Development of an Enzymatic Recombinase Amplification Assay for the Rapid Detection of Plasmodium Nucleic Acids
Funding: This work was supported by a National Key Research and Development Plan Project (2021YFC2301801).
Xinxin Yang and Xiaoxue Lu contributed equally to this work and shared the co-first authorship.
ABSTRACT
Background
Malaria remains a global health challenge, with 249 million cases and 608,000 deaths reported in 2022. While China achieved malaria elimination, imported cases surged by 194.4% in 2023, underscoring the need for rapid diagnostics. Traditional methods like microscopy and rapid diagnostic tests (RDTs) face limitations in sensitivity and infrastructure requirements. This study aimed to establish and optimize a “one-pot” enzymatic recombinase amplification (ERA) assay for the molecular detection of Plasmodium falciparum and Plasmodium vivax, and to evaluate the efficacy of this assay through methodological verification and clinical performance.
Methods
We designed a specific ERA assay targeting the conserved regions of P. falciparum and P. vivax genetic material. We evaluated the sensitivity and specificity of this assay using synthetic plasmids and genomic material. Additionally, we tested the stability of the reaction by incorporating potential interfering substances into the reaction system. Finally, we analyzed the detection performance of the ERA method against real-time fluorescent quantitative PCR and rapid diagnostic tests using clinical samples.
Results
The detection process could be completed within 25 min at 35°C–40°C, and the results could be interpreted either under UV light or using a GeneScope instrument. The detection limit of the ERA assay was 250 copies/mL, which was 40 times more sensitive than fluorescent quantitative PCR. When evaluating the clinical performance using 75 clinical specimens, the detection rate of the ERA method was 94.54% compared with 89.09% for fluorescent quantitative PCR. The ERA assay and fluorescent quantitative PCR can achieve positive detection when blood samples were diluted 1024 times or even 4096 times. Comparatively, the detection capabilities of rapid diagnostic tests were significantly lower than that of the ERA assay.
Conclusion
The ERA method shows good performance in the detection of P. falciparum and P. vivax, and can be used as a complementary tool for malaria screening and clinical diagnosis.
Abbreviations
-
- Ct
-
- cycle threshold
-
- ERA
-
- enzymatic recombinase amplification
-
- LOD
-
- the limit of detection
-
- P. falciparum
-
- Plasmodium falciparum
-
- P. vivax
-
- Plasmodium vivax
-
- RDT
-
- rapid diagnostic test
-
- ROC
-
- receiver operating characteristic
-
- RPA
-
- recombinase polymerase amplification
1 Introduction
Malaria, a parasitic disease caused by Plasmodium infection, continues to pose a significant threat to public health worldwide, particularly in tropical and subtropical regions. According to the latest World Malaria Report 2023, there were 249 million malaria infections and 608,000 deaths in 85 countries worldwide in 2022 [1]. Despite the World Health Organization officially declaring that China had eliminated malaria, the threat of imported malaria cases remains a critical concern. For example, in 2023, there were 2488 imported malaria cases, which was a 194.4% increase compared with 2022 (845 cases) [2]. Accurate and rapid diagnosis, followed by prompt and effective treatment with antimalarial drugs, is crucial to preventing recrudescence and re-transmission of malaria in China [3-5].
Microscopy examination has been considered the “gold standard” method for laboratory diagnosis of malaria infection because of its simplicity of operation and cost-effectiveness [6, 7]. However, low-quality blood smears or inexperienced personnel may lead to misdiagnosis or missed diagnoses [8]. Moreover, morphological examination presents challenges in identifying multispecies infections [9]. The rapid diagnostic test (RDT), based on colloidal gold immunochromatography, serves as an adjunct to microscopy examination, offering a rapid and easy diagnosis method for malaria infection, which can be widely used in grass-roots and remote areas [10]. Nevertheless, the RDT has limitations in the detection of low-density Plasmodium infections and mixed infections [9, 10]. Nucleic acid tests, such as nested PCR and fluorescent quantitative PCR, play an important role in malaria detection because of their high sensitivity and specificity [11]. However, the requirement for well-equipped laboratories and trained personnel has hindered their wide-scale implementation in grass-roots areas [12].
To address these problems, several isothermal amplification techniques, such as loop-mediated isothermal amplification (LAMP) [13], recombinase polymerase amplification (RPA) [14], and recombinase-aided amplification have been developed for rapid nucleic acid detection, making it possible to perform on-site surveillance and confirm suspected cases in resource-limited settings [15, 16]. Recently, enzymatic recombinase amplification (ERA) assay, an improved version of the RPA assay developed by GenDx Biotech (Suzhou, China), has been successfully applied for detecting diverse pathogens with high sensitivity and specificity [17-19]. Compared with traditional PCR methods, the ERA assay can rapidly detect low parasite levels at room temperature without the need for specialized instruments. Compared with the RDT, the ERA kit exhibits a higher detection rate for low-concentration samples and serves as a powerful tool for point-of-care testing. However, research is lacking in the application of the ERA assay to identify Plasmodium species.
In this study, we co-developed (with GenDx Biotech) an ERA kit for the rapid on-site detection of Plasmodium falciparum and Plasmodium vivax, the two most common species responsible for Plasmodium infections [20]. The detection process using this kit can be completed within 25 min at a temperature range of 35°C–42°C, and the results are presented as fluorescent signals, which can be directly observed either under UV light or using a GeneScope (a portable, easy-to-use instrument). Crucially, we assessed the clinical diagnostic efficacy of the ERA kit using 75 clinical samples from endemic regions and the kit achieved an accuracy rate of 98% without any false positives. The outstanding performance of the ERA assay shows the great potential for rapid malaria detection, which is invaluable not only for countries with malaria outbreaks but also for those requiring surveillance to monitor imported malaria cases.
2 Materials and Methods
2.1 Experimental Materials
Plasmodium-positive whole blood samples were obtained from patients with suspected malaria fever who were admitted to the Sierra Leone Military Medical Team of the Fifth Medical Center of Chinese PLA General Hospital. The samples had been previously characterized according to clinical symptoms, microscopy examination, and antimalarial treatment. For fluorescent quantitative PCR, febrile patients who responded positively to antimalarial treatment were also included as malaria-positive samples. In addition, whole blood samples from healthy people (based on physical examination) were included as malaria-negative samples. The above blood samples were collected in 2-mL EDTA anticoagulant tubes and 0.5 mL/tube was stored at −80°C. The genomic nucleic acids of Babesia microti, Trypanosoma cruzi, Leishmania donovani, Dengue virus, Cytomegalo virus, and Leptospira were obtained from Suzhou GenDx Biotech Co. Ltd. The genomic nucleic acids of Streptococcus pneumoniae, Staphylococcus aureus, Plasmodium ovale, and Plasmodium malariae were provided by the Department of Clinical Microbiology and Immunology of The Army Medical University. The Plasmodium Nucleic Acid Detection Kit (fluorescent quantitative PCR method) (Cat. No: SKY-8611, Lot No: 24052111/2) was purchased from Shenzhen MabSky Biotech Co. Ltd. The Plasmodium (Pf/Pan) detection kit (colloidal gold method) (Cat. No: XJWF-pan, Lot No: 2701230614) was purchased from Xinjiang Yufu Biotech Co. Ltd. This study was approved by the Ethics Committee of Chinese PLA General Hospital with approval number KY-2022-6-44-1. During the conduct of the research and the writing of the article, we strictly adhered to relevant regulations regarding research ethics. This study did not involve any direct participation of individuals throughout the entire process; therefore, it was not necessary to obtain informed consent.
2.2 The Procedure for the ERA Method
In this study, the primers and probes for the ERA kit were designed based on the conserved 18S rRNA sequence of P. falciparum and P. vivax. The primers, probes, recombinase, single-stranded DNA binding protein, and DNA polymerase were all lyophilized into powder microspheres and placed in a reaction tube. For blood samples, 10 μL of whole blood sample was added to 1 mL of sample lysis buffer in the sample vial of the ERA kit. The sample and buffer were mixed and then incubated for 5 min at room temperature. Then, 40 μL of lysed sample and 10 μL of reconstitution solvent M were added to the reaction tube and incubated at 40°C for 15 min. Next, 50 μL of reconstitution solvent M was added to the reaction tube, which was incubated for another 5 min at 40°C. Finally, the reaction tube was immediately placed into a preheated GD200 isothermal nucleic acid amplifier (GenDx Biotech, Suzhou). A fluorescence value ≥ 1000 was considered positive and a fluorescence value < 1000 was considered negative.
2.3 Preparation of Standard Plasmids
The recombinant standard plasmids containing Plasmodium target sequences were provided by Suzhou GenDx Biotech Co. Ltd. The concentration of the recombinant plasmid was quantified using a NanoDrop One spectrophotometer (Thermo Fisher Scientific, USA). The plasmid copy number was calculated using the following formula: number of copies = (plasmid concentration × 6.02 × 1023)/(length (bp) × 660) as previously reported [21]. To evaluate the ERA assay sensitivity, the recombinant plasmid was diluted 10-fold with sample lysis buffer to acquire a series of concentrations ranging from 1 × 107 to 1 × 102 copies/mL. To evaluate the limit of detection (LOD) of the ERA assay and fluorescent quantitative PCR, the standard plasmids were diluted to 1.0 × 103, 5.0 × 102, 2.5 × 102, 1.0 × 102, 5.0 × 101, and 1.0 × 101 copies/mL. Each dilution was tested with 10 replicates. The LOD was defined as the lowest concentration at which ≥ 95% of replicates showed positive signals.
2.4 Testing the Specificity of the ERA Assay
The genomic nucleic acids of P. falciparum, P. vivax. B. microti, T. cruzi, L. donovani, Dengue virus, Cytomegalo virus, Leptospira, S. pneumoniae, S. aureus, P. ovale, and P. malariae, at a concentration of 1.0 × 107 copies/mL, were, respectively, added to 1 mL of sample processing solution as a template, and were then tested by the ERA method. The assay was repeated three times for each pathogen to verify whether there was any cross-reactivity for the detection of Plasmodium.
2.5 Effect of Interfering Substances on the ERA Assay
Common interfering substances, such as artesunate, budesonide, azithromycin, tobramycin, triglyceride, hemoglobin, bilirubin, and leukocytes, were selected to investigate the effects of interference on the ERA assay. Negative interference verification was conducted by placing different interfering substances into the sample processing solution and positive interference verification was conducted by placing different interfering substances into recombinant plasmids at a concentration of 1.0 × 104 copies/mL. Then, all samples were tested by the ERA assay three times.
2.6 Application of the ERA Assay to Clinical Samples
Nucleic acids (5 μL) extracted from the whole blood of 75 patients with fever and suspected malaria infection were tested by ERA and fluorescent quantitative PCR. Laboratory test results, in conjunction with clinical diagnoses, served as the standard. The receiver operating characteristic (ROC) curve generated by the SPSS 26 software, and the sensitivity and specificity of the ERA method were evaluated by the area under the curve (AUC) with a 95% confidence interval (CI).
2.7 Fluorescent Quantitative PCR
Nucleic acid was extracted from 200 μL of blood according to the instructions of the Easy Pure Viral DNA/RNA kit (TransGen Biotech, Beijing, China). The fluorescent quantitative PCR assay was performed according to the manufacturer’s (MabSky Biotech, Shenzhen, China) protocol with 19.5 μL of PCR reaction solution, 0.5 μL of enzyme solution, and 5 μL of nucleic acid template. The cycling program was set as follows: 50°C for 2 min, 9°C for 3 min, 95°C for 5 s, 55°C for 1 min, for 40 cycles, followed by single-site fluorescence detection at 60°C. A positive result was reported when a distinct amplification curve was observed with a cycle threshold (Ct) value of less than 36. A negative result was indicated when no Ct value was detected or when the Ct value was equal to or greater than 39.
2.8 Rapid Diagnostic Test (RDT)
For the RDT, whole blood (5 μL) was pipetted directly into the center of the sample hole in the spiking area. Then, two drops of lysate were immediately added and the result was read within 30 min. A positive result was indicated by the presence of a distinct color change, typically a visible line or dot in the test region, indicating the presence of the target.
3 Results
3.1 Screening of the Optimal Primer for the ERA Assay
We designed three primer combinations within the conserved 18S RNA regions of P. falciparum and P. vivax. First, we evaluated the amplification efficiency of the three primer combinations using real-time ERA tests. The results showed that each primer pair demonstrated efficient PCR amplification. Among them, primer pair 1(NJTYF1/R1) had the lowest and most stable Ct value (Figure 1a). Then, we tested various concentrations of the primer (0.3, 0.4, 0.5 μM) using a real-time ERA assay. The results showed that concentrations of 0.4 μM each for the ERA forward and reverse primers yielded the best amplification efficiency (Figure 1b). Finally, we verified the specificity of the primer using a positive control plasmid and a negative control (Figure 1c). In summary, the primer pair NJTYF1/R1 was chosen as the optimal primer pair for the ERA method.
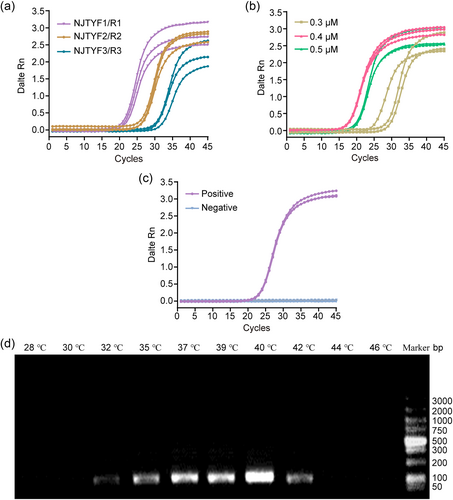
Screening for the optimal primer combinations and reaction temperature for ERA assay. (a) Identification of highly active prime pairs (NJTYF1/R1, NJTYF2/R2, and NJTYF3/R3) using real-time ERA tests. (b) Real-time ERA assay performed with different concentrations of primers (0.3, 0.4, 0.5 μM) to evaluate the optimal concentration of primer sets. (c) Analysis of the specificity of primer (NJTYF1/R1) amplification using a positive control plasmid and a negative control. (d) Agarose gel electrophoresis (1%) of the ERA products obtained at different assay temperatures (28°C–46°C) to analyze the optimal reaction temperature. ERA, enzymatic recombinase amplification.
To determine the optimal reaction temperature, the real-time ERA assay was performed at a series of temperatures (28, 30, 32, 35, 37, 39, 40, 42, 44, and 46°C), and the optimal amplification curve and highest fluorescence value were observed at 40°C (Figure 1d). Thus, we chose 40°C as the optimal reaction temperature for the follow-up experiments.
3.2 Testing the Specificity of the ERA Assay
To investigate the specificity of the ERA assay, which is the primary feature of this detection platform, we used genomic nucleic acids at a concentration 1.0 × 107 copies/mL from other common pathogens (B. microti, T. cruzi, L. donovani, Dengue virus, Cytomegalo virus, Leptospira, S. pneumoniae, S. aureus, P. ovale, P. malariae). All of the common pathogens tested, except for P. falciparum and P. vivax, showed no fluorescence, even P. ovale and P. malariae. This result indicated that the ERA assay for P. falciparum and P. vivax detection has no cross-reactivity with any other common pathogens (Figure 2a,b).
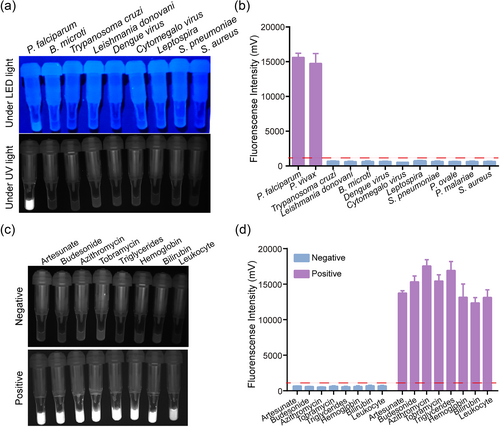
Specificity and stability analysis of ERA assay. (a and b) Specificity analysis of the ERA assay for the detection of other common pathogens. (c and d) Stability analysis of the ERA assay by the addition of interfering substances. ERA, enzymatic recombinase amplification.
Whole blood contains substances that might interfere with the detection capacity of the ERA assay for P. falciparum and P. vivax. These interfering substances can be divided into endogenous interfering substances, such as the various components of blood and genetic material, and exogenous interfering substances, such as drugs. To investigate the stability of the ERA assay, we added substances such as the antimalarial drug artesunate, the anti-inflammatory drug budesonide, antibiotic drugs azithromycin and tobramycin, as well as triglycerides, hemoglobin, bilirubin, and leukocytes to the reaction system. The results indicated that the ERA assay was neither positively nor negatively influenced by these interfering substances (Figure 2c,d).
3.3 The Sensitivity of the ERA Assay
To verify the sensitivity of the ERA assay for P. falciparum and P. vivax detection, synthetic recombinant standard plasmids were serially diluted 10-fold from 1 × 107 to 1 × 102 copies/mL and used as template. As the concentration increased, the fluorescence intensity gradually increased within less than 30 min (Figure 3a,b). Additionally, considering that false negative results were detected when the concentration reached 1 × 102 copies/mL, we further studied the detection rate of the ERA assay at low concentrations of plasmid by diluting the plasmids to 1 × 103, 5 × 102, 2.5 × 102, 1 × 102, 5 × 101, and 1 × 101 copies/mL. The results showed that 100% of the replicates yielded a positive fluorescent signal at a concentration of 2.5 × 102 copies/mL, whereas 80% and 60% of replicates yielded positive signals at concentrations of 1 × 102 and 5 × 101 copies/mL, respectively. The LOD for standard plasmids was therefore 2.5 × 102 copies/mL for the ERA assay, which was 40 times more sensitive than the standard fluorescent quantitative PCR method (Figure 3c,d).
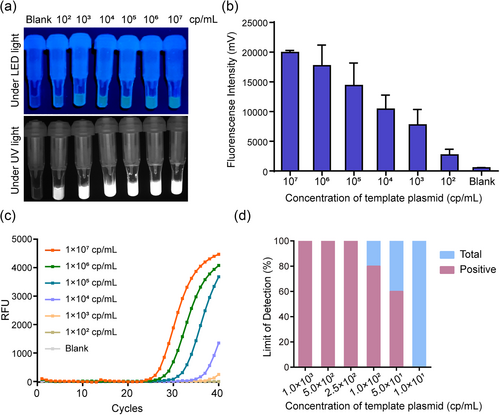
Comparison of the LOD of the ERA assay and fluorescent quantitative PCR. (a and b) Analytical sensitivity of the ERA assay. Blank control was included with RNase-free ddH2O as the template. (c) Analytical sensitivity of fluorescent quantitative PCR. (d) Determination of the LOD of the ERA assay with 10 replicates of different concentrations of plasmid. ERA, enzymatic recombinase amplification; LOD, limit of detection.
3.4 Application of the ERA Assay in Clinical Diagnosis
Next, we tested the applicability of the detection platform for the accurate diagnosis of clinical samples. According to the laboratory test results combined with clinical symptoms, the whole blood samples from 75 patients with fever were classified as malaria-positive (55 patients) or malaria-negative (20 patients). Among them, all the malaria-positive cases were identified as P. falciparum infections. DNA extracted from the whole blood of 75 patients was tested using the ERA method and fluorescent quantitative PCR. As shown in Figure 4a, 52 cases were detected as positive by the ERA method with a detection rate of 94.54%, whereas only 49 cases were detected as positive by fluorescent quantitative PCR with a detection rate of 89.09%. No false positives were detected among the 20 malaria-negative samples by the ERA assay (Figure 4b), which indicated that the specificity and predictive value for this method were 100%.
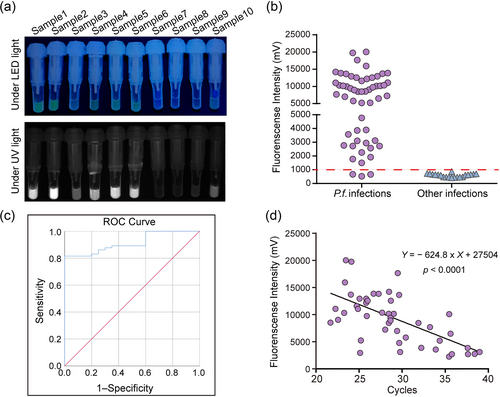
Detection of clinical samples by the ERA assay. (a) Detection of clinical samples infected with Plasmodium falciparum by the ERA assay. (b) The detection results of the ERA assay. (c) ROC curve analysis of the ERA assay. The AUC is 0.915 (95% CI: 0.856–0.973; p < 0.0001). (d) The linear relationship between the ERA assay and fluorescent quantitative PCR. ERA, enzymatic recombinase amplification.
An ROC curve is a graphical representation that illustrates diagnostic ability. We further evaluated the diagnostic performance of the ERA assay for P. falciparum detection by ROC curve analysis. The AUC value for the ERA assay was determined to be 91.5%, with a confidence interval (CI) of 0.85–0.97, indicating a high level of diagnostic accuracy (Figure 4c). In addition, the consistency of the results between the ERA method and fluorescent quantitative PCR was measured by the fluorescence of the ERA assay and the Ct value of the PCR. The results indicated a strong linear relationship between the two methods, confirming highly consistent results (Figure 4d). In summary, the sensitivity and specificity of the ERA assay were comparable to those of the fluorescent quantitative PCR assay, indicating that the newly developed assay has the potential to replace existing detection methods in the field.
3.5 Comparison of the Clinical Performance of the ERA Assay With Fluorescent Quantitative PCR and the RDT
The whole blood samples of three patients with P. falciparum infection were diluted 1∶4, 1∶16, 1∶64, 1∶256, 1∶1024, and 1∶4096, and tested by the ERA assay, RDT, and fluorescent quantitative PCR. The results are shown in Table 1. The original blood samples were all identified as positive using the three methods. However, once the blood samples were diluted, the detection rates of the three methods varied. The ERA assay and fluorescent quantitative PCR exhibited similar detection capabilities, both positively detecting blood samples diluted 1024 times or even 4096 times. However, the detection capability of the RDT was significantly lower than those of the ERA assay and fluorescent quantitative PCR. The strong specificity, repeatability, and stability of the ERA assay confirm it as a promising alternative to the RDT for the rapid field detection of P. falciparum and P. vivax infection and the surveillance of imported malaria cases.
| Blood sample | Dilution factor | ERA | RDT | Fluorescence quantitative PCR | |
|---|---|---|---|---|---|
| Result | Ct value | ||||
| 1 | No dilution | + | + | + | 37.66 |
| 4 | + | + | + | 35.55 | |
| 16 | + | − | + | 36.71 | |
| 64 | + | − | − | 39.61 | |
| 256 | − | − | − | 39.77 | |
| 1024 | − | − | − | N/A | |
| 4096 | − | − | − | N/A | |
| 2 | No dilution | + | + | + | 30.87 |
| 4 | + | + | + | 27.05 | |
| 16 | + | + | + | 29.77 | |
| 64 | + | + | + | 32.37 | |
| 256 | + | − | + | 34.16 | |
| 1024 | + | − | + | 36.94 | |
| 4096 | + | − | + | 38.55 | |
| 3 | No dilution | + | + | + | 29.10 |
| 4 | + | + | + | 29.02 | |
| 16 | + | + | + | 31.13 | |
| 64 | + | + | + | 32.50 | |
| 256 | + | + | + | 35.27 | |
| 1024 | + | − | + | 36.07 | |
| 4096 | − | − | + | 38.55 | |
- Note: “+” indicates positive; “−” indicates negative; N/A indicates no fluorescent signal detected.
- Abbreviations: Ct, cycle threshold; ERA, enzymatic recombinase amplification; RDT, rapid diagnostic test.
4 Discussion
The persistence and spread of imported malaria cases is a major threat to China's biosecurity [22]. Malaria screening is crucial for reducing hotspots of parasite infection and interrupting transmission, especially for infected individuals who have taken anti-malarial drugs and are therefore asymptomatic or low-density carriers with mild disease symptoms [22-24]. At present, the most commonly used methods for the detection of Plasmodium include microscopy, the RDT, and fluorescent quantitative PCR [25, 26]. However, these methods each have their limitations and the development of a convenient, cost-effective, and rapid detection method for early on-site detection of Plasmodium infection is still needed [27, 28]. In this study, we developed and validated an ERA assay for the screening of P. falciparum and P. vivax infection that provides a rapid and accurate tool for malaria diagnosis, epidemiological investigation, and the surveillance of imported cases.
As a novel nucleic acid detection technology, our established ERA assay demonstrated superior performance compared with other methods. After screening and optimization, the lower LOD of the ERA assay was 2.5 × 102 copies/mL, which was at least 40 times more sensitive than fluorescent quantitative PCR (LOD: 1 × 104 copies/mL). Nguyen et al. developed a colorimetric LAMP assay targeting the 18S rRNA gene of P. falciparum with a lower LOD of 0.21 parasites/μL [21]. It has been reported that the 18S rRNA gene is expressed at high levels with approximately 7.4 × 103 copies/parasite [29]. Wei et al. developed a rapid and ultrasensitive detection method for Plasmodium parasites via an RPA-CRISPR/Cas12a platform with a lower LOD of 1 copy/μL (1 × 103 copies/mL) [30]. The sensitivity of the ERA assay was similar to or higher than that of the above methods. Importantly, the results of the ERA assay were not influenced by interfering substances, such as blood or sample lysis buffer, allowing for the direct detection of lysed blood samples without the need for nucleic acid extraction.
Nonetheless, the ERA assay has some limitations. First, the reagents for the ERA assay require low-temperature storage and transportation, with inappropriate storage conditions potentially leading to false negative results. This can be mitigated by freeze-drying to enhance the stability of the reaction reagents. Second, although no false positives were observed in our testing of 75 clinical specimens, the ERA reaction may still potentially yield false positive results due to cross-reactivity. To further enhance the accuracy of the ERA assay, integrating technologies such as a CRISPR/Cas assay may be a viable solution. The CRISPR/Cas assay relies on the non-specific endonuclease activity of Cas13 or Cas12 proteins [31, 32]. It has emerged as an attractive alternative to traditional point-of-care testing methods, but its high cost and time-consuming procedure are limiting factors [17]. Finally, although the ERA assay can be used for rapid screening of the two most common Plasmodium species, it is not able to further distinguish between the two parasites. Our ongoing work focuses on further developing an ERA approach that can differentiate between various malaria parasite species, enabling detailed subtype classification. This may be achieved by designing specific primers for different types of Plasmodium species coupled with microfluidic technology and lateral flow chromatography techniques.
Despite these limitations, we have established an ERA method capable of concurrently detecting P. vivax and P. falciparum within a single reaction. This newly designed method is quick and effective for clinical diagnosis and custom screening of mixed or single Plasmodium species infections. It may therefore enable a rapid response upon identifying positive cases and facilitate subsequent subtype testing, which could significantly minimize resource wastage and increase detection accuracy [33].
Author Contributions
Xinxin Yang: data curation (lead), methodology (equal), visualization (equal), writing – original draft (equal). Xiaoxue Lu: data curation (equal), formal analysis (equal), methodology (equal), visualization (equal), writing – review and editing (lead). Junlian Yang: data curation (supporting), investigation (equal). Wen Xu: data curation (supporting), methodology (supporting), supervision (supporting). Qian Li: data curation (supporting), investigation (supporting). Weiwei Chen: conceptualization (lead), funding acquisition (lead), methodology (equal), project administration (lead), supervision (lead), writing – original draft (equal), writing – review and editing (equal).
Acknowledgments
The authors would like to thank Mr Wenzhu Chen at Suzhou GenDx Biotech Co. Ltd for his technological support and all patients who contributed data and samples in the 34th Military Hospital of Republic of Sierra Leone Armed Forces and the Fifth Medical Center of Chinese PLA General Hospital.
Ethics Statement
This study was approved by the Ethics Committee of Chinese PLA General Hospital with approval number KY-2022-6-44-1. During the conduct of the research and the writing of the article, we strictly adhered to relevant regulations regarding research ethics.
Consent
This study did not involve any direct participation of individuals throughout the entire process; therefore, it was not necessary to obtain informed consent.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The datasets used or analyzed in this study are available from the corresponding author upon reasonable request. The data is not publicly available due to privacy or ethical restrictions.




