Rosettes in a matrix: Predicting spatial variation in density of a large felid in a forest-production mosaic
多用途景观中的“玫瑰花纹”:预测森林-生产镶嵌区大型猫科动物密度的空间变化
Editor-in-Chief & Handling Editor: Ahimsa Campos-Arceiz
Abstract
enLarge carnivores are keystone for ecosystems and flagships for conservation efforts but face severe threats globally. Protected areas are vital for the conservation of these charismatic species along with a host of ecological processes. However, the extent and scope of protected areas for conservation of all threatened species is limited, especially in the global south. Considering larger landscapes that can be compatible with large carnivore conservation goals is an alternative approach to ensure their persistence. This study explores the potential of multi-use landscapes for the persistence of a globally threatened large felid, the Indian leopard (Panthera pardus fusca). This study investigated the spatial variability of leopard densities across a land-use gradient using spatially explicit capture-recapture framework in a tea-plantation-dominated forest-production landscape mosaic in the Duars region of northeastern India. While the density of leopards in this landscape was estimated to be 7.96 ± 1.56 (SE) per 100 km2, significant (p = .048, t = 2.02, df=61) differences in estimates were observed between tea-plantations (11.53 ± 2.72 (SE) leopards per 100 km2) and the forested habitats (4.67 ± 2.07 (SE) per 100 km2). Densities between tea plantations and protected areas (a subset of the forested habitat) were found to be comparable (9.19 ± 4.55 (SE) per 100 km2). The study posits that conservation-compatible land use in landscapes shared with people can host a higher density of adaptable large felids like leopard than forested areas and that conservation planning needs to move beyond the dominant PA-centric paradigm. The study also reinforces the importance of multi-use landscapes for wildlife conservation, especially for an adaptable large felid.
摘要
zh大型食肉动物是生态系统的基石和保护的旗舰物种,但在全球范围内面临着严重威胁。保护区对于保护这些富有魅力的物种以及一系列生态过程至关重要。然而,用于保护所有濒危物种的保护区的范围和程度有限,尤其是在南半球地区。考虑与大型食肉动物保护目标相适应的更大范围的景观,是确保大型食肉动物持续生存的另一种方法。本研究探讨了全球濒危大型猫科动物印度豹(Panthera pardus fusca)在多用途景观中的生存潜力。本研究采用空间显性捕获再捕获框架,在印度东北部杜拉斯(Duars)地区以茶叶种植为主的森林-生产景观镶嵌体中调查了豹密度在土地利用梯度上的空间变化。据估计,该景观中的豹密度为每100平方公里7.96 ± 1.56(SE)只,茶叶种植园(每100平方公里11.53 ± 2.72(SE)只)与森林栖息地(每100平方公里4.67 ± 2.07(SE)只)之间的豹密度有显著差异(p = .048,t = 2.02,df=61)。茶种植园和保护区(森林栖息地的一个子区域)之间的密度相当(每 100平方公里 9.19 ± 4.55 (SE))。本研究认为,与森林地区相比,与人类共享的景观中与保护相容的土地利用,可以承载更多适应性强的大型猫科动物,如豹,因此保护规划需要超越以保护区为中心的主流范式。研究还强调了多用途景观对野生动物保护的重要性,尤其对于适应性较强的大型猫科动物。【审阅 :周聪】
Plain language summary
enLarge carnivores are often considered to be flagships of ecosystems. Their density estimates are often regarded as an effective tool for measuring the success of conservation efforts allocated to a specific region. Protected areas play a significant role in the conservation of large carnivores. However, comprising only 16% of the global landmass, the extent to which protected areas can serve this purpose is limited, especially in the global south. Globally, human-dominated areas are being reported to be conservation landscapes for adaptable species, including large-bodied carnivores. In the present study, we explored the potential of human-dominated tea-production lands to serve as conservation areas for the Indian leopard, a globally threatened large felid. Our results suggest that tea production areas support higher densities of the species than forests of the same region. The results highlight the need to consider human-dominated areas with suitable land use as conservation-compatible lands. Our study also underscores the need to move beyond the protected area-centric paradigm of wildlife conservation to foster human-wildlife coexistence in shared landscapes.
zh
大型食肉动物通常被认为是生态系统的旗舰物种。其密度估算常被认为是衡量特定区域的保护工作是否成功的有效工具。保护区在大型食肉动物保护中发挥着重要作用。然而,由于保护区仅占全球陆地面积的16%,其效力范围有限,尤其是在南半球地区。全球范围的报道发现,人类主导的区域也是包括大型食肉动物在内的适应性物种的保护地。在本研究中,我们探索了人类主导的茶叶种植地作为印度豹(全球受威胁的大型猫科动物)的保护区的潜力。结果表明,茶叶种植地比同一地区的森林能够承载更高的印度豹种群密度。研究结果强调,有必要将人类主导的适宜土地利用的地区视为保护相容的土地。进一步,我们需要重新思考以保护区为中心的野生动物保护范式,在共享的景观中促进人类与野生动物的共存。
Practitioner points
en
-
Globally, there is increasing evidence of large carnivore presence in human-dominated multi-use landscapes.
-
The study attempts to predict spatial variation in the densities of an adaptable large carnivore in a forest-human use landscape mosaic.
-
Human-use areas overshadow the forested areas in terms of leopard density, highlighting the immense conservation potential of human-dominated landscapes having suitable land use and management regimes.
实践者要点
zh
-
在全球范围内,越来越多的证据表明大型食肉动物生存在人类主导的多用途土地上。
-
本研究试图预测在森林和人类利用的景观镶嵌体中可适应的大型食肉动物密度的空间变化。
-
豹在人类活动区的密度超过了森林区,这凸显了人类主导的景观在适当的土地利用和管理制度下具有巨大的保护潜力。
1 INTRODUCTION
Large-bodied felids, owing to their charismatic nature, ecological role and profound cultural veneration, have always been under immense research focus (Chaudhary et al., 2019). They play vital roles in maintaining the ecosystem's health and function as flagship species of their habitat (Ripple et al., 2014). Over the past century of the Anthropocene, many large-bodied carnivore species have experienced a drastic reduction in their global population and distribution (Ripple et al., 2014). Conventional conservation and wildlife management planning revolved around earmarking a piece of habitat as a protected area for wildlife to roam around without considering any possibility of human-wildlife co-occurrence in shared spaces (Woodroffe, 2000). However, protected areas account for only 16% of the world's total land area (Palfrey et al., 2022) and even less in developing countries (Ghosh-Harihar et al., 2019). Even though protected areas play a crucial role in maintaining the population of endangered animals, they fail to conserve large-bodied species if the area is too small to meet the ecological needs of wide-ranging species (Brashares et al., 2001; Ghosh-Harihar et al., 2019; Packer et al., 2013). Moreover, increasing developmental pressures on protected areas, especially in the global south further threaten the paradigm of protected areas as inviolate parks (Ghosh-Harihar et al., 2019). Conversely, the PA-centric conservation paradigm automatically excludes shared landscapes that hold the potential to conserve biodiversity along with human lives and livelihoods.
Production landscapes with appropriate management regimes can host biodiversity beyond the protected area boundaries (Ghosh & Basu, 2022; Wright et al., 2012). There is increasing evidence of the survival and persistence of carnivores in human-modified multi-use landscapes across their global distribution (Alexander et al., 2016; Bateman & Fleming, 2012; Kshettry et al., 2020; Majgaonkar et al., 2019; Riley et al., 2021; Schuette et al., 2013; Valeix et al., 2012; Warrier et al., 2020; Wilmers et al., 2013). While it creates opportunities for wildlife conservation, it presents challenges in terms of spatial and temporal overlap between humans and wildlife that can lead to negative interactions between the two. Hence, carnivore conservation now calls for a landscape-level approach by considering spaces beyond protected area boundaries while proposing wildlife management plans that consider larger landscapes that are shared with people. However, this approach is still largely missing from traditional wildlife and conservation management plans. Moreover, the current ecological knowledge about large carnivores is primarily based on the information gathered from studies conducted within protected areas, and, therefore, may be inadequate to propose carnivore management strategies outside protected areas (Ghosal et al., 2013).
Globally, several large carnivore species have increased their range in the recent past and are distributed well beyond the boundaries of protected areas (Chapron et al., 2014; López-Bao et al., 2017). Mountain lions (Puma concolor) and Indian leopards (Panthera pardus fusca) have been known to persist in densely populated peri-urban and urban areas (Riley et al., 2021; Surve et al., 2022). Even with a high density of humans, production landscapes in India have been known to provide refuge for large-bodied carnivores (Athreya et al., 2013; Kshettry et al., 2020; Warrier et al., 2020). In the presence of abundant prey base, vegetation cover, and compatible land-use practices, leopards are known to persist in areas with high human densities (Jacobson et al., 2016; Kshettry et al., 2020). However, the relative importance of natural and shared (human-dominated) landscapes has rarely been investigated for large carnivores leading to a gap in knowledge of how population densities vary across such landscapes. Understanding such variation in densities across shared landscapes and ‘natural habitats’ for adaptable large carnivores could have widespread implications for their conservation as well as to devise appropriate conflict mitigation measures. The leopard is an ideal model species to study the effects of land use on its density owing to its widespread distribution and adaptability (Jacobson et al., 2016). This study attempts to understand the variation in leopard density across a land-use gradient to understand the relative importance of natural habitats (with varying protection regimes) and habitats shared with people. The paper also discusses the need to balance human safety and carnivore conservation in shared landscapes for equitable and inclusive conservation models.
In India, leopards mostly share their space with the other charismatic large carnivore, the Bengal tiger (Panthera tigris tigris), in most protected areas (Chaudhary et al., 2019). Hence, most of the information available about the ecology of leopards is a bycatch of studies focused on tigers, and they mainly deal with the estimation of abundance (Harihar et al., 2011; Jhala et al., 2008; Kalle et al., 2011). Very few studies have been focused on the species ecology (but see Borah et al., 2013; Mandal et al., 2017; Noor et al., 2020; Surve et al., 2022; Thapa et al., 2014), and fewer attempted to study their ecology in human-modified multi-use landscapes despite a recent emphasis (Athreya et al., 2016; Gubbi et al., 2020; Kshettry et al., 2018, 2020; Majgaonkar et al., 2019; Naha et al., 2021; Sidhu et al., 2017). However, no study has attempted to understand the variation in leopard densities across land-use gradients from the same landscape thereby leaving a lacuna which has been addressed by the current study which was carried out in a forest-production landscape mosaic.
The Duars region of northern West Bengal represents a heterogeneous matrix of protected areas, reserve forests, tea estates, agricultural fields, human settlements, and waterbodies. The region has been proven to be extensively used by leopards to reside, reproduce, and hunt (Kshettry et al., 2017; Naha et al., 2020). Thus, the landscape was deemed appropriate to investigate the habitat factors influencing the species density in this shared landscape. The study also provides a framework for estimating carnivore population density in shared landscapes by incorporating the knowledge and experiences of local communities and stakeholders into camera-trapping methodology. The study investigates the relative importance of natural and shared spaces for conserving a threatened and charismatic felid through estimating their densities across a land-use gradient.
2 MATERIALS AND METHODS
2.1 Study area
The study was conducted in an area of ~400 km2 situated in the Duars region of northern West Bengal (Figure 1). The region constitutes a portion of the East-Himalayan Biodiversity Hotspot and is historically represented by naturally occurring Sal (Shorea robusta) and Simul (Bombax ceiba) dominated forests (Champion & Seth, 1968; Myers et al., 2000). The region is represented by two major forest types: Northern Tropical Semi-evergreen forests and Tropical Moist Deciduous forests, along with some remnant patches of Eastern Alluvial Grasslands (Bhattacharjee & Parthasarathy, 2013; Roy, 2014). Apart from naturally occurring vegetation, artificial monoculture plantations of several economically important species like sal (Shorea robusta), teak (Tectona grandis), and rubber fig (Ficus elastica) are present in a patchy distribution within the entire landscape (Roy, 2009). During the late 1800s, vast stretches of these forests were converted to tea plantations by the British Colonials (Kshettry et al., 2018). Presently, the remaining forest patches stand isolated, interspersed with numerous tea estates, agricultural fields, and human habitations (Kshettry et al., 2020). Currently, a significant portion of the naturally occurring forests is represented by two protected areas, namely Chapramari Wildlife Sanctuary (9.5 km2) and Gorumara National Park (80 km2), and the rest falls under the Reserve Forests of Jalpaiguri Territorial Division. The protected areas and Reserve Forests in the study area have differing protection and human activity regimes and hence, were included as two separate classes of ‘natural habitats’ for the leopard density estimation. The protected areas have lower human activity and higher habitat management and wildlife conservation efforts compared to the reserve forests.
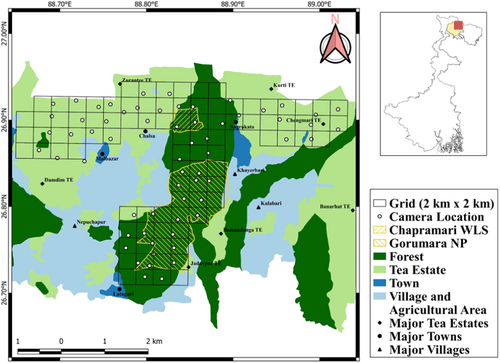
As per the 2011 census report, the region has a population density of 701 persons per km2 (According to Census of India, 2011, https://jalpaiguri.gov.in/district-statistics/). The daily per-capita income of people is often less than 1 USD, and agriculture and livestock rearing constitute the most predominant source of livelihood apart from tea production (Kshettry et al., 2017). Leopard is the apex predator of the landscape and uses both forests and tea estates as its habitat (Kshettry et al., 2017; Naha et al., 2021). The last confirmed tiger sighting in the study area was in the 1990s and subsequently, there was one tiger photo-captured in Gorumara National Park in 2020, the tiger was moving from Buxa Tiger Reserve to Mahananda Wildlife Sanctuary. However, neither of these two protected areas has an established tiger population.
Other species from the area include Asian elephant (Elephas maximus), gaur (Bos gauras), one-horned rhinoceros (Rhinoceros unicornis), sambar (Rusa unicolor), chital (Axis axis), muntjac (Muntiacus muntjac), rhesus macaque (Macaca mulata), wild boar (Sus scrofa), jungle cat (Felis chaus), leopard cat (Prionailurus bengalensis) and Indian peafowl (Pavo cristatus) (Bhattacharjee & Parthasarathy, 2013).
2.2 Data collection
Carnivore population estimation using the photographic capture-recapture technique was first implemented in India for tigers in Nagarhole National Park (Karanth, 1995). Since then, with the slightest of modifications, this method has been widely used to estimate densities of animals bearing distinct natural markings like tiger (Panthera tigris) (Karanth et al., 2006; Rather et al., 2021), lion (Panthera leo) (Elliot & Gopalaswamy, 2017; Gogoi et al., 2020), snow leopard (Panthera uncia) (Alexander et al., 2015; Sharma et al., 2021), jaguar (Panthera onca) (Maffei et al., 2004; Silver et al., 2004), leopards (Athreya et al., 2013; Harihar et al., 2009; Surve et al., 2022), ocelots (Leopardus pardalis) (Maffei & Noss, 2008; Trolle & Kéry, 2003), hyena (Hyaena sp.) (Singh et al., 2014) as well as of species which lack distinct natural marks, such as rusty-spotted cat (Prionailurus viverrinus) (Chatterjee et al., 2020) and dhole (Cuon alpinus) (Punjabi et al., 2022).
The entire study area was divided into grid cells of 2 km × 2 km. For grid cells with forest cover, we determined potential camera trap locations based on information gained from exploratory sign surveys (Karanth & Nichols, 1998), prior knowledge of the site and information from the forest officials. We conducted preliminary sign surveys to identify all possible trails frequently used by leopards for the tea estates. Due to heavy traffic of people and vehicles, it becomes hard to detect animal signs in tea-estate trails. Hence, we consulted with the tea estate authorities, workers and residents to identify the areas of frequent leopard sightings. Field visits were conducted with staff of the respective tea estate to note areas where leopards are frequently seen and where previous incidents of human injuries due to leopards have occurred. Some residents and the guards of the estates were involved to ensure the safety and security of the cameras. The purpose of placing the cameras was also explained to the workers so that there are no local apprehensions regarding the presence of cameras in their workplace. Furthermore, we also explained best practices that can be adopted to avoid accidental encounters with leopards. Three local community members were also engaged in regular monitoring and maintenance of the cameras and in downloading the images regularly. The dry season in the study area overlapped with the pruning season of the tea bushes, leading to the loss of vegetation cover, which leads to lesser leopard activities in the pruned portions (Kshettry et al., 2017; Naha et al., 2021). Hence, we avoided sections earmarked for pruning while selecting camera locations. The sampling area was divided into four blocks, and the exercise was carried out from December 2021 to March 2022 in four trapping sessions of 20 days each. A total of 63 trap locations were used to sample the four blocks. Block 1 had 14 locations, Block 2 had 15 locations, Block 3 had 23 locations, and Block 4 had 11 locations. The average spacing between all the trapping sites was 1.8 km, which ensured that all leopard individuals had an equal probability of getting photo-captured without leaving major holes in the trap array (Athreya et al., 2013; Balme et al., 2009; Odden & Wegge, 2005). Each site was provided with two camera traps deployed opposite each other to obtain photographs of both the flank of the animal (Karanth & Nichols, 1998). Cameras deployed within the forests were kept operational for 24 h daily and checked every 7-10 days. Camera traps operating within tea estates were attended to daily and were removed at 06:00 h from the location and reinstalled at 17:00 h at the same place to avoid damage and theft (Athreya et al., 2013; Surve et al., 2022).
We (AP and NK) manually tagged and classified all the photographs using Digikam 7.0.0 software. To maintain sampling homogeneity between forests and tea estates, we considered 17:00 to next morning 06:00 to be one trap night and only used the photographs captured between this time window for further analysis. Photographs with any distortions or lack of clarity were discarded from any further use. A single author (AP) manually identified all the photo-captured leopards and prepared a capture history file. Due to technical issues, both flanks of a few individuals could not be obtained. Hence, we used only right-flanked images to primarily identify individuals as it was higher in the number unless the left flank matched with a previously photographed leopard whose both flanks were profiled (Athreya et al., 2013; Harihar et al., 2009).
2.3 Analysis
Each trap night was treated as an independent sampling occasion (Otis et al., 1978). Data from all four blocks were pooled together to construct individual capture histories. The cumulative number of leopard individuals captured was plotted against the camera trap occasions to check for sampling adequacy. We used sequential photo-captures of individual leopards across locations to examine the extent of movement. The analysis was done using the package “secr” (Efford, 2023) v4.5.10 in Program R (R Core Team, 2018). The “suggest.buffer” function was used to create a habitat mask around the trap sites over which the density would be estimated (Sharma et al., 2021). This function uses the movement patterns of individual leopards from the capture history to predict a buffer width, which ensures that any animal having activity centres beyond that distance from the outermost trap will have a negligible probability of getting photo-captured in any of the detectors. All the non-habitat areas, like towns and villages, were removed from the mask layer. Agricultural lands, although known to constitute habitats for large carnivores in parts of the country (Warrier et al., 2020), were also removed from the final mask layer as during the study period, there were no standing crops in these areas to provide cover to the leopards. Furthermore, a previous study in the landscape found a low habitat-use probability of leopards in agriculture fields in the region (Kshettry et al., 2020).
Null models (D~1, g0~1, sigma~1) were fitted with three different detection functions, that is, half normal (HN), negative exponential (EX) and hazard rate (HR). Among the null models, four models were tested for the study, that is, null model (D~1, g0~1, sigma~1), global learned response model (D~1, g0~b, sigma~1), site-specific learned response model (D~1, g0~bk, sigma~1) and 2-part individual heterogeneity model (D~1, g0~h2, sigma~1). Global learned response model (g0~b) assumes that the detection probability of all the individuals changes after their first capture (Karanth & Nichols, 1998; Rather et al., 2021). Site-specific learned response model (g0~bk) is a modified version of it and assumes that individuals respond differently at each site after their first capture, that is, detection probability varies across sites along with a behavioural response to being captured for the first time (Rather et al., 2021). Individual heterogeneity model (g0 ~ h2) considers the variation in capture probability from one individual to another, without assuming any behavioural response to the first capture (Karanth & Nichols, 1998).
2.4 Covariate development
The leopard population density in the study area was modelled as a function of ecological and anthropogenic covariates. We referred to available literature to choose suitable covariates and hypothesize their possible relationships with leopard density (Supporting Information Table S2). A habitat mask layer of ~6 km was prepared using the “secr” package (Efford, 2023) v4.1.2 in R (R Core Team, 2018). All the points of the mask layer were given their land use-land cover identity, and the non-habitat points were removed to prepare the final mask layer (Supporting Information Figure S4).
We tested the influence of five covariates on the detection probability (g0). Detection probability (g0) was hypothesized to be a function of Euclidian distance to nearest waterbody, nearest human settlement and nearest road from each camera trap location. Proportions of the total operational trap night of each camera trap location on which humans and prey species were photo-captured were also added as a covariate of detection function (g0). We modelled leopard density (D) using three covariates (Supporting Information Table S2). Euclidian distance of the nearest waterbody and nearest human settlement from each point of the mask layer. QGIS v3.16.15 was used to estimate all the distances. We standardized all covariate values before using them in the model.
Habitat type was added as a covariate to estimate density separately for entire forested habitat (protected area + reserve forests) and tea estates (Supporting Information Figure S4). Furthermore, only the protected areas were included as another habitat type, which was a subset of the forested habitat, and we compared the density between the tea plantations and protected forests. Akaike Information Criterion corrected for small sample size (AICc) was calculated for all the competing models, and the model with a better relative fit in each case was selected based on the difference between the corresponding AICc values (∆AICc) (Burnham & Anderson, 2001).
3 RESULTS
3.1 Leopard photo capture
A total of 71 photographic detections of 32 individuals were obtained during the period of 1205 trap nights. Camera traps at two locations became nonfunctional due to theft. Some of the cameras were damaged by elephants, while some appeared nonfunctional for a few nights due to technical errors. Out of 63 camera locations, 35 had leopard detections.
The number of unique individuals detected did not reach an asymptote during the study period (Supporting Information Figure S2). However, even after a prolonged sampling effort, it is unlikely to capture all the individuals present in a landscape (Sharma et al., 2010). The extent of movement determined based on photo-captured individuals showed that almost 77% of all movements occurred within the range of 0–3000 m from focal camera traps (Supporting Information Figure S3). A relatively low percentage (7.7%) of all movements occurred beyond 6000 m. The “suggest.buffer” function in “secr” produced a buffer of 6075 m around each camera trap. Altogether, it created a mask layer with an area of 1036.31 km2. All the non-habitat areas removed from the suggested mask layer finally yielded a state space with a sampling area of 759.64 km2 (Supporting Information Figure S4). The state space comprised 416.23 km2 of tea-plantations and 343.41 km2 of forested area (comprising of Reserve forests and protected areas. The area under protected area habitat (a subset of the forested area) was 82.4 km2.
3.2 Model fitting and density estimation
Models were fitted under the assumption of a null model using three different detection functions, that is, HN, EX and HR, to estimate the density. The HN model had the lower AICc score and was used for the subsequent model-building process (Supporting Information Tables S3 and S4; Supporting Information Figure S5).
The density estimation models have two key parameters, first is the detection probability (g0) and the second is the density (D). The models were built in two steps where in the first step, variables that affected detection probability were identified and then these predictors were kept constant while identifying the best predictors of density. The set of models included the null model (D~1, g0~1, sigma~1) and three models hypothesized to influence the detection function (g0): (a) global learned response model (D~1, g0~b, sigma~1), (b) site-specific learned response model (D~1, g0~bk, sigma~1), and (c) 2-part individual heterogeneity model (D~1, g0~h2, sigma~1). The estimated density of leopards using the Null model was found to be 7.96 ± 1.56 per 100 km2 (Mean ± SE) (Supporting Information Table S5). The 2-part individual heterogeneity model (h2) was the second-ranked, following the null model (Table 1). As none of the other covariates influenced the detection function significantly (Supporting Information Table S6), the null model of detection function (g0~1) was retained following the principle of parsimony.
| Model | detectfn | npar | logLik | AIC | AICc | dAICc | AICcwt |
|---|---|---|---|---|---|---|---|
| D~1 g0~1 sigma~1 | halfnormal | 3 | −161.1481 | 328.296 | 329.153 | 0.000 | 0.7721 |
| D~1 g0~h2 sigma~1 pmix~h2 | halfnormal | 5 | −159.6429 | 329.286 | 331.593 | 2.440 | 0.2279 |
| D~1 g0~bk sigma~1 | halfnormal | 4 | −359.9167 | 727.833 | 729.315 | 400.162 | 0.0000 |
| D~1 g0~b sigma~1 | halfnormal | 4 | −359.8814 | 727.763 | 729.244 | 400.091 | 0.0000 |
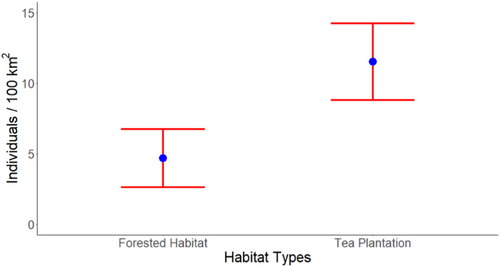
In the second step category, the density (D) was hypothesized to be influenced by additive effects of three habitat covariates- Habitat Type, Distance to Waterbody, and Distance to Human Settlements. AICc scores comparison suggested the top model for density to be a function of both Habitat Type and Distance to Human Settlements, followed by the one with Habitat Type alone (Table 2). The model with Habitat Type as a covariate of density revealed that leopard density was highest in tea estates with an estimate of 11.53 ± 2.72 per 100 km2 (Mean ± SE). Estimated density for the forested area was 4.67 ± 2.07 per 100 km2 (Mean ± SE) (Table 3, Figure 2). Density for the protected areas (a subset of the forested habitat) was estimated to be 9.19 ± 4.55 per 100 km2 (Mean ± SE) (Table 3).
| Model | detectfn | npar | logLik | AIC | AICc | dAICc | AICcwt | |
|---|---|---|---|---|---|---|---|---|
| Habitat type + distance to human settlement | D~HabType + DisHS g0~1 sigma~1 | halfnormal | 5 | −157.1902 | 324.380 | 326.688 | 0.000 | 0.4107 |
| Habitat type | D~HabType g0~1 sigma~1 | halfnormal | 4 | −159.5617 | 327.123 | 328.605 | 1.917 | 0.1575 |
| Distance to waterbody | D~DisWB g0~1 sigma~1 | halfnormal | 4 | −159.7859 | 327.572 | 329.053 | 2.365 | 0.1259 |
| Null | D~1 g0~1 sigma~1 | halfnormal | 3 | −161.1481 | 328.296 | 329.153 | 2.465 | 0.1197 |
| Global | D~HabType + DisWB + DisHS g0~1 sigma~1 | halfnormal | 6 | −157.1211 | 326.242 | 329.602 | 2.914 | 0.0957 |
| Habitat type + distance to waterbody | D~HabType + DisWB g0~1 sigma~1 | halfnormal | 5 | −159.3184 | 328.637 | 330.945 | 4.257 | 0.0489 |
| Distance to human settlement | D~DisHS g0~1 sigma~1 | halfnormal | 4 | −160.8896 | 329.779 | 331.261 | 4.573 | 0.0417 |
- Note: HabType = Habitat type, DisHS = distance to nearest human settlement, DisWB = distance to nearest waterbody. Three model parameters are D = density per 100 km2, g0 = probability detection and sigma = distance over which detection probability gradually decreases.
| Habitat | Effective sampling area (km2) | Density | SE | lcl | ucl |
|---|---|---|---|---|---|
| Protected area | 82.4 | 9.19 | 4.55 | 3.67 | 23.02 |
| Tea estate | 416.23 | 11.53 | 2.72 | 7.31 | 18.21 |
| Forested habitat | 343.4 | 4.67 | 2.07 | 2.03 | 10.72 |
4 DISCUSSION
We estimated leopard densities from a tea-plantation-dominated landscape of north-eastern India and investigated the reasons for the spatial variability in density. The study also underscored including local knowledge for carrying out such large-scale exercises in human-dominated landscapes. Our density models indicated that variation in the leopard density was a function of land-use type. The best-fit model also included distance to human settlements, indicating fine-scale patterns of leopard density within the different habitat types. Distance to human settlements was found to be negatively correlated with leopard density (Supporting Information Figure S6). This can result from the high prey availability near the human settlements in the form of livestock. In the landscape, domestic prey makes up almost 60% of the prey biomass in the leopard's diet (Kshettry et al., 2018).
Habitat-specific density estimate showed leopard density in the tea estate to be 11.53 ± 2.72 leopards per 100 km2 (Table 3). The only other leopard density estimation study from a human-dominated multi-use landscape reported a density of 4.8 ± 1.2 individuals per 100 km2 (Athreya et al., 2013) which is much less than the current estimate. The current estimate of leopard density in tea estates of the study area is even greater than the same from many of the protected areas of the country, such as Achanakmar Tiger Reserve, Bandhavgarh Tiger Reserve, Kanha Tiger Reserve, Kaziranga Tiger Reserve, Manas National Park, Nagarhole Tiger Reserve, Tadoba Andhari Tiger Reserve and Pakke Tiger Reserve and Tungareshwar Wildlife Sanctuary (Qureshi et al., 2024; Rather et al., 2021; Surve et al., 2022). Only a few other protected areas in the country, such as Bhadra Tiger Reserve, Katarniaghat Wildlife Sanctuary, Panna Tiger Reserve, Pench Tiger Reserve, Rajaji Tiger Reserve, Sariska Tiger Reserve, and Sanjay Gandhi National Park (Qureshi et al., 2024; Surve et al., 2022) have been known to have a comparable or greater density of leopards than this estimate. However, this comparison is relevant only to underscore the conservation potential of a production landscape mosaic for leopards and exploring the reasons for this variation in densities is beyond the scope of this study. However, we can speculate that the variation in densities discussed in this and subsequent sections can be a function of the variety of habitats in these areas, prey availability, and the presence of sympatric carnivores. Even within the study area, spatial variation in leopard density was observed across land-use types.
Density of leopards within the forested areas (reserve forests and protected areas) was significantly lower than in the tea plantations. This could be speculated to result from very low prey densities in the reserve forest areas. Reserve forests of the region experience a greater extent of deforestation, greater anthropogenic pressure and scarcity of prey animals, which can make them incapable of supporting a substantial population of large carnivores or herbivores (Nagendra et al., 2009). Despite that, the density is still greater than the estimates reported from protected areas such as Dachigam National Park, Kaziranga Tiger Reserve, Kudremukh National Park, and Nameri Tiger Reserve (Noor et al., 2020; Qureshi et al., 2024).
The higher density of leopards in this landscape can be attributed to the absence of tigers. Leopards tend to occur at greater density in the absence of tigers, which often diminishes once the tiger is reintroduced (Harihar et al., 2011; Mondal et al., 2012). However, some of the country's highest estimates of leopard density have been reported from a landscape where tigers and leopards co-occur (Kalle et al., 2011; Qureshi et al., 2024). Similarly, the lowest leopard density estimate has been reported from a region where tiger does not occur, though another sympatric species, the snow leopard (Panthera uncia), is present in the landscape (Noor et al., 2020). Our findings from the protected areas, however, stand in contrast with estimates from the country-wide leopard population survey (Qureshi et al., 2024) but are in conjunction with estimates from a previous study carried out in the same landscape (Rawat & Sathyakumar, 2020). Most previous studies have only considered the protected areas in the landscape as leopard habitats. However, leopards in our study site are known to occur over the larger landscape beyond the protected area boundaries (Kshettry et al., 2017, 2020). Hence, we speculate that the buffer sizes may have been inadequate for those studies, leading to a positive bias in the density estimates. Our study addresses this bias of the previous estimates by sampling across different land-cover types in the landscape.
Another possible reason for such a high density of leopards is prey availability at the study site. Large-bodied carnivores can survive in areas closer to high anthropogenic disturbance if the prey availability is sufficient (Alexander et al., 2016). In human-dominated areas, if the availability of wild prey species is limiting, leopards are known to prey on livestock and free-ranging dogs, which is one of the reasons that makes them capable of inhabiting human-use areas (Athreya et al., 2016; Kshettry et al., 2018; Shehzad et al., 2015). Livestock availability in the tea plantations of this region is up to seven times higher than wild prey availability inside the forests (Kshettry et al., 2018). Estimating the relative availability of prey using their detection in the camera traps was beyond the scope of the current study due to the inability to keep the cameras operational in the tea estates during the daytime when livestock are most active. Moreover, this high density of leopards is also a function of local acceptance of the species, a strong legislature that accords them high protection irrespective of land use and the ecological history of the landscape (Kshettry et al., 2020). Studies conducted elsewhere in India have also confirmed leopards (and other big cats) to be part of human imagination and culture and explained how people can share space with this charismatic felid (Dhee et al., 2019; Nair et al., 2021). However, the presence of a large felid in these shared landscapes is not without significant challenges to local lives and livelihoods.
The forest-production landscape mosaic of the Duars region in West Bengal reports one of the highest frequencies of human-leopard negative interaction globally (Kshettry et al., 2020). The tea estates of this region, which leopards use as their habitats, have been reporting incidents of human injuries due to leopards for a long time, and the numbers are increasing (Bhattacharjee & Parthasarathy, 2013; Kshettry et al., 2017). During 1990–1997, there were 121 reported incidents of leopard attacks on humans, whereas, between 2001 and 2008, 243 incidents of humans being injured by leopards were reported from the region (Bhattacharjee & Parthasarathy, 2013). However, during 2009–2018, an average of 56 cases of human injury by leopard per year was reported from the area (Kshettry et al., 2020), and the spatiotemporal pattern of the attacks showed that most of such attacks took place in the tea estates during the dry season, that is, December-April (Kshettry et al., 2017). During this time, the vegetation cover in tea estates is partially lost due to the pruning of the tea bushes, and tea workers carry out intensive work within the small tea-bearing sections where the leopards also rest during the day (Kshettry et al., 2017). Livestock loss has also been high in the landscape, incurring additional costs to local communities (Naha et al., 2020). However, despite heavy losses, the communities have strong cultural and religious reverence towards local wildlife; the people of northern West Bengal still do not practice persecution of leopards and retaliatory killings of leopards are infrequent (Naha et al., 2021).
The high leopard densities in the tea-plantation habitat indicate the tremendous conservation potential of the species in such habitats and present conservation opportunities and challenges. In the case of our study area, the effective sampling area under tea was approximately 416.23 km2, and with an average leopard density of 11.5 individuals per 100 km2, the area could host more than 45 leopards. The region has approximately 1000 km2 under tea and the combined tea plantation area in West Bengal and Assam is more than 4000 km2, which dwarfs the area under formal protection in the region. There are also reports of leopard presence in tea estates of southern India (Navya et al., 2014; Sidhu et al., 2017) and Sri Lanka (Kittle et al., 2012). Similar studies in these regions will also help to assess the potential of tea plantations as conservation landscapes for highly adaptable species like leopards. The conservation potential of such landscapes is immense, and yet they are outside the purview of traditional conservation and management plans (Kshettry et al., 2020). Furthermore, large felids such as jaguars, lions, puma and snow leopards are also known to persist in shared landscapes (Alexander et al., 2016; Devlin et al., 2023; Riley et al., 2021; Schuette et al., 2013; Sharma et al., 2021). Investigating the variability in densities in shared landscapes could throw up similar patterns, thereby underscoring the conservation potential for these species. Our study attempts to take a small step towards highlighting that species conservation plans, even for large carnivores, can consider shared landscapes but need to be planned in a socially just and equitable manner. Balancing conservation goals with local safety and food security can enable space sharing in such Conservation Compatible Landscapes (CCL) and needs to be the primary focus for all relevant stakeholders (Kshettry et al., 2020). The first step towards identifying such landscapes would need the estimation of the population of the species of interest and the social carrying capacity for the species.
5 CONCLUSION
The present study tries to evaluate the potential of a highly populated production landscape in supporting an apex carnivore population. The study is only the second attempt in India after Athreya et al. (2013) to study the density of leopards in a human-dominated landscape and the first ever to study leopard density in a tea-plantation landscape. The study reveals that human-modified landscapes can host significantly higher population densities of certain large carnivores than forests, even the protected areas. However, this pattern does not disregard the importance of protected areas for a host of other biodiversity and ecosystem processes; rather, it highlights the conservation potential of shared landscapes for global carnivore conservation. Nevertheless, the persistence of a large carnivore in a shared landscape with a very high density of humans can lead to negative outcomes for people and wildlife if appropriate safeguards are not instituted. This study highlights the need for evidence-based planning for large carnivore conservation beyond protected area boundaries.
AUTHOR CONTRIBUTIONS
Anish Paul: Conceptualization; data curation; formal analysis; investigation; methodology; software; validation; visualization; writing—original draft; writing—review and editing. Nitish Kumar: Data curation; investigation; methodology. Tonmoy Mukherjee: Data curation; investigation; methodology. Amir Kumar Chhetri: Data curation; investigation; methodology. Aritra Kshettry: Conceptualization; funding acquisition; investigation; methodology; project administration; resources; supervision; validation; writing—review and editing.
ACKNOWLEDGMENTS
We would like to thank West Bengal Forest Department for providing the permit to conduct the exercise within the protected areas. We would like to thank Anshu Yadav (IFS), Mridul Kumar (IFS), and all the staff of Gorumara Wildlife Division and Jalpaiguri Territorial Division for providing the necessary logistic support during the fieldwork. We would like to thank the authorities and residents of all the tea estates where we conducted the study. We would like to thank Centre for Wildlife Studies and Wildlife Conservation Society-India for providing institutional support. Support for this study was provided by Rufford Foundation (Grant no: 33632-D to AK) and Department of Science and Technology, Government of India (INSPIRE Fellowship, Grant no: IF160391 to AK and INSPIRE Scholarship for Higher Education, Registration No: 201700022793 to AP). We would like to thank Priyanka Das, Motahar Rahaman, and Ramesh Mahali for helping with the fieldwork. We thank Dr. Koustubh Sharma and Dr. Devcharan Jathanna for their help with the analysis. We would also like to thank Dr. Varun R Goswami for his comments and suggestions on the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The raw data supporting the conclusions of this article will be made available by the authors upon request from Anish Paul, without undue reservation. Contact: [email protected]




