A cross-taxon study in Thailand demonstrates significance of vertical stratification and seasonality in monitoring tropical forest insects
昆虫垂直分层和季节性在泰国热带森林监测中的重要性
การศึกษาข้ามกลุ่มทางอนุกรมวิธานในประเทศไทย แสดงให้เห็นถึงความสำคัญของการแบ่งชั้นตามแนวตั้งและฤดูกาล ในการติดตามแมลงในป่าเขตร้อน
Editor-in-Chief & Handling Editor: Ahimsa Campos-Arceiz
Abstract
enIn tropical rainforests, trees and associated plants create vertically heterogeneous habitats mediated by seasonal changes in climate and plant phenology. Despite extensive studies on the spatiotemporal dynamics of tropical forest ecosystems, insect monitoring programs often neglect the vertical and seasonal dimensions because the relative importance of muti-dimensional dynamics of insect diversity has not been well understood. In this study, we employed a spatially explicit sampling design to understand the distribution patterns of Coleoptera, Hymenoptera, and two families (Noctuidae and Nolidae) of Lepidoptera in the canopy and understory during the dry and wet seasons in two tropical rainforests located at lower and higher latitudes in Thailand. We compared the vertical stratification of gamma diversity, alpha diversity, total abundance, and beta turnover and nestedness. We also identified the insect species characteristic of certain vertical strata and/or seasons. Our samples resulted in a total of 4,452 insects (1,622 coleopterans, 1,763 hymenopterans, and 1,067 lepidopterans) representing 437 Coleoptera species, 694 Hymenoptera species, and 98 Lepidoptera species. All insect groups generally displayed moderately higher diversity in the canopy than in the understory, but the significance of this pattern varied among seasons, forests, and insect groups. Over 50% of significant habitat indicator species were restricted to certain vertical strata but were only found in certain seasons. Our findings demonstrate that spatiotemporal dynamics of insect diversity are highly context-dependent and are not as clearly discernible as we hypothesized in three major insect groups, suggesting the importance of monitoring insects across vertical strata and seasons, even in low-latitude tropical rainforests.
Abstract
zh在热带雨林中,树木及其它林下植物形成了由季节性气候变化和植物物候所调节的垂直生境异质性。尽管热带森林生态系统的时空动态已得到广泛研究,但昆虫监测计划往往忽略了垂直结构和季节性的维度,因为昆虫多样性的多维动态尚未得到充分理解。在泰国的两片分别位于低纬度和高纬度的热带雨林中,我们采用空间显式采样设计探讨了鞘翅目(Coleoptera)、膜翅目(Hymenoptera)以及鳞翅目(Lepidoptera)的两个科(夜蛾科Noctuidae和叶蛾科Nolidae)昆虫在林冠层和林下层中的分布格局及其季节动态。我们比较了垂直分层的Gamma多样性、Beta多样性(物种周转率)、Alpha多样性、总丰富度以及物种嵌套性。此外,我们还识别出特定垂直层次和/或季节的昆虫种类特征。我们共采集到4,452只昆虫(1,622只鞘翅目、1,763只膜翅目和1,067只鳞翅目),代表了437种鞘翅目、694种膜翅目和98种鳞翅目的昆虫。总体而言,所有昆虫类群在林冠层中的多样性略高于林下层,但这一格局在不同季节、不同森林和不同昆虫类群之间的显著性有所差异。超过50%的重要生境指示种仅出现在特定的垂直层次和特定季节中。我们的研究结果表明,这三类昆虫多样性的时空动态高度依赖于环境背景,并未表现出明确不变的规律,暗示即使在低纬度的热带雨林中,也应该开展昆虫在森林的垂直结构层面和季节性变化方面的监测。【翻译 :曹敏】
บทคัดย่อ
thต้นไม้และพันธุ์พืชที่อยู่ร่วมกันในระบบนิเวศป่าฝนเขตร้อนทำให้เกิดแหล่งที่อยู่อาศัยที่หลากหลายตามลำดับชั้นความสูงในแนวดิ่ง โดยอาศัยการเปลี่ยนแปลงตามฤดูกาลในด้านสภาพภูมิอากาศและสรีระวิทยาของพืช แม้ว่าจะมีการศึกษาอย่างกว้างขวางเกี่ยวกับพลวัตของสิ่งต่าง ๆ ในบริบทของพื้นที่และเวลาของระบบนิเวศป่าเขตร้อน แต่การติดตามและตรวจสอบการเปลี่ยนแปลงของแมลงในแง่มุมทางแนวตั้งและฤดูกาลมักเป็นประเด็นที่ถูกละเลย อีกทั้งยังมีความรู้ความเข้าใจที่ไม่ชัดเจนของพลวัตหลายมิติ ความสำคัญเชิงสัมพันธ์ของปัจจัยที่เกี่ยวข้องกันในบริบทต่าง ๆ ต่อความหลายหลายแมลง
การศึกษานี้ ใช้การสุ่มตัวอย่างเชิงพื้นที่ที่ชัดเจนของเรือนยอดและใต้เรือนยอด และในฤดูแล้งและฤดูฝน เพื่อทำความเข้าใจรูปแบบการกระจายของแมลงในกลุ่มด้วง (Coleoptera), กลุ่ม ผึ้ง ต่อ แตน มด (Hymenoptera) และ กลุ่มผีเสื้อกลางคืน (Lepidoptera) ใน วงศ์ Noctuidae และ Nolidae ในป่าฝนเขตร้อนสองแห่งที่อยู่ในระดับละติจูดที่ต่ำและละติจูดที่สูงในประเทศไทย ทำการเปรียบเทียบความหลากหลายของแมลงทั้งหมด (gamma diversity) ความหลากหลายภายในชนิด (alpha diversity), ความหลากชนิดทั้งหมด (total abundance) และความหลากหลายทางชนิดระหว่างที่อยู่อาศัยต่างกัน (beta turnover) และ การทับซ้อน (nestedness) ตามการแบ่งชั้นในแนวตั้ง นอกจากนี้ยังได้ระบุชนิดแมลงจำเพาะของชั้นแนวตั้งและฤดูกาลบางฤดูกาล
ผลการศึกษา พบแมลงทั้งหมด 4,452 ตัว ประกอบด้วย กลุ่มด้วง 1,622 ตัว 437 ชนิด, กลุ่ม ผึ้ง ต่อ แตน มด 1,763 ตัว 694 ชนิด, และ กลุ่มผีเสื้อกลางคืน 1,067 ตัว 98 ชนิด โดยทั่วไปกลุ่มแมลงทั้งหมดมีความหลากหลายบนชั้นเรือนยอดมากกว่าชั้นใต้เรือนยอดเล็กน้อย แต่ความสำคัญของรูปแบบนี้แตกต่างกันไปตามฤดูกาล ชนิดป่า และกลุ่มแมลง นอกจากนี้ มากกว่า 50% ของชนิดของแมลงที่จำเพาะต่อที่อยู่อาศัยยังถูกจำกัดให้อยู่ในชั้นแนวตั้งบางชั้น และพบได้เฉพาะในบางฤดูกาลเท่านั้น แสดงให้เห็นว่า พลวัตเชิงพื้นที่และเวลาของความหลากหลายของแมลงทั้งสามกลุ่มแมลง ขึ้นอยู่กับบริบทในขณะนั้นเป็นอย่างมาก และไม่ชัดเจนเท่าที่ได้ตั้งสมมติฐานไว้ ซึ่งชี้ให้เห็นถึงความสำคัญของการติดตามแมลงในชั้นแนวตั้งและฤดูกาล แม้แต่ในป่าฝนเขตร้อนละติจูดต่ำก็ตาม (Translator: Ekgachai Jeratthitikul)
Plain language summary
enIn tropical rainforests, trees and associated plants form vertical layers, providing habitats for insects and other animals. Seasonal changes affect plant growth and life cycles, which in turn influence when and where insects are most active. While insect diversity in tropical forests has been studied extensively, research often overlooks the importance of vertical layers and seasons. Here we investigated vertical and seasonal patterns of insect diversity by sampling beetles, wasps, and moths in the forest canopy and the understory during the wet and dry seasons in two monsoonal tropical rainforests located at lower and higher latitudes in Thailand. Overall, we found that insect diversity was slightly higher in the canopy than in the understory, although this pattern changed depending on the season, forest, and type of insect. Over half of the insect species were unique to specific vertical layers but were found in certain seasons. Our study suggests that insect diversity depends strongly on the vertical position and the time of year, suggesting that insect studies and monitoring should consider these factors in tropical rainforests.
Plain language summary
zh在热带雨林中,树木和其它植物形成垂直分层,为昆虫和其它动物提供了不同的栖息地。旱季和雨季的季节变化也会影响植物的生长节律和生命周期,从而影响昆虫的空间分布。前期的研究忽视了垂直分层和季节性的重要性。在泰国的两个热带雨林中,我们采集了林冠层和林下层的甲虫、黄蜂和蛾类样本,调查了它们的垂直分层和季节变化。我们发现林冠层的昆虫多样性略高于林下层,但这种模式会因季节、森林类型和昆虫类群不同而有所差异。超过一半的昆虫种类仅出现在某些垂直层面上,并且也只会出现在某些季节。我们发现,热带雨林的昆虫多样性具有垂直层面和季节性的变化,在开展昆虫研究和监测时要考虑这些因素。
Plain language summary
thในป่าฝนเขตร้อน ต้นไม้และพืชที่เกี่ยวข้องจะสร้างชั้นแนวดิ่งซึ่งเป็นที่อยู่อาศัยสำหรับแมลงและสัตว์อื่น ๆ การเปลี่ยนแปลงตามฤดูกาลส่งผลต่อการเจริญเติบโตและวงจรชีวิตของพืช ซึ่งจะมีผลต่อช่วงเวลาที่แมลงมีความเคลื่อนไหวมากที่สุด แม้ว่าความหลากหลายของแมลงในป่าฝนเขตร้อนจะได้รับการศึกษาอย่างกว้างขวาง แต่การวิจัยมักมองข้ามความสำคัญของชั้นแนวดิ่งและฤดูกาล การศึกษาครั้งนี้ได้ตรวจสอบรูปแบบเชิงแนวดิ่งและฤดูกาลของความหลากหลายของแมลง โดยสุ่มตัวอย่างด้วง ต่อ แตน และผีเสื้อ ในชั้นเรือนยอดและชั้นใต้เรือนยอดในฤดูฝนและฤดูแล้งของป่าฝนมรสุมเขตร้อนสองแห่งที่ ตั้งอยู่ที่ละติจูดต่ำและสูงในประเทศไทย โดยรวมแล้ว พบว่าความหลากหลายของแมลงในชั้นเรือนยอดสูงกว่าชั้นใต้เรือนยอดเล็กน้อย แม้ว่ารูปแบบนี้จะเปลี่ยนแปลงไปตามฤดูกาล ประเภทของป่า และชนิดของแมลง ชนิดของแมลงมากกว่าครึ่งพบได้เฉพาะในชั้นแนวดิ่งที่เฉพาะเจาะจง แต่เกิดขึ้นในบางฤดูกาลเท่านั้น การศึกษานี้ยังชี้ให้เห็นว่าความหลากหลายของแมลงขึ้นอยู่กับตำแหน่งแนวดิ่งและช่วงเวลาของปีอย่างมาก ซึ่งบ่งบอกว่าการศึกษาและการติดตามความหลากหลายของแมลงในป่าฝนเขตร้อนควรคำนึงถึงปัจจัยเหล่านี้
Practitioner points
en
-
To better understand the complex factors that shape tropical forest insect diversity, it is essential to monitor insects in both the canopy and understory vertical layers.
-
Monitoring during both dry and wet seasons offers valuable insights into how seasonal climate changes impact insect distribution. This knowledge is crucial for predicting how environmental shifts may affect insect populations.
-
Understanding these seasonal influences on insect diversity is especially important in monsoon-driven tropical forests, even in low-latitude regions of tropical Asia.
Practitioner points
zh
-
为了更好地理解热带森林昆虫多样性的影响因素,我们在开展昆虫监测时要分别在林冠层和林下层中进行。
-
昆虫的物种组成在旱季和雨季不同,因此也要开展季节性变化的监测,这些数据对于我们预测环境变化对昆虫种群的影响至关重要。
-
即使是在热带亚洲的低纬度地区,研究这些因素对昆虫多样性的影响也至关重要。
Practitioner points
th
-
เพื่อให้เข้าใจปัจจัยซับซ้อนที่กำหนดความหลากหลายของแมลงในป่าฝนเขตร้อนได้ดียิ่งขึ้น การติดตามแมลงในทั้งชั้นเรือนยอดและชั้นใต้เรือนยอดเป็นสิ่งสำคัญ
-
การติดตามในทั้งฤดูแล้งและฤดูฝนจะให้ข้อมูลเชิงลึกที่มีคุณค่าเกี่ยวกับวิธีการเปลี่ยนแปลงของภูมิอากาศตามฤดูกาลที่ส่งผลต่อการกระจายตัวของแมลง การศึกษานี้สำคัญอย่างยิ่งสำหรับการคาดการณ์ว่าการเปลี่ยนแปลงของสิ่งแวดล้อมอาจมีผลต่อประชากรแมลงอย่างไร
-
การทำความเข้าใจอิทธิพลตามฤดูกาลที่มีต่อความหลากหลายของแมลงมีความสำคัญอย่างยิ่งในป่าฝนเขตร้อนที่ได้รับอิทธิพลจากมรสุม แม้ในพื้นที่ละติจูดต่ำของเอเชียเขตร้อนก็ตาม
1 INTRODUCTION
The prominent feature of forests is their structural heterogeneity (Moore, 2008), which spans horizontally and vertically, forming three-dimensional niche spaces (Gámez & Harris, 2022). Trees of variable sizes and forms, together with lianas, epiphytes, and parasitic plants, create layers of foliage at multiple vertical strata (Iida & Swenson, 2020; Lowman & Rinker, 2004). This complex structural heterogeneity provides a variety of microhabitats, each with its own unique microclimatic conditions, creating variations in temperature, humidity, light, and wind (Álvarez et al., 2024; Anhuf & Rollenbeck, 2001; Lindo & Winchester, 2013; Lowman & Rinker, 2004). The presence of different microhabitats and microclimates results in a greater diversity of resources, which in turn creates a wider range of ecological niches (De Frenne et al., 2021; Moore, 2013; Scheffers et al., 2014). The availability of niche spaces ultimately influences the distributions and interactions of species across vertical and horizontal spaces within forest ecosystems (MacArthur & Levins, 1964; Nakamura et al., 2017).
Due to more abundant and diverse resources available in the canopy, earlier estimates of forest diversity assumed that the forest canopy harbors twice as many insect species as the forest floor (Erwin, 1982). However, one of the most comprehensive surveys of tropical forests showed that although overall insect diversity was greater in the canopy than in the understory and ground strata, the canopy diversity was not as high as previously estimated (Basset et al., 2015). Other studies investigating individual taxonomic groups also showed similar results (e.g., wasps and bees, Sobek et al., 2009; Ulyshen et al., 2010; beetles and flies, Maguire et al., 2014; beetles, Charles & Basset, 2005; Stork & Grimbacher, 2006). However, some invertebrates (e.g., butterflies, DeVries et al., 2012; moths, Ashton et al., 2016; Brehm, 2007) and vertebrates (Basham et al., 2023) showed no significant differences or even greater diversity in the understory than the canopy. These studies, however, focused on one or two taxonomic groups at a given time and place, and the relative importance of vertical stratification cannot be explicitly compared across different taxa. As vertical diversity likely depends on the focal taxa (de Souza Amorim et al., 2022), the relative importance of vertical stratification can only be robustly compared across taxa based on data derived from the same forest collected at the same time.
Vertical stratification of diversity is often structured by a set of unique species found only in certain vertical strata (Knuff et al., 2020; Wardhaugh et al., 2013). Stork & Grimbacher (2006) reported that approximately a quarter of beetle species were found only in either the canopy or understory, whereas the remaining half were found in both strata. In contrast, over 60% of Diptera species in the Central Amazon tropical forest were found above ground (de Souza Amorim et al., 2022). Unsurprisingly, a large number of unique species were found for phytophages as they depend on abundant and diverse food resources in the canopy, whereas other feeding guilds, such as predators and scavengers, were not as clearly stratified vertically (Basset et al., 2015). Certain taxa, such as mostly herbivorous Lepidoptera, are expected to have more unique species in the canopy compared with other taxonomic groups, such as Coleoptera, which represent various feeding guilds (Basset et al., 2015).
The distribution of insects and other organisms is also highly heterogeneous in horizontal space (Basset et al., 2015; Beck and Khen, 2007; Kitching et al., 2013). Both vertical and horizontal beta diversities ultimately contribute to the overall gamma diversity of ecosystems (Xing et al., 2022). The horizontal distribution of insects is, however, rarely studied in the canopy (Nakamura et al., 2017; Wardhaugh, 2014). Higher horizontal beta diversity in the canopy than in the understory is expected because the canopies have higher and more spatially heterogeneous resource availability (e.g., foliage density, flowers, pollen, nectar, and fruits) than within the understory (Basham et al., 2019; Basset et al., 2003). Mosaics of different crown sizes, leaf surface areas, and connectivities among canopies increase habitat heterogeneity and promote the coexistence of more species (Tews et al., 2004). Past research on fruit-feeding nymphalid assemblages found higher species turnover in the canopy than in the understory due to the presence of diverse plant species, which provide spatially variable resources in a forest (Fordyce & DeVries, 2016). Similarly, a much greater horizontal beta diversity of ants was found in the canopy than in the understory of tropical forests (Xing et al., 2022). These studies, however, utilized taxa that are highly dependent on canopy resources (fruits and flowers for nymphalids) or wingless (ant workers). It is not surprising that horizontal beta diversity is much greater for wingless canopy organisms as they are confined to individual trees (i.e., trees are effectively “island” habitats for wingless organisms) unless canopies are connected by lianas or other structures (Adams et al., 2017). The vertical and horizontal distribution patterns of other taxonomic groups have rarely been studied.
Although forests are now more accurately viewed as having three-dimensional space with the inclusion of vertical strata (Gámez & Harris, 2022), the diversity of forest organisms cannot be fully captured unless another dimension, temporal dynamics, is also considered (Basset et al., 2015; Grimbacher & Stork, 2009; Wardhaugh, 2014). Seasonality is the major driver of temporal dynamics, which influences plant phenology and life cycles, mediating ecological processes such as plant-pollinator interactions (Duchenne et al., 2021), herbivory (Kuchenbecker et al., 2021), frugivory (Ramos-Robles et al., 2018), seed predation (Basset et al., 2018), and, indirectly, insect predation and parasitism (Wenda et al., 2023). Organisms, especially poikilotherms (e.g., insects), are not only influenced by changes in ecological processes but also by climate itself (Wolda, 1988). In high latitudinal regions, for example, the available thermal budget for growth and reproduction is limited, which generally results in high seasonality in the phenology of many insect species (Bale et al., 2002; Lowman, 1982; Wolda, 1988). In contrast, tropical rainforests in lower latitudes generally present more stable climatic conditions, but precipitation regimes are often seasonally variable (Allaby, 2006). Furthermore, precipitation seasonality in tropical forests generally increases with increasing latitude (Kumagai et al., 2009). Hence the strength of seasonality may be variable among tropical forests at different latitudes. Current evidence is mixed, however, with some studies suggesting the seasonal variability of tropical forest insect diversity across latitudes (Beck & Khen, 2007; Hernández-Ortiz et al., 2022; Oliveira et al., 2021), whereas others reporting no consistent seasonal patterns (Grimbacher & Stork, 2009; Wolda, 1992). While there is no consensus, seasonal influence is expected to be even stronger in forest canopies compared to the understory, where climatic fluctuations are buffered by the canopies (De Frenne et al., 2019). In addition, the seasonal phenology of tropical forest insects is likely to be variable among taxa whose ecological traits, such as physiological and behavioral thermal tolerance and voltinism, differ (Wardhaugh, 2014; Wolda, 1988). More studies are clearly needed to understand how seasonality influences different insect groups across tropical forests at different latitudes.
This lack of “four-dimensional” understanding of the spatiotemporal dynamics of insects has serious implications for the monitoring and conservation of insect diversity. An increasing number of studies have reported the decline of insect abundance (or biomass) and richness worldwide (Dirzo et al., 2014; Klink et al., 2023), but these studies are based primarily on long-term data from Europe and North America, and we know little about the fate of tropical insect diversity. A relatively recent initiative to monitor insect diversity using a standardized protocol has been carried out in a number of tropical forests, including Asia and Central America (Lamarre et al., 2020). Despite the importance of canopy diversity, these monitoring initiatives and other monitoring programs elsewhere have not adequately addressed the spatiotemporal dynamics of canopy diversity (Lamarre et al., 2020). To effectively monitor the diversity of forest organisms, it is crucial to understand the spatiotemporal drivers of insect diversity, and monitoring protocols should be designed accordingly.
Here, we investigate the vertical stratification and spatiotemporal dynamics of insect diversity using a spatially explicit sampling protocol in two tropical rainforests at lower and higher latitudes in Thailand. We focused on three taxonomic groups (viz., Coleoptera, Hymenoptera, and two families of Lepidoptera), as these three groups represent: the largest proportions of insect diversity (Stork, 2018); nocturnal and diurnal species (Warrant & Dacke, 2011); species sensitive to environmental changes (Lamarre et al., 2020); and different compositions of feeding guilds (Coleoptera consists of mixed guilds, Hymenoptera mainly consists of parasitoids, predators, and omnivores (ants), and Lepidoptera mainly consists of herbivores) (Schowalter, 2011). Due to the extremely high diversity of Lepidoptera, we decided to focus on two moth families, Noctuidae and Nolidae, because of their ubiquity across the vertical strata (De Smedt et al., 2019).
Using these three focal insect groups, we tested the following hypotheses: 1. Insect diversity is greater in the canopy than in the understory, with more species uniquely found in the canopy; 2. The horizontal distribution of insects is more heterogeneous in the canopy than understory (i.e., beta diversity is higher in the canopy than the understory); 3. Spatiotemporal dynamics of insect diversity are more pronounced for herbivorous taxa such as Lepidoptera, as they depend on plant phenology; 4. Spatiotemporal dynamics of insect diversity are more pronounced in a higher than lower latitude forest due to stronger seasonal changes in climatic conditions. Based on the results of our study, we discuss implications for monitoring tropical forest insect diversity.
2 MATERIALS AND METHODS
2.1 Study site
Our study was conducted in two tropical rainforests distributed at lower and higher latitudes in Thailand. One rainforest site was located at Klongnaka Wildlife Sanctuary, in southern Thailand (9° 27′ N, 98° 30′ E), and the other at Mosingto, Khao Yai National Park (14° 26′ N, 101° 21′ E) in eastern Thailand. Klongnaka is a wet seasonal evergreen tropical forest with an average annual rainfall of 4067 mm (Thai Meteorological Department, https://www.tmd.go.th/en/), whereas Mosingto is a dry seasonal evergreen tropical forest with an average annual rainfall of 2338 mm (Brockelman et al., 2017). Based on available 2020 (Klongnaka) and 2019 (Mosingto) meteorological data that correspond to our sampling periods, the rainfall regime was more variable in Klongnaka, with monthly rainfall ranging from 10 mm in dry and 789 mm in wet seasons, while monthly rainfall in Mosingto ranges from 13 mm in dry seasons to 426 mm in wet seasons (Figure S1). Temperatures were more variable in Mosingto than Klongnaka, with mean daily minimum temperatures dropping below 20°C (Figure S1). Elevation ranges between 120 and 340 m above sea level in Klongnaka and between 725 and 815 m above sea level in Mosingto. The dominant tree families in Klongnaka are Dipterocarpaceae, Meliaceae, Lauraceae, and Myrtaceae, whereas the dominant families in Mosingto are Dipterocarpaceae, Myrtaceae, Rosaceae, and Annonaceae. Some canopy trees in Mosingto can reach over 40 m high; however, trees in Klongnaka are generally taller than those in Mosingto, with many canopy trees reaching 30–50 m high.
In each forest, an area of 400 × 500 m (Klongnaka) or 500 × 600 m (Mosingto) has been designated as a forest dynamics monitoring plot where forest diversity has been surveyed regularly to study spatiotemporal dynamics of forest organisms and ecological processes (Anderson-Teixeira et al., 2015). Within each forest dynamics plot, we established four adjoining 150 × 150 m square grids, and insect sampling was conducted at the intersections of the grids (N = 9) (Figure S2). Insect surveys in Klongnaka were conducted in February-March 2020 (dry season) and in September-October 2020 (wet season). Insect surveys in Mosingto were conducted in June-July 2019 (wet season) and in December-January 2019–2020 (dry season) (Figure S1).
2.2 Insect trapping and identification
At each sampling point, we employed three sampling methods to collect the three target groups of insects (Coleoptera, Hymenoptera, and Lepidoptera) from the canopy and understory forest strata (Figure S3). SLAM (Sea, Land, and Air Malaise) traps were used to sample beetles (Coleoptera) (Skvarla et al., 2020). SLAM traps were left hanging for ten consecutive days at each sampling point. Yellow pan traps were used to collect wasps, bees, and ants (Hymenoptera). Yellow pan traps were left in the field for ten consecutive days at each sampling point, and collected insects were transferred to 95% ethanol. Pennsylvania light traps modified for forest use (Kitching et al., 2005; Nakamura et al., 2016) were used to sample moths (Lepidoptera). Light traps ran for 10 h at night, from 08:00 pm to 06:00 am of the next day, for three consecutive nights. All light-trapped insects were killed on-site by dichlorvos-saturated cotton wick left inside the collecting buckets. The batteries and collecting buckets were replaced every day, and daily light trap catches were brought back to the station and stored in −20°C freezers before processing and sorting. Detailed descriptions of the three traps are provided in the Supporting Information (Trap design).
At each sampling point, we selected a tree with a canopy height of at least 12 meters to set traps. For the canopy traps, we used a giant slingshot to set up ropes on tree branches. All understory traps were suspended by ropes hung over low-hanging branches at 2 m aboveground, and canopy traps were hoised by ropes hung just below the canopy surface. Trap heights in the canopy varied between 12 and 30 m aboveground (measured by a range finder), depending on the height of the canopy surface at a given sampling point. Although the actual heights of the canopy traps varied across locations, we decided to use the same reference point (i.e., just below the forest canopy surface) rather than the same height, as the height of trees at a given location determines the nature of microhabitat conditions and insect diversity (McCaig et al., 2020). At each sampling point at a given time, we set only one of the three traps in the canopy and in the understory, so that different traps did not interfere with each other within and between vertical strata. Light traps were visited daily, while yellow pan traps and SLAM traps were visited every 5 days to check whether they were in proper working order. If traps malfunctioned due to a failed timer (for light traps), damage by arboreal mammals, or collection jars filled with rainwater, we redeployed the traps until the trapping was completed without trap malfunctioning.
We first extracted all lepidopterans from light traps, hymenopterans from yellow pan traps, and coleopterans from SLAM traps. Only adult specimens were considered in this study. We sorted specimens of the three target groups into families using Triplehorn & Johnson (2005), and, where possible, to subfamilies, genera, and species using available literature and online resources (see Table S1 for the list of references used for each of the three target groups). When specimens were not identified to described species, we gave morphospecies codes. All adult specimens of Coleoptera and Hymenoptera were identified to species or morphospecies and used for the data analyses described below, whereas adult specimens of only two Lepidoptera families (Noctuidae and Nolidae) were identified to species or morphospecies. Sexual dimorphism of the moth species was considered where possible using the literature provided in Table S1 (Moths of Thailand). Coleopterans were identified by LP, hymenopterans by PY, BB, and AR, and lepidopterans by RT and EJ. All dry and wet specimens used in this study were deposited in the Faculty of Science at Mahidol University, Thailand.
2.3 Environmental measurements
We measured elevation and temperature at each sampling point. Elevation was measured by a hand-held GPS device. Temperature (°C) and light intensity (lux) were measured using HOBO pendant data loggers (UA-200-08, Temp/light,8 K, Onset Computer Corp., Bourne, MA) attached to the ropes just above the canopy and understory traps (Figure S3D). Data loggers were set to measure temperature and light intensity every hour for the whole duration of insect sampling. Mean daily temperature and light intensity measurements in March (dry) and October (wet) 2020 were used for Klongnaka, and in July 2019 (wet) and January 2020 (dry) for Mosingto. Plant species richness was taken from the recent plant surveys (2020 in Klongnaka and 2019 in Mosingto), which identified woody plants greater than 1 cm DBH at 20 ha (Klongnaka) and 30 ha (Mosingto) forest dynamics plots. We used plant data taken from a 20 × 20 m quadrat adjacent to each of our sampling points.
2.4 Statistical analyses
Before data analyses, we pooled specimens collected from each sampling point at each stratum in each season; thus, our data consisted of a total of 72 samples (two forests × two seasons × two strata × nine sampling points). Specimens of the three target taxa (Coleoptera, Hymenoptera, and Lepidoptera) were analyzed separately. Due to substantial differences in abundance and species composition of insects collected from the two forests, we analyzed samples collected from the two forests separately. All analyses described below were performed using the packages available in R version 4.2.3 (R Core Team, 2023).
We first used the iNEXT package (Hsieh et al., 2016) to generate individual-based rarefaction curves and to calculate the total species richness (gamma diversity) and sampling coverage in the two forests. Samples collected from the canopy and understory in the wet and dry seasons were used separately to generate rarefaction curves. Rarefaction curves were based on Hill number with q = 0 (species richness). Interpolation and extrapolation of the richness with 95% confidence intervals were made using Chao richness estimation with 100-sample bootstrapping. Sample coverage is a measure of sample completeness representing the proportion of the number of individuals in a given local community represented by observed samples (Chao et al., 2014).
We tested the differences in species richness (alpha diversity), horizontal heterogeneity (beta diversity), and abundance between the vertical strata (canopy vs. understory) and seasons (dry vs. wet) within each of the two forests. Beta diversity was calculated as the measure of species turnover and nestedness, as we were interested in the complementarity of samples collected within the canopy or understory. To this end, beta diversity (measured as Bray-Curtis dissimilarity) was decomposed into turnover (β.bray.bal: the balanced variation component) and nestedness (β.bray.gra: the abundance-gradient component) components (Baselga, 2017; Baselga & Orme, 2012) using the beta.pair.abund() function in the betapart package (Baselga and Orme, 2012). The distance between each sample and its group-wise centroid was used as a measure of beta diversity.
To test the effects of vertical stratum and season, generalized linear models (GLMs, for species richness and abundance) and linear models (LMs, for beta diversity) were employed. GLMs were fitted for species richness and abundance using negative binomial error distribution using the glm.nb() function in the MASS package (Finch et al., 2014) because overdispersion was detected with the Poisson distribution. LMs were fitted for beta turnover and nestedness as the data were normally distributed. We first built a full model, which included vertical stratum, season, and their interaction as fixed effects. We also included elevation, mean temperature, light intensity, and plant species richness as additional fixed effects to test if these predictors can explain the response variables in addition to the main effects (vertical stratum and season). We ran the backward model elimination (the step() function) to test which additional predictors to be included in the final model, while keeping vertical strata, season, and their interaction. We also tested if the addition of trap height improved the model (based on AIC values), as the trap height varied among samples within the canopy. The significance of fixed effects in the final models was evaluated using Wald chi-square (for GLMs) and F-values (for LMs) calculated by the ANOVA() function in the car package (Fox & Weisberg, 2019). Post-hoc pairwise contrasts between the canopy and understory were made using estimated marginal means calculated by the emmeans package (Lenth, 2024).
To identify species that were restricted to and characteristic of a certain vertical stratum, season, or combination of stratum and season (e.g., species characteristic of the canopy but only in the dry season), we generated Venn diagrams to check how many species were unique to specific habitats and how many species were shared among them. To quantitatively identify species characteristic of certain habitats, we used the indicator value (IndVal) protocol available from the multipatt() function in the indicspecies package (Cáceres & Legendre, 2009). The IndVal protocol quantifies the relative abundance (specificity) and relative frequency (fidelity) of a species found in a given habitat (Dufrêne & Legendre, 1997) and expresses the relative importance of a species for the habitat of interest in percentage. The significance of species as indicators of certain habitats was assessed by their indicator values and associated P-values as calculated from 999 permutations of samples.
3 RESULTS
3.1 Temperature in the canopy and understory
Hourly temperatures (measured for 1 month during the surveys) substantially increased in the canopy during the day in both forests across the seasons, whereas temperatures converged at night. The coefficient of variations (CVs) in temperature was greater in the dry than wet seasons in both forests (Figure S4). The CVs were consistently greater in the canopy than in the understory within each season and forest (Figure S4). Linear models (Table S2) and post-hoc pairwise contrasts (Table S3) confirmed the significant effects of both stratum (F = 43.92, df = 1, P = <0.001 in Klongnaka, F = 24.02, df = 1, P = <0.001 in Mosingto) and season (F = 31.37, df = 1, P = <0.001 in Klongnaka, F = 212.80, df = 1, P = <0.001 in Mosingto) but no significant interaction was found between these factors.
3.2 Insect diversity in the canopy and understory
We sampled a total of 4452 insects (1,622 coleopterans, 1763 hymenopterans, and 1067 lepidopterans) representing 437 Coleoptera species, 694 Hymenoptera species, and 98 Lepidoptera species from the two tropical rainforests. Greater numbers of individuals were collected from Mosingto (1058 coleopterans, 1166 hymenopterans, and 629 lepidopterans) than from Klongnaka (564 coleopterans, 597 hymenopterans, and 438 lepidopterans). The species richness of Coleoptera and Hymenoptera (but not Lepidoptera) were greater in Mosingto (314 Coleoptera species, 524 Hymenoptera species, and 56 Lepidoptera species) than Klongnaka (185 Coleoptera species, 179 Hymenoptera species, and 61 Lepidoptera species) (Table S4). Rarefaction curves of the three taxonomic groups showed no signs of full asymptote at the end of the observed number of individuals (Figure 1). Sample coverage of Coleoptera and Hymenoptera was lower than that of Lepidoptera, and ranged between 0.43 and 0.87 (Table S4), suggesting that some data did not fully represent the local communities of Coleoptera and Hymenoptera.
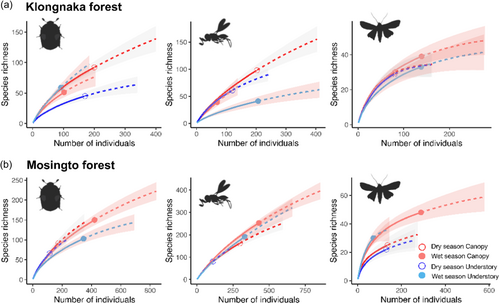
The total number of species was generally greater in the canopy than in the understory (Table S4). Rarefaction curves and their 95% confidence intervals showed that only Coleoptera showed significantly greater gamma diversity (no overlapping confidence intervals) in the canopy than in the understory in the dry season in Klongnaka (Figure 1a) and in the wet season in Mosingto (Figure 1b). Extrapolations suggested that the gamma diversity of Hymenoptera was significantly greater in the canopy than in the understory in the wet season in Klongnaka (Figure 1a).
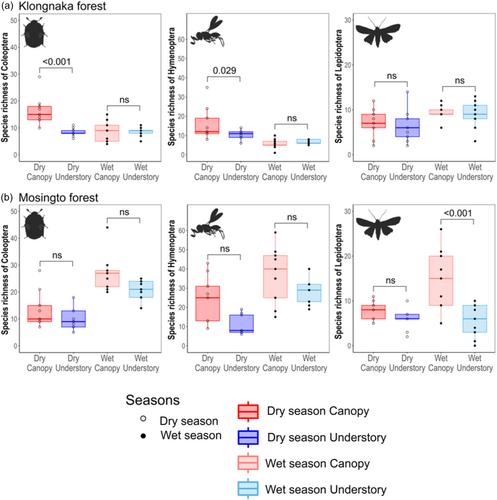
Species richness (alpha diversity) was generally greater in the canopy than in the understory across the three taxonomic groups (Figure 2). The results of GLMs (Table 1) showed the significant effects of vertical stratum on the species richness of Coleoptera in Klongnaka, Hymenoptera in Klongnaka, and Lepidoptera in Klongnaka and Mosingto (Table 1). The seasonal influence was significant for all but Hymenoptera in Klongnaka. However, we also found a significant interaction between stratum and season for Coleoptera in Klongnaka, Hymenoptera in Klongnaka, and Lepidoptera in Mosingto (Table 1). Post-hoc pairwise comparisons showed significantly greater species richness in the canopy than in the understory for Coleoptera and Hymenoptera during the dry season in Klongnaka and Lepidoptera during the wet season in Mosingto (Figure 2, Tables S5–S7). None of the pairwise comparisons showed greater richness in the understory than in the canopy. The final models included light intensity and temperature as significant predictors for Hymenoptera and Lepidoptera in Mosingto, respectively (Table 1). Trap height improved the final model for Hymenoptera in both Klongnaka and Mosingto (Table 1).
| Source of variation | Species richness (Klongnaka) | Species richness (Mosingto) | Abundance (Klongnaka) | Abundance (Mosingto) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Est. | χ2 | p | Est. | χ2 | p | Est. | χ2 | p | Est. | χ2 | p | |
| Coleoptera | ||||||||||||
| Stratum | −0.66 | 13.67 | <0.001 | −0.09 | 0.25 | 0.617 | 0.04 | 0.23 | 0.631 | 0.01 | 0.002 | 0.966 |
| Season | −0.63 | 11.50 | 0.001 | 0.73 | 53.49 | <0.001 | 0.26 | 0.36 | 0.547 | −0.55 | 16.34 | <0.001 |
| Stratum × Season | 0.64 | 8.70 | 0.003 | 0.01 | 0.01 | 0.953 | 0.09 | 0.13 | 0.718 | −0.03 | 0.01 | 0.920 |
| Elevation | - | - | - | −0.003 | 4.03 | 0.045 | ||||||
| Temperature | - | - | 0.30 | 4.06 | 0.044 | - | ||||||
| Light intensity | - | <0.001 | 2.13 | 0.145 | - | <0.001 | 0.79 | 0.375 | ||||
| Plant species | - | - | - | - | ||||||||
| Trap heighta | - | - | - | - | ||||||||
| Hymenoptera | ||||||||||||
| Stratum | −0.43 | 36.78 | <0.001 | −0.41 | 0.13 | 0.724 | −0.52 | 1.82 | 0.180 | −0.36 | 0.03 | 0.869 |
| Season | −1.04 | 3.33 | 0.068 | 0.50 | 33.00 | <0.001 | −1.07 | 1.53 | 0.210 | 0.38 | 15.26 | <0.001 |
| Stratum × Season | 0.58 | 4.91 | 0.027 | 0.51 | 4.00 | 0.045 | 1.61 | 19.12 | <0.001 | 0.56 | 4.18 | 0.041 |
| Elevation | - | - | - | - | ||||||||
| Temperature | - | - | - | 0.15 | 2.41 | 0.121 | ||||||
| Light intensity | - | <0.001 | 5.29 | 0.021 | - | <0.001 | 2.74 | 0.098 | ||||
| Plant species | −0.004 | 3.36 | 0.067 | - | - | 0.02 | 2.34 | 0.126 | ||||
| Trap heighta | + (ΔAIC = 1.6) | + (ΔAIC = 4.8) | + (ΔAIC = 0.5) | + (ΔAIC = 3.8) | ||||||||
| Lepidoptera | ||||||||||||
| Stratum | −0.35 | 5.37 | 0.021 | −0.27 | 19.26 | <0.001 | −0.33 | 3.80 | 0.051 | −0.23 | 18.62 | <0.001 |
| Season | −0.87 | 4.52 | 0.034 | 0.65 | 6.07 | 0.014 | −0.76 | 2.43 | 0.119 | 0.68 | 0.26 | 0.612 |
| Stratum × Season | −0.05 | 0.05 | 0.822 | −0.71 | 5.44 | 0.020 | −0.07 | 0.07 | 0.793 | −1.27 | 12.33 | <0.001 |
| Elevation | - | 0.01 | 2.12 | 0.146 | - | 0.01 | 4.35 | 0.037 | ||||
| Temperature | −0.38 | 8.70 | 0.003 | - | −0.43 | 7.62 | 0.006 | - | ||||
| Light intensity | - | - | - | - | ||||||||
| Plant species | - | - | - | 0.02 | 2.61 | 0.106 | ||||||
| Trap heighta | - | - | - | - | ||||||||
- Note: χ2 and p-values were calculated by ANOVA type II tests. The intercept was set as: stratum = canopy; and season = dry. Significant p-values are shown in bold.
- a + symbols indicate that the addition of trap height improved the final model with ΔAIC values in brackets.
The effect of vertical stratum on abundance was only significant for Lepidoptera in Mosingto (Table 1). Significant seasonal effects were observed for Coleoptera and Hymenoptera in Mosingto, where their abundances increased in the wet season (Figure 3b). The interaction of stratum and season was significant for Hymenoptera in Klongnaka and Mosingto, and Lepidoptera in Mosingto. Post-hoc pairwise comparisons (Tables S5–S7) showed a significantly greater abundance of Lepidoptera in the canopy than in the understory during the wet season in Mosingto, whereas a greater abundance of Hymenoptera was found in the understory than in the canopy during the wet season in Klongnaka (Figure 3). Final models included temperature as a significant additional predictor for Coleoptera and Lepidoptera in Klongnaka, and elevation as a significant additional predictor for Coleoptera in Mosingto (Table 1). Trap height improved the final model for Hymenoptera in both Klongnaka and Mosingto (Table 1).
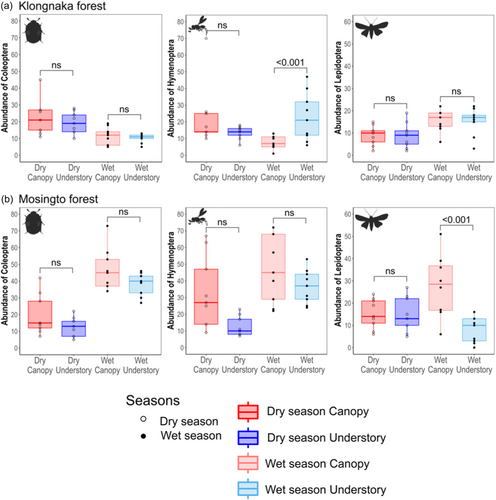
Unlike species richness and abundance, the effect of the vertical stratum on beta turnover was not significant for any of the taxonomic groups (Table S8). However, the interaction effect of stratum and season was significant for Coleoptera in Klongnaka, Hymenoptera in Klongnaka and Mosingto, and Lepidoptera in Mosingto (Table S8). Post-hoc pairwise comparisons (Tables S9–S11) showed that the beta turnover of Coleoptera was significantly greater in the canopy than understory in the dry season in Klongnaka (Figure 3a). In contrast, beta turnover of Lepidoptera was greater in the understory in the wet season in Mosingto (Figure 3b). Although some of the final models included additional environmental predictors, none of them were significant for any of the three taxonomic groups (Table S8). The addition of trap height marginally improved the final model for Coleoptera in Klongnaka (Table S8).
Similarly, the effect of vertical stratum on beta nestedness was not significant for any of the taxonomic groups (Table S8), whereas the interaction effect of stratum and season was significant for Coleoptera and Hymenoptera in Klongnaka. Post-hoc pairwise comparisons (Tables S9–S11) showed significantly greater understory beta nestedness for Hymenoptera in the wet season in Klongnaka (Figure S6A). Elevation, temperature, and light intensity were included as significant additional predictors for Coleoptera in Klongnaka, whereas light intensity and plant species richness were significant for Coleoptera in Mosingto and Hymenoptera in Klongnaka, respectively (Table S8). The addition of trap height improved the final model for Coleoptera in Klongnaka (Table S8).
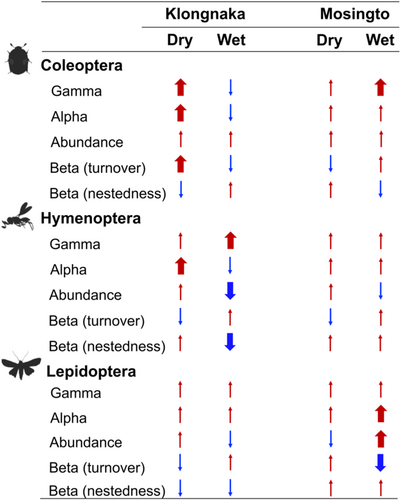
Figure 4 summarizes the differences in gamma diversity, alpha diversity, beta diversity (turnover and nestedness), and abundance of the three taxonomic groups between the canopy and understory. The overall effect of vertical stratification was inconsistent with many nonsignificant vertical trends. When significant differences were found, gamma and alpha diversities were always greater in the canopy than in the understory, whereas abundance and beta diversity were often significantly greater in the understory (Figure 4).
3.3 Indicator species
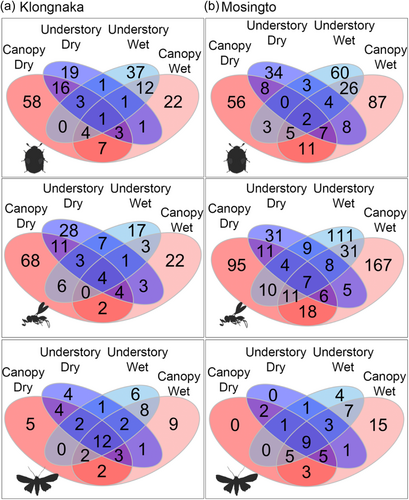
Larger numbers of species were generally restricted to the canopy than the understory for all of the three insect groups (Figure 5). Among the species whose occurrences were restricted to the canopy or understory, few of them were found throughout the two seasons (Figure 5). For Coleoptera in Klongnaka, for example, many species were restricted to the dry season canopy (58 species), dry season understory (19), wet season canopy (22), or wet season understory (37), whereas only one canopy and seven understory species were found in both seasons (Figure 5). Similarly, the indicator value protocol showed that many significant indicator species were characteristic of the canopy or understory in certain seasons, but few species were characteristic of the canopy or understory across the two seasons (Figure 4, Table S14).
4 DISCUSSION
Our study employed a spatially explicit sampling design to investigate the diversity of three insect groups between the canopy and understory across two seasons in two tropical forests. In forests, canopies provide the dominant structural influence on the microclimatic conditions, movement of insects, and the interaction between species (Schulze & Fiedler, 2003). Despite the differences in climatic conditions between the canopy and understory, we found that the overall effect of vertical stratification on insect diversity was weak and inconsistent, with significantly greater species richness (gamma and alpha diversity) in the canopy than in the understory in certain seasons and forests (Figure 4). Our results corroborate previous studies that found slightly greater richness in the canopy for beetles (Stork & Grimbacher, 2006) and hymenopterans (Sobek et al., 2009). Other studies that found substantially greater canopy richness focused on specific groups or feeding guilds of insects such as leaf beetles (Charles & Basset, 2005), bees (Ulyshen et al., 2010), and an herbivore guild (Basset et al., 2015). In contrast to these studies, Lepidoptera, one of the major herbivore guilds, did not show greater richness in the canopy except for Mosingto forest in the wet season (Figure 4). Other moth studies similarly found no vertical differences (Ashton et al., 2016; Beck & Khen, 2007) or even greater richness in the understory (Beck et al., 2002). It is also important to note that weak vertical stratification was reported from studies targeting actively flying insects (as was done in our study, see also Ashton et al., 2016), whereas much greater canopy richness was reported when utilizing other methods, such as plant beating that actively collects insects from foliage (Basset et al., 2015; Charles & Basset, 2005). The vertical stratification of flying insects may be “diluted” as the winged insects can fly across vertical strata throughout their adult life stages. That being said, across all three major insect taxa, the canopy stratum consistently presented greater gamma and alpha diversity when significant differences were found (Figure 4).
The effect of vertical stratification was further complicated by differences in seasons and forests. We hypothesized that insect diversity in the canopy increased in the wet season when more resources were presumably available in the canopy (Basset et al., 2003; Ulyshen, 2011; Wolda, 1978). We also hypothesized that seasonal influence was greater in high-latitude Mosingto. In Mosingto, the wet season significantly increased the alpha diversity of Coleoptera and Hymenoptera in both the canopy and understory (Figure 3b, Table 1), reflecting the cold seasonal influence in high-latitude tropical rainforests. Furthermore, significantly greater canopy than understory gamma (Coleoptera) and alpha (Lepidoptera) diversities were observed in the wet season, suggesting that the seasonal influence may be more consistent in high-latitude Mosingto. In contrast, Coleoptera and Hymenoptera in low-latitude Klongnaka presented significantly greater alpha diversity in the canopy during the dry season (Figure 4). These results did not follow our hypothesis (i.e., we expected stronger vertical differences in the wet season), but a study conducted in the Australian tropics also suggested that beetle diversity may not necessarily follow patterns of precipitation, temperature, or solar radiation (Grimbacher & Stork, 2009), even though insects are generally known to be sensitive to temperature fluctuations over time (Schowalter, 2011). The lack of vertical differences in the wet season may suggest that the availability of understory resources, such as saplings, seedlings, and fungi, and generally warm and humid climatic conditions may support similar diversity to that in the canopy in low-latitude tropical forests (Stork & Grimbacher, 2006). This is further reinforced by a study conducted in Bornean tropical rainforests that demonstrated that liana and tree foliage availability was not necessarily limited to the upper canopy layers, and arthropod abundance followed the vertical distribution of such resources (Dial et al., 2006).
The mechanisms of greater diversity in the canopy (or understory) can be explained by the heterogeneity of resources (the habitat heterogeneity hypothesis, MacArthur, 1965), which creates more niches for different species to coexist across space. An alternative mechanism explains that the provision of more resources, which are not necessarily heterogeneous in space, simply attracts more individuals, leading to a greater diversity of organisms (the more-individuals hypothesis, Srivastava & Lawton, 1998). The diversity of beetles in Klongnaka seems to support the habitat heterogeneity hypothesis: greater beta diversity is related to the heterogeneous distribution of beetle species that ultimately increases overall species richness (Kraft et al., 2011), while not affecting their abundance (Figure 5). However, none of our environmental variables, namely temperature, light intensity, and plant species richness, explained beetle diversity. In contrast, the significantly greater diversity of moths in Mosingto during the wet season seems to portray the more-individuals hypothesis, where the abundance of moths was significantly greater in the canopy than understory, causing the observed increase in their alpha diversity. Similar patterns were also observed for Sphigidae and Arctiinae moths in Bornean forests (Schulze et al., 2001). It is important to note that the extrapolation of the rarefaction curves indicated that the greater diversity of moths may be caused by insufficient sampling in the understory (Figure 1). However, the sample coverage of moths reached over 80% (Table 1), and the differences in abundance likely reflect the true density of moths as the sampling method and intensity were the same between the two strata.
In contrast to our hypothesis, horizontal beta diversity was not greater in the canopy than in the understory (except for Coleoptera in the dry season in Klongnaka). The lack of greater beta diversity in the canopy may be attributable to insects' low host plant specificity, as tropical forests were found to harbor phylogenetically diverse plant species that proliferated more polyphagous herbivore species (Novotny et al., 2002). Indeed, we found that the effect of plant species richness was not significant for any diversity metrics, except for the beta nestedness of Hymenoptera in Klongnaka. We also found that, across all three taxonomic groups, beta turnover was higher than beta nestedness in both the canopy and understory (Figures S5 and S6), suggesting that spatially replicated samples complement each other for capturing the horizontal heterogeneity of insect diversity. None of the environmental variables, however, explainrd the differences in horizontal beta turnover. Our results suggest that the distribution of insect diversity cannot be explained simply by the differences in temperature, light intensity (a proxy for canopy cover), or plant species richness.
We found many species whose occurrences were restricted to the canopy. However, both the canopy and understory harbored equally large numbers of unique species, suggesting that both the canopy and understory strata are important for monitoring tropical forest insect diversity (Stork & Grimbacher, 2006). Critically, most species characteristic of a particular vertical stratum were only found in the dry or wet season, suggesting that many species may be missed if the monitoring only takes place in a certain season. Our study highlights the significance of all four dimensions (3D physical space represented by the horizontal and vertical dimensions and time represented by season) for insect monitoring, even in low-latitude tropical forests where seasonal influence is considered weak. Perhaps the only exception was Lepidoptera, which presented a very small number of species characteristic of the dry season in both Klongnaka and Mosingto. This supports the justification of more season-limited studies of moths, which are often conducted by sampling in warm, wet seasons to reveal their ecological patterns (e.g., Ashton et al., 2016; Axmacher et al., 2004; Odell et al., 2016).
The lack of consistent results in our study may be attributable to our simple study design, which represented only two ends of spectrums in vertical stratification (the canopy vs. understory), and the height of canopy traps varied depending on the height of the trees and available branches on which we set the traps (following McCaig et al., 2020). The addition of trap height improved the final models for Hymenoptera species richness and abundance (both Klongnaka and Mosingto) as well as Coleoptera beta turnover and nestedness (Klongnaka), suggesting that the diversity metrics of certain insect groups may be sensitive to the height of the traps in the canopy. The changes in insect diversity across vertical gradients can be fully captured if multiple traps are set at different heights from the ground on the same trees or in the same area (e.g., De Smedt et al., 2019; McCaig et al., 2020), but this presents a tradeoff between horizontal and vertical distributions of traps given the limited resources for insect monitoring programs.
Furthermore, the lack of consistent results may be related to insufficient sampling intensity, especially for Coleoptera and Hymenoptera, whose sample coverage ranged between 42% and 87% (Table 1). Rarefaction curves of tropical insect species are unlikely to reach asymptotes, as the diversity is generally much greater than in other biomes (Ashton et al., 2015). One way to alleviate the insufficient sampling is to focus on a subset of a few common groups and exclude rarer taxa. This, however, will unavoidably cause the loss of valuable diversity information. Increased sampling intensity, however, burdens our limited resources. The use of meta-barcoding may well address this trade-off by reducing the cost of insect monitoring (Ji et al., 2013), but this promising approach is not yet as useful in tropical forests due to the lack of local DNA barcode libraries (Lamarre et al., 2020).
In conclusion, our study showed that flying insect richness was greater in the canopy than in the understory when significant differences were found. However, the overall patterns in vertical stratification were inconsistent among the insect groups, seasons, and forests. The influence of seasonality differed from our hypothesis and the observed patterns were not as clearly discernible as we hypothesized. The complex spatiotemporal dynamics of insect diversity warrant long-term monitoring of three-dimensional insect diversity across seasons in tropical forests.
AUTHOR CONTRIBUTIONS
Laksamee Punthuwat: Data curation; formal analysis; methodology; software; validation; visualization; writing—original draft; writing—review and editing. Ronnarot Taveesri: Data curation; formal analysis; methodology; software; validation; visualization; writing—review and editing. Alyssa B. Stewart: Conceptualization; data curation; funding acquisition; methodology; project administration; resources; supervision; writing—review and editing. Ponrujee Yongsiri: Data curation; methodology; validation; writing—review and editing. Achariyaporn Janmaneeporn: Data curation; investigation; methodology; resources; writing—review and editing. Pattharawadee Waitayachart: Data curation; investigation; methodology; project administration; resources; writing—review and editing. Pitoon Kongnoo: Investigation; methodology; resources; writing—review and editing. Alexey Reshchikov: Data curation; investigation; methodology; writing—review and editing. Mark Jun M. Alcantara: Formal analysis; software; supervision; writing—original draft; writing—review and editing. Min Cao: Conceptualization; methodology; project administration; supervision; writing—review and editing. Luxiang Lin: Conceptualization; funding acquisition; methodology; project administration; supervision; writing—review and editing. Benjamin David Blanchard: Data curation; formal analysis; software; supervision; validation; writing—original draft; writing—review and editing. Ekgachai Jeratthitikul: Conceptualization; data curation; funding acquisition; investigation; project administration; resources; supervision; writing—review and editing. Akihiro Nakamura: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; software; supervision; validation; writing—original draft; writing—review and editing.
ACKNOWLEDGMENTS
We thank the Chinese Academy of Sciences Southeast Asian Biodiversity Research Institute (SEABRI, grant number: Y4ZK111B01) and Biodiversity Information Fund for Asia (BIFA), the Global Biodiversity Information Facility (GBIF, BIFA5_026) for providing financial support. AN was supported by the National Natural Science Foundation of China International (Regional) Cooperation and Exchange Project (32161160324) and the 14th 5-Year Plan of Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences (E3ZKFF1K). LL was funded by Southeast Asia Biodiversity Research Institute, Chinese Academy of Sciences (151C53KYSB20200019). We thank Cheng Wenda, Putita Jiranuntskul and Kongkit Macharoenboon for assistance with statistical analyses, laboratory and fieldwork. We acknowledge the Royal Thai Forest Department, the National Parks Wildlife and Plant Conservation Department, the National Research Council of Thailand (NRCT), the National Center for Genetic Engineering and Biotechnology (BIOTEC), the Department of Forest Biology, Faculty of Forestry, and the Andaman Coastal Research Station for Development, Faculty of Fisheries, Kasetsart University for their support and collaboration.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
The ethical clearance was approved by the Faculty of Science, Mahidol University, Thailand (MUSC61-030-432).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in the Global Biodiversity Information Facility at https://doi.org/10.15468/92ca8u (Coleoptera), https://doi.org/10.15468/bne2yc (Hymenoptera), and https://doi.org/10.15468/yge43c (Lepidoptera).




