD-L1 and kidney | [71] |
Immunobiological responses to human cancers are not always well represented in routinely used mouse tumor models. The shortage of cross-reactivity limits the usefulness of evaluating candidate drug efficacy in immunocompetent mouse models. Therefore, the use of monoclonal antibodies with human and mouse cross-reactivity could provide unique insights for studying the distribution of monoclonal antibodies in the body, thereby bridging preclinical and clinical research. Radiolabeled anti-PD-L1 antibodies with mouse and human cross-reactivity were specifically deposited in human cell lines and tumors expressing various levels of PD-L1 [64-66]. Monoclonal antibody probes targeting PD-L1 can detect not only PD-L1-positive tumors, but also normal lymphoid organs, including the spleen, thymus, lymph nodes, and brown adipose tissue (BAT) [63, 64, 66, 75]. The combined use of unlabeled monoclonal antibodies blocked the uptake of tracers in normal lymphoid organs [63, 75].
Monoclonal antibodies have proven to be very reliable as imaging agents for molecular imaging. However, they cannot be used to produce high-contrast images in a short period of time because of their large size and long half-life. Therefore, it is important that we aim to find a developer with a faster removal rate that can provide high-contrast images within hours of injection [76]. Some monoclonal antibody fragments, including single domain antibodies (sdAbs) [71], nanobodies [70, 72], and small proteins from adnectin [74] and the PD-1 external domain [77], have been applied in preclinical models. For example, the increased tissue permeability and shorter half-life of single-domain nanobodies enable high-contrast imaging at 1–2 h post-injection. The expression of PD-L1 in the body can be measured in real time, quantitatively and dynamically, using the radiolabeled nanobody 99mTc-MY1523 in SPECT/CT. Furthermore, anti-PD-L1 immunotherapy obviously improves treatment outcomes guided by PD-L1 imaging [70]. This strategy to better manage the combination therapy of radiotherapy or chemotherapy deserves to be translated into clinical practice.
Some researchers are committed to the study of smaller low-molecular-weight drugs, such as small molecules and peptides, which have better performance in tissue penetration, tumor deposition, metabolism, and other areas. Some researchers have developed the 64Cu-labeled DOTAGA-binding peptide 64Cu-WL12, which can rapidly and specifically detect the PD-L1 expression in tumors after tracer injection [73].
Some studies have been used in clinical research. A PET study using 18F-BMS-98619210, anti-PD-L1 adnectin labeled with 18F [74] and 89zirconium (Zr)-labeled nivolumab [78], demonstrated successful imaging of the tumor PD-L1 expression by assessing the heterogeneity between and within tumors. On the basis of the above research, an article reported the results of the first human study using 18F-BMS-986192 and 89Zr-nivolumab to perform whole-body PET imaging of patients with advanced NSCLC before nivolumab treatment. There was a heterogeneity in the uptake of tracer between patients and among different tumor lesions within patients [79]. Preclinical studies of 89Zr-atezolizumab PET have shown that this imaging method may be useful to select patients [69]. A clinical study performed 89Zr-atezolizumab imaging of three tumor types in 22 patients before starting atezolizumab treatment. The PET signal was higher in tumors, lymphoid organs, and areas of inflammation. However, the tracer uptake varied between different patients and different tumor types. Interestingly, compared with predicted biomarkers based on immunohistochemistry or RNA sequencing, pre-processing PET signals were more strongly correlated with the patient's clinical response [80]. In a published clinical study, 16 NSCLC patients received 99mTc-labeled NM-01, the PD-L1 single-domain antibody, for SPECT imaging. This first study in humans showed that 99mTc labeling of an anti-PD-L1 single-domain antibody for SPECT/CT imaging was reliable with easy uptake in tumors and background tissues, especially 2 h post-injection of the tracer [81]. The tracer uptake could be seen in the kidneys, spleen, liver, and bone marrow.
Unlike tumors and various immune cells that widely express PD-L1, PD-1 is mainly expressed on T cells, B cells, and macrophages [82]. Therefore, studies have focused on imaging of PD-1-expressing TILs using molecular probes that target PD-1. These include 64Cu-labeled anti-PD-1 mouse antibody PET probe imaging in a mouse melanoma model [83], 89Zr-Df-nivolumab probe imaging in a humanized mouse lung cancer model [84], and 89Zr-labeled pembrolizumab PET probe imaging in a mouse model of humanized melanoma [85].
6 IMAGING OF CTLA-4
CTLA-4 is an important participant of the immune resistance and antitumor response, which is predominantly found in activated T cells and regulatory T cells (Tregs) [86]. CTLA-4 negatively regulates T-cell activation in multiple ways [87]. Recent studies have shown that many types of tumor cells also express CTLA-4 [88]. In 2011, the FDA approved ipilimumab, an anti-CTLA-4 monoclonal antibody, for the treatment of advanced melanoma. Anti-CTLA-4 monoclonal antibodies have been used in preclinical and clinical trials to treat other tumors, including colon, breast, ovarian, lung, and prostate cancers [89]. However, few studies have focused on targeted CTLA-4 imaging.
PET revealed that a 64Cu-DOTA-anti-CTLA-4 monoclonal antibody exhibited significant high aggregation in colon cancer in BALB/c mice, making the noninvasive visualization of CTLA-4 in tumors a reality [90]. In vitro studies have shown that T cells were associated with a high expression of CTLA-4. The tumor uptake of 64Cu-DOTA-ipilimumab has been confirmed in NSCLC tumors transplanted in mice [91]. Two tracers that target CTLA-4—64Cu-NOTA-ipilimumab (a full antibody) and 64Cu-NOTA-ipilimumab-F(ab')2 (an antibody fragment)—can be used to locate CTLA-4+ tissue. Additionally, F(ab’)2 reagent was cleared from the blood faster than full antibodies [92]. These preclinical studies demonstrate the feasibility of using anti-CTLA-4 tracers for PET imaging to visualize CTLA-4.
7 RADIOMICS AND RADIOGENOMICS IN IMMUNOTHERAPY
Thus far, biomarkers that have been studied in relation to immunotherapy have involved tumor mutational burden/neo-antigens, microsatellite instability, interferon-γ gene signature, tumor immunophenotype, degree of T-cell infiltration in tumors, PD-L1 expression, T-cell activation status, and other areas. However, because of the heterogeneity of tumors, these features vary in space and time, relying on a variety of complex interactions, rather than a single dominant determinant; thus, they cannot be used to accurately identify which patients will respond [93]. For instance, although the PD-L1 expression has been related to increased immunotherapy response, there have also been cases of nonresponses to immunotherapy in tumors with a high PD-L1 expression as well as a low PD-L1 expression. Therefore, fruitful cooperation between radiologists and oncologists is necessary to navigate the increasing reliance on imaging results as a therapeutic biomarker.
The advancement of artificial intelligence has led to the development of radiomics, an application of machine learning and deep learning in radiology [94, 95]. Radiomics technologies automatically extract image features that can be mined to describe tumor phenotypes. Relationships between radiological image features, tumor biological characteristics, and clinical outcomes are established through computational procedures. Sun et al. applied machine learning to assess tumor infiltration of CD8 cells by conjoint analysis of contrast-enhanced CT images of 135 patients with advanced solid malignancies and RNA sequencing genomic data from tumor biopsies [96]. In another study of 203 patients with advanced melanoma and NSCLC who received anti-PD1 treatment, Trebeschi et al. performed an artificial intelligence-based characterization of the CT enhanced imaging data that proved that the imaging features of lesions could serve as noninvasive biomarkers of the response to immunotherapy [97]. Furthermore, artificial intelligence has been used to associate imaging features with biomarkers [98], pathological images [99], and tumor microenvironment features [100]. Finally, radiogenomics, which correlates imaging features with the genome atlas, is beginning to be used to assess the response to immunotherapy in patients with NSCLC and glioblastoma [101, 102].
Despite the great potential shown by machine learning-based radiomics and radiogenomics, significant limitations remain in the application of immunotherapeutic imaging biomarkers. A major limitation is poor reproducibility due to the lack of consistency at each step [103]. The complexity of the process, including image acquisition, target lesion recognition and segmentation, feature extraction, and data analysis, is another limitation. To ensure the clinical utility of biomarker imaging, rigorous standardized procedures are required [104]. Additionally, many studies have used local software programs and different algorithms, making these studies almost impossible to reproduce and repeat. The lack of traditional genetic data and the nonstandardization of tissue sampling limit the clinical application of radiogenomics.
Great potential has been demonstrated by deep learning, which facilitates automatic feature learning in areas ranging from tumor segmentation to survival prediction [105]. After training, the neural network can automatically complete the key steps in the radiomics processes, such as tumor segmentation and multi-image feature extraction, which have significant clinical values.
The purpose of radiomics and radiogenomics is to improve the accuracy of disease diagnosis and help clinicians develop precision treatment strategies. To develop and accelerate clinical applications of radiomics and radiogenomics, data acquisition methods that are standardized and more reproducible must be developed, publicly available radiology databases must be generated, and prospective large-scale multi-institutional clinical trials must be conducted.
8 CONCLUSION
The development of immunotherapy has led to significant tumor responses and improved patient survival. The variability in response to immunotherapy of different tumors in different patients is due to differences in the tumor immune microenvironment (TME). Molecular imaging plays a significant role in patient stratification and response assessment. More precise visualization of the complex interactions between tumor cells and the TME may be an important step in the development of immunotherapy. However, only a small number of receptors and ligands related to immune response suppression have been identified and imaged. Recent studies have identified new potential immunotherapy targets, such as CD47, nuclear receptor subfamily 2 group F member 6, and LAG-3 [106]. Further studies are needed to monitor the dynamic imaging of these targets in vivo, so as to guide the implementation of immunotherapy strategies more accurately. Additionally, due to limitations that include safety assessment and manufacturing process validation, translating preclinical research results of animal tumor models into clinical results remains challenging. The true picture of the human immune response to cancer requires not only humanized tumor models but also the TME of humanized tumors. Furthermore, most preclinical studies are conducted in 4- to 6-week-old mice, whereas patients tend to be older in age at the time of onset. This may be related to changes in the immune and lymphatic systems that occur with age. This review article provides only a preliminary discussion on the latent value of tracers and artificial intelligence. The main challenge ahead is to implement these imaging methods in clinical practice, which will require uniformity in procedures and proper validation across larger cohorts. Collaboration between multidisciplinary oncology teams may allow us to overcome the challenges posed by these technologies, facilitating their transformation into the clinical setting and promoting the potentially valuable clinical application of molecular imaging in tumor immunotherapy.
AUTHOR CONTRIBUTIONS
Jing Yu contributed to the drafting of the manuscript and collation and analysis of the literature. Bo Gao contributed to the conception and critical revision of the manuscript and approved the final version of the submitted manuscript. Both authors read and approved the final manuscript.
ACKNOWLEDGMENTS
We are grateful for the Brain Science Laboratory team of Guizhou Medical University for their support.
CONFLICT OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could appear to influence the work reported in this paper.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




